1. Introduction
Increasing the efficiency and quality of pharmaceutical supply to the population is an urgent task of pharmacy. Due to the limited and steadily decreasing natural resources, there is an emergent tendency to discover new medicines through the complex processing of raw plant materials [
1,
2,
3]. This approach makes it possible to extend the range of herbal medicines, to use rationally natural resources, to increase the profitability of production, and to reduce its negative impact on the environment [
1,
4].
The pharmaceutical industry, as a rule, gets only one drug product from plant raw materials using either hydrophobic or hydrophilic substances in the final product. The remaining meal of the plant material is thrown away as a waste, although it still contains a significant number of biological active substances (BAS) with an opposite solubility [
5,
6,
7]. It is evident, however, that combining these substances in one final drug product can increase the effectiveness of pharmacotherapy.
The genus
Eucalyptus L'Her (
Myrtaceae) is characterized by a wide variety of almost 500 species. Eucalypt leaves have been used as medicinal raw materials for preparing hydrophobic BAS containing essential oils, tinctures, and “Chlorophyllipt” soft extracts (GNCLS Research Plant LLC, Kharkiv, Ukraine) [
8]. The state-of-the-art literature reveals that the species of this genus form mainly terpenes, polyphenolic compounds, flavonoids, stilbenes, polysaccharides, resins and waxes. The essential oil component is known in many species of
Eucalyptus, although monoterpenoids predominate in all essential oils [
9,
10,
11]. The composition of phenolic compounds in
E. globulus,
E. viminalis,
E. incrassata,
E. blakelyi,
E. rubida, and
E. grandis has been partially clarified [
12,
13,
14,
15,
16,
17,
18,
19,
20,
21].
The main therapeutic activity of eucalypt medicinal preparations is antiseptic. However, eucalypt preparations have also a pronounced anti-inflammatory effect [21, 22]. Furthermore, eucalypt preparations have shown to increase tonic activity, promote rapid healing of wounds, have analgesic and weak sedative activity, and act as a mild expectorant. In addition, eucalypt medicinal products are widely used for the treatment of infectious and inflammatory diseases of the upper respiratory tract, SARS, complex therapy of flu, neuralgia, myalgia, arthritis, rheumatism, sciatica, sports injuries, infections caused by
Staphylococcus aureus, etc [
21,
22,
23].
The Ukrainian pharmaceutical industry produces the anti-staphylococcal plant medicine "Chlorophyllipt" in various dosage forms, such as 1% ethanolic and 2% oil solutions, 0.25% solution for injections, spray, tablets and suppositories [
8]. The main active substances in these medicinal products are chlorophylls
a,
b and terpenes. The extraction of active substances from eucalypt leaves is carried out with 96% ethanol by using a six-fold re-percolation method with infusion for 18 hours at each stage. In the modification stage of extract, a 4% copper sulfate solution and benzene are used [
24]. Annually, 25–30 tons of eucalypt leaves are imported to Ukraine for the production of "Chlorophyllipt", while the residual plant meal becomes waste, although it still contains a significant amount of hydrophilic BAS. Thus, only isoprenoid compounds can be extracted in this six-fold re-percolation process. Therefore, there is an urgent need to study the BAS of eucalypt leaves for developing potential new medicines with antimicrobial and anti-inflammatory activity. Consequently, combining the hydrophobic and hydrophilic BAS of eucalypt leaves through a complex processing, enables to formulate appropriate dosage forms, and thus to accomplish this goal.
Three-dimensional (3D) printing is a promising new approach for the extemporaneous formulation of plant extracts, since it enables preparing individualized dosage forms, dosage accuracy and flexibility of the composition of the final dosage form. Such 3D-printed plant products are also highly innovative and sophisticated. To date, synthetic drug substances have been mainly used as an active agent in 3D-printed drug products, and only little is known about the 3D printing of plant-origin extracts and substances. Plant-origin materials are very often sensitive to organic solvents and high temperatures, which provides an additional challenge for a 3D printing process. Only a few studies on the pharmaceutical 3D printing of plant extracts have been published to date [
25,
26,
27]. A semi-solid extrusion (SSE) 3D-printing method is the most suitable for plant-origin materials because it can be performed at room temperature without any heating [
28,
29,
30].
The aim of this study was to promote the usability of plant materials in the pharmaceutical formulation by developing a new non-wasting extraction method for eucalypt leaves for preparing both hydrophobic soft eucalypt extracts and hydrophilic dry extracts. Moreover, the phytochemical and pharmacological properties of such extracts and their SSE 3D-printed dosage forms were investigated. The antimicrobial and anti-inflammatory activity of extracts and 3D-printed preparations were studied.
2. Results
The hydrophobic soft eucalypt extract was prepared using the “Chlorophyllipt” technological scheme. The extract was characterized as a semi-solid viscous mass with a dark green color and with a specific smell of 1,8-cineol.
The hydrophilic dry eucalypt extract was prepared from the wastes of the raw materials which was obtained after the extraction of an above-mentioned soft extract. The present dry extract was a hygroscopic powder with a color ranging from light-brown to brown and with a specific smell. The color depended on the quality of raw materials and drying conditions. The yield of hydrophilic dry extract was 9%.
2.1. Phytochemical study of the eucalypt extracts
The main terpenoids of hydrophobic soft eucalypt extract were quantitatively determined by means of gas chromatography (GC) equipped with a mass-spectrometric (MS) detector. The results are presented in
Table 1.
Total 28 substances were identified in the hydrophobic soft eucalypt extract. The quantitative content of chlorophyll derivatives in the soft extract was 3.31 ± 0.01%. The content of terpenes was 4.3 ± 0.02%, and essential oil (by hydro-distillation) 3.2 ± 0.1%.
