1. Introduction
Type 2 diabetes mellitus (T2DM) is a disease with a worldwide high and increasing prevalence [
1]. In the course of recent decades, cardiovascular and renal risk has become the focus of treatment of T2DM [
2]. However, we know that improvements in pharmacological and non-pharmacological therapeutic approaches have ameliorated CVD outcomes [
3]. Therefore, CVD-related mortality decreased faster than the change in cancer-related mortality [
4]. In fact, stated in a paper, ‘Cancer is becoming the leading cause of death in diabetes’ [
5]. In Australia, between 1997 and 2010, CVD-related mortality decreased while cancer-related mortality increased [
6]. A study from Hong-Kong found that in the age group of 45-74 years, cancer became the leading cause of mortality [
7]. A Swedish national observation study showed that CVD-related mortality decreased, whereas cancer-related mortality increased both in patients with T2DM and in controls between 1998 and 2012. The study suggests that cancer would expectedly be the leading contributor to mortality in patients with T2DM by 2030 and in the control group by 2040 [
8]. Researchers from England found that in the age groups of 75-85 and 85+ years, the risk of cancer increased by 1.2% and 1.6% annually [
9]. Data from Scotland verified that mortality due to CVD decreased, while mortality related to cancer became the leading cause of mortality in diabetes with a 28% value by 2018 [
10]. Data of the Global Burden of Disease show that worldwide, cancer is the second most common cause of mortality, and in fact, in high-income countries, cancer was the most frequent cause [
11]. Thus, analyses related to diabetes and cancer is required to become a focus of interest.
In the present study, we performed a nationwide retrospective analysis related to incidence of cancer in patients with T2DM and in controls. We intended to find information about the odds of development of cancer in general and in a site-specific manner, and about the temporal trends related to cancer.
2. Materials and Methods
The study was carried out in accordance with the Helsinki declaration [
12]. The study was approved by the Scientific and Research Ethics Committee of the Medical Research Council of Hungary (BMEÜ/325-1/2022/EKU). During preparation of the manuscript, the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline was followed.
The study population was also investigated according to decades of age. Furthermore, comparison of 18-59 and 60+ year-old subgroups were also performed. The study population was divided at 60 years of age due to two reasons: i) this is the cut-off age suggested by WHO to be used for the definition of the elderly population [
13]; ii) the 60+ year-old subgroup provides approximately 25% part of the total population and nearly 33% of the general adult population [
14], providing sufficient data for analysis.
Data sources
Data on annual total population figures were obtained from the Central Statistical Office (CSO) of Hungary, while data of patients associated to diabetes or cancer were obtained from the National Health Insurance Found (NHIF). Due to data protection rules, the NHIF provided only aggregated data for subgroups with a number of cases ≥ 10, and the results of the statistical models.
Cancer-related cases were identified according to previously applied methods [
15,
16]. T2DM cases were identified similar to those described in our previous papers [
17,
18,
19,
20]. The details are available in the supplement of part 1.
Analysis of the incidence of cancer was carried out by using R version 4.0.4. We used binomial logistic regression where the population in each year included people without cancer on 1st of January, who had T2DM already or did not get T2DM in a year. Prevalent T2DM patients and non-diabetic subjects who did not develop T2DM during the calendar year were considered. We analyzed new T2DM cases in another paper (part 1). The dependent variable was the incidence of cancer during the calendar year, while the independent variables were presence of T2DM, the age group, the gender and all of their interactions.
Odds ratio (OR) and annual percent changes in the incidence rate (APC) were calculated by bootstrap with one billion repetitions shown together with their 95% confidence intervals. During the statistical analysis, adjustment for age and gender was carried out using the average number of baseline population of the subgroups during the study period.
3. Results
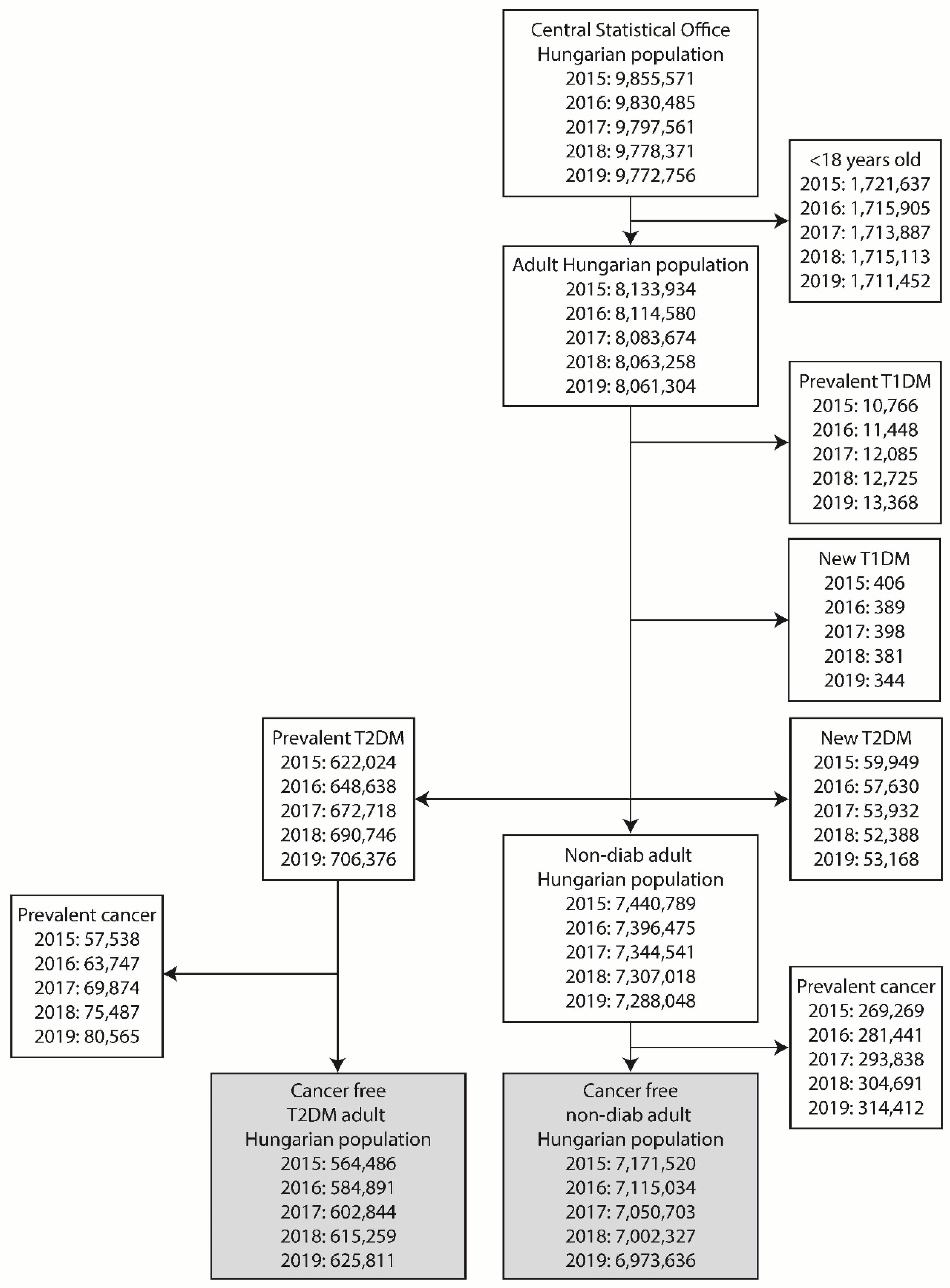
Cases with an age of less than 18 years were excluded from the study. Similarly, cases with Type 1 diabetes mellitus, and subsequently, cases with incident T2DM were excluded from the data of adult population. The remaining population was divided into groups of cases with prevalent T2DM and non-diabetic controls (
Figure 1).
