1. Introduction
It is well established that diet [
1] and exercise [
2] influence the gut microbiota. Numerous studies have suggested that athletes, as a result of their specific diet (e.g., high-protein) and regular physical training, often exhibit a different gut microbiota compared with sedentary subjects [
3,
4]. Specifically, the gut microbiota of athletes has been reported to have higher alpha-diversity [
3] and an abundance of short-chain fatty acid (SCFA)-producing bacteria (e.g.,
Faecalibacterium) [
5,
6]. Interestingly, several studies have proposed a potential correlation between gut microbiota and exercise performance [
7,
8]. For example, certain characteristics of the gut microbiota, such as higher alpha-diversity and butyrate-producing bacteria, have been linked with maximal oxygen uptake (VO
2max), an indicator of aerobic capacity [
8]. Additionally, a significant positive correlation between
Bacteroides and leg extension power has been reported in Japanese older individuals [
9]. Therefore, understanding the gut microbiota is crucial because it could potentially influence athletic performance.
Despite these findings, most studies have focused on athletes from Europe, the United States, and China, with limited research on Japanese athletes [
1,
3,
5,
6]. Notably, Japanese individuals have been shown to have a unique gut microbiota compared with individuals from China and Western countries [
10]. It remains unclear whether the gut microbiota of athletes also exhibits ethnic differences. Furthermore, recent studies have suggested that the gut microbiota may vary among athletes depending on the type of sport (e.g., aerobics, wrestling, rowing) [
11]. To address these gaps in knowledge, it is necessary to accumulate microbiome data from athletes of diverse ethnicities from various sports.
Exercise has been suggested to increase the alpha-diversity and the relative abundance of butyrate-producing bacteria in the gut microbiota [
2]. However, high-intensity or prolonged exercise might increase the presence of inflammation-associated gut microbiota (e.g.,
Haemophilus,
Rothia,
Mucispirillum, and
Ruminococcus gnavus) [
12], potentially negatively affecting the gut health of athletes who perform frequent high-intensity exercise. Athletes experience high levels of mental and physical stress during the athletic season, while the off-season is characterized by a decrease in training frequency. However, few studies have investigated the impact of these seasonal differences on the gut microbiota of athletes.
This study aimed to compare the gut microbiotas between Japanese male handball players, as athletic subjects (AS), and age-matched, healthy males, as non-athletic subjects (NS). Handball is a sport that demands both speed and endurance, and Japanese male handball is a seasonal sport with distinct athletic and off-season periods. Therefore, the gut microbiotas were also compared between the athletic season and the off-season.
2. Materials and Methods
2.1. Dataset Construction
This study used two datasets, referred to below as datasets 1 and 2, derived from the analysis of fecal samples given to the MORINAGA cohort. The cohort comprised healthy adults with no prior history of cancer, cardiovascular disease, liver disease, or gastrointestinal disease [
13]. The participants included players and team staff members of an elite Japanese men’s handball team. Informed consent was obtained from all participants. The cohort study was approved by the ethics committees of the National Institutes of Biomedical Innovation, Health and Nutrition (Osaka, Japan) and the Ethics Committee of the Japan Clinical Research Association (Tokyo, Japan), and all guidelines were followed.
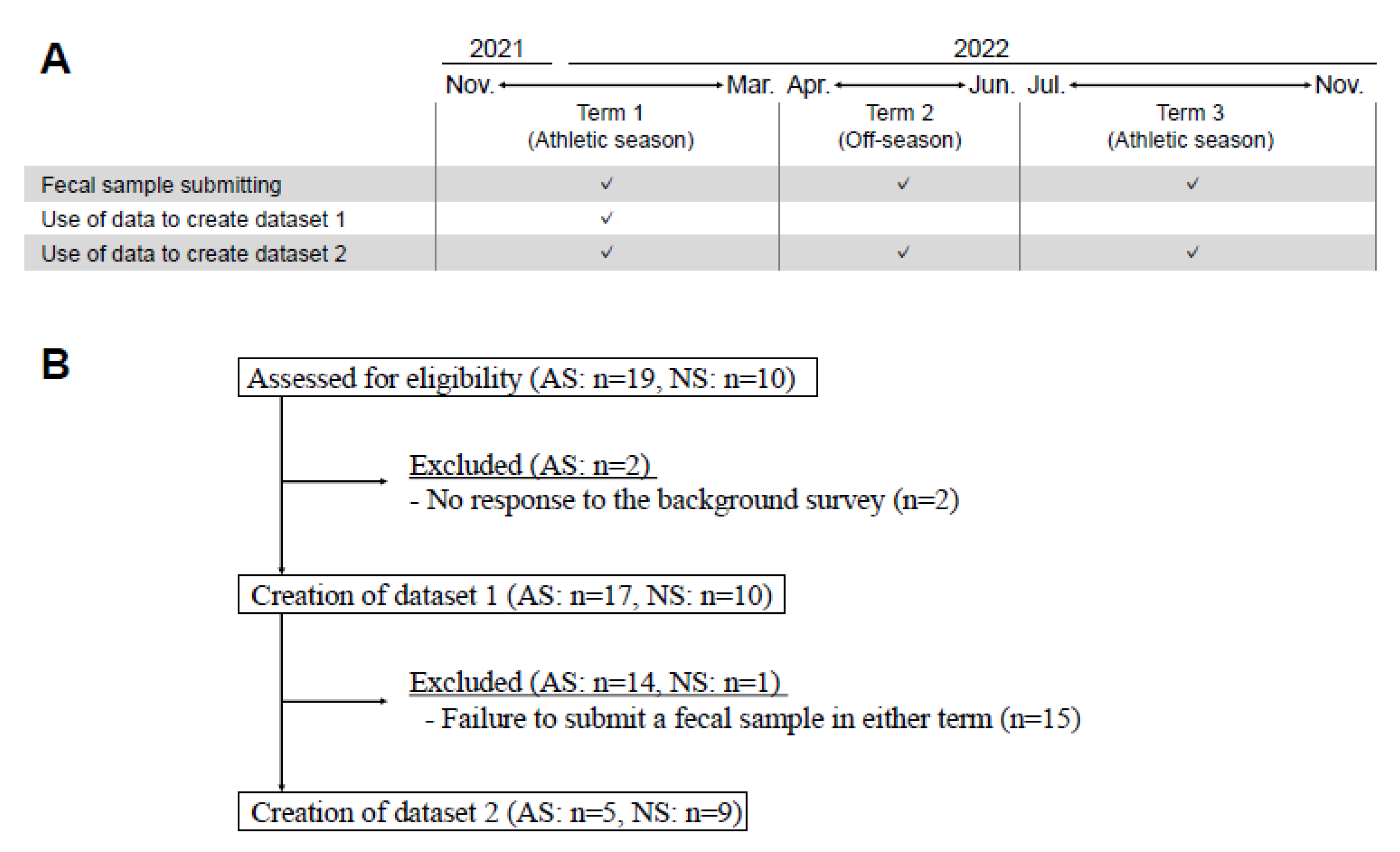
Datasets 1 and 2 were constructed from selected samples of male participants in their 20s and 30s with the aim of matching the age and sex of the players. Dataset 1 comprised samples submitted from November 2021 to March 2022. Dataset 2 comprised samples from three distinct terms: Term 1 (T1), spanning from November 2021 to March 2022; Term 2 (T2), from April 2022 to June 2022; and Term 3 (T3), from July 2022 to November 2022 (
Figure 1A). In cases where multiple samples were available within a term, one was randomly selected. The selected samples were divided into two groups, AS and NS, for comparative analysis. Subjects who met the aforementioned criteria were included in the study, resulting in the recruitment of 27 and 14 subjects for datasets 1 and 2, respectively (
Figure 1B).
