Submitted:
18 February 2024
Posted:
19 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
Aims of the study
2. Materials and Methods
Patients’ enrolment
Electrocardiographic screening
Laboratory and instrumental tests
Genetic Testing
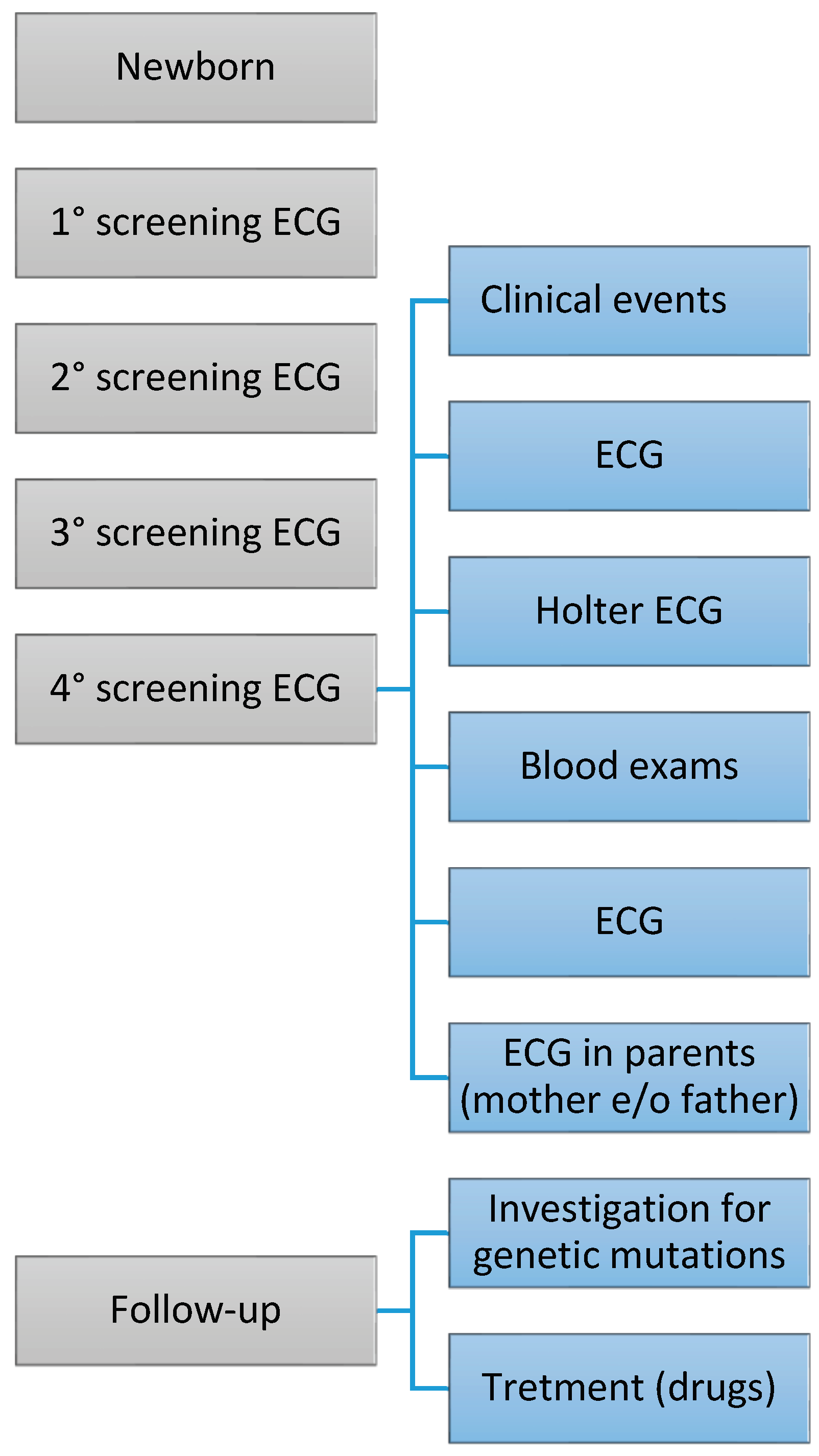
Statistical analysis
3. Results
Electrocardiographic screening
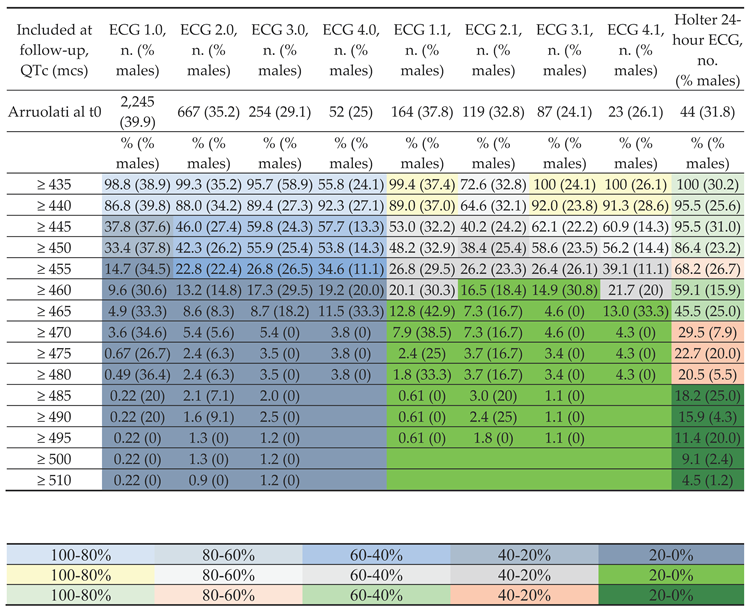 |
| Included at follow-up (QTc, mcs) | ECG 1.0,QTc mean ± S.D. (min-max) mcs | ECG 2.0,QTc mean ± S.D. (min-max) mcs | ECG 3.0,QTc mean ± S.D. (min-max) mcs | ECG 4.0,QTc mean ± S.D. (min-max) mcs | ECG 1.1,QTc mean ± S.D. (min-max) mcs | ECG 2.1,QTc mean ± S.D. (min-max) mcs | ECG 3.1,QTc mean ± S.D. (min-max) mcs | ECG 4.1,QTc mean ± S.D. (min-max) mcs | Holter 24-hour ECG, mean QTc ± S.D. (min-max) mcs |
| Arruolati al t0 | 445.9 ± 9.4 (392-513) | 448.1 ± 12.1 (427-513) | 450.2 ± 12.7 (428-520) | 450.5 ± 11.2 (435-482) | 449.6 ± 11.9(433-513) | 451.7 ± 14.8(435-513) | 450.4 ± 12.1(435-520) | 451.3 ± 11.6(435-482) | 465.7 ± 19.2(430-511) |
| ≥ 435 | 446.1 ± 9.2 | 448.3 ± 12.1 | 450.3 ± 12.6 | 449.9 ± 10.9 | 449.7 ± 11.9 | 451.7 ± 14.8 | 450.4 ± 12.1 | 451.3 ± 11.6 | 466.5 ± 18.6 |
| ≥ 440 | 447.5 ± 9.0 | 449.8 ± 11.9 | 452.0 ± 12.2 | 451.8 ± 10.6 | 451.3 ± 11.5 | 453.6 ± 14.6 | 451.7 ± 11.7 | 452.9 ± 11.0 | 467.1 ± 18.3 |
| ≥ 445 | 455.2 ± 8.7 | 457.5 ± 12.1 | 457.0 ± 12.0 | 458.0 ± 8.7 | 457.9 ± 10.7 | 460.9 ± 14.1 | 456.3 ± 11.7 | 458.5 ± 9.0 | 467.2 ± 18.3 |
| ≥ 450 | 456.3 ± 8.6 | 458.4 ± 12.2) | 457.7 ± 12.2 | 458.8 ± 8.5 | 459.0 ± 10.6 | 461.5 ± 14.1 | 456.9 ± 11.8 | 459.4 ± 8.7 | 469.4 ± 17.8 |
| ≥ 455 | 463.7 ± 8.3 | 465.2 ± 13.3 | 465.6 ± 13.7 | 463.1 ± 7.6 | 465.8 ± 9.8 | 466.7 ± 14.4 | 464.7 ± 14.1 | 463.1 ± 7.8 | 474.1 ± 17.2 |
| ≥ 460 | 467.0 ± 8.6 | 470.8 ± 15.1 | 470 ± 15.4 | 457.2 ± 8.2 | 468.5 ± 10.0 | 472.5 ± 15.7 | 470.4 ± 16.9 | 467.2 ± 8.7 | 476.9 ± 16.7 |
| ≥ 465 | 472.6 ± 8.9 | 479.2 ± 16.2 | 479.3 ± 17.4 | 471.3 ± 8.3 | 472.7 ± 10.3 | 484.7 ± 16.5 | 489.5 ± 20.7 | (466-482) | 481.6 ± 16.4 |
| ≥ 470 | 475.0 ± 9.3 | 483.7 ± 16.4 | 488.5 ± 17.4 | 482 | 476.9 ± 11.3 | 484.7 ± 16.5 | 489.5 ± 20.7 | 482 | 489.9 ± 14.4 |
| ≥ 475 | 488.4 ± 15.7 | 499.1 ± 13.3 | 495.4 ± 16.7 | 482 | 487.3 ± 17.3 | 497.5 ± 14.2 | (482-520) | 482 | 495.1 ± 12.2 |
| ≥ 480 | 492.9 ± 16.2 | 499.1 ± 13.3 | 495.4 ± 16.7 | 482 | 513 | 497.5 ± 14.2 | 520 | 482 | 497.3 ± 10.5 |
| ≥ 485 | 508.4 ± 10.3 | 501 ± 11.8 | 506 ± 15.2 | 513 | 501 ± 12.7 | 520 | 499.5 ± 8.8 | ||
| ≥ 490 | 508.4 ± 10.3 | 506.4 ± 8.6 | 506 ± 15.2 | 513 | 505 ± 10.4 | 520 | 501.6 ± 7.1 | ||
| ≥ 495 | 513 | 510 ± 3.1 | (511-520) | 513 | (506-513) | 520 | 504.6 ± 5.9 | ||
| ≥ 500 | 513 | 510 ± 3.1 | (511-520) | 506.0 ± 5.8 | |||||
| ≥ 510 | 513 | 512.0 ± 1.1 | (511-520) | 511 |
Genetic investigation
| N | Mean ± SD | Minimum – maximum | |
| ECG 1.1 QTc msec | 27 | 452,5 ±13,1 | 435 – 476 |
| ECG 2.1 QTc msec | 25 | 458,5 ±19,1 | 435 – 511 |
| ECG 3,1 QTc msec | 21 | 462,2 ±20,9 | 441 – 520 |
