Submitted:
20 February 2024
Posted:
20 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Total Phenolics and Oil Content
2.2. Phenolic Compounds Profiles
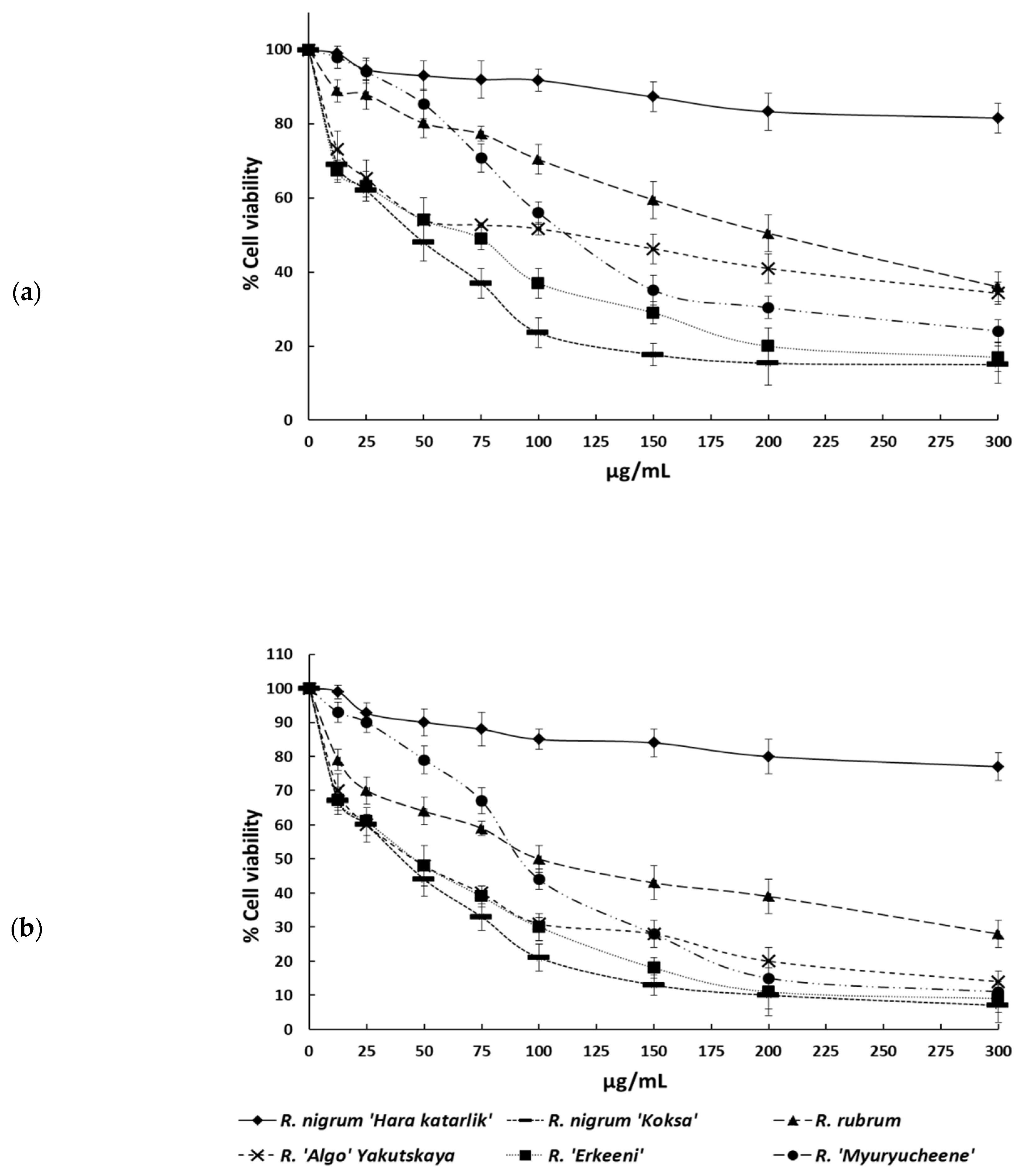
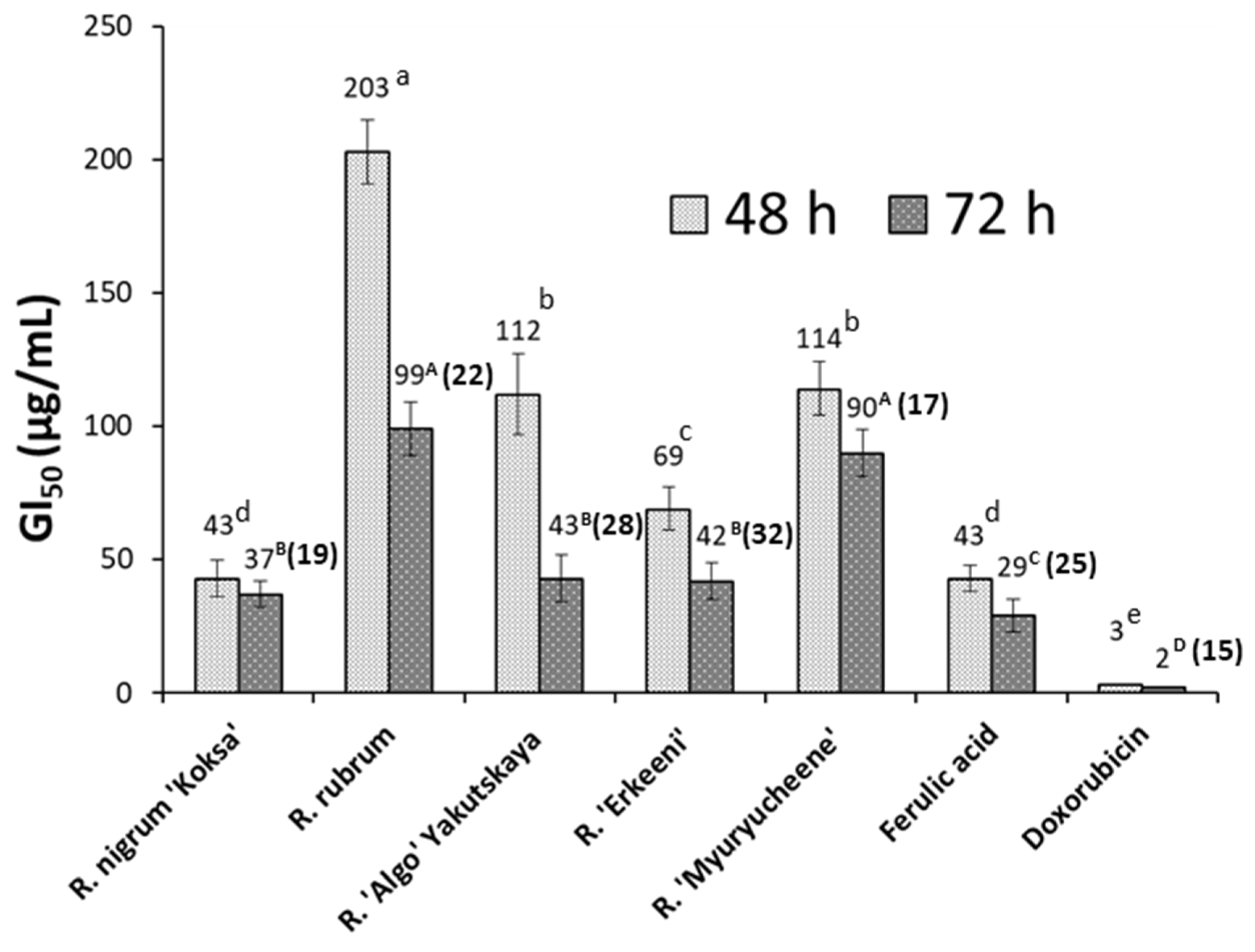
2.3. Antiproliferative Activity of the Water:Methanol Seed Extracts on HT-29 Cancer Cells
Conclusions
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Plant Material
3.3. Seed oil Extraction
3.4. Extraction of Phenolics from Ribes Seeds
3.5. Determination of Total Phenol Content
3.6. Characterization of Phenolics by Liquid Chromatography-Mass Spectrometry
3.7. Cell assays on Cancer and Normal Cell Lines
3.8. Statistical Analysis
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AOAC (2000). Fat (crude) in nuts and nut products. Gravimetric method (AOAC Official Method 948.22) W. Horwitz (Ed.), Officials methods of analysis of AOAC International (17th ed.), AOAC, Gaithersburg, MD.
- Bakowska-Barczak, A. M., Schieber, A., & Kolodziejczyk, P. (2009). Characterization of Canadian black currant (Ribes nigrum L.) seed oils and residues. Journal of agricultural and food chemistry, 57, 11528-11536. [CrossRef]
- Behbahani, M., Sayedipour, S., Pourazar, A., & Shanehsazzadeh, M. (2014). In vitro anti-HIV-1 activities of kaempferol and kaempferol-7-O-glucoside isolated from Securigera securidaca. Research in pharmaceutical sciences, 9, 463.
- Brennan, R. M. (1996). Currants and Gooseberries. Chapter 3 pp. 191-295 in: J. Janick and J. N. Moore (eds.) Fruit Breeding, Vol. II Vine and Small Fruit Crops. John Wiley & Sons. Inc. N.Y.
- Brennan, J., & Graham, J. (2009). Improving Fruit Quality in Rubus and Ribes Through Breeding. Functional Plant Science and Biotechnology, 3 (1), 22-29.
