1. Introduction
Angiogenesis is the process in which new blood vessels are formed from pre-existing vessels. It is crucial in many physiological contexts, such as in wound healing, and as an adaptive response to hypoxia and ischemia [
1]. However, imbalance in angiogenic regulation can cause deleterious effects, as it accelerates inflammation-driven pathologies, as seen in atherosclerosis and cancer [
2,
3]. New vessels provide additional conduits for the delivery of inflammatory cells and cytokines that promote atherosclerotic plaque development and rupture. They also deliver the oxygen and nutrients necessary to sustain tumor growth and serve as potential routes for metastatic spread [
4].
Angiogenesis-associated conditions are highly prevalent globally, with cardiovascular disease (CVD) and cancer among the leading causes of morbidity and mortality worldwide. Current anti-angiogenic agents are limited, as they can interfere with physiological angiogenic processes, while pro-angiogenic therapies can potentially exacerbate chronic inflammation and inadvertently precipitate tumorigenesis [
5,
6]. Given the critical role of angiogenesis across such diverse pathologies, any agent capable of differentially modulating angiogenesis in a context-specific manner would be of great therapeutic value.
We previously identified a novel angiogenic role for tripartite motif-containing protein 2 (TRIM2) [
7]. Lentiviral short hairpin (sh)RNA knockdown of TRIM2 impaired endothelial cell tubule formation in both hypoxia and inflammatory conditions
in vitro [
7]. We have also shown that TRIM2 knockdown attenuates the ability of HCAECs to migrate and proliferate in response to hypoxic and inflammatory stimuli. However, whether these effects are translated
in vivo, and the molecular mechanisms underlying these, remains unknown.
In this study, we used CRISPR/Cas9-generated homozygous Trim2 null (Trim2–/–) mice to evaluate the functional importance of TRIM2 in two well-validated models of pathological inflammation-driven angiogenesis and physiological hypoxia-mediated, namely the periarterial cuff and hindlimb ischemia models respectively. In Trim2–/– mice, we report markedly attenuated infiltration of adventitial macrophages in response to femoral artery cuff placement, when compared to wildtype (WT) littermates concomitant with a reduction in mRNA levels of the macrophage marker cluster of differentiation 68 (Cd68). Mechanistically, we show that TRIM2 knockdown in human coronary artery endothelial cells (HCAECs) attenuates the induction of key mediators involved in the classical inflammation-driven angiogenic signalling pathway, including nuclear translocation of hypoxia-inducible factor (HIF)-1α and phosphorylation of downstream mediator endothelial nitric oxide synthase (eNOS).
In contrast, we find no significant differences in blood flow reperfusion despite a reduction in proliferating neovessels and arterioles in the ischemic hindlimbs of Trim2–/– and WT mice. In vitro, while the hypoxia-mediated induction of HIF-1α was tempered by TRIM2 knockdown, further downstream activation of angiogenic signalling proteins were unaffected. These findings collectively highlight a novel role for TRIM2 in the regulation of inflammation-driven angiogenesis and delineate the mechanistic basis for these effects. We propose TRIM2 to be a potential therapeutic target for diseases driven by pathological angiogenesis, unlimited by the usual adverse effects associated with inhibiting physiological angiogenesis.
3. Discussion
Dysregulated angiogenesis crucially underpins a wide range of chronic and debilitating diseases including atherosclerotic CVD and cancer. TRIM2 has emerged as a promising novel target that may differentially modulate both inflammation-driven pathological angiogenesis and hypoxia-stimulated physiological angiogenesis, as our previous studies have demonstrated impaired endothelial tubule formation in HCAECs with TRIM2 knockdown
in vitro [
7]. Here, we report markedly reduced adventitial macrophage infiltration following
Trim2 deletion in a murine periarterial cuff model of inflammation-driven angiogenesis, concomitant with reduced proliferating adventitial neovessels and attenuated the induction of the inflammatory response. Correspondingly, we find that TRIM2 knockdown in HCAECs suppresses the TNFα-driven induction of several classical angiogenic mediators, particularly nuclear HIF-1α and reduced activation of eNOS angiogenic signalling pathway.
Trim2 deletion, however, did not alter the capacity for blood flow reperfusion nor the extent of neovascularization in the murine hindlimb ischemia model despite a reduction in proliferating neovessels and arterioles. While TRIM2 knockdown
in vitro suppressed the hypoxia-driven stimulation of nuclear HIF-1α, it did not affect downstream expression and activation of pro-angiogenic signalling pathways.
Our findings, particularly from the periarterial cuff model and the mechanistic studies, are consistent with our previous work showing inhibition of inflammation-induced endothelial tubule formation after TRIM2 knockdown [
7]. The reduction of CD68
+ macrophage infiltration into the inflamed arteries of
Trim2–/– mice suggests that TRIM2 may be involved in broader mechanisms of inflammatory activation, which enhance vessel growth by stimulating a wealth of pro-angiogenic growth factors and mediators [
8]. The concomitant reduction in
Cd68 mRNA levels in the cuffed arteries of
Trim2–/– mice further support the idea that TRIM2 plays a key role in regulating inflammation-driven pathological angiogenesis, particularly in the early stages of macrophage recruitment to the site of injury. The reduction in proliferating adventitial neovessels in the cuffed arteries of
Trim2–/– mice is reflective of this. This was associated with a trend towards reduced total neovessels. Had the study duration been extended beyond 3 weeks, it could be hypothesized that a larger reduction in adventitial neovessels would be observed, as the blunted macrophage response would lead to fewer pro-angiogenic factors being released. No differences were observed in the presence of CD34
+ endothelial tip cells. The intima-to-media ratio, though, which assesses the formation of a thickened neointima as an ‘outside-in’ response to adventitial inflammation, was not affected by
Trim2 deletion, indicating that the reduction in adventitial neovessels was a specific effect on angiogenesis and not a consequence of the development of a smaller neointimal or media. Furthermore, recent clinical studies have identified TRIM2 as a potential oncogene in human cancer cell lines including colorectal carcinoma, epithelial ovarian carcinoma, and osteosarcoma [
9,
10,
11]. These observations fit with a postulated role for TRIM2 in inflammation-driven angiogenesis, a hallmark of cancer development and progression.
Our findings also suggest a potential mechanistic pathway by which TRIM2 may be directing angiogenic responses to inflammation. Direct comparisons of TNFα-stimulated cells
in vitro showed a significant reduction in nuclear HIF-1α levels and eNOS activation. Intriguingly, when compared to their respective baseline unstimulated controls, we found that the extent of inflammatory induction of nuclear NF-κB, PHD3 and VEGFA were less pronounced in shTRIM2 cells. Under stimulation with cytokines like TNFα, it is possible that TRIM2, functioning as a ubiquitin ligase [
12], may contribute to the stabilization and nuclear translocation of the transcription factors NF-κB and HIF-1α, likely by promoting proteasomal degradation of their cytosolic inhibitors, such as PHD3 in the latter case [
13]. These transcription factors, in turn, promote VEGFA expression, which activates endothelial cell migration and tubule formation through numerous intracellular pathways [
14,
15], including the phosphorylation of p38 MAPK and eNOS. NF-κB p65 and HIF-1α may also stimulate angiogenesis downstream of TRIM2 by upregulating inflammatory cytokines and chemokines like CCL2, leading to the recruitment and activation of macrophages that help to potentiate the inflammation-driven angiogenic response.
