1. Introduction
Because of the depletion and environmental issues caused by the usage of fossil fuels, renewable raw materials have become the preferred option for meeting material and energy needs. Thus, biomass is the most abundant organic material on Earth. Colombia has favorable agroclimatic conditions for establishing agro-industrial activity [
1]; one of the crops with the highest productivity and participation in the country is sugarcane. Sugarcane is used to produce refined sugar, panela, and bioethanol. Sugarcane production is the second-most important agroindustry in the country. Sugarcane processing in Colombia generates around 16.5 million tons of bagasse each year [
2,
3,
4]; approximately 70% of the unprocessed raw material becomes waste products [
4,
5]. For this reason, the development of agro-industry aims not only to provide food security for the population, but also to recover waste and reduce its environmental impact [
6,
7].
Hydrothermal treatments are appropriate and efficient systems for the conversion of wet biomasses because water under hydrothermal conditions favors the reaction mechanisms that break down the cellulose, hemicellulose, and lignin present in the biomass [
8]. In this sense, hydrothermal carbonization is a thermochemical process that consists of the conversion of biomass by the action of an aqueous reaction medium, in which hydrolysis is favored by ionization reactions. The system conditions should range from 180 to 260 °C and 1 to 5 MPa; these conditions keep the water in its liquid state [
9].
The main products obtained in hydrothermal carbonization systems are an aqueous phase in which a wide variety of water-soluble platform chemicals can be found, such as monomeric sugars (glucose, xylose, galactose, and arabinose, among others) [
10]; products of the hydrolysis of cellulose and hemicellulose carried out at 180 °C. In turn, at temperatures above 200 °C, monomers can produce new compounds such as 5-hydroxymethyl-2-furfural and fructose [
11]. Other reaction mechanisms, such as dehydration, decarboxylation, condensation, polymerization, and aromatization, can occur simultaneously. Dehydration and decarboxylation of HMF and furfural derivatives can produce acetic acid, formic acid, levulinic acid, aldehydes, water, and gaseous products such as dioxide. Also, polymerization reactions by aldol condensation and aromatization produce a lignite-like solid called a hydrochar [
11,
12,
13].
Platform chemicals have varied applications in the industry of additives, solvents, reagents, and lubricants, among other products of high commercial value [
14]. While hydrocarbons are currently under study, it has been demonstrated that these carbonaceous materials have a wide variety of applications. Some of these include catalyst supports, biofuels, activated carbon and adsorbent materials for wastewater treatment, soil remediation, CO
2 sequestration, and some other applications [
15,
16].
This study is expected to contribute knowledge to the optimization of the conversion parameters of an HTC reaction system for the valorization of sugarcane bagasse from the Colombian industry. In this sense, as a starting point, the yields of the possible products obtained from the optimization of the parameters (particle size of the raw material and B:W ratio of the system) at two working temperatures were determined. The results obtained are expected to serve as a basis for future research, with the objective of achieving higher yields in the products and thus increasing their value in the agroindustrial production chain.
2. Materials and Methods
2.1. Sampling of Residual Biomass.
The biomass selected for this research was sugar cane bagasse. 2 kg of this raw material were donated by a producer associated with the National Federation of Panela Producers (Fedepanela). The sampling was carried out on a farm in the municipality of La Peña, Cundinamarca, in the province of Gualivá, located 140 km northwest of Bogotá. Biomass was collected fifteen days after milling.
2.2. Sugarcane Bagasse Is the Primary Pretreatment. Biomass Characterization Techniques
The primary pretreatment of the biomass was performed in accordance with the 2008 technical report NREL/TP-510-42620, "Preparation of samples for compositional analysis.". This technical standard describes how to convert the biomass sample into a material suitable for reproducible analysis and processing. The pretreatment applied to this biomass was grinding and sieving. The grinding process was carried out in a SM100 cutting mill with a 1 mm-diameter screen. This mill has a parallel cutting rotor that passes close to the sieve ring; thus, the particles are crushed by the collision of the rotating cutting disc, shear forces, and friction exerted by the sieve ring and rotor blades on the particles. After grinding, sieving was carried out with sieves meeting ASTM E-12 specifications (600, 212 y 106 µm).
2.3. Biomass Characterization Techniques
2.3.1. Proximal Analysis
Proximal analysis includes the determination of moisture, ash, volatile matter, and fixed carbon content. From technical standards: moisture NREL/TP-510-42621, March 2008; "Determination of total solids in biomass and total dissolved solids in process liquid samples," ash NREL/TP-510-42622, January 2008. "Determination of ash in biomass," volatile matter ASTM E872-82, 2019, "Standard test method for volatile matter in wood fuel particulate analysis," and fixed carbon were calculated by the difference between the determination of the properties described above.
2.3.2. Ultimate Analysis.
Based on the ASTM-D5373 technical standard, the raw material underwent elemental analysis. The purpose of this analysis is to determine the percentage of carbon, hydrogen, nitrogen, sulfur, and elemental oxygen; the latter was determined by the difference in its elemental composition, as explained in ASTM D3173. The main basis of this process is to perform a combustion of the raw material under controlled conditions.
2.3.3. Structural Analysis.
This analysis made it possible to determine the cellulose, hemicellulose, and lignin content present in the sugarcane bagasse. These structural components were determined based on the protocols FNA-AOAC 973.18, "Official methods of analysis, acid detergent fiber," FND-AOAC 200.04, "Official methods of analysis, neutral detergent fiber," and Lignin-AOAC 973.18, "Official methods of analysis, lignin," respectively.
2.4. Hydrothermal Treatment of Biomass.
The hydrothermal carbonization was carried out in a high-pressure synthesis batch reactor in stainless steel with an autoclave and a capacity of 500 ml. Twelve experiments were carried out at two temperatures, 220 and 260 °C, with self-generated pressure, residence time was 1 hour, without the use of catalysts or any other type of solvent. The biomass-to-water ratio and particle size parameters were varied. To evaluate the biomass: water ratio, 1:10 and 1:50 ratios were taken; for the 1:10 ratio, 10 g of dry biomass per 100 g of water were taken, and for this 1:50 ratio, about 5 g of biomass on a dry basis per 250 g of water were weighed; while the sizes used were: small (106 µm), medium (212 µm), and large (600 µm). The amount of water was measured based on the percentage of biomass moisture plus the amount of additional water.
Table 1 shows the experimental design implemented. The product of the reactions was vacuum filtered, and two products were obtained: an aqueous phase and a solid phase.
2.4.1. Aqueous Phase.
The pH and conductivity were measured in the aqueous phase to monitor the formation of acidic compounds characteristic of the decomposition of lignocellulosic biomass. The equipment used to measure these properties was a Hanna Instruments pH and conductivity meter reference HI-HI5522.
Identification and quantification of the formation of platform chemicals: The aqueous products were analyzed by High Performance Liquid Chromatography (HPLC) on a Hitachi LaChrom Elite® HPLC System by Hitachi High Technologies America. Characteristics of the chromatographic method: Stationary phase: Shodex, Sugar SH1821 8.0 x 300 mm, particle size: 6 µm; mobile phase: 0.005M sulfuric acid solution, flow rate 0.5 ml/min; column temperature: 60°C, injection volume 20 µL; method run time 60 min and detection system: Hitachi RI (Refractive Index) L-2490 detector. The standards used for the determination of the platform chemicals obtained from the HTC treatment applied to sugarcane bagasse were glucose, xylose, formic acid, levulinic acid, HMF, and furfural. Finally, with equation 1, the performance of the platform chemicals was determined.