- Chelh, T. C., Lyashenko, S., Lahlou, A., Belarbi, E. H., Rincón-Cervera, M. Á., Rodríguez-García, I., ... & Guil-Guerrero, J. L. (2022). Buglossoides spp. seeds, a land source of health-promoting n-3 PUFA and phenolic compounds. Food Research International, 157, 111421. [CrossRef]
- https://doi.org/10.1016/j.foodres.2022.111421. [CrossRef]
- Chernonosov, A. A., Karpova, E. A., & Lyakh, E. M. (2017). Identification of phenolic compounds in Myricaria bracteata leaves by high-performance liquid chromatography with a diode array detector and liquid chromatography with tandem mass spectrometry. Revista Brasileira de Farmacognosia, 27, 576-579. [CrossRef]
- Dantas, C. A. G., Abreu, L. S., da Cunha, H. N., Veloso, C. A. G., Souto, A. L., de Fatima Agra, M., ... & Tavares, J. F. (2021). Dereplication of phenolic derivatives of three Erythroxylum species using liquid chromatography coupled with ESI-MSn and HRESIMS. Phytochemical Analysis, 32, 1011-1026. [CrossRef]
- Dos Santos, C., Galaverna, R. S., Angolini, C. F., Nunes, V. V., De Almeida, L. F., Ruiz, A. L., ... & Eberlin, M. N. (2018). Antioxidative, antiproliferative and antimicrobial activities of phenolic compounds from three Myrcia species. Molecules, 23, 986. [CrossRef]
- https://doi.org/10.3390/molecules23050986. [CrossRef]
- Fabre, N., Rustan, I., de Hoffmann, E., & Quetin-Leclercq, J. (2001). Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. Journal of the American society for mass spectrometry, 12, 707-715. [CrossRef]
- Fabrikov, D., Guil-Guerrero, J. L., González-Fernández, M. J., Rodríguez-García, I., Gómez-Mercado, F., Urrestarazu, M., ... & Lyashenko, S. (2019). Borage oil: Tocopherols, sterols and squalene in farmed and endemic-wild Borago species. Journal of Food Composition and Analysis, 83, 103299. [CrossRef]
- https://doi.org/10.1016/j.jfca.2019.103299. [CrossRef]
- Golovenko, E., Lyashenko, S., Akimova, S., Mitina, L., Mulenkova, E., Belarbi, E. H., & Guil-Guerrero, J. L. (2021). Gamma-linolenic acid from fifty-seven Ribes species and cultivars. Plant Foods for Human Nutrition, 76, 385-393. [CrossRef]
- Granato, D., Fidelis, M., Haapakoski, M., dos Santos Lima, A., Viil, J., Hellström, J., ... & Pap, N. (2022). Enzyme-assisted extraction of anthocyanins and other phenolic compounds from blackcurrant (Ribes nigrum L.) press cake: From processing to bioactivities. Food Chemistry, 391, 133240. [CrossRef]
- https://doi.org/10.1016/j.foodchem.2022.133240. [CrossRef]
- Jiménez-Aspee, F., Thomas-Valdés, S., Schulz, A., Ladio, A., Theoduloz, C., & Schmeda-Hirschmann, G. (2015). Antioxidant activity and phenolic profiles of the wild currant Ribes magellanicum from Chilean and Argentinean Patagonia. Food Science & Nutrition, 4(4), 595–610. [CrossRef]
- Jurgoński, A., Fotschki, B., & Juśkiewicz, J. (2015). Disparate metabolic effects of blackcurrant seed oil in rats fed a basal and obesogenic diet. European Journal of Nutrition, 54, 991–999. [CrossRef]
- Kapasakalidis, P. G., Rastall, R. A., & Gordon, M. H. (2006). Extraction of polyphenols from processed black currant (Ribes nigrum L.) residues. Journal of agricultural and food chemistry, 54, 4016-4021. [CrossRef]
- Liu, B., & Li, Z. (2016). Black currant (Ribes nigrum L.) extract induces apoptosis of MKN-45 and TE-1 cells through MAPK-and PI3K/Akt-mediated mitochondrial pathways. Journal of medicinal food, 19, 365-373. [CrossRef]
- Lyashenko, S., González-Fernández, M. J., Gomez-Mercado, F., Yunusova, S., Denisenko, O., & Guil-Guerrero, J. L. (2019). Ribes taxa: A promising source of γ-linolenic acid-rich functional oils. Food chemistry, 301, 125309. [CrossRef]
- Lyashenko, S., Fabrikov, D., González-Fernández, M. J., Gómez-Mercado, F., Ruiz, R. L., Fedorov, A., ... & Guil-Guerrero, J. L. (2021). Phenolic composition and in vitro antiproliferative activity of Borago spp. seed extracts on HT-29 cancer cells. Food Bioscience, 42, 101043. [CrossRef]
- Ma, Y. L., Li, Q. M., Van den Heuvel, H., & Claeys, M. (1997). Characterization of flavone and flavonol aglycones by collision-induced dissociation tandem mass spectrometry. Rapid communications in mass spectrometry, 11, 1357-1364. [CrossRef]
- https://doi.org/10.1002/(SICI)1097-0231(199708)11:12<1357::AID-RCM983>3.0.CO;2-9. [CrossRef]
- March, R. E., & Miao, X. S. (2004). A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. International Journal of Mass Spectrometry, 231, 157-167. [CrossRef]
- Marcum, C. L. (2016). Fundamental studies of collision-activated dissociation (CAD) of deprotonated model compounds relevant to lignin degradation products (Doctoral dissertation, Purdue University).