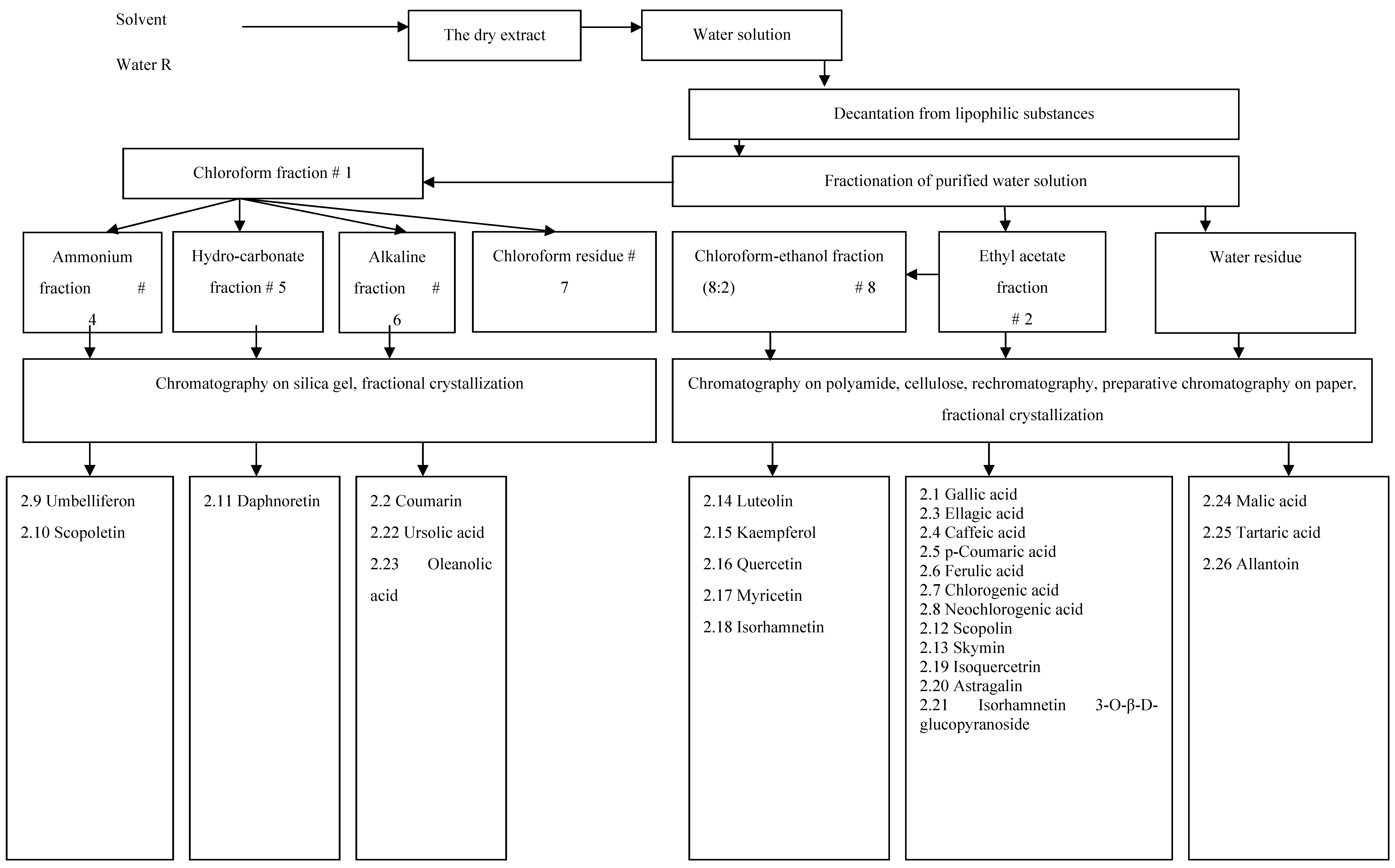
Figure 1 shows the scheme applied in the isolation and identification of the main substances of hydrophilic (aqueous) dry extract of eucalypt leaves. All substances that were isolated and identified in the dry eucalypt extract are listed in
Table 2. Total 57 substances were identified in the hydrophilic (aqueous) dry eucalypt extract, and 26 of them were isolated in an individual state. From the dry extract of eucalypt leaves, two phenolic acids (gallic and ellagic acid), five hydroxycinnamic acids (
p-coumaric, caffeic, ferulic, chlorogenic and neochlorogenic acid), six coumarins (coumarin, umbelliferon, scopoletin, daphnoretin, skymin and scopoline), eight flavonoids and their glycosides (luteolin, myricetin, quercetin, kaempferol, isorhamnetin, isoquercitrin, astragalin and isorhamnetin 3-O-β-D-glucopyranoside), two triterpenoids (ursolic and oleanolic acids), two organic acids (tartaric and malic acid), and one urea derivative (allantoin) were isolated. In addition, four sugars and 22 amino acids were identified.
The present results allowed us to select and adapt the methods for the assay of polysaccharides, amino acids, flavonoids and tannins in the dry extract of eucalypt leaves, and also enabled to select standards for calculation. The BAS groups and individual substances found in the dry eucalypt extract were shown to have antimicrobial (tannins, flavonoids) and anti-inflammatory activity (polyphenolic compounds, polysaccharides).
For further standardization of the dry eucalypt extract, the assay of amino acids, polysaccharides, hydroxycinnamic acids, flavonoids and polyphenolic compounds in the extract were carried out (
Table 3).
In our previous study, we developed the composition for a SSE 3D-printable PEO-eucalypt extract gel based on 20% aqueous PEO solution [
31,
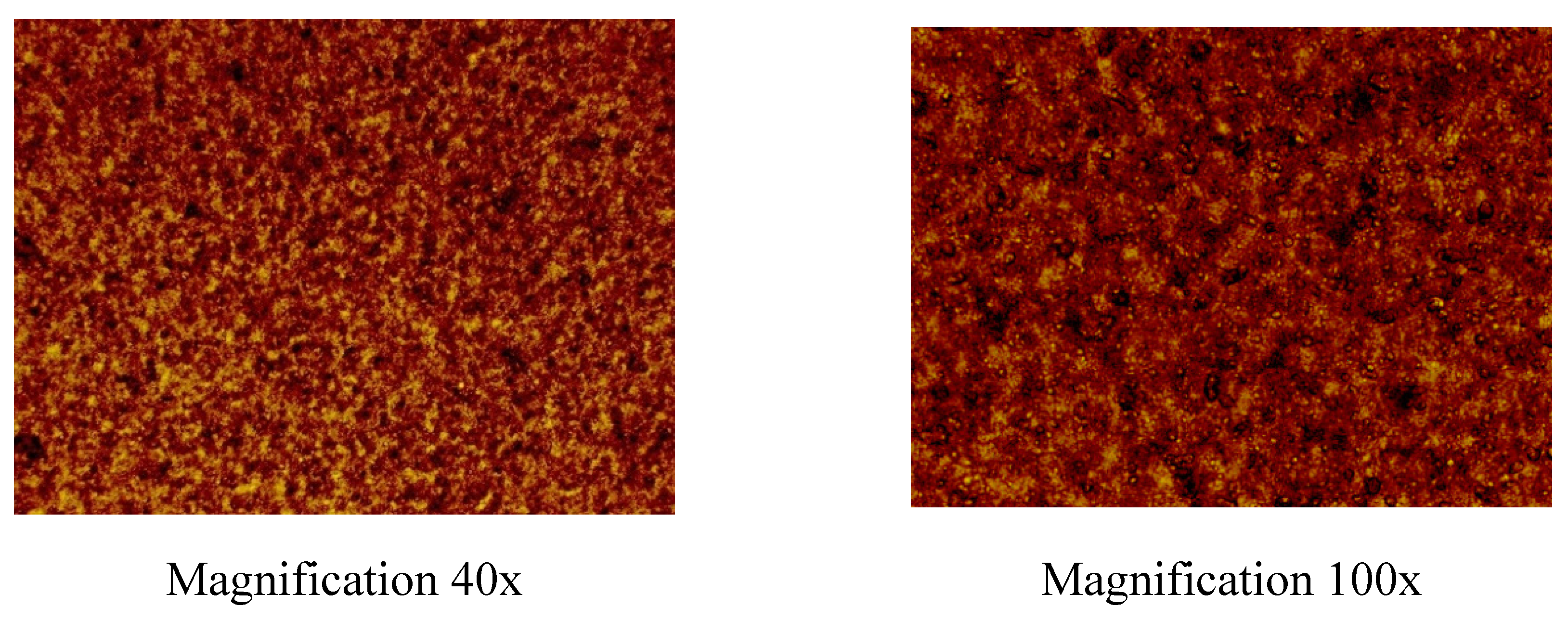
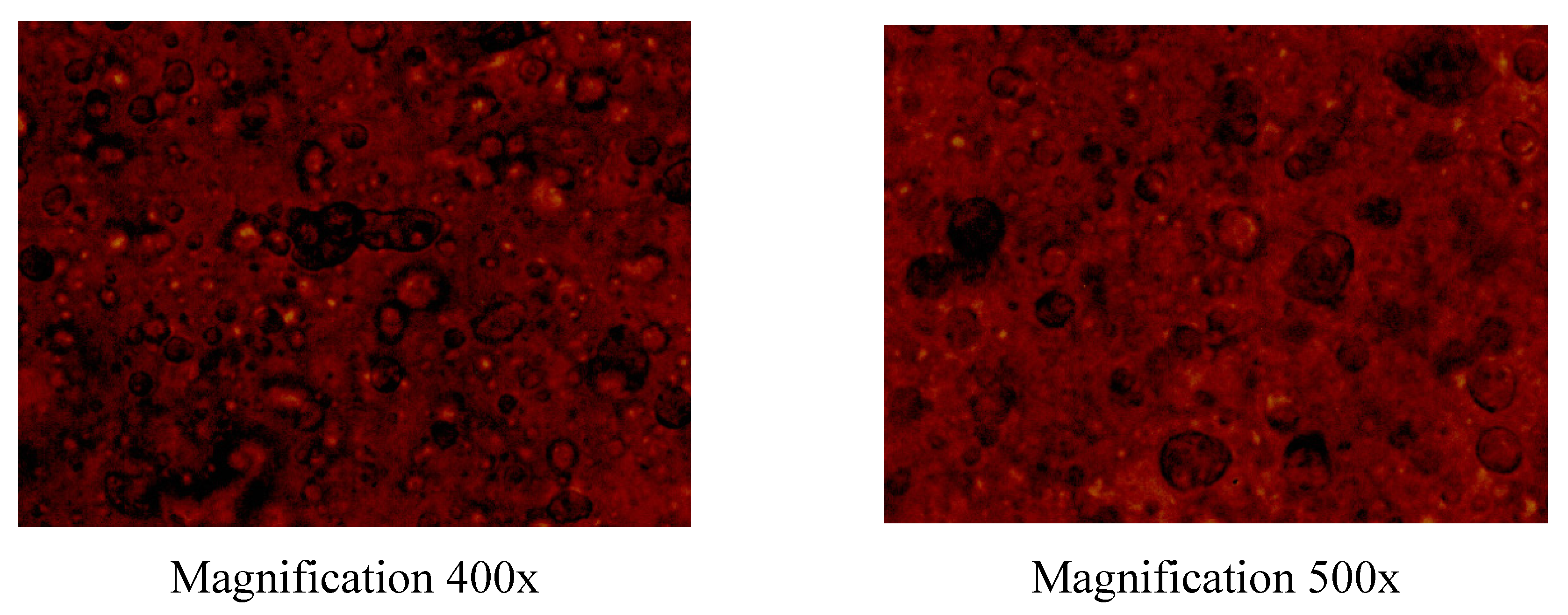
32]. In this study, the hydrophilic dry eucalypt extract, which has a pronounced anti-inflammatory activity, was added in this gel composition at the concentration of 10 mg/ml. The physical appearance and homogeneity of aqueous PEO-eucalypt extract gels were investigated by a visual inspection and light microscopy. As shown in
Figure 2, the aqueous PEO-eucalypt extract gels based on 20% PEO solution were homogeneous, and also uniform in structure.