2.2. Data Collection
The gut microbiota data were collected in accordance with established protocols, which included fecal sampling, DNA extraction, and 16S rRNA sequencing. Detailed descriptions of these procedures can be found in a previous study [
13]. Briefly, fecal samples were collected in containers with guanidine thiocyanate as a preservative solution, supplied by Techno Suruga Laboratory (Shizuoka, Japan). The fecal sample mixtures were then mechanically disrupted using the bead beating method with glass beads. DNA was then extracted using an automated extraction machine (Kurabo Industries, Osaka, Japan). The amplified V3–V4 region of the bacterial 16S rRNA gene was amplified by PCR and sequenced using the paired-end method on an Illumina MiSeq instrument, with the MiSeq v3 Reagent Kit (Illumina, Inc., Foster City, CA, USA).
2.3. Bioinformatics Analysis
The obtained paired-end FASTQ data from the registered data in the cohort study were trimmed and merged before selection of the amplicon sequence variants (ASVs). Classification and diversity analysis of the ASVs were performed using the QIIME2 software package, version 2017.10 (
https://qiime2.org/), as described previously [
14]. After assignment of each ASV to a bacterial species, alpha-diversities were calculated using QIIME2 software. Moreover, principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity was performed using R software (ver. 4.3.1) using the vegan and ape packages.
2.4. Statistical Analysis
All statistical analyses were performed using R software and EZR (ver. 4.2.2) [