| ECG 4.1 QTc msec | 12 | 457,0 ±11,2 | 442 – 482 |
| ECG Holter, QTc msec | 18 | 452,9 ±26,2 | 417 – 538 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krous, H.F.; Byard, R.W.; Rognum, T.O. Pathology research into sudden infant death syndrome: where do we go from here? Pediatrics 2004, 114, 492–494. [Google Scholar] [CrossRef]
- Moon, R.Y. SIDS and Other Sleep-Related Infant Deaths: Evidence Base for 2016 Updated Recommendations for a Safe Infant Sleeping Environment. Pediatrics 2016, 138. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, G.F.; Oddy, L.; Holcroft, C.A.; Abenhaim, H.A. Incidence and determinants of sudden infant death syndrome: a population-based study on 37 million births. World J Pediatr 2015, 11, 41–47. [Google Scholar] [CrossRef]
- Haas, E.A. Sudden Unexplained Death in Childhood: An Overview. In SIDS Sudden Infant and Early Childhood Death: The Past, the Present and the Future, Duncan, J.R., Byard, R.W., Eds.; University of Adelaide Press © 2018 The Contributors, with the exception of which is by Federal United States employees and is therefore in the public domain.: Adelaide (AU), 2018.
- Hauck, F.R.; Hunt, C.E. Sudden infant death syndrome in 2000. Current Problems in Pediatrics 2000, 30, 237–261. [Google Scholar] [CrossRef]
- Garcia, A.J., 3rd; Koschnitzky, J.E.; Ramirez, J.M. The physiological determinants of sudden infant death syndrome. Respir Physiol Neurobiol 2013, 189, 288–300. [Google Scholar] [CrossRef]
- Opdal, S.H.; Rognum, T.O. Gene variants predisposing to SIDS: current knowledge. Forensic Sci Med Pathol 2011, 7, 26–36. [Google Scholar] [CrossRef]
- Tfelt-Hansen, J.; Winkel, B.G.; Grunnet, M.; Jespersen, T. Cardiac channelopathies and sudden infant death syndrome. Cardiology 2011, 119, 21–33. [Google Scholar] [CrossRef]
- Fifer, W.P.; Fingers, S.T.; Youngman, M.; Gomez-Gribben, E.; Myers, M.M. Effects of alcohol and smoking during pregnancy on infant autonomic control. Dev Psychobiol 2009, 51, 234–242. [Google Scholar] [CrossRef]
- Richardson, H.L.; Walker, A.M.; Horne, R.S. Maternal smoking impairs arousal patterns in sleeping infants. Sleep 2009, 32, 515–521. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Task Force on Sudden Infant Death, S. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics 2005, 116, 1245-1255. [CrossRef]
- Carlin, R.F.; Moon, R.Y. Risk Factors, Protective Factors, and Current Recommendations to Reduce Sudden Infant Death Syndrome: A Review. JAMA Pediatr 2017, 171, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.S.; Mitchell, E.A.; Heckstall-Smith, E.M.A.; Fleming, P.J. Head covering - a major modifiable risk factor for sudden infant death syndrome: a systematic review. Archives of Disease in Childhood 2008, 93, 778–783. [Google Scholar] [CrossRef]
- Hauck, F.R.; Thompson, J.M.; Tanabe, K.O.; Moon, R.Y.; Vennemann, M.M. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics 2011, 128, 103–110. [Google Scholar] [CrossRef]