The present PEO gels loaded with both hydrophobic soft eucalypt extract and hydrophilic dry extract were successfully 3D printed to composite small discs (10 mm diameter). The discs were used for study antimicrobial activity of these dosage forms.
2.2. Pharmacological Studies of the Eucalypt Extracts
2.2.1. Study of Antimicrobial Activity
Total 13 standard (culture collection) strains of microorganisms and 8 clinical strains obtained from patients with inflammatory diseases were used in the experiments. Six trials were conducted (n=6). The dry eucalypt extract was dissolved in water. The test concentrations were 1% and 10%.
The results of the antimicrobial activity study based on a serial-dilutions method and a diffusion-in-agar method are summarized in
Table 4 and
Table 5, respectively.
The eucalypt extracts showed antimicrobial activity against various taxonomic groups of microorganisms. The hydrophilic dry eucalypt extract has a significant antimicrobial activity against S. aureus, P. aeruginosa, B. subtilis, C. albicans, C. diphtheriae gravis and C. diphtheriae mitis.
The SSE 3D-printed round discs loaded with eucalypt extracts showed different growth-inhibition effects on the
S. aureus and
S. pyogenes strains used in an agar diffusion test (the maximum growth-free zone around the substance was 4 mm and 3 mm, respectively). As seen in
Table 6, a similar (but weaker) effect was observed with
S. mutans and
S. sobrinus strains (the growth inhibition zones up to 1 mm). The eucalypt extract discs did not have any effect on the growth of E. coli and
C. albicans. The two most effective eucalypt extract preparations were the one that contained 10 mg/ml of hydrophobic soft eucalypt extract and the other one with a combination of 10 mg/ml of both the eucalypt extracts in 20% PEO platform (
Table 6).
3. Discussion
The present noval binary non-wasting extraction method for eucalypt leaves enabled to get two extracts, one containing hydrophobic substances (a soft extract) and the other one containing hydrophilic substances (a dry extract). The proposed method is simple and it can be readily implemented in the pharmaceutical industry plants (e.g., in Ukraine) using standard equipment. Purified water is used as an extractant, thus making the process cheap and environmentally safe, since it does not require working with poisonous, flammable and harmful solvents and reagents. The extraction method utilizes (as a raw material) the meal of eucalypt leaves after extracting the hydrophobic fraction. The implementation of such binary extraction developed for this plant material, results in more rational use of natural resources, increases the profitability of production and reduces a negative impact on the environment. Moreover, the extracts prepared by the present non-wasting method are most likely applicable as a BAS rich material for the preparing various medicinal and food supplement products.
In the present study, we found that the main BAS-s of the hydrophobic soft extract are terpenes and chlorophylls, which have been reported to have a significant antimicrobial activity [
22,
33,
34]. Our results indicated that such BAS-s have a significant antimicrobial activity against
S. aureus.
The following BAS-s were isolated from the hydrophilic dry extract of
Eucalyptus viminalis Labill. leaves for the first time ever: two phenolic acids (gallic and ellagic acid), five hydroxycinnamic acids (p-coumaric, caffeic, ferulic, chlorogenic and neochlorogenic acid), six coumarins (coumarin, umbelliferon, scopoletin, daphnoretin, skymin and scopolin), eight flavonoids and their glycosides (luteolin, myricetin, quercetin, kaempferol, isorhamnetin, isoquercitrin, astragalin and isorhamnetin 3-O-β-D-glucopyranoside), two triterpenoids (ursolic and oleanolic acids), two organic acids (tartaric and malic acid), and one urea derivative (allantoin). In addition, four sugars and 22 amino acids were identified. The BAS-s found in the hydrophilic eucalypt extract have shown to possess antimicrobial and anti-inflammatory activity.[
3,
4,
21]. In the present study, we confirmed experimentally that such BAS-s have above-mentioned activities.
The hydrophilic dry eucalypt extract showed a broader range antimicrobial activity compared to that observed with the hydrophobic soft extract. The hydrophilic dry extract (unlike hydrophobic soft extract) had a clear antimicrobial activity against, e.g. P. aeruginosa, B. subtilis, C. albicans, C. diphtheriae gravis and C. diphtheriae mitis. Moreover, the hydrophilic dry extract had a pronounced anti-inflammatory activity. These results indicate the feasibility of combining these extracts in one dosage form. Such medicinal product could enhance, e.g. wound healing and relieve the inflammation of damaged tissue.
In this study, we used SSE 3D printing technology for preparing novel eucalypt extract –loaded dosage forms potentially for medicinal applications. Previously, we found the optimal printing parameters and developed the optimized composition of the basic PEO gel loaded with a hydrophobic soft eucalypt extract for SSE 3D printing [
31,
32]. Based on the previous formulation development, we were able to successfully 3D print the novel types of round scaffolds (small discs) loaded with the hydrophilic dry eucalypt extract at different concentrations. In addition, the 3D-printed composite scaffolds (small discs) loaded with both hydrophilic dry extract and hydrophobic soft extract were prepared and their antimicrobial activity were evaluated in vitro.
The antimicrobial effect of the 3D-printed preparations of eucalypt extracts was found to be microbial species -specific being more expressed against gram-positive cocci. Eucalypt extracts inhibited the growth of main pathogens causing wound and upper respiratory tract infections, such as
S. aureus and
S. pyogenes. The extracts showed also inhibiting activity against caries-causing pathogens, such as
S. mutans and
S. sobrinus. Similar activity against
S. aureus was observed in our previous study [
32]. According to the literature, eucalypt possesses some growth inhibition capacity against different streptococci [
33,
35]. However, to date virtually nothing is known about the effect of 3D-printed preparations (scaffolds) loaded with eucalypt extracts on the viability of
S. pyogenes,
S. mutans and
S. sobrinus.
In this study, we used the reference strains and clinical strains of several bacteria and one yeast in order to investigate the antimicrobial effect of eucalypt extracts. It is well known that the selected set of target microorganisms can cause several infections of oral cavity and upper respiratory tract. For example, S. pyogenes is associated with tonsillitis, and S. mutans and S. sobrinus with dental caries. Candida albicans causes candidiasis of oral cavity and other locations, and C. diphtheriae is causative agent of diphtheria. The microorganisms used in our study can also cause intestinal infections (S. enterica, S. flexneri), and wound infections and other opportunistic infections (S. aureus, E. coli, K. pneumoniae, P. vulgaris, P. aeruginosa, B. subtilis). In this study, we found that the 3D-printed discs (scaffolds) loaded with a hydrophilic dry eucalypt extract showed a weaker antimicrobial activity compared to that observed with the corresponding 3D-printed preparations loaded with a hydrophobic soft extract. However, since the hydrophilic dry extract has a significant anti-inflammatory activity, it would be justified to combine these two extracts in one 3D-printed preparation to achieve the dual synergistic effect of such extracts.
