1. Introduction
Biomonitoring emerges as the most sensitive approach for assessing the quality of human milk across diverse populations globally [
1]. It facilitates the evaluation of breastfed infants’ exposure to various lipophilic toxicants. Despite substantial publications on contaminants in breast milk, the data remains fragmented and sometimes questionable, primarily due to the lack of standardized methods for sample collection and storage [
2]. This scenario underscores the necessity for ongoing epidemiological and biological studies to deepen our understanding of pollutant variations over future decades. Given the passive diffusion mechanism by which most contaminants, owing to their high lipophilicity, reach breast milk, the importance of scrutinizing their presence becomes paramount [
3].
Recent investigations have spotlighted the critical need to assess the impact of pollutants, specifically metals and persistent organic pollutants (POPs), on child health. However, this crucial aspect has often been overlooked or insufficiently addressed. This review aims to bridge this gap by meticulously evaluating the most recent data on breast milk pollutants, with a particular focus on the 2023 cut-off, thereby providing a contemporary perspective on this pressing issue.
In light of the above, our study endeavors to extend the introduction with a comprehensive overview of the current state of research on breast milk contamination. We aim to contextualize the significance of our study against the backdrop of existing literature, thereby justifying the need for our research. Furthermore, we will summarize prior findings related to contaminants in breast milk, delineating how our study contributes new insights to this critical field.
2. Objectives
A scoping review was conducted in order to systematically map the research done on persistent POPs in breast milk, as well as to identify any existing gaps in knowledge about POP in breast milk.
3. Materials and Methods
3.1. Data Sources and Search Strategy
To identify potentially relevant documents, the bibliographic databases MEDLINE and Google Scholar were searched from 1995 to June 2023. Peer-reviewed journal articles were included if they were published between 1995 and 2023 in the English language, involved human participants and reported a measure of POP and heavy metals in breast milk, including the assay methods. Papers comparing infant formula and breast milk were also included.
Papers were excluded if they did not fit into the conceptual framework of the study.
The search strategies were drafted by us the researchers and further refined through team discussion. The final search strategy for MEDLINE can be found in Additional file 1. The final search results were exported into Zotero. Three reviewers evaluated the titles, abstracts and the full text of all publications identified by our search as potentially relevant publications. We resolved disagreements on study selection and data extraction by consensus and discussion with other reviewers if needed. A data-charting form was jointly developed by two reviewers to determine which variables to extract. When we identified a systematic review, we counted the number of studies included in the review that potentially met our inclusion criteria and noted how many studies had not been considered by our search.
3.2. Inclusion and Exclusion Criteria
Peer-reviewed journal articles were included if they were published between 1995 and 2023 in the English language, involved human participants and reported a measure of POP and heavy metals in breast milk, and included the methodology of the assays used. Articles comparing infant formula and breast milk were also included.
Articles were excluded if they did not fit into the conceptual framework of the study.
Our findings indicate that there is little recent research specifically on breastfeeding women, and a limited number of studies on implementation in this field. We also found that the training of professionals and the development of knowledge need to be pursued. Our exploratory study has certain limitations. To make our study more feasible, we were only able to include a sample of the articles published in MEDLINE. Further bibliographic research would be necessary to obtain a more exhaustive bibliographic search in a future exploratory review in the field of pollutants in breast milk. The aim of this scoping review was to identify gaps in the literature that may guide a future systematic review. This study has not received any funding.
4. Results:
4.1. Study Selection
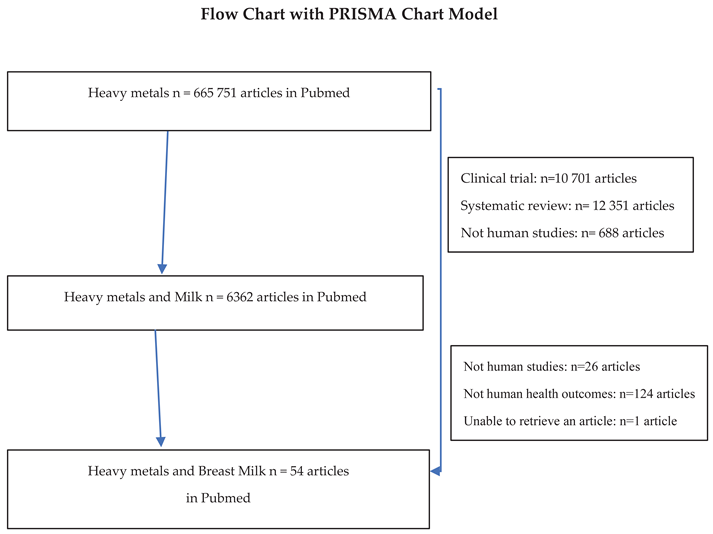
In this exploratory study, we identified 341 primary studies on the presence of persistent organic pollutants in breast milk, published between 1998 and 2023. A total of 54 articles were ultimately selected.
4.2. POPs into Breast Milk:
Table 1: Contaminants in Breast Milk
Polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) are persistent organic pollutants (POPs) regulated by the Stockholm Convention. They originate from industrial activities such as heavy industry, paper manufacture, fertilizer and pesticide production. POPs are highly resistant in the environment and can travel long distances by air; PCBs and PBDEs, both POPs, can travel very long distances [
20]. They are present in the soil, water, earth and eventually accumulate in our foods. Organochlorines (OCs) can accumulate in children via breast milk but they are expected to decline be lower in high income countries [
21].
PCDDs (frequently called “dioxins”) have effects on the endocrine system, certain liver enzymes, but also on the immune and cardiovascular systems. Because they are lipophilic, PCDDs accumulate in our organism in tissues rich in fat and in breast milk and therefore can contaminate infants at the very early stages of their lives. The transfer of PCDD and PCDF is also a function of the composition of breast milk which changes depending on the stage of lactation, nutritional status of the mother, time of day and milk protein content. Thus, it varies from one woman to another and from one breastfeeding to another for the same woman [
21,
22,
23].