3.1. Data on total cancer incidence with trends
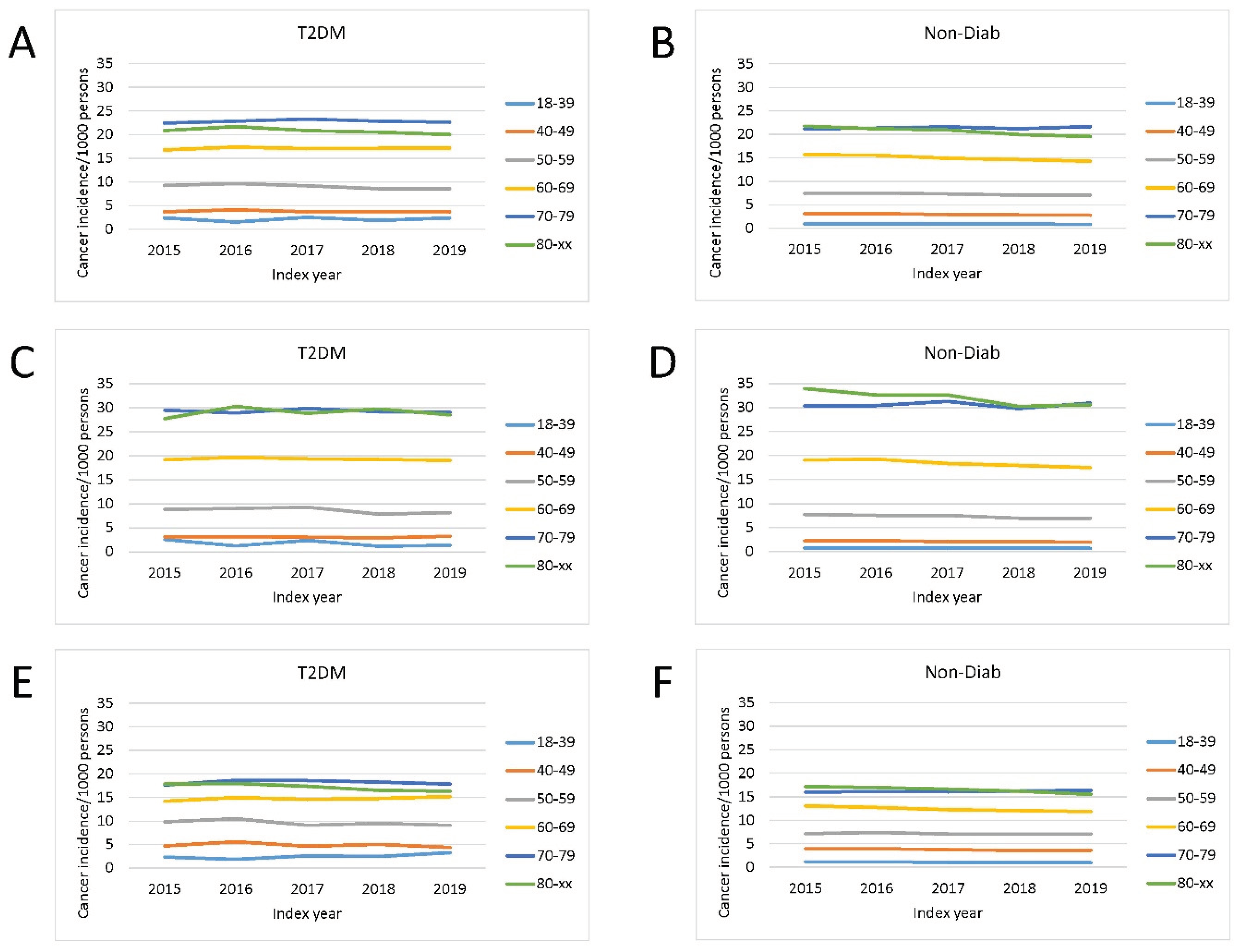
Total cancer incidence in patients with type 2 diabetes mellitus and in controls was investigated in the period of 2015-2019 in different age groups. The crude cancer incidence was highest in the 80+ and 70–79-year-old groups. The same was observed in analyses of male and female patients separately, however, overall cancer incidence was higher in males than in females in the 60-69, 70-79 and 80+ age groups (
Figure 2).
3.2. Age group distribution of cancer
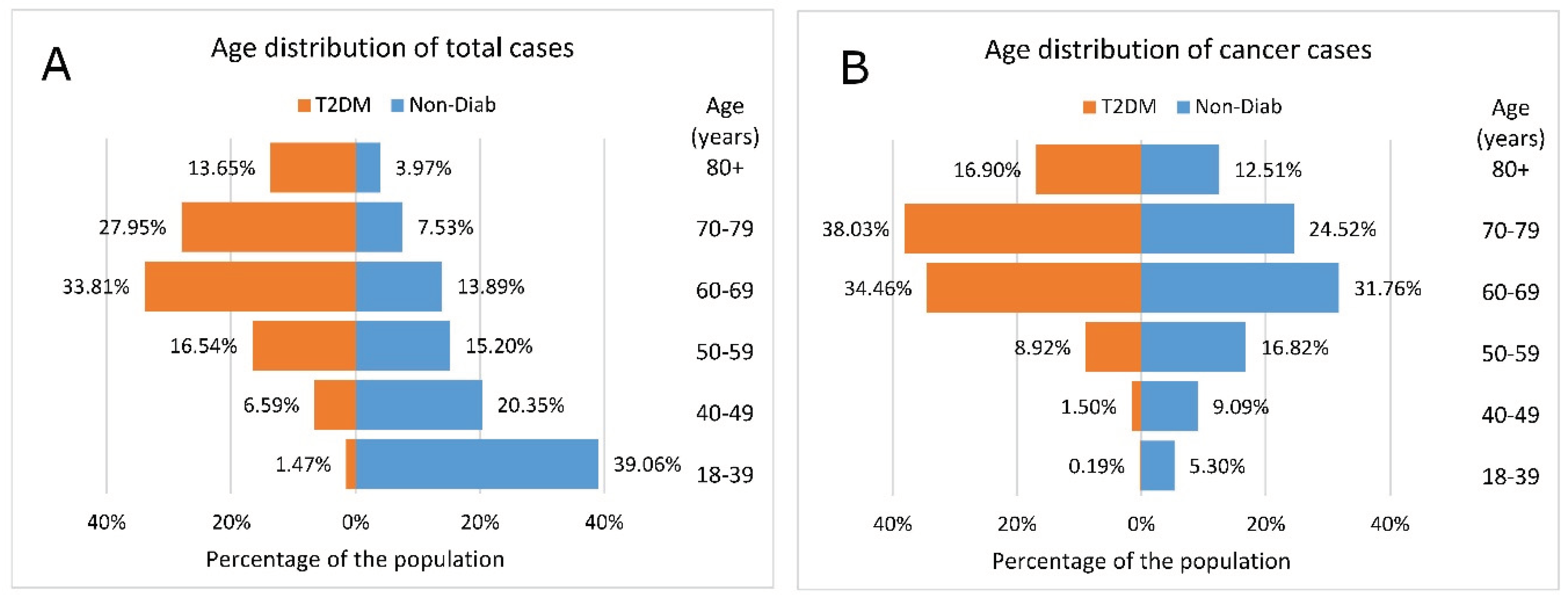
Comparison of age distribution of the total cases showed that the 60+ age groups dominated in the T2DM group. Conversely, in the Non-Diab group, the <60 year-old groups accounted for nearly 75% of all cases (
Figure 3A).
The age pyramid of cancer cases shows that the age distribution was markedly different in the two groups, with more young patients in the Non-Diab group and more elderly patients in the T2DM group (
Figure 3B).