It is not clear whether TRIM2 mediates VEGFA-related effects via VEGFR2, as only a modest decrease in Tyr
1175 phosphorylation was seen following TRIM2 knockdown. TRIM2 could be targeting alternative VEGFR2 phosphorylation sites, or perhaps neuropilin-1 (NRP1), one of the key co-receptors for VEGFR2. NRP1 is highly expressed in endothelial cells and neurons and can bind to both VEGFA and the class 3 semaphorins, a family of axonal guidance proteins, thus forming a key link between angiogenesis and neurogenesis [
15,
16]. Given the association of TRIM2 with axonal outgrowth and development [
12,
17], and now angiogenesis, it is possible that NRP1 and/or its semaphorin ligands may be involved in regulating angiogenic function by TRIM2. Further studies examining a possible link between NRP1 and TRIM2 would be useful to clarify the mechanistic pathway.
Consistent with our previous work which demonstrated impairment of hypoxia-stimulated tubule formation
in vitro with TRIM2 knockdown [
7],
Trim2 deletion reduced the number of proliferating neovessels and arterioles in the ischemic tissue
in vivo. Interestingly, there was an increase in total CD31
+ vessels in the ischemic hindlimbs of
Trim2–/– mice, suggestive of a potential negative feedback loop. However, while there were significant changes at a cellular/tissue level, this did not seem to impact the recovery of blood flow reperfusion to the ischemic hindlimb. These incongruous findings may reflect inherent angiogenic compensatory mechanisms
in vivo that may be activated in response to
Trim2 deletion, perhaps starting early in embryonic development and thereby rendering TRIM2 redundant. Mechanistically, while the hypoxia-driven increase in nuclear HIF-1α was attenuated in TRIM2-deficient HCAECs, downstream angiogenic signalling was not altered. The paucity of effects on these intracellular mediators may explain the lack of differences between WT and
Trim2–/– mice in their angiogenic responses to hypoxia. This could prove clinically useful, as anti-TRIM2 therapies may be developed to suppress pathological inflammatory angiogenesis, without the adverse effects of impairing hypoxia-driven physiological angiogenesis.
The discordant
in vitro and
in vivo findings may also reflect activation of compensatory angiogenic mechanisms in response to
Trim2 deletion
in vivo. Future studies could explore inducible and endothelial cell-specific
Trim2 silencing to exclude such effects as angiogenic responses may be countered by
Trim2 deletion in other cell types, like vascular smooth muscle cells, pericytes and immune cells [
18]. It is also plausible that TRIM2 may target non-classical pathways downstream of VEGFA to confer its angiogenic effects in hypoxia, or it could be modulating other angiogenic factors like the fibroblast growth factors or angiopoietins. Future studies could evaluate a broader range of signalling targets to better elucidate the mechanistic basis by which TRIM2 may be modulating endothelial responses to hypoxia.
4. Materials and Methods
4.1. Animal Studies
All experimental procedures were conducted with approval from the SAHMRI Animal Ethics Committee (#SAM335) and conformed to the Australian Code for the Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council, Australia). A Trim2–/– mouse line was generated by the South Australian Genome Editing facility using a CRISPR/Cas9 approach. In brief, Cas9 protein was injected into C57BL/6J murine embryos along with two guide RNA sequences. These guide RNAs were designed such that non-homologous end joining of the DNA following CRISPR/Cas9 activity would result in excision of a DNA fragment containing exon 2 of Trim2, leading to a frameshift in the coding sequence and an early stop codon in exon 3. The founder male carrying this mutant Trim2 allele was back-crossed to wildtype (WT) female C57BL/6J mice, generating identical heterozygous offspring which were subsequently crossed to generate homozygous Trim2 knockout (Trim2–/–) mice. Male wildtype (WT) and Trim2–/– mouse littermates were housed in a temperature and humidity-controlled environment under a 12-hour light/dark cycle with ad libitum access to water and standard mouse chow. They underwent surgery at 8 weeks of age.
4.2. Plasma Glucose and Lipid Analyses
Plasma glucose concentrations were determined using a glucometer (Accu-Chek® Performa, Roche), while total plasma and HDL cholesterol concentrations were measured enzymatically (439-17501, Wako Diagnostics). HDL cholesterol concentrations were determined following polyethylene glycol precipitation of apoB-containing lipoproteins, while LDL cholesterol concentrations were calculated by subtracting HDL from total cholesterol concentrations. Triglyceride concentrations were determined using a colorimetric assay (290-63701, Wako Diagnostics).
4.3. Periarterial Cuff Model
The femoral periarterial cuff model is an established model of inflammation-driven neointima formation and adventitial angiogenesis [
19,
20], processes which are known to contribute to atherosclerotic plaque development. A non-occlusive 2-mm length of polyethylene cuff was placed around the left femoral artery to trigger a localized inflammatory response, while a sham operation was performed on the right femoral artery as a parallel control. The animals were sacrificed 21 days post-surgery by overdose of isoflurane and intracardiac puncture, followed by perfusion with phosphate buffered saline (PBS) via the left ventricle. The femoral arteries (complete with cuff) were excised for histochemical analyses.
Excised femoral arteries were paraffin-embedded and the angiogenic responses to cuff placement assessed by immunohistochemistry on 5-μm sections, probing for CD68 (Bio-Rad, Cat# MCA1957GA, RRID:AB_324217) to assess macrophage infiltration and CD31 (Abcam, Cat# ab28365, RRID:AB_726365) to detect adventitial vessels. Proliferating neovessels were determined by co-staining tissue sections with proliferation marker Ki-67 (Thermo Fisher Scientific, Cat# 14-5698-82, RRID:AB_10854564) and CD31. Endothelial tip cells were determined by staining sections with CD34 (Abcam, Cat# ab8158, RRID:AB_306316). Masson’s trichrome staining was performed with a Trichrome Stain Kit (ab150686, Abcam) to assess intima-to-media ratio as a measure of neointimal responses to inflammatory stimulation. All histological sections were photographed with a Zeiss Axio Scan.Z1 Digital Slide Scanner (Carl Zeiss Microscopy) and image analysis performed using Image-Pro Premier software (v9.0.4, Media Cybernetics).
An additional cohort of mice underwent the same procedure and were sacrificed 24 hours post-surgery for gene expression analysis. Total RNA was isolated from the femoral arteries with TRI® reagent (Sigma-Aldrich) and quantitated spectrophotometrically. 200 ng of total RNA was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR was performed for: Cd68 (F: 5’GGACAGCTTACCTTTGGATTCAA–3’; R: 5’–CTGTGGGAAGGACACATTGTATTC–3’), Ccl2 (F: 5’–GCTGGAGCATCCACGTGTT–3’; R: 5’–ATCTTGCTGGTGAATGAGTAGCA–3’), NF-κB p65 (Rela, forward [F]: 5’–AGTATCCATAGCTTCCAGAACC–3’; reverse [R]: 5’–ACTGC-ATTCAAGTCATAGTCC–3’) and 36B4 (F: 5’–CAACGGCAGCA-TTTATAACCC–3’; R: 5’–CCCATTGATGATGGAGTGTGG–3’). Relative gene expression was calculated using the ΔΔCt method, normalized to 36B4 and WT non-cuffed arteries.
