1. Introduction
With factors such as global aging, military and civilian trauma, orthopedic surgeries are increasing [
1]. In 2019, the number of total knee and hip replacement surgeries in China reached more than 950,000 cases [
2]. In the United States, more than 1 million joint replacement surgeries are performed annually [
3], and it is predicted that by 2030, the demand for total hip and total knee arthroplasties in the United States will increase to over 4 million cases [
4].
Despite preoperative and prophylactic antibiotic treatment, infections related to invasive orthopedic surgeries are common, exceeding 25% in complex open fractures or revision surgeries [
5]. In addition, data show that 5% to 10% of joint replacements require revision within 7 years, with prosthetic joint infection (PJI) being one of the most common reasons for revision [
6]. PJI is a severe complication of prosthetic replacement with a recurrence rate of 16 % and a mortality rate of 2.5 % [
7,
8]. In the treatment of bone infections, achieving effective concentrations of antibiotics at the site of infection through intravenous or oral routes can be challenging due to the poor blood supply to the infected area. This has led to the implantation of antibacterial bone cements (ABCs) as a significant treatment option for orthopedic infections [
9]. Among them, antibiotic-loaded bone cements (ALBCs) are the most widely used and studied type of antibacterial bone cement as it is the gold standard for use in orthopaedic procedures [
10,
11].
Bone cement is an injectable orthopedic biomaterial with self-setting properties. According to the type of material, bone cement can be divided into two categories: 1) organic bone cement, mainly polymethylmethacrylate (PMMA) bone cement; 2) inorganic bone cement, represented by calcium phosphate cement (CPC), calcium sulfate cement (CSC), etc. PMMA bone cement has the advantages of high mechanical strength and good injectability, while CPC and CSC have poor mechanical strength but better biocompatibility and biodegradability[
12,
13]. PMMA based antibiotic-eluting bone cement has gradually become the gold standard for ALBCs due to its excellent mechanical properties and injectability[
14,
15]. Currently, bone cements loaded with vancomycin, gentamicin, tobramycin, or clindamycin are approved by the US Food and Drug Administration [
16].
While ALBCs have many appealing features, they also have their own drawbacks. One such drawback is that the most common bacteria causing orthopedic infections,
Staphylococcus aureus (
S. aureus ) and
Staphylococcus epidermidis (S. epidermidis), have gradually developed antibiotic resistance, particularly to gentamicin, which makes it difficult for the clinically common gentamicin bone cement to completely kill them [
17,
18]. Another fact is that ALBCs lack biological activity, which is not only an inherent property of the polymer itself. Studies have shown that gentamicin, vancomycin, and other antibiotics have inhibitory effects on osteoblasts [
19,
20]. Another drawback is the severe burst release of antibiotics in ALBCs, which exacerbates biological toxicity. In addition, there will be long-term low-dose antibiotic release after the burst release, which can exacerbate the occurrence of bacterial resistance. In addition, the addition of antibiotics can also lead to problems such as deterioration of mechanical properties and prolongation of setting time [
21,
22]. Currently, to address the above-mentioned problems, there have been many studies on novel antimicrobial agents contained ABCs, such as ABCs containing silver can kill drug-resistant bacteria, some ABCs containing quaternary ammonium polymer do not have the problem of burst release, and have good cell compatibility [
23,
24]. These studies on ABCs have greatly pushed forward the new horizon of orthopedic infection treatment.
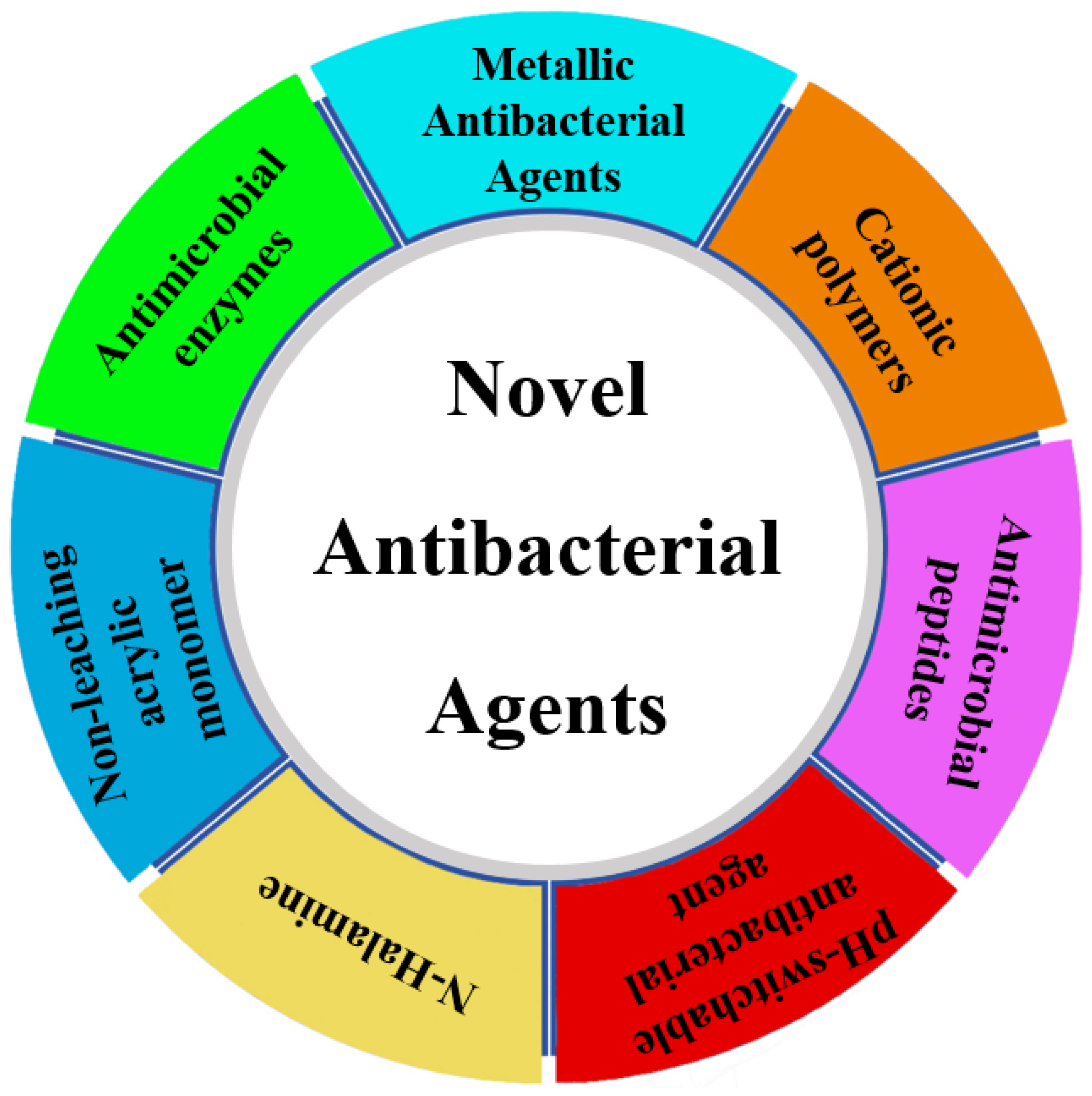
Figure 1.
Antimicrobial agents discussed in this review.
Figure 1.
Antimicrobial agents discussed in this review.
The observations made above underscore the urgent need for the development of ABCs incorporating novel antimicrobial agents. In this review, we aim to present an overview and prospect of bone cement loaded with novel antimicrobial agents, encompassing metal ions, pH-switchable antibacterial agents, positively charged polymers, N-halamines, non-leaching antibacterial agents, antimicrobial peptides, and antimicrobial enzymes. Our objective is to provide a comprehensive survey of novel antibacterial agents that have been utilized in bone cement or have the potential to be incorporated into bone cement research, highlighting the ongoing issues in this area.
