1. Introduction
Ovarian cancer is a significant health concern, ranking as the eight most common cancer in woman. Ovarian cancer, more appropriately epithelial ovarian carcinoma (EOC) accounts for approximately 2% of all malignancies affecting women, thus might seem to be a very rare disease, nevertheless its prognosis remains poor. This translates to the fact that EOC is the fifth leading cause of cancer-related deaths among women. Ovarian cancer is a silent cancer which prognosis and survival mainly relies in early stage detection. However, the majority of cases are diagnosed as late-stage disease (III-IV stage according to the current classification), mainly due to the lack of clinical symptoms and lack of a specific early diagnostic laboratory marker. From the histological point of view, EOC is classified into five histological subtypes: most frequent is high-grade serous ovarian carcinoma (HGSOC). Other subtypes include low-grade serous ovarian carcinoma, endometrioid ovarian carcinoma, mucinous ovarian carcinoma and finally ovarian clear cell carcinoma. Each of these subtypes differ not only from the point of view of cellular origin, but also have different molecular profiles. Despite advances in the complex management of patients suffering from cancer generally, the prognosis remains poor. For advanced disease, the platinum-based chemotherapy is still the first-choice treatment. Administration of chemotherapy leads to the remission in majority of patients, but recurrence rates are high. Poor outcomes in HGSOC are mainly due to the early dissemination to the peritoneal cavity. Micro and macrometastases into omentum result in the formation of malignant ascites (1). At this stage, the disease resists all available approved therapies.
Immunotherapy has revolutionized the treatment of many solid tumors, yet its efficacy in ovarian cancer has been limited (2-4)}. Antiangiogenic therapies, such as bevacizumab, have shown restricted effectiveness (5). Similarly, immune checkpoint inhibitors (ICIs) targeting PD-1, PD-L1, and CTLA-4, which have been approved for various cancers, have not demonstrated significant survival improvement in ovarian cancer (2). A comprehensive review of 20 clinical trials, including phase I, II, and III studies, revealed no reported improvement in survival with ICIs, and some trials were terminated early due to toxicity or lack of response. Combining ICIs with chemotherapy, anti-VEGF therapy, or PARP inhibitors did modestly improve response rates and survival, albeit with a worse safety profile (6). The identification of predictive biomarkers for ICI efficacy and genomic and immune profiling of ovarian cancer are crucial for developing better treatment options and designing tailored trials (3).
In this review, we describe the contribution of our scientific and clinical team to the field of ovarian cancer. During past 20 years, we tried to understand immune contexture of ovarian cancer and we focused on developing an immunotherapy based on autologous dendritic cell vaccine. DC-based vaccine called DCVAC/OvCa was then tested in several clinical trials. The partial achievements both in basic research as well as in clinical trials were published and discussed in deep in corresponding papers. Here we bring a comprehensive review of the twenty-years scientific march from bench to bedside referring to individual published articles.
2. Dendritic Cell Vaccines in Ovarian Cancer
Dendritic cell (DC)-based immunotherapy has been a long-studied approach for treating ovarian cancer (7). DC vaccines are generated using autologous DCs derived from peripheral blood monocytes yielded from the patients by leukapheresis. Immature DCs are exposed to tumor-associated antigens from different sources (autologous tumor cell lysates, allogenic cells derived from tumor cell lines killed by various methods, tumor-derived mRNA, tumor-derived or synthetic peptides etc.). Exposition of iDC to tumor antigens is called pulsation. During this process, several immunostimulatory molecules should be added to induce maturation of pulsed immature DC. The final product containing mDC pulsed with tumor antigens are reinfused into patients, most often via subcutaneous administration (8-11). DC-based vaccines can induce tumor-specific CD8+/CD4+ T cell responses in vivo (12). Unfortunately, immune response to tumor antigens do not usually reflect the clinical efficacy which is often suboptimal as monotherapy. Nevertheless, immunogenicity and efficacy of DC vaccines can be augmented by a combinatorial chemo-immunotherapy regimens (13,14), (15). Further combinations were tested in several clinical trials which included concomitant therapy of DC with ICI, radiation, hormonal therapy, kinase inhibitors, antiangiogenic therapies and others (16,17).
We conducted a feasibility study on the ex vivo generation of DCs, using autologous tumor cells for pulsation already in 2006. The study demonstrated the technical feasibility of preparing individual DC-based vaccines. In vitro generated DC were able to induce T lymphocyte responses. From the methodological point of view, we generated monocyte-derived DCs cultivated with GM-CSF and IL-4 and pulsed with autologous tumor-derived apoptotic bodies (18). Tumor cells were acquired from the ascites of patients with ovarian carcinoma during surgery. The tumor cells were killed by UV irradiation. Immature DC were matured by the addition of poly-IC and finally cocultured with autologous lymphocytes to test the ability of T cell to proliferate and produce IFN-gamma detected by ELISPOT. Results showed that maturation of DCs and induction of T cell response were achieved in 75% of patients tested. Thus, we proved a limited feasibility of this approach (18-20).