4.4. Hindlimb Ischemia Model
The hindlimb ischemia model is a well-validated model of physiological angiogenesis in response to tissue ischemia [
21]. Hindlimb ischemia was induced by ligation and excision of the left superficial and deep femoral arteries, along with the left femoral vein down to the saphenous artery. A sham procedure was performed on the contralateral hindlimb as a parallel control. Hindlimb blood reperfusion was determined by laser Doppler imaging (moorLDI2-IR, Moor Instruments), performed prior to and immediately following surgery, then at days 1, 3, 6, 8 and 10 post-surgery. Animals were sacrificed 10 days post-surgery by isoflurane overdose and intracardiac puncture, and the gastrocnemius muscles of both hindlimbs were collected for histological analyses.
Gastrocnemius muscles from both ischemic and non-ischemic hindlimbs were OCT-embedded and frozen on dry ice. To histologically assess angiogenic responses to ischemia, immunofluorescence was performed on 5-µm tissue sections, staining with CD31 (Abcam, Cat# ab28364, RRID:AB_726362) to detect neovessels, α-smooth muscle actin (α-SMA, Sigma-Aldrich, Cat# F3777, RRID:AB_476977) to detect arterioles and laminin (Millipore, Cat# MAB1905, RRID:AB_94392) to detect myocytes. Proliferating neovessels and arterioles were determined by co-staining tissue sections with Ki-67 (Thermo Fisher Scientific, Cat# 11-5698-82, RRID:AB_11151330) and either CD31 or α-SMA respectively. Images were taken using an Eclipse Ni-E fluorescent microscope (Nikon Instruments). CD31+ neovessels and α-SMA+ arterioles were quantified using CellProfiler software (Broad Institute), while the myocytes were manually quantified using ImageJ (National Institutes of Health).
4.5. Lentiviral shRNA Knockdown of TRIM2 In Vitro
Human coronary artery endothelial cells (HCAECs, Cell Applications, USA) were cultured in MesoEndo Cell Growth Medium (212-500, Cell Applications) and used at passages 3–4. HCAECs were seeded at 5×10
4 cells/well in 6-well plates and cultured at 37°C and 5% CO
2 overnight. The cells were exposed to 1x10
4 infectious units (IFU)/mL of lentiviral particles containing shRNA against TRIM2 (shTRIM2) or a random control sequence (shControl) for 24 hours in the presence of polybrene. Transduced HCAECs were trypsinized, counted and seeded at a density of 1.5×10
5 cells/well and 8×10
4 cells/well for the inflammation and hypoxia experiments respectively. HCAECs were then either incubated for 4.5 hours with 0.6 ng/mL TNFα (to mimic inflammation) or for 6 hours at 5% CO
2 and 1.2% O
2 balanced with N
2 (to mimic hypoxia). To measure phosphorylated proteins, HCAECs were stimulated with 10 ng/mL recombinant human VEGF
165 protein (R&D Systems) 15 min prior to harvest. Nuclear proteins were isolated from cell lysates using the NE-PER
® Nuclear and Cytoplasmic Extraction kit (ThermoFisher Scientific). Whole cell protein lysates were extracted using RIPA buffer [
7,
22]. Each experiment was performed at least four times independently with triplicates for each condition.
4.6. Protein Expression
Nuclear and whole-cell protein extracts were subjected to Western blot analysis and probed with primary antibodies for: NF-κB p65 (Abcam, Cat# ab16502, RRID:AB_443394), HIF-1α (Novus, Cat# NB100-105, RRID:AB_10001154), PHD3 (Novus, Cat# NB100-303, RRID:AB_10003302), VEGFA (Abcam, Cat# ab46154, RRID:AB_2212642), phosphorylated (Tyr1175) VEGFR2 (Cell Signaling Technology, Cat# 2478, RRID:AB_331377), total VEGFR2 (Cell Signaling Technology, Cat# 2479, RRID:AB_2212507), phosphorylated (Thr180/Tyr182) p38 MAPK (Cell Signaling Technology, Cat# 4511, RRID:AB_2139682), total p38 MAPK (Cell Signaling Technology, Cat# 8690, RRID:AB_10999090), phosphorylated (Ser1177) eNOS (BD Biosciences, Cat# 612393, RRID:AB_399751) and total eNOS (BD Biosciences, Cat# 610297, RRID:AB_397691). Even protein loading was confirmed with lamin B1 (Abcam, Cat# ab16048, RRID:AB_443298) for nuclear fractions or α-tubulin (Abcam, Cat# ab40742, RRID:AB_880625) for whole-cell lysates.
4.7. Statistics
Data is expressed as mean±SEM. Comparisons were made using unpaired student’s t-tests or two-way ANOVA followed by post hoc analysis using Bonferroni’s multiple comparison tests. Significance was set at a two-sided P<0.05.
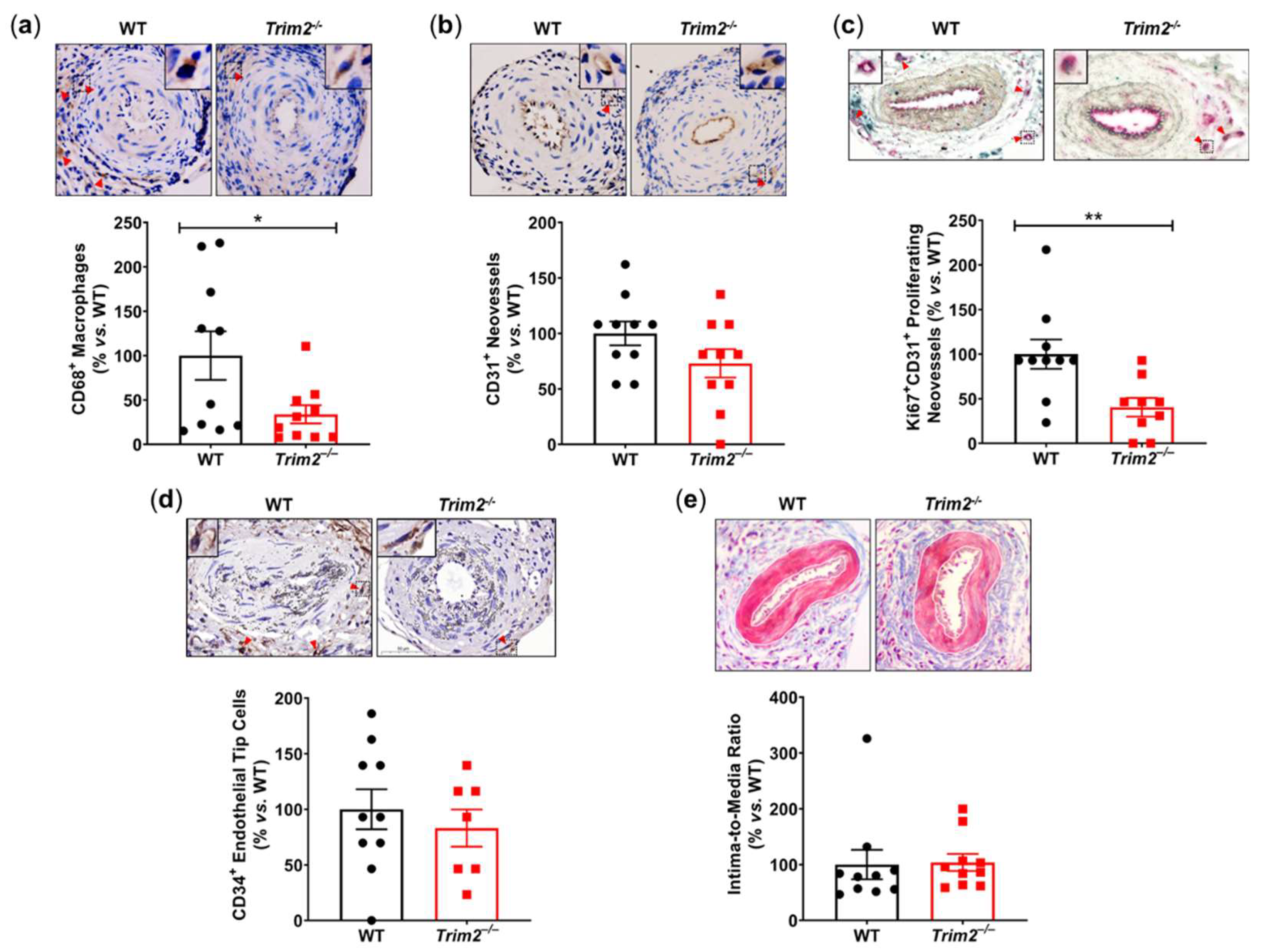
Figure 1.