- Yiallourou, S.R.; Poole, H.; Prathivadi, P.; Odoi, A.; Wong, F.Y.; Horne, R.S. The effects of dummy/pacifier use on infant blood pressure and autonomic activity during sleep. Sleep Med 2014, 15, 1508–1516. [Google Scholar] [CrossRef]
- Weese-Mayer, D.E.; Berry-Kravis, E.M. Genetics of congenital central hypoventilation syndrome: lessons from a seemingly orphan disease. Am J Respir Crit Care Med 2004, 170, 16–21. [Google Scholar] [CrossRef]
- Getahun, D.; Amre, D.; Rhoads, G.G.; Demissie, K. Maternal and obstetric risk factors for sudden infant death syndrome in the United States. Obstet Gynecol 2004, 103, 646–652. [Google Scholar] [CrossRef]
- Highet, A.R. An infectious aetiology of sudden infant death syndrome. Journal of Applied Microbiology 2008, 105, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Pryce, J.W.; Weber, M.A.; Heales, S.; Malone, M.; Sebire, N.J. Tandem mass spectrometry findings at autopsy for detection of metabolic disease in infant deaths: postmortem changes and confounding factors. Journal of Clinical Pathology 2011, 64, 1005–1009. [Google Scholar] [CrossRef]
- Jaeggi, E.; Öhman, A. Fetal and Neonatal Arrhythmias. Clin Perinatol 2016, 43, 99–112. [Google Scholar] [CrossRef]
- Mazzanti, A.; Kanthan, A.; Monteforte, N.; Memmi, M.; Bloise, R.; Novelli, V.; Miceli, C.; O’Rourke, S.; Borio, G.; Zienciuk-Krajka, A.; et al. Novel insight into the natural history of short QT syndrome. J Am Coll Cardiol 2014, 63, 1300–1308. [Google Scholar] [CrossRef]
- Liebrechts-Akkerman, G.; Liu, F.; van Marion, R.; Dinjens, W.N.M.; Kayser, M. Explaining sudden infant death with cardiac arrhythmias: Complete exon sequencing of nine cardiac arrhythmia genes in Dutch SIDS cases highlights new and known DNA variants. Forensic Sci Int Genet 2020, 46, 102266. [Google Scholar] [CrossRef]
- Sarquella-Brugada, G.; Campuzano, O.; Cesar, S.; Iglesias, A.; Fernandez, A.; Brugada, J.; Brugada, R. Sudden infant death syndrome caused by cardiac arrhythmias: only a matter of genes encoding ion channels? Int J Legal Med 2016, 130, 415–420. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Priori, S.G.; Dumaine, R.; Napolitano, C.; Antzelevitch, C.; Stramba-Badiale, M.; Richard, T.A.; Berti, M.R.; Bloise, R. A molecular link between the sudden infant death syndrome and the long-QT syndrome. The New England Journal of Medicine 2000, 343, 262–267. [Google Scholar] [CrossRef]
- Aktaa, S.; Tzeis, S.; Gale, C.P.; Ackerman, M.J.; Arbelo, E.; Behr, E.R.; Crotti, L.; d’Avila, A.; de Chillou, C.; Deneke, T.; et al. European Society of Cardiology quality indicators for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2023, 25, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Stramba-Badiale, M.; Segantini, A.; Austoni, P.; Bosi, G.; Giorgetti, R.; Grancini, F.; Marni, E.D.; Perticone, F.; Rosti, D.; et al. Prolongation of the QT interval and the sudden infant death syndrome. The New England Journal of Medicine 1998, 338, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Arnestad, M.; Crotti, L.; Rognum, T.O.; Insolia, R.; Pedrazzini, M.; Ferrandi, C.; Vege, A.; Wang, D.W.; Rhodes, T.E.; George, A.L., Jr.; et al. Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation 2007, 115, 361–367. [Google Scholar] [CrossRef]