Obtaining autologous cells from a patient’s tumor during surgery can be logistically challenging. To address this issue, we explored the use of allogenic tumor cell lines for DC pulsation. An analysis of patient samples was performed to compare the expression of tumor antigens with available cell lines. In order to select a suitable combination of cancer cell lines as an appropriate source of antigens for dendritic cell-based immunotherapy of ovarian cancer, we analyzed the expression level of 21 tumor associated antigens (BIRC5, CA125, CEA, DDX43, EPCAM, FOLR1, Her-2/neu, MAGE-A1, MAGE-A2, MAGE-A3, MAGE-A4, MAGE-A6, MAGE-A10, MAGE-A12, MUC-1, NY-ESO-1, PRAME, p53, TPBG, TRT, WT1) in 4 established ovarian cancer cell lines and in primary tumor cells isolated from the high-grade serous epithelial ovarian cancer tissue. More than 90% of tumor samples expressed very high levels of CA125, FOLR1, EPCAM and MUC-1 and elevated levels of Her-2/neu, similarly to OVCAR-3 cell line. The combination of OV-90, SK-OV3 and OVCAR-3 cell lines showed the highest overlap with patients’ samples in the TAA expression profile. Finally, we selected the OV-90 and SK-OV cell lines as the most suitable for pulsation of DCs not only due to their high overlap with patients’ samples in the tumor-associated antigen (TAA) expression profile, but also due to the technical feasibility to obtain a license for their use (21).
We introduced a new physical modality, high hydrostatic pressure (HHP), as a method for inducing immunogenic cell death (ICD) in tumor cell lines (14,20)}. HHP induced rapid expression of immunogenic markers like HSP70, HSP90 and calreticulin on the cell surface, as well as the release of “danger signaling” molecules HMGB1 and ATP. Interaction of DCs with HHP-treated tumor cells led to enhanced DC phagocytosis, upregulation of maturation and activation surface markers CD83, CD86, and HLA-DR, and the release of proinflammatory cytokines IL-6, IL-12p70, and TNF-α. DCs pulsed with HHP-treated tumor cells induced high numbers of tumor-specific CD8+ and CD4+ T cells, but on the other hand, the lowest number of regulatory T cells (FoxP3+), establishing HHP as a reliable and potent inducer of immunogenic cell death in human tumor cells (14,22).
Additionally, we developed a fast DC protocol by comparing standard DCs (Day 5 DCs) and fast DCs (Day 3 DCs) generated in CellGro media and subsequently activated by Poly (I:C) or LPS. We found that Day 3 DCs activated using Poly (I:C) were similarly potent in most functional aspects as DCs produced by the standard 5-day protocol. This fact provides rationale for faster protocols for DC generation in clinical trials (Day 3 Poly (I:C)-activated dendritic cells generated in CellGro for use in cancer immunotherapy trials are fully comparable to standard Day 5 DCs) (19).
In order to improve the performance of DC-based vaccines, we are currently working on further modifications of the manufacturing process. These modifications aim to skew the differentiation of DC progenitors and maturation of DCs into the phenotype that could effectively induce Th1 response and effectively translate into much stronger proliferation of tumor-reactive lymphocytes, namely the CD8+ T cells. One of the modifications is the implementation of LL-37 into the manufacturing process. LL-37 is an antimicrobial that can either suppress or stimulate immune responses based on the actual conditions. Due to this immunomodulatory duality, we have tested multiple algorithms of its implementation into different phases of the production of monocyte-derived DCs. We found that monocyte-derived DCs differentiated in the presence of LL-37 minimally improved the ability of DCs to induce the proliferation of CD8+ T cells. However, the implementation of LL-37 also during the phases of the DC antigen pulsation (loading) and maturation markedly enhanced the ability of the produced DCs to expand CD8+ T cells, downregulate their expression of PD-1, and significantly enhance the frequency of tumor cell-reactive CD8+ T cells. These attributes also translated into superior in vitro cytotoxicity of the expanded cells against tumor cells. These data surprisingly demonstrated that whereas a partial implementation of LL-37 into the cell production process has no desired impact on the anti-tumor performance of the produced DCs, LL-37 implementation into the whole process of their ex vivo production could elicit the desired impact, leading to significantly enhanced anti-tumor performance of the produced DCs (23). However, introducing such a change into the GMP manufacturing process requires months of administrative work and “new” DC product should be tested from scratch in new clinical trials. Compliance with all legislative requirements have significant financial impact and substantially slower the clinical use.