4. Materials and Methods
4.1. Raw Materials
Eucalyptus viminalis Labill. leaves (Ltd “LIKTRAVY”, Zhytomyr, Ukraine) were used as a raw material for preparing the plant extracts. It complies with the requirements of the State Pharmacopeia of Ukraine [
36].
4.2. Preparation of Eucalypt Extracts
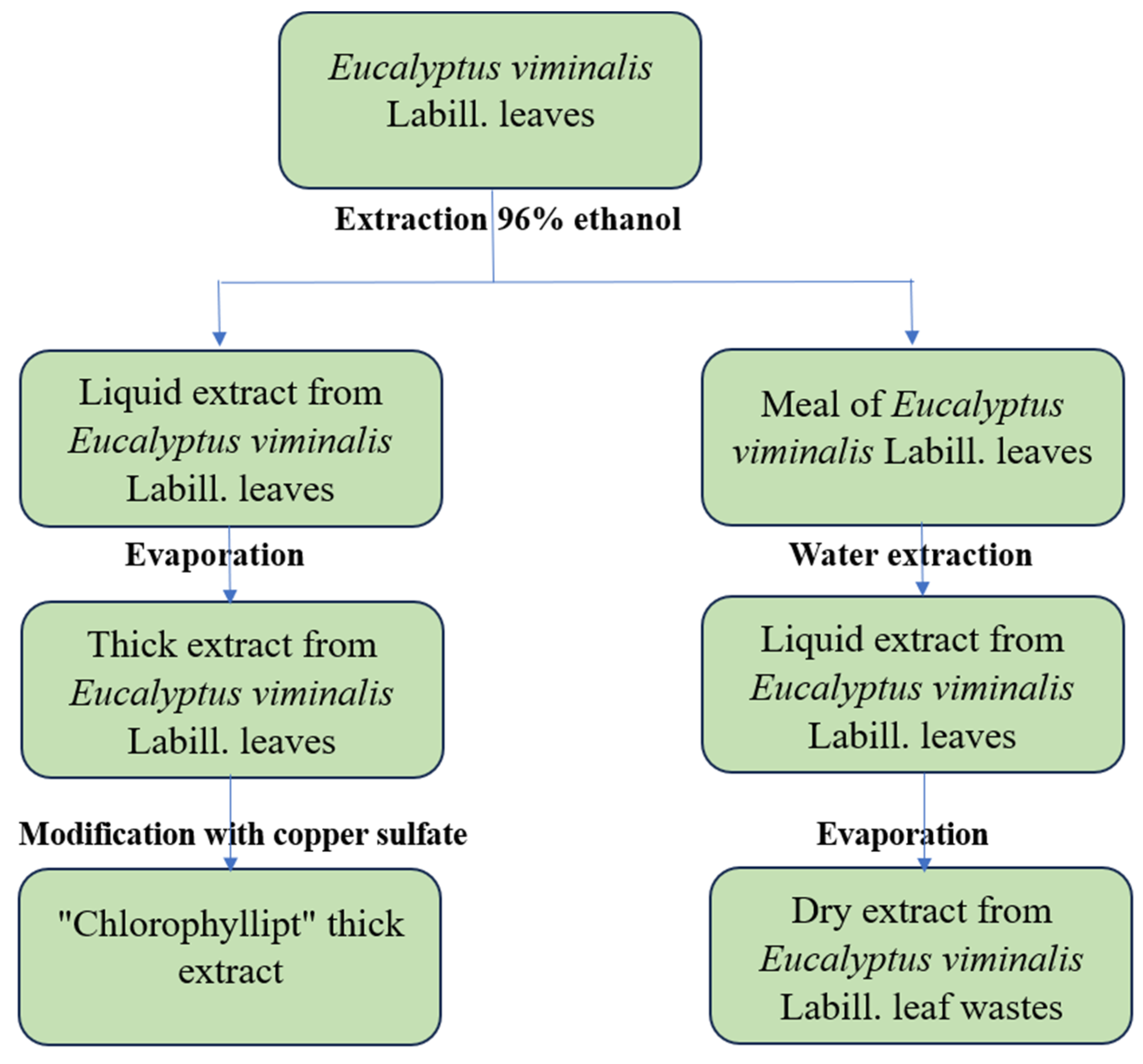
The hydrophobic soft and hydrophilic (aqueous) dry extracts of eucalypt leaves were prepared according to the scheme shown in
Figure 4.
First, 7 kg of dry eucalypt leaves was crushed by rolling to particle sizes of 2.5-3 mm, and then the crushed leaves were mixed with 21 liters of 96% ethanol. A total of 82 liters of eucalypt ethanolic extract was obtained after a 4-fold extraction. From this ethanolic extract, “Chlorophyllipt” soft extract was subsequently obtained [
24]. After the regeneration of ethanol from the powdered raw material, it was extracted with 21 liters of purified water at 95 °C for 2 hours and infused for subsequent 10-12 hours. The extraction was repeated three times at the same way [
37]. The combined extract (60.5 liters) was evaporated at 85 °C under vacuum in a vacuum circulation apparatus at a dilution of 690 mm Hg to a volume of water residue 20 : l. This residue was a semi-solid, transparent dark brown liquid, which was kept for 4 days at 4 ± 2 °C. The separated supernatant was sterilized and dried in a spray dryer (EVZ-01-RC-1,2-09-NK-21, "50th anniversary of October" factory, 1989, USSR) with a coolant temperature of 95 °C at the inlet and 65 °C at the outlet to the dry extract.
4.3. Preparation of eucalypt extracts –loaded gels for 3D printing
The aqueous gels of polyethylene oxide (PEO) (MW approx. 900,000, Sigma-Aldrich, USA) at concentrations of 20% were used as a formulation platform for the SSE 3D printing of the eucalypt extracts. For preparing such gels, PEO (2.0 g) was dissolved in water
R (5 ml) approximately for at least 13–15 hours at an ambient room temperature to form a viscous gel [
31,
32,
38]. Eumulgin SMO 20 (polyethylene lycol 40–hydrogenated castor oil, Polysorbate 80) was used to enhance the release of the eucalypt extracts from the 3D-printed preparations [
31,
32]. The soft eucalypt extract (100.0 mg) was solved in 1 ml of ethanol, mixed carefully with Eumulgin SMO 20 and added to 2 ml of water. The dry eucalypt extract (100.0 mg) was dissolved in 2 ml of water. The solution of a soft extract was added to the solution of dry extract and mixed carefully. Finally, this mixture was added to PEO gels.
4.4.3. D printing of the eucalypt extracts
The PEO gels loaded with the eucalypt extracts were directly printed using a bench-top SSE 3D printing system (System 30 M, Hyrel 3D, Norcross, GA, USA). The printing head consists of a steel syringe with a plunger connected to a stepper motor. A blunt needle (Gauge, 21G) connected to a syringe serves as a printing nozzle. The printing head (i.e., a syringe with a nozzle) was not heated. The printing plate temperature was 30 °C.