Trim2 Deletion Inhibits Inflammatory-Driven Adventitial Macrophage Infiltration In Vivo. A non-occlusive polyethylene cuff (2 mm) was placed around the left femoral arteries of wildtype (WT) and Trim2–/– mice (N=10/group) for 21 days to trigger localized inflammatory responses. Femoral arteries were sectioned for immunohistochemical detection of adventitial (a) CD68+ macrophages, (b) CD31+ neovessels, (c) Ki67+CD31+ proliferating neovessels and (d) CD34+ endothelial tip cells. (e) Masson’s trichrome staining was performed to assess intima-to-media ratio. Representative images of cuffed artery sections from WT and Trim2–/– mice were taken at 40X magnification. Results are mean±SEM. *P<0.05, **P<0.01 by unpaired student’s t-test.
Figure 1.
Trim2 Deletion Inhibits Inflammatory-Driven Adventitial Macrophage Infiltration In Vivo. A non-occlusive polyethylene cuff (2 mm) was placed around the left femoral arteries of wildtype (WT) and Trim2–/– mice (N=10/group) for 21 days to trigger localized inflammatory responses. Femoral arteries were sectioned for immunohistochemical detection of adventitial (a) CD68+ macrophages, (b) CD31+ neovessels, (c) Ki67+CD31+ proliferating neovessels and (d) CD34+ endothelial tip cells. (e) Masson’s trichrome staining was performed to assess intima-to-media ratio. Representative images of cuffed artery sections from WT and Trim2–/– mice were taken at 40X magnification. Results are mean±SEM. *P<0.05, **P<0.01 by unpaired student’s t-test.
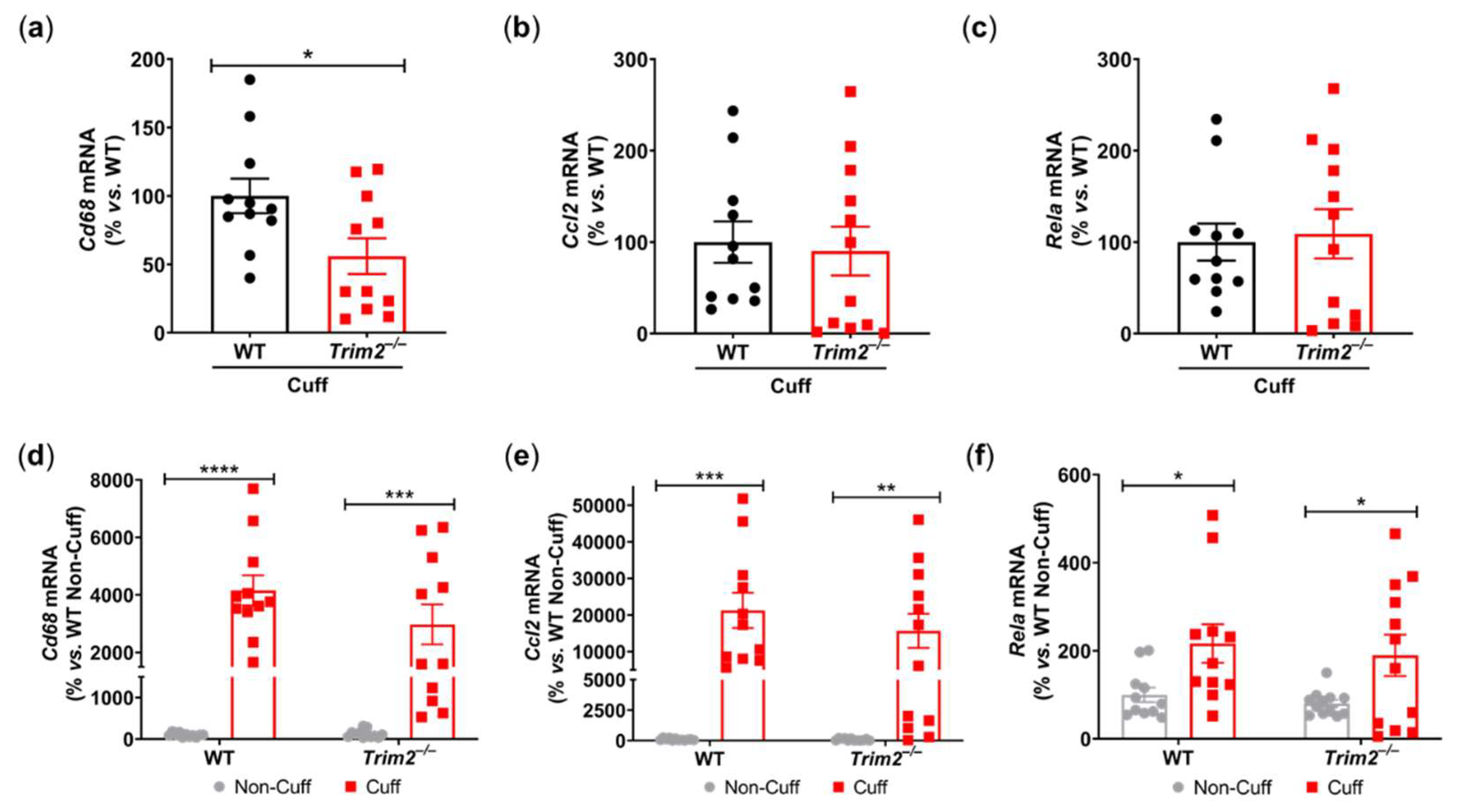
Figure 2.