3. Tumor Microenvironment in Ovarian Cancer and Its Impact to the Disease Outcome
In parallel with preparation of manufacturing processes for the generation of dendritic cell vaccine, we studied immunological markers in the blood and within primary tumors as well as within metastases with the aim to understand better the immunological contexture of the tumor and its impact on disease outcome (24-26). The immunological configuration of ovarian carcinoma was analyzed, highlighting the poor infiltration by immune cells and active immunosuppression within the tumor microenvironment (27). Comparative analysis of the humoral and cellular features of primary and metastatic epithelial ovarian carcinoma (EOC) was performed, proposing measures to alter them in support of treatment sensitivity and superior patient survival (26).
We also described the dynamics of T-cell infiltration during the course of ovarian cancer. We studied immune cells that infiltrated the tumor tissues and circulated in the peripheral blood of ovarian cancer patients at different stages of disease. Patients with the early stages of development of ovarian cancer (stage I-II) were characterized by a strong Th17 immune response. In stage II patients, we observed recruitment of high numbers of Th1 cells. In disseminated tumors (stages III-IV), we found a dominant population of activated regulatory T cells (Tregs) expressing the molecule Helios and also high numbers of myeloid dendritic cells (mDCs) as well as monocytes/macrophages. Tumor-infiltrating Tregs had markedly lower expression of chemokine receptor CCR4 than circulating Tregs. The number of tumor-infiltrating Tregs significantly correlated with the amount of chemokine CCL22 in ovarian tumor cell culture supernatants, suggesting their recruitment via a CCR4/CCL22 interaction. We demonstrated that the chemokine CCL22 was mainly produced by tumor cells, monocytes/macrophages and mDCs in the primary ovarian tumors, and its expression markedly increased in response to IFNγ. On the basis of these experiments, we suppose that recruitment of Tregs was triggered by inflammatory stimuli in advanced stages of the disease which finally led to a significant immune suppression in the late stages of ovarian cancer. Gradual shift from a Th17/Th1 effector cell response to a predominant infiltration by regulatory T-cells in advanced stages reflects the decline of effective antitumor immune response to a significant immune suppression enabling the loss of the immune surveillance against tumor and consequently a disease progression (28).
Furthermore, we investigated the expression of classical (PD-1/PD-L1) and more recently described check-point coinhibitory molecules (TIM-3) in relation to the functional orientation of the immune infiltrate in ovarian cancer (29). High levels of PD-L1 and high densities of PD-1+ cells in the microenvironment of high-grade serous ovarian cancer were associated with an immune contexture characterized by robust Th1 polarization and cytotoxic orientation, which enabled superior clinical benefits. However, PD-1+TIM-3+CD8+ T cells presented features of functional exhaustion and correlated with poor disease outcome. The amount of TIM-3+ cells contributes to the patient stratification based on the intratumoral abundance of CD8+ T cells (29).
The potential impact of mature dendritic cells (DCs) in shaping the immune contexture of high-grade serous ovarian carcinoma, their role in the establishment of T cell-dependent antitumor immunity, and their potential prognostic value for HGSC patients were also investigated. A high density of tumor-infiltrating DC-LAMP+ DCs was robustly associated with an immune contexture characterized by Th1 polarization and cytotoxic activity. Both mature DCs and CD20+ B cells played a critical role in generating a clinically favourable cytotoxic immune response in the HGSC microenvironment. Robust tumor infiltration by both DC-LAMP+ DCs and CD20+ B cells was associated with the most favourable overall survival in two independent cohorts of chemotherapy-naïve HGSC patients (30).
4. Clinical Trials with DC-Based Vaccine in Patients with Ovarian Cancer
After fulfilling all legislative requirements, including GMP premises and regulatory approval, we conducted the first-in-human investigator-initiated clinical trial phase I of DCVAC/OvCa (EudraCT: 2010–021462-30) in patients with ovarian cancer. This small study included 10 patients with recurrent platinum sensitive ovarian cancer stage III-IV. Primary end-points were safety and immune response. DC-vaccination was safe. Administration of DCVAC/OvCa lead to the increased numbers of NY-ESO-1-, MAGE-A1-, and MAGE-A3-specific T cells in the peripheral blood.