2. Metallic Antibacterial Agents
The widespread antibacterial activities of silver are well-known, and it has been utilized in various medical fields for years [
25,
26]. Research on the antibacterial activities of silver, particularly silver salts, has persisted for decades [
27]. For instance, Dueland et al. found that PMMA bone cement loaded with silver sulfate exhibited good in vivo antibacterial activity, enhancing survival rates in experimental animals [
28]. However, numerous studies have also demonstrated that although silver salts possess antibacterial properties, they exhibit strong cytotoxicity when used internally. Consequently, recent research has focused on the development of nano-silver-containing antibacterial bone cement with reduced biotoxicity [
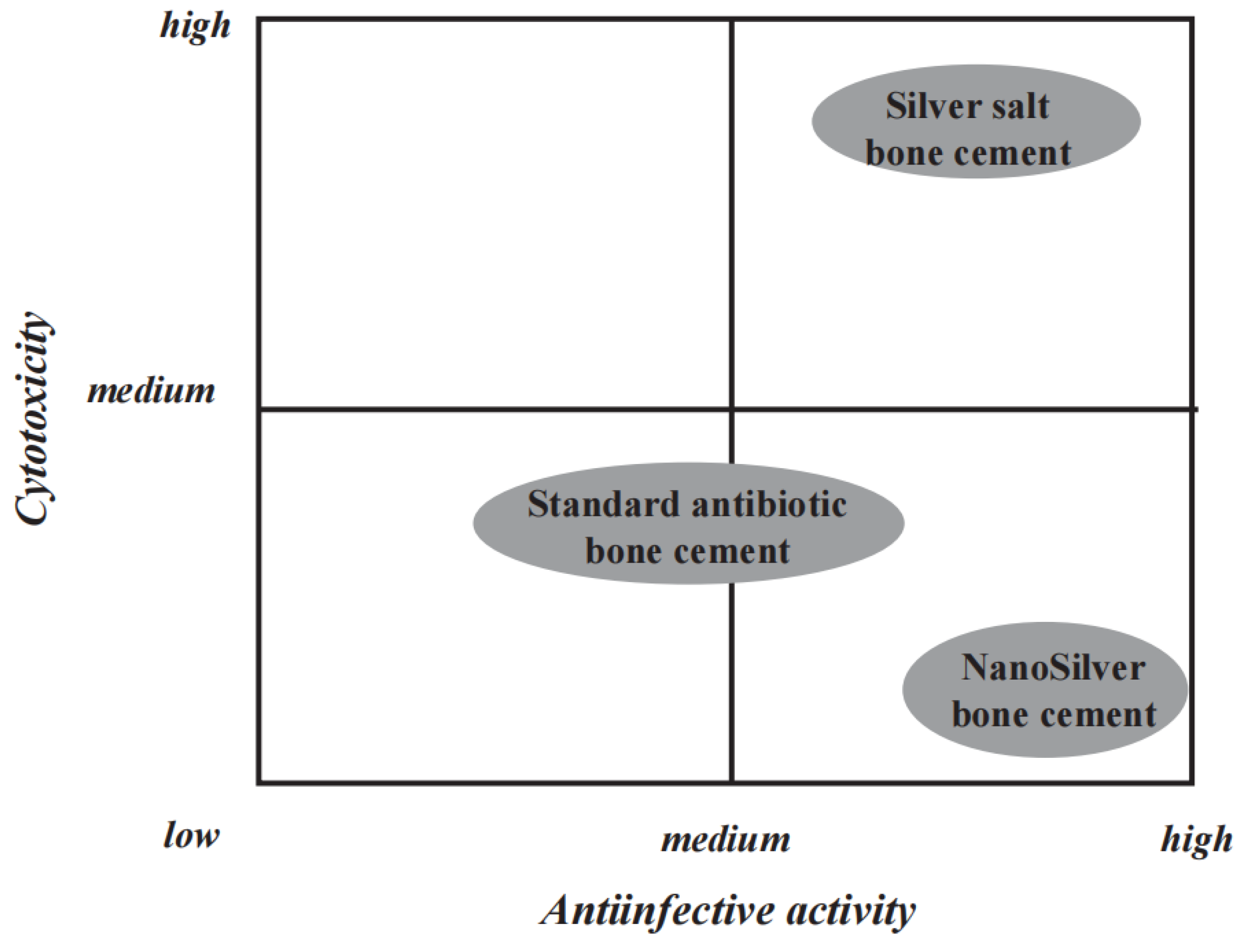
29]. Alt et al. discovered that acrylic bone cement loaded with nano-silver exhibited excellent antibacterial activity. Researchers compared the antibacterial activity and cytotoxicity of silver salt bone cement, gentamicin-loaded bone cement, and nano-silver bone cement (
Figure 2). This nano-silver cement showed good antibacterial activity against methicillin-resistant
S. epidermidis (MRSE) and methicillin-resistant
S. aureus (MRSA), while traditional gentamicin bone cement was ineffective against these resistant bacteria. Remarkably, this new nano-silver bone cement exhibited no cytotoxicity, marking a significant distinction from previously reported high cytotoxicity associated with silver salt bone cement. This may be due to the primarily non-leaching bactericidal effect of nano-silver, although the mechanism is currently unclear and may involve the generation of reactive oxidative species or cationic damage to the bacterial cell membrane. This differs from the bactericidal action of silver ions released by silver salts.
Beyond antibacterial properties, scientists are also interested in the bioactivity of silver-containing bone cement. Miola et al. prepared a silver-containing bioactive glass powder (SBAG) and studied its antibacterial properties when added to bone cement [
30]. Additionally, they examined the ability of this composite bone cement to form hydroxyapatite on its surface. They found that bone cement containing SBAG could release Ag ions, exhibiting good antibacterial activity, while also depositing hydroxyapatite on its surface, demonstrating certain bioactivity. Later, they doped silver ions with silica-based glass powder (SBA) to create a new bioactive glass, Ag-SBA2, which was then added to bone cement [
31]. They discovered that this bone cement exhibited good antibacterial and bioactive properties, similar to those of SBAG-containing bone cement. Remarkably, bone cement containing Ag-SBA2 also exhibited optimized mechanical strength.
In addition to silver, other metals such as copper and gold also possess antibacterial properties. Wekwejt et al. studied the cell compatibility of bone cement containing nano-silver and nano-copper [
32]. They found that both silver and copper enhanced the antibacterial properties of the bone cement, but nano-silver-containing bone cement exhibited excellent cell compatibility, while the cell compatibility of the copper-containing group was poorer. For example, nano-silver-containing bone cement had almost no hemolytic activity, while nano-copper-containing bone cement exhibited higher hemolytic activity. Russo found that bone cement containing gold nanoparticles exhibited good antibacterial properties [
33].
Although PMMA bone cement is the most commonly used clinically, it has some limitations, such as its non-degradability. Therefore, recent research has focused on the development of silver-containing degradable inorganic bone cement (such as CPC, brushite cement, etc.) [
34,
35,
36,
37,
38,
39]. Unlike the introduction of silver in PMMA bone cement, silver agents can be introduced through both the liquid and solid phases of inorganic bone cement. For instance, Jacquart et al. showed that the introduction of silver salts into the liquid or solid phase of calcium carbonate-calcium phosphate cement produced antibacterial and non-cytotoxic bone cement [
35]. They found that using carboxymethylcellulose microspheres as a carrier for silver ions effectively avoided burst release of silver ions, thus minimizing their toxic effects [
38]. This is an impressive recent advancement.
Liu et al. comprehensively compared the antibacterial properties, cell compatibility, and injectability of silver ions and silver nanoparticles [
39]. Their studies demonstrated that bone cement containing silver ions exhibited good injectability and cell compatibility. The higher cell compatibility of silver salts compared to nano-silver seems inconsistent with some previous understandings. We speculate that this may be due to the use of starch-modified CPC by Liu et al., which optimized the release profile of silver ions, avoiding burst release.
In conclusion, antibacterial bone cement containing metals, particularly silver (in the form of silver salts or nano-silver particles), exhibits good antibacterial properties. In this active research field, once-inherent drawbacks, such as the biotoxicity of silver salts, are being overcome through innovative delivery methods. We believe that there will be more exciting metal-containing antibacterial bone cements developed in the future.
