Submitted:
05 February 2024
Posted:
19 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3.1. Context
3.2. Participants
3.3. Data sources
3.4. Procedure
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease 2019 Cancer Collaboration, Kocarnik JM, Compton K, et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8(3):420-444. [CrossRef]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 Feb 4. Epub ahead of print. PMID: 33538338. [CrossRef]
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021 Apr 5. Epub ahead of print. PMID: 33818764. [CrossRef]
- He, Fang; Huang, Haiying; Ye, Liyan; Wen, Xiulan; Cheng, Andy S. K.1. Meta-analysis of neurocognitive rehabilitation for cognitive dysfunction among pediatric cancer survivors. Journal of Cancer Research and Therapeutics 18(7):p 2058-2065, December 2022. |. [CrossRef]
- Krull KR, Hardy KK, Kahalley LS, Schuitema I, Kesler SR. Neurocognitive Outcomes and Interventions in Long-Term Survivors of Childhood Cancer [published correction appears in J Clin Oncol. 2019 Mar 1;37(7):612]. J Clin Oncol. 2018;36(21):2181-2189. [CrossRef]
- Semendric I, Pollock D, Haller OJ, George RP, Collins-Praino LE, Whittaker AL. "Chemobrain" in childhood cancer survivors-the impact on social, academic, and daily living skills: a qualitative systematic review. Support Care Cancer. 2023;31(9):532. Published 2023 Aug 22. [CrossRef]
- WHO. World health statistics 2023: monitoring health for the SDGs, sustainable development goals https://www.who.int/publications/i/item/9789240074323.
- Kusi-Mensah K, Nuamah ND, Wemakor S, et al. Assessment Tools for Executive Function and Adaptive Function Following Brain Pathology Among Children in Developing Country Contexts: a Scoping Review of Current Tools. Neuropsychol Rev. 2022;32(3):459-482. [CrossRef]
- Sun Z, Yuan Y, Xiong X, Meng S, Shi Y, Chen A. Predicting academic achievement from the collaborative influences of executive function, physical fitness, and demographic factors among primary school students in China: ensemble learning methods. BMC Public Health. 2024;24(1):274. Published 2024 Jan 23. [CrossRef]
- Kopp B, Lange F, Steinke A. The Reliability of the Wisconsin Card Sorting Test in Clinical Practice. Assessment. 2021;28(1):248-263. [CrossRef]
- Kusi-Mensah K, Nuamah ND, Wemakor S, et al. A Systematic Review of the Validity and Reliability of Assessment Tools for Executive Function and Adaptive Function Following Brain Pathology among Children and Adolescents in Low- and Middle-Income Countries [published correction appears in Neuropsychol Rev. 2022 Apr 30;:]. Neuropsychol Rev. 2022;32(4):974-1016. [CrossRef]
- Departamento Nacional de Estadística (DANE) https://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/proyecciones-de-poblacion.
- BERG EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39:15-22. [CrossRef]
- GRANT DA, BERG EA. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J Exp Psychol. 1948;38(4):404-411. [CrossRef]
- Steinke A, Lange F, Kopp B. Parallel model-based and model-free reinforcement learning for card sorting performance. Sci Rep. 2020;10(1):15464. Published 2020 Sep 22. [CrossRef]
- Kopp B, Al-Hafez B, Steinke A. Habits, Goals, and Behavioral Signs of Cognitive Perseveration on Wisconsin Card-Sorting Tasks. Brain Sciences. 2023; 13(6):919. [CrossRef]
- The jamovi project (2022). jamovi. (Version 2.3) [Computer Software]. Retrieved from https://www.jamovi.org.
- R Core Team (2021). R: A Language and environment for statistical computing. (Version 4.1) [Computer software]. Retrieved from https://cran.r-project.org. (R packages retrieved from MRAN snapshot 2022-01-01).
- Kazuki, Y. (2019). tableone: Create 'Table 1' to Describe Baseline Characteristics. [R package]. Retrieved from https://CRAN.R-project.org/package=tableone.