In 2010, the biotech company Sotio was founded and took over the further development of dendritic cell immunotherapy. Sotio sponsored two phase II randomized clinical trials in patients with ovarian cancer. The first study was an open-label, parallel-group, phase 2 trial (NCR02107950) study which included patients with platinum-sensitive ovarian cancer relapsing after first-line chemotherapy. DCVAC/OvCa was administered every 3–6 weeks up to 10 doses to patients randomized to in the arm A who received DCVAC/OvCa and chemotherapy. Into arm B were randomized patients treated with chemotherapy alone. The endpoints of this clinical trial included safety, progression-free survival and overall survival (PFS being the primary efficacy endpoint and OS was the secondary efficacy endpoint).
A total of 71 patients were randomized to chemotherapy in combination with DCVAC/OvCa or to chemotherapy alone. Adverse events were mainly related to the chemotherapy. Progression free survival was not improved significantly (hazard ratio 0.73, P = 0.274), while median OS was significantly prolonged (by 13.4 months) in the DCVAC/OvCa group (HR 0.38, 95% CI 0.20–0.74, P = 0.003; data maturity 56.3%). A tendency to enhanced antigen-specific T-cell activity was seen in patients assigned to the arm chemotherapy+ DCVAC/OvCa (31).
In parallel, another phase II study (NCT02107937) was run to assess the safety and efficacy of dendritic cell-based immunotherapy in patients with recently diagnosed ovarian cancer. DCVAC/OvCa was added to first-line chemotherapy (carboplatin plus paclitaxel) after debulking surgery. Ninety-nine patients with stage III EOC (serous, endometrioid or mucinous) who underwent cytoreductive surgery up to 3 weeks prior to randomization and were scheduled for first-line platinum-based CT, were eligible. Patients were stratified by tumor residuum (0 or <1 cm) and were randomized (1:1:1) to DCVAC/OvCa parallel to chemotherapy (Group A), DCVAC/OvCa sequential to CT (Group B), or chemotherapy alone (Group C). Primary endpoints were safety and progression free survival (PFS), secondary endpoint was overall survival (OS). The modified intent to treat population included 31, 29, and 30 patients in Groups A, B, and C, respectively. There were no differences in the baseline characteristics between the treatment arms. Median PFS was 20.3, not reached, and 21.4 months in Groups A, B, and C, respectively. The hazard ratio for Group A versus Group C was 0.98 (0.48 to 2.00; p=0.9483) and the hazard ratio for Group B versus Group C was 0.39 (0.16 to 0.96; p=0.0336). Median OS was not reached in any group after a median follow-up of 66 months (34% of events), but a non-significant trend of improved OS in Groups A and B was noted. DCVAC/OvCa application and the process of leukapheresis itself was not associated with significant safety concerns. Overall, DCVAC/OvCa administration showed a good safety profile. Thus, this study found that DCVAC/OvCa administration sequential to platinum-based first line chemotherapy led to a statistically significant improvement in progression-free survival in patients with epithelial ovarian cancer (32).
Deeper analysis of data from this trial revealed that patients with so-called cold tumors, which typically have a poor prognosis, most benefited from the DC immunotherapy. We analyzed pretreatment tumor samples taken from primary surgery and pretreatment and posttreatment peripheral blood samples from 82 patients enrolled in this trial. The aim was to identify biomarkers that would predict clinical outcome of patients with epithelial ovarian cancer treated with DCVAC/OvCa. Samples were analysed with the use of several methods, including immunohistochemistry, flow cytometry, sequencing and multispectral immunofluorescence microscopy. We found that patients with low mutational burden and cold tumors (low numbers of CD8+ T cells infiltrating the tumor) benefited from DCVAC/OvCa administration both in terms of overall survival as well as in the induction of antitumor immunity. Patients with hot tumors (characterized by the high numbers of CD8+ T cells infiltrating the tumor) had quite a good prognosis with the application of chemotherapy only. Adding immunotherapy by DCVAC/OvCa did not further improve the outcome of the disease in this subgroup of patients. DCVAC/OvCa administration to patients with cold tumors improved the initially poor prognosis to the level of better prognosis of patients with pre-treatment hot tumors. Thus, DC-based vaccination seems to initiate clinically relevant anticancer immune responses in patients with cold tumors. Based on this data, numbers of CD8+ T cells infiltrating tumor together with the level of mutation burden might serve as a good biomarker for the selection of patients who will benefit from the immunotherapy by DC-based vaccines (33).
Administration of DC vaccines developed by our team showed efficacy also in patients with non-small cell lung cancer in phase II clinical trials (34). In addition, a subgroup of patients with metastatic castrate-resistant prostate cancer in phase III clinical trial benefited from this treatment (35). The list of clinical trials with DCVAC includes the
Table 1.