2.4.1. Solid Phase (Hydrochar).
The solid was dried at 105 °C to a constant weight, then analyzed by infrared spectroscopy using the ATR analysis method, Nicolet iS10 Spectrometer, Thermo Fisher Scientific equipment, to study and follow up on the breakdown of the characteristic chemical bonds in the sugarcane bagasse. To characterize the hydrocarbons, the morphology was evaluated by means of a scanning electron microscope, Bruker Nano GmbH Berlin, Germany, VEGA 3 TESCAN Easy Probe. The elemental composition of the hydrocarbons was also determined by energy dispersive spectroscopy with an x-ray detector (EDX). In addition, equation 2 was used to determine the percent biomass conversion rate.
3. Results
In the conversion of sugarcane bagasse, the biomass, water, and particle size ratio parameters were evaluated at various temperatures. The goal is to identify and describe the conditions that result in higher yields in the production of value-added products through the hydrothermal carbonization process.
3.1. Biomass Characterization
3.1.1. Proximal Analysis
Table 2 shows the results of the proximate analysis of sugarcane bagasse and data from other biomasses reported in the literature. The percentage of moisture refers to the water content present in the biomass. The sugarcane bagasse has 7.9105% moisture; the value obtained is comparably close to that reported for another sample of sugarcane bagasse, whereas, when compared to other biomasses, this is a source with a high moisture content. This is a great advantage because the high moisture content represents energy savings during the primary pretreatment, avoiding drying prior to the biomass conversion process, and it also reduces the amount of water needed to carry out the hydrothermal reaction system.
The proportion of ash indicates the amount of minerals and inorganic compounds that are not combustible. Sugarcane bagasse thus contains 1.556% inorganic and mineral compounds following combustion. This raw material is found to have a low ash content when compared to other biomasses and another sample of sugarcane bagasse. Compared to raw materials with a higher percentage of ash, lignocellulosic biomass with a lower percentage of ash is thought to be a more advantageous raw material for thermal conversion processes like pyrolysis, combustion, and gasification because it will result in a lower formation of inorganic solid residues. Conversely, a lower production of inorganic solid residues is seen as advantageous since it lessens the harm that residue generation causes to the environment and can help create biofuels that are more sustainably produced and efficient [
17].
The percentage of volatile matter in the sample indicates how much condensable and non-condensable gaseous products are released during heating.
Table 2 shows that the percentage of volatile matter in bagasse made from sugarcane is 80.932%. This number is within the range of information that has been published in other studies. Because of its high volatile matter content, the biomass burns quickly, producing a variety of combustible gaseous, liquid, and solid products. On the other hand, fixed carbon represents the portion of solid carbon that is left over after biomass is burned. This type of carbon is made up of the elemental carbon that remains after volatile matter decomposes and is found in biomass and other carbonaceous compounds. Stated differently, the percentage of fixed carbon is a variable quantity because it depends on the formation products of the volatile matter as well as the rate of heating [
20].
However, the formation of fixed carbon, which is measured by counting the percentage of volatile matter under controlled conditions, is one parameter that provides information about the combustion reaction of the biomass under study.
Table 2 demonstrates that bagasse derived from sugarcane has the lowest percentage of fixed carbon (9.601%), indicating that the amount of solid product produced by this raw material is less than that of other biomasses [
21,
22].
3.1.2. Ultimate Analysis.
The elemental composition of sugarcane bagasse is shown in
Table 3 as follows: 45.22% carbon, 5.94% hydrogen, 0.292% nitrogen, and 48.56% oxygen. There is no sulfur content. We can see that this biomass’s elemental composition falls between the average range of other lignocellulosic residual biomasses when compared to other biomasses. Regarding composition, bagasse’s low nitrogen and sulfur-free levels make it appropriate for use in thermochemical conversion processes like hydrothermal, as these low concentrations lessen the production of greenhouse gases and other harmful gases like sulfur oxides and nitrogen [
21,
23].
3.1.3. Structural Analysis.
Details about the structural makeup of sugarcane bagasse are shown in
Table 4. According to the data, this raw material has a higher cellulose percentage (61.4%) than other biomasses that have been reported, and its hemicellulose content (23.6%) is consistent with other samples made from sugarcane bagasse and other biomasses that have been reported in the past. However, lignin (8.1%) comprises less of this raw material than all the other biomasses put together.
Because they boost the conversion of value-added products, sugarcane bagasse’s high cellulose and hemicellulose contents imply that it is the ideal raw material to use in the HTC process. It is anticipated that under the HTC conversion system’s operating conditions, cellulose and hemicellulose will hydrolyze to fermentable sugars. These sugars can then serve as precursors in other reaction mechanisms to produce chemical compounds with a broad range of applications, including furfural derivatives and organic acids.
This raw material’s low lignin content, when compared to the other structural components of the biomass and the lignin percentage in other samples mentioned in
Table 4, is a significant advantage in terms of the yield of products derived from cellulose and hemicellulose. It is well known that lignin is more resistant to heat breakdown; however, because it makes up a smaller portion of the structure, its impact on the conversion process is lessened. Similarly, the fragmentation of the sample and its reduced lignin content make it suitable for effective HTC conversion processes. This is because fragmentation increases the surface area of the particles, which in turn increases access to the structural components. Therefore, the milling process indirectly affects the conversion of the biomass’s structural components.
3.2. Hydrothermal Treatment of Biomass.
2.4.1. Aqueous Phase.
The Experiments Included Conductivity Monitoring, pH Measurement, and Particle Size Variation.
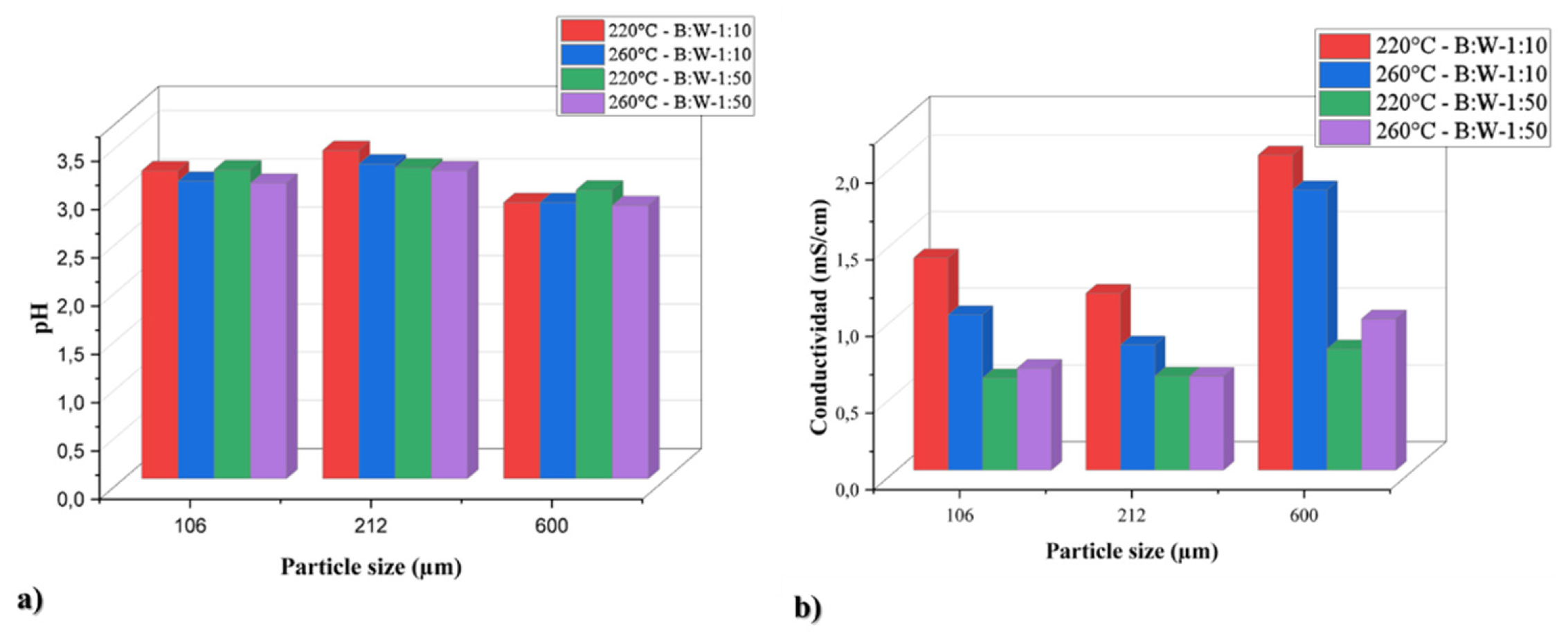
To monitor the evolution of the pertinent compounds generated in the aqueous phase, measurements of pH and conductivity were made. Consequently, a plot of the conductivity and pH results is displayed in
Figure 1. First, the difference in conductivity was found to be approximately one unit throughout the whole data set, and the variation in pH was found to be less than one unit based on the data displayed by the pH graph. Based on the findings, it was clear that sugarcane bagasse tends to produce more
ions at 600 μm than at 106 and 212 μm when compared to treated biomass. Similar trends could also be seen in the conductivity graph, where an increase in conductivity values was seen for reaction operating parameters carried out with a 600 μm size. Possibly this is since small particle sizes can favor different reaction mechanisms with the medium, such as polymerization due to the increase in the surface area of the particle, which leads to a lower formation of acidic species in the aqueous medium [
12,
28].