Trim2 Deletion Attenuates Angiogenic Responses to Inflammation In Vivo. A non-occlusive polyethylene cuff (2 mm) was placed around the left femoral arteries of wildtype (WT) and Trim2–/– mice (N=10/group) for 24 hours to trigger localized inflammatory responses. (a) Cd68, (b) Ccl2 and (c) NF-κB p65 (Rela) mRNA levels in cuffed arteries of WT and Trim2–/– mice, normalized using the ΔΔCt method to 36B4 and WT cuffed arteries. (d) Cd68, (e) Ccl2 and (f) NF-κB p65 (Rela) mRNA levels in non-cuffed and cuffed arteries of WT and Trim2–/– mice, normalized using the ΔΔCt method to 36B4 and WT non-cuffed arteries. Results are mean±SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 by unpaired student’s t-test or two-way ANOVA with Bonferroni’s post hoc analysis.
Figure 2.
Trim2 Deletion Attenuates Angiogenic Responses to Inflammation In Vivo. A non-occlusive polyethylene cuff (2 mm) was placed around the left femoral arteries of wildtype (WT) and Trim2–/– mice (N=10/group) for 24 hours to trigger localized inflammatory responses. (a) Cd68, (b) Ccl2 and (c) NF-κB p65 (Rela) mRNA levels in cuffed arteries of WT and Trim2–/– mice, normalized using the ΔΔCt method to 36B4 and WT cuffed arteries. (d) Cd68, (e) Ccl2 and (f) NF-κB p65 (Rela) mRNA levels in non-cuffed and cuffed arteries of WT and Trim2–/– mice, normalized using the ΔΔCt method to 36B4 and WT non-cuffed arteries. Results are mean±SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 by unpaired student’s t-test or two-way ANOVA with Bonferroni’s post hoc analysis.
Figure 3.
Inflammation-Induced Activation of Angiogenic Signalling Mediators is Attenuated by TRIM2 Knockdown In Vitro. Lentiviral-transduced shControl and shTRIM2 HCAECs were incubated without and with TNFα (0.6 ng/mL, 4.5 hours). Protein levels of (a) nuclear NF-κB p65, (b) nuclear HIF-1α, (c) PHD3, (d) VEGFA, (e) Phospho:Total VEGFR2, (f) Phospho:Total p38 MAPK and (g) Phospho:Total eNOS, presented as percent change relative to TNFα treated shControl cells. Protein levels of (h) nuclear NF-κB p65, (i) nuclear HIF-1α, (j) PHD3, (k) VEGFA, (l) Phospho:Total VEGFR2, (m) Phospho:Total p38 MAPK and (n) Phospho:Total eNOS, presented as percent change relative to No TNFα shControl cells. Dotted lines separate noncontiguous lanes from the same gel. The cropped blots are used in the figure, and the full blots are presented in Supplemental Figures 3 – 4. Each experiment was conducted at least three times independently with triplicates for each condition. Results are mean±SEM. *P<0.05, **P<0.01 ***P<0.001 by unpaired student’s t-test or two-way ANOVA with Bonferroni’s post hoc analysis.
Figure 3.
Inflammation-Induced Activation of Angiogenic Signalling Mediators is Attenuated by TRIM2 Knockdown In Vitro. Lentiviral-transduced shControl and shTRIM2 HCAECs were incubated without and with TNFα (0.6 ng/mL, 4.5 hours). Protein levels of (a) nuclear NF-κB p65, (b) nuclear HIF-1α, (c) PHD3, (d) VEGFA, (e) Phospho:Total VEGFR2, (f) Phospho:Total p38 MAPK and (g) Phospho:Total eNOS, presented as percent change relative to TNFα treated shControl cells. Protein levels of (h) nuclear NF-κB p65, (i) nuclear HIF-1α, (j) PHD3, (k) VEGFA, (l) Phospho:Total VEGFR2, (m) Phospho:Total p38 MAPK and (n) Phospho:Total eNOS, presented as percent change relative to No TNFα shControl cells. Dotted lines separate noncontiguous lanes from the same gel. The cropped blots are used in the figure, and the full blots are presented in Supplemental Figures 3 – 4. Each experiment was conducted at least three times independently with triplicates for each condition. Results are mean±SEM. *P<0.05, **P<0.01 ***P<0.001 by unpaired student’s t-test or two-way ANOVA with Bonferroni’s post hoc analysis.
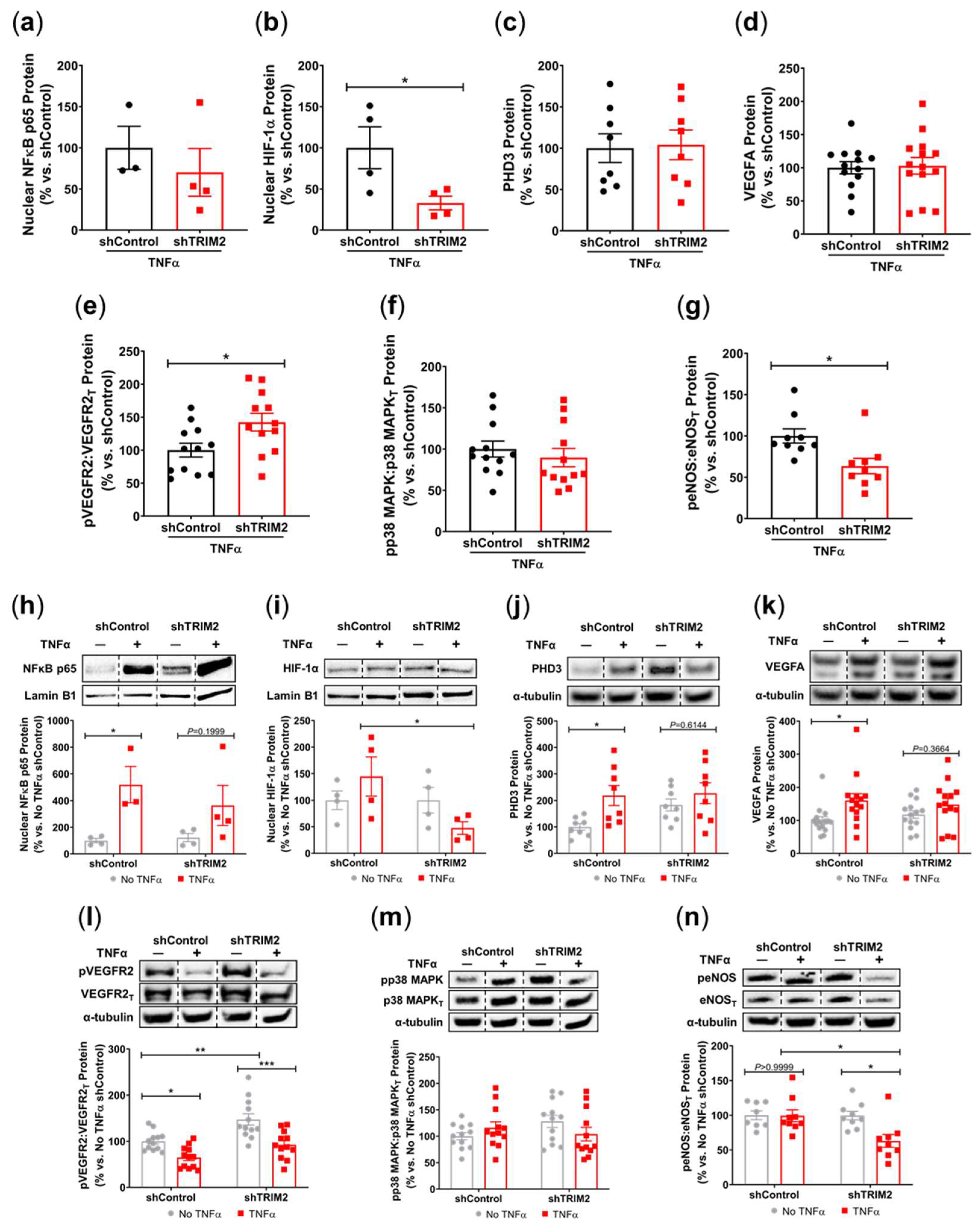
Figure 4.