3.3. Cancer risk and temporal changes of incidence of cancer
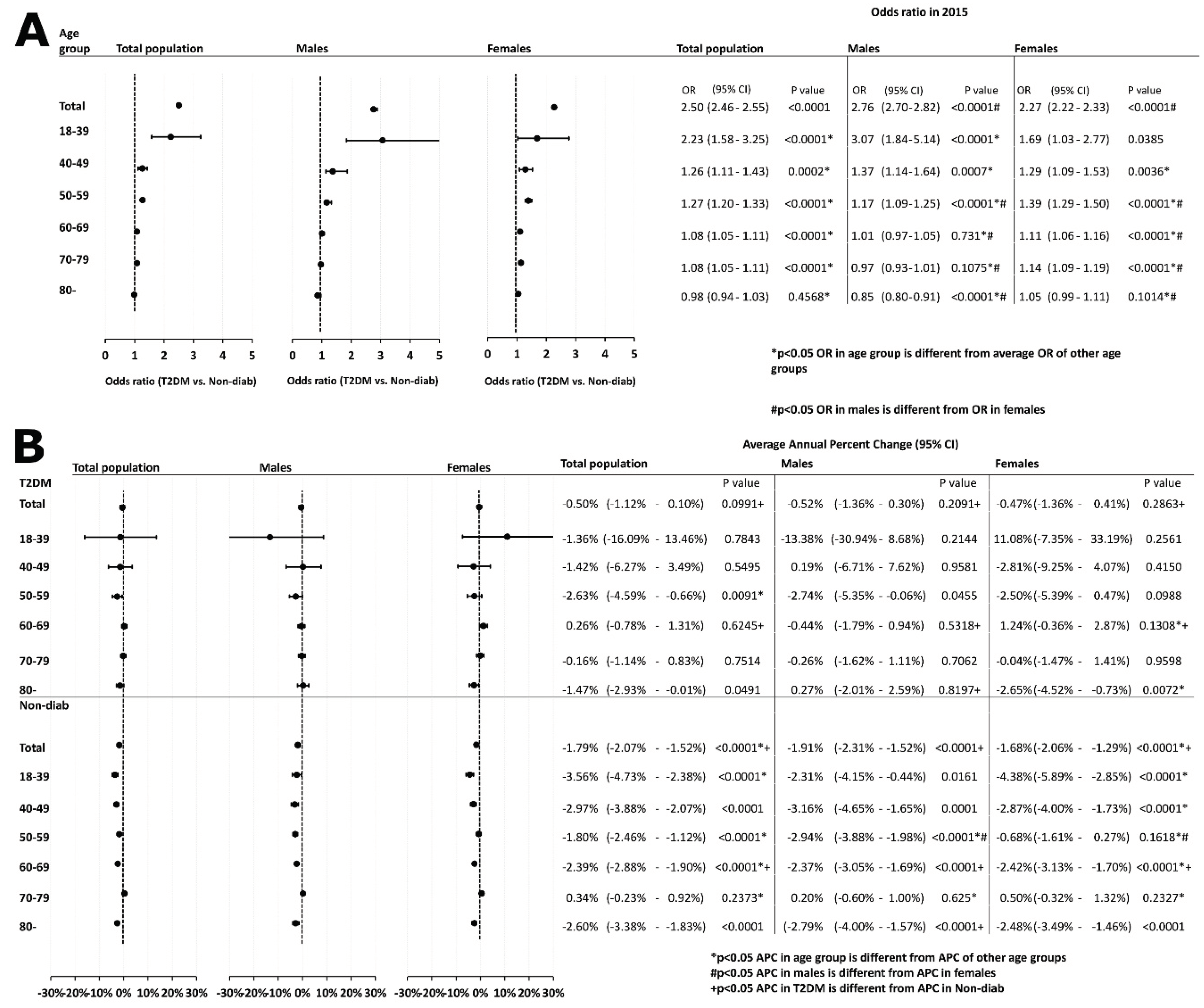
Furthermore, we analyzed the odds of developing cancer and found that patients with T2DM had a significantly higher cancer risk than those in the Non-Diab group. When analyzing the risk in different age groups, we found that the elevated OR was present in all age groups, except for the 80+ group, although the excess risk decreased as the age increased. Moreover, the overall cancer risk of male patients was higher than that of female patients. When analyzing the risk in the different age groups, we found that the OR was highest in young (18-39 years) males, but this was not significantly different form the risk of females in the same age group. The OR was higher in females than in males in the 50+ age groups but was not different in the group of 40-49-year-olds (
Figure 4A).
Next, we studied the temporal change of overall cancer in the total population and in different age groups. In the total population, no significant trend could be observed in the T2DM group, while a decreasing trend was found in the Non-Diab group. When analyzing different age groups separately, in the T2DM group, there was a decreasing trend in the 50-59 and the 80+ groups only. In the Non-Diab group, there was a decreasing trend in all of age groups, except for the 70-79-year-old group. No difference could be detected between males and females in the APC, neither in patients with T2DM nor in the Non-Diab group. Overall, among patients with T2DM, there was no difference in the APC between males and females in any age group, whereas in the Non-Diab group, the APC showed a more marked decrease in the 50-59 year-old males than in females. (
Figure 4B).
3.4. Data of site-specific incidence of cancer
3.4.1. Site-specific distribution of cancer types in the T2DM vs. Non-Diab groups
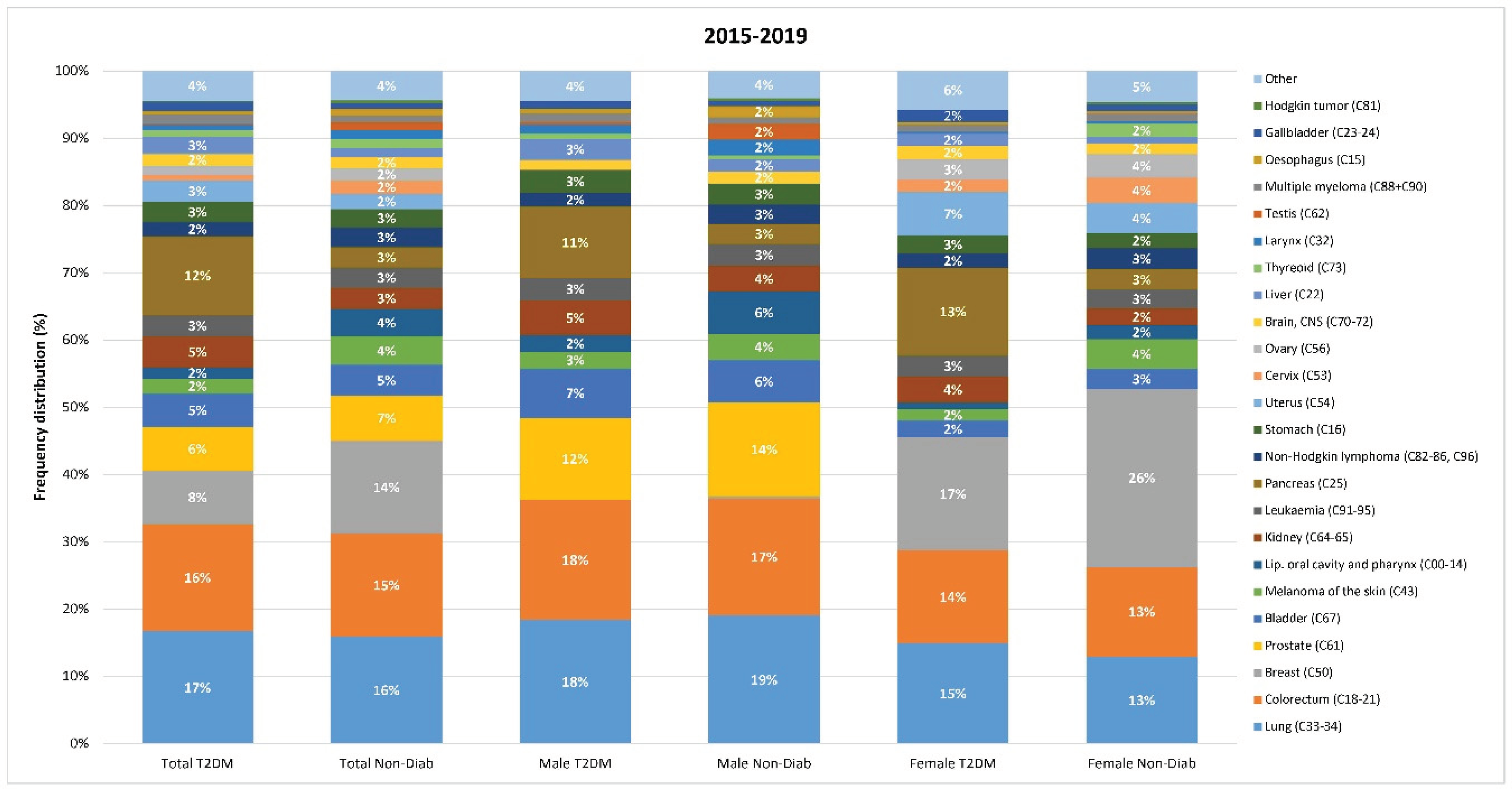
In both groups, the four most common cancer types (lung, colorectal, breast and prostate) accounted for approx. 50% of cancer cases (
Figure 5).