The experiments included conductivity monitoring, pH measurement, and particle size variation.
Experiments with B:W Ratio Variation, pH, and Conductivity Monitoring.
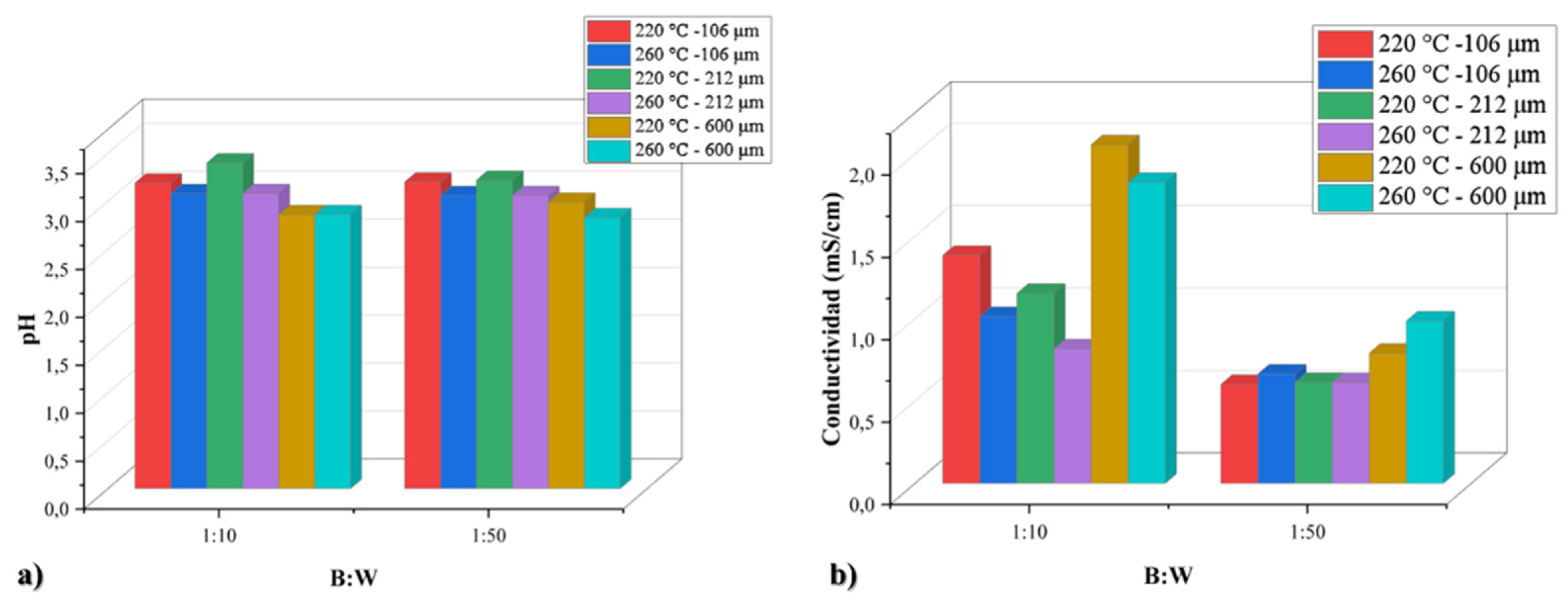
In
Figure 2, the variation of the concentration of acid species as a function of the B:W ratio was analyzed. (From the pH graph, numeral a). It can be seen that for the two B:W ratios (1:10 and 1:50), the conditions at which the highest concentrations of
acidic species are obtained are for particle sizes of 600 μm at temperatures of 220 and 260 °C. Likewise, in the measurement of conductivity (number b) in this same figure, it is found that for the two B:W ratios, the parameters that present higher conductivities are at the particle size of 600 μm and at a temperature of 220 °C in the ratio 1:10 and at 260 °C in the ratio 1:50.
Quantification of Platform Chemicals
The results of the yields of the platform chemicals obtained in the aqueous phase are recorded in
Table 3. In this table, the concentrations of the platform chemicals identified from an external standard calibration method using the standards: carbohydrates (glucose and xylose), chromic acid, levulinic acid, HMF, and furfural, were recorded to determine the total yield of the products in the aqueous phase.