In a study from the Czech Republic, POPs were analyzed in 231 breast milk samples from 2019 to 2021 [
20]. for the presence of 94 organohalogen pollutants. Specifically, 6 polychlorinated biphenyls (PCBs), 10 organochlorine pesticides (OCPs), 34 halogenated flame retardants (HFRs), 29 perfluoroalkyl and polyfluoroalkyl substances (PFASs) and 15 polychlorinated naphthalenes (PCNs) were analyzed. PCBs, OCPs, most HFRs and PCNs were analyzed by (tandem) mass spectrometry (GC-MS(/MS), while PFASs, HBCDs, brominated phenols and tetrabromobisphenol A (TBBPA) were quantified by UHPLC-MS/MS. The average value for the sum of the 6 indicator PCBs was 123.12 nanograms per gram of lipid weight (ng/g). PFAS concentrations were also low, perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) being the main congeners in this group (averages of 22 pg/ml and 21 pg/ml, respectively). These values indicate that these contaminants are still present in breast milk, however they also show a decrease in these pollutants over time [
20].
Concentrations of 29 POPs in breast milk were found to be significantly reduced in 90 countries. Dioxin-like POPs and polychlorinated biphenyls (PCBs) were the ones that had declined the most over the past 30 years [
9].
A study carried out in Turkey showed that PCB and OCP levels were still present in the breast milk of nursing mothers living in Istanbul. These pollutants decreased more in multiparous women than in primiparous mothers [
24].
PCBs, PCDD/Fs, chlorinated pesticides and brominated flame retardants, with the exception of the polybrominated diphenyl ethers (PBDEs) BDE-153 and BDE-209, were measured in breast milk in Uppsala, Sweden, and a decline was observed in breast milk, averaging -4 to 14% per year from 1996 to 2017 [
18].
Key factors in the passage of these pollutants into the breast milk are maternal age and the long half-life of these pollutants in the mother’s body [
25]. The POP concentration is correlated with the mother’s age. The older the mother is, the higher the POP concentrations in her body. After the age of 30, the amount of dioxins in the primipara maternal body is higher and higher with time. For an age difference of five years, the levels of PCDD and PCDF are on average 13 to 38% higher depending on the studies [
26]; Contrary to what was previously thought, lipophilic pollutants do not decrease significantly after the first breastfeeding. It no longer appears necessary to pump and discard milk prior to a first breastfeeding in order to reduce the infant’s exposure to these lipophilic pollutants.
4.3. What is the Origin of Major Contaminants in Breast Milk?
4.3.1. Dietary Origin.
Substances, which pollute the environment, are found worldwide, in soil, water and the air [
27]. Mothers are contaminated through food, particularly eggs, meat and dairy products.
In 90% of cases, the mother’s diet is the primary source of dioxins in breast milk. These dioxins originate from the consumption of animal fats, mainly from fatty fish and meat. Despite the quest for optimal organic nutrition, the consumption of free-range eggs does not prevent egg contamination by dioxins originating in the earthworms consumed by the hens [
27,
28].
In Pakistan, a research team found that contamination of chicken eggs could be observed via the presence of heavy metals in the poultry feeding process [
29]. Women in Lebanon who ate cereals at least twice a week had higher levels of DDE-type pollutants in their breast milk [
30]. Lebanese women who also consumed potatoes and beans at least once a week had a significant presence of DDE in their milk [
30].
A study carried out among women in several West African countries (Cameroon, Democratic Republic of Congo, Nigeria, Zimbabwe, Côte d’Ivoire and Uganda) found that maternal diet was contaminated by pollutants such as PCDDs/Fs and dl-PCBs. The foods consumed by pregnant women were reported to have a maximum concentration of 103 pg TEQ/g [
15,
31], and a great variability in the concentrations of POPs such as PCDFs, PCBs and polybrominated diphenyl ethers (PBDEs) in breast milk. This variability persisted even though the tested women lived in the same geographical area. This study included 33 breastfeeding women, all primiparous and living close to industrial areas [
31,
32].
An Italian study showed that women consumed around 1.9 µg of carcinogenic PAHs per day in their diet. Almost half of the total intake of carcinogenic PAHs came from cereal products, meats, oils and fats. Barbecued meats accounted for less than 13% of carcinogenic PAH intake [
15].
Heavy metals are ubiquitous, present everywhere in air, water, soil and sediments, in plants, animals and fish, and therefore in all food sources for humans [
33]. Exposure to heavy metals occurs via the diet (over 90% for cadmium in non-smokers, 100% for methylmercury). The proportion attributable to atmospheric pollution remains negligible compared with the contamination of pollutants via the diet observed in breast milk [
34].
4.3.2. Environmental Origin.
Apart from diet, which remains the major factor in contamination, living close to factories or structures with a reputation for pollution increases the level of POPs in milk. An Australian team analyzed the presence of POPs in the breast milk of mothers exposed to mega forest fires. They measured the concentration of polycyclic aromatic hydrocarbons (PAHs) in breast milk samples. PAHs are not POPs, but they are lipophilic and tend to be localized in breast milk. They found that PAHs were only present in the milk of mothers exposed to the fires; they detected fluoranthene (median concentration: 0.015 mg/kg) and pyrene (median concentration: 0.008 mg/kg). There was a correlation between the quantities of fluoranthene and pyrene measured and the duration of exposure of nursing mothers to these fires [
35]. Pesticides are another cause of contamination of breast milk. They assessed the exposure of breastfed infants to these pesticides in Ethiopia.
Three agricultural regions in south-west Ethiopia (Asendabo, Deneba and Serbo), were studied at three different times: at the start of the study (at 1 month), midway through (at 6 months) and at the end of the study (at 12 months). 168 mothers gave milk samples at three different times for pesticide analysis. DDT was found in 447 milk samples, as were its metabolites: p,p’-DDE. During the first month of breastfeeding, the estimated daily intake of infants was 11.24 µg/kg body weight/day. This was higher than the provisional tolerable daily intake (PTDI) for total DDT set by the FAO/WHO. This TDI is set at 10 µg/kg body weight/day [
16].