15]. Data collected as background information were statistically analyzed using Welch’s t-test or Fisher’s exact test. The Mann–Whitney U test was used to compare alpha-diversity and the genera of bacteria between AS and NS. When analyzing relative abundance at the genus level, the false discovery rate (FDR) was calculated using the Benjamini–Hochberg procedure after the Mann–Whitney U test, with a significance threshold set at q<0.05. Permutational analysis of variance (PERMANOVA) for PCoA was conducted in R using the adonis2 function from the vegan package. The Friedman test was used for the longitudinal analysis using dataset 2.
2.5. Data Availability
The data pertaining to background information, alpha-diversity, and relative abundance at the genus level are presented in
Supplemental Table 1, Table 2 and Table 3. DNA sequences corresponding to the 16S rRNA gene data have been deposited in the DDBJ database under accession number DRA017698 (
Supplemental Table 4).
3. Results
3.1. Description of Subjects and Background Information in Datasets
The gut microbiota data from 17 AS and 10 NS were included in dataset 1. Dataset 2 comprised data from 5 AS and 9 NS, resulting in a total of 42 unique data entries related to gut microbiota across both datasets (
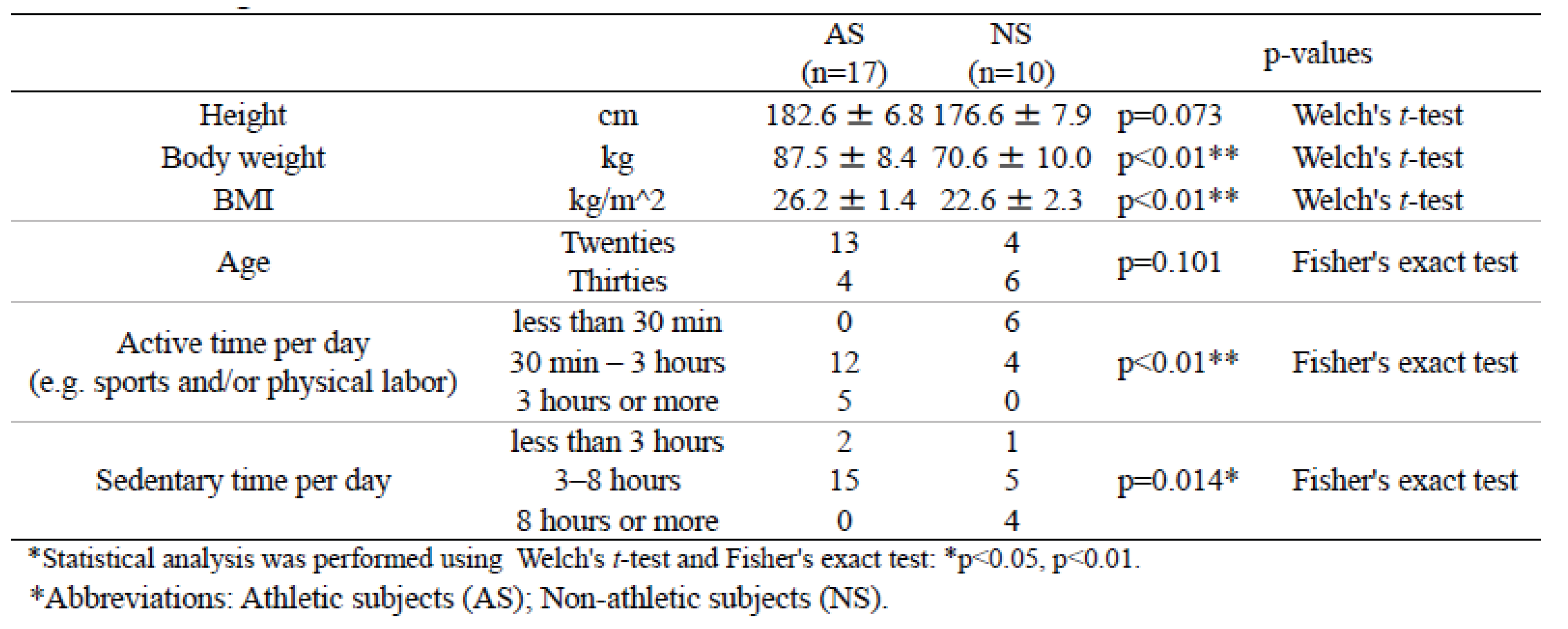
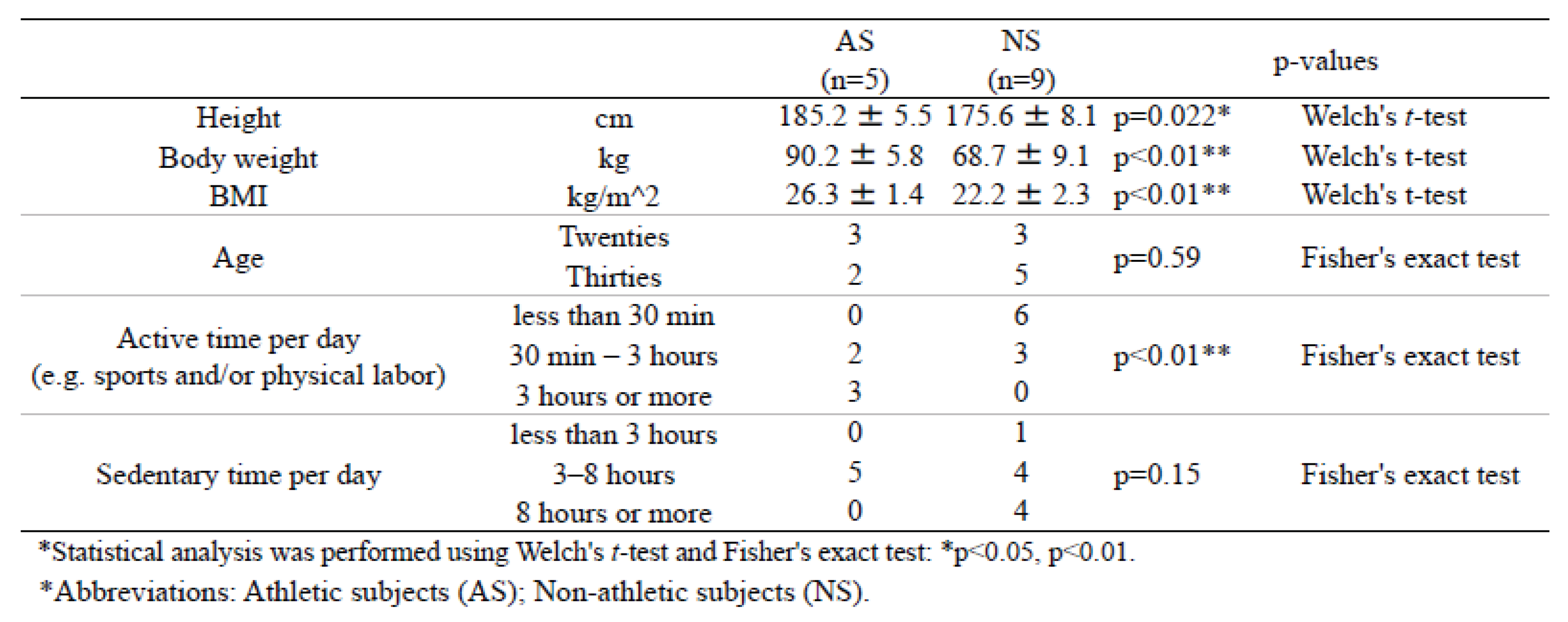
Supplemental Table 1). DNA sequencing of the 42 samples yielded 270,660 reads (mean 6,444 reads per sample, min 2,662, max 12,191), which after de-noising, resulted in a total of 744 ASVs. In both datasets, AS exhibited significantly higher values for height, weight, and body mass index (BMI) compared with NS. Significant differences were also observed in the duration of activity (“active time”) per day, including sports and/or physical labor (
Table 1 and
Table 2). Furthermore, the duration of inactivity (“sedentary time”) per day was significantly lower in AS than in NS for dataset 1 (
Table 1). A similar result was also observed in dataset 2, but this was not statistically significant (
Table 2).