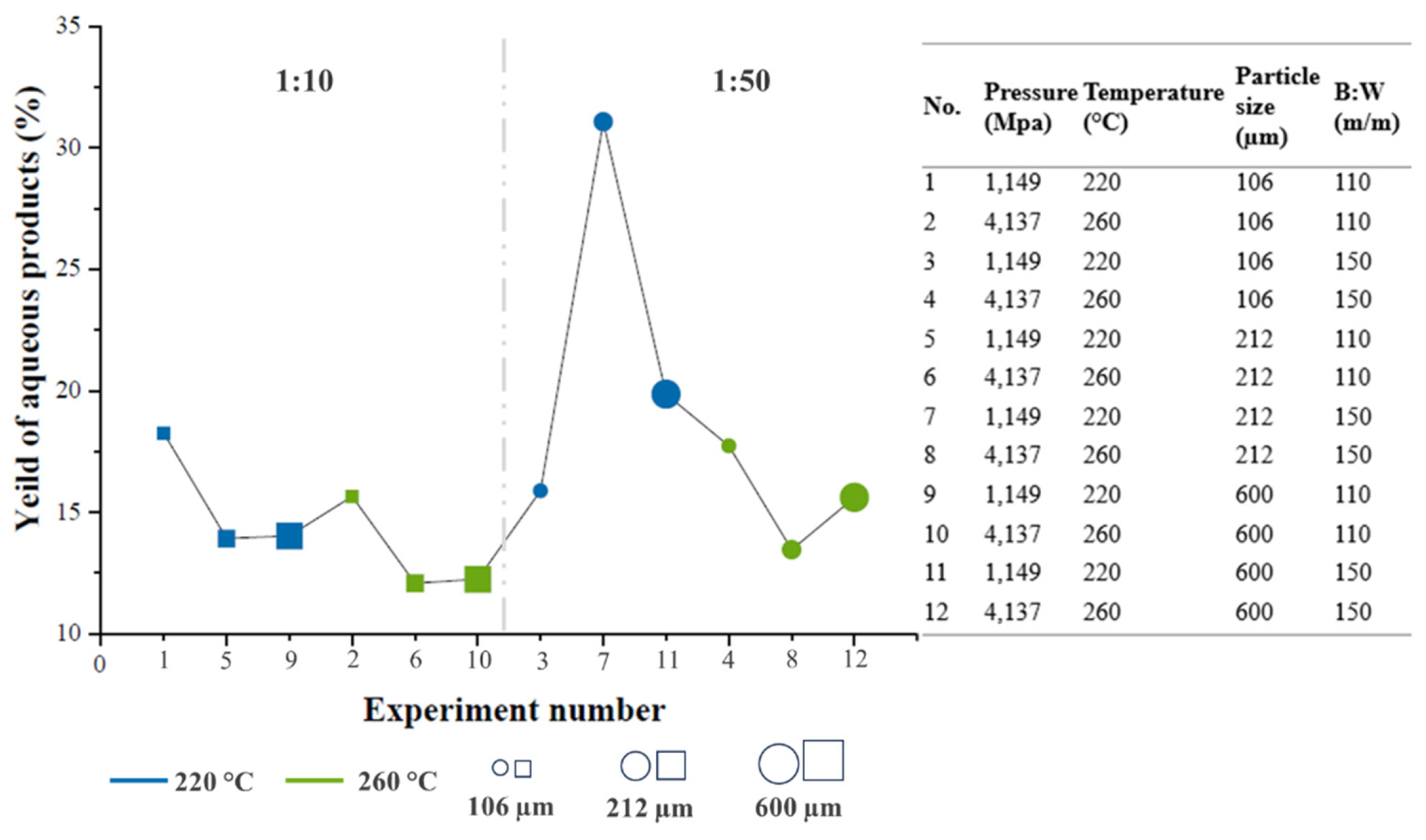
Figure 3 shows graphically the yields of the aqueous fractions, through which it was observed that in experiment 7, the best yield in weight of the aqueous products was obtained, being 31.073%. The experimental conditions in this trial were a B:W ratio of 1:50, a temperature of 220 °C, and a particle size of 212 μm (as presented in the Materials and Methods section). When comparing the effect of particle size on the results obtained, it was observed that the experiments performed at a B:W ratio of 1: 10, better yields were achieved when the particle size of 106 μm was used (18.25 and 15.65 % at 220 °C and at 260 °C, respectively); this is because smaller particle sizes undergo higher conversions since reducing the size of the biomass favors a greater diffusion of water in the biomass, since with the reduction of the size of the surface By which a faster decomposition of the lignocellulosic material occurs, resulting in a decrease in yields [
13,
29].
Likewise, in the ratio B:A 1:50 at 260 °C, it was observed that the particle size of 106 μm has a higher yield (17.73%) with respect to the other two particle sizes. On the contrary, in the experiments carried out with the B:W ratio of 1:50, it was evidenced that at 220 °C, the best yields were reached with the largest particle sizes, 212 and 600 μm, with respect to that of 106 μm (31.72; 19.85; 15.88%, respectively). When comparing the yields with respect to the B:W ratio, it was observed that in the 1:50 ratio, it tends to increase the yield with respect to the results obtained in the 1:10 ratio, since the amount of water is a key factor in guaranteeing the dispersion of the biomass in the reaction system, which results in more efficient processes [
14,
29]
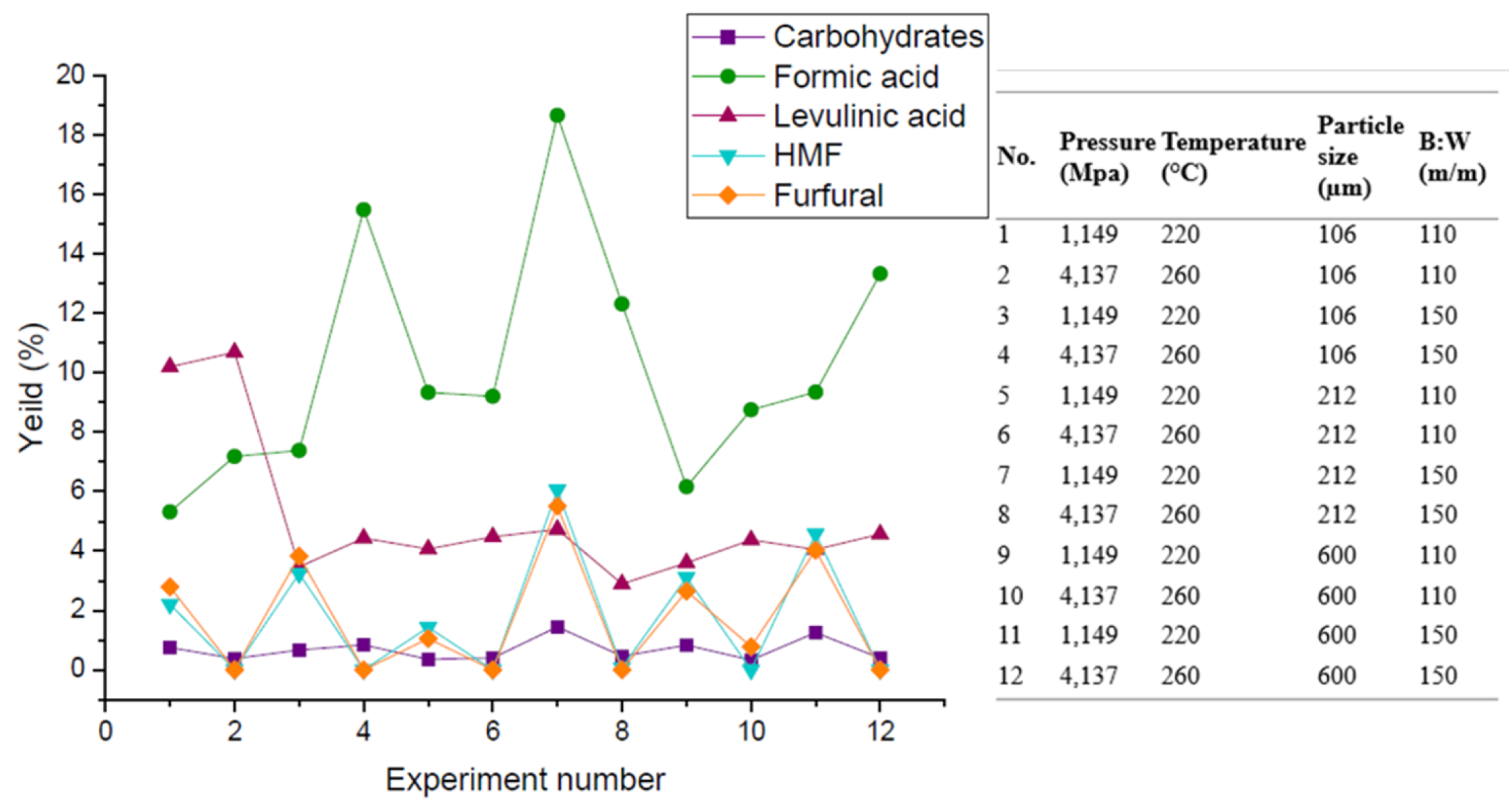
Also,
Figure 4 shows the percentage yield for each of the platform chemicals that constitute the total yield of the aqueous phase products. From the information provided by this graph, it was observed that formic acid is the chemical product with the highest formation from sugarcane bagasse under HTC conditions, followed by levulinic acid with respect to the other compounds. According to what has been reported by other studies, cellulose and hemicellulose in a hydrothermal system are known to hydrolyze at temperatures above 180 °C [
30]. However, when the reaction system reaches 220 °C, further decomposition of cellulose and hemicellulose occurs. This occurs through a hydrolysis reaction where the ester and ether bonds (mainly the O-glycosidic bond) are broken to produce soluble oligomers such as saccharides and monosaccharides. Sugarcane bagasse is characterized by containing mainly glucose, galactose, xylose, and arabinose [
31,
32].