During SSE 3D printing, a printing head moved at a set speed on the X-Y axis (= printing speed) and extruded printing material at a specified speed through a nozzle system (= extrusion speed) onto a thermostated printing plate. Following every printed layer, a printing plate was lowered by a predefined distance (layer height), thus allowing a printing head to create another layer of material on the top of a printed object. The printing head speed used was 0.5 mm/s. The software of an SSE 3D printer controls the temperature of the printing head and plate, the moving speed of the printing head, the gel extrusion rate, and other settings. Total five (5) layers were printed in preparing each round-shaped disc, and the present 3D-printed discs were used in a microbiological study. A round-shaped disc preparation (10 mm in diameter) was designed using FreeCAD software (vers. 0.19/release date 2021) [
39]. The 3D-printed round-shaped discs of PEO were weighed with an analytical scale (Scaltec SBC 33, Scaltec, Germany) and subsequently photographed.
4.5. Phytochemical analysis
The three different plates, "Silufol UV-254", "Silufol UV-366" and "Sorbfil" - PTSH-A-UV, were used for thin-layer chromatography (TLC). The ascending and descending one-dimensional, two-dimensional and multiple TLC methods were used. The results of the Rf value on the chromatograms are the average values of 5-6 determinations.
The solvents used in TLC were applied in volume units. The following solvent systems were used for TLC: # 1 n-butanol – acetic acid –water (4:1:2); # 2 30% acetic acid; # 3 15% acetic acid; # 4 hexane (formamide 25%); # 5 chloroform (formamide 25%); # 6 – toluene – n-butanol (3:1)/water (35%); # 7 chloroform – acetic acid – water (13:6:2); # 8 toluene – ethyl acetate –acetic acid (12:4:0.5); # 9 ethyl acetate – formic acid – water (3:1:1).
Cellulose (0.25-0.5 mm fraction), KSK brand silica gel with a particle size of 0.25 to 0.75 mm, LS 100/250 brand, and polyamide sorbent powder with a particle size ranging from 0.25 to 0.75 mm, were used for column chromatography.
The substances were analyzed after two- and three-fold crystallization from the appropriate solvents and after drying in a vacuum at 10-2 mm Hg over phosphoric anhydride for 4 hours at 110-115 °С. The melting point was determined by a capillary method [
36]. UV absorption spectra and optical density of solutions were recorded with a Hewlett Packard 8453 spectrophotometer (Santa Clara, CA, USA) and using the cuvettes with a layer thickness of 10 mm. IR spectra were recorded with a UR-20 spectrometer (Сarl Zeiss, Jena, Germany) using potassium bromide tablets (1 mm high) at a ratio of substance to filler of 1:200-1:400. The optical activity of glycosides was measured with a SPU-E polarimeter (Schmidt+Haensch GmbH & Co., Berlin, Germany).
Qualitative and quantitative analysis of terpenoids. The terpenoids of the soft eucalypt extract were quantitatively determined by means of gas chromatography, GC (Agilent Technology 6890, Santa Clara, CA, USA) equipped with a 5973 mass-spectrometric (MS) detector under standard conditions: HP-5 on fused silica capillary columns with bonded stationary phases SPB-5 (30 m × 0.25 mm, Supelco), film thickness of stationary phases was 0.25 μm. The temperature program was from 50°C to 250°C at 4°C/ min, and the injector temperature was 250°C. Injection volume: 2.0 μl, two injections were carried out. Carrier gas He with split ratio 150:1, and the flow rate of 1 ml/min was applied. The temperature of the interface: 250°C; ion source temperature: 230°C. The mass spectrometry (MS) detector was operated in the electron ionization mode 70 eV at a scan rate of 2 scans/sec with an acquisition mass range of 29–450 a.m.u. The identification of compounds was performed based on the comparison of the mass spectra obtained with the data from the NIST05-WILEY library (approximately 500,000 mass spectra) [
40]. The retention indices of the components were calculated based on the results of control analyses of compounds when adding a mixture of normal alkanes (С10– С18).
Qualitative and quantitative analysis of free amino acids in the dry eucalypt extract was carried out using an amino acid analyzer T 339 (Mikrotekhnika, Prague, Czech Republic) [
41]. In the analysis, we used the columns with a water jacket (0.37 x 25 cm) and with ionite osteon LGANB, and a sequence of lithium-citrate buffer solutions of different acidity and ionic strength (pH 2.9+0.01; 2.95+0.01; 3.2+0.02; 3.8+0.02 and 5 +0.02) was used as a mobile phase. The flow rate of the buffer solutions was 12 ml/h, the reagent rate was 8-10 ml/h. Detection was carried out using post-column staining with a solution of ninhydrin in DMSO at a wavelength of 570 nm. Quantification was performed using standard solutions of amino acids (TU 6-09-3147-83).
Study of the quantitative content of amino acids by a spectrophotometric method. A spectrophotometric method was used to determine the quantitative content of amino acids in the dry eucalypt extract. Since the average molecular weight of amino acids in eucalypt leaf extract is 137 g/mol, which is the closest to the value of the molecular weight of leucine (131 g/mol), the quantitative determination was carried out in terms of this amino acid [
42].
Quantitative determination of polysaccharides by a gravimetric method. Approximately 1.0 g (exact weight) of the extract was added to 10 ml of water. The flask was then connected to a reflux condenser and boiled with stirring for 30 min. Polysaccharides were precipitated three times using ethanol, and the precipitate was separated by centrifugation at a rotation speed of 5000 rpm for 10 min. Finally, the precipitate was dried to a constant mass and weighed on an analytical balance [
36].
Quantitative determination of polysaccharides by spectrophotometric method. Determination of polysaccharides content in the extract of eucalypt leaves was carried out by the spectrophotometric method in terms of glucose units after sulfuric acid hydrolysis for 2.5 hours, neutralization and reaction with a 1% aqueous solution of picric acid [
43].
Quantitative determination of hydroxycinnamic acids. Caffeic,
p-coumaric, ferulic, neochlorogenic and chlorogenic acids were identified in the extract of eucalypt leaves by a TLC method. The largest area of spots referred to the highest content of chlorogenic acid in TLC. The content of hydroxycinnamic acid derivatives in the dry extract of eucalypt leaves was determined by a spectrophotometric method in terms of chlorogenic acid. The absorption maximum of chlorogenic acid standard was observed at 327 nm, so the measurements were performed at this wavelength [
3,
42,
44].
Quantitative determination of flavonoids. Previous studies have shown the presence of total eight (8) flavonoid compounds (one flavone and seven flavonols,
Table 1) in the dry extract. For verifying the correlation of the methods and the feasibility of the standard used (and a conversion validity), the content of flavonoids was determined by a spectrophotometric method in terms of rutin and quercetin [
42,
45,
46,
47].
Determination of tannins content by a complexometric method. Complexonometric method was selected for the determination of tannins, because it is more selective for tannins in a mixture with accompanying substances (flavonoids, phenolic acids, etc.) [
48,
49,
50].
Determination of the total content of polyphenolic compounds by a spectrophotometric method in terms of gallic acid. The present method was selected, since gallic acid is the main component of polyphenolic compounds [
42,
44,
51].
Quantitative determination of chlorophylls. Chlorophyll content was determined by a spectrophotometric method at the wavelengths of 649 and 665 nm [
31,
32,
34].
4.6. Study of antimicrobial activity
The study of the antimicrobial activity of the eucalypt extracts was carried out by the method of serial dilutions in a liquid nutrient medium and by the method of diffusion in agar at the I.I. Mechnikov Institute of Microbiology and Immunology in the Laboratory of Biochemistry of Microorganisms and Nutrient Media (Kharkov, Ukraine) under the supervision of Ph.D. Osolodchenko T.P. [
52,
53,
54]
In accordance with the WHO recommendations, the reference strains Staphylococcus aureus ATCC 25923, Staphylococcus aureus 6538 ATCC, Escherichia coli ATCC 25922, Proteus vulgaris NSTC 4636, Pseudomonas aeruginosa ATCC 27853, Pseudomonas aeruginosa 9027 ATCC, Bacillus subtilis ATCC 6633, Candida albicans 885/653 ATCC, were used to evaluate the antimicrobial activity of the extracts. In addition, the study was conducted on clinical strains that were in the laboratory: Salmonella enterica Typhimurium 144, Salmonella enterica Paratyphi A-290, Shigella flexneri 170, Corynebacterium diphtheriae gravis 14 tox+, C. diphtheriae mitis 6 tox+. Nutrient broth was used for the cultivation of microorganisms, and for cultivation glucose was added at the rate of 3 ml per 100 ml of broth.