The relative distribution of individual cancer sites was slightly different in the T2DM and in the Non-Diab groups: colorectal, prostate, bladder, kidney, pancreas, stomach, uterus corpus, liver cancers accounted for a larger proportion of all cancer cases in the T2DM than in the Non-Diab group. On the contrary, lung, breast, melanoma, oral cancers, leukemia, non-Hodgkin lymphoma, ovary, brain, thyroid, larynx, testis accounted for a larger proportion of cases in the non-Diab group than in the T2DM groups (
Figure 5).
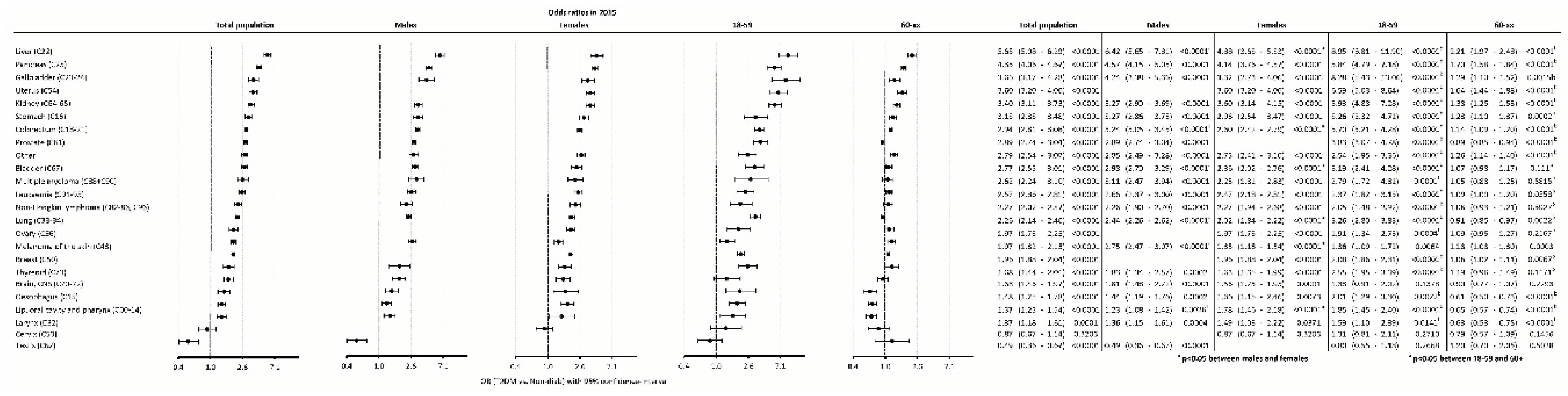
The odds of developing new cancer were highest for liver cancer, followed by pancreas, gallbladder, uterus, kidney cancer] and other types of cancer. On the contrary, the odds were lower in T2DM for testis cancer as compared to controls (
Figure 6).
When comparing males to females, the risk in males was higher for liver, colorectal, bladder, lung cancers and for melanoma, while it was higher in females than in males for cancers of lip, oral cavity and pharynx combined (
Figure 6).
In younger patients (18-59 years), T2DM was associated with significant higher odds of developing cancer for most cancer types except for brain, cervix and testis cancers. In the older age group (60+), T2DM was associated with higher odds for several cancer sites, except for bladder, myeloma, leukemia, non-Hodgkin lymphoma, ovary, thyroid, brain, cervix and testis cancers (
Figure 6).
When comparing the 18-59 and the 60+ age groups, the excess risk of diabetic patients in the younger group was higher for nearly all cancer types (including colorectal, lung, prostate and breast cancer) (
Figure 6).
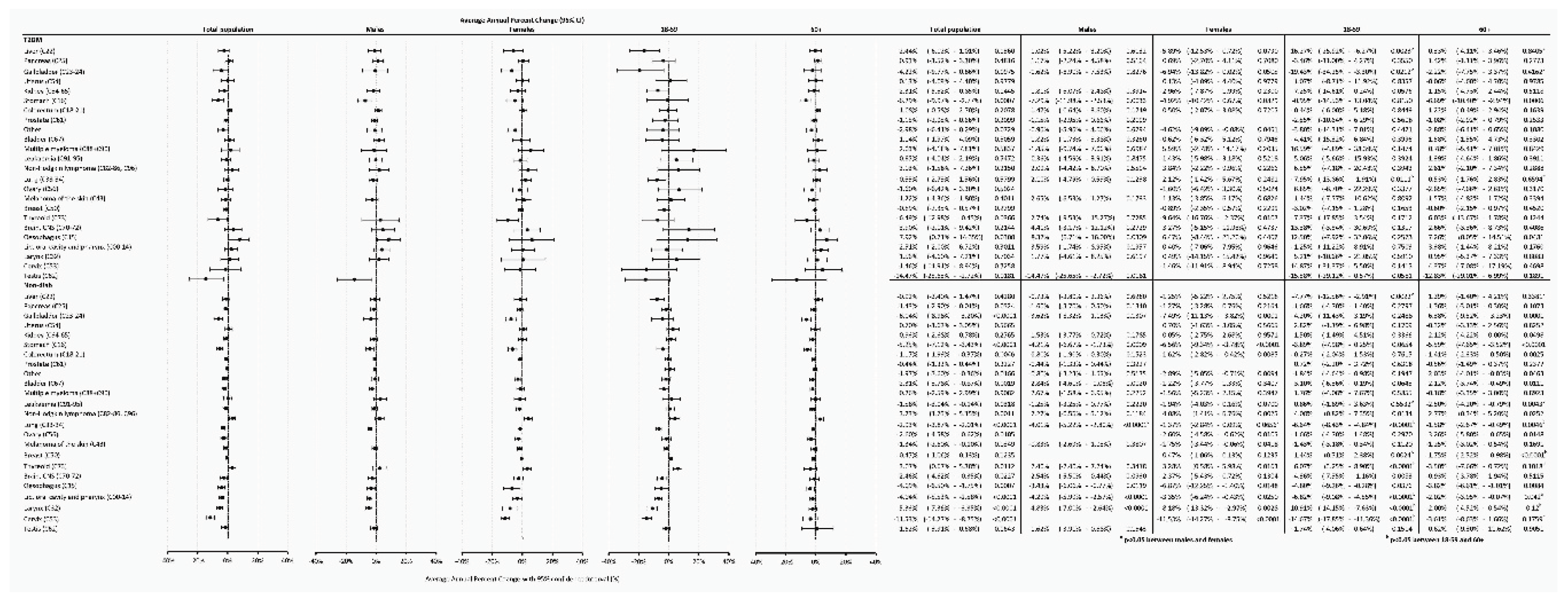
3.4.2. Temporal trends on site-specific cancer incidence
Analyzing temporal trends using the APC for the total population in the T2DM group, we found a significant increasing tendency for esophagus cancer, a significant decreasing tendency for stomach, thyroid and testis cancers. As for the Non-Diab group, we found an increasing trend for non-Hodgkin lymphoma, thyroid cancer, and a decreasing trend for gallbladder, stomach, colorectum, lung, brain, esophagus, pharynx, larynx, and cervix cancers (
Figure 7).