Trim2 Does Not Affect Angiogenic Responses to Ischemia In Vivo. Wildtype (WT) and Trim2–/– mice (N=10/group) underwent ligation and excision of the left femoral artery and vein. (a) Blood flow reperfusion was measured by laser Doppler imaging over 10 days. Representative images show high (red) to low (blue) blood flow in the ischemic (ISC) and non-ischemic (NON) hindlimbs. Laser Doppler perfusion index (LDPI) was calculated as the ratio of flow in the ISC:NON hindlimbs. (b) Representative sections from the ischemic hindlimbs of WT and Trim2–/– mice taken at 20X magnification showing CD31+ neovessels (red/purple staining, arrows), α-SMA+ arterioles (green staining, arrowheads) and laminin+ myocytes (blue staining). (c) The density of CD31+ neovessels per myocyte, normalized to WT mice. (d) The density of α-SMA+ arterioles per myocyte, normalized to WT mice. Immunofluorescent co-staining was performed to detect (e) Ki67+CD31+ proliferating neovessels and (F) Ki67+α-SMA+ proliferating arterioles. Results are mean±SEM. *P<0.05 by unpaired student’s t-test.
Figure 4.
Trim2 Does Not Affect Angiogenic Responses to Ischemia In Vivo. Wildtype (WT) and Trim2–/– mice (N=10/group) underwent ligation and excision of the left femoral artery and vein. (a) Blood flow reperfusion was measured by laser Doppler imaging over 10 days. Representative images show high (red) to low (blue) blood flow in the ischemic (ISC) and non-ischemic (NON) hindlimbs. Laser Doppler perfusion index (LDPI) was calculated as the ratio of flow in the ISC:NON hindlimbs. (b) Representative sections from the ischemic hindlimbs of WT and Trim2–/– mice taken at 20X magnification showing CD31+ neovessels (red/purple staining, arrows), α-SMA+ arterioles (green staining, arrowheads) and laminin+ myocytes (blue staining). (c) The density of CD31+ neovessels per myocyte, normalized to WT mice. (d) The density of α-SMA+ arterioles per myocyte, normalized to WT mice. Immunofluorescent co-staining was performed to detect (e) Ki67+CD31+ proliferating neovessels and (F) Ki67+α-SMA+ proliferating arterioles. Results are mean±SEM. *P<0.05 by unpaired student’s t-test.

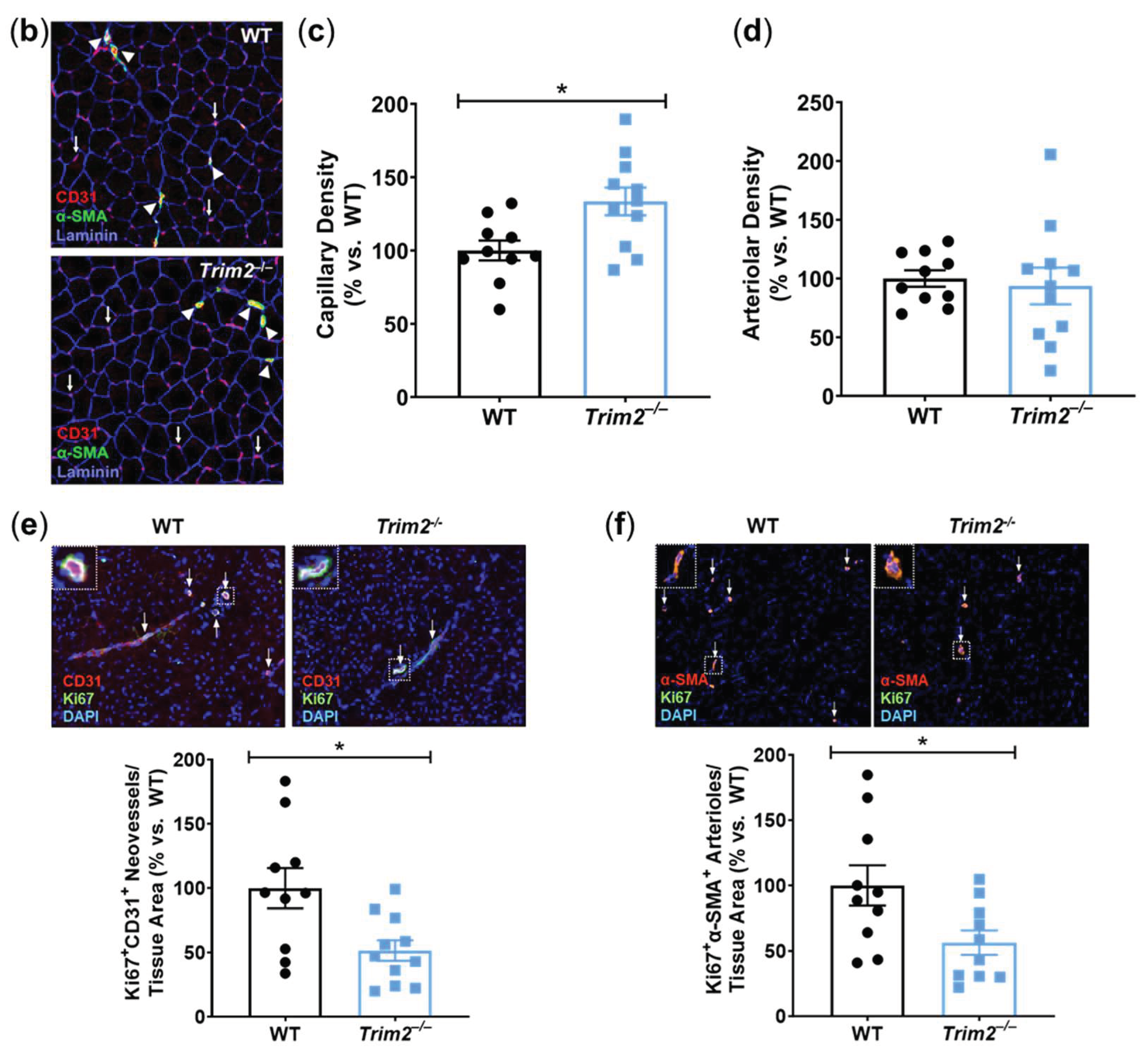
Figure 5.
