Submitted:
18 February 2024
Posted:
20 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Conventional 2-Dimensional (2-D) Sonographic Examinations
2.3. Volume Acquisition
2.4. Determination of Power Doppler Indices
2.5. Amniocentesis Procedure
2.6. Samples
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Data and Statistical Analysis
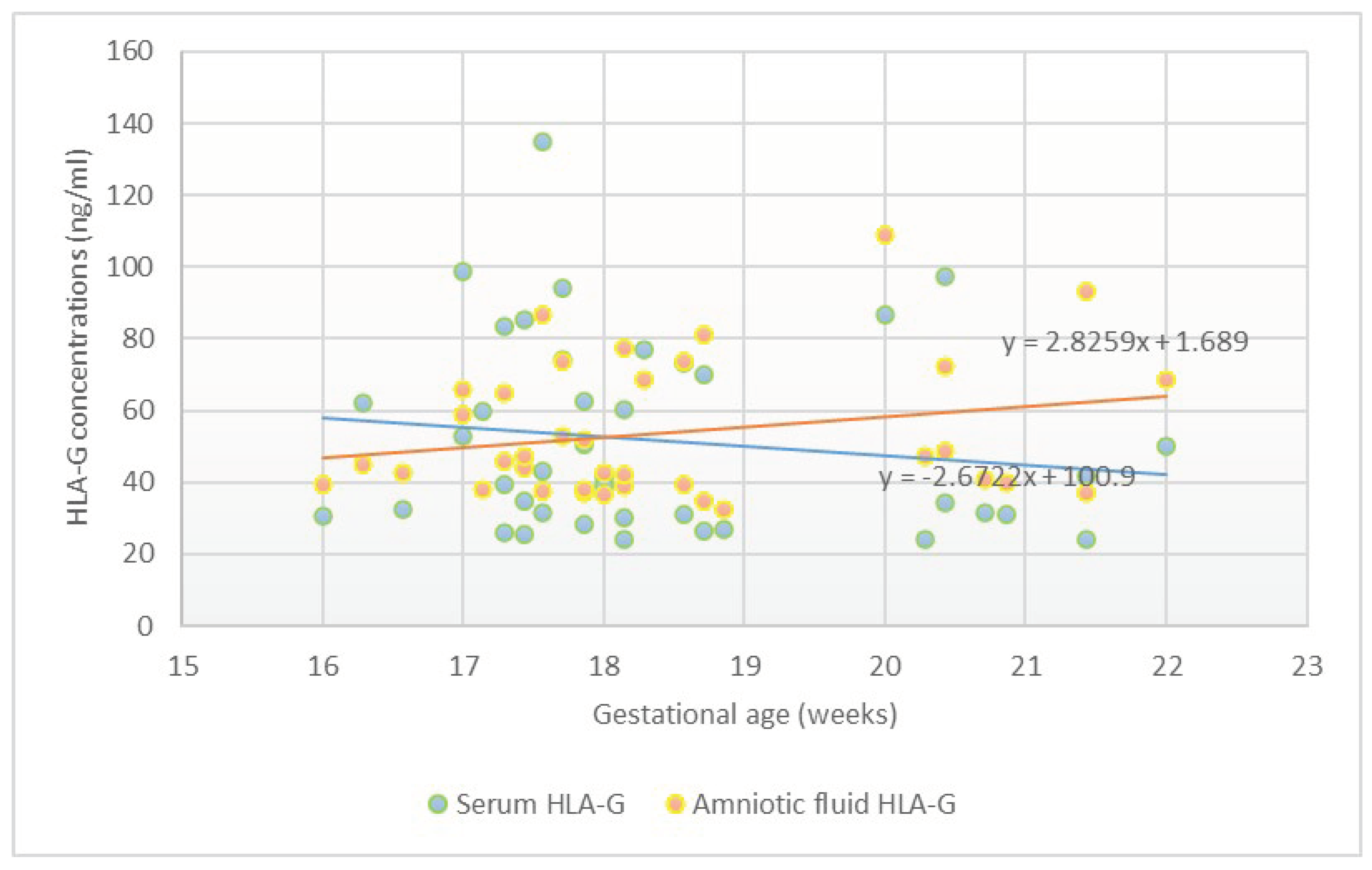
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu X, Zhou Y, Wei H. Roles of HLA-G in the Maternal-Fetal Immune Microenvironment. Front Immunol. 2020, 11. [CrossRef]
- Tantengco OAG, Richardson L, Lee A, Kammala A, Silva M de C, Shahin H, et al. Histocompatibility antigen, class i, g (Hla-g)’s role during pregnancy and parturition: A systematic review of the literature. Life 2021, 11(10). [CrossRef]
- Mao J, Feng Y, Zhu X, Ma F. The Molecular Mechanisms of HLA-G Regulatory Function on Immune Cells during Early Pregnancy. Biomolecules 2023, 13(8). [CrossRef]
- Li C, Houser BL, Nicotra ML, Strominger JL. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc Natl Acad Sci U S A. 2009, 106(14). [CrossRef]
- Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, Van Der Meer A, Joosten I, et al. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006, 4(1). [CrossRef]
- Pazmany L, Mandelboim O, Valés-Gómez M, Davis DM, Reyburn HT, Strominger JL. Protection from natural killer cell-mediated lysis by HLA-G expression on target cells. Science (1979). 1996, 274(5288). [CrossRef]
- Rajagopalan S, Long EO. KIR2DL4 (CD158d): An activation receptor for HLA-G. Front Immunol. 2012, 3(AUG). [CrossRef]
- Le Bouteiller, P. HLA-G in human early pregnancy: Control of uterine immune cell activation and likely vascular remodeling. Biomed J. 2015, 38(1). [CrossRef]
- Murphy SP, Tayade C, Ashkar AA, Hatta K, Zhang J, Croy BA. Interferon gamma in successful pregnancies. Biol Reprod. 2009;80(5). [CrossRef]
- Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci U S A. 2003, 100(15). [CrossRef]