2.4.1. Solid Phase (Hydrochar).
Infrared Spectroscopy
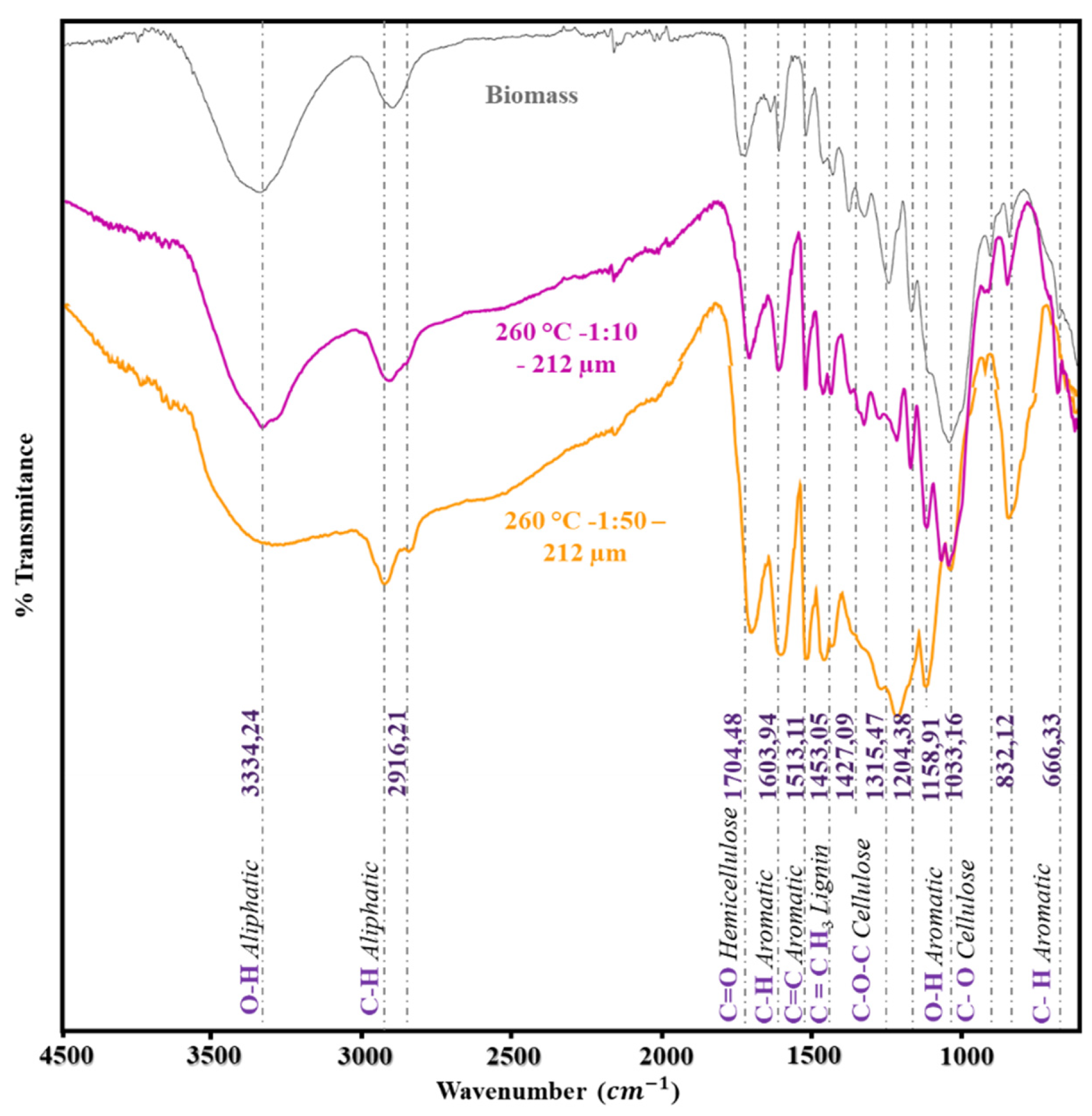
One analysis that was done to see structural alterations in the lignocellulosic material’s chemical composition was infrared spectroscopy. For this reason, the IR spectra of the untreated biomass and those found in the solid products of the hydrothermal treatment were compared in
Figure 5. The unaltered biomass is represented by the gray spectrum. Owing to the material’s heterogeneity, numerous distinguishing peaks of the bonds pertaining to the biopolymers that make up the lignocellulosic biomass were visible. Thus, the cellulose, hemicellulose, and lignin bond characteristic peaks were found in the fingerprint region and are as follows (cm
-1): 666.33 – 833.12 – 1033.16 – 1158.91 – 1204.38 – 1315.47 – 1427.09 – 1453.05 – 1513.11 – 1603.94 – 1704.48.
The 666.33 and 832.12 cm
-1 peaks may correspond to bending vibrations between the hydrogen-carbon bonds of the aromatic compounds of lignin, as well as vibrations of the (C-H) groups of the glycopyranose ring of cellulose and hemicellulose. The peaks at 1033.16 cm
1 are characteristic of the stretching vibration of the single (C-O) bonds present in the polysaccharides and oligosaccharides of cellulose and hemicellulose; these signals serve to describe the deformation of the (C-O) bonds of some primary alcohols and of the (C-H) bonds of polysaccharides and lignin [
33,
34]. The peak at 1158.91 cm
-1 describes the stretching vibrations of the glycosidic bonds in cellulose and hemicellulose (C-O-C). In addition, it is typical of the aliphatic bending of the (C-H) bond of the syringyl ring.
The absorption peak in the range of 1204.38 cm
-1 is caused by the stretching vibrations of the (C-O) bonds of aliphatic compounds, which may correspond to the oxygen-containing functional groups in cellulose and hemicellulose. The peak corresponding to wavenumber 1315.47 cm
-1 corresponds to the stretching vibration of the aryl alkyl ester groups of the syringyl and condensed guaiacyl rings present in lignin (O-CH
3); at 1427.09 and 1453.05 cm
-1 are the characteristic peaks of the asymmetric vibrations of aromatic groups of hemicellulose (CH, CH
2, and CH
3). At 1513.11 and 1603.94 cm
-1, these peaks may correspond to the vibrations and stretching of the bonds of the ketone groups of hemicellulose (C=O), as well as the vibrations of the bonds (C=C) belonging to the aromatic compounds. And at 1704.48 cm
-1, this region is distinctive of the bond stretches (C=O) of ketones, carbonyls, unconjugated ester groups, and xylan acetates of hemicellulose [
33,
35]. As for the 3334.24 and 2916.21 cm
-1 signals, the first signal is characteristic of aliphatic (O-H) hydroxyl bonds, while the other may correspond to (C-H) bond stretching of aliphatic hydrocarbons present in cellulose.
Comparing the spectra of ratio 1:10 y 1:50 in
Figure 5 to the untreated biomass levels reveals modifications to a few of the peaks. In this sequence of concepts, a minor deformation is discernible in the peak of 2916.21 cm
-1 upon comparing the biomass with the solid product of ratio 1:10. This could be the result of cellulose and hemicellulose’s C-H bonds breaking down. Similar variation is seen in ratio 1:50 signal, but with a more pointed peak. While suggests that dehydration during the hydrothermal process may be the cause of the peak’s broadening at 3334.21 cm
-1 [
36]. Conversely, that the peaks located at 1704.48, 1603.91, and approximately 832.12 cm
-1 appeared to be more intense than the unaltered biomass; based on the literature these peaks support a modification in the cellulose and hemicellulose structures, potentially as a result of degradation reactions.The experiment’s peaks 1513.11, 1427.09, 1204.38, and 1158.91 A greater modification in the peaks is observed, mainly in the 1:50 ratio, with respect to the unmodified biomass.