3. Cationic polymers (Quaternary Ammonium Polymers)
Cationic polymers primarily refer to polymers containing quaternary ammonium compounds (QACs), which are extensively studied as antibacterial compounds [
40,
41,
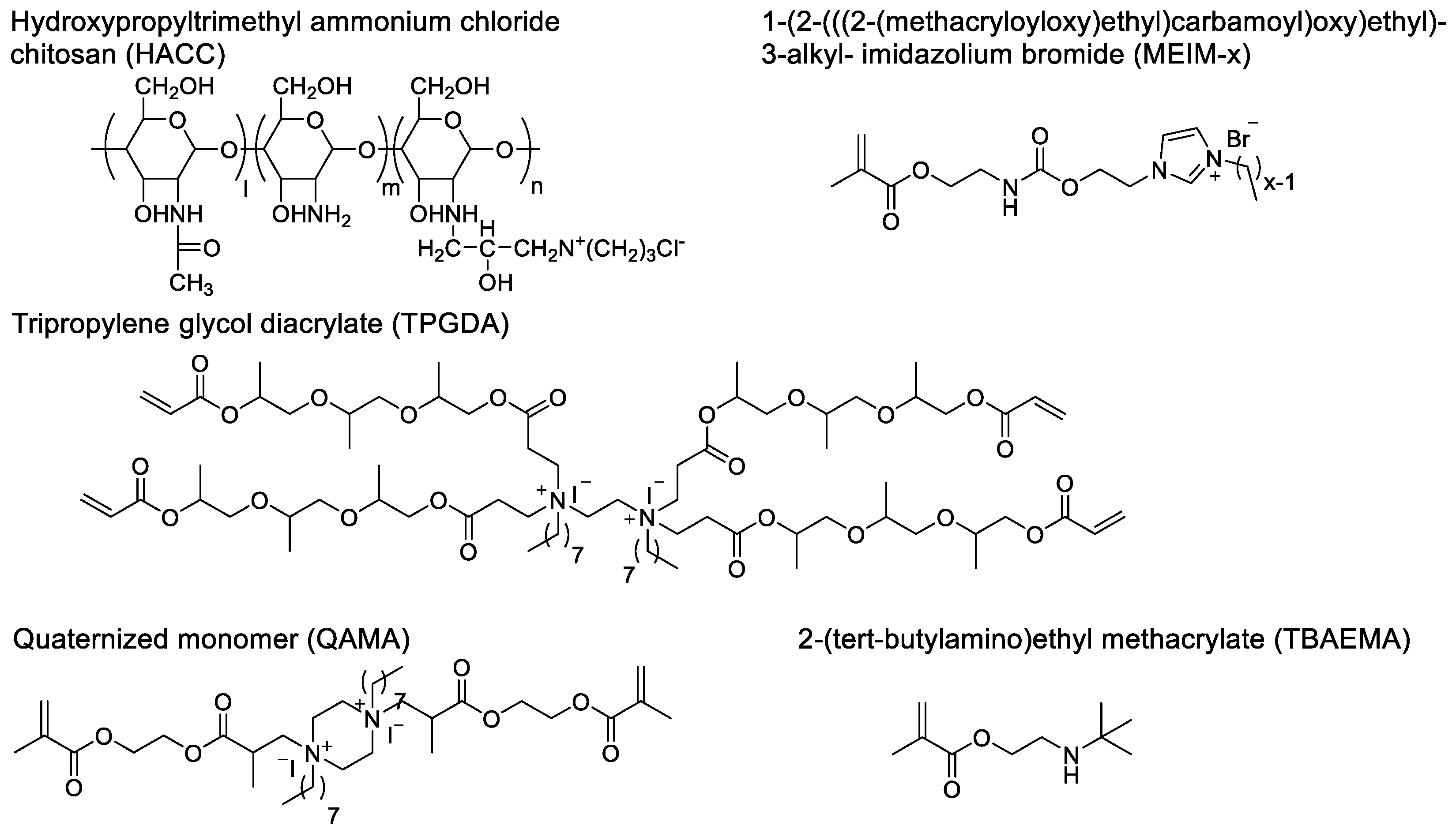
42]. The quaternary ammonium motifs carry positive charges and interact with the lipid membranes of Gram-negative and -positive bacteria. This electrostatic interaction with the negatively charged bacterial cell membranes leads to physical damage to the cells, conferring broad-spectrum antibacterial activity to these quaternary ammonium functionalized polymers. In recent years, QACs-containing antibacterial bone cement has made significant progress in the prevention and treatment of bone infections. Currently, the introduction of quaternary ammonium motifs can be achieved either through physical doping or covalent polymerization. Tang et al. designed hydroxypropyltrimethyl ammonium chloride chitosan (HACC) containing quaternary ammonium motifs (
Figure 3 and
Figure 4) [
43,
44,
45]. The addition of a certain amount of HACC to PMMA bone cement exhibits good antibacterial activity. Tang et al. found that HACC with a quaternary ammonium substitution degree of 26% exhibits good antibacterial activity and biocompatibility, while higher substitution degrees may cause biotoxicity. Furthermore, HACC bone cement exhibits superior ability to inhibit the formation of
Staphylococcus aureus biofilm on the bone cement surface compared to gentamicin bone cement. Surprisingly, they also observed that this novel bone cement can downregulate the expression of virulence-related genes in antibiotic-resistant
Staphylococcus aureus. To investigate the potential of this bone cement, Tang et al. conducted a dedicated biomechanical and biocompatibility study. The study showed that the optimal mass ratio of 26% substitution degree HACC added to PMMA bone cement is 20%, at which point the mechanical properties of the bone cement decrease significantly. After soaking for 4 weeks, the mechanical properties of the bone cement decrease slightly. Encouragingly, the mechanical strength of the HACC-loaded bone cement is close to that of human cancellous bone. Biocompatibility studies further revealed that HACC-loaded PMMA bone cement has good bone integration with bone tissue. Compared to chitosan or gentamicin-loaded bone cement, more new bone formation was observed around the HACC-loaded PMMA bone cement.
In Tang et al.'s study, the quaternary ammonium motifs were first integrated into the glycan molecules and then non-covalently doped with PMMA bone cement. Many studies have also designed acrylic monomers containing quaternary ammonium motifs. These monomers are added to the liquid phase of the bone cement during use, forming bone cement containing QACs. Deb et al. developed an acrylic monomer, quaternized EGDMA-piperazine octyl ammonium iodide (QAMA), which has two polymerizable acrylic structures and can cross-link with PMMA bone cement [
46]. It also contains two quaternary ammonium motifs, exhibiting antibacterial activity. When QAMA monomer is added as a component of the liquid phase to acrylic bone cement, the results show that bone cement containing 15% QAMA does not have a bacteriostatic zone, but there is no bacterial growth on the surface of the bone cement, demonstrating non-leaching antibacterial activity. The working mechanism of this antibacterial monomer is to eliminate bacteria through contact without releasing bioactive agents.
Singh et al. developed a 4-arm dendrimer quaternary ammonium salt acrylic acid comonomer with tri-propylene glycol diacrylate (TPGDA), which indicates that it has four acrylic acid polymerizable structures (
Figure 4) [
47]. Studies have shown that the powder of TPGMA cement has antibacterial activity. Although the surface area of the bone cement powder obtained by this grinding pretreatment is increased, which may be different from the morphology of the bone cement during use, it demonstrates its potential efficacy as an antimicrobial agent in bone cement. He et al. developed acrylic acid copolymers containing imidazole quaternary ammonium salt, 1-(2-(((2-(methacryloyloxy)ethyl)carbamoyl)oxy)ethyl)-3-alkyl-imidazolium bromide (MEIM-x), which have different alkane chain lengths [
48]. The study found that the monomers have antibacterial activity against
Staphylococcus aureus and
Escherichia coli, and the antibacterial activity of the monomers is not positively correlated with the length of the chain. In addition, the antibacterial activity of the monomers is inconsistent with that of the cement containing the monomers. For example, MEIM-14 has the highest antibacterial activity against
Escherichia coli, while the antibacterial activity of cement containing MEIM-14 against
Escherichia coli is lower than that of cement containing MEIM-10 and MEIM-12.
Miyazak et al. developed an acrylic acid monomer 2-(tert-butylamino)ethyl methacrylate (TBAEMA) containing a secondary amine motif in the side chain [
49]. In this study, the authors believed that TBAEMA is a quaternary ammonium salt. We believe that when the external environment is acidic, the secondary amine motif may carry a positive charge and can exhibit similar functions to QACs (
Figure 4). Experimental results showed that as the content of TBAEMA in the cement increased, the antibacterial activity also increased. In this work, the authors fully discussed the mechanism of antibacterial activity, and they found TBAEMA and amino motifs produced by hydrolysis through mass spectrometry, which may be the cause of antibacterial activity. At the same time, we believe that these low-dose compounds may produce antibacterial activity, but the secondary amine motif on the surface of the cement also contributes to the reduction of bacteria.
Bone cement containing QACs has good antibacterial activity, but the destruction of the cell membrane by QACs lacks selectivity, which may also cause damage to normal cells, manifesting as high hemolytic activity. Therefore, when using it, it is necessary to carefully consider the amount of QACs used or the degree of substitution, achieving a balance between antibacterial activity and safety.
4. Antimicrobial peptides
Antimicrobial peptides (AMPs) are a class of peptides with antibacterial activity [
50,
51,
52]. The number of amino acid residues in antibacterial peptides ranges from 10 to 60, and almost all AMPs contain cationic amino acid residues such as lysine and arginine. Unlike antibiotics that target intercellular functions, AMPs mainly interact with the lipid membrane surface of Gram-negative and -positive bacteria. The cationic groups allow electrostatic interaction with the negatively charged bacterial cell membrane, while the hydrophobic segments facilitate insertion and disruption of the cell membrane, leading to physical damage to the cell. This unique mechanism has been shown to be unlikely to cause antibiotic resistance. Due to their broad antibacterial spectrum and difficulty in developing resistance, they have become a highly promising new class of antibacterial agents. In recent years, researchers have conducted many studies on the effectiveness of AMPs in bone cement, and the results show that both PMMA bone cement and calcium phosphate bone cement loaded with antibacterial peptides have antibacterial activity.
