Submitted:
20 February 2024
Posted:
20 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
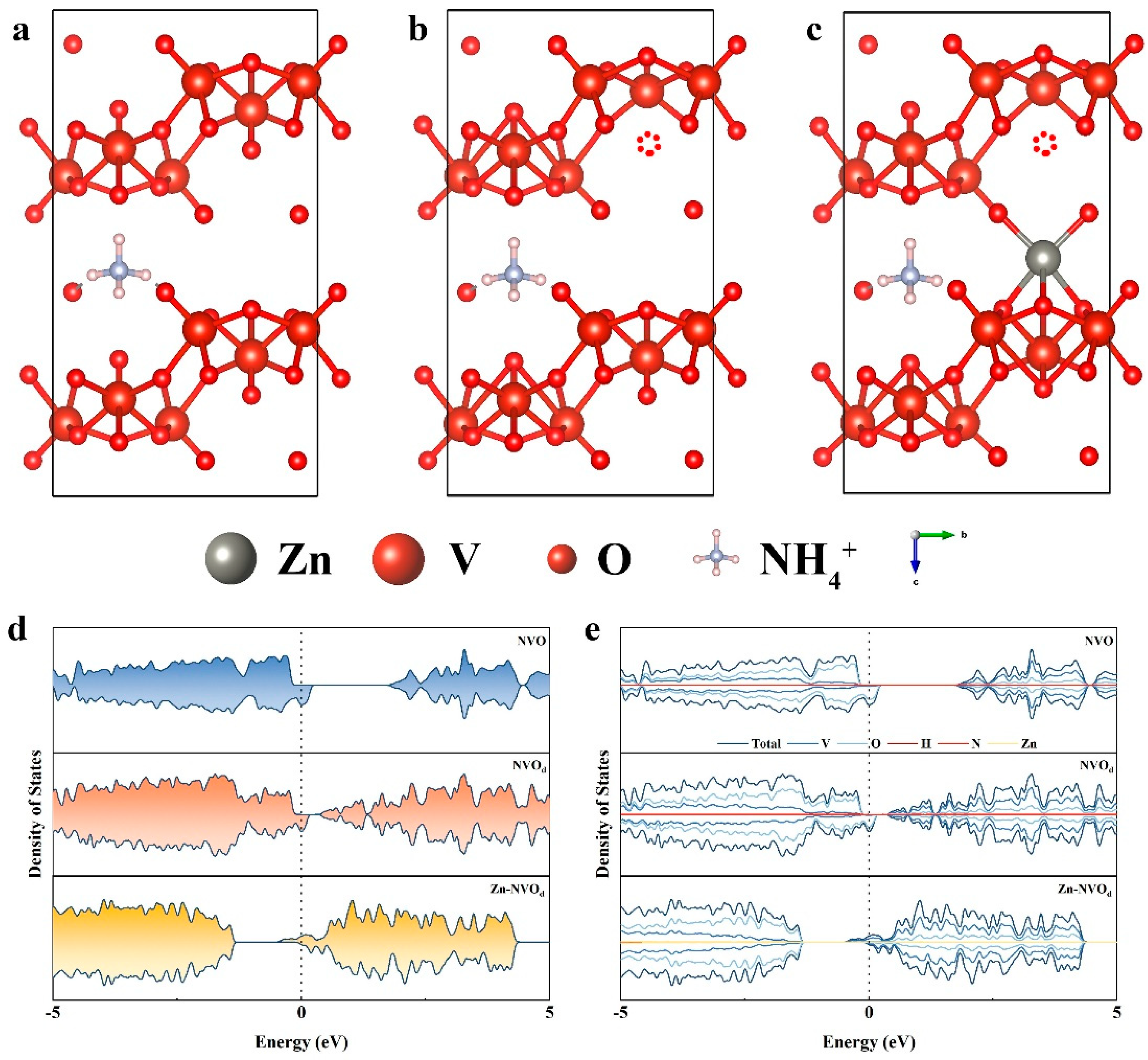
2.1. DFT Calculations
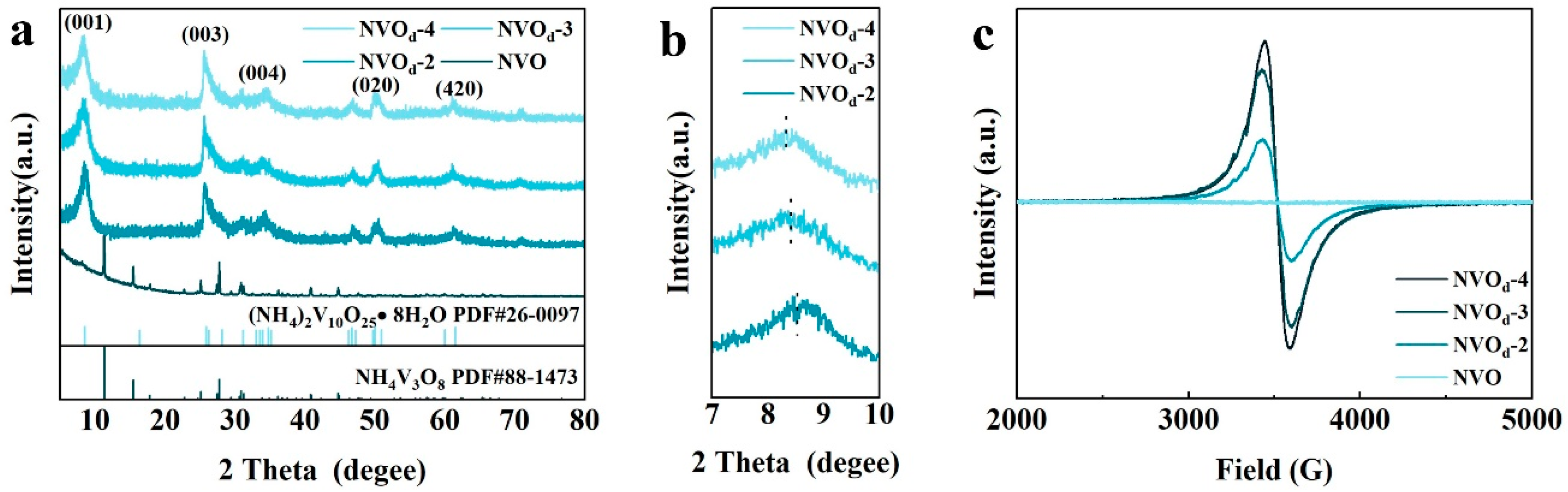
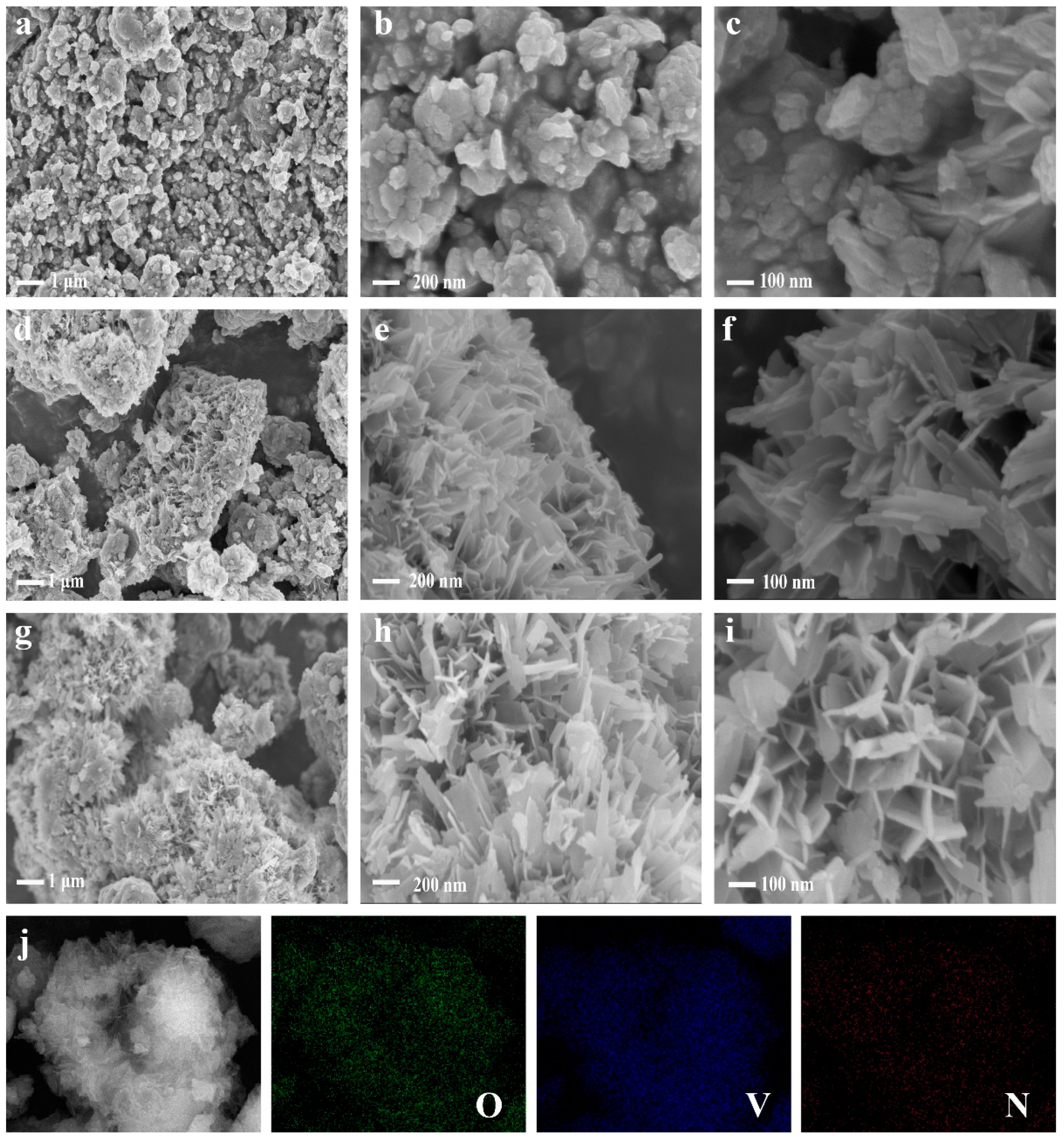
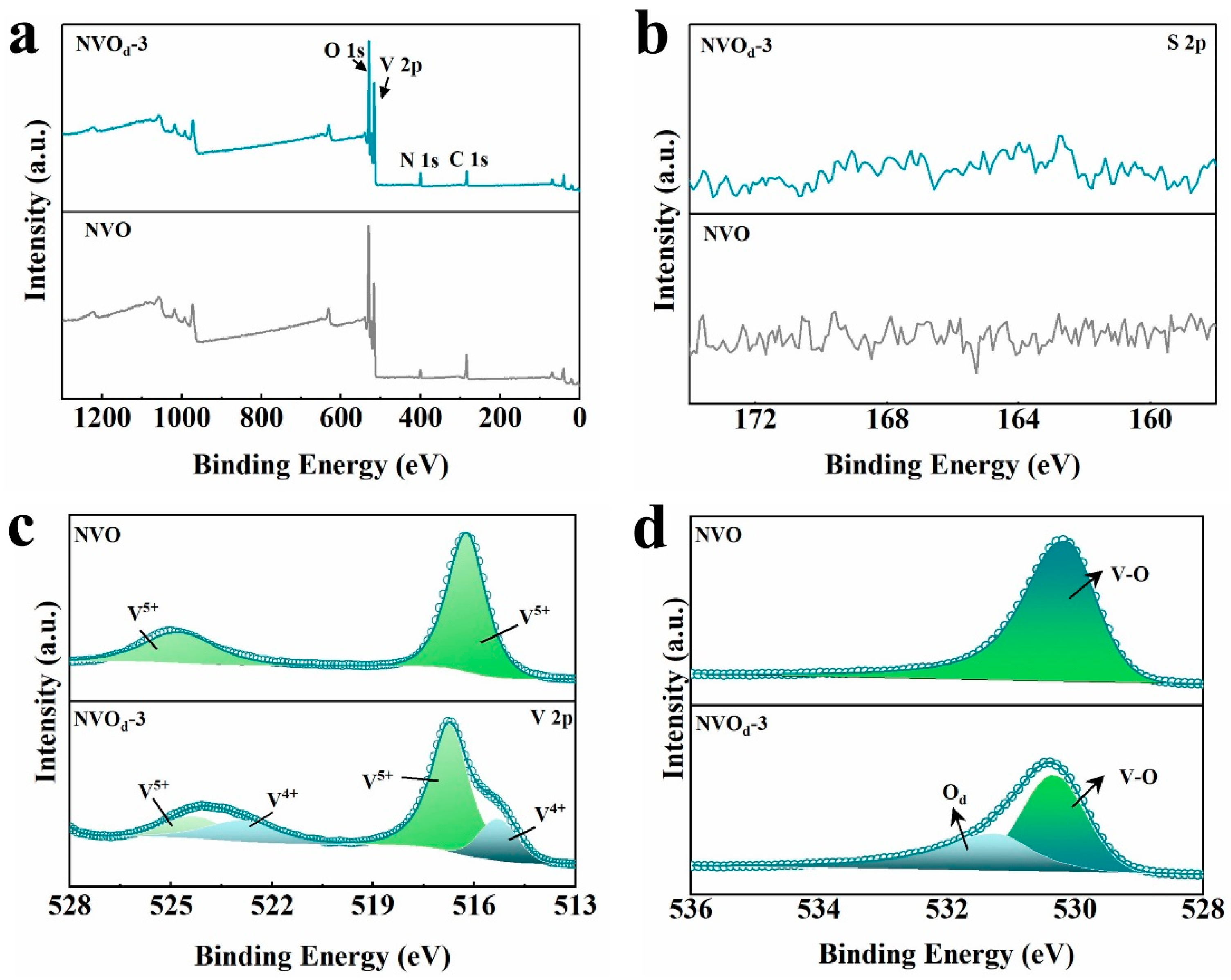
2.2. Composition and Structural Characterization
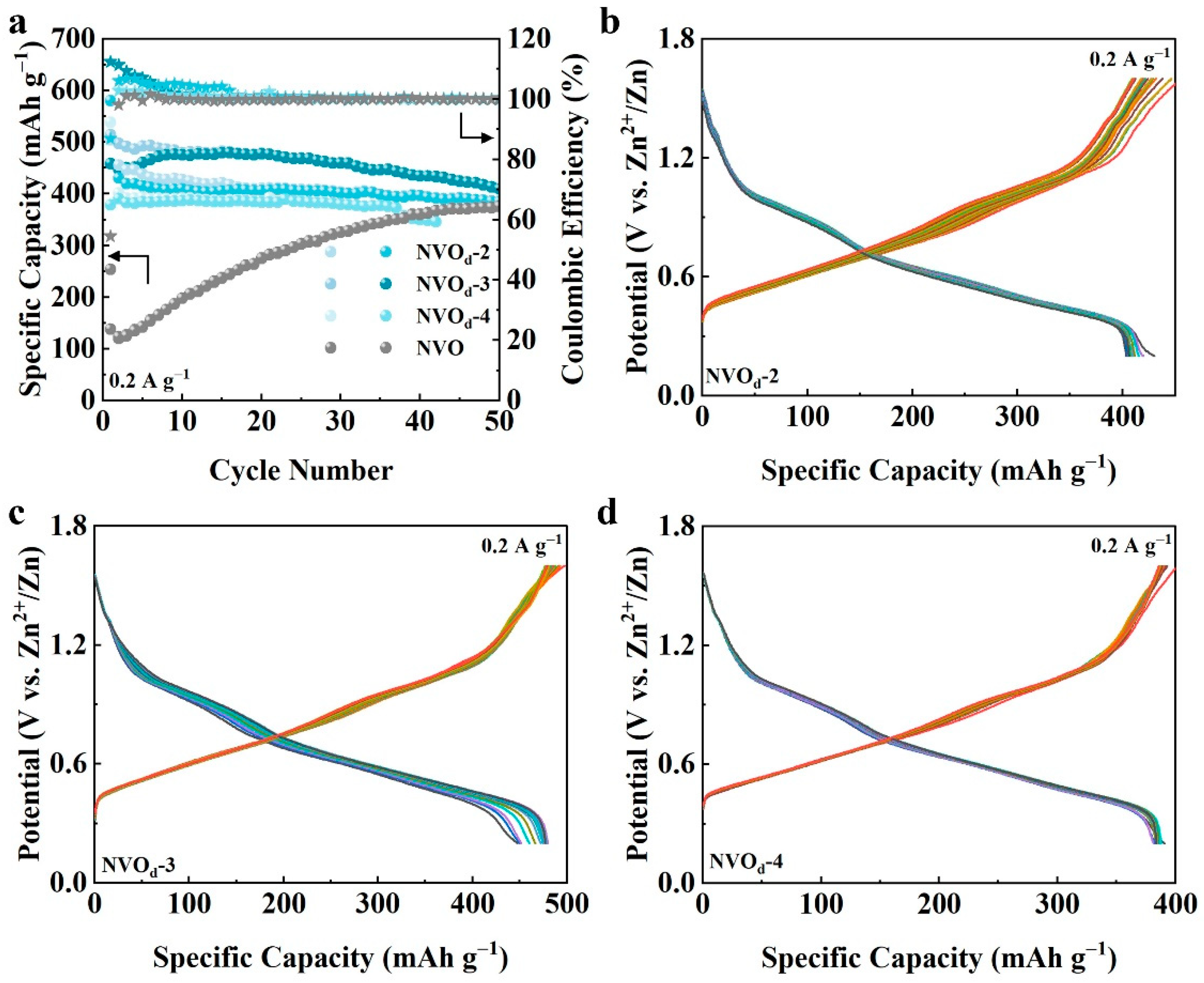
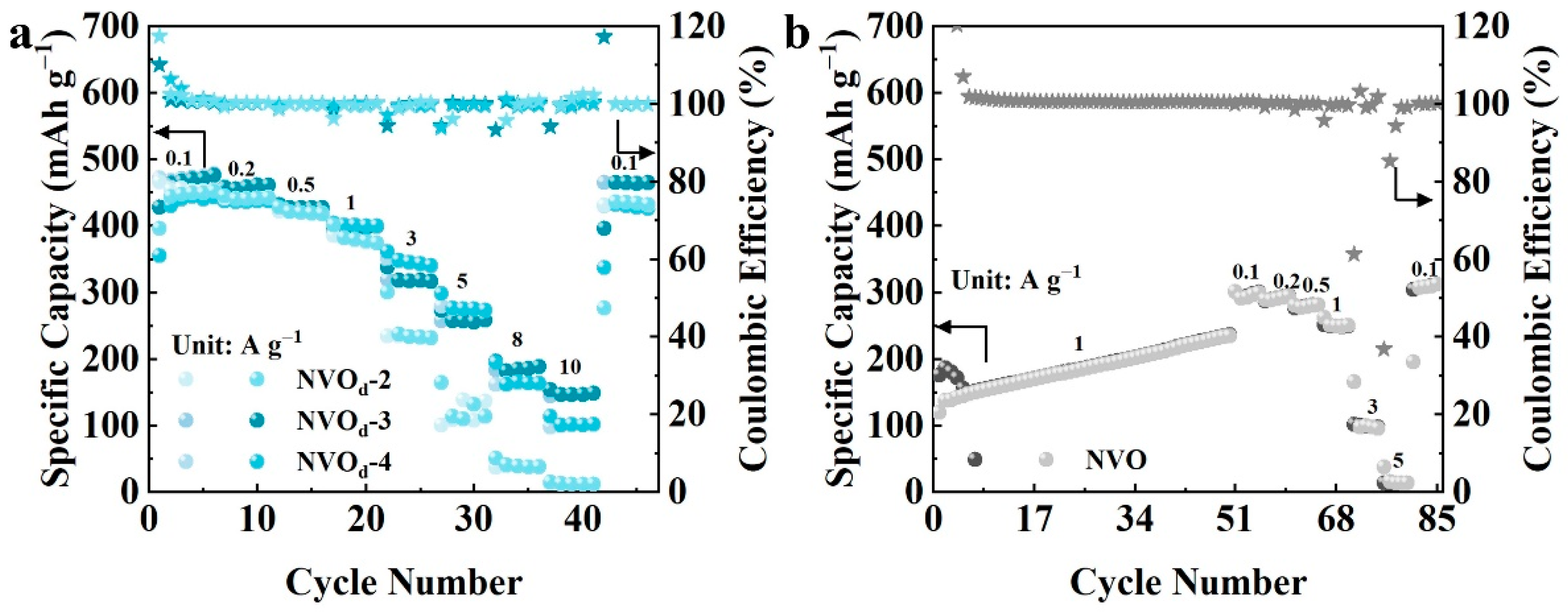
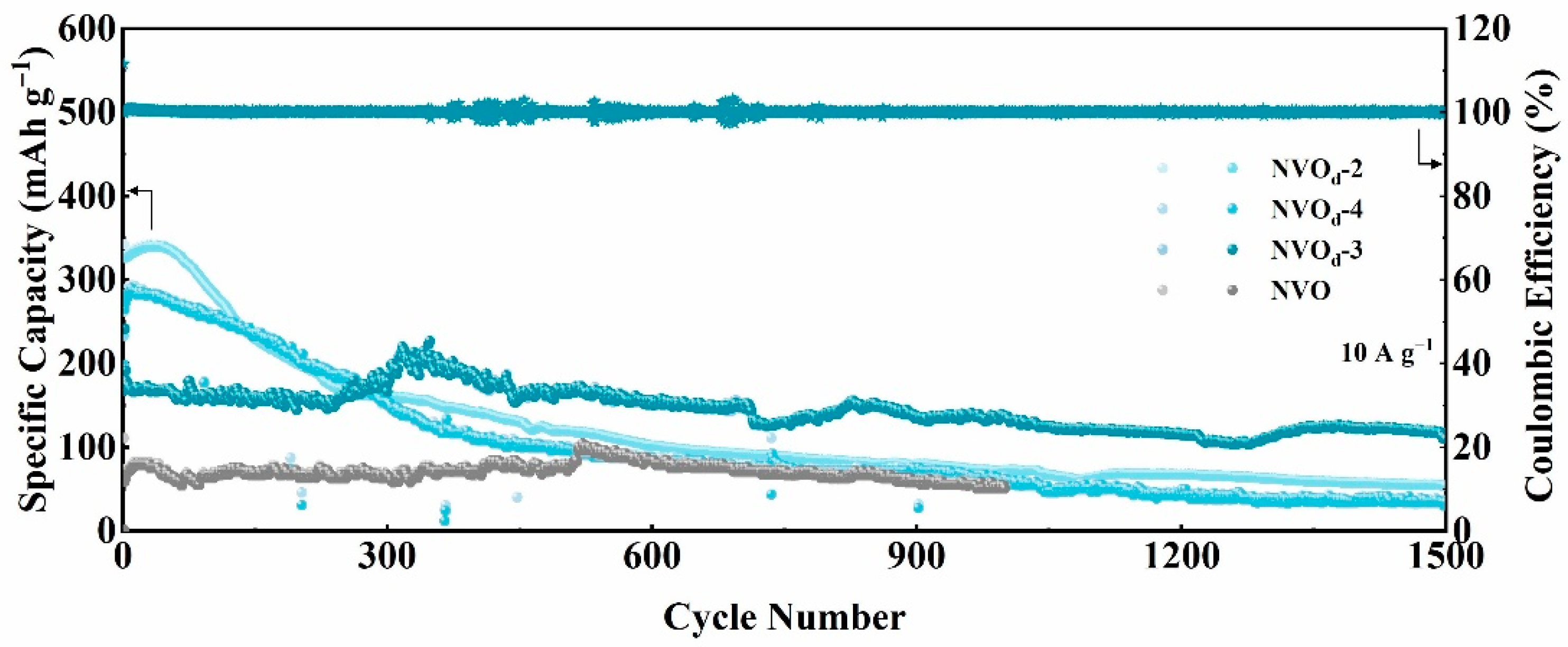
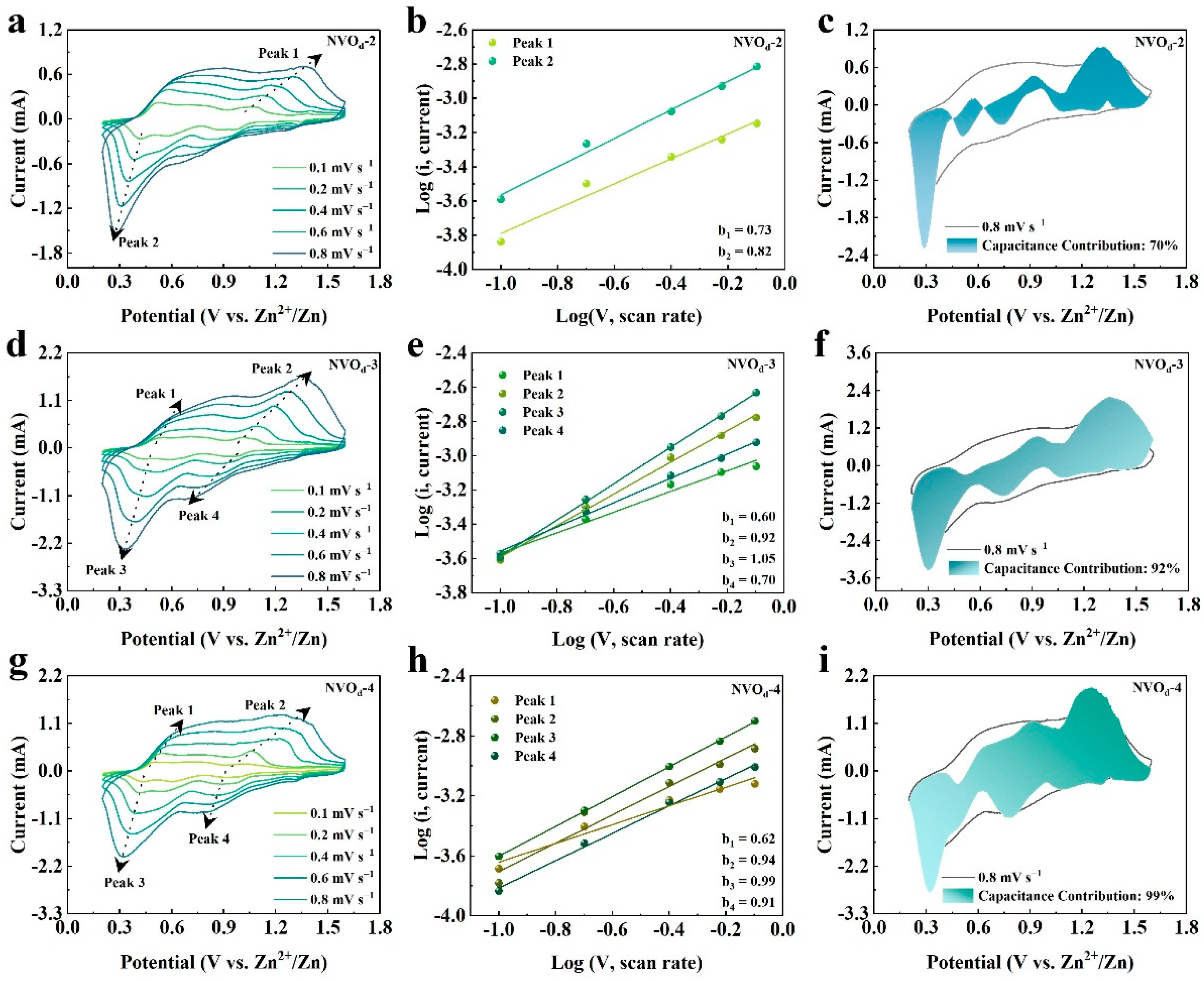
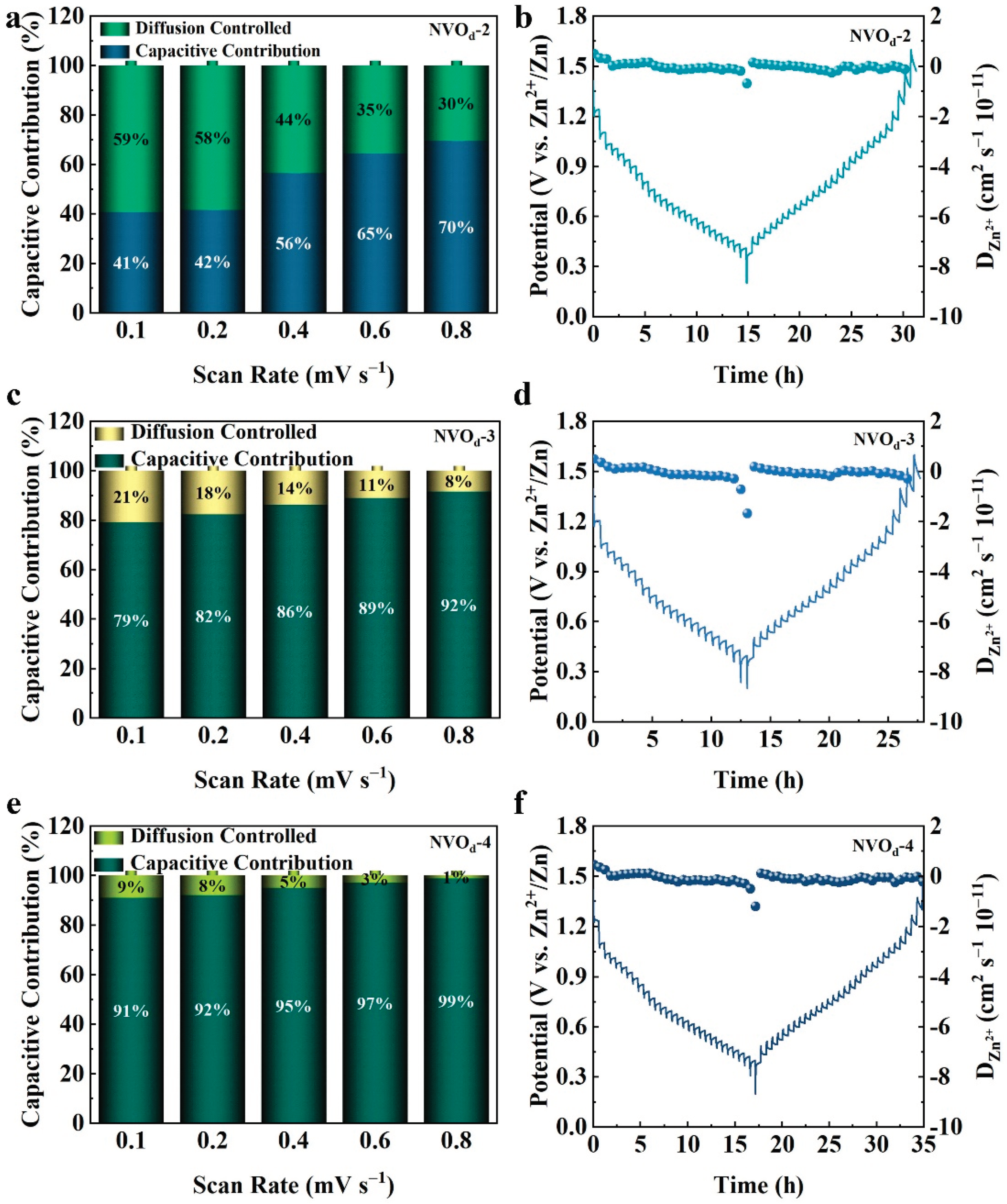
2.3. Electrochemical Properties Characterization
3. Materials and Methods
3.1. Calculation Method
3.2. Preparation of Material
3.3. Structure and Morphology Characterization
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active materials for aqueous zinc ion batteries: synthesis, crystal structure, morphology, and electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef]