The peaks mentioned above are mainly associated with cellulose and hemicellulose, so it is inferred that degradation of these biopolymers occurs under HTC conditions [
12]. As for the 1453.05 cm
-1 peak, this peak is characteristic of lignin, so being sharper, it can be deduced that a possible lignin enrichment or hydrolysis of lignin occurs. The peak of 1315.47 cm
-1 disappears with the increase in the variation of the B:W ratio during the HTC reaction. Also, at the peak of 1204.38 cm
-1, the formation of a shoulder is evident. This is characteristic of the deformation of primary alcohols and the C-O stretching of the ether bond in lignin [
34,
37].
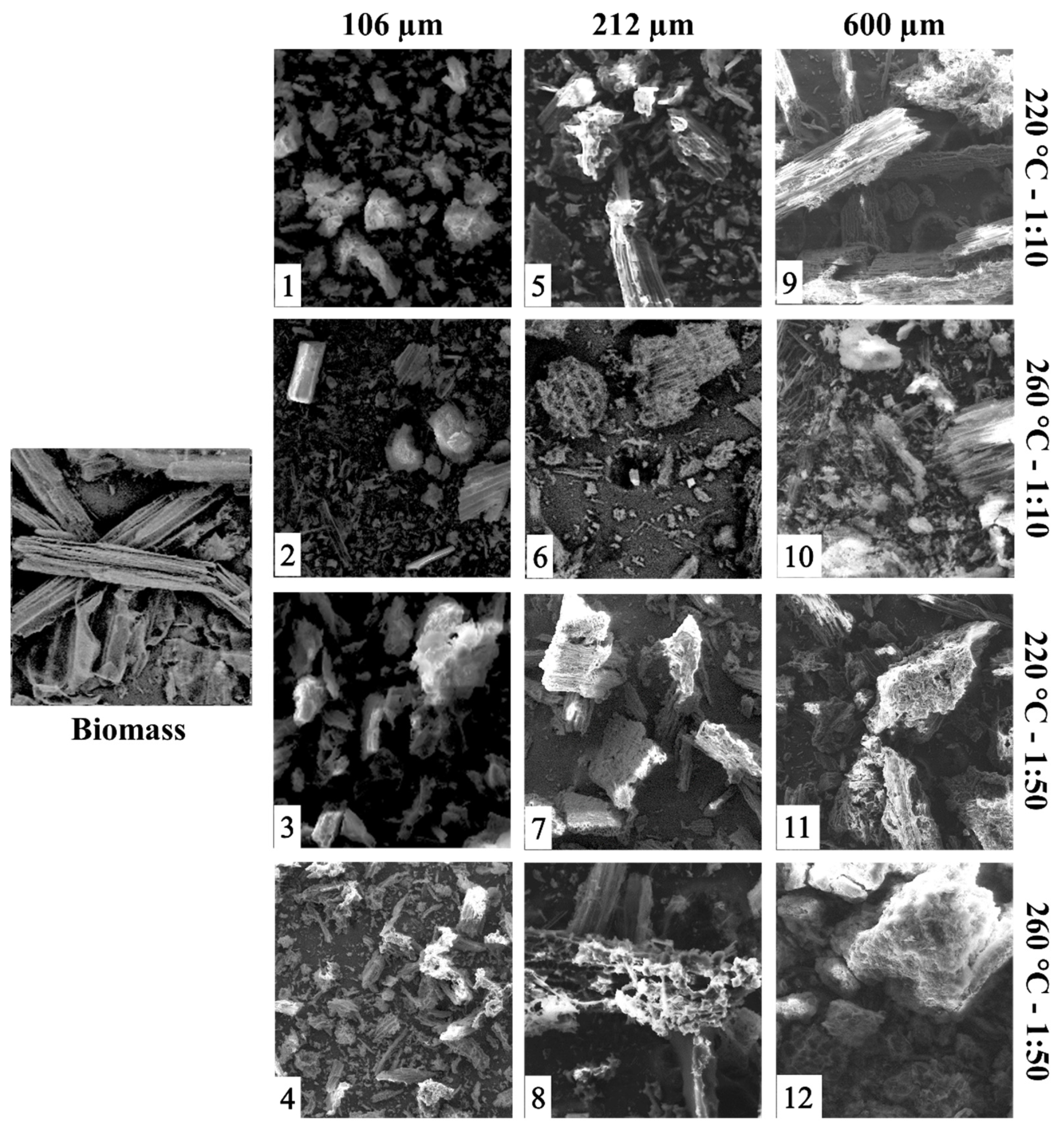
The Morphology of the Samples Microscopically.
SEM is used to observe the morphology of the samples microscopically.
Figure 6 shows the photographs of the 12 hydrocarbons obtained and compares them according to the variation of particle size and B:W ratio. The morphology of sugarcane bagasse before treatment is characterized by having a fibrous and compact structure [
38]. First, when comparing the hydrocarbons obtained by varying the particle sizes and maintaining the same operating conditions (B:W ratio and temperature), it was observed that when particle sizes were increased from 106 to 600 μm, hydrocarbons with more cracks and pores on the surface of the solid material were produced. It was also observed that most of the solids obtained at 260 °C had a more porous surface with respect to those at 220 °C. In addition, in the photographs of hydrocarbons 2, 6, and 10, some solid structures were identified. As reported in the literature, this may be due to a modification in the surface characteristics of the material during HTC conversion because chemical changes occur in the lignocellulosic structural composition of the biomass. Likewise, at high temperatures, mechanisms of polymerization of the monomers that constitute the lignocellulose and degradation of the compounds present in the aqueous phase have been associated with the formation of products with a high carbon content and a morphology similar to a microsphere [
38,
39].
With respect to the variation in the proportions of biomass to water within the reaction system, it was observed that the hydrocarbons obtained from the proportions 1:50 were characterized by having a surface with more porosities and cracks compared to those obtained in the proportions 1:10. This may be due to the characteristics of sugarcane bagasse, remembering that given its medullary and fibrovascular structure, it has the capacity to absorb and retain water. For this reason, a greater amount of water guarantees better heat and mass transfer in the reaction medium [
40].
Elemental Analysis.
From the information provided in Table 4-4, it could be observed that the biomass conversion by HTC treatment generated a greater amount of solid products when the working temperature was 260 °C. In the table, these data correspond to rows 2, 4, 6, 8, 10, and 12. From which it was observed that in the operating conditions of experiment 8, the ones that showed the best percentage of solid product conversion on a dry basis were 85.851%, and the operating conditions were: reaction temperature of 260 °C, 212 μm particle size, and a B:A ratio of 1:50.
Regarding elemental composition, it was observed that %C and %N of hydrocarbons increased with respect to the original biomass, while %O decreased. When evaluating the O/C ratio with respect to the untreated biomass, a decrease in the O/C ratio was observed. According to the Van Krevelen diagrams, it can be inferred that the material is carbonizing and is losing oxygen. Therefore, hydrocarbons obtained at higher temperatures have an O/C ratio similar to that of lignite. Therefore, hydrocarbons obtained at higher temperatures have an O/C ratio similar to that of lignite [
41,
42].
Table 6.
Elemental composition EDX-SEM of the hydrochars.
Table 6.