Comparing males to females, we only found a difference in the Non-Diab group and only for lung cancer, but not in the T2DM group and not for other cancer sites (
Figure 7).
When comparing the 18-59 to the 60+ age groups, in the T2DM group, a decreasing tendency was more expressed in the 18-59-year-old group for liver, gallbladder and lung cancer, but not for other types of cancer. In the Non-Diab group, the decreasing tendency was more expressed in the 18-59 year-old group for liver, lung, pharynx, larynx and cervix cancers, while it occurred to be more expressed in the 60+ age-group for leukemia. In the case of breast cancer, the incidence significantly increased in the younger and significantly decreased in the older non-diabetic patients. For thyroid cancer, the incidence increased significantly in the younger but did not change in older non-diabetic patients. (
Figure 7.)
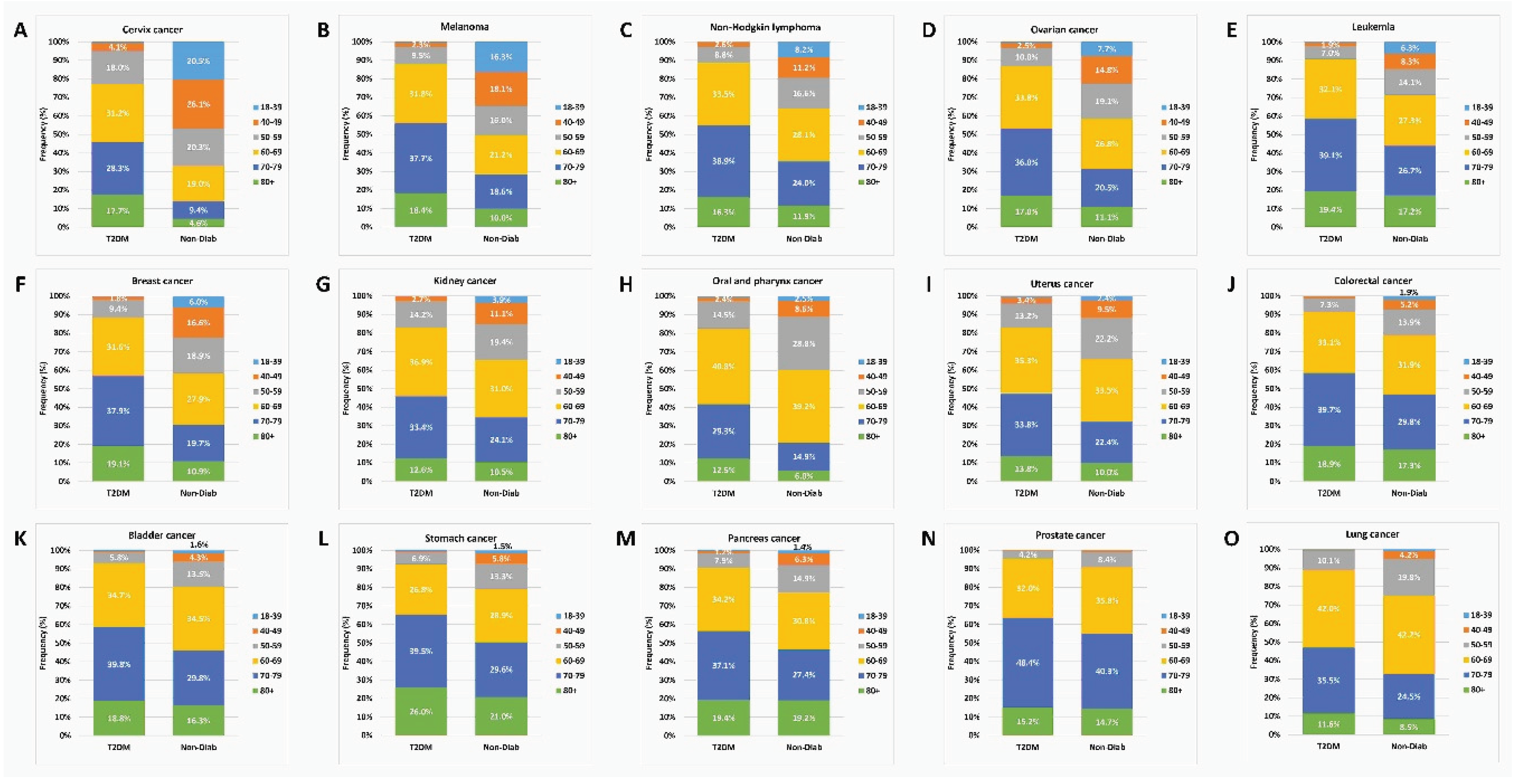
3.5. Relative age distribution
We also analyzed the percentage of the age groups within the cancer cases in the two groups, and found striking differences, especially for cervix, melanoma, non-Hodgkin lymphoma, ovarian, leukemia and breast cancer types, where the proportion of younger age groups was higher in the controls than in the T2DM cases. On the other hand, 70+ patients dominated in the T2DM group for all individual cancer sites, while the relative proportion of these patients was lower in the Non-Diab group (
Figure 8).
4. Discussion
The present study found that the incidence of cancer is generally higher in males than in females in both patients with T2DM and in the Non-Diab groups. Cancer affects different age groups differently in patients with T2DM and in controls, the odds ratio is highest in the young patients with T2DM, especially in males. In Non-Diab cases, the incidence of cancer decreased overall as well as in several age groups. On the contrary, there was no decrease in the incidence in the T2DM group. Also, the site-specific distribution of cancer types is different e.g., the relative contribution of kidney, pancreas, liver cancers is higher in T2DM, whereas the relative contribution of breast, oropharyngeal and testis cancers is higher in the Non-Diab groups. When analyzing the OR of cancer of individual sites in the T2DM vs the Non-Diab groups, we found that the odds were highest for liver, pancreas, gallbladder, uterus, kidney and colorectal cancers. For most cancer types, the OR of developing cancer in T2DM was higher in the <60-year-old group vs. the 60+ age group. In the Non-Diab group, the annual incidence declined for gallbladder, stomach, colorectal, lung, brain, esophagus, pharynx, larynx and cervix cancers, while in the T2DM group, only the incidence of stomach, thyroid and testis cancer decreased. In the Non-Diab group, the annual incidence increased for non-Hodgkin lymphoma and thyroid cancer, while in the Non-Diab group it increased only for esophagus cancer.
One of the most important observations of our study is the high relative cancer risk in young patients with T2DM. In the general population, probability of developing invasive cancer increases with increasing age in the US [
21].
When calculating the OR of developing cancer for different age groups, we found that the OR is highest (2.23) in the youngest age group, and the risk decreases with increasing age to a nonsignificant value. Similar results were found in a recent paper, where analysis of data of the US National Cancer Institute showed that the incidence of early-onset cancer rose in the 30-39 year-old group, while it decreased in the 50+ age groups [
22]. Concerning individual cancer sites, we found higher odds in younger patients with T2DM for several cancer types of high importance and high frequency such as colorectal, lung, prostate, liver, pancreas cancers, with some of them being screenable in high-risk individuals.
In a previous nationwide analysis by our workgroup, a high hazard ratio of mortality, myocardial infarction and stroke was found in the younger (19-30 and 31-40 year-old groups), much higher than in the elderly [
23]. This is very similar to the present observation on cancer. These data suggest that we should focus on the young T2DM patients.