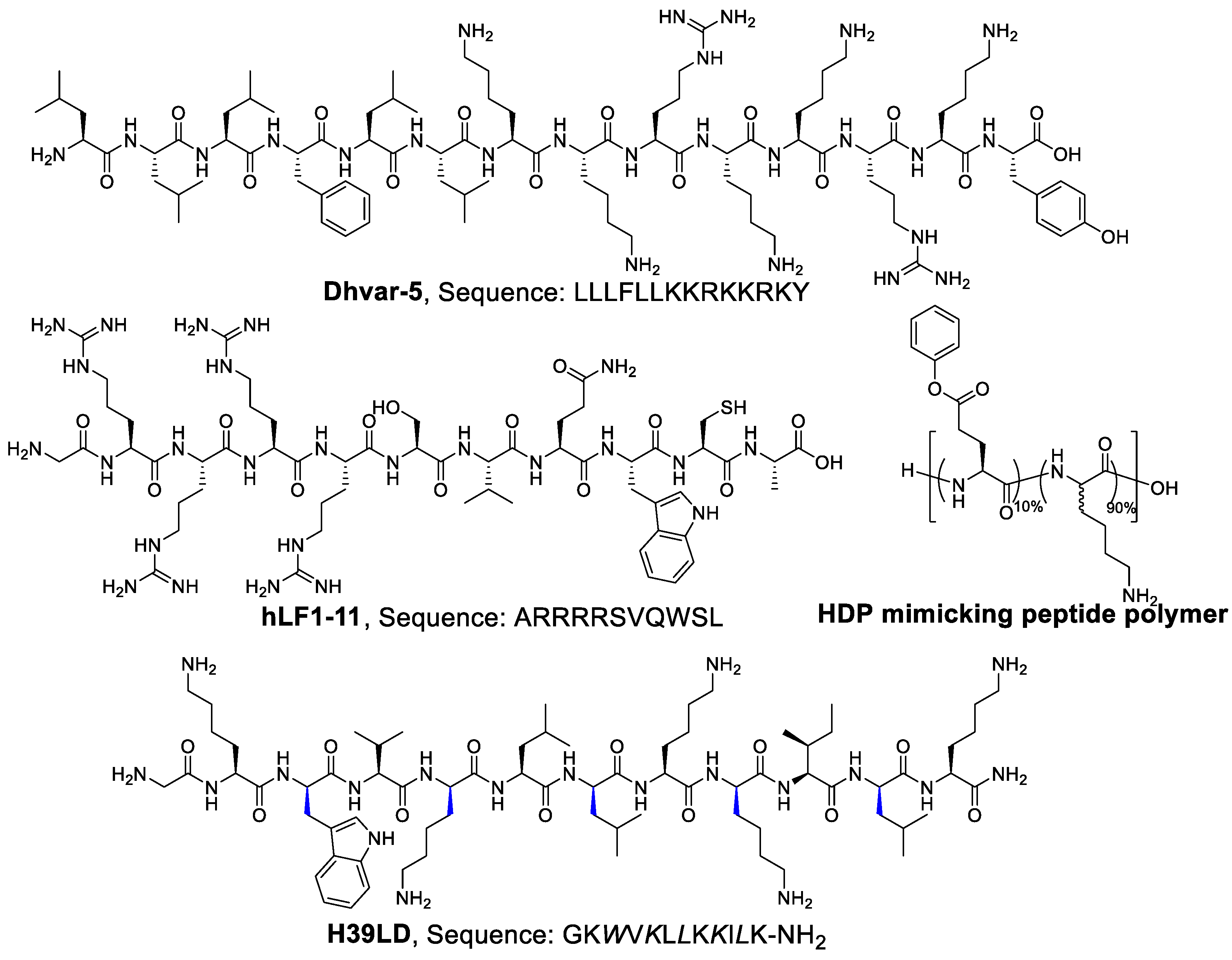
Wuisman and his team were pioneers in research related to antimicrobial peptide-loaded bone cement [
53,
54,
55,
56,
57,
58]. In 2003, Wuisman et al. studied the release kinetics of the antimicrobial peptide Dhvar-5 (
Figure 5) loaded into PMMA bone cement. The results showed that the release kinetics of the peptide depended on the amount of peptide added, i.e., the higher the peptide incorporation rate, the higher the proportion of peptide released. Subsequently, they conducted studies demonstrating that Dhvar-5 can increase the porosity of bone cement and enhance the release of gentamicin from gentamicin-loaded bone cement. We believe that this may also be related to drug cluster interconnectivity [
3,
59,
60].
In addition, they conducted in vitro release studies and in vivo antibacterial studies on calcium phosphate bone cement loaded with the antibacterial peptide hLF1-11 (
Figure 5). The results showed that this degradable bone cement loaded with antimicrobial peptides is feasible for antibacterial use. Studies have shown that hLF1-11 can not only be released but also effectively reduce the development of osteomyelitis in a rabbit model when loaded into bone cement. AMPs are effective against MRSA, and Wuisman et al. conducted infection treatment studies using hLF1-11-loaded bone cement in a rabbit model of MRSA osteomyelitis. The results showed that the AMPs were effective against infection, with antibacterial activity comparable to gentamicin.
Subsequently, to further investigate the basis of the antibacterial activity of antimicrobial peptide-loaded bone cement, Wuisman et al. also conducted in vivo release studies of hLF1-11-loaded bone cement. The studies showed that there were similar burst release characteristics in vivo as in vitro. We believe that the reason why antimicrobial peptides do not exhibit antibacterial activity comparable to gentamicin may have less to do with the antibacterial activity of the peptides themselves and more to do with their stability in vivo.
While antimicrobial peptides have good application prospects, they also have some inherent shortcomings, such as high production costs and poor stability, which can be easily degraded by proteases in vivo. To address the issue of high production costs of AMPs, Liu et al. designed antibacterial peptide polymer, a method suitable for large-scale and low-cost preparation of antibacterial peptides. Liu et al. studied the mechanical strength and antibacterial activity of HDP mimicking peptide polymer-loaded bone cement (
Figure 5) [
61]. They found that the addition of peptide polymer had little effect on mechanical strength, and both in vitro and in vivo studies demonstrated good anti-MRSA activity of the bone cement loaded with antibacterial peptide polymer. The study by Cěrovský et al. considered the stability issue of natural antimicrobial peptides and introduced D-amino acids into the peptides to enhance their stability against proteases [
62,
63,
64]. Their study found that although the MIC value of the antimicrobial peptides was higher than that of vancomycin and gentamicin in vitro, the calcium phosphate bone cement loaded with antibacterial peptides exhibited higher antibacterial activity in vivo than bone cement loaded with antibiotics. In addition, they also found that PMMA bone cement loaded with antimicrobial peptides could prevent the formation of bacterial biofilms on the surface of the bone cement.