Elemental composition EDX-SEM of the hydrochars.
| Experiment number |
% |
O/C |
| C |
O |
N |
Other |
conversion by weight of solid product |
| Biomass |
44.22 |
45.56 |
0.292 |
|
|
1.030 |
| 1 |
68.605 |
25.655 |
5.140 |
Si=0.39; F=0.21 |
49.592 |
0.375 |
| 2 |
70.255 |
20.335 |
68.605 |
Si=4.76 |
59.132 |
0.297 |
| 3 |
61.965 |
33.165 |
4.420 |
F=0.45 |
52.921 |
0.536 |
| 4 |
77.565 |
15.975 |
6.465 |
|
72.187 |
0.206 |
| 5 |
68.520 |
25.890 |
5.465 |
Si=0.12 |
56.712 |
0.380 |
| 6 |
74.920 |
10.730 |
3.650 |
|
58.490 |
0.142 |
| 7 |
64.110 |
27.330 |
9.235 |
|
65.860 |
0.426 |
| 8 |
74.310 |
19.540 |
6.150 |
|
85.851 |
0.263 |
| 9 |
67.540 |
27.345 |
5.115 |
|
51.196 |
0.405 |
| 10 |
74.180 |
19.570 |
6.250 |
|
62.759 |
0.264 |
| 11 |
63.200 |
31.825 |
4.985 |
|
57.454 |
0.504 |
| 12 |
75.865 |
17.910 |
6.225 |
|
74.213 |
0.236 |
4. Conclusions
According to the results obtained, it can be inferred that the conditions that can most favor the formation of platform chemicals are: Experiment 9 and 10, 220 and 260 °C, 600 μm, B:W 1:10, pH 2.86, 2.056, and 1.828 mS/cm. Similarly, the solid phase was monitored by IR spectroscopy, which showed that at 260 °C, the structural composition of the sugarcane bagasse showed a greater degradation of the lignocellulosic components. With respect to the B:W ratio, a greater effect of the decomposition pattern was observed using the 1:50 ratio, while when evaluating the effect on particle size, it is evident that this parameter has a different tendency for each of the biomass and water ratios.
By HPLC-RI chromatographic analysis, it was determined that the most appropriate conditions for obtaining platform chemicals were at a biomass: water ratio 1:50, temperature 220 °C, particle size 212 μm, for a yield of 31.073% on a dry basis of aqueous products. The platform chemicals identified in the aqueous fractions were carbohydrates (glucose, xylose), formic acid, levulinic acid, HMF, and furfural. Formic acid was the platform chemical with the highest yield, 15.926% by weight on a dry basis. Regarding the solid product, it was evidenced that the hydrocarbons that presented greater porosities and cracks on the surface of the solid were those obtained from the biomass at a size of 600 μm, with a reaction temperature of 260 °C, and with a B:A ratio of 1:50. And in the elemental composition of the hydrocarbons, it was evidenced that the carbon content increased with respect to the untreated biomass while the amount of oxygen decreased. By observing the decrease in the O/C ratio, it can be inferred that, through different reaction mechanisms, the solid products are carbonizing.
Author Contributions
Conceptualization, L.N.M.-C; methodology, L.N.M.-C and A.S.L.-P.; validation, L.N.M.-C and A.S.L.-P; formal analysis L.N.M.-C and A.S.L.-P.; investigation, L.N.M.-C and A.S.L.-P; resources, C.A.G.-F.; data curation, L.N.M.-C and A.S.L.-P.; writing— original draft preparation, L.N.M.-C and A.S.L.-P.; writing—review and editing, C.A.G.-F.; supervision, C.A.G.-F.; project administration, C.A.G.-F.; funding acquisition, A.S.L.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MINCIENCIAS, Contrato de financiamiento de recuperación contingente No. 80740-101-2022
Data Availability Statement
Data are contained within the article
Acknowledgments
We thank the Universidad Nacional de Colombia and the Departamento de Química for their support and the possibility of using the equipment and techniques that allowed for the development of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FINAGRO, “Crecimiento del sector agropecuario y AgroExpo 2023, un reto hacia el desarrollo del campo | Finagro.” Accessed: Dec. 07, 2023. [Online]. Available: https://www.finagro.com.co/noticias/articulos/crecimiento-del-sector-agropecuario-agroexpo-2023-reto-desarrollo-del-campo-0.
- DANE, “Boletín Encuesta Nacional Agropecuaria 2019,” DANE.
- DANE, “DANE - PIB Información técnica,” DANE. Accessed: Dec. 07, 2023. [Online]. Available: https://www.dane.gov.co/index.php/estadisticas-por-tema/cuentas-nacionales/cuentas-nacionales-trimestrales/pib-informacion-tecnica.
- DANE, “Boletín mensual INSUMOS Y FACTORES ASOCIADOS A LA PRODUCCIÓN AGROPECUARIA,” 2020. Accessed: Nov. 06, 2021. [Online]. Available: http://www.agronet.gov.co/.
- DANE, “Un camino para la inclusión, la equidad y el reconocimiento.” Accessed: Dec. 08, 2023. [Online]. Available: https://www.mineducacion.gov.co/1759/articles-362822_recurso.pdf.
- J. Felipe and L. Bustamante, “LA CAÑA DE AZUCAR (Saccharum officinarum) PARA LA PRODUCCIÓN DE PANELA. CASO: NORDESTE DEL DEPARTAMENTO DE ANTIOQUIA,” 2015.
- Minagricultura, “Cadena Agroindustrial de la panela,” Colombia, 2019. Accessed: Jan. 02, 2022. [Online]. Available: https://sioc.minagricultura.gov.co/Panela/Documentos/2019-12-30 Cifras Sectoriales.pdf.
- H. S. Kambo and A. Dutta, “A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications,” Renewable and Sustainable Energy Reviews, vol. 45, pp. 359–378, May 2015. [CrossRef]
- A. A. Peterson, F. Vogel, R. P. Lachance, M. Fröling, M. J. Antal, Jr., and J. W. Tester, “Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies,” Energy Environ Sci, vol. 1, no. 1, p. 32, Jul. 2008. [CrossRef]
- Y. Shen, “A review on hydrothermal carbonization of biomass and plastic wastes to energy products,” Biomass Bioenergy, vol. 134, p. 105479, Mar. 2020. [CrossRef]
- X. Zhuang et al., “Insights into the evolution of chemical structures in lignocellulose and non-lignocellulose biowastes during hydrothermal carbonization (HTC),” Fuel, vol. 236, pp. 960–974, Jan. 2019. [CrossRef]
- C. J. Coronella, J. G. Lynam, M. T. Reza, and M. H. Uddin, “Hydrothermal Carbonization of Lignocellulosic Biomass,” pp. 275–311, 2014. [CrossRef]
- M. Heidari, A. Dutta, B. Acharya, and S. Mahmud, “A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion,” Journal of the Energy Institute, vol. 92, no. 6, pp. 1779–1799, Dec. 2019. [CrossRef]
- T. A. H. Nguyen, T. H. Bui, W. S. Guo, and H. H. Ngo, “Valorization of the aqueous phase from hydrothermal carbonization of different feedstocks: Challenges and perspectives,” Chemical Engineering Journal, vol. 472, p. 144802, Sep. 2023. [CrossRef]
- J. Fang, L. Zhan, Y. S. Ok, and B. Gao, “Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass,” Journal of Industrial and Engineering Chemistry, vol. 57, pp. 15–21, Jan. 2018. [CrossRef]
- A. Rehman et al., “A focused review on lignocellulosic biomass-derived porous carbons for effective pharmaceuticals removal: Current trends, challenges and future prospects,” Sep Purif Technol, vol. 330, p. 125356, Feb. 2024. [CrossRef]
- A. O. Onokwai, E. S. A. Ajisegiri, I. P. Okokpujie, R. A. Ibikunle, M. Oki, and J. O. Dirisu, “Characterization of lignocellulose biomass based on proximate, ultimate, structural composition, and thermal analysis,” Mater Today Proc, vol. 65, pp. 2156–2162, 2022. [CrossRef]
- E. M. A. Edreis, G. Luo, and H. Yao, “Investigations of the structure and thermal kinetic analysis of sugarcane bagasse char during non-isothermal CO2 gasification,” J Anal Appl Pyrolysis, vol. 107, pp. 107–115, May 2014. [CrossRef]
- A. A. Castro Vega, “Estudio de la naturaleza química de biocrudos obtenidos mediante licuefacción hidrotérmica de biomasa lignocelulósica,” 2011. Accessed: Dec. 08, 2021. [Online]. Available: https://repositorio.unal.edu.co/handle/unal/8651.