170 nursing mothers were included in a prospective study. A correlation was identified between arsenic and the mother’s place of residence if she lived in an urban area (p = 0.013), lead and smoking (p = 0.024), and lead and consumption of well water (p = 0.046). For infants born prematurely in north-western Spain, the toxicity of pollutants was combined with malnutrition factors [
36]. In the southern Spanish region of Murcia, 50 breastfeeding women donated their milk for analysis of heavy metals. The highest concentrations of aluminum, zinc, arsenic, lead, mercury and nickel were found in the milk of women living in mining areas, while the highest concentrations of manganese, chromium and iron were found in the milk of women living in agricultural areas [
10]. Samples of breast milk were measured at 3 months of breastfeeding. The levels of heavy metals found were higher than the legal doses allowed by the WHO, and As, Hg and Pb were consumed by 46.4%, 33.3% and 4.4% of breastfed babies [
11]. PCDD and PCDF levels in breast milk are correlated with urban density (Focant et al. 2013), place of residence (city or country), and time spent in a polluted area. These geographical factors are significant in explaining the amounts of these pollutants in breast milk. Studies in Slovakia [
37] and Africa have shown that total PCDD/Fs concentrations range from 0.5 ng/g fat to 12 ng/g fat. Inter-laboratory evaluations have been carried out by African teams on various persistent organic pollutants (POPs), including two specifically for PCDD/Fs analysis.
4.4. Impact of the Mother’s Obesity.
Significant weight loss in women resulted in increased circulation of POPs in maternal plasma and, within 6-12 months, a significant 15% decrease in total PCB body burden [
38]. These accumulated POPs are slowly released into the bloodstream, and more so during weight loss. Adipose tissue (AT) or adiposity is a continuous source of internal exposure to POPs. The burden of POPs I obese individuals is higher than in lean individuals. 45 breast milk samples were collected from 24 obese women (BMI > 30) in the USA and from 21 women with a normal BMI < 25 (18.5-24.9, normal) from 14 different counties (18-34 years). 69% of samples were positive for PAHs. Phenanthrene was the most frequently detected PAH, followed by pyrene and fluoranthene. The average individual PAH concentration for all samples ranged from 0 to 25.1 ng/g milk fat; the sum of all individual PAH averages was 146.9 ng/g milk fat. The mean concentration of total PAHs in the BMI > 30 group was 224.8 ng/g milk fat, 4 times the PAH levels measured in women with a normal BMI. Benzo(b)fluoranthene is one of the most carcinogenic PAHs (32.08%), not found in the group of mothers with a BMI between 18.5-24.9. These results suggest that breastfed babies of obese mothers are potentially more exposed to carcinogenic PAHs [
39].
In obese breastfeeding women the duration of breastfeeding is reduced compared to breastfeeding women with a normal BMI. This could have an impact on the development of breastfed newborns [
40]. In a Portuguese study, there was a relationship between age of the mother over 30 and her child’s low birth weight of less than 3 kg. Breastfed children had PAH levels of 1.41 μg/kg body weight. Unmetabolized and metabolized PAHs must be considered when calculating contamination [
41]. Bariatric surgery leads to significant weight loss in the mother, which may be associated with a sustained increase in circulating lipophilic POPs in that mother. In the case of obese women of childbearing age, it is important to inform them of the risk and to quantify these potential risks for the health of the future breastfed child [
42].
4.5. Impact of Mother’s Age.
There is an interaction between age and sex, and the most significant interaction was observed for hexachlorobenzene (HCB) concentrations in serum and breast milk. For β-hexachlorocyclohexane (β-HCH) concentrations, interactions with time and pollutants were confirmed [
43]. In primiparous women, HCB excretion is due to placental transfer and breastfeeding [
44]. In older women, this factor is no longer relevant, as discussed by Salihovic et al. [
45].
4.6. Toxicokinetic Modeling.
There is a correlation between the concentration of contaminants in the mother’s body and the concentration of contaminants in breast milk [
46]. A toxicokinetic (TK) model has been developed to quantify the amount of pollutant that has been transferred from mother to child via breast milk (LPEC) during pregnancy and lactation. The model was created to facilitate internal dosimetry calculations to assess the developmental toxicity risks associated with LPEC. We used the model to estimate whole-body concentrations in mothers and children following maternal exposures to hexachlorobenzene (HCB)N. The toxicokinetic model was tested and validated. Their analyses revealed that half-life was the most influential parameter on children’s plasma pollutant concentrations, followed by the pharmacokinetic parameters of milk/plasma partition coefficient and volume of distribution [
47,
48].
4.7. The Effect of Parity.
Parity was studied as a factor that could influence dichlorodiphenyltrichloroethane (DDT) concentrations in women living in southern Mexico. The median DDE/DDT ratio was 14.7. Primiparity was one of the explanatory factors that could explain the doubling of concentrations (0.010 mg/kg and 0.868 mg/kg) than in multiparous women (0.005 mg/kg and 0.583 mg/kg) (p < 0.05) [
49]. In a Spanish study, this same parity factor was found to be correlated in POP measurements [
50].
4.8. What Are Their Effects on Child Health, in Particular Neurotoxicity?
The most «critical window of vulnerability” is the period of the first 1,000 days of life [
51]. The metabolic pathways of xenobiotics are immature, suggesting a different efficiency of metabolic and detoxification systems. the toxicity of pollutants can be explained by the very large exposure ratio in infants, given their body surface (Landrigan 2004); Carcinogenicity, developmental and endocrine disruption, reproductive toxicity and neurotoxicity are the main known serious adverse effects of POPs. Recently, Caspersen IH, et al. observed that maternal dietary exposure to dioxins and polychlorinated biphenyls (PCBs) could be associated with language delay in Norwegian children aged 3 [
51].
To study the effects of low-level Hg exposure on brain development through fish consumption and the interaction between Hg and Se, a prospective study was carried out on Italian children aged 40 months, to assess cumulative effects. 900 pregnant women were included; 767 remained in the study at the time of delivery and 470 children at the age of 40 months. After excluding premature births, 456 children were analyzed. The greatest difference in terms of risk of sub-optimal neurodevelopment was observed for the category with high Total Hg and low Se, with an OR = 2.55 (90% CI 1.02; 6.41) in the multiplicative model and an OR = 1.33 (90% CI 0.80; 1.87) in the additive model. The high THg and high Se categories showed a very slight fit of the additive model (OR = 1.07, 90% CI 0.65; 1.50) compared with the multiplicative model (OR = 1.66, 90% CI 0.73; 1.77). A ne
gative-antagonistic-interaction term for this category was estimated in the multiplicative model, giving an OR = 1.17 (90% CI 0.42; 3.28). Assessment of the effect of fish consumption on human health should also consider the various ratios between Se and Hg concentrations in different fish species [
51].