5. Discussion and Conclusions
Despite significant progress in the complex management of oncological diseases including immunotherapy of many tumors, quite little progress has been achieved in ovarian cancer management and prognosis. The majority of women with EOC reach complete remission after primary or interval cytoreductive surgery combined with chemotherapy based on a platinum-taxane doublet, but almost all experience relapse of the disease (36). Understanding of genetic and molecular background of the disease led only to partial improvement of the prognosis of patients. This is the case of homologous recombination (HR) defects imposed by germline or somatic BRCA1 DNA repair-associated (BRCA1) or BRCA2 mutations which are key determinants of platinum sensitivity in EOC patients and provide a strong rationale for maintenance therapy based on poly(ADP-ribose) polymerase (PARP) inhibitors, which is generally associated with improved progression-free survival (PFS). The hope that improvement of PFS with PARP inhibitors will translate into overall survival benefit finally concerns only population of HR deficient patients which represent a minority of ovarian cancer patients. Even HR deficient patients finally develop recurrence of the disease and resistance to PARP inhibitors. Development of novel therapies for patients with ovarian cancer is thus very urgent.
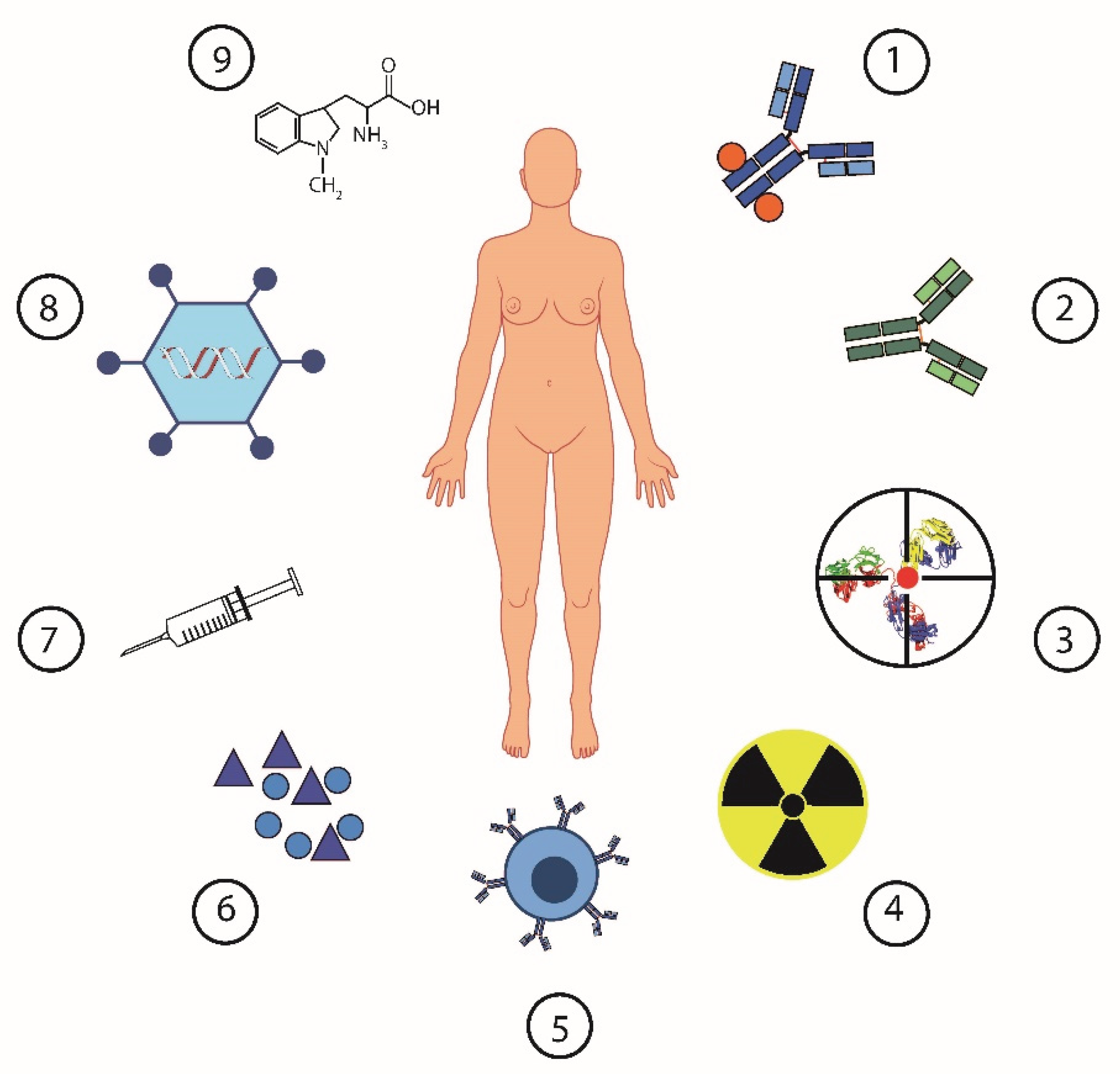
Approval of immune checkpoint inhibitors (ICIs) for the treatment of various tumor types such as melanoma, non-small cell lung cancer or renal cancer has created hope also for patients with EOC. Unfortunately, efficiency expectations have not been met in the case of ovarian cancer. EOC is little sensitive to ICIs administered both as monotherapy or in combination with chemotherapy or PARP inhibitors (37-39) The reason for ineffectiveness of ICI in EOC is related to absent or low anticancer immunity in majority of patients and highly immunosuppressive tumor microenvironment at baseline. Based on our data and other published scientific articles, multiple mechanisms are involved in the formation of an immunosuppressive environment in EOC. We and other described increased levels of proinflammatory or immunosuppressive cytokines, including interleukines IL-6, IL-10, macrophage migration inhibitory factor (MIF), vascular endothelial growth factor A or immunosuppressive metabolites like indoleamine 2,3-dioxygenase 1 (IDO1), lactate and arginase 1. With the progression of the disease, these factors contribute to the accumulation of immunosuppressive cell including regulatory T cells, tolerogenic dendritic cells and various types of myeloid suppressor cells (MDSCs) and tumor-associated macrophages. Targeting these factors and cells by various pharmacologic and therapeutic approaches represents attempts to break the immunosuppression in EOC in order to improve the prognosis of patients suffering from this disease (13,40). The possible approaches in the current treatment of ovarian cancer show the