- Mikulic-Petkovsek, M., Rescic, J., Schmitzer, V., Stampar, F., Slatnar, A., Koron, D., & Veberic, R. (2015). Changes in fruit quality parameters of four Ribes species during ripening. Food Chemistry, 173, 363-374. [CrossRef]
- Peña-Morán, O. A., Villarreal, M. L., Álvarez-Berber, L., Meneses-Acosta, A., & Rodríguez-López, V. (2016). Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules, 21, 1013. [CrossRef]
- Ramos-Bueno, R. P., González-Fernández, M. J., & Guil-Guerrero, J. L. (2016). Various acylglycerols from common oils exert different antitumor activities on colorectal cancer cells. Nutrition and Cancer, 68, 518-529. [CrossRef]
- Schulz, E., Tohge, T., Zuther, E., Fernie, A. R., & Hincha, D. K. (2016). Flavonoids are determinants of freezing tolerance and cold acclimation in Arabidopsis thaliana. Scientific reports, 6, 34027. [CrossRef]
- https://doi.org/10.1038/srep34027. [CrossRef]
- Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in enzymology (Vol. 299, pp. 152-178). Academic press. [CrossRef]
- Śliwka-Kaszyńska, M., Anusiewicz, I., & Skurski, P. (2022). The Mechanism of a Retro-Diels–Alder Fragmentation of Luteolin: Theoretical Studies Supported by Electrospray Ionization Tandem Mass Spectrometry Results. Molecules, 27, 1032. [CrossRef]
- Tuli, H. S., Garg, V. K., Bhushan, S., Uttam, V., Sharma, U., Jain, A., ... & Sethi, G. (2023). Natural flavonoids exhibit potent anticancer activity by targeting microRNAs in cancer: A signature step hinting towards clinical perfection. Translational Oncology, 27, 101596. [CrossRef]
- https://doi.org/10.1016/j.tranon.2022.101596. [CrossRef]
- Van Hoed, V., Barbouche, I., De Clercq, N., Dewettinck, K., Slah, M., Leber, E., & Verhé, R. (2011). Influence of filtering of cold pressed berry seed oils on their antioxidant profile and quality characteristics. Food Chemistry, 127, 1848-1855. [CrossRef]
- Vichitsakul K, Laowichuwakonnukul K, Soontornworajit B, Poomipark N, Itharat A, & Rotkrua P. (2023). Anti-proliferation and induction of mitochondria-mediated apoptosis by Garcinia hanburyi resin in colorectal cancer cells. Heliyon 22, e16411. [CrossRef]