Endocrine effects: BDE209, a widely used industrial brominated flame retardant
-1(BFR), is a pollutant increasin
gly present in breast milk. It is a highly toxic pollutant for the thyroid gland and thyroid function and may promote thyroid cancer at multiple levels. For example, BDE209 interferes directly with thyroid, hypothalamic-pituitary-thyroid (HPT) axis and thyroid enzyme activities [
52], which may explain its effect on neurocognitive functions. A Chinese survey studied the average estimated daily intakes (EDIs) of TBBPA, HBCD and BDE-209 via human milk for infants aged 1-6 months were 39.2, 51.7 and 3.65 ng/kgbw/day respectively [
53].
4.9. Can We Compare Breast and Formula Milk? Which One Is Safer?
Breast milk is the best source of nutrition for newborns, and has been shown to provide nutritional, immunological, metabolic, organic and neurological benefits. Indeed, breast milk contains specific antibodies to protect the child, lipids beneficial for brain maturation and a reduced risk of obesity in children [
54]. For all these reasons, breast milk is superior to formula milk in terms of health benefits for the child. However, as a complex biological fluid, it consists not only of nutritional compounds but also contains environmental contaminants. From this point of view, breast milk may represent a risk. However, the real challenge lies in assessing the extent to which artificial milk is also contaminated, and in comparing different types of heterogeneous contamination [
3].
Artificial milk comes from cow’s milk, which is not immune to POP and heavy metal contamination, given that the cow’s diet is polluted by these same contaminants. Artificial milk can also be contaminated by plastic by-products during processing, which can reach the child via this mode of feeding [
55]. The advantages of breastfeeding are well established, but it is difficult to find studies comparing contamination levels in breast milk and artificial milk. The scientific community is awaiting more well-constructed studies to provide mothers with more transparent information about their choice of diet [
56]. The absence of such comparative data hinders a better approach to the benefits of breastfeeding [
58].
5. Discussion:
5.1. Can We Predict Chemical Load in Breast Milk?
Smith and colleagues (1987) described a method for quantifying POP contamination in breast milk. They estimated the mother’s average daily intake, an estimate of the half-life (t 1/2) of POPs as a function of body weight (BW). The Smith team’s approach calculates the biological half-life of POPs that are poorly metabolized and lipophilic. This concerns POPs that are bound in breast milk due to its high fat content. The biomonitoring of breastfeeding mothers through this study in the milk matrix is interesting because it’s a sensitive, reproducible method that allows comparisons over time and across different countries. This makes it possible to see the impact of public health policies on POPs reduction over the long term, particularly in developing and developed countries [
59].
5.2. Some Recommendations to the Mothers and Decision-Makers.
International recommendations are unanimous and concordant in justifying breastfeeding up to the child’s 2nd birthday, with exclusive breastfeeding up to 6 months of age. Breastfeeding is the only exclusive food recommended for newborns up to 6 months of age by the WHO and EFSA [
60,
61]. EFSA is a European scientific authority that issues recommendations based on literature analysis. It examines the question of whether foods other than breast milk or formula should be introduced, and what the consequences are for the child’s health. Based on nearly 300 scientific publications over the chosen period, it analyzes the impact of the addition of foods containing eggs, cereals, fish, soy or peanuts on children’s health. In particular, EFSA monitors the risk of obesity, sleep disorders, infections, iron deficiency and the occurrence of potential allergies. In its opinions for breastfeeding mothers, EFSA recommends that mothers refrain from smoking tobacco, THC [
62,
63], refrain from using narcotics while breastfeeding, use organic food, reduce cosmetics, reduce high-risk workplaces in line with legislation, refrain from using insecticides and pesticides at home for indoor plants.
6. Conclusion:
This comprehensive review underscores the pervasive presence of Persistent Organic Pollutants (POPs) and heavy metals in breast milk, highlighting the critical need for ongoing vigilance and research in this area. Our findings reveal that despite global efforts to reduce environmental contamination, pollutants such as Polychlorinated Biphenyls (PCBs) and Dichloro-Diphenyl-Trichloroethane (DDT) continue to be detected in breast milk samples worldwide. The implications of these contaminants on infant health remain a significant concern, given the potential for adverse developmental and health outcomes.
Significantly, our analysis indicates that while breast milk remains the most beneficial source of nutrition for infants, the detection of these substances points to a broader environmental issue that requires comprehensive policy and regulatory action. The WHO’s endorsement of exclusive breastfeeding for the first six months of life underscores the importance of reducing environmental pollutant exposure to protect the health of both mothers and infants.
Future research should focus on longitudinal studies to assess the long-term health impacts of early-life exposure to these pollutants. Additionally, further efforts are needed to enhance the monitoring and regulation of environmental pollutants, aiming to minimize their presence in human milk. By addressing these challenges, we can safeguard the health benefits of breastfeeding and ensure a healthier start for future generations.
In light of these findings, it is imperative to continue risk reduction strategies and policies at both national and international levels to protect mothers and infants from the potential risks posed by contaminants in breast milk. Our review calls for a united effort from policymakers, healthcare providers, and researchers to address this critical public health issue.
7. Perspectives:
POPs are characterized by their lipophilicity and bioaccumulation, which accounts for the putative presence of these pollutants in breast milk. The main objectives of this literature review were to update the origins of these pollutants and the mechanisms leading to their transfer to breast milk, the factors that may contribute to their occurrence and their possible impact on breastfed children.
The main recommendation that can be made is to inform mothers about the benefits of breastfeeding and the risks of not breastfeeding. It is critical to always compare two conditions for benefits and risks: breastfeeding vs not breastfeeding. The current knowledge argues in favor of breastfeeding despite the risks represented by the presence of pollutants and this is due to the important nutritional and immunological benefits of breastfeeding. Furthermore, formula-based diets may also contain contaminants which are often poorly characterized. The impact of the duration of breastfeeding is often raised, however, very few women actually breastfeed for a long time. Parents should also be aware that children can be contaminated at different periods of their development, from the prenatal period, the in utero period and via total oral feeding. The more informed they are, based on objective, scientific data, the better informed their choice will be. We propose to conduct a comparative scoping review exploring pesticide and other environmental exposures that formula-fed infants are exposed to.