3.2. Comparative Analysis of Gut Microbiota Diversity and Composition between AS and NS in Dataset 1
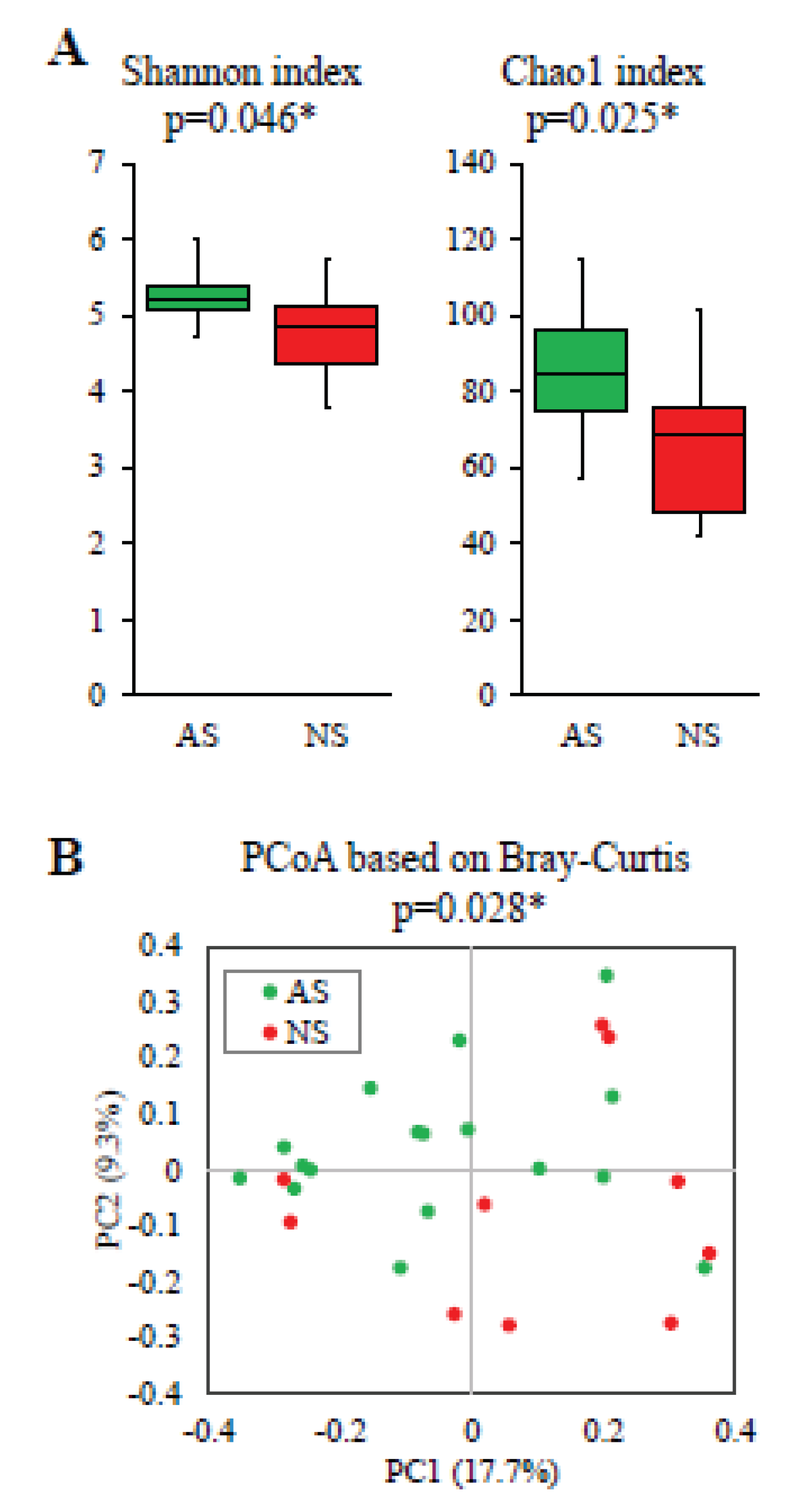
The Shannon (p=0.046) and Chao1 (p=0.025) indices, representing alpha-diversity, were significantly higher in AS compared with NS (
Figure 2A). Furthermore, PCoA based on Bray-Curtis dissimilarity metrics also indicated significant differences (p=0.028,
Figure 2B).
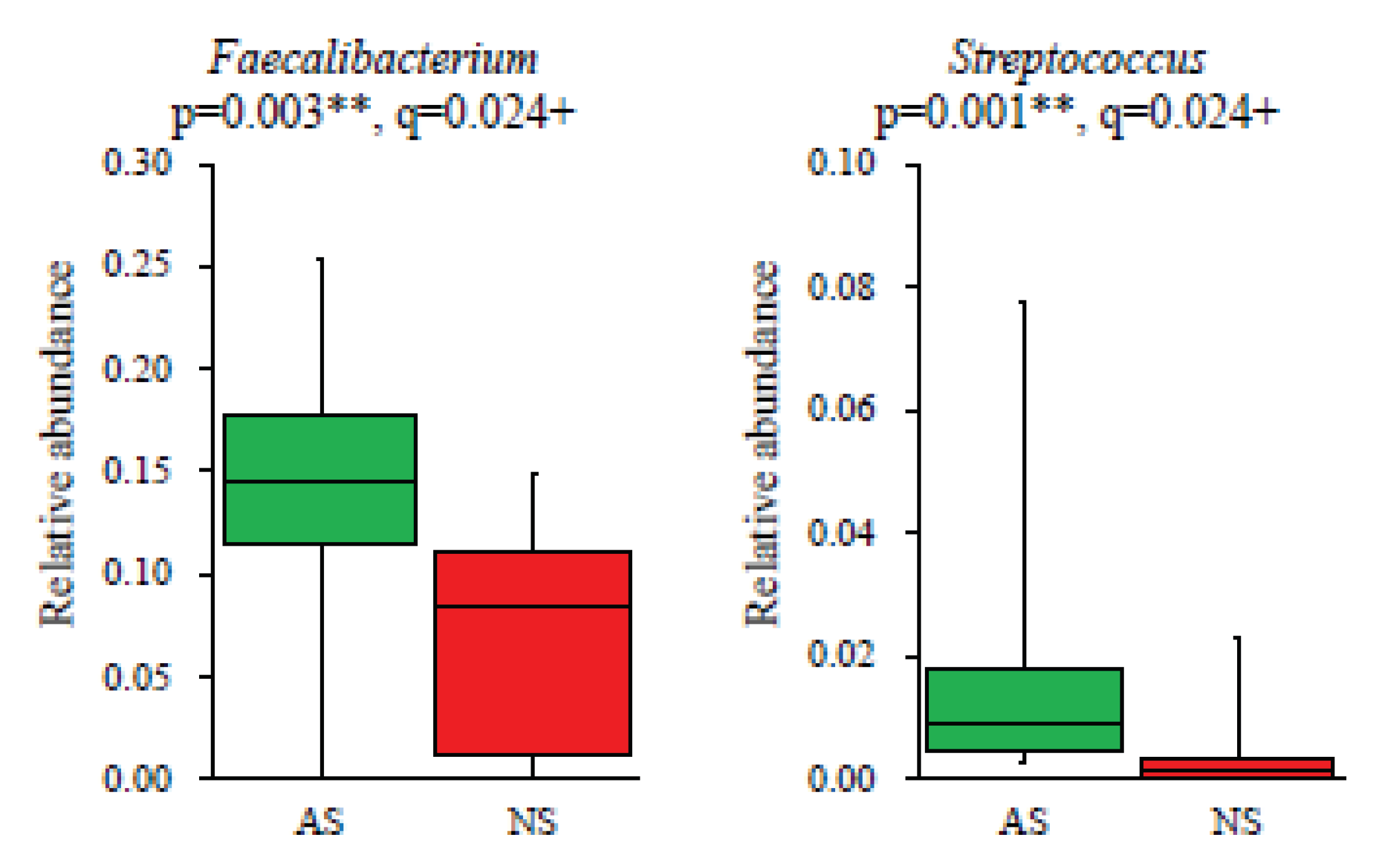
In terms of the composition at the genus level, the relative abundance of 19 dominant genera (>1%) was compared between AS and NS. The results showed that
Faecalibacterium (p=0.003, q=0.024) and
Streptococcus (p=0.001, q=0.024) were significantly more abundant in AS than NS (
Figure 3). Additionally,
Fusicatenibacter and
Anaerostipes tended to be higher in AS than NS, although this difference did not reach statistical significance (p<0.05 but q>0.05,
Supplemental Figure 1). The detection rate of
Streptococcus was higher in AS (100%) compared with NS (60%). However, after applying FDR correction, this difference was not statistically significant (p=0.012, q=0.228,
Supplemental Table 5).