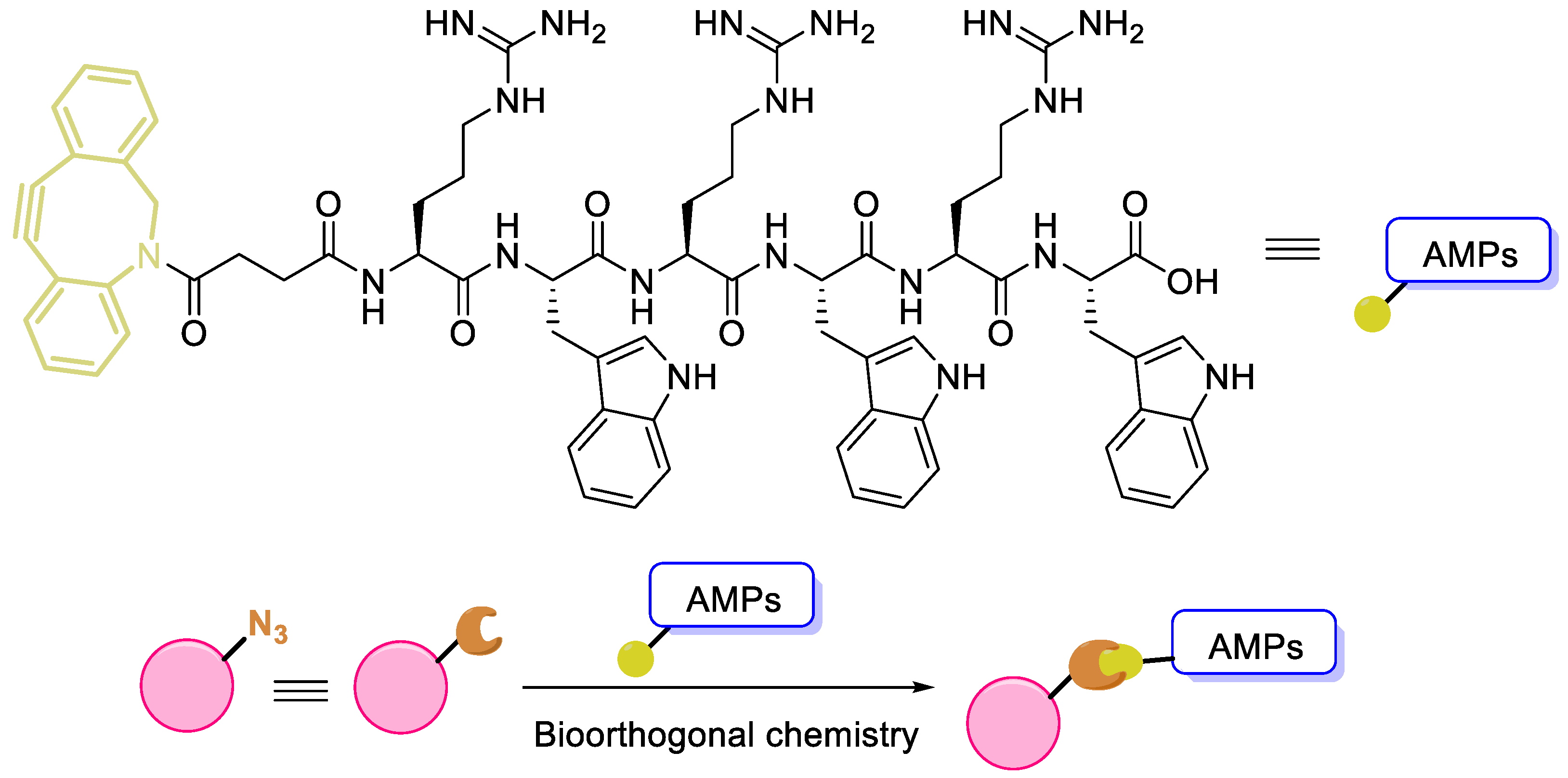
In addition to introducing D-amino acids to enhance stability, immobilization of antimicrobial peptides is also an effective method to enhance stability or duration of action [
65]. Geng et al. covalently conjugated antimicrobial peptides to the surface of bone implant PEEK materials through bioorthogonal chemistry (
Figure 6). Studies have shown that this material conjugated with peptides exhibits antibacterial activity. AMPs contain many reactive functional groups, and although there is currently no bone cement modified through covalent bonding peptides, we believe that the cross-linking of peptides with bone cement based on bioorthogonal chemistry can help promote the development of ABCs.
5. pH-switchable antibacterial agent
As mentioned in previous sections, positively charged QACs and AMPs can disrupt cell membranes, but these positively charged antibacterial agents also act on the cell membranes of normal cells, often manifesting as hemolytic activity. To address this issue, researchers have developed pH-switchable antibacterial agents. Bacterial infection creates a locally acidic special pathological environment. Based on this special pH change, researchers have designed antibacterial agents that can respond to pH changes, and pH reduction can enhance the antibacterial activity of the agents. Currently, this novel antibacterial agent has not been studied in bone cement, but we believe that such antibacterial agents are likely to play an important role in the research and application of antibacterial activity of bone cement.
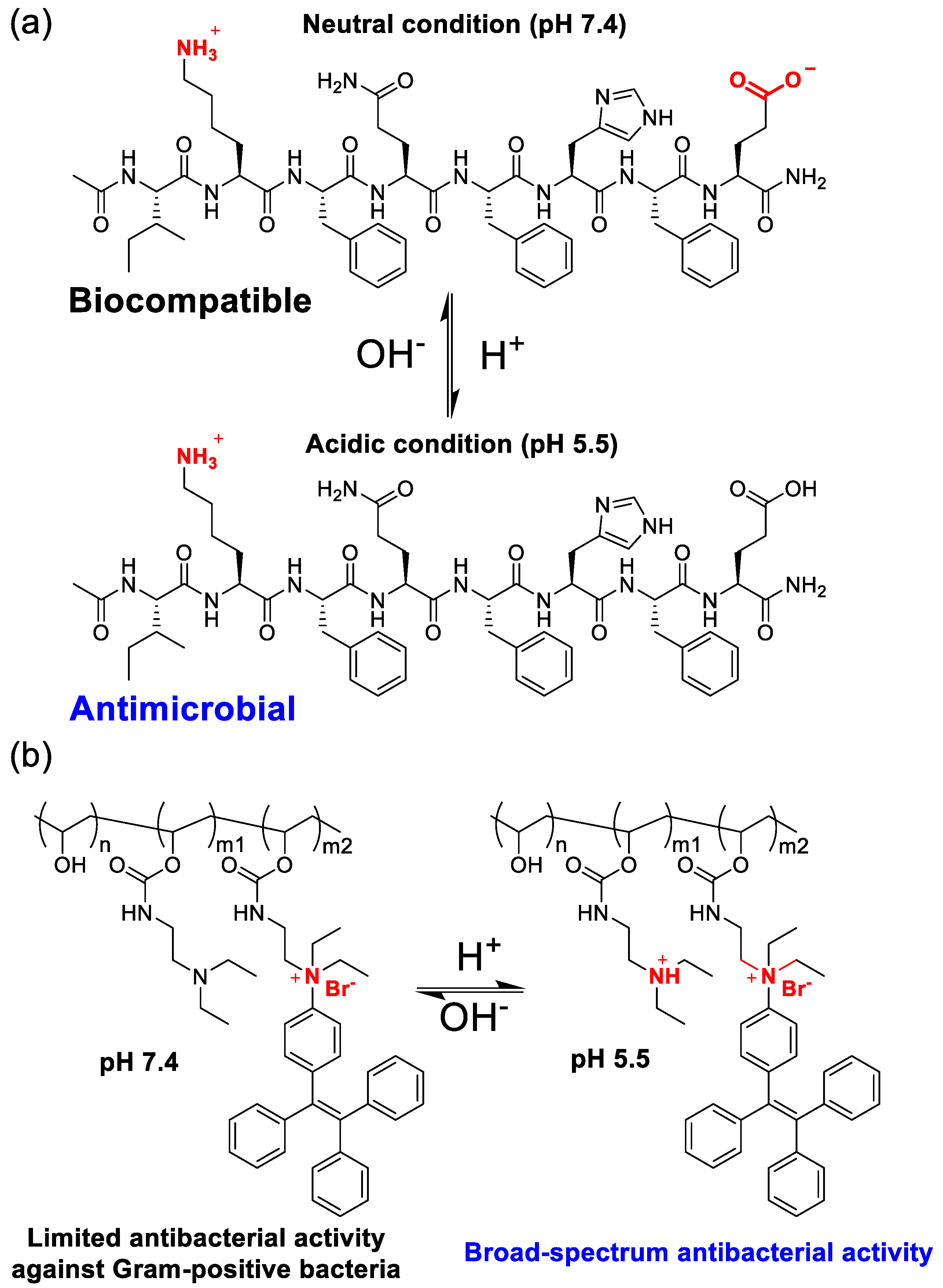
Li et al. designed a pH-responsive adjustable antibacterial octapeptide [
66]. We can notice that this peptide has an N-terminal amidation structure and contains lysine and aspartic acid residues in the main chain (
Figure 7a). Under neutral pH conditions (pH 7.4) in physiological conditions, the octapeptide exhibits an overall electrical neutrality (zero net charge) due to the electrostatic neutralization between the amino cations of the lysine residue side chain and the carboxyl anions of the glutamic acid residue side chain, and the peptide has the best biocompatibility.
Under acidic conditions caused by bacterial infection (pH 5.5), the charge of the peptide undergoes a sharp change. First, the carboxylic acid group of the glutamic acid residue side chain is protonated, resulting in the transformation of the electrically neutral octapeptide into a positively charged peptide, exhibiting antibacterial activity similar to that of antibacterial peptides. Based on this novel pH-switchable antibacterial peptide, they prepared hydrogels and used them for wound infection treatment studies. Similar to this research approach, researchers have also developed pH-switchable antibacterial peptides such as antibacterial 5-peptides [
67]. As orthopedic implants increasingly emphasize biosafety, it is necessary to incorporate pH-switchable antibacterial peptides into the related research of antibacterial bone cement.
In addition to novel peptide-based pH-switchable antibacterial agents, Ren et al. designed poly(vinyl alcohol) derivatives (PVA–TPE) that can regulate antibacterial activity with pH changes [
68]. PVA–TPE exhibits antibacterial activity against Gram-positive bacteria only under neutral pH conditions, but when the pH is reduced to 5.5, the tertiary amine on the polymer side chain is protonated, increasing the overall positive charge, making PVA–TPE exhibit broad-spectrum antibacterial activity against both Gram-positive and negative bacteria (
Figure 7b). Their studies have also shown that PVA-TPE has low cytotoxicity to mammalian cells L929, HepG2, and red blood cells.