The method of serial dilutions enabled the quantitative assessment of the antimicrobial activity for the extracts. The meat-peptone nutrient medium was poured into 10 test tubes of 2 ml each. Total 2 ml of the prepared eucalypt extract solution was added to the first test tube and mixed, and 2 ml was taken and transferred to the next test tube, etc. Then 2 ml of the mixture was drained from the last test tube. Next 0.2 ml of the prepared culture was added to each test tube (including the control one) at the rate of 107-108 CFU/ml. The standard of the seed crop was established according to the optical standard of turbidity of DIX named after L. O. Tarasevich. The crops were placed in a thermostat for 18-24 hours at 37 °С. The results were noted by the presence or absence of turbidity of the medium in test tubes containing different dilutions of the experimental extract. The concentration of the drug in the last test tube with a transparent medium (absence of growth of the test microbe) corresponds to the minimum inhibitory concentration (MIC) of the drug.
For determining a bactericidal concentration, 0.1 ml of a transparent medium from the last two-three tubes (absence of visible growth), was placed on the cups with a dense nutrient medium or tubes with broth. Incubation was carried out for 18-24 hours at 37 °C and the minimum concentration of the drug was registered (the seed from which did not grow on agar or in broth). The present amount of drug corresponds to its minimum bactericidal concentration.
The method of the extracts diffusion in agar was carried out by using "wells". Determination of the activity of the extracts was carried out on two layers of a dense nutrient medium poured into a Petri dish. "Hungry" non-seeded media (agar-agar, water, salts) were used in the lower layer. The bottom layer is a substrate (10 mm in height) on which 3-6 thin-walled stainless steel cylinders with a diameter of 8 mm and a height of 10 mm are installed strictly horizontally. The cylinders were covered with an upper layer consisting of nutrient agar medium, and then melted and cooled to 40 °C. In this medium, the appropriate standard of daily culture of the test microbe was introduced. Previously, the upper layer was well mixed until a homogeneous mass was formed. After hardening, the cylinders were pulled out of the formed hole with sterile tweezers, and the tested substance was introduced considering its volume. The thickness of the medium for the upper layer ranged from 14 to 16 mm. The cups were dried for 30-40 minutes at an ambient room temperature and placed in a thermostat for 18-24 hours.
For investigating new antibacterial agents and antibiotic-resistant strains, the following criteria were used: the absence of growth inhibition zone around the hole and the growth inhibition zone up to 10 mm, indicate that the microorganism is not sensitive to the drug into the hole or its concentration; the growth inhibition zone with a diameter of 10-15 mm indicates the low sensitivity of culture to the tested concentration of the antibacterial substance; the growth inhibition zone with a diameter of 15-25 mm indicates the sensitivity of the microorganism to the tested medicinal product; the growth inhibition zone with the diameter exceeding 25 mm, indicates a high sensitivity of microorganisms to experimental drugs.
The method for investigating antimicrobial activity of the 3D-printed forms.
The antimicrobial effect of eucalypt extract was investigated on total six (6) microbial species each representing with three culture collection strains. The microbial species used were Staphylococcus aureus (ATCC 29213, HUMB 19417, HUMB 5630); Escherichia coli (ATCC 25922, HUMB 7024, HUMB 5666); Candida albicans (ATCC 10231, HUMB 05355, HUMB 19373); Streptococcus pyogenes (DSM 25943, HUMB 18939, HUMB 18966); Streptococcus mutans (HUMB 13076, HUMB 13034, HUMB 13033); Streptococcus sobrinus (HUMB 13087, HUMB 13104, HUMB 13038).
A suspension of microbes was prepared in PBS buffer with a density of 0.5 McFarland and applied to the following agar plates: Mueller-Hinton agar (S. aureus, E. coli, C. albicans), and Mueller-Hinton agar with blood (Streptococcus spp.). The 3D-printed eucalypt extract moulds were placed on the top of agar and incubated in a 10% CO2 incubator for 22 hours. The diameter of a growth-free zone around the eucalypt 3D-printed preparation was measured.
4.7. Study of anti-inflammatory activity
The pharmacological studies were conducted in compliance with the rules of the “European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes” (Strasbourg, 1986), Directive 2010/63/EU of the European Parliament, and the Council of the European Union (2010) on the protection of animals used for scientific purposes. The Order of the Ministry of Health of Ukraine No. 944 “On Approval of the Procedure for Preclinical Study of Medicinal Products and Examination of Materials for Preclinical Study of Medicinal Products” (2009) and the Law of Ukraine No. 3447-IV “On the protection of animals from cruel treatment” (2006) were also strictly followed. The present research work was approved by the Bioethics Commission of the National University of Pharmacy (protocol №4 from 03.10.2023) [
55,
56,
57,
58,
59]
The anti-inflammatory activity of dry extracts was studied with white mice (17-22 g) and using a formalin oedema model [
52,
60]. Diclofenac sodium (Voltaren
®) was chosen as a reference drug. The mice were divided into three groups (n = 8): a control group, the group treated with an eucalypt extract, and the group treated with a reference drug.
The degree of anti-inflammatory activity of the extract was evaluated based on the anti-exudative effect. To reproduce acute aseptic exudative inflammation, a 2% formalin solution was injected (0.05 ml) subplantarly one hour after the oral administration of dry extract, the reference drug (diclofenac sodium), and in the control group - water. The anti-inflammatory activity of the experimental agents was studied by their ability to reduce the development of oedema in comparison with the control.
4.8. Statistical Analysis
The statistical properties of random variables (with an n-dimensional normal distribution) are given by their correlation matrices, which can be calculated from the original matrices. A statistical assessment of the data was performed using MS Excel (Microsoft Excel 2016, version 16.0, Microsoft Corporation, Redmond, DC, USA). P values less than 0.05 were considered as statistically significant [
36,
61].
5. Conclusions
The present study introduces a new non-wasting extraction method for eucalypt leaves, which enables to prepare both hydrophobic soft extracts and hydrophilic dry extracts with a potential pharmacological activity. Such extraction method could support the pharmaceutical industry to use plant materials in a formulation development by more comprehensive way. Total 28 terpenes were identified in the hydrophobic soft extract. In the hydrophilic dry extract, total 57 substances were identified, and 26 of them were isolated in an individual state. The eucalypt extracts and their 3D-printed dosage forms have antimicrobial and anti-inflammatory activity. The eucalypt extracts (especially, hydrophobic soft extract) present an antimicrobial activity against various taxonomic groups of microorganisms such as S. aureus, P. aeruginosa, B. subtilis, C. albicans, C. diphtheriae gravis and C. diphtheriae mitis. The hydrophilic dry extract has in turn a pronounced anti-inflammatory activity. The maximum anti-exudative effect of hydrophilic dry extract (61.5%) was observed with mice at a dose of 20 mg/kg. The composite gels of polyethylene oxide (PEO) and eucalypt extract were developed, and the key process parameters for semi-solid extrusion (SSE) 3D printing of such gels were verified. The SSE 3D-printed preparations of synergistically acting eucalypt extracts could have uses in antimicrobial and anti-inflammatory medicinal applications.