Figure 1.
Our research contributed to a better understanding of the role of different cells of the immune system in the tumor microenvironment. We found that pre-existing immunity in the ovarian TME has a major impact on the sensitivity of EOC to immunotherapy by dendritic cell vaccine. Identification of further immune biomarkers which might be integrated into common diagnostic assessments will guide appropriate treatment selection in the future.
We developed and tested DC-based immunotherapy in patients with EOC. Our data showed that the administration is safe and translate into clinical benefit both in patients with recurrent ovarian cancer in the combination with second-line chemotherapy, as well as in patients with early disease after cytoreductive surgery in the combination with the first-line platinum-based chemotherapy. Patients with cold tumors characterized by low mutation burden and low T cell infiltrate most clinically benefited from the therapy. Based on these observations and other research data, combination of treatment modalities respecting the individual baseline immune characteristics of the tumor microenvironment seems to be the best therapeutic strategy applied in order to reverse the natural course of the disease.
Based on the promising data from phase II clinical studies, we planned to initiate phase III study in patients with recurrent platinum sensitive ovarian cancer. However, the registration study for the DC-based vaccine did not commence. Initially delayed by the COVID-19 pandemic, subsequent changes in the ownership structure of Sotio led to the decision not to continue the vaccine’s development due to the lengthy and risky nature of a phase III trial. The current regulatory environment suggests that a phase III trial would take approximately eight years to yield statistically relevant results for product registration. Consequently, there is no business case for pharmaceutical companies to co-develop such a product, and the investment would be too high and risky for a single sponsor. In light of the recent failure of PARP inhibitors in HRP ovarian cancer (41) and the inefficacy of approved checkpoint inhibitors in this disease, there is currently no immunotherapy available in the near future for ovarian cancer patients. It is hoped that the right combination, timing, and/or sequence of current therapies, along with a personalized approach based on relevant biomarkers, will improve the prognosis for patients with this deadly disease.
Acknowledgments
Supported partially by grant NU22-03-00300. All clinical studies were realized in compliance with GCP.
Conflicts of Interest
Author is minority shareholder of the biotech company Sotio, a.s.