TRIM2 Knockdown In Vitro Attenuates Hypoxia-Driven Induction of Nuclear HIF-1α, PHD3 and VEGFA but not Downstream Signalling Pathways. Lentiviral-transduced shControl and shTRIM2 HCAECs were incubated in normoxia or hypoxia (1.2% O2, 6 hours). Protein levels of (a) nuclear HIF-1α, (b) PHD3, (c) VEGFA, (d) Phospho:Total VEGFR2, (e) Phospho:Total p38 MAPK and (f) Phospho:Total eNOS, presented as percent change relative to hypoxia stimulated shControl cells. Protein levels of (g) nuclear HIF-1α, (h) PHD3, (i) VEGFA, (j) Phospho:Total VEGFR2, (k) Phospho:Total p38 MAPK and (l) Phospho:Total eNOS, presented as percent change relative to Normoxia shControl cells. Dotted lines separate noncontiguous lanes from the same gel. The cropped blots are used in the figure, and the full blots are presented in Supplemental Figures 5 – 6. Each experiment was conducted at least three times independently with triplicates for each condition. Results are mean±SEM. *P<0.05 by unpaired student’s t-test or two-way ANOVA with Bonferroni’s post hoc analysis.
Figure 5.
TRIM2 Knockdown In Vitro Attenuates Hypoxia-Driven Induction of Nuclear HIF-1α, PHD3 and VEGFA but not Downstream Signalling Pathways. Lentiviral-transduced shControl and shTRIM2 HCAECs were incubated in normoxia or hypoxia (1.2% O2, 6 hours). Protein levels of (a) nuclear HIF-1α, (b) PHD3, (c) VEGFA, (d) Phospho:Total VEGFR2, (e) Phospho:Total p38 MAPK and (f) Phospho:Total eNOS, presented as percent change relative to hypoxia stimulated shControl cells. Protein levels of (g) nuclear HIF-1α, (h) PHD3, (i) VEGFA, (j) Phospho:Total VEGFR2, (k) Phospho:Total p38 MAPK and (l) Phospho:Total eNOS, presented as percent change relative to Normoxia shControl cells. Dotted lines separate noncontiguous lanes from the same gel. The cropped blots are used in the figure, and the full blots are presented in Supplemental Figures 5 – 6. Each experiment was conducted at least three times independently with triplicates for each condition. Results are mean±SEM. *P<0.05 by unpaired student’s t-test or two-way ANOVA with Bonferroni’s post hoc analysis.
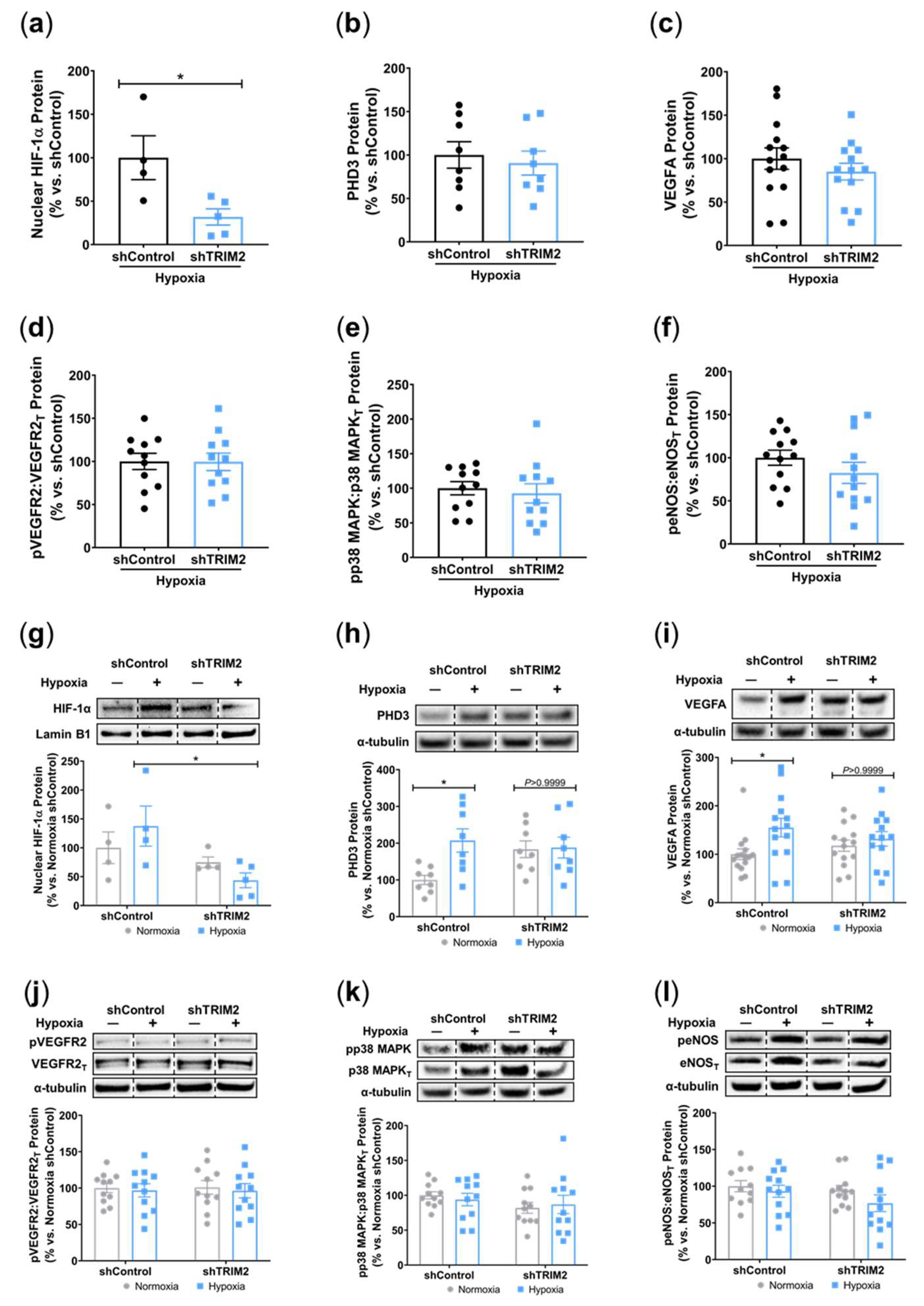
Figure 6.
Proposed Mechanistic Role of TRIM2 in Inflammatory-Driven Angiogenesis. (
a) In response to an inflammatory stimulus such as TNFα, nuclear translocation of the key inflammatory transcription factor NF-κB occurs. The NF-κB complex is comprised of both the p50/p65 subunits, forming a dimer that translocates into the nucleus. The p50/p65 dimer binds to NF-κB response elements (NRE) and upregulates a range of inflammatory and angiogenic targets including HIF-1α and VEGFA. Nuclear HIF-1α (nHIF-1α) dimerises with HIF-1β and together bind to hypoxia-response elements (HRE), leading to upregulation of VEGFA, among many other pro-angiogenic genes. VEGFA binds to the VEGFR2 receptor, resulting in autophosphorylation of tyrosine residues in the cytoplasmic domain of VEGFR2. This leads to the phosphorylation and activation of downstream signalling mediators including eNOS and p38 MAPK, resulting in angiogenesis. Additionally, NF-κB activates pro-inflammatory cytokines such as CCL2, that facilitate the recruitment of macrophages. Macrophages contribute to inflammatory-driven angiogenesis by secreting pro-inflammatory angiogenic factors. The solid arrows indicate activation, dashed arrows indicate translocation. (
b) Our study found that
Trim2–/– mice that underwent a periarterial collar model of inflammation-induced angiogenesis exhibited significantly less adventitial macrophage infiltration, concomitant with decreased
Cd68 mRNA levels.