- A. A. Vega Castro, L. I. Rodríguez Varela, and J. Díaz Velásquez, “Subcritical hydrothermal conversion of organic wastes and biomass. Reaction pathways,” ABRIL DE, vol. 27, no. 1, pp. 41–50, 2007.
- C. Qian, Q. Li, Z. Zhang, X. Wang, J. Hu, and W. Cao, “Prediction of higher heating values of biochar from proximate and ultimate analysis,” Fuel, vol. 265, p. 116925, Apr. 2020. [CrossRef]
- A. O. Ayeni, O. A. Adeeyo, O. M. Oresegun, and T. E. Oladimeji, “Compositional analysis of lignocellulosic materials: Evaluation of an economically viable method suitable for woody and non-woody biomass,” American Journal of Engineering Research, 2015, Accessed: Jan. 14, 2024. [Online]. Available: www.ajer.org.
- P. Zhang, W. Liao, A. Kumar, Q. Zhang, and H. Ma, “Characterization of sugarcane bagasse ash as a potential supplementary cementitious material: Comparison with coal combustion fly ash,” J Clean Prod, vol. 277, p. 123834, Dec. 2020. [CrossRef]
- R. Khatami, C. Stivers, K. Joshi, Y. A. Levendis, and A. F. Sarofim, “Combustion behavior of single particles from three different coal ranks and from sugar cane bagasse in O2/N2 and O2/CO2 atmospheres,” Combust Flame, vol. 159, no. 3, pp. 1253–1271, Mar. 2012. [CrossRef]
- A. O. Onokwai, I. P. Okokpujie, E. S. Ajisegiri, M. Oki, A. O. Adeoyeb, and E. T. Akinlabi, “Characterization of Lignocellulosic Biomass Samples in Omu-Aran Metropolis, Kwara State, Nigeria, as Potential Fuel for Pyrolysis Yields,” International Journal of Renewable Energy Development, vol. 11, no. 4, pp. 973–981, Nov. 2022. [CrossRef]
- G. Hincapié, A. Soto, and D. López, ‘Pre-tratamiento ácido y básico de bagazo de caña y de compuestos modelo para la producción de bio-aceite vía licuefacción hidrotérmica,’ Energética, vol. 47, pp. 23–30, Jan. 2016, Accessed: Jan. 14, 2024. [Online]. Available: https://repositorio.unal.edu.co/handle/unal/64263.
- S. Yao, S. Nie, Y. Yuan, S. Wang, and C. Qin, “Efficient extraction of bagasse hemicelluloses and characterization of solid remainder,” Bioresour Technol, vol. 185, pp. 21–27, Jun. 2015. [CrossRef]
- D. Wüst, C. R. Correa, D. Jung, M. Zimmermann, A. Kruse, and L. Fiori, “Understanding the influence of biomass particle size and reaction medium on the formation pathways of hydrochar,” Biomass Convers Biorefin, vol. 10, no. 4, pp. 1357–1380, Dec. 2020. [CrossRef]
- M. Heidari, S. Salaudeen, A. Dutta, and B. Acharya, “Effects of Process Water Recycling and Particle Sizes on Hydrothermal Carbonization of Biomass,” Energy & Fuels, vol. 32, no. 11, pp. 11576–11586, Nov. 2018. [CrossRef]
- Y. Zhou, J. Remón, X. Pang, Z. Jiang, H. Liu, and W. Ding, “Hydrothermal conversion of biomass to fuels, chemicals and materials: A review holistically connecting product properties and marketable applications,” Science of The Total Environment, vol. 886, p. 163920, Aug. 2023. [CrossRef]
- M. Usman et al., “Characterization and utilization of aqueous products from hydrothermal conversion of biomass for bio-oil and hydro-char production: a review,” Green Chemistry, vol. 21, no. 7, pp. 1553–1572, Apr. 2019. [CrossRef]
- Y. Zhou, M. Li, Y. Chen, and C. Hu, “Conversion of polysaccharides in Ulva prolifera to valuable chemicals in the presence of formic acid,” J Appl Phycol, vol. 33, no. 1, pp. 101–110, Feb. 2021. [CrossRef]
- K. Krysanova, A. Krylova, M. Kulikova, A. Kulikov, and O. Rusakova, “Biochar characteristics produced via hydrothermal carbonization and torrefaction of peat and sawdust,” Fuel, vol. 328, p. 125220, Nov. 2022. [CrossRef]
- R. Zhu and V. Yadama, “Effects of hot water extraction pretreatment on physicochemical changes of Douglas fir,” Biomass Bioenergy, vol. 90, pp. 78–89, Jul. 2016. [CrossRef]
- T. M. Sabry, S. A. E.-H. El-Korashy, H. E. S. Jahin, G. M. Khairy, and N. F. A. Aal, “Hydrothermal carbonization of Calotropis procera leaves as a biomass: Preparation and characterization,” J Mol Struct, vol. 1302, p. 137397, Apr. 2024. [CrossRef]
- M. Sevilla and A. B. Fuertes, “The production of carbon materials by hydrothermal carbonization of cellulose,” Carbon N Y, vol. 47, no. 9, pp. 2281–2289, Aug. 2009. [CrossRef]
- Y. H. Ju, L. H. Huynh, N. S. Kasim, T. J. Guo, J. H. Wang, and A. E. Fazary, “Analysis of soluble and insoluble fractions of alkali and subcritical water treated sugarcane bagasse,” Carbohydr Polym, vol. 83, no. 2, pp. 591–599, Jan. 2011. [CrossRef]
- D. A. Iryani, S. Kumagai, M. Nonaka, K. Sasaki, and T. Hirajima, “Characterization and Production of Solid Biofuel from Sugarcane Bagasse by Hydrothermal Carbonization,” Waste Biomass Valorization, vol. 8, no. 6, pp. 1941–1951, Sep. 2017. [CrossRef]
- L. Mendoza Martinez et al., “Hydrothermal carbonization of lignocellulosic agro-forest based biomass residues,” Biomass Bioenergy, vol. 147, p. 106004, Apr. 2021. [CrossRef]
- K. O. Iwuozor, E. Chizitere Emenike, J. O. Ighalo, F. O. Omoarukhe, P. E. Omuku, and A. George Adeniyi, “A Review on the thermochemical conversion of sugarcane bagasse into biochar,” Cleaner Materials, vol. 6, p. 100162, Dec. 2022. [CrossRef]
- S. A. Nicolae et al., “Recent advances in hydrothermal carbonisation: from tailored carbon materials and biochemicals to applications and bioenergy,” Green Chemistry, vol. 22, no. 15, pp. 4747–4800, Aug. 2020. [CrossRef]
- M. Sevilla and A. B. Fuertes, “The production of carbon materials by hydrothermal carbonization of cellulose,” Carbon N Y, vol. 47, no. 9, pp. 2281–2289, Aug. 2009. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).