Submitted:
19 February 2024
Posted:
20 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Perovskite Deposition
2.4. Characterizations
3. Results and Discussion
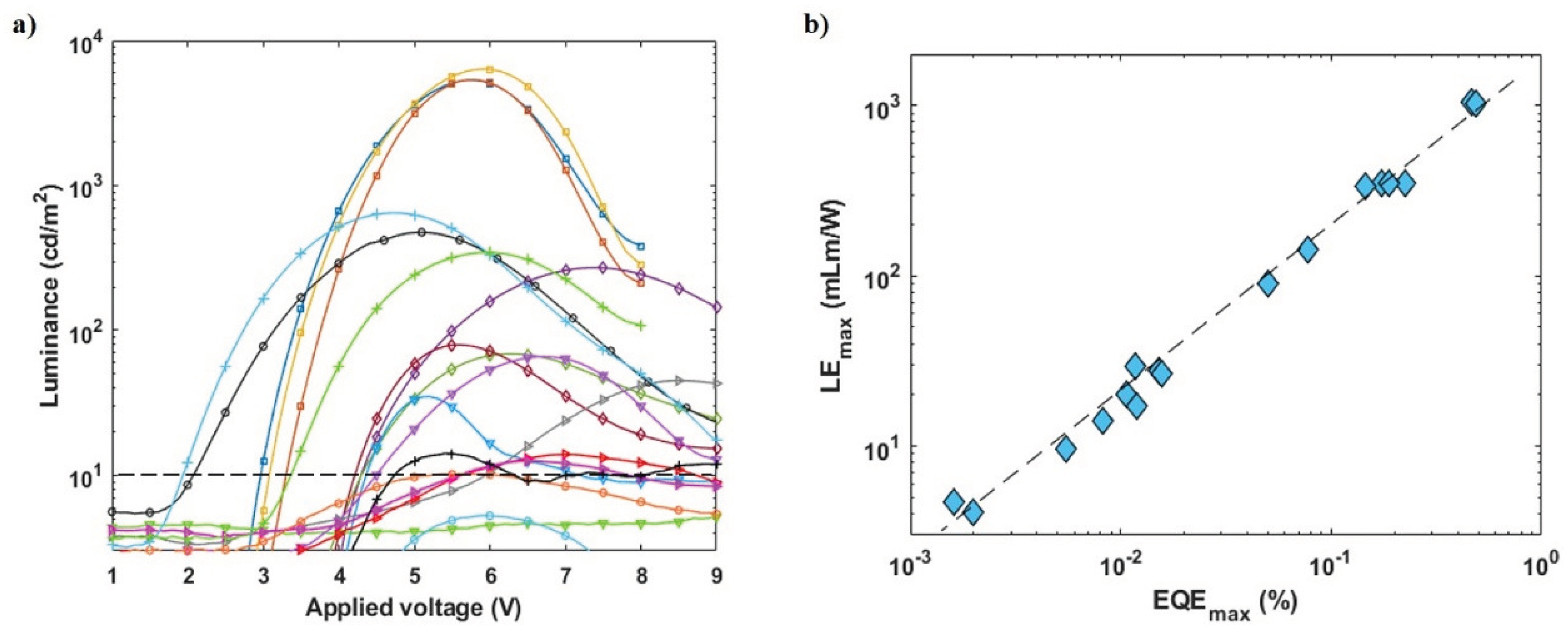
3.1. Reproducibility Challenge in PeLEDs Based on Layered Perovskites
- Fluctuation in the temperature of the deposition environment;
- The presence of a small quantity of uncontrolled O2 and H2O inside the glovebox;
- Chemical contamination from evaporated solvents in the glovebox atmosphere;
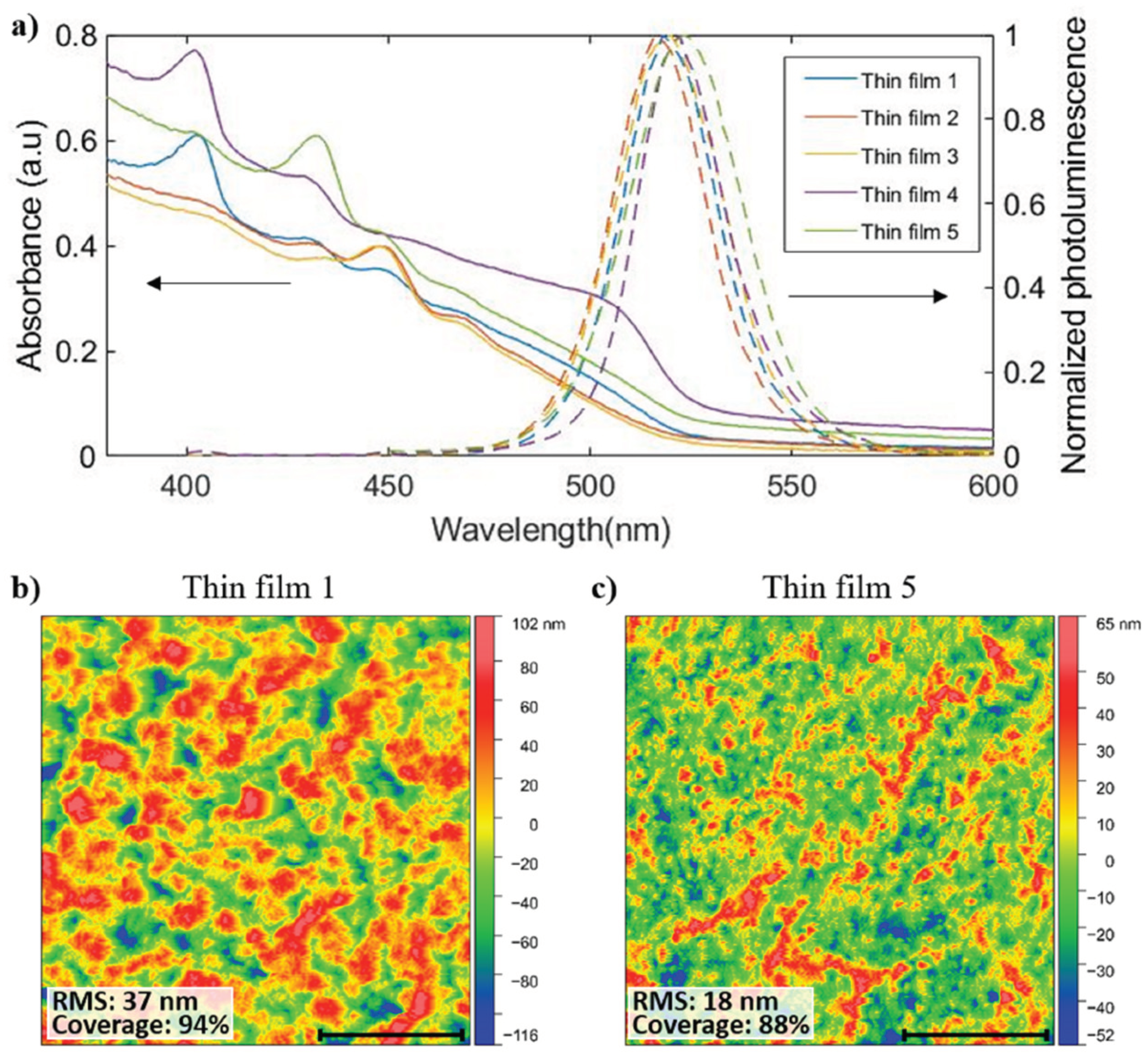
3.2. Effects of Thermal Fluctuation Inside Deposition Chamber and Chemical Contamination on Quasi-2D Perovskite Composition
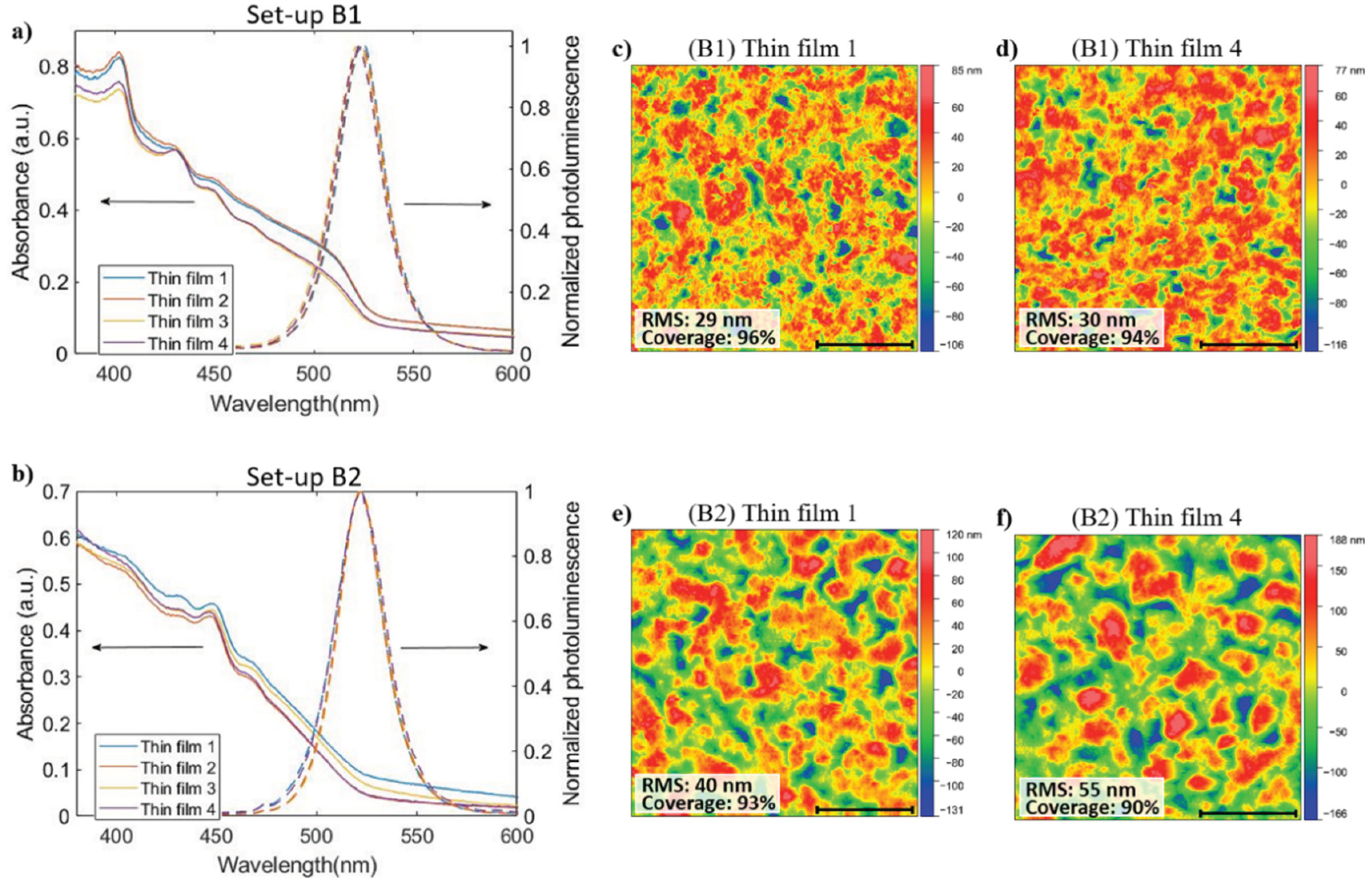
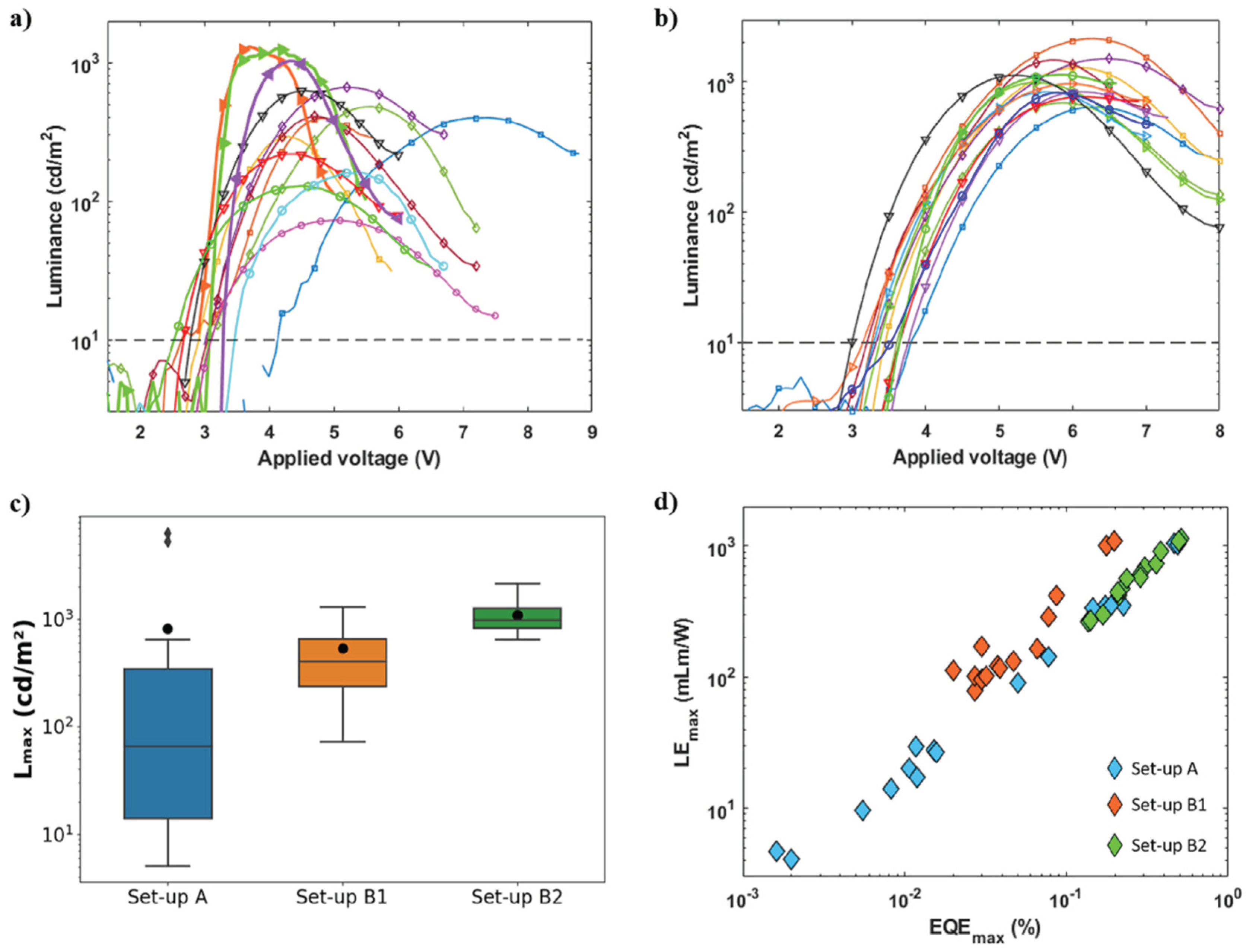
3.3. Improvement in PeLED Reproducibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, H.; Wang, H. 2D/3D Halide Perovskites for Optoelectronic Devices. Front. Chem. 2021, 9, 715157. [Google Scholar] [CrossRef]
- Wang, H.-P.; Li, S.; Liu, X.; Shi, Z.; Fang, X.; He, J.-H. Low-Dimensional Metal Halide Perovskite Photodetectors. Adv. Mater. 2021, 33, 2003309. [Google Scholar] [CrossRef]
- Liu, X.-K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal Halide Perovskites for Light-Emitting Diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Gangishetty, M.K.; Abdi-Jalebi, M.; Chin, S.-H.; bin Mohd Yusoff, A.R.; Congreve, D.N.; Tress, W.; Deschler, F.; Vasilopoulou, M.; Bolink, H.J. Perovskite Light-Emitting Diodes. Nat. Electron. 2022, 5, 203–216. [Google Scholar] [CrossRef]