Trim2–/– mice also had reduced adventitial proliferating neovessels. Mechanistically, our
in vitro findings show that TRIM2 knockdown inhibits nuclear HIF-1α translocation and eNOS phosphorylation. Figure adapted from [
22] and [
23]. Created with
BioRender.com.
Figure 6.
Proposed Mechanistic Role of TRIM2 in Inflammatory-Driven Angiogenesis. (
a) In response to an inflammatory stimulus such as TNFα, nuclear translocation of the key inflammatory transcription factor NF-κB occurs. The NF-κB complex is comprised of both the p50/p65 subunits, forming a dimer that translocates into the nucleus. The p50/p65 dimer binds to NF-κB response elements (NRE) and upregulates a range of inflammatory and angiogenic targets including HIF-1α and VEGFA. Nuclear HIF-1α (nHIF-1α) dimerises with HIF-1β and together bind to hypoxia-response elements (HRE), leading to upregulation of VEGFA, among many other pro-angiogenic genes. VEGFA binds to the VEGFR2 receptor, resulting in autophosphorylation of tyrosine residues in the cytoplasmic domain of VEGFR2. This leads to the phosphorylation and activation of downstream signalling mediators including eNOS and p38 MAPK, resulting in angiogenesis. Additionally, NF-κB activates pro-inflammatory cytokines such as CCL2, that facilitate the recruitment of macrophages. Macrophages contribute to inflammatory-driven angiogenesis by secreting pro-inflammatory angiogenic factors. The solid arrows indicate activation, dashed arrows indicate translocation. (
b) Our study found that
Trim2–/– mice that underwent a periarterial collar model of inflammation-induced angiogenesis exhibited significantly less adventitial macrophage infiltration, concomitant with decreased
Cd68 mRNA levels.
Trim2–/– mice also had reduced adventitial proliferating neovessels. Mechanistically, our
in vitro findings show that TRIM2 knockdown inhibits nuclear HIF-1α translocation and eNOS phosphorylation. Figure adapted from [
22] and [
23]. Created with
BioRender.com.
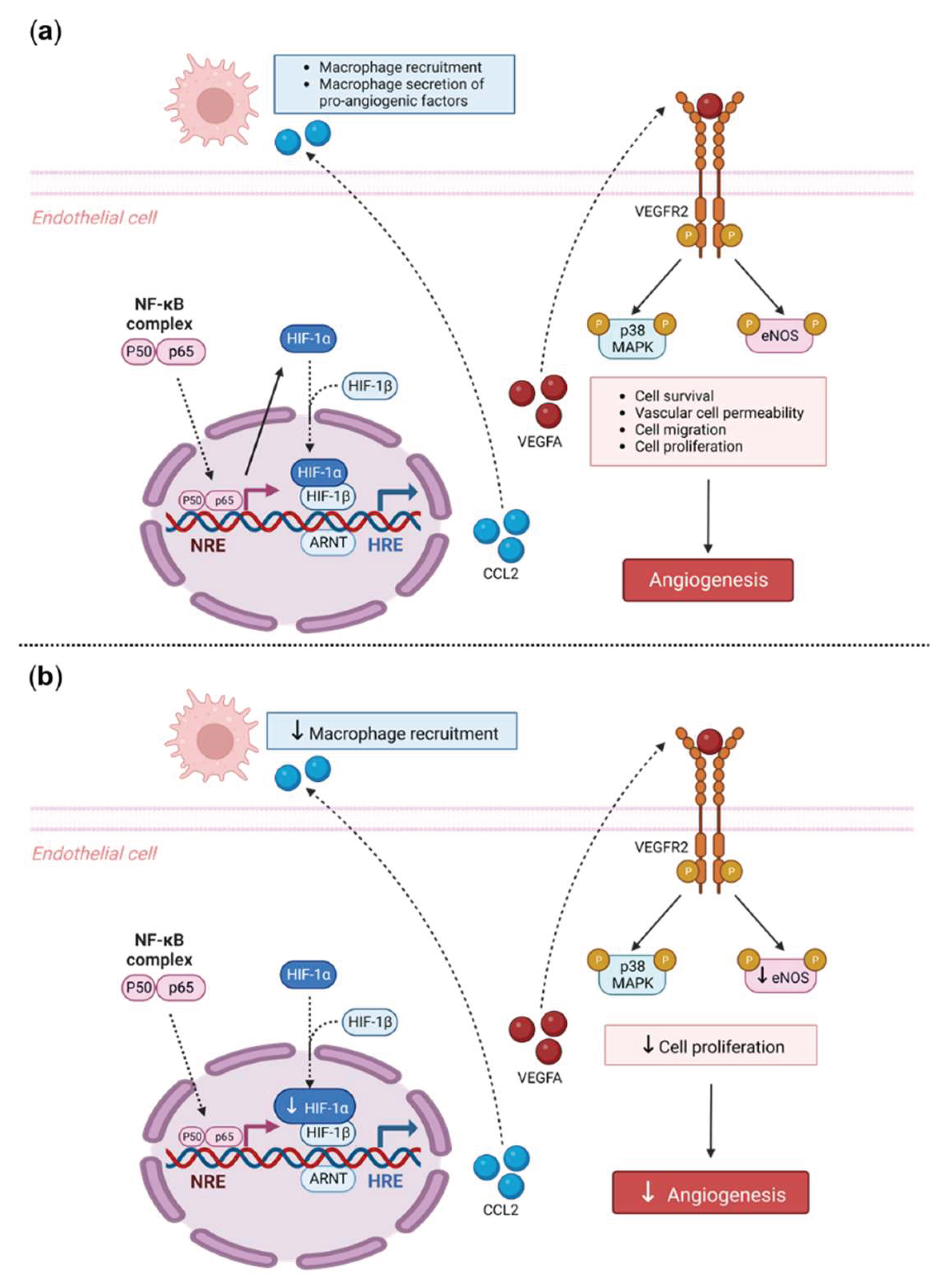
Table 1.
Plasma Glucose and Lipids Measured in WT and Trim2–/– Mice.
Table 1.
Plasma Glucose and Lipids Measured in WT and Trim2–/– Mice.
| Plasma Parameter |
WT (N=23) |
Trim2–/– (N=23) |
| Glucose (mM) |
14.5±0.3 |
14.0±0.4 |
| Total cholesterol (mg/dL) |
256.3±7.4 |
239.7±7.2 |
| HDL cholesterol (mg/dL) |
122.2±4.1 |
118.2±4.4 |
| LDL cholesterol (mg/dL) |
134.1±6.0 |
121.5±5.7 |
| Triglycerides (mg/dL) |
100.4±7.6 |
97.0±6.2 |