- Amiot L, Ferrone S, Grosse-Wilde H, Seliger B. Biology of HLA-G in cancer: A candidate molecule for therapeutic intervention? Cellular and Molecular Life Sciences. 2011, 68(3). [CrossRef]
- Poehlmann TG, Schaumann A, Busch S, Fitzgerald JS, Aguerre-Girr M, Le Bouteiller P, et al. Inhibition of term decidual NK cell cytotoxicity by soluble HLA-G1. American Journal of Reproductive Immunology. 2006, 56(5–6). [CrossRef]
- Wagner SN, Rebmann V, Willers CP, Grosse-Wilde H, Goos M. Expression analysis of classic and non-classic HLA molecules before interferon alfa-2b treatment of melanoma. Lancet 2000, 356(9225). [CrossRef]
- Lemaoult JL, Ne Krawice-Radanne I, Dausset J, Carosella ED. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4 T cells. 2004. Available from: www.pnas.orgcgidoi10.1073pnas.0401922101.
- De Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006, 6(1). [CrossRef]
- Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005, 106(1). [CrossRef]
- Mordoh J, Levy EM, Roberti MP. Natural killer cells in human cancer: From biological functions to clinical applications. J Biomed Biotechnol. 2011, 2011. [CrossRef]
- Yie SM, Li LH, Li YM, Librach C. HLA-G protein concentrations in maternal serum and placental tissue are decreased in preeclampsia. Am J Obstet Gynecol. 2004, 191(2). [CrossRef]
- Peng B, Zhang L, Xing A yun, Hu M, Liu S yun. The expression of human leukocyte antigen G and E on human first trimester placenta and its relationship with recurrent spontaneous abortion. Journal of Sichuan University (Medical Science). 2008, 39(6).
- Kusanovic JP, Romero R, Jodicke C, Mazaki-Tovi S, Vaisbuch E, Erez O, et al. Amniotic fluid soluble human leukocyte antigen-G in term and preterm parturition, and intra-amniotic infection/inflammation. Journal of Maternal-Fetal and Neonatal Medicine. 2009, 22(12). [CrossRef]
- Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol. 1985 Feb, 151(3), 333–337.