- Wójciak, M., Mazurek, B., Tyśkiewicz, K., Kondracka, M., Wójcicka, G., Blicharski, T., & Sowa, I. (2022). Blackcurrant (Ribes nigrum L.) seeds—a valuable byproduct for further processing. Molecules, 2, 8679. [CrossRef]
- Yang, B., Zheng, J., Laaksonen, O., Tahvonen, R., & Kallio, H. (2013). Effects of latitude and weather conditions on phenolic compounds in currant (Ribes spp.) cultivars. Journal of Agricultural and Food Chemistry, 61, 3517-3532. [CrossRef]
- Yang, Y., Lei, Z., Zhao, M., Wu, C., Wang, L., & Xu, Y. (2020). Microwave-assisted extraction of an acidic polysaccharide from Ribes nigrum L.: Structural characteristics and biological activities. Industrial Crops and Products, 147, 112249. [CrossRef]
| Code | Samples | Sample location | Total oil content g/100 g seeds |
TPC (mg CAE/g seeds)abc |
TPC (mg CAE/g oil)abc |
| Subgenus Ribes (Currants) | |||||
| Sect. Berisia Spach (Alpine currants) | |||||
| 1A | R. alpinum | Sukachev Institute of Forest of the Siberian Branch of the RAS, Krasnoyarsk, Russia | 19.9±0.5b | 36.9±1.8d | 7.3±0.3e |
| 1B | R. alpinum | Sierra de Baza, Granada, Spain | 12.7±0.4f | 33.4±0.9de | 4.2±0.1hi |
| 2 | R. pulchellum | Sukachev Institute of Forest of the Siberian Branch of the RAS, Krasnoyarsk, Russia | 23.0±1.0a | 34.2±1.2de | 7.9±0.2de |
| Sect. Coreosma (Spach) Jancz. (Black Currants) | |||||
| 3 | R. dikuscha | Botanical Garden of North-Eastern Federal University, Yakutsk, Russia | 17.8±0.2c | 30.5±2.4e | 5.4±0.0g |
| 4 | R. hudsonianum | Botanical Garden of North-Eastern Federal University, Yakutsk, Russia | 25.6±0.8a | 46.1±3.2c | 11.8±0.1b |
| 5A | R. nigrum ‘Hara katarlik’ | Botanical Garden of North-Eastern Federal University, Yakutsk, Russia | 18.4±0.1b | 53.4±2.5b | 9.8±0.2c |
| 5B | R. nigrum ‘Koksa’ | Botanical Garden of North-Eastern Federal University, Yakutsk, Russia | 16.3±0.0de | 94.8±3.4a | 15.5±0.1a |
| 6 | R. ‘Algo’ Yakutskaya | Botanical Garden of North-Eastern Federal University, Yakutsk, Russia | 17.0±0.3cd | 48.9±2.8bc | 8.3±0.2d |
| 7 | Ribes ‘Erkeeni’ | Botanical Garden of North-Eastern Federal University, Yakutsk, Russia | 18.3±0.2bc | 49.0±2.6b | 9.0±0.2cd |
| 8 | Ribes ‘Myuryucheene’ | Botanical Garden of North-Eastern Federal University, Yakutsk, Russia | 17.7±0.6c | 34.4±1.9de | 6.1±0.4f |
| Sect. Ribes (Red Currants) | |||||