- Serdar Balci (2022). ClinicoPath jamovi Module. [R package]. Retrieved from https://github.com/sbalci/ClinicoPathJamoviModule. link. [CrossRef]
- Kerby, D. S. (2014). The simple difference formula: An approach to teaching nonparametric correlation. Comprehensive Psychology, 3, 2165–2228. [CrossRef]
- Sing, T., Sander, O., Beerenwinkel, N., & Lengauer, T. (2015). ROCR: Visualizing the Performance of Scoring Classifiers. [R package]. Retrieved from https://cran.r-project.org/package=ROCR.
- Hernández Martínez Montserrat, Pastor Hernández Nuria. Afectación neuropsicológica como secuela del tratamiento oncológico. Rev Pediatr Aten Primaria [Internet]. 2020 Mar [citado 2024 Feb 02] ; 22(85): e27-e30. Disponible en: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1139-76322020000100010&lng=es. Epub 03-Ago-2020.
- Arce B, Grañana N. Alteraciones cognitivas por quimioterapia en pacientes con cáncer de mama: una revisión bibliográfica [Cognitive deficits with chemotherapy in women with breast cancer: a bibliographic revisión]. Rev Fac Cien Med Univ Nac Cordoba. 2023 Jun 30;80(2):126-133. Spanish. PMID: 37402295; PMCID: PMC10443418. [CrossRef]
- García Gómez, R. Estudio de las funciones cognitivas en sujetos con leucemia infantil. 2017. http://hdl.handle.net/10366/137319.
- González, A., et al. "Perfil cognitivo de niños con meduloblastoma que culminaron su tratamiento oncológico: caracterización de los procesos mnésicos." Med. infant 2011: 190-197.
- Jacola LM, Litten M, Reddick WE, Krull KR. Structural and Functional Brain Imaging in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia Treated With Chemotherapy: A Systematic Review. JNCI Cancer Spectr. 2021;5(5):pkab069. Published 2021 Aug 11. [CrossRef]
- Cheung YT, Sabin ND, Reddick WE, et al. Leukoencephalopathy and long-term neurobehavioral, neurocognitive, and brain imaging outcomes in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy: a longitudinal analysis. Lancet Hematol. 2016;3(10):e456-e466. [CrossRef]
- Laaker CJ, Cantelon C, Davis AB, et al. Early life cancer and chemotherapy lead to cognitive deficits related to alterations in microglial-associated gene expression in prefrontal cortex. Brain Behav Immun. 2023;113:176-188. [CrossRef]
- Kafadar, Hatice, Hasibe Arıcan Süren, and Selin Yılmaz. "A comparison of individuals whose capacity of working memory is high and low from the perspective of WCST performance." Anatomy: International Journal of Experimental & Clinical Anatomy 16 (2022).
- Mackey, WE, Devinsky, O., Doyle, WK, Meager, MR, & Curtis, CE. The Human Dorsolateral Prefrontal Cortex Is Not Necessary for Spatial Working Memory. Journal of Neuroscience, 2016, 36 (10), 2847–2856. [CrossRef]
- Faustino, B., Oliveira, J., & Lopes, P. (2019). Diagnostic precision of the Wisconsin Card Sorting Test in assessing cognitive deficits in substance use disorders. Applied Neuropsychology: Adult, 1–8. [CrossRef]
- Joly F, Giffard B, Rigal O et al. Impact of cancer and its treatments on cognitive function: advances in research from the Paris International Cognition and Cancer Task Force Symposium and update since 2012. J Pain Symptom Manage 2015; 50 (6): 830–841.