Author Contributions
The Author Contributions statement must describe the contributions of individual authors referred to by their initials and, in doing so, all authors agree to be accountable for the content of the work. RS has made the scoping review, RS has written all the article, VR has written the discussion.
Funding
No fundings were provided for this scoping review.
Acknowledgments
RS: VR will acknowledge the Ile de France milk bank and ANSES to collaborate for the “ContaLait study” which was concerned with mercury measurements in breast milk from 180 women. RS, YT and VR will acknowledge Prof. Robert Barouki for his reviewing. We thank Prof. Robert Barouki for fruitful discussions on this topic. We thank Ethan and Aharona Freedman for English reviewing.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional File 1. Search Strategy
POP and Heavy metals into breast milk – Medline Search Strategy (Literature Search performed: June 15, 2023)
Persistent Organic Pollutant (n= 8042)
Persistent Organic Pollutant Milk (n= 341)
Persistent Organic Pollutant Breast Milk (n= 16)
Heavy metals (n= 445 470)
Heavy metals Milk (n = 6362)
Heavy metals Persistent Organic pollutant (n= 779)
Heavy metals Maternal Breast Milk (n= 54)
References
- Vacchina, V., Séby, F., Chekri, R., Verdeil, J., Dumont, J., Hulin, M., Sirot, V., Volatier, J.-L., Serreau, R., Rousseau, A., Simon, T., & Guérin, T. (2017). Optimization and validation of the methods for the total mercury and methylmercury determination in breast milk. Talanta, 167, 404-410. [CrossRef]
- LaKind, J. S., Brent, R. L., Dourson, M. L., Kacew, S., Koren, G., Sonawane, B., Tarzian, A. J., & Uhl, K. (2005). Human milk bio-monitoring data : Interpretation and risk assessment issues. Journal of Toxicology and Environmental Health. Part A, 68(20), 1713-1769. [CrossRef]
- Street, M. E., Shulhai, A.-M., Rotondo, R., Giannì, G., & Caffarelli, C. (2023). Current knowledge on the effects of environmental contaminants in early life nutrition. Frontiers in Nutrition, 10, 1120293. [CrossRef]
- Parizek, O., Gramblicka, T., Parizkova, D., Polachova, A., Bechynska, K., Dvorakova, D., Stupak, M., Dusek, J., Pavlikova, J., Topinka, J., Sram, R. J., & Pulkrabova, J. (2023). Assessment of organohalogenated pollutants in breast milk from the Czech Republic. The Science of the Total Environment, 871, 161938. [CrossRef]
- European Union. (2022). Regulation (EU) 2022/2002 of the European Parliament and of the Council of 25 October 2022 amending Regulation (EC) No 1881/2006 as regards maximum levels of dioxins and PCBs in certain foodstuffs. Official Journal of the European Union.
- U.S. Environmental Protection Agency. (2016). Drinking Water Health Advisories for PFOA and PFOS. EPA 822-R-16-005.
- European Commission. (2005). Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Official Journal of the European Union.
- European Union. (2021). Commission Regulation (EU) 2021/1317 of 9 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs. Official Journal of the European Union.
- Fiedler, H., Li, X., & Zhang, J. (2023). Persistent organic pollutants in human milk from primiparae—Correlations, global, re-gional, and national time-trends. Chemosphere, 313, 137484. [CrossRef]
- Motas, M., Jiménez, S., Oliva, J., Cámara, M. Á., & Pérez-Cárceles, M. D. (2021). Heavy Metals and Trace Elements in Human Breast Milk from Industrial/Mining and Agricultural Zones of Southeastern Spain. International Journal of Environmental Research and Public Health, 18(17), 9289. [CrossRef]
- Bansa, D. K., Awua, A. K., Boatin, R., Adom, T., Brown-Appiah, E. C., Amewosina, K. K., Diaba, A., Datoghe, D., & Okwabi, W. (2017). Cross-sectional assessment of infants’ exposure to toxic metals through breast milk in a prospective cohort study of mining communities in Ghana. BMC Public Health, 17(1), 505. [CrossRef]
- European Union. (2021). Regulation (EU) 2021/1317 of 9 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs. Official Journal of the European Union.
- European Union. (2006). Regulation (EC) No 1881/2006 of the European Parliament and of the Council of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union.
- European Union. (2021). Regulation (EU) 2021/1323 of 10 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in certain foodstuffs. Official Journal of the European Union.
- Lodovici, M., Dolara, P., Casalini, C., Ciappellano, S., & Testolin, G. (1995). Polycyclic aromatic hydrocarbon contamination in the Italian diet. Food Additives and Contaminants, 12(5), 703-713. [CrossRef]
- Mekonen, S., Ambelu, A., Wondafrash, M., Kolsteren, P., & Spanoghe, P. (2021). Exposure of infants to organochlorine pesticides from breast milk consumption in southwestern Ethiopia. Scientific Reports, 11(1), 22053. [CrossRef]
- Ministry of Agriculture and Food Sovereignty. (2024). Control of phytosanitary products: Maximum Residue Limits (MRLs). Retrieved February 12, 2024, from https://agriculture.gouv.fr/maitrise-des-produits-phytosanitaires-limites-maximales-de-residus-lmr.
- Gyllenhammar, I., Aune, M., Fridén, U., Cantillana, T., Bignert, A., Lignell, S., & Glynn, A. (2021). Are temporal trends of some persistent organochlorine and organobromine compounds in Swedish breast milk slowing down? Environmental Re-search, 197, 111117. [CrossRef]
- Public Health France. (2019). French population exposure to brominated flame retardants: National biomonitoring program Esteban 2014-2016. Public Health France. Retrieved from www.santepubliquefrance.fr.