Author Contributions
Conceptualization, O.K., A.K., T.O., J.H., R.M. and A.R.; methodology, O.K., A.K., T.O., J.H., R.M. and A.R..; software, O.K., M.K., and S.K.; validation, O.K. and A.R.; formal analysis, M.K., T.O., S.K. and O.K.; investigation, M.K., T.O., S.K. and O.K.; resources, O.K., A.K., R.M., J.H. and A.R.; data curation, M.K., O.K. and A.R.; writing—original draft preparation, O.K., R.M., S.K., A.K., J.H. and A.R.; writing—review and editing, O.K., J.H., R.M. and A.R.; visualization, M.K., T.O., S.K. and O.K.; supervision, O.K., A.K., R.M., J.H. and A.R.; project administration, O.K., R.M., J.H. and A.R.; funding acquisition, J.H. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Estonian Research Council grant (PRG1903), CurifyLabs project (VMVFA22189), Estonian Ministry of Education and Research (KOGU-HUMB), and the European Union in the MSCA4Ukraine project “Design and development of 3D-printed medicines for bioactive materials of Ukrainian and Estonian medicinal plants origin” [ID number 1232466].
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors sincerely thank all the defenders of Ukraine who made the performance of this study possible. The authors sincerely appreciate the support of the Partners who stand with Ukraine.
Conflicts of Interest
The authors declare no conflicts of interest
References
- Martin, N.; Madrid-López, C.; Villalba-Méndez, G.; Talens-Peiró, L. New Techniques for Assessing Critical Raw Material Aspects in Energy and Other Technologies. Environ. Sci. Technol. 2022, 56, 17236–17245. [Google Scholar] [CrossRef]
- Sepp, J.; Koshovyi, O.; Jakstas, V.; Žvikas, V.; Botsula, I.; Kireyev, I.; Tsemenko, K.; Kukhtenko, O.; Kogermann, K.; Heinämäki, J.; et al. Phytochemical, Technological, and Pharmacological Study on the Galenic Dry Extracts Prepared from German Chamomile (Matricaria Chamomilla L.) Flowers. Plants 2024, 13, 350. [Google Scholar] [CrossRef]
- Koshovyi, O.; Vovk, G.; Akhmedov, Ey.; Komissarenko, AN. The Study of the Chemical Composition and Pharmacological Activity of Salvia Officinalis Leaves Extracts Getting by Complex Processing. Azerbaijan Pharmaceutical and Pharmacotherapy Journal 2015, 15, 30–34. [Google Scholar]
- Shanaida, M.; Hudz, N.; Jasicka-Misiak, I.; Wieczorek, P.P. Polyphenols and Pharmacological Screening of a Monarda Fistulosa L. Dry Extract Based on a Hydrodistilled Residue By-Product. Front. Pharmacol. 2021, 12, 563436. [Google Scholar] [CrossRef]
- Kapadia, P.; Newell, A.S.; Cunningham, J.; Roberts, M.R.; Hardy, J.G. Extraction of High-Value Chemicals from Plants for Technical and Medical Applications. Int. J. Mol. Sci. 2022, 23, 10334. [Google Scholar] [CrossRef]
- Offiah, V.; Kontogiorgos, V.; Falade, K.O. Extrusion Processing of Raw Food Materials and By-Products: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2979–2998. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Patra, J.K.; Kang, S.-S.; Shin, H.-S. Pharmaceutical Importance of Some Promising Plant Species with Special Reference to the Isolation and Extraction of Bioactive Compounds: A Review. Curr. Pharm. Biotechnol. 2022, 23, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, V.N. Compendium 2020. Medicines; MORION: Kyiv, Ukraine, 2020. [Google Scholar]
- Branco, S.; Videira, N.; Branco, M.; Paiva, M.R. A Review of Invasive Alien Species Impacts on Eucalypt Stands and Citrus Orchards Ecosystem Services: Towards an Integrated Management Approach. J. Environ. Manage. 2015, 149, 17–26. [Google Scholar] [CrossRef]
- Mansfield, S. New Communities on Eucalypts Grown Outside Australia. Front. Plant Sci. 2016, 7, 1812. [Google Scholar] [CrossRef] [PubMed]
- Poke, F.S.; Vaillancourt, R.E.; Potts, B.M.; Reid, J.B. Genomic Research in Eucalyptus. Genetica 2005, 125, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Eschler, B.M.; Foley, W.J. A New Sideroxylonal from Eucalyptus Melliodora. Aust. J. Chem. 1999, 52, 157. [Google Scholar] [CrossRef]
- Osawa, K.; Yasuda, H.; Morita, H.; Takeya, K.; Itokawa, H. Eucalyptone from Eucalyptus Globulus. Phytochemistry 1995, 40, 183–184. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Kozuka, M.; Haruna, M.; Ito, K.; Crow, W.D.; Paton, D.M. Euglobal-In-1, a New Euglobal from Eucalyptus Incrassata. Chem. Pharm. Bull. (Tokyo) 1994, 42, 2113–2116. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Kozuka, M.; Haruna, M.; Ito, K.; Yoshida, S. Four Euglobals from Eucalyptus Blakelyi. Chem. Pharm. Bull. (Tokyo) 1994, 42, 2177–2179. [Google Scholar] [CrossRef]
- Murata, M.; Yamakoshi, Y.; Homma, S.; Aida, K.; Hori, K.; Ohashi, Y. Macrocarpal A, a Novel Antibacterial Compound from Eucalyptus Macrocarpa. Agric. Biol. Chem. 1990, 54, 3221–3226. [Google Scholar] [CrossRef]
- Guan, X.; Guo, Q.; Huang, X.; Wang, Y.; Ye, W. [A new flavonoid glycoside from leaves of Eucalyptus robusta]. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2015, 40, 4868–4872. [Google Scholar]
- Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S.; Neto, C.P.; Domingues, P. New Glucosides from Eucalyptus Globulus Wood, Bark and Kraft Pulps. Holzforschung 2004, 58, 501–503. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Kozuka, M.; Haruna, M.; Ito, K.; Shingu, T. Structures of Euglobals-G1, -G2, -G3, -G4, and -G5 from Eucalyptus Grandis. ChemInform 1995, 26, chin–199535229. [Google Scholar] [CrossRef]
- Begum, S.; Sultana, I.; Siddiqui, B.S.; Shaheen, F.; Gilani, A.H. Structure and Spasmolytic Activity of Eucalyptanoic Acid from Eucalyptus Camaldulensis Var. Obtusa and Synthesis of Its Active Derivative from Oleanolic Acid. J. Nat. Prod. 2002, 65, 1939–1941. [Google Scholar] [CrossRef]
- Mani, J.S.; Johnson, J.B.; Hosking, H.; Ashwath, N.; Walsh, K.B.; Neilsen, P.M.; Broszczak, D.A.; Naiker, M. Antioxidative and Therapeutic Potential of Selected Australian Plants: A Review. J. Ethnopharmacol. 2021, 268, 113580. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, Medicinal and Toxicological Significance of Eucalyptus Leaf Essential Oil: A Review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Mieres-Castro, D.; Ahmar, S.; Shabbir, R.; Mora-Poblete, F. Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses. Pharm. Basel Switz. 2021, 14, 1210. [Google Scholar] [CrossRef] [PubMed]
- Nadtoka, V.L.; Bozhko, N.G.; Grizhko, A.O. The Method of Obtaining Chlorophyllipt 1994, 1–7.