- K. Joubert. Magyar születéskori testtömeg- és testhossz-standardok az 1990-96. évi országos élveszületési adatok alapján. Magy Noorv Lapja. 2000, 63(12), 155–163.
- Suranyi A, Kozinszky Z, Molnar A, Nyari T, Bito T, Pal A. Placental three-dimensional power Doppler indices in mid-pregnancy and late pregnancy complicated by gestational diabetes mellitus. Prenat Diagn. 2013 Oct, 33(10), 952–958.
- Molnar A, Suranyi A, Nyari T, Nemeth G, Pal A. Examination of placental three-dimensional power Doppler indices and perinatal outcome in pregnancies complicated by intrauterine growth restriction. Int J Gynaecol Obstet. 2015 Apr, 129(1), 5–8.
- Lai PK, Wang YA, Welsh AW. Reproducibility of regional placental vascularity/perfusion measurement using 3D power Doppler. Ultrasound Obstet Gynecol. 2010 Aug, 36(2), 202–209.
- Központi Statisztikai Hivatal (Central Statistical Office H. KSH database 22.1.1.7. Live births by main characteristics of mother and newborn 1980-2022] [Internet]. 2023.
- Beneventi F, Locatelli E, De Amici M, Simonetta M, Cavagnoli C, Bellingeri C, et al. Soluble HLA-G concentrations in maternal blood and cervical vaginal fluid of pregnant women with preterm premature rupture of membranes. J Reprod Immunol. 2016, 116. [CrossRef]
- Vincze M, Sikovanyecz J, Molnár A, Földesi I, Surányi A, Várbíró S, et al. Predictive Capabilities of Human Leukocyte Antigen-G and Galectin-13 Levels in the Amniotic Fluid and Maternal Blood for the Pregnancy Outcome. Medicina (B Aires). 2024 Jan, 60(1), 85.
- Umapathy A, Chamley LW, James JL. Reconciling the distinct roles of angiogenic/anti-angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies. Angiogenesis. 2020, 23(2). [CrossRef]
- Rizzo R, Andersen AS, Lassen MR, Sørensen HC, Bergholt T, Larsen MH, et al. Soluble Human Leukocyte Antigen-G isoforms in maternal plasma in early and late pregnancy. American Journal of Reproductive Immunology. 2009, 62(5). [CrossRef]
- Steinborn A, Varkonyi T, Scharf A, Bahlmann F, Klee A, Sohn C. Early detection of decreased soluble HLA-G levels in the maternal circulation predicts the occurrence of preeclampsia and intrauterine growth retardation during further course of pregnancy. American Journal of Reproductive Immunology. 2007, 57(4). [CrossRef]
- Fu B, Zhou Y, Ni X, Tong X, Xu X, Dong Z, et al. Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors. Immunity. 2017, 47(6). [CrossRef]
- Yockey LJ, Iwasaki A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity. 2018, 49(3). [CrossRef]
- Andreotti JP, Paiva AE, Prazeres PHDM, Guerra DAP, Silva WN, Vaz RS, et al. The role of natural killer cells in the uterine microenvironment during pregnancy. Cell Mol Immunol. 2018, 15(11). [CrossRef]
- Vincze M, Sikovanyecz J, Molnár A, Földesi I, Surányi A, Várbíró S, et al. Predictive Capabilities of Human Leukocyte Antigen-G and Galectin-13 Levels in the Amniotic Fluid and Maternal Blood for the Pregnancy Outcome. Medicina (B Aires). 2024 Jan, 60(1), 85.
- Nardi F da S, König L, Wagner B, Giebel B, Santos Manvailer LF, Rebmann V. Soluble monomers, dimers and HLA-G-expressing extracellular vesicles: the three dimensions of structural complexity to use HLA-G as a clinical biomarker. HLA. 2016, 88(3). [CrossRef]
- Merce LT, Barco MJ, Bau S. Reproducibility of the study of placental vascularization by three-dimensional power Doppler. J Perinat Med. 2004, 32(3), 228–233.