- Zareba, W. Genotype-specific ECG patterns in long QT syndrome. J Electrocardiol 2006, 39, S101–106. [Google Scholar] [CrossRef]
- Tester, D.J.; Benton, A.J.; Train, L.; Deal, B.; Baudhuin, L.M.; Ackerman, M.J. Prevalence and spectrum of large deletions or duplications in the major long QT syndrome-susceptibility genes and implications for long QT syndrome genetic testing. Am J Cardiol 2010, 106, 1124–1128. [Google Scholar] [CrossRef]
- Ackerman, M.J.; Priori, S.G.; Willems, S.; Berul, C.; Brugada, R.; Calkins, H.; Camm, A.J.; Ellinor, P.T.; Gollob, M.; Hamilton, R.; et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Europace 2011, 13, 1077–1109. [Google Scholar] [CrossRef]
- Johannsen, E.B.; Baughn, L.B.; Sharma, N.; Zjacic, N.; Pirooznia, M.; Elhaik, E. The Genetics of Sudden Infant Death Syndrome-Towards a Gene Reference Resource. Genes (Basel) 2021, 12. [Google Scholar] [CrossRef]
- Dai, Y.; Yin, R.; Yang, L.; Li, Z.H. Clinical and genetic spectrum of neonatal arrhythmia in a NICU. Transl Pediatr 2021, 10, 2432–2438. [Google Scholar] [CrossRef]
- van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011, 58, 2241–2247. [Google Scholar] [CrossRef]
- Wu, W.; He, J.; Shao, X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990-2017. Medicine (Baltimore) 2020, 99, e20593. [Google Scholar] [CrossRef] [PubMed]
- McKenna, W.J.; Judge, D.P. Epidemiology of the inherited cardiomyopathies. Nat Rev Cardiol 2021, 18, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Ioakeimidis, N.S.; Papamitsou, T.; Meditskou, S.; Iakovidou-Kritsi, Z. Sudden infant death syndrome due to long QT syndrome: a brief review of the genetic substrate and prevalence. J Biol Res (Thessalon) 2017, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Stramba-Badiale, M.; Crotti, L.; Pedrazzini, M.; Besana, A.; Bosi, G.; Gabbarini, F.; Goulene, K.; Insolia, R.; Mannarino, S.; et al. Prevalence of the congenital long-QT syndrome. Circulation 2009, 120, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Quaglini, S.; Rognoni, C.; Spazzolini, C.; Priori, S.G.; Mannarino, S.; Schwartz, P.J. Cost-effectiveness of neonatal ECG screening for the long QT syndrome. Eur Heart J 2006, 27, 1824–1832. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Garson, A.; Paul, T.; Stramba-Badiale, M.; Vetter, V.L.; Wren, C.; European Society of, C. Guidelines for the interpretation of the neonatal electrocardiogram. A task force of the European Society of Cardiology. European Heart Journal 2002, 23, 1329–1344. [Google Scholar] [CrossRef]
- Schwartz, A.R.; O’Donnell, C.P.; Baron, J.; Schubert, N.; Alam, D.; Samadi, S.D.; Smith, P.L. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med 1998, 157, 1051–1057. [Google Scholar] [CrossRef]