References
- Lengyel, E. Ovarian cancer development and metastasis. Am J Pathol 2010,177,1053-64. [CrossRef]
- Kandalaft LE, Odunsi K, Coukos G. Immunotherapy in Ovarian Cancer: Are We There Yet? J Clin Oncol 2019,37,2460-71. [CrossRef]
- Maiorano BA, Maiorano MFP, Lorusso D, Maiello E. Ovarian Cancer in the Era of Immune Checkpoint Inhibitors: State of the Art and Future Perspectives. Cancers (Basel) 2021,13. [CrossRef]
- Coukos G, Tanyi J, Kandalaft LE. Opportunities in immunotherapy of ovarian cancer. Ann Oncol 2016,27 Suppl 1:i11-i5. [CrossRef]
- Haunschild CE, Tewari KS. Bevacizumab use in the frontline, maintenance and recurrent settings for ovarian cancer. Future Oncol 2020,16,225-46. [CrossRef]
- Stewart RA, Pilie PG, Yap TA. Development of PARP and Immune-Checkpoint Inhibitor Combinations. Cancer Res 2018,78,6717-25. [CrossRef]
- Zhang X, He T, Li Y, Chen L, Liu H, Wu Y, et al. Dendritic Cell Vaccines in Ovarian Cancer. Front Immunol 2020,11:613773. [CrossRef]
- Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 2020,20,7-24. [CrossRef]
- Sahin U, Tureci O. Personalized vaccines for cancer immunotherapy. Science 2018,359,1355-60. [CrossRef]
- Chow S, Berek JS, Dorigo O. Development of Therapeutic Vaccines for Ovarian Cancer. Vaccines (Basel) 2020,8(4). [CrossRef]
- Zhong R, Ling X, Cao S, Xu J, Zhang B, Zhang X, et al. Safety and efficacy of dendritic cell-based immunotherapy (DCVAC/LuCa) combined with carboplatin/pemetrexed for patients with advanced non-squamous non-small-cell lung cancer without oncogenic drivers. ESMO Open 2022,7,100334. [CrossRef]
- Tanyi JL, Bobisse S, Ophir E, Tuyaerts S, Roberti A, Genolet R, et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med 2018,10(436). [CrossRef]
- Truxova I, Hensler M, Skapa P, Halaska MJ, Laco J, Ryska A, et al. Rationale for the Combination of Dendritic Cell-Based Vaccination Approaches With Chemotherapy Agents. Int Rev Cell Mol Biol 2017,330:115-56. [CrossRef]
- Fucikova J, Moserova I, Truxova I, Hermanova I, Vancurova I, Partlova S, et al. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int J Cancer 2014,135,1165-77. [CrossRef]
- Vacchelli E, Vitale I, Eggermont A, Fridman WH, Fucikova J, Cremer I, et al. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology 2013,2,e25771. [CrossRef]
- Zsiros E, Duttagupta P, Dangaj D, Li H, Frank R, Garrabrant T, et al. The Ovarian Cancer Chemokine Landscape Is Conducive to Homing of Vaccine-Primed and CD3/CD28-Costimulated T Cells Prepared for Adoptive Therapy. Clin Cancer Res 2015,21,2840-50. [CrossRef]
- Xiao Z, Wang R, Wang X, Yang H, Dong J, He X, et al. Impaired function of dendritic cells within the tumor microenvironment. Front Immunol 2023,14:1213629. [CrossRef]
- Fucikova J, Rozkova D, Ulcova H, Budinsky V, Sochorova K, Pokorna K, et al. Poly I: C-activated dendritic cells that were generated in CellGro for use in cancer immunotherapy trials. J Transl Med 2011,9:223. [CrossRef]
- Truxova I, Pokorna K, Kloudova K, Partlova S, Spisek R, Fucikova J. Day 3 Poly (I:C)-activated dendritic cells generated in CellGro for use in cancer immunotherapy trials are fully comparable to standard Day 5 DCs. Immunol Lett 2014,160,39-49. [CrossRef]
- Adkins I, Fucikova J, Garg AD, Agostinis P, Spisek R. Physical modalities inducing immunogenic tumor cell death for cancer immunotherapy. Oncoimmunology 2014,3,e968434. 10.4161/21624011.2014.968434.