- Coomans, M. B., van der Linden, S. D., Gehring, K., & Taphoorn, M. J. B. Treatment of cognitive deficits in brain tumor patients. Current Opinion in Oncology, 2019. 1. [CrossRef]
- Lange, M., Joly, F., Vardy, J., Ahles, T., Dubois, M., Tron, L., Winocur, G., De Ruiter, M. B., & Castel, H. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Annals of Oncology, 2019. 30(12), 1925–1940. [CrossRef]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012 Jul 10;30(20):2500-8. Epub 2012 Jun 4. PMID: 22665542; PMCID: PMC3397784. [CrossRef]
- Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015 Mar;65(2):123-38. Epub 2014 Dec 5. PMID: 25483452; PMCID: PMC4355212. [CrossRef]
- Lange M, Joly F, Vardy J, Ahles T, Dubois M, Tron L, Winocur G, De Ruiter MB, Castel H. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol. 2019 Dec 1;30(12):1925-1940. PMID: 31617564; PMCID: PMC8109411. [CrossRef]
- Wouters H, Baars JW, Schagen SB. Neurocognitive function of lymphoma patients after treatment with chemotherapy. Acta Oncol. 2016 Sep-Oct;55(9-10):1121-1125. Epub 2016 Jun 22. PMID: 27333078. [CrossRef]
- Firoozi, M., & Azadfar, Z. Working Memory Performance, Attention Maintenance and Executive Function in Children with Acute Lymphoblastic Leukemia. International Journal of Cancer Management, 2017. 10(7). [CrossRef]
- Janelsins MC, Heckler CE, Peppone LJ, Kamen C, Mustian KM, Mohile SG, Magnuson A, Kleckner IR, Guido JJ, Young KL, Conlin AK, Weiselberg LR, Mitchell JW, Ambrosone CA, Ahles TA, Morrow GR. Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared With Age-Matched Controls: An Analysis From a Nationwide, Multicenter, Prospective Longitudinal Study. J Clin Oncol. 2017 Feb 10;35(5):506-514. Epub 2016 Dec 28. PMID: 28029304; PMCID: PMC5455314. [CrossRef]
- Vannorsdall TD. Cognitive Changes Related to Cancer Therapy. Med Clin North Am. 2017 Nov;101(6):1115-1134. Epub 2017 Aug 25. PMID: 28992858. [CrossRef] [PubMed]
- Krull KR, Brinkman TM, Li C, Armstrong GT, Ness KK, Srivastava DK, Gurney JG, Kimberg C, Krasin MJ, Pui CH, Robison LL, Hudson MM. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J Clin Oncol. 2013 Dec 10;31(35):4407-15. Epub 2013 Nov 4. PMID: 24190124; PMCID: PMC3842908. [CrossRef]