3.3. Longitudinal Analysis of Alpha-Diversity in the Gut Microbiota between the Terms in Dataset 2
To investigate longitudinal changes between the athletic season and off-season, the gut microbiota, as represented by fecal samples collected during the first athletic season (T1), the off-season (T2), and the second athletic season (T3), were compared. A significant longitudinal change in the Shannon index was observed in AS (p=0.022), whereas no such change was observed in NS (p=0.236). The Chao1 index showed similar trends to the Shannon index, but these were not statistically significant (AS: p=0.091 and NS: p=0.368). Furthermore, both the Shannon and Chao1 indices in AS were significantly higher in T1 and T3 (athletic seasons) compared with NS, while no significant difference in both indices was observed between AS and NS in T2 (off-season) (
Figure 4A).
3.4. Longitudinal Analysis of the Gut Microbiota at the Genus Level between the Terms in Dataset 2
To further examine longitudinal changes in the gut microbiota, we performed PCoA based on Bray–Curtis dissimilarity metrics, which was a significant index between AS and NS in dataset 1. No significant longitudinal changes were observed in either AS or NS (
Figure 4B). We then attempted to identify genera that showed significant changes between the athletic seasons and the off-season.
Among the 202 genera detected in dataset 2, 24 genera (11.9%) in AS showed an inverted V-shaped change, while 58 genera (28.7%) showed a V-shaped change, with higher and lower abundance in the off-season (T2) compared with the athletic seasons (T1 and T3). Although none of these changes were statistically significant,
Collinsella,
Roseburia, unclassified Peptostreptococcaceae 256921, unclassified Lachnospiraceae, and
Dorea A showed a tendency to change (p<0.05 and q>0.05,
Supplemental Figure 2). No longitudinal changes were observed in
Faecalibacterium and
Streptococcus, which exhibited significant differences between AS and NS in dataset 1 (
Supplemental Figure 3).
4. Discussion
The primary objective of this study was to investigate the gut microbiota in Japanese male handball players and to discern any potential changes in their gut microbiota between the athletic season and the off-season. In dataset 1, a comparative analysis of the gut microbiota between AS and NS revealed a significantly distinct gut microbiota in AS compared with NS, as evidenced by the results of PCoA. Specifically, the alpha-diversity in AS was significantly higher than in NS, a finding that aligns with previous studies despite differences in ethnicity and the type of sport [
4]. Moreover, AS demonstrated a significantly higher relative abundance of
Faecalibacterium and
Streptococcus, while
Fusicatenibacter and
Anaerostipes tended to be more abundant in AS than NS.
Faecalibacterium,
Fusicatenibacter, and
Anaerostipes, well-known SCFA-producing genera, have been reported to be highly prevalent in athletes [
5,
6]. In the previous study of gut microbiota changes with training periodization in Japanese elite athletes,
Fusicatenibacter and
Anaerostipes tended to increase after training periodization, and a tendency for correlation was also observed between changes in
Fusicatenibacter and anaerobic power output [
16].
Streptococcus, which has been reported to increase with high-intensity exercise lasting 95 minutes or more [
2], may be induced by intensive exercise. For example,
S. pyogenes and
S. pneumoniae have been suggested to be the major cause of upper respiratory infections, which is a common disease in athletes who undergo heavy training [
17,
18]. Therefore, further research is necessary to understand both the positive and negative impacts of the gut microbiota in athletes on their physical and mental performance.
In dataset 2, we investigated the difference in gut microbiota between the athletic season and the off-season. A significant difference was observed in alpha-diversity during the athletic season (T1 and T3) between AS and NS, while no significant difference was observed during the off-season (T2). Although not statistically significant, some genera (e.g.,
Roseburia) showed a tendency to change longitudinally between the athletic season and off-season, similar to alpha-diversity (
Supplemental Figure 2). This suggests potential changes in the gut microbiota of athletes throughout a season. Among the limited studies in this area, one report examined the changes in gut microbiota of professional soccer players during the season [
19] and found that the most variation occurred at the start of the season. Taking this into consideration, the beginning of an athletic season could be interpreted as the time when the modified gut microbiota associated with the off-season is returning to the state associated with the athletic season.
Diet and exercise are well-established factors affecting the gut microbiota [
1,
2]. We interviewed players, staff, and managers of the handball team, including the participants in this cohort study, to investigate the possible reasons for changes between the athletic season and off-season. Most of the interviewed athletic players mentioned that their dietary habits and dietary supplement (e.g., protein supplement) intake remained unchanged between the athletic season and off-season (data not shown). A systematic review investigating the impact of exercise on the gut microbiota reported that an exercise frequency of two to three times per week resulted in no significant changes in alpha-diversity, while an exercise frequency of four to five times per week and more led to an increase in alpha-diversity [
2]. Furthermore, the impact of an 8-week exercise intervention on the gut microbiota in sedentary subjects was reported to increase alpha-diversity, which returned to pre-intervention levels 3 weeks after stopping the exercise intervention [
20]. Therefore, we hypothesize that the changes during the off-season might be due to a decrease in physical activity rather than alternations in diet or nutritional status in this team.
This study had some limitations. Specifically, a small sample size was analyzed. This was particularly noticeable because dataset 2 only included samples that were submitted in all three terms. To confirm the new findings in this study, it will be necessary to conduct larger trials in the future focusing on the time before and after the athletic season. To investigate the mechanisms of impact on the gut microbiota throughout a season, data on not only the gut microbiota but also on dietary intake and physical activity will need to be collected. Data also need to be collected because lifestyle changes during the off-season vary depending on the team. Additionally, it is important to assess how these changes in the gut microbiota throughout a season influence performance and health in athletes.