| 9 | R. glabellum | Botanical Garden of North-Eastern Federal University, Yakutsk, Russia | 14.9±0.4e | 30.8±2.0e | 4.6±0.2h |
| 10 | R. triste | Dendropark “Alexandria” NAS of Ukraine, Belaja Tserkov, Ukraine | 18.5±0.5bc | 31.2±2.9e | 5.8±0.3fg |
| 11 | R. rubrum | Botanical Garden of North-Eastern Federal University, Yakutsk, Russia | 15.0±0.2e | 25.8±3.1f | 3.9±0.1i |
| aData represent means ± standard deviation of samples analyzed in triplicate; bDifferences in TPC amounts were tested according to one-way ANOVA followed by Duncan’s test; c Within a column, means followed by different letter are significantly different at P<0.05. | |||||
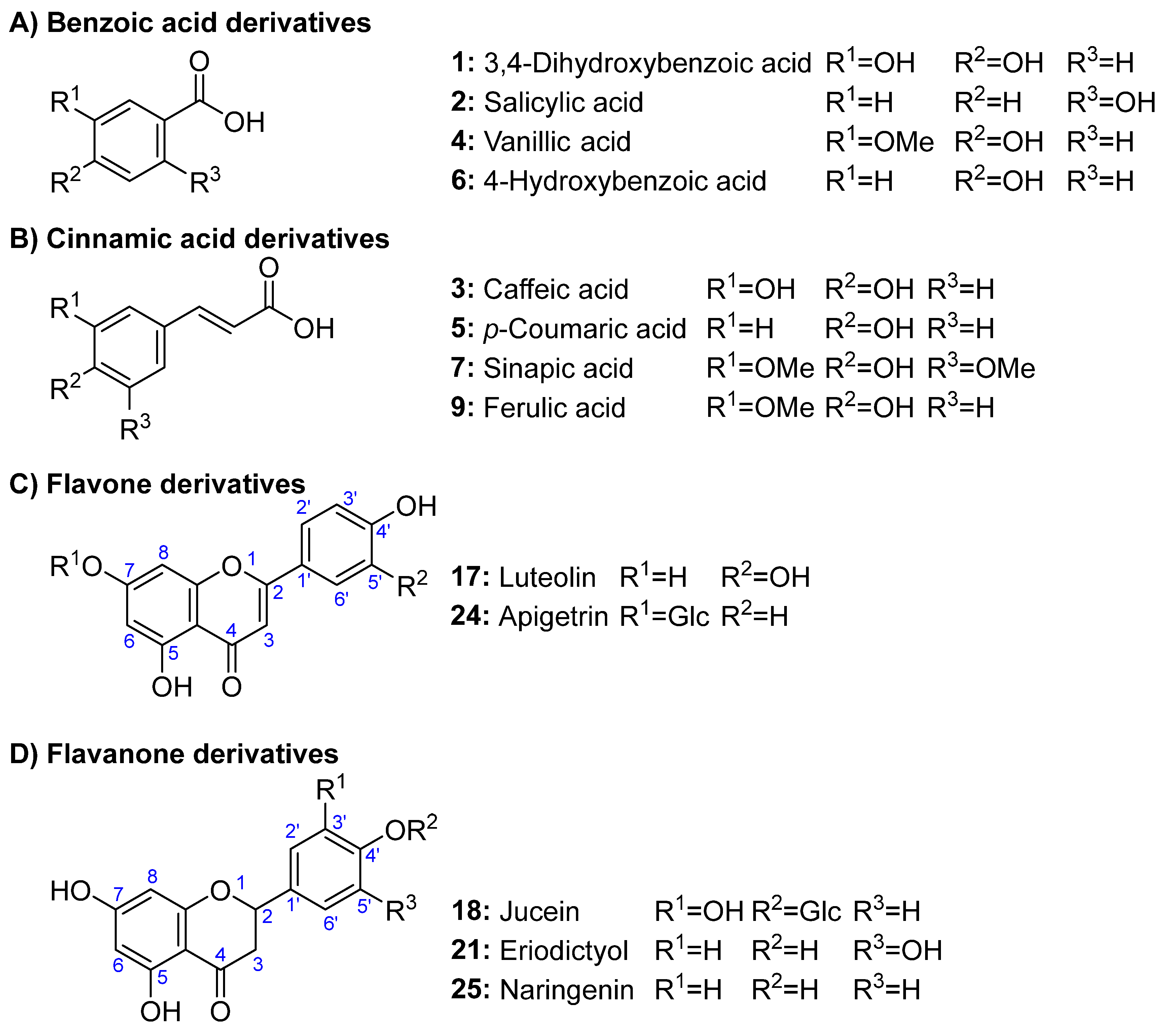
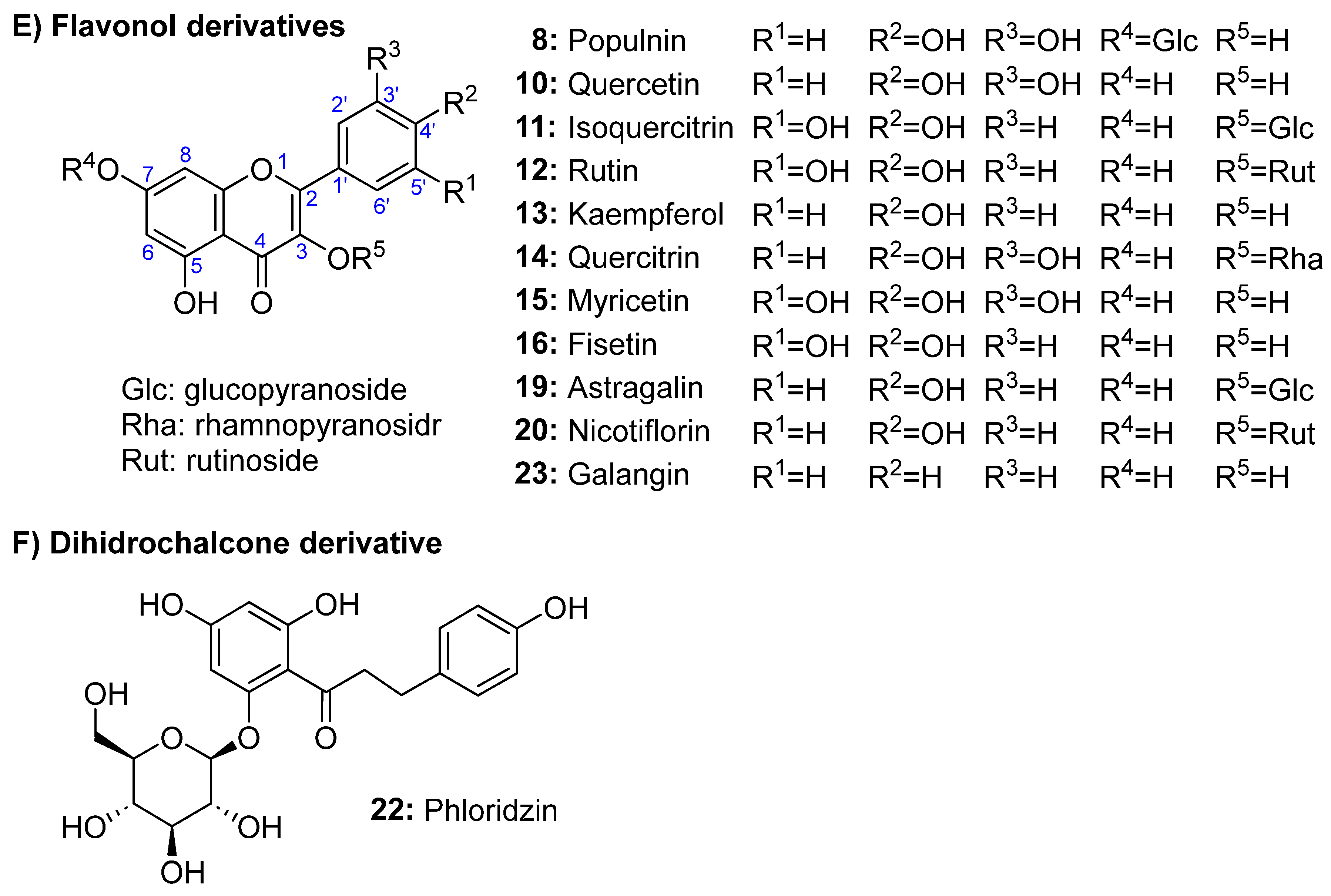
| N | Rt min | Massa m/z | Adduct | Fragmentb | Formula | Identification | Identification basis | Occurrence in samplesc |
| 1 | 3.88 | 153.01868 | [M-H]- | 109.02970 | C7H6O4 | 3,4-Dihydroxybenzoic (protocatechuic) acid | Molecular ion [M–H]− m/z 153, and at m/z 109, produced after the neutral loss of CO2 (44 Da) | 1B,5B,6,7,8,9,10 |
| 2 | 5.12 | 139.03909 | [M-H]- | 93.03460 | C7H6O3 | Salicylic acid | Molecular ion [M–H]− m/z 137, which further yielded a fragment ion at m/z 93, due to the loss of a CO2 group | 5A,5B,6,7,10,11 |
| 3 | 8.72 | 179.03498 | [M-H]- | 135.04810 | C9H8O4 | Caffeic acid | Molecular ion [M–H]− m/z 179 and its characteristic product ion 135, due to the loss of the CO2 group | 1A,1B,2,5A,5B,6,7,10 |
| 4 | 13.92 | 167.03498 | [M-H]- | 152.00996 | C8H8O4 | Vanillic acid | Molecular ion [M–H]− m/z 167 and its characteristic product ion 152, due to the loss of CH4 | 1A,1B,2,5B,6,7,8,10 |
| 5 | 16.68 | 163.04007 | [M-H]- | 119.04881 | C9H8O3 | p-coumaric acid | Molecular ion [M–H]− m/z 163 and its characteristic product ion 119, due to the loss of the CO2 group | 1A,1B,2,5A,5B,6,7,8,9,11 |
| 6 | 24.56 | 137.02442 | [M-H]- | 93.03325 | C7H6O3 | 4-hydroxybenzoic acid | Molecular ion [M–H]− m/z 137 and its characteristic product ion 93, generated by the loss of the CO2 group | 1A,1B,2,3,4,5A,5B,6,7,8,9,10,11 |
| 7 | 26.41 | 223.06120 | [M-H]- | 121.02821 | C11H12O5 | Sinapic acid | Molecular ion [M–H]− m/z 223, and the loss of 2CH3 – CO2 – CO (m/z 121) (Marcum 2016) | 1A,1B,5B,6,7,8,9,11 |