- Lin, X.; Khosravinia, K.; Hu, X.; Li, J.; Lu, W. Lithium plating mechanism, detection, and mitigation in lithium-ion batteries. Prog. Energ. Combust. 2021, 87, 100953. [Google Scholar] [CrossRef]
- Shadike, Z.; Tan, S.; Wang, Q.-C.; Lin, R.; Hu, E.; Qu, D.; Yang, X.-Q. Review on organosulfur materials for rechargeable lithium batteries. Mater. Horiz. 2021, 8, 471–500. [Google Scholar] [CrossRef] [PubMed]
- Selvakumaran, D.; Pan, A.; Liang, S.; Cao, G. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J. Mater. Chem. A 2019, 7, 18209–18236. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.-C.; Guo, Y.-F.; Wang, P.-F.; Zhu, Y.-R.; Yi, T.-F. Insights on rational design and energy storage mechanism of Mn-based cathode materials towards high performance aqueous zinc-ion batteries. Coordin. Chem. Rev. 2023, 479, 215009. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Mathew, V.; Gim, J.; Kim, S.; Song, J.; Baboo, J.P.; Choi, S.H.; Kim, J. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system. Chem. Mater. 2015, 27, 3609–3620. [Google Scholar] [CrossRef]
- Chen, D.; Lu, M.; Cai, D.; Yang, H.; Han, W. Recent advances in energy storage mechanism of aqueous zinc-ion batteries. J. Energy. Chem. 2021, 54, 712–726. [Google Scholar] [CrossRef]
- Wu, J.; Chi, X.; Liu, Y.; Yang, J.; Liu, Y. Electrochemical characterization of hollow urchin-like MnO2 as high-performance cathode for aqueous zinc ion batteries. J. Electroanal. Chem. 2020, 871, 114242. [Google Scholar] [CrossRef]
- Mathew, V.; Sambandam, B.; Kim, S.; Kim, S.; Park, S.; Lee, S.; Alfaruqi, M.H.; Soundharrajan, V.; Islam, S.; Putro, D.Y.; Hwang, J.-Y.; Sun, Y.-K.; Kim, J. Manganese and vanadium oxide cathodes for aqueous rechargeable zinc-ion batteries: a focused view on performance, mechanism, and developments. ACS Energy Lett. 2020, 5, 2376–2400. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; Wang, C. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; Mueller, K.T.; Mai, L.; Liu, J.; Yang, J. Water-lubricated intercalation in