- Ran, L.; Li, J.; Bao, L.; Chen, L. Association Between Neonatal Arrhythmia and Mortality and Recurrence: A Retrospective Study. Front Pediatr 2022, 10, 818164. [Google Scholar] [CrossRef]
- Mishra, V.; Zaidi, S.; Axiaq, A.; Harky, A. Sudden cardiac death in children with congenital heart disease: a critical review of the literature. Cardiol Young 2020, 30, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Ariagno, R.; Coons, S.; Winkle, R.; Korobkin, R.; Baldwin, R.; Souquet, M. Near-miss sudden infant death syndrome in eight infants with sleep apnea-related cardiac arrhythmias. Pediatrics 1985, 76, 236–242. [Google Scholar]
- Southall, D.P.; Richards, J.M.; Stebbens, V.; Wilson, A.J.; Taylor, V.; Alexander, J.R. Cardiorespiratory function in 16 full-term infants with sudden infant death syndrome. Pediatrics 1986, 78, 787–796. [Google Scholar] [CrossRef]
- Goldwater, P.N. A perspective on SIDS pathogenesis. the hypotheses: plausibility and evidence. BMC Med 2011, 9, 64. [Google Scholar] [CrossRef]
- Filiano, J.J.; Kinney, H.C. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biology of the Neonate 1994, 65, 194–197. [Google Scholar] [CrossRef]
- Schwartz, J.R.; Roth, T. Neurophysiology of sleep and wakefulness: basic science and clinical implications. Curr Neuropharmacol 2008, 6, 367–378. [Google Scholar] [CrossRef]
- Kline, J.; Costantini, O. Inherited Cardiac Arrhythmias and Channelopathies. Med Clin North Am 2019, 103, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Groswasser, J.; Scaillet, S.; Lanquart, J.P.; Benatar, A.; Sastre, J.P.; Chevalier, P.; Kugener, B.; Kahn, A.; Lin, J.S. QT interval prolongation in future SIDS victims: a polysomnographic study. Sleep 2008, 31, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Leren, I.S. Prevalence, Clinical Presentation, and Management of Channelopathies and Cardiomyopathies, Long QT Syndrome, Brugada Syndrome, Arrhythmogenic Cardiomyopathy, and Hypertrophic Cardiomyopathy. Current Cardiovascular Risk Reports 2019, 13, 16 %U. [Google Scholar] [CrossRef]
- Priori, S.G.; Schwartz, P.J.; Napolitano, C.; Bloise, R.; Ronchetti, E.; Grillo, M.; Vicentini, A.; Spazzolini, C.; Nastoli, J.; Bottelli, G.; et al. Risk stratification in the long-QT syndrome. N Engl J Med 2003, 348, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Gosselin-Badaroudine, P.; Moreau, A.; Chahine, M. Nav 1.5 mutations linked to dilated cardiomyopathy phenotypes: Is the gating pore current the missing link? Channels (Austin) 2014, 8, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Zaklyazminskaya, E.; Dzemeshkevich, S. The role of mutations in the SCN5A gene in cardiomyopathies. Biochim Biophys Acta 2016, 1863, 1799–1805. [Google Scholar] [CrossRef]