5. Conclusions
This study, being the first of its kind to investigate the gut microbiota in handball players, has revealed significant differences in gut microbiota between AS and NS. Notably, AS exhibited higher alpha-diversity during the athletic season, which decreased during the off-season. This suggests that the gut microbiota is likely to be influenced during the off-season when lifestyle factors, such as physical activity, are potentially changing. The gut microbiota has been shown to potentially affect the intestinal environment, immune system, and systemic metabolism, which could, in turn, impact athletic performance and health [
7,
21]. Therefore, if we consider the hypothesis that the gut microbiota can influence an athlete’s performance, our findings underscore the importance of managing the gut microbiota during the off-season. In particular, it may be crucial to guide the gut microbiota back to its balanced state during the transition from the off-season to the athletic season. This could potentially ensure a successful start to the season, optimizing the athletes’ performance. Further research is needed to validate these findings and to explore the potential strategies for managing the gut microbiota in athletes, which could open new avenues for enhancing athletic performance and health.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Supplemental Figure 1.
Fusicatenibacter and
Anaerostipes showed a tendency to differ between AS and NS in dataset 1. Among 19 dominant genera (>1%),
Fusicatenibacter and
Anaerostipes showed a tendency to differ between AS (n=17, green) and NS (n=10, red), as presented by box plots. Box plots show the median, as well as the lower and upper quartiles. Whiskers represent the minimum and maximum spread. Statistical analysis was performed using the Mann–Whitney U test and the Benjamini–Hochberg procedure.
Faecalibacterium and
Streptococcus, which were significantly different (p<0.05 and q<0.05), are presented in
Figure 3.
Supplemental Figure 2. Genera showed a tendency to differ between terms in dataset 2. We attempted to search for genera with significant changes between the athletic seasons and the off-season. Although none of the detected genera indicated significant longitudinal changes, Collinsella, Roseburia, unclassified Peptostreptococcaceae 256921, unclassified Lachnospiraceae, and Dorea A tended to change (p<0.05 and q>0.05). The p-values were assessed using the Mann–Whitney U test and the Friedman test for comparisons between groups (**p<0.01) and between terms, respectively. The q-values were analyzed using the Benjamini–Hochberg procedure. The data are expressed as the mean and standard deviation. Abbreviations: T1, term 1 (athletic season); T2, term 2 (off-season); T3, term 3 (athletic season).
Supplemental Figure 3. Longitudinal analysis of the relative abundance of Faecalibacterium and Streptococcus between terms in dataset 2 Faecalibacterium and Streptococcus, which showed a significant difference in abundance in dataset 1 (
Figure 3), are presented by the mean and standard deviation. The p-values were assessed using the Mann–Whitney U test and the Friedman test for comparisons between groups (**p<0.01) and between terms, respectively. Abbreviations: T1, term 1 (athletic season); T2, term 2 (off-season); T3, term 3 (athletic season).
Supplemental Table 1. Detailed background information for subjects
Supplemental Table 2. Individual data regarding alpha-diversity.
Supplemental Table 3. Individual data regarding relative abundance at the genus level.
Supplemental Table 4. DDBJ accession numbers corresponding to 16S rRNA gene sequence data.
Supplemental Table 5. Relative abundance at the genus level (>1%) for dataset 1
Author Contributions
K.T., T.O., H.N., J.K., and M.M. designed and scheduled the study. K.T., K. Y., E.M., and T.O. collected the raw data and analyzed the data. K.T. and T.O. wrote the paper, and S.Y., N.I., K.H., TJ.S., A.M., M.T., H.N., J.K., and M.M. revised the manuscript.
Funding
This study was supported by Morinaga Milk Industry Co., Ltd.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
We express our sincere gratitude to the participants of the cohort study for their invaluable contribution. Special thanks are extended to Ms. Yu Izumitani, the manager of the handball team, and Ms. Noriko Habara, an employee of Morinaga Milk Industry Co., Ltd., for their administrative support and assistance throughout this study. Their efforts have been instrumental in the successful completion of this research. We thank Edanz (
https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflicts of Interest
K.T., S.Y., K.Y., N.I., M.T., and T.O. are employees of Morinaga Milk Industry Co., Ltd., which markets several probiotic products worldwide. K.T., S.Y., K.Y., E.M., N.I., M.T., and T.O. declare that they have no conflicts of interest.
References
- Jang, L.; Choi, G.; Kim, S.-W.; Kim, B.; Lee, S.; Park, H. The Combination of Sport and Sport-Specific Diet Is Associated with Characteristics of Gut Microbiota: An Observational Study. J. Int. Soc. Sports Nutr. 2019, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Boytar, A.N.; Skinner, T.L.; Wallen, R.E.; Jenkins, D.G.; Dekker Nitert, M. The Effect of Exercise Prescription on the Human Gut Microbiota and Comparison between Clinical and Apparently Healthy Populations: A Systematic Review. Nutrients 2023, 15, 1534. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The Athletic Gut Microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yang, K.; Yang, P.; Zhong, C.; Chen, C.; Wang, S.; Lu, Q.; Ning, K. Stratification of Athletes’ Gut Microbiota: The Multifaceted Hubs Associated with Dietary Factors, Physical Characteristics and Performance. Gut Microbes 2020, 12, 1842991. [Google Scholar] [CrossRef] [PubMed]
- Bragina, T.V.; Elizarova, E.V.; Sheveleva, S.A. Intestinal Microbiote of Athletes. Probl. Nutr. 2021, 90, 36–52. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.T.; O’Sullivan, O.; Claesson, M.J.; Cotter, P.D. The Athlete Gut Microbiome and Its Relevance to Health and Performance: A Review. Sports Med. 2022, 52, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory Fitness as a Predictor of Intestinal Microbial Diversity and Distinct Metagenomic Functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- Iwasaka, C.; Nanri, H.; Nakagata, T.; Ohno, H.; Tanisawa, K.; Konishi, K.; Murakami, H.; Hosomi, K.; Park, J.; Yamada, Y.; et al. Association of Skeletal Muscle Function, Quantity, and Quality with Gut Microbiota in Japanese Adults: A Cross-Sectional Study. Geriatr. Gerontol. Int. 2024, 24, 53–60. [Google Scholar] [CrossRef]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.-W.; Hirose, Y.; Morita, H.; Hattori, M. The Gut Microbiome of Healthy Japanese and Its Microbial and Functional Uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, M.; Zha, Y.; Yang, K.; Tong, Y.; Wang, S.; Lu, Q.; Ning, K. Gut Microbiota and Inflammation Patterns for Specialized Athletes: A Multi-Cohort Study across Different Types of Sports. mSystems 2023, 8, e0025923. [Google Scholar] [CrossRef]