- Chiu EC, Wu WC, Hung JW, Tseng YH. Validity of the Wisconsin Card Sorting Test in patients with stroke. Disabil Rehabil. 2018 Aug;40(16):1967-1971. Epub 2017 May 11. PMID: 28494623. [CrossRef]
- Adjorlolo S. Ecological validity of executive function tests in moderate traumatic brain injury in Ghana. Clin Neuropsychol. 2016 Jan-Dec;30(sup1):1517-1537. Epub 2016 Apr 13. PMID: 27071720. [CrossRef]
- Gómez-de-Regil L. Assessment of Executive Function in Patients with Traumatic Brain Injury with the Wisconsin Card-Sorting Test. Brain Sci. 2020 Oct 1;10(10):699. PMID: 33019772; PMCID: PMC7600451. [CrossRef]
- Diercks CM, Gunther KE, Teti DM, Lunkenheimer E. Ecological validity in measuring parents' executive function. Child Dev Perspect. 2022 Dec;16(4):208-214. Epub 2022 Sep 2. PMID: 36590076; PMCID: PMC9799100. [CrossRef]
- Malik HB, Norman JB. Best Practices and Methodological Strategies for Addressing Generalizability in Neuropsychological Assessment. J Pediatr Neuropsychol. 2023;9(2):47-63. Epub 2023 May 13. PMID: 37250805; PMCID: PMC10182845. [CrossRef]
| Classification | Threshold | Score |
| Significantly deficient | Severe impairment | 0-54 |
| Severe to moderate impairment | 55-61 | |
|
Poor |
Moderate deterioration | 62-69 |
|
Borderline Expected |
Moderate to intermediate Intermediate deterioration |
70-76 77-84 |
| Below average | 85-91 | |
| Medium | 92-106 | |
| Above average | 107 + | |
| Overall |
| n 24 |
| Age (mean (SD)) 12.08 (3.98) |
| Sex = Woman (%) 5 (20.8) |
| Department (%) |
| Cauca 10 (41.7) |
| Caqueta 2 (8.3) |
| Valley 12 (50.0) |
| Study = No (%) 5 (20.8) |
| Health regime 1 = Contributory (%) 5 (20.8) |
| Typology Dx (%) |
| Leukemias 17 (70.8) |
| Lymphomas 3 (12.5) |
| Bone tumor 3 (12.5) |
| Solid tumor 1 (4.2) |
| 4 years or more Dx (%) |
| No 17 (70.8) |
| Yes 4 (16.7) |
| Does not record 3 (12.5) |
| Palliative Care Value = No. (%) 15 (62.5) |
| Radiation therapy = No. (%) 19 (79.2) |
| Step home (%) |