- Moore, J.P.; Gallotti, R.G.; Shannon, K.M.; Bos, J.M.; Sadeghi, E.; Strasburger, J.F.; Wakai, R.T.; Horigome, H.; Clur, S.A.; Hill, A.C.; et al. Genotype Predicts Outcomes in Fetuses and Neonates With Severe Congenital Long QT Syndrome. JACC Clin Electrophysiol 2020, 6, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Novelli, V.; Amin, A.S.; Abiusi, E.; Care, M.; Nannenberg, E.A.; Feilotter, H.; Amenta, S.; Mazza, D.; Bikker, H.; et al. An International, Multicentered, Evidence-Based Reappraisal of Genes Reported to Cause Congenital Long QT Syndrome. Circulation 2020, 141, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Alders, M.; Bikker, H.; Christiaans, I. Long QT Syndrome. In GeneReviews(®), Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington, Seattle. Copyright © 1993-2023, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.: Seattle (WA), 1993.
- Kondo, M.; Ohishi, A.; Baba, T.; Fujita, T.; Iijima, S. Can echocardiographic screening in the early days of life detect critical congenital heart disease among apparently healthy newborns? BMC Pediatr 2018, 18, 359. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J Am Coll Cardiol 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Dawson, A.L.; Cassell, C.H.; Riehle-Colarusso, T.; Grosse, S.D.; Tanner, J.P.; Kirby, R.S.; Watkins, S.M.; Correia, J.A.; Olney, R.S. Factors associated with late detection of critical congenital heart disease in newborns. Pediatrics 2013, 132, e604–611. [Google Scholar] [CrossRef]
- Li, J.J.; Liu, Y.; Xie, S.Y.; Zhao, G.D.; Dai, T.; Chen, H.; Mu, L.F.; Qi, H.Y.; Li, J. Newborn screening for congenital heart disease using echocardiography and follow-up at high altitude in China. Int J Cardiol 2019, 274, 106–112. [Google Scholar] [CrossRef]
| SEX | MUTATION | Familiarity | QTc msc (ECG) | QTc msec (ECG Holter) | Therapy | Dosage | SEX | QTc msc (ECG) | QTc msec (ECG holter) | Therapy | Dosage |
| F | SCN5A (LQT3; c.647C>T) | No | 427 | 438 | Propranolol | 2 mg/kg, 3 vv/die | F | 468 | 460 | Propranolol | 2 mg/kg, 2 vv/die |
| F | KCNH2 (LQT2) | Father | 458 | 461 | Inderal | 20 mg | F | 402 | 441 | Propranolol | 2 mg/kg, 1 v /die |
| F | KCNH2 (LQT2; c.1196C>T) | No | 438 + PR corto | - | - | - | F | 413 | 449 | Propranolol | 2 mg/kg, 3 vv/die |
| F | KCNH2 (LQT2; c.3367G>C) | No | 452 | 462 | Propranolol | 3 mg/kg, 3 vv/die | M | 433 | 440 | Propranolol | 2 mg/kg, 3 vv/die |
| F | KCNH2 (LQT2; c.2560T>G), SCN5A (LQT3; c.5845G>A) | No | - | - | - | F | 494 | 537 | Nadolol | ¾ cp (40 mg), 3 vv/die | |
| F | KCNQ1 (polymorphism SCN5A-H558R, KCNH2-K897K, e KCNE1-S38G) | No | 448 | 465 | Propranolol | 2 mg/kg, 3 vv/die | F | EXTENDED | EXTENDED | Metoprolol | 8 mg, 2 vv/die |
| F | KCNQ1 (LQT1) | Mother | ND | ND | Nadolol | 1,5 mg/Kg/die | F | EXTENDED | EXTENDED | Metoprolol | 8 mg, 2 vv/die |
| F | KCNQ1 (LQT1) | Mom and maternal grandfather | ND | ND | - | - | |||||
| M | KCNQ1 (LQT1) | Mom and sister | 434 | 465 | Inderal | ¾ + ½ + ½ | |||||
| M* | KCNQ1 (LQT1) | Father (asymptomatic, QTc in the norm) | ND | ND | Nadolol | 1 mg/Kg, 1 v/die | |||||
| M* | KCNQ1 (LQT1) | Father (asymptomatic QTc in the norm) | ND | ND | Nadolol | 1 mg/Kg, 1 v/die |