| 8 | 28.01 | 447.09328 | [M-H]- | 257.04496 | C21H20O11 | Populnin (kaempferol-7-O-glucoside) | Molecular ion [M–H]− m/z 447 and m/z 257, corresponding to the fragment [M–H–CO]− . The ejection of CO is notably followed by B-ring rotation and bonding with the A-ring to form the fused ring structure of m/z 257 (March and Miao, 2004). | 1A,1B,6,7,8 |
| 9 | 28.2 | 193.05063 | [M-H]- | 134.03690 | C10H10O4 | Ferulic acid | Molecular ion [M–H]− m/z 193, and m/z 134 corresponding to the loss of CO2 and CH3 | 1A,1B,2,4,5A,5B,6,7,8, 9,10,11 |
| 10 | 28.62 | 303.04993 | [M+H]+ | 178.99749 | C15H10O7 | Quercetin | Molecular ion [M–H]− m/z 303 and m/z 179, originated after cleavage of the B ring by a Retro Diels-Alder (RDA) mechanism (Dos Santos et al., 2018) | 1A,1B,5B,6,7,8,9,11 |
| 11 | 28.81 | 463.08820 | [M-H]- | 302.03696 | C21H20O12 | Isoquercitrin (quercetin-3-O-glucoside) | Molecular ion [M–H]− m/z 463, and m/z 302, corresponding to the aglycone of quercetin following the loss of a hexose ([M – H − 162]− | 1B,2,4,5A,5B,6,7,8 |
| 12 | 28.83 | 609.14611 | [M-H]- | 301.03474 | C27H30O16 | Rutin (quercetin 3-O rutinoside) | Molecular ion [M–H]− m/z 609, and fragment m/z 301 due to the loss of 308 Da (rutinose) | 1A,1B,2,3,4,5A,5B,6,7,8,11 |
| 13 | 29.57 | 287.05501 | [M+H]+ | 153.01760 | C15H10O6 | Kaempferol | Molecular ion [M–H]− m/z 287, and m/z 153 formed by RDA fragmentation wherein bonds 1 and 3 undergo scission leading to the formation of the A+ ion (m/z 153) (Ma et al., 1997) | 1A,1B,5B,6,7,8,9,11 |
| 14 | 29.75 | 447.09328 | [M-H]- | 230.98517 | C21H20O11 | Quercitrin (quercetin 3-O-rhamnoside) | Molecular ion [M–H]− m/z 447, and fragment m/z 231, corresponding to [quercetin-H-CO2-CO]- | 5B,6,7,8,9,10,11 |
| 15 | 29.77 | 317.03029 | [M-H]- | 151.00262 | C15H10O8 | Myricetin | Molecular ion [M–H]− m/z 317, and typical MS/MS fragment at m/z 151, that corresponded to retrocyclization on the A–C ring (1,2A−) and the consecutive loss of CO (1,2A−-CO) (Chernosov et al., 2017) | 1A,1B,2,3,4,5A,5B,6,7,8, 10 |
| 16 | 29.80 | 285.04046 | [M-H]- | 121.02799 | C15H10O6 | Fisetin | Molecular ion [M–H]− m/z 285, and m/z 121, that correspond to fragmentation of B ring (1,2B-), as described by Fabre et al. (2001) | 5B,6,7,9 |