- ter Schure, A. F. H., Larsson, P., Agrell, C., & Boon, J. P. (2004). Atmospheric transport of polybrominated diphenyl ethers and polychlorinated biphenyls to the Baltic Sea. Environmental Science & Technology, 38(5), 1282-1287. [CrossRef]
- Anderson, H. A., & Wolff, M. S. (2000). Environmental contaminants in human milk. Journal of Exposure Analysis and Environmental Epidemiology, 10(6 Pt 2), 755-760. [CrossRef]
- Castro, I., Arroyo, R., Aparicio, M., Martínez, M. Á., Rovira, J., Ares, S., Cunha, S. C., Casal, S., Oliveira Fernandes, J., Schuhmacher, M., Nadal, M., Rodríguez, J. M., & Fernández, L. (2021). Dietary Habits and Relationship with the Presence of Main and Trace Elements, Bisphenol A, Tetrabromobisphenol A, and the Lipid, Microbiological and Immunological Profiles of Breast Milk. Nutrients, 13(12), 4346. [CrossRef]
- Fång, J., Nyberg, E., Bignert, A., & Bergman, Å. (2013). Temporal trends of polychlorinated dibenzo-p-dioxins and dibenzofurans and dioxin-like polychlorinated biphenyls in mothers’ milk from Sweden, 1972-2011. Environment International, 60, 224-231. [CrossRef]
- Agus, S., Akkaya, H., Daglioglu, N., Eyuboglu, S., Atasayan, O., Mete, F., Colak, C., Sandal, S., & Yilmaz, B. (2022). Polychlorin-ated biphenyls and organochlorine pesticides in breast milk samples and their correlation with dietary and reproduc-tive factors in lactating mothers in Istanbul. Environmental Science and Pollution Research International, 29(3), 3463-3473. [CrossRef]
- Idowu, I. G., Megson, D., Tiktak, G., Dereviankin, M., & Sandau, C. D. (2023). Polychlorinated biphenyl (PCB) half-lives in hu-mans : A systematic review. Chemosphere, 345, 140359. [CrossRef]
- LaKind, J. S., Berlin, C. M., & Naiman, D. Q. (2001). Infant exposure to chemicals in breast milk in the United States : What we need to learn from a breast milk monitoring program. Environmental Health Perspectives, 109(1), 75-88. [CrossRef]
- Bhattacharya, S. S., Kim, K.-H., Ullah, Md. A., Goswami, L., Sahariah, B., Bhattacharyya, P., Cho, S.-B., & Hwang, O.-H. (2016). The effects of composting approaches on the emissions of anthropogenic volatile organic compounds : A comparison be-tween vermicomposting and general aerobic composting. Environmental Pollution, 208, 600-607. [CrossRef]
- Hurdzan, C. M., & Lanno, R. P. (2009). Determining exposure dose in soil : The effect of modifying factors on chlorinated benzene toxicity to earthworms. Chemosphere, 76(7), 946-951. [CrossRef]
- Kabeer, M. S., Hameed, I., Kashif, S.-U.-R., Khan, M., Tahir, A., Anum, F., Khan, S., & Raza, S. (2021). Contamination of heavy metals in poultry eggs : A study presenting relation between heavy metals in feed intake and eggs. Archives of Environ-mental & Occupational Health, 76(4), 220-232. [CrossRef]
- Bassil, M., Hassan, H., Elaridi, J., Abi, J., Mohamad, K., Abiad, G., Hassan, H., Kharma, J., & Abiad, M. (2021). Persistent Organic Pollutants in Human Milk : Exposure Levels and Determinants among Lactating Mothers in Lebanon. Journal of Food Pro-tection, 85. [CrossRef]
- Pius, C., Sichilongo, K., Koosaletse Mswela, P., & Dikinya, O. (2019). Monitoring polychlorinated diben-zo-p-dioxins/dibenzofurans and dioxin-like polychlorinated biphenyls in Africa since the implementation of the Stockholm Convention-an overview. Environmental Science and Pollution Research International, 26(1), 101-113. [CrossRef]
- Focant, J.-F., Fréry, N., Bidondo, M.-L., Eppe, G., Scholl, G., Saoudi, A., Oleko, A., & Vandentorren, S. (2013). Levels of polychlo-rinated dibenzo-p-dioxins, polychlorinated dibenzofurans and polychlorinated biphenyls in human milk from different regions of France. The Science of the Total Environment, 452-453, 155-162. [CrossRef]
- Guéguen, M., Amiard, J.-C., Arnich, N., Badot, P.-M., Claisse, D., Guérin, T., & Vernoux, J.-P. (2011). Shellfish and residual chemi-cal contaminants : Hazards, monitoring, and health risk assessment along French coasts. Reviews of Environmental Con-tamination and Toxicology, 213, 55-111. [CrossRef]
- Järup, L. (2003). Hazards of heavy metal contamination. British Medical Bulletin, 68, 167-182. [CrossRef]
- Beyene, T., Zosky, G. R., Gibson, P. G., McDonald, V. M., Holliday, E. G., Horvat, J. C., Vertigan, A. E., Van Buskirk, J., Morgan, G. G., Jegasothy, E., Hanigan, I., Murphy, V. E., & Jensen, M. E. (2023). The impact of the 2019/2020 Australian landscape fires on infant feeding and contaminants in breast milk in women with asthma. International Breastfeeding Journal, 18(1), 13. [CrossRef]
- Mandiá, N., Bermejo-Barrera, P., Herbello, P., López-Suárez, O., Fraga, J. M., Fernández-Pérez, C., & Couce, M. L. (2021). Human Milk Concentrations of Minerals, Essential and Toxic Trace Elements and Association with Selective Medical, Social, Demographic and Environmental Factors. Nutrients, 13(6), 1885. [CrossRef]
- Chovancová, J., Čonka, K., Kočan, A., & Sejáková, Z. S. (2011). PCDD, PCDF, PCB and PBDE concentrations in breast milk of mothers residing in selected areas of Slovakia. Chemosphere, 83(10), 1383-1390. [CrossRef]
- Kim, M.-J., Marchand, P., Henegar, C., Antignac, J.-P., Alili, R., Poitou, C., Bouillot, J.-L., Basdevant, A., Le Bizec, B., Barouki, R., & Clément, K. (2011). Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects be-fore and after drastic weight loss. Environmental Health Perspectives, 119(3), 377-383. [CrossRef]
- Acharya, N., Gautam, B., Subbiah, S., Rogge, M., Anderson, T., & Gao, W. (2019). Polycyclic aromatic hydrocarbons in breast milk of obese vs normal women : Infant exposure and risk assessment. Science of the Total Environment, 658-667.