Regarding the association between overall cancer risk and T2DM, we verified the positive association suggested by the literature. As for the overall incidence of cancer, the hazard ratio (HR) was 1.21 (1.16-1.26) in one analysis [
24], whereas the SIR in another paper was 1.22 for male and 1.737 for female patients with T2DM [
25]. An umbrella review of meta-analyses found a positive association with T2DM for breast, liver, endometrium and colorectal cancers [
26]. In a Swedish national study, overall cancer risk had a slight positive association with T2DM [HR: 1.10 (1.09-1.12)] [
8]. Italian authors found an incidence rate ratio (IRR) of 1.22 (1.15-1.29) in patients with DM, with the strongest association with liver, pancreas, colorectal, urinary bladder cancers, uterus corpus cancers [
27], while in our study the strongest associations were found for liver, pancreas, gallbladder, uterus and kidney cancers.
Data of the literature are quite heterogenous, and also different measures (IRR, standardized incidence rate [SIR], relative risk [RR], HR, OR) are used in different studies and for different study designs. At the same time, data can be markedly different for different cancer sites (
Table 1.).
In general, odds of developing cancer in T2DM seem higher in the present paper as compared to the literature, and this might have multiple explanations. Obesity is a well-known risk factor for both T2DM and cancer and can act as a confounder in the epidemiological studies analyzing the association of T2DM and cancer [
28]. Several of the above-mentioned studies (e.g., [
3,
10,
12,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38]) used adjustment to anthropometric markers including markers for obesity, such as BMI, which severely could have altered their results. In the present study, we did not perform any adjustment for BMI or other anthropometric parameters as BMI was not available in our database. This might have contributed to the higher OR values as compared to some studies from the literature. However, obesity is a hallmark of T2DM for the majority of the patients, and by correcting for BMI in the analysis we could get a false impression of the real excess risk caused by T2DM.
Also, demographic and ethnic markers of the Hungarian population could also have influenced the results, as compared to data taken other geographic areas.
With regard to temporal trends, our data indicate a decreasing cancer incidence trend in the non-diabetic group and nearly all age groups. In the T2DM group, however, no beneficial trend could be observed, except for the 50-59 and 80+ age subgroups. Global data to metabolic-associated cancer showed a worldwide overall increasing trend [AAPC: 0.74 (0.71-0.76)] [
39]. The Global Burden of Disease study also put Hungary into the category with a -0,9 - 0% annual percent change in cancer incidence, our present data were -1.78 for the Non-Diab and -0.5 for the T2DM group [
11].
Strengths of our paper include its nationwide character with an almost 100% coverage of the Hungarian population enabling robust results. Data also enabled simultaneous analysis of the effect of age groups, gender, individual cancer sites and temporal changes.
Limitations of our study include the lack of anthropometric, clinical and laboratory data, the retrospective nature of the study.
5. Conclusions
Prevalent T2DM represents a 150% increase in the risk of developing cancer. Especially younger age groups are susceptible. Site- and age-distribution in the two groups are different, and trends are also less favorable in the T2DM group, altogether underlining the importance of cancer surveillance in patients with T2DM, especially in the younger age groups, which should be regarded as high-risk individuals.
Author Contributions
Conceptualization, I.W., Z.K., G.R., G.J., P.K., C.L.; methodology, I.W., Z.K., G.R.; software, Z.A-T., I.F., G.A.M.; validation, Z.A-T., I.F.; formal analysis, Z. A-T, I.F., G.A.M.; investigation, Z. A-T, I.F., G.A.M., G.S., I.W.; resources, I.W., P.K., C.L.; data curation, Z. A-T., I.F., G.R,; writing—original draft preparation, Z.A-T., G.A.M., I.W.; writing—review and editing, I.W., Z.K.; visualization, Z.A-T, I.F., G.A.M.; supervision, I.W., Z.K, G.R., G.J., P.K., C.L.; project administration, X.X. Z.A-T., I.F., G.A.M.; funding acquisition, I.W., P.K., C.L. All authors have read and agreed to the published version of the manuscript.
Funding
The Hungarian Diabetes Association provided funding for data collection and research.
Institutional Review Board Statement
The study protocol was approved by the Ethical Board of the University of Pécs (9326-PTE 2022), by the National Institute of Pharmacy and Nutrition (KRID: 641936355) and by Medical Research Council (BMEÜ/95-3/2022/EKU).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data generated during the study are available upon reasonable request from the corresponding author. Individual, patient-level data are not available due to data protection rules of the NHIF.
Acknowledgments
The authors would like to thank colleagues at the National Institute of Health Insurance Fund for their help in providing the data for the study and would also like to thank Professor Dr József Andor for linguistic help and Enikő Bodor for technical assistance.
Conflicts of Interest
Authors Zsolt Abonyi-Tóth, György Rokszin and Ibolya Fábián are employed by RxTarget Ltd., Szolnok, Hungary. Dr. Kiss is also employed by MSD Pharma Hungary Ltd., but this provides no relevant conflict of interest for the current research. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Ritchey, M.D.; Wall, H.K.; George, M.G.; Wright, J.S. US Trends in Premature Heart Disease Mortality over the Past 50 Years: Where Do We Go from Here? Trends Cardiovasc. Med. 2020, 30, 364–374. [Google Scholar] [CrossRef]
- Rey-Reñones, C.; Baena-Díez, J.M.; Aguilar-Palacio, I.; Miquel, C.; Grau, M. Type 2 Diabetes Mellitus and Cancer: Epidemiology, Physiopathology and Prevention. Biomedicines 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Wang, M.; Sperrin, M.; Rutter, M.K.; Renehan, A.G. Cancer Is Becoming the Leading Cause of Death in Diabetes. Lancet 2023, 401, 1849. [Google Scholar] [CrossRef]
- Harding, J.L.; Shaw, J.E.; Peeters, A.; Guiver, T.; Davidson, S.; Magliano, D.J. Mortality Trends among People with Type 1 and Type 2diabetes in Australia: 1997-2010. Diabetes Care 2014, 37, 2579–2586. [Google Scholar] [CrossRef]
- Wu, H.; Lau, E.S.H.; Ma, R.C.W.; Kong, A.P.S.; Wild, S.H.; Goggins, W.; Chow, E.; So, W.Y.; Chan, J.C.N.; Luk, A.O.Y. Secular Trends in All-Cause and Cause-Specific Mortality Rates in People with Diabetes in Hong Kong, 2001–2016: A Retrospective Cohort Study. Diabetologia 2020, 63, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Bjornsdottir, H.H.; Rawshani, A.; Rawshani, A.; Franzén, S.; Svensson, A.M.; Sattar, N.; Gudbjörnsdottir, S. A National Observation Study of Cancer Incidence and Mortality Risks in Type 2 Diabetes Compared to the Background Population over Time. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Ling, S.; Zaccardi, F.; Issa, E.; Davies, M.J.; Khunti, K.; Brown, K. Inequalities in Cancer Mortality Trends in People with Type 2 Diabetes: 20 Year Population-Based Study in England. Diabetologia 2023, 66, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Stuttard, J.; Bennett, J.; Cheng, Y.J.; Vamos, E.P.; Cross, A.J.; Ezzati, M.; Gregg, E.W. Trends in Predominant Causes of Death in Individuals with and without Diabetes in England from 2001 to 2018: An Epidemiological Analysis of Linked Primary Care Records. Lancet Diabetes Endocrinol. 2021, 9, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019 A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- World Heath Organization Ageing. Available online: https://www.who.int/health-topics/ageing#tab=tab_1 (accessed on 29 November 2023).
- KSH Statisztikai Hivatal Magyarország Népességének Száma Nemek És Életkor Szerint Available online:. Available online: https://www.ksh.hu/interaktiv/korfak/terulet.html (accessed on 29 November 2023).