V2O5⸱nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lu, M.; Wang, B.; Cheng, H.; Yang, H.; Cai, D.; Han, W.; Fan, H.J. High-mass loading V3O7⸱H2O nanoarray for Zn-ion battery: new synthesis and two-stage ion intercalation chemistry. Nano Energy 2021, 83, 105835. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, Y.; Jia, M.; Bian, X.; Wang, Y.; Qiu, M.; Xu, J.; Liu, Y.; Jiao, L.; Cheng, F. Rechargeable aqueous Zn-V2O5 battery with high energy density and long cycle life. ACS Energy Lett. 2018, 3, 1366–1372. [Google Scholar] [CrossRef]
- Zhang, N.; Jia, M.; Dong, Y.; Wang, Y.; Xu, J.; Liu, Y.; Jiao, L.; Cheng, F. Hydrated layered vanadium oxide as a highly reversible cathode for rechargeable aqueous zinc batteries. Adv. Funct. Mater. 2019, 29, 1807331. [Google Scholar] [CrossRef]
- Zampardi, G.; Mantia, F.L. Prussian blue analogues as aqueous Zn-ion batteries electrodes: current challenges and future perspectives. Curr. Opin. Electroche. 2020, 21, 84–92. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, L.; Xie, L.; Han, Q.; Yang, X.; Chen, L.; Wang, G.; Cao, X. Cathode materials for aqueous zinc-ion batteries: a mini review. J. Colloid. Interf. Sci. 2022, 605, 828–850. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: the zinc/zinc hexacyanoferrate system. Adv. Energy Mater. 2015, 5, 1400930. [Google Scholar] [CrossRef]
- Wu, X.; Qi, Y.; Hong, J.J.; Li, Z.; Hernandez, A.S.; Ji, X. Rocking-chair ammonium-ion battery: a highly reversible aqueous energy storage system. Angew. Chem.Int. Edit. 2017, 56, 13026–13030. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and future perspective on electrode materials for rechargeable zinc-ion batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-active polymers for energy storage nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef]
- Kundu, D.; Oberholzer, P.; Glaros, C.; Bouzid, A.; Tervoort, E.; Pasquarello, A.; Niederberger, M. Organic cathode for aqueous Zn-ion batteries: taming a unique phase evolution toward stable electrochemical cycling. Chem. Mater. 2018, 30, 3874–3881. [Google Scholar] [CrossRef]