- Quan, L.N.; Rand, B.P.; Friend, R.H.; Mhaisalkar, S.G.; Lee, T.-W.; Sargent, E.H. Perovskites for Next-Generation Optical Sources. Chem. Rev. 2019, 119, 7444–7477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yi, C.; Wang, N.; Sun, Y.; Zou, W.; Wei, Y.; Cao, Y.; Miao, Y.; Li, R.; Yin, Y.; et al. Efficient Red Perovskite Light-Emitting Diodes Based on Solution-Processed Multiple Quantum Wells. Adv. Mater. 2017, 29, 1606600. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jagielski, J.; Kallikounis, N.; Kim, Y.-H.; Wolf, C.; Jenny, F.; Tian, T.; Hofer, C.J.; Chiu, Y.-C.; Stark, W.J.; et al. Ultrapure Green Light-Emitting Diodes Using Two-Dimensional Formamidinium Perovskites: Achieving Recommendation 2020 Color Coordinates. Nano Lett. 2017, 17, 5277–5284. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Gangishetty, M.K.; Quan, Q.; Congreve, D.N. Efficient Blue and White Perovskite Light-Emitting Diodes via Manganese Doping. Joule 2018, 2, 2421–2433. [Google Scholar] [CrossRef]
- Adjokatse, S.; Fang, H.-H.; Loi, M.A. Broadly Tunable Metal Halide Perovskites for Solid-State Light-Emission Applications. Mater. Today 2017, 20, 413–424. [Google Scholar] [CrossRef]
- Tan, Z.-K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite. Nat. Nanotech. 2014, 9, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Li, Y.; Xia, M.; Shi, E. Quasi-2D Halide Perovskite Crystals and Their Optoelectronic Applications. J. Mater. Chem. A 2022, 10, 19169–19183. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, C.; He, T.; Jiang, Y.; Wei, J.; Huang, Y.; Yuan, M. High-Performance Quasi-2D Perovskite Light-Emitting Diodes: From Materials to Devices. Light. Sci. Appl. 2021, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zhang, C.; Kim, Y.-H.; Lin, L.Y.; Luther, J.M. The Path to Enlightenment: Progress and Opportunities in High Efficiency Halide Perovskite Light-Emitting Devices. ACS Photonics 2021, 8, 386–404. [Google Scholar] [CrossRef]
- Chen, P.; Bai, Y.; Lyu, M.; Yun, J.-H.; Hao, M.; Wang, L. Progress and Perspective in Low-Dimensional Metal Halide Perovskites for Optoelectronic Applications. Sol. RRL 2018, 2, 1700186. [Google Scholar] [CrossRef]
- Yantara, N.; Bruno, A.; Iqbal, A.; Jamaludin, N.F.; Soci, C.; Mhaisalkar, S.; Mathews, N. Designing Efficient Energy Funneling Kinetics in Ruddlesden–Popper Perovskites for High-Performance Light-Emitting Diodes. Adv. Mater. 2018, 30, 1800818. [Google Scholar] [CrossRef]
- Yuan, M.; Quan, L.N.; Comin, R.; Walters, G.; Sabatini, R.; Voznyy, O.; Hoogland, S.; Zhao, Y.; Beauregard, E.M.; Kanjanaboos, P.; et al. Perovskite Energy Funnels for Efficient Light-Emitting Diodes. Nat. Nanotech. 2016, 11, 872–877. [Google Scholar] [CrossRef]
- Byun, J.; Cho, H.; Wolf, C.; Jang, M.; Sadhanala, A.; Friend, R.H.; Yang, H.; Lee, T.-W. Efficient Visible Quasi-2D Perovskite Light-Emitting Diodes. Adv. Mater. 2016, 28, 7515–7520. [Google Scholar] [CrossRef]