We hypothesize that the introduction of both positively charged motifs (such as quaternary ammonium salt motifs) and carefully considered negative charged motifs on the surface of bone cement may reduce the hemolytic activity of quaternary ammonium salts and facilitate the development of novel pH-switchable antibacterial bone cement with good biosafety.
6. N-Halamine
N-Halamines are a novel class of antibacterial agents that can be typically categorized into three types: amine
N-halamines, amide
N-halamines, and imide
N-halamines [
69,
70]. The structural stability hierarchy among these three classes is
N-halamines > amide
N-halamines > imide
N-halamines. The halogen in
N-halamines can include chlorine, bromine, or iodine, with chlorine being the most studied and applied. Additionally, halamines can be further classified as cyclic or acyclic based on their parent nuclear structure, with cyclic halamines exhibiting better stability. The antibacterial mechanism of
N-halamines is believed to involve release killing, contact killing, and transfer killing [
69]. We tend to believe that transfer killing can be encompassed within the first two mechanisms, therefore favoring release killing and contact killing as the primary antibacterial mechanisms of
N-halamines.
Due to their excellent antibacterial properties, materials containing halamines have been widely studied and applied in fields such as textiles and coatings. For example, Goddard et al. utilized Antimicrobial
N-halamine Modified Polyethylene to fabricate food packaging materials [
70], while Sun et al. designed Acyclic N-halamine-immobilized polyurethane, which demonstrated antibacterial activity and the prevention of biofilm formation [
71]. In another study by Sun et al., they developed polymeric
N-halamine latex emulsions for the production of antibacterial coatings, which involved the use of cyclic N-halamines with good stability that can maintain antibacterial activity for over a year [
72]. These studies fully demonstrate the excellent antibacterial applicability and acceptable safety of
N-halamines.
Halamines also have potential applications in the biomedical field. For instance, Li et al. reported the use of halamine-containing materials for antibacterial purposes in dental implant materials [
73]. In another study, researchers functionalized the surface of Ti metal with
N-halamine, resulting in implants that exhibited good antibacterial properties and osteogenic activity in vitro [
74].
These studies have collectively demonstrated the excellent potential of
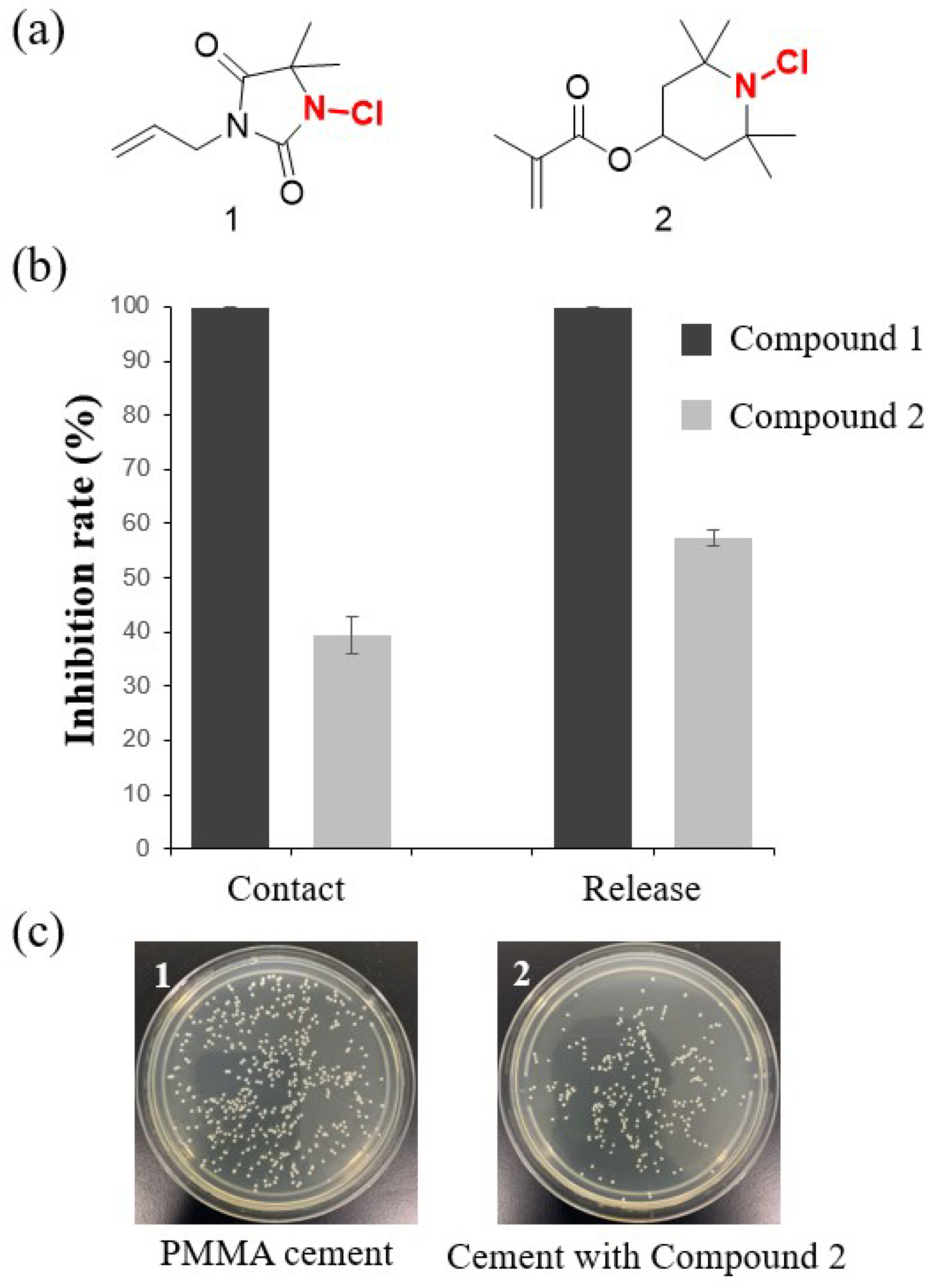
N-halamine-containing materials for use in antibacterial implantable materials. Therefore, we believe that halamines have the potential to serve as antibacterial agents for bone cement. Based on this consideration, our research group has discovered two promising polymerizable monomers containing halamines and used them to prepare halamine-containing antibacterial bone cement. Compound 1 incorporates the amide
N-halamines, while compound 2 contains relatively stable
N-halamines (
Figure 8). We aim to investigate whether these two different halamine structures exhibit antibacterial properties in bone cement. We added 15 wt% monomer to the liquid phase in the manner reported in the literature to produce bone cement [
75]. Initial research findings suggest that these new cements exhibit excellent contact antibacterial activity and release antibacterial activity against
S. aureus. As expected, amide
N-halamines, due to the unstable nature of halogens, exhibited higher antibacterial activity at the same dosage, both in terms of contact and release antibacterial activity. More detailed research on the antibacterial activity, mechanical strength, and biosafety of the
N-halamine-containing bone cement is the focus of future studies.