| SINGLE ECG ABNORMALITY | Patients, No. (Partial %) | MULTIPLE ECG ABNORMALITY | Patients, No. (Partial %) |
| Right bundle branch focal block | 983 (54.5) | Right Bundle Branch Focal Block + Right Ventricular Prevalence | 61 |
| Left axial deviation | 166 (9.2) | Right Bundle Branch Focal Block + Left Axial Deviation | 26 |
| Nonspecific abnormalities of ventricular repolarization | 128 (7.1) | Right bundle branch focal block + Non-specific alterations of repolarization | 8 |
| Ventricular extrasystole | 83 (4.6) | Right Bundle Branch Focal Block + Supraventricular Extrasystole | 6 |
| Supraventricular extrasystole | 82 (4.5) | Right Bundle Branch Focal Block + Ventricular Extrasystole | 6 |
| Complete right bundle branch block | 66 (3.7) | Right bundle branch focal block + High voltages of QRS | 5 |
| High Voltage QRS | 46 | Right Bundle Branch Focal Block + PQ at Upper Limits | 5 |
| Ventricular pre-excitation | 32 | Right bundle branch focal block + negative T wave | 2 |
| Increased P wave amplitude | 28 | Right Bundle Branch Focal Block + Inf Q Waves | 2 |
| FP at Upper Limits | 27 | Right Bundle Branch Focal Block + Right Ventricular Head + Left Axial Deviation | 2 |
| Low QRS voltages | 20 | Right bundle branch focal block + Sinus tachycardia | 2 |
| Tachycardia sinusale | 20 | Blocco focale di branca dx + Deviazione assiale sx + Extrasistolia sopraventricolare | 1 |
| Bradicardia sinusale relativa | 18 | Right Bundle Branch Focal Block + Supraventricular Extrasystole + Ventricular Extrasystole | 1 |
| FP at Lower Limits | 16 | Right bundle branch focal block + Ventricular parasystole | 1 |
| AV conduction at the sup limits | 10 | Right Bundle Branch Focal Block + Right Ventricular Prevalence + PQ at Lower Limits | 1 |
| Positive T | 10 | Right Branch Focal Block + Migrant Steplight | 1 |
| Ectopic atrial rhythm | 9 | Right bundle branch focal block + Right ventricular and atrial prevalence | 1 |
| Positive T-wave | 8 (97.1) | Right Bundle Branch Focal Block + Right Ventricular Prevalence + PQ at Upper Limits | 1 |
| Migrant step marker | 8 | TOTAL Right bundle branch focal block | 132 (66.7%) |
| Right Axial Deviation | 7 | Right ventricular head + left axial deviation | 17 |
| Left front hemiblock | 5 | Right Ventricular Head + Deep Q Waves | 7 |
| Delayed right intraventricular conduction | 4 | Right ventricular prevalence + PR at the experimental limits | 6 |
| Respiratory sinus arrhythmia | 3 | Right Ventricular Head + High QRS Voltages | 4 |
| ST Elevation | 3 | Right Ventricular Prevalence + Non-Specific Abnormalities of Ventricular Repolarization | 4 |
| Negative T | 3 | Right Ventricular Prevalence + Ventricular Extrasystole | 4 |
| Dextrocardia | 2 | Right Ventricular Head + Low QRS Voltages | 4 |
| QTc and PQ at the lower limits | 2 | Right Ventricular Prevalence + Nonspecific Abnormalities of Ventricular Repolarization | 3 |
| Ventricular hyperkinetic arrhythmia | 1 | Right Ventricular Prevalence + Supraventricular Extrasystole | 3 |