- Sun, C.; Jiang, Y.; Cui, M.; Qiao, L.; Wei, J.; Huang, Y.; Zhang, L.; He, T.; Li, S.; Hsu, H.-Y.; et al. High-Performance Large-Area Quasi-2D Perovskite Light-Emitting Diodes. Nat. Commun. 2021, 12, 2207. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, W.; Peng, X.; Sun, G.; Liu, X.; Liu, D.; Li, Z.; He, F.; Shen, C.; Gu, Q.; et al. Perovskite Light-Emitting Diodes with EQE Exceeding 28% through a Synergetic Dual-Additive Strategy for Defect Passivation and Nanostructure Regulation. Adv. Mater. 2021, 33, 2103268. [Google Scholar] [CrossRef]
- Han, T.-H.; Jang, K.Y.; Dong, Y.; Friend, R.H.; Sargent, E.H.; Lee, T.-W. A Roadmap for the Commercialization of Perovskite Light Emitters. Nat. Rev. Mater. 2022, 7, 757–777. [Google Scholar] [CrossRef]
- Xing, J.; Zhao, Y.; Askerka, M.; Quan, L.N.; Gong, X.; Zhao, W.; Zhao, J.; Tan, H.; Long, G.; Gao, L.; et al. Col-or-Stable Highly Luminescent Sky-Blue Perovskite Light-Emitting Diodes. Nat. Commun. 2018, 9, 3541. [Google Scholar] [CrossRef] [PubMed]
- Laxmi; Kabra, D. Optimization of Composition with Reduced Phase Impurity in Quasi-2D Perovskite for Electroluminescence. Adv. Photonics Res. 2021, 2, 2000164. [Google Scholar] [CrossRef]
- Han, Y.; Wang, J.; Bischak, C.G.; Kim, S.; Lee, K.; Shin, D.; Lee, M.J.; Ginger, D.S.; Hwang, I. Significance of Ambient Temperature Control for Highly Reproducible Layered Perovskite Light-Emitting Diodes. ACS Photonics 2020, 7, 2489–2497. [Google Scholar] [CrossRef]