- Bonomini-Gnutzmann, R.; Plaza-Díaz, J.; Jorquera-Aguilera, C.; Rodríguez-Rodríguez, A.; Rodríguez-Rodríguez, F. Effect of Intensity and Duration of Exercise on Gut Microbiota in Humans: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kato, K.; Murakami, H.; Hosomi, K.; Tanisawa, K.; Nakagata, T.; Ohno, H.; Konishi, K.; Kawashima, H.; Chen, Y.A.; et al. Comprehensive Analysis of Gut Microbiota of a Healthy Population and Covariates Affecting Microbial Variation in Two Large Japanese Cohorts. BMC Microbiol. 2021, 21, 151. [Google Scholar] [CrossRef]
- Hiraku, A.; Nakata, S.; Murata, M.; Xu, C.; Mutoh, N.; Arai, S.; Odamaki, T.; Iwabuchi, N.; Tanaka, M.; Tsuno, T.; et al. Early Probiotic Supplementation of Healthy Term Infants with Bifidobacterium Longum Subsp. Infantis M-63 Is Safe and Leads to the Development of Bifidobacterium-Predominant Gut Microbiota: A Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, N.; Nakamura, M.; Eda, N.; Murakami, H.; Nakagata, T.; Nanri, H.; Park, J.; Hosomi, K.; Mizuguchi, K.; Kunisawa, J.; et al. Gut Microbiota Alternation with Training Periodization and Physical Fitness in Japanese Elite Athletes. Front. Sport. Act. living 2023, 5, 1219345. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Decker, C.F. Respiratory Tract Infections in Athletes. Dis. Mon. 2010, 56, 407–413. [Google Scholar] [CrossRef]
- Jama-Kmiecik, A.; Frej-Mądrzak, M.; Sarowska, J.; Choroszy-Król, I. Pathogens Causing Upper Respiratory Tract Infections in Outpatients. Adv. Exp. Med. Biol. 2016, 934. [Google Scholar] [CrossRef]
- Viciani, E.; Barone, M.; Bongiovanni, T.; Quercia, S.; Di Gesu, R.; Pasta, G.; Manetti, P.; Iaia, F.M.; Trecroci, A.; Rampelli, S.; et al. Fecal Microbiota Monitoring in Elite Soccer Players Along the 2019-2020 Competitive Season. Int. J. Sports Med. 2022, 43, 1137–1147. [Google Scholar] [CrossRef]
- Bycura, D.; Santos, A.C.; Shiffer, A.; Kyman, S.; Winfree, K.; Sutliffe, J.; Pearson, T.; Sonderegger, D.; Cope, E.; Caporaso, J.G. Impact of Different Exercise Modalities on the Human Gut Microbiome. Sport. (Basel, Switzerland) 2021, 9, 1–22. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between Diet, Gut Microbiota Composition and Gut Metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
Figure 1.
Study scheme and the generation of datasets. (A) Fecal samples were submitted from three distinct terms: Term 1 (T1), spanning from November 2021 to March 2022; Term 2 (T2), from April 2022 to June 2022; and Term 3 (T3), from July 2022 to November 2022. Fecal samples could be submitted more than once throughout this trial. In cases where multiple samples were available within a term, one was randomly selected. Two datasets were created; dataset 1 comprised samples submitted from T1; dataset 2 comprised samples from the three distinct terms. (B) Data from subjects who met the criteria were selected, and 27 and 14 subjects were finally recruited for datasets 1 and 2, respectively. Abbreviations: Athletic subjects (AS), Non-athletic subjects (NS).
Figure 1.
Study scheme and the generation of datasets. (A) Fecal samples were submitted from three distinct terms: Term 1 (T1), spanning from November 2021 to March 2022; Term 2 (T2), from April 2022 to June 2022; and Term 3 (T3), from July 2022 to November 2022. Fecal samples could be submitted more than once throughout this trial. In cases where multiple samples were available within a term, one was randomly selected. Two datasets were created; dataset 1 comprised samples submitted from T1; dataset 2 comprised samples from the three distinct terms. (B) Data from subjects who met the criteria were selected, and 27 and 14 subjects were finally recruited for datasets 1 and 2, respectively. Abbreviations: Athletic subjects (AS), Non-athletic subjects (NS).
Figure 2.
Comparative analysis of alpha and beta diversity between AS and NS. Alpha (A) and beta diversity plots (B) to visualize the differences in gut microbiota between AS (n=17, green) and NS (n=10, red). Alpha-diversity was measured using the Shannon and Chao1 indices (A). Box plots show the median, as well as the lower and upper quartiles. Whiskers represent the minimum and maximum spread. PCoA plots show the beta-diversity with Bray–Curtis dissimilarity (B). Each dot represents an individual sample. Statistical differences in alpha and beta diversity were analyzed for significance using the Mann–Whitney U test and PERMANOVA, respectively (*p<0.05).
Figure 2.
Comparative analysis of alpha and beta diversity between AS and NS. Alpha (A) and beta diversity plots (B) to visualize the differences in gut microbiota between AS (n=17, green) and NS (n=10, red). Alpha-diversity was measured using the Shannon and Chao1 indices (A). Box plots show the median, as well as the lower and upper quartiles. Whiskers represent the minimum and maximum spread. PCoA plots show the beta-diversity with Bray–Curtis dissimilarity (B). Each dot represents an individual sample. Statistical differences in alpha and beta diversity were analyzed for significance using the Mann–Whitney U test and PERMANOVA, respectively (*p<0.05).
Figure 3.
Significant differences in genera present in the gut microbiota between AS and NS. Among 19 dominant genera (>1%), Faecalibacterium and Streptococcus showed significant differences between AS (n=17, green) and NS (n=10, red), as presented by box plots. Statistical analysis was performed using the Mann–Whitney U test and the Benjamini–Hochberg procedure, with p<0.05 and q<0.05 considered statistically significant: **p<0.01, ‡q<0.05.
Figure 3.
Significant differences in genera present in the gut microbiota between AS and NS. Among 19 dominant genera (>1%), Faecalibacterium and Streptococcus showed significant differences between AS (n=17, green) and NS (n=10, red), as presented by box plots. Statistical analysis was performed using the Mann–Whitney U test and the Benjamini–Hochberg procedure, with p<0.05 and q<0.05 considered statistically significant: **p<0.01, ‡q<0.05.