| Appearance S1-Q3 | 1 | Right ventricular prevalence + PQ at upper limits | 2 |
| A-V Dissociation | 1 | Right Ventricular Head + Appearance S1-Q3 | 1 |
| Right atrial engagement | 1 | Right Ventricular Prevalence + AV Conduction at Upper Limits | 1 |
| Increased amplitude P waves | 1 | TOTAL Right ventricular prevalence | 56 (28.3%) |
| Septal Q waves | 1 | Supraventricular Extrasystole + Ventricular Extrasystole | 7 |
| Flat T Waves | 1 | Ventricular Pre-Excitation + Right Bundle Branch Focal Block | 2 |
| AV Conduction Extension | 1 | Ventricular Pre-Excitation + Right Ventricular Head | 2 |
| qR in inferolateral site | 1 (2.5) | TOTAL Ventricular pre-excitation | 4 (2%) |
| Biventricular overload s. | 1 | Left Axial Deviation + Sinus Tachycardia | 1 |
| Signs of bi-atrial engagement | 1 | Left front hemiblock + Left axial deviation | 1 |
| Right Overload | 1 | Biventricular Hypertrophy + Supraventricular Extrasystole | 1 |
| Right ventricular overload | 1 | PQ at Lower Limits + Supraventricular Extrasystole | 1 |
| Diphasic T | 2 | FP at Lower Limits + Probable Junctional Rhythm in Migrant Stepper | 1 |
| Paroxysmal supraventricular tachycardia | 1 | Ventricular Pre-Excitation + Supraventricular Extrasystoa | 1 |
| Total | 1805 | Migrant Steplight + Supraventricular Extrasystole | 1 |
| TOTAL | 198 | ||
| Structural alteration of the heart associated with ECG abnormalities | Patients, n. (%) | Multiple structural alterations of the heart associated with a prolongation of the QTc interval | Patients, n. (%) |
| PFO | 164 (61.4) | PFO | 72 (62.1) |
| PFO / DIA OS | 25 (9.4) | PFO + DIV | 6 (5.2) |
| PFO + Mitral insufficiency | 21 (7.9) | PFO + PDA | 4 (3.4) |
| PFO + PDA | 12 (4.5) | PFO / DIA OS | 3 |
| PFO + DIV | 6 | PFO + Mitral insufficiency | 3 |
| PFO / DIA OS + Mitral insufficiency | 3 | PFO + Aortic insufficiency | 1 |
| PFO + PDA + Mitral insufficiency | 3 | PFO + Tricuspid insufficiency | 1 |
| PFO + Aortic insufficiency | 2 | DIA OS | 12 (10.3) |
| PFO / DIA OS + DIV | 1 | DIA OS + IM | 1 |
| PFO / DIA OS + PDA | 1 | DIA OS + Itric | 1 |
| PFO + Aortic Coarctation + Mitral Insufficiency | 1 | DIA OS + Aortic dysplasia | 1 |
| PFO + Tricuspid insufficiency | 1 | PDA | 4 |
| PFO + Flow acceleration at the level of the aortic isthmus without obstructive gradient + Mitral insufficiency | 2 | DIV | 3 |
| Mitral insufficiency | 12 (4.5) | Mitral insufficiency | 2 |
| DIA OS | 2 | Aortic Insufficiency | 1 |
| DIA OS + Tricuspid insufficiency | 2 | Pulmonary stenosis | 1 (11) |
| PDA | 2 | TOTAL | 125 |
| PDA + Mitral insufficiency | 2 | ||
| DIV | 1 | ||
| Pulmonary insufficiency | 1 | ||
| Tricuspid insufficiency | 1 | ||
| Mild aortic insufficiency in apparently tricuspid valve | 1 | ||
| Multiple ventricular echodense neoformations, referable in the first hypothesis to rhabdomyomas | 1 | ||
| TOTAL | 267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