- Bae, Y.; Ryu, J.; Yoon, S.; Kang, D.-W. Recent Progress in Quasi-Two-Dimensional and Quantum Dot Perovskite Light-Emitting Diodes Harnessing the Diverse Effects of Ligands: A Review. Nano Res. 2022, 15, 6449–6465. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Deng, J.; Chu, Z.; Jiang, Q.; Meng, J.; Wang, P.; Zhang, L.; Yin, Z.; You, J. Efficient Green Light-Emitting Diodes Based on Quasi-Two-Dimensional Composition and Phase Engineered Perovskite with Surface Passivation. Nat. Commun. 2018, 9, 570. [Google Scholar] [CrossRef]
- Ye, Z.; Xia, J.; Zhang, D.; Duan, X.; Xing, Z.; Jin, G.; Cai, Y.; Xing, G.; Chen, J.; Ma, D. Efficient Quasi-2D Per-ovskite Light-Emitting Diodes Enabled by Regulating Phase Distribution with a Fluorinated Organic Cation. Nanomaterials 2022, 12, 3495. [Google Scholar] [CrossRef]
- Cheng, T.; Qin, C.; Watanabe, S.; Matsushima, T.; Adachi, C. Stoichiometry Control for the Tuning of Grain Passivation and Domain Distribution in Green Quasi-2D Metal Halide Perovskite Films and Light-Emitting Diodes. Adv. Funct. Mater. 2020, 30, 2001816. [Google Scholar] [CrossRef]
- Yuan, Z.; Miao, Y.; Hu, Z.; Xu, W.; Kuang, C.; Pan, K.; Liu, P.; Lai, J.; Sun, B.; Wang, J.; et al. Unveiling the Synergistic Effect of Precursor Stoichiometry and Interfacial Reactions for Perovskite Light-Emitting Diodes. Nat. Commun. 2019, 10, 2818. [Google Scholar] [CrossRef]