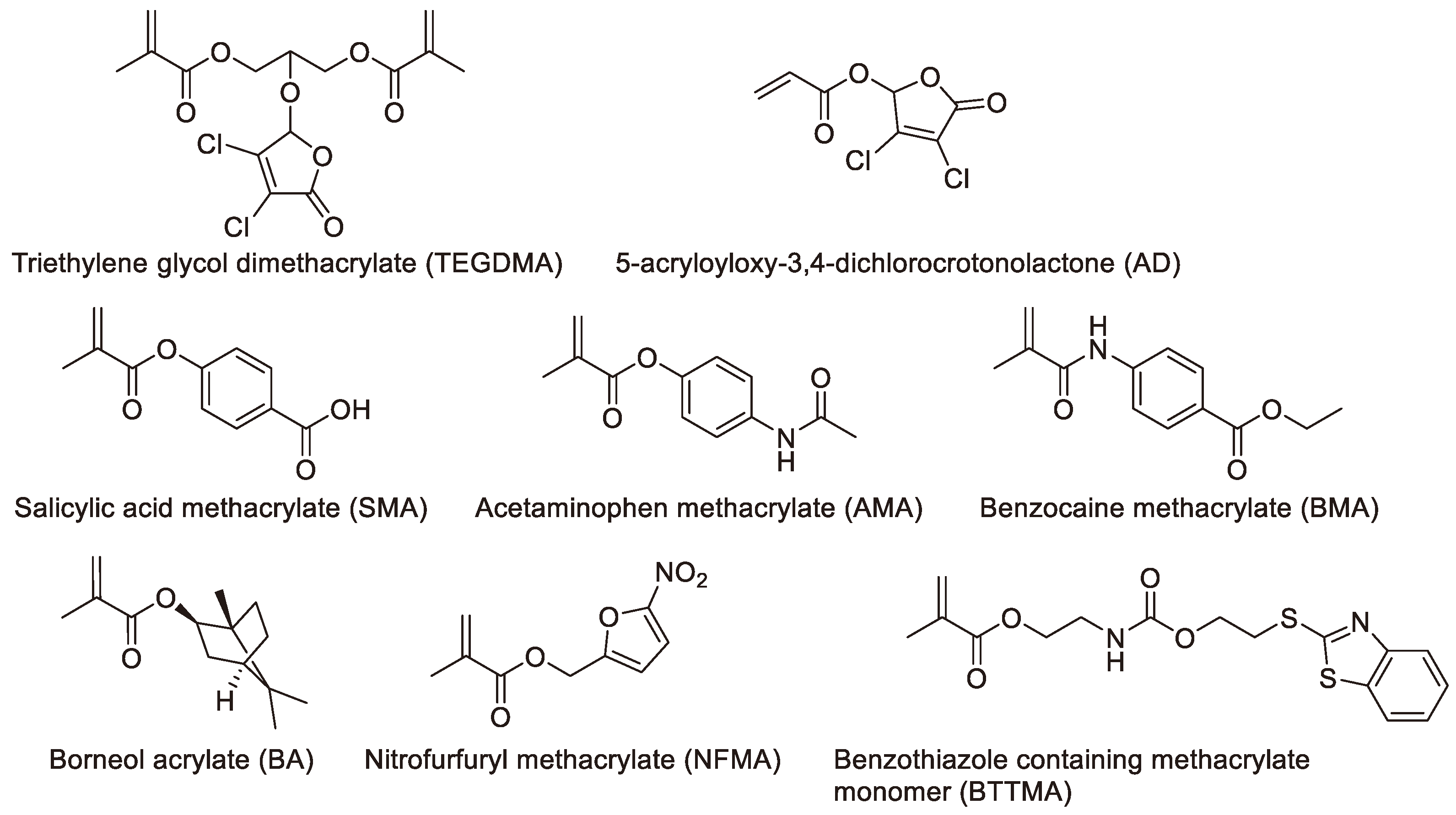
7. Non-leaching acrylic monomer
The bone cement prepared using non-leaching acrylic monomer is referred to as non-leaching bone cements (NLBCs) [
75]. The antibacterial mechanism of NLBCs is believed to be contact killing. For example, QACs antibacterial agents discussed in the section 3 can be classified as non-leaching antibacterial acrylic monomer, and their antibacterial mechanism involves destroying cell membranes. The ABCs with covalently attached antimicrobial peptides that we envisioned in
Section 4, if they are designed and manufactured, can also be classified as NLBCs. However, many reported non-leaching acrylic monomers are cyclic organic molecules (
Figure 9), and the antibacterial mechanisms of these agents are still unclear [
75,
76,
77,
78,
79,
80,
81,
82,
83]. Some believe that they may affect the activity of certain enzymes on the cell surface [
75]. Surprisingly, research has shown that the addition of non-leaching agents can enhance the mechanical strength of bone cement [
81,
82]. We have discussed these in detail in our previously published review articles [
75], so we will not elaborate further here.
8. Antimicrobial enzymes
Antimicrobial enzymes refer to proteins or enzymes with antibacterial activity, such as lysozyme and Lysostaphin. Research and applications of antimicrobial enzymes in fields such as food and medicine have been ongoing. Therefore, this review will only briefly discuss some recent work instead of providing a comprehensive overview. Researchers have prepared complexes of lysozyme and Poly(lactide-co-glycolide) using hot-melt extrusion, and studied the stability and release properties of the enzyme [
84]. The results showed that lysozyme could be completely released while maintaining its activity. Similar studies have involved the preparation of block copolymers containing lysozyme through the solvent extrusion method [
85]. In these studies, lysozyme was physically incorporated into the polymer, resulting in non-injectable enzyme-loaded materials. Some studies have designed injectable polymers, in which antimicrobial enzymes are chemically crosslinked to the polymer. Lysostaphin is a metallo-endopeptidase produced by
Staphylococcus simulans, and García et al. designed a Lysostaphin and BMP-2 co-delivery gel [
86]. This hydrogel, constructed using four-arm maleimide–terminated PEG (PEG-4MAL) macromere, can be used to reduce S. aureus infection and regenerate critical-sized segmental bone defects. Recently, Qu et al. used 4-arm-PEG-NHS to construct an injectable lysozyme hydrogel, which was successfully used for corneal stroma defect repair and rapid vision restoration [
87].
To date, antimicrobial enzymes have not been incorporated into the research of antibacterial bone cement. We believe that this may be due to the severe preparation conditions (high temperature, hydrophobic environment, etc) of PMMA bone cement, which hinder the use of antimicrobial enzymes. At the same time, we also note that the use of mild, water-based liquid phase agents such as calcium phosphate for the research of antimicrobial enzyme-containing bone cement has great feasibility. In addition, we can also consider integrating antimicrobial enzymes into bone cement through hydrogels to ensure that lysozyme does not denature. In conclusion, antimicrobial enzymes are efficient and biocompatible antibacterial agents that have the potential to play a role in the research of antibacterial bone cement.
9. Future Perspectives
For decades, antibacterial bone cements have been widely studied, both in academic and medical communities. Since the first report of antibacterial bone cements in 1970 [
88], with the continuous development of materials science, pharmacology, etc., various new ABCs have been designed, manufactured and clinical used, with better antibacterial activities, mechanical strength, biocompatibility. These studies provide more choices and confidence for humans to overcome orthopedic infections.
Bacterial resistance has become a global challenge. Increasing the dosage of antibiotics can enhance the antibacterial activity of ABCs, but it also leads to mechanical degradation, drug toxicity and even more severe bacterial resistance. Therefore, the design and use of new antibacterial agents are important ways to solve this war against bacterial infections. In this review, we categorize the antibacterial agents currently used or potentially used in cement into seven categories. Some ancient antibacterial agents, such as silver salts, have achieved a balance between antibacterial activity and biosafety by combining the latest drug delivery methods suppressing the burst release of silver ions. Antimicrobial peptides are attractive new antibacterial agents, and bone cement containing antimicrobial peptides has been studied for decades. Some inherent disadvantages of antimicrobial peptides, such as easy proteolysis by proteases and hemolytic activity, can be overcome by combining new technologies. For example, by immobilizing the peptide on the surface of the implanted material via bioorthogonal chemistry, its stability is improved; by precise design, pH-switchable antibacterial peptides can be produced to improve the biosafety of antibacterial peptides. These new technologies urgently need to be introduced to the research of antimicrobial peptide-containing bone cement. Other types of new materials also promote the development of ABCs, such as dental materials and nano materials [
89,
90,
91,
92]. Quaternary ammonium compounds and antimicrobial enzymes are also new antibacterial agents that have attracted increasing attention in recent years. The knowledge gained from these studies provides powerful support for the research of new ABCs. These new discoveries have provided a fresh perspective and pushed forward the horizon for addressing orthopedic infections.
We firmly believe that the investigation of novel antibacterial agents, coupled with their comprehensive evaluation in conjunction with bone cement, holds significant potential in propelling orthopedic science forward and revolutionizing surgical procedures. The development and utilization of bone cement formulations enriched with these innovative antibacterial agents are poised to significantly enhance the management of orthopedic infections.