- Boudet-Berquier, J., Salanave, B., Desenclos, J.-C., & Castetbon, K. (2018). Association between maternal prepregnancy obesity and breastfeeding duration : Data from a nationwide prospective birth cohort. Maternal & Child Nutrition, 14(2), e12507. [CrossRef]
- Oliveira, M., Duarte, S., Delerue-Matos, C., Pena, A., & Morais, S. (2020). Exposure of nursing mothers to polycyclic aromatic hydrocarbons : Levels of un-metabolized and metabolized compounds in breast milk, major sources of exposure and infants’ health risks. Environmental Pollution (Barking, Essex: 1987), 266(Pt 3), 115243. [CrossRef]
- Fénichel, P., Coquillard, P., Brucker-Davis, F., Marchand, P., Cano-Sancho, G., Boda, M., Antignac, J.-P., Iannelli, A., Gugenheim, J., Le Bizec, B., & Chevalier, N. (2021). Sustained bloodstream release of persistent organic pollutants induced by extensive weight loss after bariatric surgery : Implications for women of childbearing age. Environment International, 151, 106400. https://doi.org/. [CrossRef]
- Thomas, A., White, N. M., Leontjew Toms, L.-M., & Mengersen, K. (2019). Application of ensemble methods to analyse the de-cline of organochlorine pesticides in relation to the interactions between age, gender and time. PloS One, 14(11), e0223956. [CrossRef]
- LaKind, J. S., Berlin, C. M., Sjödin, A., Turner, W., Wang, R. Y., Needham, L. L., Paul, I. M., Stokes, J. L., Naiman, D. Q., & Patterson, D. G. (2009). Do human milk concentrations of persistent organic chemicals really decline during lactation? Chemical concentrations during lactation and milk/serum partitioning. Environmental health perspectives, 117(10), 1625-1631. [CrossRef]
- Salihovic, S., Lampa, E., Lindström, G., Lind, L., Lind, P. M., & van Bavel, B. (2012). Circulating levels of persistent organic pollu-tants (POPs) among elderly men and women from Sweden : Results from the Prospective Investigation of the Vascula-ture in Uppsala Seniors (PIVUS). Environment International, 44, 59-67. [CrossRef]
- Lehmann, G. M., Verner, M.-A., Luukinen, B., Henning, C., Assimon, S. A., LaKind, J. S., McLanahan, E. D., Phillips, L. J., Davis, M. H., Powers, C. M., Hines, E. P., Haddad, S., Longnecker, M. P., Poulsen, M. T., Farrer, D. G., Marchitti, S. A., Tan, Y.-M., Swartout, J. C., Sagiv, S. K., … Simmons, J. E. (2014). Improving the risk assessment of lipophilic persistent environ-mental chemicals in breast milk. Critical Reviews in Toxicology, 44(7), 600-617. [CrossRef]
- Kapraun, D. F., Zurlinden, T. J., Verner, M.-A., Chiang, C., Dzierlenga, M. W., Carlson, L. M., Schlosser, P. M., & Lehmann, G. M. (2022). A Generic Pharmacokinetic Model for Quantifying Mother-to-Offspring Transfer of Lipophilic Persistent Envi-ronmental Chemicals. Toxicological Sciences: An Official Journal of the Society of Toxicology, 189(2), 155-174. [CrossRef]
- Verner, M.-A., Plouffe, L., Kieskamp, K. K., Rodríguez-Leal, I., & Marchitti, S. A. (2017). Evaluating the influence of half-life, milk:plasma partition coefficient, and volume of distribution on lactational exposure to chemicals in children. Environ-ment International, 102, 223-229. [CrossRef]
- Chávez-Almazán, L. A., Saldarriaga-Noreña, H. A., Díaz-González, L., Garibo-Ruiz, D., & Waliszewski, S. M. (2023). Relationship Between DDT Concentrations with Multiparity and Breastfeeding History. Bulletin of Environmental Contamination and Toxicology, 111(3), 27. [CrossRef]
- Rovira, J., Martínez, M. Á., Mari, M., Cunha, S. C., Fernandes, J. O., Marmelo, I., Marques, A., Haug, L. S., Thomsen, C., Nadal, M., Domingo, J. L., & Schuhmacher, M. (2022). Mixture of environmental pollutants in breast milk from a Spanish cohort of nursing mothers. Environment International, 166, 107375. [CrossRef]
- Lorenzetti, S., Plösch, T., & Teller, I. C. (2021). Antioxidative Molecules in Human Milk and Environmental Contaminants. An-tioxidants (Basel, Switzerland), 10(4), 550. [CrossRef]
- Castriotta, L., Rosolen, V., Biggeri, A., Ronfani, L., Catelan, D., Mariuz, M., Bin, M., Brumatti, L. V., Horvat, M., & Barbone, F. (2020). The role of mercury, selenium and the Se-Hg antagonism on cognitive neurodevelopment : A 40-month fol-low-up of the Italian mother-child PHIME cohort. International Journal of Hygiene and Environmental Health, 230, 113604. [CrossRef]
- Wang, Y., Wang, X., Sui, S., & Liu, Z. (2023). Endocrine disrupting and carcinogenic effects of decabromodiphenyl ether. Frontiers in Endocrinology, 14, 1183815. [CrossRef]
- Shi, Z., Zhang, L., Zhao, Y., Sun, Z., Zhou, X., Li, J., & Wu, Y. (2017). A national survey of tetrabromobisphenol-A, hexabromocy-clododecane and decabrominated diphenyl ether in human milk from China : Occurrence and exposure assessment. The Science of the Total Environment, 599-600, 237-245. [CrossRef]
- van den Berg, M., Kypke, K., Kotz, A., Tritscher, A., Lee, S. Y., Magulova, K., Fiedler, H., & Malisch, R. (2017). WHO/UNEP global surveys of PCDDs, PCDFs, PCBs and DDTs in human milk and benefit-risk evaluation of breastfeeding. Archives of Toxi-cology, 91(1), 83-96. [CrossRef]
- Zhang, D., Xiao, J., Xiao, Q., Chen, Y., Li, X., Zheng, Q., Ma, J., Xu, J., Fu, J., Shen, J., Xiao, L., & Lu, S. (2023). Infant exposure to parabens, triclosan, and triclocarban via breastfeeding and formula supplementing in southern China. Science of The To-tal Environment, 858, 159820. https://doi.org/10.1016/j.scitotenv.2022.159820.