- Chen, C.; Zeng, L.; Jiang, Z.; Xu, Z.; Chen, Y.; Wang, Z.; Chen, S.; Xu, B.; Mai, Y.; Guo, F. Vacuum-Assisted Preparation of High-Quality Quasi-2D Perovskite Thin Films for Large-Area Light-Emitting Diodes. Adv. Funct. Mater. 2022, 32, 2107644. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Mu, L.; Li, M.; Luo, Y.; Zhang, B.; Mai, C.; Guo, B.; Lan, L.; Wang, J.; et al. Inkjet-Printed Full-Color Matrix Quasi-Two-Dimensional Perovskite Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2021, 13, 41773–41781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yuan, F.; Zhao, W.; Jiao, B.; Ran, C.; Zhang, W.; Wu, Z. High Performance Organo-Lead Halide Perovskite Light-Emitting Diodes via Surface Passivation of Phenethylamine. Org. Electron. 2018, 60, 57–63. [Google Scholar] [CrossRef]
- Ma, J.; Yang, L.; Zhang, Y.; Kuang, Y.; Shao, M. Rearranging the Phase Distribution of Quasi-2D Perovskite for Efficient and Narrow Emission Perovskite Light-Emitting Diodes. J. Phys. Chem. Lett. 2022, 13, 4739–4746. [Google Scholar] [CrossRef] [PubMed]
- Worku, M.; He, Q.; Xu, L.; Hong, J.; Yang, R.X.; Tan, L.Z.; Ma, B. Phase Control and In Situ Passivation of Quasi-2D Metal Halide Perovskites for Spectrally Stable Blue Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2020, 12, 45056–45063. [Google Scholar] [CrossRef] [PubMed]
- Molenda, Z.; Chambon, S.; Bassani, D.M.; Hirsch, L. Assessing the Impact of Ambient Fabrication Temperature on the Performance of Planar CH3NH3PbI3 Perovskite Solar Cells. Eur. J. Inorg. Chem. 2021, 2021, 2533–2538. [Google Scholar] [CrossRef]