- Suto, G.; Molnár, G.A.; Rokszin, G.; Fábián, I.; Kiss, Z.; Szekanecz, Z.; Poór, G.; Jermendy, G.; Kempler, P.; Wittmann, I. Risk of Morbidity and Mortality in Patients with Type 2 Diabetes Treated with Sodium-Glucose Cotransporter-2 Inhibitor and/or Dipeptidyl Peptidase-4 Inhibitor: A Nationwide Study. BMJ Open Diabetes Res. Care 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Rokszin, G.; Kiss, Z.; Sütő, G.; Kempler, P.; Jermendy, G.; Fábián, I.; Szekanecz, Z.; Poór, G.; Wittmann, I.; Molnár, G.A. Sodium-Glucose Co-Transporter 2 Inhibitors May Change the Development of Urinary Tract and Hematological Malignancies as Compared With Dipeptidyl Peptidase-4 Inhibitors: Data of the Post-Hoc Analysis of a Nationwide Study. Front. Oncol. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bodner, K.; Irvine, M.A.; Kwong, J.C.; Mishra, S. Observed Negative Vaccine Effectiveness Could Be the Canary in the Coal Mine for Biases in Observational COVID-19 Studies. Int. J. Infect. Dis. 2023, 131, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Statistics Notes: How to Obtain the P Value from a Confidence Interval. BMJ 2011, 343, 1–2. [Google Scholar] [CrossRef]
- Jermendy, G.; Kiss, Z.; Rokszin, G.; Abonyi-Tóth, Z.; Wittmann, I.; Kempler, P. Decreasing Incidence of Pharmacologically Treated Type 2 Diabetes in Hungary from 2001 to 2016: A Nationwide Cohort Study. Diabetes Res. Clin. Pract. 2019, 155. [Google Scholar] [CrossRef]
- Jermendy, G.; Kiss, Z.; Rokszin, G.; Abonyi-Tóth, Z.; Wittmann, I.; Kempler, P. Decreasing Incidence of Pharmacologically Treated Type 2 Diabetes in Hungary from 2001 to 2016: A Nationwide Cohort Study. Diabetes Res. Clin. Pract. 2019, 155. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA. Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Koh, B.; Tan, D.J.H.; Ng, C.H.; Fu, C.E.; Lim, W.H.; Zeng, R.W.; Yong, J.N.; Koh, J.H.; Syn, N.; Meng, W.; et al. Patterns in Cancer Incidence among People Younger Than 50 Years in the US, 2010 to 2019. JAMA Netw. Open 2023, 6, E2328171. [Google Scholar] [CrossRef]
- Kiss, Z.; Rokszin, G.; Abonyi-Tóth, Z.; Jermendy, G.; Kempler, P.; Aradi, D.; Wittmann, I. Dissimilar Impact of Type 2 Diabetes on Cardiovascular Outcomes According to Age Categories: A Nationwide Population Study from Hungary. Cardiovasc. Diabetol. 2018, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, X.; Ma, Y.; Yuan, C.; Wang, M.; Wu, K.; Tabung, F.K.; Tobias, D.; Hu, F.B.; Giovannucci, E.; et al. Incident Type 2 Diabetes Duration and Cancer Risk: A Prospective Study in Two US Cohorts. J. Natl. Cancer Inst. 2021, 113, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Riaz, S. Type 2 Diabetes and Cancer. Ann. Clin. Endocrinol. Metab. 2020, 4, 001–006. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P.A. Type 2 Diabetes and Cancer: Umbrella Review of Meta-Analyses of Observationlal Studies. BMJ 2015, 350, 1–11. [Google Scholar] [CrossRef]
- Ballotari, P.; Vicentini, M.; Manicardi, V.; Gallo, M.; Chiatamone Ranieri, S.; Greci, M.; Giorgi Rossi, P. Diabetes and Risk of Cancer Incidence: Results from a Population-Based Cohort Study in Northern Italy. BMC Cancer 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and Cancer: A Consensus Report. In Proceedings of the Diabetes Care; 2010; Vol. 33; pp. 1674–1685. [Google Scholar]
- European Commission; Eurostat; Corselli-Nordblad, L.; Strandell, H. Ageing Europe - Looking at the Lives of Older People in the EU : 2020 Edition; 2020. [Google Scholar]
- Zhou, R.; Huang, C.; Luo, Z.; Wang, T. The Association between the Risk of Esophageal Cancer and Type 2 Diabetes Mellitus: An Updated Meta-Analysis. Biomed Res. Int. 2022, 2022. [Google Scholar] [CrossRef]
- GLOBOCAN World Health Organization Estimated Age-Standardized Incidence Rates (World) in 2020, World, Both Sexes, All Ages (Excl. NMSC). World Heal. Organ. 2020, 2020.
- Kang, C.; LeRoith, D.; Gallagher, E.J. Diabetes, Obesity, and Breast Cancer. Endocrinology 2018, 159, 3801–3812. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Papadimitriou, N.; Markozannes, G.; Cividini, S.; Kakourou, A.; Gill, D.; Rizos, E.C.; Monori, G.; Ward, H.A.; Kyrgiou, M.; et al. Type 2 Diabetes and Cancer: An Umbrella Review of Observational and Mendelian Randomization Studies. Cancer Epidemiol. Biomarkers Prev. 2021, 30, 1218–1228. [Google Scholar] [CrossRef]
- Jordt, N.; Kjærgaard, K.A.; Thomsen, R.W.; Borgquist, S.; Cronin-Fenton, D. Breast Cancer and Incidence of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Breast Cancer Res. Treat. 2023, 202, 11–22. [Google Scholar] [CrossRef]
- Elena, J.W.; Steplowski, E.; Yu, K.; Hartge, P.; Tobias, G.S.; Brotzman, M.J.; Chanock, S.J.; Stolzenberg-Solomon, R.Z.; Arslan, A.A.; Amundadottir, L.; et al. Diabetes and Risk of Pancreatic Cancer: A Pooled Analysis from the Pancreatic Cancer Cohort Consortium. Cancer Causes Control 2013, 24, 13–25. [Google Scholar] [CrossRef]
- Muilwijk, M.; Ho, F.; Waddell, H.; Sillars, A.; Welsh, P.; Iliodromiti, S.; Brown, R.; Ferguson, L.; Stronks, K.; Van Valkengoed, I.; et al. Contribution of Type 2 Diabetes to All-Cause Mortality, Cardiovascular Disease Incidence and Cancer Incidence in White Europeans and South Asians: Findings from the UK Biobank Population-Based Cohort Study. BMJ Open Diabetes Res. Care 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Kuo, H.-Y.; Lin, Z.-Z.; Kuo, R.; Shau, W.-Y.; Lai, C.-L.; Yang, Y.-Y.; Shao, Y.-Y.; Hsu, C.; Cheng, W.-F.; Cheng, A.-L.; et al. The Prognostic Impact of Type 2 Diabetes Mellitus on Early Cervical Cancer in Asia. Oncologist 2015, 20, 1051–1057. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Zhu, N.; Mi, M.; Lu, Y.; Zheng, J.; Weng, S.; Yuan, Y. Emerging Patterns and Trends in Global Cancer Burden Attributable to Metabolic Factors, Based on the Global Burden of Disease Study 2019. Front. Oncol. 2023, 13, 1–12. [Google Scholar] [CrossRef]
Figure 1.
Flowchart depicting case numbers at each step of the study. The two major groups compared (prevalent T2DM cases and non-diabetic controls) are highlighted with bold style.
Figure 1.
Flowchart depicting case numbers at each step of the study. The two major groups compared (prevalent T2DM cases and non-diabetic controls) are highlighted with bold style.
Figure 2.
Change in incidence of cancer cases per 1.000 prevalent T2DM cases (a, c, e) and in nondiabetic controls (b, d, f) in the time interval of 2015-2019 in distinct age groups. (a, b) total population, (c, d) male patients, (e, f) female patients. Crude incidence data are shown.
Figure 2.
Change in incidence of cancer cases per 1.000 prevalent T2DM cases (a, c, e) and in nondiabetic controls (b, d, f) in the time interval of 2015-2019 in distinct age groups. (a, b) total population, (c, d) male patients, (e, f) female patients. Crude incidence data are shown.