| 17 | 29.80 | 285.04046 | [M-H]- | 175.03898 | C15H10O6 | Luteolin | Molecular ion [M–H]− m/z 285, and m/z 175, corresponding to the loss of C3O2 – C2H2O (Śliwka-Kaszyńska et al., 2022) | 1A,1B,5B,6,7,8,9 |
| 18 | 29.90 | 447.09328 | [M-H]- | 285.03995 | C21H20O11 | Juncein (luteolin-4′-O-glucoside) | Molecular ion [M–H]− m/z 447, and m/z 285 corresponding to luteolin aglycone, indicating the loss of a hexose | 1A,1B,5B,6,7,8,11 |
| 19 | 29.92 | 447.09328 | [M-H]- | 255.02924 | C15H10O6 | Astragalin (kaempferol-3-O-glucoside) | Molecular ion [M–H]− m/z 447, and m/z 255, corresponding to the loss of the CH2O from the aglycone (30 Da) (Dantas et al., 2021). | 1A,1B,5B,6,7,10,11 |
| 20 | 29.95 | 593.15119 | [M-H]- | 285.03973 | C27H30O15 | Nicotiflorin (kaempferol-3-O-rutinoside) | Molecular ion [M–H]− m/z 593, and m/z 285 corresponding to a deprotonated kaempferol aglycone, and further loss of the rutinoside moiety | 1A,1B,5B,6,7,8,10,11 |
| 21 | 30.06 | 287.05611 | [M-H]- | 135.04382 | C15H12O6 | Eriodictyol | Molecular ion [M–H]− m/z 287, and m/z 135 corresponding to fragmentation of the B ring (1,3B-), as described by Fabre et al. (2001) | 5B,6,7 |
| 22 | 30.53 | 435.12967 | [M-H]- | 273.07598 | C21H24O10 | Phloridzin (phloretin-2′-O-glucoside) | Molecular ion [M–H]− m/z 435, and m/z 273 corresponding to phloretin (dihydronaringenin), after the losses of a hexosyl (glucose, 162 Da) | 5B,6,7 |
| 23 | 30.78 | 269.04555 | [M-H]- | 213.0545 | C15H10O5 | Galangin | Molecular ion [M–H]− m/z 269, and m/z 213 corresponding to the loss of 2CO (56 Da) | 1A,3,4,5A,5B,6,7,8,9,10,11 |
| 24 | 30.87 | 433.11292 | [M+H]+ | 271.05908 | C21H20O10 | Apigetrin (apigenin-7-O-glucoside) | Molecular ion [M+H]− m/z 433, and m/z 271 corresponding to the aglycon apiginin, by the loss of a glucose (162 Da) | 5B,6,11 |
| 25 | 31.14 | 271.06120 | [M-H]- | 119.04879 | C15H12O5 | Naringenin | Molecular ion [M–H]− m/z 271, and m/z 119 that correspond to fragmentation of the B ring (1,3B-), as described by Fabre et al. (2001) | 1A,1B,2,4,5A,5B,6,7,10 |
| a mass error lower than 5 ppm; b mass error lower than 10 ppm; c Sample codes as in Table 1 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