- Ravanbakhsh, H.; Bao, G.; Luo, Z.; Mongeau, L.G.; Zhang, Y.S. Composite Inks for Extrusion Printing of Biological and Biomedical Constructs. ACS Biomater. Sci. Eng. 2021, 7, 4009–4026. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Y.; Zhou, Y.; Li, R.; Jiang, Y.; Alomgir Hossen, M.; Dai, J.; Qin, W.; Liu, Y. Facile Fabrication of Sandwich-like Anthocyanin/Chitosan/Lemongrass Essential Oil Films via 3D Printing for Intelligent Evaluation of Pork Freshness. Food Chem. 2022, 370, 131082. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, D.; Zang, Z.; Sun, X.; Tan, H.; Si, X.; Tian, J.; Teng, W.; Wang, J.; Liang, Q.; et al. 3D Food Printing: Applications of Plant-Based Materials in Extrusion-Based Food Printing. Crit. Rev. Food Sci. Nutr. 2022, 62, 7184–7198. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, J.; He, Y. A Review of 3D Printing Technologies for Soft Polymer Materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- El Aita, I.; Rahman, J.; Breitkreutz, J.; Quodbach, J. 3D-Printing with Precise Layer-Wise Dose Adjustments for Paediatric Use via Pressure-Assisted Microsyringe Printing. Eur. J. Pharm. Biopharm. 2020, 157, 59–65. [Google Scholar] [CrossRef]
- Elbadawi, M.; Nikjoo, D.; Gustafsson, T.; Gaisford, S.; Basit, A.W. Pressure-Assisted Microsyringe 3D Printing of Oral Films Based on Pullulan and Hydroxypropyl Methylcellulose. Int. J. Pharm. 2021, 595, 120197. [Google Scholar] [CrossRef]
- Koshovyi, O.; Heinämäki, J.; Laidmäe, I.; Topelius, N.S.; Grytsyk, A.; Raal, A. Semi-Solid Extrusion 3D-Printing of Eucalypt Extract-Loaded Polyethylene Oxide Gels Intended for Pharmaceutical Applications. Ann. 3D Print. Med. 2023, 12, 100123. [Google Scholar] [CrossRef]
- Koshovyi, O.; Heinämäki, J.; Raal, A.; Laidmäe, I.; Topelius, N.S.; Komisarenko, M.; Komissarenko, A. Pharmaceutical 3D-Printing of Nanoemulsified Eucalypt Extracts and Their Antimicrobial Activity. Eur. J. Pharm. Sci. 2023, 187, 106487. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Gago, C.; Antunes, M.; Lagoas, S.; Faleiro, M.; Megías, C.; Cortés-Giraldo, I.; Vioque, J.; Figueiredo, A. Antibacterial, Antioxidant, and Antiproliferative Activities of Corymbia Citriodora and the Essential Oils of Eight Eucalyptus Species. Medicines 2018, 5, 61. [Google Scholar] [CrossRef]
- Tumanov, V.N.; Chiruk, S.L. Qualitative and Quantitative Methods for Studying Photosynthesis Pigments; GrGU im., I. Kupala: Grodno. 2007. [Google Scholar]
- Balhaddad, A.A.; AlSheikh, R.N. Effect of Eucalyptus Oil on Streptococcus Mutans and Enterococcus Faecalis Growth. BDJ Open 2023, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- 36. State Pharmacopoeia of Ukraine, 2nd ed.; Ukrainian Scientific Pharmacopoeial Center of Drugs Quality: Kharkiv, Ukraine, 2015.
- Marzullo, L.; Ochkur, O.; Orlandini, S.; Renai, L.; Gotti, R.; Koshovyi, O.; Furlanetto, S.; Del Bubba, M. Quality by Design in Optimizing the Extraction of (Poly)Phenolic Compounds from Vaccinium Myrtillus Berries. J. Chromatogr. A 2022, 1677, 463329. [Google Scholar] [CrossRef] [PubMed]
- Viidik, L.; Seera, D.; Antikainen, O.; Kogermann, K.; Heinämäki, J.; Laidmäe, I. 3D-Printability of Aqueous Poly(Ethylene Oxide) Gels. Eur. Polym. J. 2019, 120, 109206. [Google Scholar] [CrossRef]
- Riegel, J.; Mayer, W.; Havre, Y.V. FreeCAD 2001.
- Koshovyi, O.; Raal, A.; Kovaleva, A.; Myha, M.; Ilina, T.; Borodina, N.; Komissarenko, A. The Phytochemical and Chemotaxonomic Study of Salvia Spp. Growing in Ukraine. J. Appl. Biol. Biotechnol. 2020, 8, 29–36. [Google Scholar] [CrossRef]
- Koshevoi, O.N. Amino-Acid and Monosaccharide Compositions of Salvia Officinalis Leaves. Chem. Nat. Compd. 2011, 47, 492–493. [Google Scholar] [CrossRef]
- Kovaleva, A.M.; Georgievskyi, G.V.; Kovalev, V.M.; et al. Development of the Method of Standardization of the New Medicinal Product Piflamin. Pharmacom 2002, 92–97. [Google Scholar]
- Xue, T.; Ruan, K.; Tang, Z.; Duan, J.; Xu, H. Isolation, Structural Properties, and Bioactivities of Polysaccharides from Althaea Officinalis Linn.: A Review. Int. J. Biol. Macromol. 2023, 242, 125098. [Google Scholar] [CrossRef] [PubMed]
- Koshovyi, O.M.; Zagayko, A.L.; Kolychev, I.O.; Akhmedov, E.Yu.; Komissarenko, A.N. Phytochemical Study of the Dry Extract from Bilberry Leaves. Azerbaijan Pharmaceutical and Pharmacotherapy Journal 2016, 16, 18–23. [Google Scholar]
- Shinkovenko, I.L.; Kashpur, N.V.; Ilyina, T.V.; et al. The Immunomodulatory Activity of the Extracts and Complexes of Biologically Active Compounds of Galium Verum L. Herb. Ceska a Slovenska Farmacie 67, 25-29.
- Vlasova, I.; Gontova, T.; Grytsyk, L.; Zhumashova, G.; Sayakova, G.; Boshkayeva, A.; Shanaida, M.; Koshovyi, O. Determination of Standardization Parameters of Oxycoccus Macrocarpus (Ait.) Pursh and Oxycoccus Palustris Pers. Leaves. Sci. Pharm. Sci. 2022, 48–57. [Google Scholar] [CrossRef]
- Khokhlova, K.; Zdoryk, O.; Vyshnevska, L. Chromatographic Characterization on Flavonoids and Triterpenes of Leaves and Flowers of 15 Crataegus L. Species. Nat. Prod. Res. 2020, 34, 317–322. [Google Scholar] [CrossRef]
- Belikov, V.V. Method for Quantitative Determination of Tannin 1980.
- GOST 4565-79. Sumac Leaf. 02/26/1979, No. 754.
- Khvorost, O.P.; Belikov, V.V.; Serbin, A.G.; et al. Comparative Quantitative Assessment of the Content of Tannins in Alnus Glutinosa (L) Gaerth. Rastit. resources 1986, 22, 258–262. [Google Scholar]
- Huzio, N.; Grytsyk, A.; Raal, A.; Grytsyk, L.; Koshovyi, O. Phytochemical and Pharmacological Research in Agrimonia Eupatoria L. Herb Extract with Anti-Inflammatory and Hepatoprotective Properties. Plants 2022, 11, 2371. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, O.V. Preclinical Studies of Drugs; Avicenna: Kyiv, Ukraine, 2001. [Google Scholar]
- Lapach, S.N.; Chubenko, A.V.; Babich, P.N. Statistical Methods in Biomedical Research Using Excel; MORION: Kyiv, 2000. [Google Scholar]
-
Methodological Recommendations for Experimental (Preclinical) Study of Drugs for Local Treatment of Purulent Wounds; Ministry of Health: Moscow, USSR, 1989.
-
European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes, 1986; Vol. Official Journal L 222, p. P 0031-0037.
- 56. Council Directive 2010/63/EU of 22 September 2010 on the Protection of Animals Used for Scientific Purposes, 22 September.
- 57. On the Protection of Animals from Cruel Treatment.
- 58. On Approval of the Procedure for Preclinical Study of Medicinal Products and Examination of Materials of Preclinical Study of Medicinal Products.
-
Regulating the Application of Principles of Good Laboratory Practice and the Verification of Their Applications for
Tests on Chemical Substances, 1986; Vol. 1, pp. 145–146.
- Ihnatova, T.; Kaplaushenko, A.; Frolova, Y.; Pryhlo, E. Synthesis and Antioxidant Properties of Some New 5-Phenethyl-3-Thio-1,2,4-Triazoles. Pharmacia 2021, 68, 129–133. [Google Scholar] [CrossRef]
-
European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, 2022.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).