- Lehmann, G. M., LaKind, J. S., Davis, M. H., Hines, E. P., Marchitti, S. A., Alcala, C., & Lorber, M. (2018). Environmental Chemi-cals in Breast Milk and Formula : Exposure and Risk Assessment Implications. Environmental Health Perspectives, 126(9), 96001. [CrossRef]
- LaKind, J. S., Lehmann, G. M., Davis, M. H., Hines, E. P., Marchitti, S. A., Alcala, C., & Lorber, M. (2018). Infant Dietary Exposures to Environmental Chemicals and Infant/Child Health : A Critical Assessment of the Literature. Environmental Health Per-spectives, 126(9), 96002. [CrossRef]
- Smith, A. H. (1987). Infant exposure assessment for breast milk dioxins and furans derived from waste incineration emissions. Risk Analysis: An Official Publication of the Society for Risk Analysis, 7(3), 347-353. [CrossRef]
- Aburto, T. C., Romieu, I., Stern, M. C., Barquera, S., Corvalán, C., Hallal, P. C., Reynales-Shigematsu, L. M., Barnoya, J., Cavalcante, T. M., Canelo-Aybar, C., Santero, M., Feliu, A., Espina, C., & Rivera, J. A. (2023). Latin American and the Caribbean Code Against Cancer 1st edition : Weight, physical activity, diet, breastfeeding, and cancer. Cancer Epidemiol-ogy, 86 Suppl 1, 102436. [CrossRef]
- Harris, M., Schiff, D. M., Saia, K., Muftu, S., Standish, K. R., & Wachman, E. M. (2023). Academy of Breastfeeding Medicine Clinical Protocol #21 : Breastfeeding in the Setting of Substance Use and Substance Use Disorder (Revised 2023). Breastfeeding Medicine: The Official Journal of the Academy of Breastfeeding Medicine, 18(10), 715-733. [CrossRef]
- Navarrete, F., García-Gutiérrez, M. S., Gasparyan, A., Austrich-Olivares, A., Femenía, T., & Manzanares, J. (2020). Cannabis Use in Pregnant and Breastfeeding Women : Behavioral and Neurobiological Consequences. Frontiers in Psychiatry, 11, 586447. [CrossRef]
- Shenkoya, B., Yellepeddi, V., Mark, K., & Gopalakrishnan, M. (2023). Predicting Maternal and Infant Tetrahydrocannabinol Exposure in Lactating Cannabis Users : A Physiologically Based Pharmacokinetic Modeling Approach. Pharmaceutics, 15(10), 2467. [CrossRef]
Table 1.
Contaminants in Breast Milk.
Table 1.
Contaminants in Breast Milk.
| Contaminant Category |
Specific Contaminants |
Average Concentrations |
Max Allowed Levels |
Health Risks |
| Polychlorinated Biphenyls (PCBs) |
Indicator PCBs |
0.123 µg/g (123.12 ng/g) lipid weight [4] |
0.002 µg/g bw/week (2 pg TEQ/kg bw/week, EU) [5] |
Endocrine disruption, cancer, effects on nervous system development, immunotoxicity [4] |
| Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) |
PFOA, PFOS |
0.022 µg/L, 0.021 µg/L respectively [4] |
0.07 µg/L combined (70 ppt combined, PFOA + PFOS, EPA - Water) [6] |
Immune system effects, developmental delays, endocrine disruptions, fertility effects, increased risk of certain cancers [4] |
| Organochlorine Pesticides (OCPs) |
Various |
Not done |
0.01 mg/kg (10 µg/kg, general MRL for pesticides in EU) [7] |
Neurological effects, cancer, endocrine disruptions, reproductive and developmental effects [4] |
| Halogenated Flame Retardants (HFRs) |
Various |
Not done |
Exposure in children: 0.237-0.320 ng/kg bw/day [8] |
Hormone-dependent cancers, endocrine disruptions, developmental toxicity, effects on the nervous system [4] |
| Dioxins (PCDDs) and Furans (PCDFs) |
Various |
Observed decrease [9] |
0.002 µg/g bw/week (2 pg TEQ/kg bw/week, EU) [5] |
Cancer, reproductive and developmental problems, endocrine disruption, immune effects, chloracne [9] |
| Heavy Metals |
Lead, Cadmium |
Higher levels in mining and agricultural areas [10,11] |
Lead: 0.020 mg/kg (Milk, EU) [12], 0.015 mg/L (15 µg/L, EPA - Water) [13] ; Cadmium: 0.010 mg/kg (Infant food, EU), 0.005 mg/L (5 µg/L, EPA - Water) [14] |
Neurotoxicity, hypertension, reproductive issues. Cadmium: renal toxicity, osteoporosis, increased risk of certain cancers [10] |
| Polycyclic Aromatic Hydrocarbons (PAHs) |
PAHs 4 (group of four PAHs including Benzo[a]pyrene) |
0.0019 mg/kg in diet [15] |
Benzo(a)pyrene: 0.002 mg/kg, PAHs 4: 0.010 mg/kg [15] |
Cancer, genetic damage, reproductive effects, immunosuppression [15] |
| Dichlorodiphenyltrichloroethane (DDT) |
DDT, p,p’-DDE |
0.01124 mg/kg bw/day in infants [16] |
0.1 mg/kg body weight/day [17] |
Cancer, reproductive and developmental effects, endocrine disruptions, immunotoxicity [16] |
| Polybrominated Diphenyl Ethers (PBDEs) |
BDE-153, BDE-209 |
Observed decrease [18]0.00354 mg/kg of lipids [19] |
0.00102 mg/kg of lipids [19] |
Endocrine disruption, toxic effects on nervous system development in young children, reproductive effects [19] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).