- Cui, H.; Ma, L.; Huang, Z.; Chen, Z.; Zhi, C. Organic materials-based cathode for zinc ion battery. SmartMat 2022, 3, 565–581. [Google Scholar] [CrossRef]
- Deng, Y.-P.; Liang, R.; Jiang, G.; Jiang, Y.; Yu, A.; Chen, Z. The current state of aqueous Zn-based rechargeable batteries. ACS Energy Lett. 2020, 5, 1665–1675. [Google Scholar] [CrossRef]
- Chen, L.; Ruan, Y.; Zhang, G.; Wei, Q.; Jiang, Y.; Xiong, T.; He, P.; Yang, W.; Yan, M.; An, Q.; Mai, L. Ultrastable and high-performance Zn/VO2 battery based on a reversible single-phase reaction. Chem. Mater. 2019, 31, 699–706. [Google Scholar] [CrossRef]
- Shang, Y.; Kundu, D. Aqueous Zn-ion batteries: cathode materials and analysis. Curr. Opin. Electroche. 2022, 33, 100954. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X. Defect engineering of vanadium-based electrode materials for zinc ion battery. Chinese Chem. Lett. 2023, 34, 107839. [Google Scholar] [CrossRef]
- Lai, J.; Tang, H.; Zhu, X.; Wang, Y. A hydrated NH4V3O8 nanobelt electrode for superior aqueous and quasi-solid-state zinc ion batteries. J. Mater. Chem. A 2019, 7, 23140–23148. [Google Scholar] [CrossRef]
- Wang, X.; Xi, B.; Feng, Z.; Chen, W.; Li, H.; Jia, Y.; Feng, J.; Qian, Y.; Xiong, S. Layered (NH4)2V6O16⸱1.5H2O nanobelts as a high-performance cathode for aqueous zinc-ion batteries. J. Mater. Chem. A 2019, 7, 19130–19139. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Cheng, H.; Cai, X.; Jia, D.; Lin, H. “Dual-engineering” strategy to regulate NH4V4O10 as cathodes for high-performance aqueous zinc ion batteries. Small 2023, 19, 2301870. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; Mueller, K.T.; Mai, L.; Liu, J.; Yang, J. Introducing Ce ions and oxygen defects into V2O5 nanoribbons for efficient aqueous zinc ion storage. Nano Res. 2023, 16, 2445–2453. [Google Scholar]
- Liu, N.; Wu, X.; Yin, Y.; Chen, A.; Zhao, C.; Guo, Z.; Fan, L.; Zhang, N. Constructing the efficient ion diffusion pathway by introducing oxygen defects in Mn2O3 for high-performance aqueous zinc-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 28199–28205. [Google Scholar] [CrossRef]
- Ding, J.; Du, Z.; Gu, L.; Li, B.; Wang, L.; Wang, S.; Gong, Y.; Yang, S. Ultrafast Zn2+ intercalation and deintercalation in vanadium dioxide. Adv. Mater. 2018, 30, 1800762. [Google Scholar] [CrossRef]
- Ding, J.; Du, Z.; Gu, L.; Li, B.; Wang, L.; Wang, S.; Gong, Y.; Yang, S. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar]
- Zhang, Y.; Jiang, H.; Xu, L.; Gao, Z.; Meng, C. Ammonium vanadium oxide [(NH4)2V4O9] sheets for high capacity electrodes in aqueous zinc ion batteries. ACS Appl. Energy Mater. 2019, 2, 7861–7869. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Jiang, H.; Zheng, J.; Dong, X.; Hu, T.; Meng, C. Facile hydrothermal synthesis and electrochemical properties of (NH4)2V6O16 nanobelts for aqueous rechargeable zinc ion batteries. Colloid. Surface. A 2020, 593, 124621. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