- Merce LT, Barco MJ, Bau S, Kupesic S, Kurjak A. Assessment of placental vascularization by three-dimensional power Doppler “vascular biopsy” in normal pregnancies. Croat Med J. 2005 Oct, 46(5), 765–771.
- Rizzo G, Capponi A, Pietrolucci ME, Aiello E, Arduini D. First trimester placental volume and three dimensional power doppler ultrasonography in type I diabetic pregnancies. Prenat Diagn. 2012 May;32(5):480–4.
- de Paula CFS, Ruano R, Campos JADB, Zugaib M. Quantitative analysis of placental vasculature by three-dimensional power Doppler ultrasonography in normal pregnancies from 12 to 40 weeks of gestation. Placenta. 2009 Feb, 30(2), 142–148.
- Metzenbauer M, Hafner E, Schuchter K, Philipp K. First-trimester placental volume as a marker for chromosomal anomalies: Preliminary results from an unselected population. Ultrasound in Obstetrics and Gynecology. 2002, 19(3). [CrossRef]
- Barbaro G, Inversetti A, Cristodoro M, Ticconi C, Scambia G, Di Simone N. HLA-G and Recurrent Pregnancy Loss. Int J Mol Sci. 2023, 24(3). [CrossRef]
- Arnaiz-Villena A, Juarez I, Suarez-Trujillo F, López-Nares A, Vaquero C, Palacio-Gruber J, et al. HLA-G: Function, polymorphisms and pathology. Int J Immunogenet. 2021, 48(2). [CrossRef]
- Bilardo CM, Pajkrt E, De Graaf I, Mol BW, Bleker OP. Outcome of fetuses with enlarged nuchal translucency and normal karyotype. Ultrasound in Obstetrics and Gynecology. 1998, 11(6). [CrossRef]
| Maternal age (years)* | 33.63 ± 6.51 |
|---|---|
| Number of nulliparous women in the study** | 12 (29.3) |
| BMI at the time of genetic consultation (kg/m2)* | 26.35 ± 6.19 |
| Birth weight (grams)* | 3351.22 ± 370.10 |
| Birth weight (percentile)* | 54.34 ± 24.39 |
| Gestational age at the time of delivery (weeks)* | 39.01 ± 1.32 |
| Data on genetic ultrasound examination in the first trimester | |
|---|---|
| NT (mm) | 1.88 ± 0.66 |
| CRL at NT (mm) | 63.90 ± 6.54 |
| Gestational age at nuchal translucency (weeks) | 12.62 ± 0.55 |
| Fetal biometry at the time of amniocentesis | |
| Gestational age at the time of amniocentesis (weeks) | 18.37 ± 1.49 |
| Head circumference (mm) | 153.62 ± 15.89 |
| Head circumference (percentile) | 56.70 ± 29.21 |
| Abdominal circumference (mm) | 134.10 ± 17.06 |
| Abdominal circumference (percentile) | 55.25 ± 27.57 |
| Femur length (mm) | 27.71 ± 4.99 |
| Femur length (percentile) | 57.54 ± 27.46 |
| Estimated fetal weight (grams) | 260.71 ± 81.26 |
| Estimated fetal weight (percentile) | 53.50 ± 26.11 |
| Placental sonography | |
| Placental volume (mm3) | 214.80 ± 94.67 |
| VI | 14.38 ± 5.67 |
| FI | 43.27 ± 8.76 |
| VFI | 8.46 ± 4.20 |
| sHLA-G concentration in amniotic fluid (ng/ml) | 53.39 ± 19.00 |
|---|---|
| sHLA-G concentration in serum (ng/ml) | 51.05 ± 26.99 |