- Kloudova K, Hromadkova H, Partlova S, Brtnicky T, Rob L, Bartunkova J, et al. Expression of tumor antigens on primary ovarian cancer cells compared to established ovarian cancer cell lines. Oncotarget 2016,7,46120-6. [CrossRef]
- Mikyskova R, Indrova M, Stepanek I, Kanchev I, Bieblova J, Vosahlikova S, et al. Dendritic cells pulsed with tumor cells killed by high hydrostatic pressure inhibit prostate tumor growth in TRAMP mice. Oncoimmunology 2017,6,e1362528. [CrossRef]
- Stakheev D, Taborska P, Kalkusova K, Bartunkova J, Smrz D. LL-37 as a Powerful Molecular Tool for Boosting the Performance of Ex Vivo-Produced Human Dendritic Cells for Cancer Immunotherapy. Pharmaceutics 2022,14(12). [CrossRef]
- Hensler M, Kasikova L, Fiser K, Rakova J, Skapa P, Laco J, et al. M2-like macrophages dictate clinically relevant immunosuppression in metastatic ovarian cancer. J Immunother Cancer 2020,8(2). [CrossRef]
- Kasikova L, Hensler M, Truxova I, Skapa P, Laco J, Belicova L, et al. Calreticulin exposure correlates with robust adaptive antitumor immunity and favorable prognosis in ovarian carcinoma patients. J Immunother Cancer 2019,7,312. [CrossRef]
- Fucikova J, Coosemans A, Orsulic S, Cibula D, Vergote I, Galluzzi L, et al. Immunological configuration of ovarian carcinoma: features and impact on disease outcome. J Immunother Cancer 2021,9(10). [CrossRef]
- Demuytere J, Ernst S, van Ovost J, Cosyns S, Ceelen W. The tumor immune microenvironment in peritoneal carcinomatosis. Int Rev Cell Mol Biol 2022,371:63-95. [CrossRef]
- Fialova A, Partlova S, Sojka L, Hromadkova H, Brtnicky T, Fucikova J, et al. Dynamics of T-cell infiltration during the course of ovarian cancer: the gradual shift from a Th17 effector cell response to a predominant infiltration by regulatory T-cells. Int J Cancer 2013,132,1070-9. [CrossRef]
- Fucikova J, Rakova J, Hensler M, Kasikova L, Belicova L, Hladikova K, et al. TIM-3 Dictates Functional Orientation of the Immune Infiltrate in Ovarian Cancer. Clin Cancer Res 2019,25,4820-31. [CrossRef]
- Truxova I, Kasikova L, Hensler M, Skapa P, Laco J, Pecen L, et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J Immunother Cancer 2018,6,139. [CrossRef]
- Cibula D, Rob L, Mallmann P, Knapp P, Klat J, Chovanec J, et al. Dendritic cell-based immunotherapy (DCVAC/OvCa) combined with second-line chemotherapy in platinum-sensitive ovarian cancer (SOV02): A randomized, open-label, phase 2 trial. Gynecol Oncol 2021. [CrossRef]
- Rob L, Cibula D, Knapp P, Mallmann P, Klat J, Minar L, et al. Safety and efficacy of dendritic cell-based immunotherapy DCVAC/OvCa added to first-line chemotherapy (carboplatin plus paclitaxel) for epithelial ovarian cancer: a phase 2, open-label, multicenter, randomized trial. J Immunother Cancer 2022,10(1). [CrossRef]
- Fucikova J, Hensler M, Kasikova L, Lanickova T, Pasulka J, Rakova J, et al. An autologous dendritic cell vaccine promotes anticancer immunity in ovarian cancer patients with low mutational burden and cold tumors. Clin Cancer Res 2022. [CrossRef]
- Zemanova M, Cernovska M, Havel L, Bartek T, Lukesova S, Jakesova J, et al. Autologous dendritic cell-based immunotherapy (DCVAC/LuCa) and carboplatin/paclitaxel in advanced non-small cell lung cancer: A randomized, open-label, phase I/II trial. Cancer Treat Res Commun 2021,28:100427. [CrossRef]
- Vogelzang NJ, Beer TM, Gerritsen W, Oudard S, Wiechno P, Kukielka-Budny B, et al. Efficacy and Safety of Autologous Dendritic Cell-Based Immunotherapy, Docetaxel, and Prednisone vs Placebo in Patients With Metastatic Castration-Resistant Prostate Cancer: The VIABLE Phase 3 Randomized Clinical Trial. JAMA Oncol 2022. [CrossRef]
- Kampan NC, Madondo MT, McNally OM, Quinn M, Plebanski M. Paclitaxel and Its Evolving Role in the Management of Ovarian Cancer. Biomed Res Int 2015,2015:413076. [CrossRef]
- Farkkila A, Gulhan DC, Casado J, Jacobson CA, Nguyen H, Kochupurakkal B, et al. Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat Commun 2020,11,1459. [CrossRef]
- Higuchi T, Flies DB, Marjon NA, Mantia-Smaldone G, Ronner L, Gimotty PA, et al. CTLA-4 Blockade Synergizes Therapeutically with PARP Inhibition in BRCA1-Deficient Ovarian Cancer. Cancer Immunol Res 2015,3,1257-68. [CrossRef]
- Shen J, Zhao W, Ju Z, Wang L, Peng Y, Labrie M, et al. PARPi Triggers the STING-Dependent Immune Response and Enhances the Therapeutic Efficacy of Immune Checkpoint Blockade Independent of BRCAness. Cancer Res 2019,79,311-9. [CrossRef]
- Truxova I, Cibula D, Spisek R, Fucikova J. Targeting tumor-associated macrophages for successful immunotherapy of ovarian carcinoma. J Immunother Cancer 2023,11(2). [CrossRef]
- Olivier T, Prasad V. PARP inhibitors and overall survival in ovarian cancer, reevaluation advised in all settings. J Cancer Res Clin Oncol 2023,149,9509-12 . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).