Figure 4.
Longitudinal analysis of alpha and beta diversity between the athletic season and the off-season. (A) Alpha-diversity in AS (n=5, green) and NS (n=9, red) was measured using the Shannon and Chao1 indices. The data are expressed as the mean and the standard deviation. Statistical significance was assessed using the Mann–Whitney U test and the Friedman test for comparisons between groups (*p<0.05, **p<0.01) and between terms (#p<0.05), respectively. (B) PCoA plots showing the beta-diversity with Bray–Curtis dissimilarity. The samples are represented by individual dots, with shades of green representing AS (n=5: Nimo5002, Nimo5004, Nimo5005, Nimo5011, and Nimo5016), shades of red representing NS (n=9: Nimo5009, Nimo5017, Nimo5023, Nimo5027, Nimo5029, Nimo5031, Nimo5032, Nimo5034, and Nimo5035), circles (●) representing T1, triangles (▲) representing T2, and squares (■) representing T3. The differences between the terms were analyzed using PERMANOVA. Abbreviations: T1, term 1 (athletic season); T2, term 2 (off-season); T3, term 3 (athletic season).
Figure 4.
Longitudinal analysis of alpha and beta diversity between the athletic season and the off-season. (A) Alpha-diversity in AS (n=5, green) and NS (n=9, red) was measured using the Shannon and Chao1 indices. The data are expressed as the mean and the standard deviation. Statistical significance was assessed using the Mann–Whitney U test and the Friedman test for comparisons between groups (*p<0.05, **p<0.01) and between terms (#p<0.05), respectively. (B) PCoA plots showing the beta-diversity with Bray–Curtis dissimilarity. The samples are represented by individual dots, with shades of green representing AS (n=5: Nimo5002, Nimo5004, Nimo5005, Nimo5011, and Nimo5016), shades of red representing NS (n=9: Nimo5009, Nimo5017, Nimo5023, Nimo5027, Nimo5029, Nimo5031, Nimo5032, Nimo5034, and Nimo5035), circles (●) representing T1, triangles (▲) representing T2, and squares (■) representing T3. The differences between the terms were analyzed using PERMANOVA. Abbreviations: T1, term 1 (athletic season); T2, term 2 (off-season); T3, term 3 (athletic season).
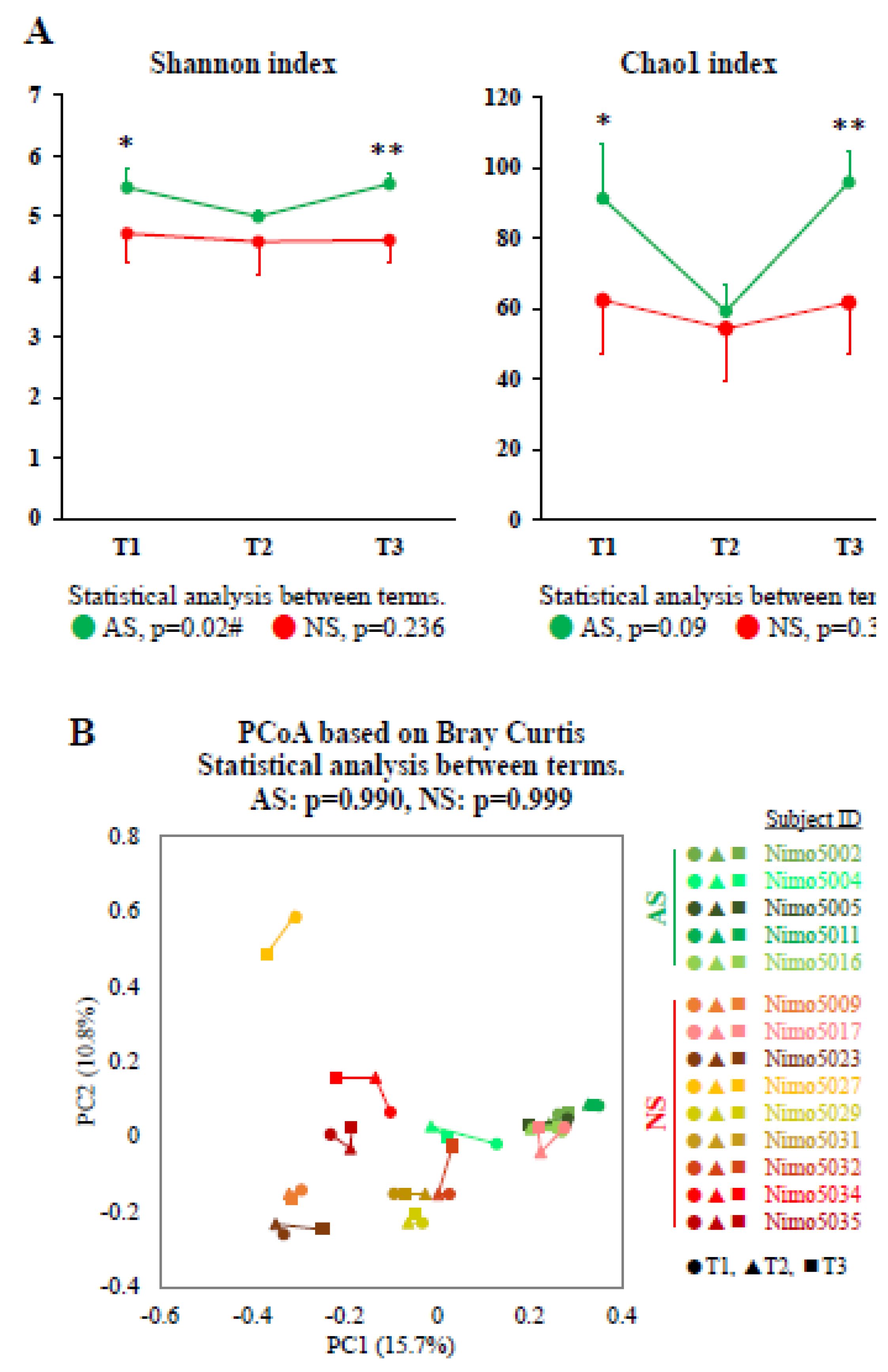
Table 1.
Background information for dataset 1.
Table 1.
Background information for dataset 1.
Table 2.
Background information for dataset 2.
Table 2.
Background information for dataset 2.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).