- Ji, K.; Anaya, M.; Abfalterer, A.; Stranks, S.D. Halide Perovskite Light-Emitting Diode Technologies. Adv. Opt. Mater. 2021, 9, 2002128. [Google Scholar] [CrossRef]
- Zhao, B.; Lian, Y.; Cui, L.; Divitini, G.; Kusch, G.; Ruggeri, E.; Auras, F.; Li, W.; Yang, D.; Zhu, B.; et al. Efficient Light-Emitting Diodes from Mixed-Dimensional Perovskites on a Fluoride Interface. Nat. Electron. 2020, 3, 704–710. [Google Scholar] [CrossRef]
- Anaya, M.; Rand, B.P.; Holmes, R.J.; Credgington, D.; Bolink, H.J.; Friend, R.H.; Wang, J.; Greenham, N.C.; Stranks, S.D. Best Practices for Measuring Emerging Light-Emitting Diode Technologies. Nat. Photonics 2019, 13, 818–821. [Google Scholar] [CrossRef]
- Mai, C.; Cun, Y.; Luo, Y.; Zhang, B.; Li, M.; Li, H.; Mu, L.; Wang, J.; Li, D.; Wang, J. Dependence of the Radiative Efficiency of Quasi-2D Perovskite Light-Emitting Diodes on the Multiquantum-Well Composition. J. Phys. Chem. C 2021, 125, 12241–12250. [Google Scholar] [CrossRef]
- Yan, C.; Lin, K.; Lu, J.; Wei, Z. Composition Engineering to Obtain Efficient Hybrid Perovskite Light-Emitting Diodes. Front. Optoelectron. 2020, 13, 282–290. [Google Scholar] [CrossRef]
- Li, Z.; Cao, K.; Li, J.; Du, X.; Tang, Y.; Yu, B. Modification of Interface between PEDOT:PSS and Perovskite Film Inserting an Ultrathin LiF Layer for Enhancing Efficiency of Perovskite Light-Emitting Diodes. Org. Electron. 2020, 81, 105675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