Figure 3.
Age distribution of (a) total cases and (b) incidental cases of cancer in patients with type 2 diabetes mellitus and in nondiabetic controls.
Figure 3.
Age distribution of (a) total cases and (b) incidental cases of cancer in patients with type 2 diabetes mellitus and in nondiabetic controls.
Figure 4.
Data related to incidental cancer development in different age groups, irrespective of location and histological type. (a) odds ratio of cases with T2DM vs. controls is shown for the total population, both males and females, respectively. (b) average annual percent changes in total cancer incidence in cases with T2DM and controls. Also here, data of the total population, as well as male and female cases are shown.
Figure 4.
Data related to incidental cancer development in different age groups, irrespective of location and histological type. (a) odds ratio of cases with T2DM vs. controls is shown for the total population, both males and females, respectively. (b) average annual percent changes in total cancer incidence in cases with T2DM and controls. Also here, data of the total population, as well as male and female cases are shown.
Figure 5.
Cumulative frequency (crude, non-adjusted data) of different cancer types in non-diabetic controls and in cases with Type 2 diabetes. (a) total data, (b) cancer site distribution in males, (c) cancer site distribution in female patients.
Figure 5.
Cumulative frequency (crude, non-adjusted data) of different cancer types in non-diabetic controls and in cases with Type 2 diabetes. (a) total data, (b) cancer site distribution in males, (c) cancer site distribution in female patients.
Figure 6.
Odds ratio of development of cancer in T2DM cases compared to controls. Detailed data for individual cancer sites are shown. The panel shows data for the total population, for a male-to-female comparison as well as comparison of 18-59 vs. 60+ age groups.
Figure 6.
Odds ratio of development of cancer in T2DM cases compared to controls. Detailed data for individual cancer sites are shown. The panel shows data for the total population, for a male-to-female comparison as well as comparison of 18-59 vs. 60+ age groups.
Figure 7.
Average annual percent changes in incidence for individual cancer types in cases with T2DM and in controls. Detailed data for individual cancer sites. Data for the total population, for a male-to-female comparison as well as comparison of 18-59 vs. 60+ age groups are shown.
Figure 7.
Average annual percent changes in incidence for individual cancer types in cases with T2DM and in controls. Detailed data for individual cancer sites. Data for the total population, for a male-to-female comparison as well as comparison of 18-59 vs. 60+ age groups are shown.
Figure 8.
Age distribution of site-specific incident cancer types in non-diabetic controls and in patients with type 2 diabetes mellitus. Cancer sites are ordered according to the relative frequency of the 18-39 year-old group in non-diabetic controls.
Figure 8.
Age distribution of site-specific incident cancer types in non-diabetic controls and in patients with type 2 diabetes mellitus. Cancer sites are ordered according to the relative frequency of the 18-39 year-old group in non-diabetic controls.
Table 1.
Site-specific cancer risk in the literature and in our study.
Table 1.
Site-specific cancer risk in the literature and in our study.
| Cancer type |
Data from the literature |
Our data |
| Lung cancer |
OR: 1.16 (1.03-1.31)28
|
HR: 1.21 (1.07-1.38)24
|
|
|
OR: 2.26 (2.14-2.40) |
| Colorectal cancer |
meta HR: 1.21 (1.06-1.38)29
|
RR: 2.05 (1.79-2.34)29
|
|
|
OR: 2.94 (2.81-3.08) |
| Breast cancer |
meta RR: 1.20 (1.12–1.28) |
meta RR: 1.72 (1.47–2.00) |
RR: 1.25 (1.20–1.29)]30
|
OR: 0.55 (0.45-0.66)]28
|
OR: 1.96 (1.88-2.04) |
| |
HR: 1.30 (1.20-1.41)24
|
|
|
|
|
| Prostate cancer |
OR: 1.14 (0.93-1.40)28
|
RR: 0.90 (0.80-1.02) |
|
|
OR: 2.89 (2.74-3.04) |
| Bladder cancer |
HR: 1.17 (1.05-1.30)31
|
HR: 1.04 (0.85-1.28)24
|
HR: 0.84 (0.63-1.13)]32
|
|
OR: 2.77 (2.55-3.01) |
| Melanoma |
RR: 0.93 (0.64-1.36)25
|
|
|
|
OR: 1.97 (1.82-2.15) |
| Oropharyngeal cancer |
OR: 0.89 (0.76-1.04)]28
|
|
|
|
OR: 1.37 (1.23-1.54) |
| Kidney cancer |
OR: 1.7 (1.3-2.1)]33
|
HR: 1.36 (1.05-1.76)24
|
|
|
OR: 3.40 (3.11-3.73) |
| Pancreas cancer |
rev. RR: 0.83-6.90*34
|
rev. RR: 1.73-6.08**34
|
HR: 2.13 (1.76-2.58)24
|
OR: 1.40 (1.07-1.84)]35
|
OR: 4.35 (4.06-4.67) |
| Stomach cancer |
OR: 1.19 (0.97-1.46)28
|
|
|
|
OR: 3.13 (2.83-3.48) |
| Endometrial cancer |
meta: RR: 2.74 (1.87-4.00)30
|
HR: 1.85 (1.36-2.50)30
|
HR: 1.79 (1.51-2.13)24
|
rev. RR: 2.10 (1.75-2.53)36
|
OR: 3.60 (3.20-4.00) |
| |
rev. RR: 1.81 (1.38-2.37)36
|
rev. HR: 1.81 (1.37-2.41)36
|
IRR: 1.84 (1.33-2.56)27
|
SIR: 1.75 (1.67-1.83)37
|
|
| |
OR: 1.38 (1.07-1.80)]28
|
|
|
|
|
| Cervix cancer |
SIR: 1.18 (1.06-1.32)37
|
SIR: 1.18 (1.06-1.32)37
|
OR: 1.24 (1.19-1.29)38a
|
OR: 1.00 (0.95-1.05)38b
|
OR: 0.87 (0.67-1.14) |
| Ovarian cancer |
meta: OR: 1.17 (1.02–1.33)]30
|
HR: 0.84 (0.61-1.15)24
|
RR: 1.05 (0.75-1.46)36
|
OR: 0.83 (0.61-1.13)28
|
OR: 1.97 (1.73-2.25) |
| Liver cancer |
OR: 1.81 (1.66-1.97) |
HR: 3.73 (2.50-5.56) |
OR: 2.19 (1.76-2.72) |
|
OR: 5.65 (5.08-6.29) |
| Thyroid cancer |
OR: 0.70 (0.46-1.06)39
|
OR: 0.40 (0.20-0.81)39
|
OR: 0.36 (0.23-0.58)28
|
HR: 1.63 (1.14-2.34)24
|
OR: 1.68 (1.44-2.01) |
| Non-Hodgkin lymphoma |
RR: 1.21 (0.99-1.48)25
|
|
|
|
OR: 2.27 (2.02-2.57) |
| Myeloma |
RR: 1.27 (0.98-1.66)25
|
HR: 1.14 (0.80-1.65)24
|
|
|
OR: 2.62 (2.24-3.10) |
| Leukemia |
RR: 0.88 (0.71-1.10)25
|
HR: 1.07 (0.77-1.49)24
|
|
|
OR: 2.57 (2.36-2.81) |
| Esophagus cancer |
met: RR: 1.28 (1.05-1.57)40
|
RR: 1.28 (1.10-1.49)c40
|
HR: 1.96 (1.36-2.82)24
|
OR: 0.50 (0.35-0.71)28
|
OR: 1.48 (1.25-1.78) |
| Gallbladder cancer |
HR: 1.47 (0.91-2.39)24
|
RR: 1.56 (1.36-1.79)41
|
|
|
OR: 3.66 (3.17-4.29) |
| Testis cancer |
SIR: 0.94 (0.75-1.17)37
|
|
|
|
OR: 0.49 (0.36-0.67) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).