| Yes 12 (50.0) |
| No 10 (41.7) |
| Does not record 2 (8.3) |
| Family function (%) |
| Functional 17 (70.8) |
| Dysfunctional 3 (12.5) |
| Does not record 4 (16.7) |
| Treatment goal = Palliative (%) 2 (8.3) |
| Laterality = Left-handed (%) 2 (8.3) |
| Correct answers (mean (SD)) 74.12 (13.02) |
| Response Deficit = No (%) 18 (75.0) |
| Complete categories (mean (SD)) 4.46 (1.79) |
| Deficit Categories = No. (%) 18 (75.0) |
| Perseverative errors (mean (SD)) 22.75 (16.17) |
| Perseverative error deficit = No (%) 18 (75.0) |
| Maintaining attitude (mean (SD)) 1.58 (1.72) |
| Maintenance attitude deficit = No (%) 20 (83.3) Maintenance action (mean (SD)) 78.58 (14.19) |
| Action inhibition (mean (SD)) -24.33 (15.95) |
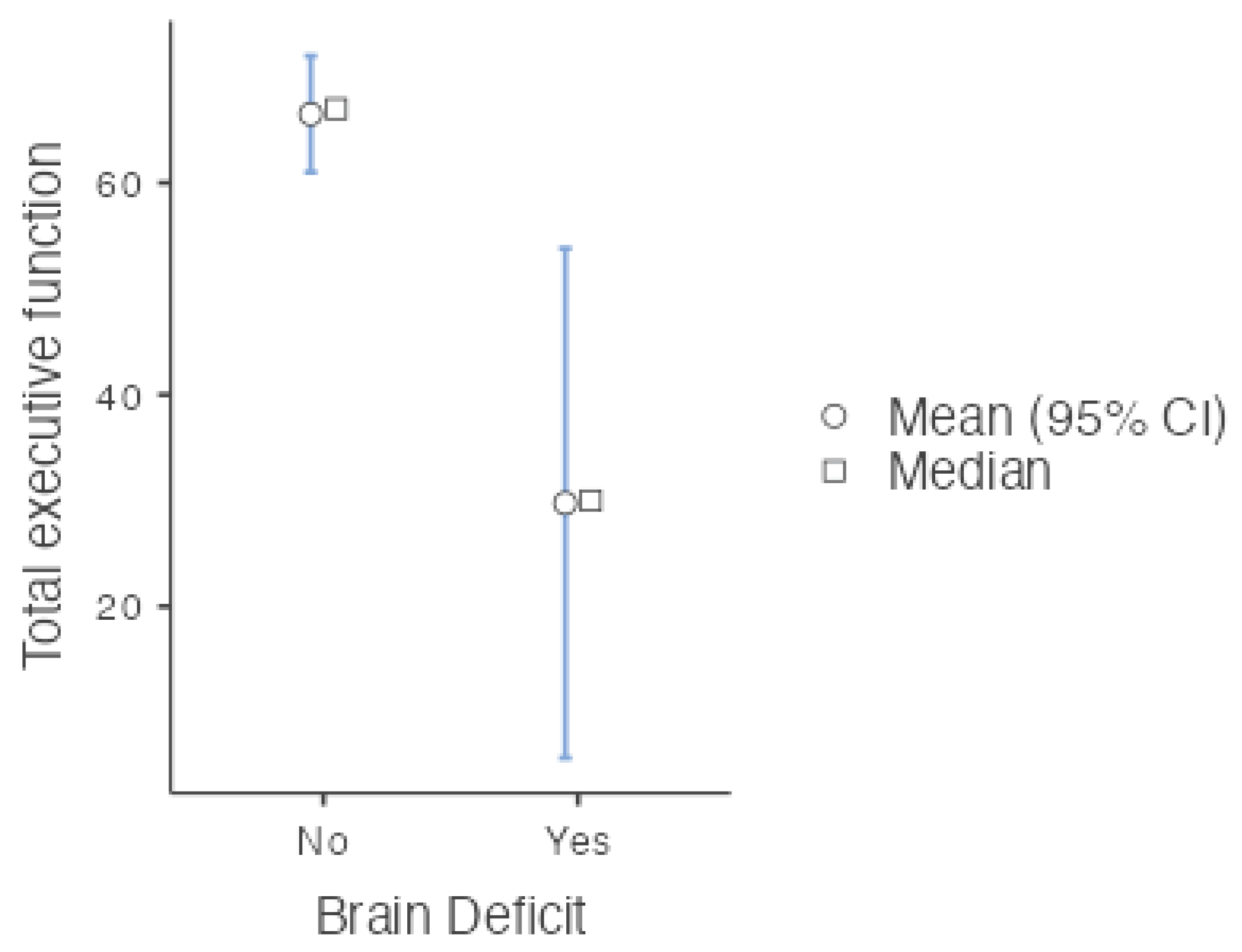
| Total executive function (mean (SD)) 54.25 (27.61) |
| Brain Deficit = Yes (%) 8 (33.3) |
| Subscale and total | χ2 | gl | p | ε2 | |
|---|---|---|---|---|---|
| Total executive function | 5.23 | 3 | 0.156 | 0.227 | |
| Inhibition action | 5.50 | 3 | 0.139 | 0.239 | |
| Maintenance Action | 4.61 | 3 | 0.203 | 0.200 | |
| Maintain attitude | 3.02 | 3 | 0.388 | 0.131 | |
| Perseverative mistakes | 5.04 | 3 | 0.169 | 0.219 | |
| Complete categories | 8.08 | 3 | 0.044 | 0.351 | |
| Correct answers | 4.07 | 3 | 0.254 | 0.177 | |
| 95% Confidence Interval | |||||||
|---|---|---|---|---|---|---|---|
| Statistic | p | Effect Size | Lower | Upper | |||
| Total executive function | Mann‒Whitney U | 17.5 | 0.002 | Rank biserial correlation | 0.727 | ||
| Brain Deficit | ||||
|---|---|---|---|---|
| Type of diagnosis | No | Yes | Total | |
| Leukemias | Observed | 12 | 5 | 17 |
| % within row | 70.6 % | 29.4 % | 100.0 % | |
| Lymphomas | Observed | 1 | 2 | 3 |
| % within row | 33.3 % | 66.7 % | 100.0 % | |
| Bone tumor | Observed | 2 | 1 | 3 |
| % within row | 66.7 % | 33.3 % | 100.0 % | |
| Solid tumor | Observed | 1 | 0 | 1 |
| % within row | 100.0 % | 0.0 % | 100.0 % | |
| Total | Observed | 16 | 8 | 24 |
| % within row | 66.7 % | 33.3 % | 100.0 % | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