| sHLA-G level in serum | sHLA-G in amniotic fluid | |||||||
| Univariate linear regression | Multivariate linear regression | Univariate linear regression | Multivariate linear regression | |||||
| β | CI | β | CI | β | CI | β | CI | |
| Clinical and obstetric characteristics | ||||||||
| Maternal age | 0.01 | -1.31-1.38 | 0.01 | -1.71-1.74 | -0.20 | -1.51-0.39 | -0.17 | -1.75-0.79 |
| Previous parity | -0.12 | -13.18-6.00 | -0.15 | -16.17-7.50 | -0.08 | -8.54-5.33 | 0.08 | -7.21-10.43 |
| BMI at the time of genetic consultation (kg/m2) | 0.19 | -0.57-2.21 | 0.15 | -0.82-2.16 | -0.04 | -1.22-0.93 | 0.00 | -1.10-1.11 |
| Birth weight (grams) | -0.02 | -0.03-0.02 | -0.01 | -0.03-0.02 | 0.01 | -0.02-0.02 | -0.01 | -0.02-0.02 |
| Birth weight (percentile) | 0.05 | -0.30-0.40 | 0.18 | -0.31-0.42 | -0.02 | -0.27-0.24 | -0.00 | -0.27-0.27 |
| NT | -0.11 | -17.87-8.79 | -0.17 | -22.29-8.50 | -0.30 | -17.78-0.86 | -0.38* | -21.73-0.04* |
| CRL at NT | 0.30 | -0.08-2.52 | 0.76 | -0.38-2.69 | 0.10 | -0.71-1.32 | 0.14 | -0.75-1.60 |
| GA at the time of delivery | -0.11 | -8.85-4.34 | -0.06 | -1.14-0.81 | 0.02 | -4.45-5.08 | 0.03 | -0.63-0.77 |
| GA at the time of amniocentesis (weeks) | -0.13 | -8.22-3.45 | 0.46 | -1.30-0.55 | 0.24 | -1.12-7.10 | 0.20 | -0.31-1.02 |
| Fetal sonography at the time of amniocentesis | ||||||||
| Head circumference (mm) | -0.12 | -0.75-0.35 | -0.09 | -1.12-0.83 | 0.26 | -0.08-0.69 | 0.19 | -0.49-0.95 |
| Head circumference (percentile) | -0.03 | -0.32-0.28 | -0.16 | -0.51-0.21 | -0.08 | -0.27-0.17 | 0.08 | -0.24-0.34 |
| Abdominal circumference (mm) | 0.01 | -0.71-0.71 | 0.70 | -0.30-2.77 | 0.33 | -0.08-0.86 | 0.93 | -0.14-2.32 |
| Abdominal circumference (percentile) | 0.41* | -0.08-0.75* | 0.35 | -0.07-0.84 | 0.26 | -0.12-0.51 | 0.35 | -0.08-0.61 |
| Femur length (mm) | 0.07 | -2.54-1.74 | -0.18 | -7.88-5.78 | 0.23 | -0.61-2.53 | -0.28 | -6.89-4.61 |
| Femur length (percentile) | 0.20 | -0.18-0.59 | -0.02 | -0.49-0.45 | -0.00 | -0.30-0.29 | -0.12 | -0.49-0.31 |
| Estimated fetal weight (grams) | -0.04 | -0.16-0.14 | 0.64 | -0.22-0.69 | 0.26 | -0.04-0.17 | 0.73 | -0.17-0.53 |
| Estimated fetal weight (percentile) | 0.41* | -0.02-0.84* | 0.31 | -0.15-0.85 | 0.24 | -0.14-0.52 | -0.31 | -0.12-0.63 |
| Placental sonography at the time of amniocentesis | ||||||||
| Placental volume (mm3) | 0.02 | -0.09-0.10 | -0.03 | -0.11-0.10 | -0.09 | -0.09-0.05 | 0.01 | -0.07-0.08 |
| VI | -0.10 | -2.00-1.08 | -0.16 | -2.56-1.03 | -0.34* | -2.13-0.06* | -0.38* | -2.47-0.03* |
| FI | 0.05 | -0.86-1.14 | 0.04 | -0.92-1.17 | -0.18 | -1.12-0.34 | -0.13 | -1.06-0.50 |
| VFI | -0.34* | -3.58-0.46* | -0.32 | -4.32-0.20 | -0.44* | -3.28-0.63* | -0.52* | -3.79-0.72* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