Author Contributions
Conceptualization, Y.X., J.-J.C., F.H.; Methodology, Y.X., J.-J.C., F.H.; validation, H.L., A.A., G.Z., Y.X., R.G., L.-Y.H., W.-H.B. and Z.W.; formal analysis, Y.X., Z.G., H.L.; investigation, H.L., A.A., Y.-C.K., Z. W., Y.-Y.Q. L.-X.H.; resources, Y.X., H.L., Z.G. and A.A.; writing-original draft preparation, Y.X., Z.G.; writing-review and editing, Y.X., H.L., Z.G., A.A.; data curation, Y.X., H.L., G.Z.; visualization, Y.X., Z.G.; supervision, J.-J.C., Y.X., F.H.; funding acquisition, J.-J.C., F.H.; Y.X.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Anhui Provincial Natural Science Foundation (2308085QB47), the Health research Project of Anhui Province (AHWJ2022b091), Hefei Health and Medical Science Research Project (Hwk2023zd011), University Natural Science Project of Anhui Province (KJ2021A0348), Fund of Anhui Medical University (2022xkj108).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Greenstein, A.S.; Gorczyca, J.T. Orthopedic surgery and the geriatric patient. Clin. Geriatr. Med. 2019, 35, 65–92. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.Y.; Cheng, K.Y.; Chang, X.; Weng, X.S. Reports and analysis of amount of hip and knee arthroplasty in China from 2011 to 2019. Chin. J. Orthop. 2020, 40, 1453–1460. [Google Scholar]
- Suhardi, V.J.; Bichara, D.A.; Kwok, S.; Freiberg, A.A.; Rubash, H.; Malchau, H.; Yun, S.H.; Muratoglu, O.K.; Oral, E. A fully functional drug-eluting joint implant. Nat. Biomed. Eng. 2017, 1, 0080. [Google Scholar] [CrossRef]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J. Bone Joint. Surg. Am. 2018, 100, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sinha, M.; Samanta, R.; Sadhasivam, S.; Bhattacharyya, A.; Nandy, A.; Saini, S.; Tandon, N.; Singh, H.; Gupta, S.; et al. A potent antibiotic-loaded bone-cement implant against staphylococcal bone infections. Nat. Biomed. Eng. 2022, 6, 1180–1195. [Google Scholar] [CrossRef] [PubMed]
- Paxton, E.W.; Inacio, M.; Slipchenko, T.; Fithian, D.C. The kaiser permanente national total joint replacement registry. Perm. J. 2008, 12, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Lentino, J.R. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin. Infect. Dis. 2003, 36, 1157–1161. [Google Scholar] [CrossRef]
- Kubista, B.; Hartzler, R.U.; Wood, C.M.; Osmon, D.R.; Hanssen, A.D.; Lewallen, D.G. Reinfection after two-stage revision for periprosthetic infection of total knee arthroplasty. Int. Orthop. 2012, 36, 65–71. [Google Scholar] [CrossRef]
- Wall, V.; Nguyen, T.H.; Nguyen, N.; Tran, P.A. Controlling Antibiotic Release from Polymethylmethacrylate Bone Cement. Biomedicines 2021, 9, 26. [Google Scholar] [CrossRef]
- Parvizi, J.; Saleh, K.J.; Ragland, P.S.; Pour, A.E.; Mont, M.A. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008, 79, 335–341. [Google Scholar] [CrossRef]
- Forsberg, J.A.; Potter, B.K.; Cierny III, G.; Webb, L. Diagnosis and management of chronic infection. J. Am. Acad. Orthop. Surg. 2011, 19, S8–S19. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.M. A review of calcium phosphate cements and acrylic bone cements as injectable materials for bone repair and implant fixation. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019872594. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhang, B.; Yan, L. A preliminary review of modified polymethyl methacrylate and calcium-based bone cement for improving properties in osteoporotic vertebral compression fractures. Front. Mater. 2022, 9, 912713. [Google Scholar] [CrossRef]
- Lee, S.H.; Tai, C.L.; Chen, S.Y.; Chang, C.H.; Chang, Y.H.; Hsieh, P.H. Elution and Mechanical Strength of Vancomycin-Loaded Bone Cement: In Vitro Study of the Influence of Brand Combination. PloS one 2016, 11, e0166545. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Su, C.Y.; Nien, W.H.; Huang, T.T.; Huang, C.H.; Lu, Y.C.; Chen, Y.J.; Huang, G.C.; Fang, H.W. Influence of Antibiotic-Loaded Acrylic Bone Cement Composition on Drug Release Behavior and Mechanism. Polymers 2021, 13, 2240. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Oh, S.H.; Lee, I.S.; Kwon, O.S.; Lee, J.H. Antibiotic-eluting hydrophilized PMMA bone cement with prolonged bactericidal effect for the treatment of osteomyelitis. J. Biomater. Appl. 2016, 30, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Thomes, B.; Murray, P.; Bouchier-Hayes, D. Development of resistant strains of Staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J. Bone Joint Surg. Br. 2002, 84, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef]
- Isefuku, S.; Joyner, C.J.; Simpson, A.H. Gentamicin may have an adverse effect on osteogenesis. J. Orthop. Trauma 2003, 17, 212–216. [Google Scholar] [CrossRef]
- Braun, J.; Eckes, S.; Rommens, P.M.; Schmitz, K.; Nickel, D.; Ritz, U. Toxic Effect of Vancomycin on Viability and Functionality of Different Cells Involved in Tissue Regeneration. Antibiotics(Basel) 2020, 9, 238. [Google Scholar] [CrossRef]
- Lewis, G. Properties of antibiotic-loaded acrylic bone cements for use in cemented arthroplasties: a state-of-the-art review. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cui, Y.; Liu, H. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis. Chinese Journal of Tissue Engineering Research, 2021, 25, 614. [Google Scholar]
- Beyth, S.; Polak, D.; Milgrom, C.; Weiss, E.I.; Matanis, S.; Beyth, N. Antibacterial activity of bone cement containing quaternary ammonium polyethyleneimine nanoparticles. J. Antimicrob. Chemother. 2014, 69, 854–855. [Google Scholar] [CrossRef]
- Prokopovich, P.; Köbrick, M.; Brousseau, E.; Perni, S. Potent antimicrobial activity of bone cement encapsulating silver nanoparticles capped with oleic acid. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Bechert, T.; Steinrücke, P.; Guggenbichler, J.P. A new method for screening anti-infective biomaterials. Nat. Med. 2000, 6, 1053–1056. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bechert, T.; Steinrücke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. In vitro testing of antimicrobial activity of bone cement. Antimicrob. Agents Chemother. 2004, 48, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, J.A.; Webster, D.A.; Becker, R.O. Silver polymethyl methacrylate antibacterial bone cement. Clin. Orthop. Relat. Res. 1979, 266–270. [Google Scholar] [CrossRef]
- Dueland, R.; Spadaro, J.A.; Rahn, B.A. Silver antibacterial bone cement. Comparison with gentamicin in experimental osteomyelitis. Clin. Orthop. Relat. Res. 1982, 264–268. [Google Scholar]
- Alt, V.; Bechert, T.; Steinrücke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef]
- Miola, M.; Bruno, M.; Maina, G.; Fucale, G.; Lucchetta, G.; Vernè, E. Antibiotic-free composite bone cements with antibacterial and bioactive properties. A preliminary study. Mater. Sci. Eng. C. Mater. Biol. Appl. 2014, 43, 65–75. [Google Scholar] [CrossRef]
- Miola, M.; Fucale, G.; Maina, G.; Verné, E. Antibacterial and bioactive composite bone cements containing surface silver-doped glass particles. Biomed. Mater. 2015, 10, 055014. [Google Scholar] [CrossRef]
- Wekwejt, M.; Michno, A.; Truchan, K.; Pałubicka, A.; Świeczko-Żurek, B.; Osyczka, A.M.; Zieliński, A. Antibacterial Activity and Cytocompatibility of Bone Cement Enriched with Antibiotic, Nanosilver, and Nanocopper for Bone Regeneration. Nanomaterials (Basel) 2019, 9, 1114. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; Gloria, A.; De Santis, R.; D'Amora, U.; Balato, G.; Vollaro, A.; Oliviero, O.; Improta, G.; Triassi, M.; Ambrosio, L. Preliminary focus on the mechanical and antibacterial activity of a PMMA-based bone cement loaded with gold nanoparticles. Bioact. Mater. 2017, 2, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.; Hösel, D.; Patel, S.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Silver-doped calcium phosphate cements with antimicrobial activity. Acta Biomater. 2011, 7, 4064–4070. [Google Scholar] [CrossRef] [PubMed]
- Jacquart, S.; Siadous, R.; Henocq-Pigasse, C.; Bareille, R.; Roques, C.; Rey, C.; Combes, C. Composition and properties of silver-containing calcium carbonate-calcium phosphate bone cement. J. Mater. Sci. Mater. Med. 2013, 24, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Fosca, M.; Graziani, V.; Egorov, A.A.; Zobkov, Y.V.; Fedotov, A.Y.; Ortenzi, M.; Caminiti, R.; Baranchikov, A.E.; Komlev, V.S. Silver-Doped Calcium Phosphate Bone Cements with Antibacterial Properties. J. Funct. Biomater. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, I.V.; Goldberg, M.A.; Preobrazhensky, I.I.; Mamin, G.V.; Davidova, G.A.; Agafonova, N.V.; Fosca, M.; Russo, F.; Barinov, S.M.; Cavalu, S.; Rau, J.V. Improved cytocompatibility and antibacterial properties of zinc-substituted brushite bone cement based on β-tricalcium phosphate. J. Mater. Sci. Mater. Med. 2021, 32, 99. [Google Scholar] [CrossRef] [PubMed]
- Jacquart, S.; Girod-Fullana, S.; Brouillet, F.; Pigasse, C.; Siadous, R.; Fatnassi, M.; Grimoud, J.; Rey, C.; Roques, C.; Combes, C. Injectable bone cement containing carboxymethyl cellulose microparticles as a silver delivery system able to reduce implant-associated infection risk. Acta Biomater. 2022, 145, 342–357. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Tang, Z.; Liu, H.; Zhang, R.; Ge, J.; Yang, H.; Ni, X.; Lin, X.; Yang, L. Study on injectable silver-incorporated calcium phosphate composite with enhanced antibacterial and biomechanical properties for fighting bone cement-associated infections. Colloids surf. B Biointerfaces 2023, 227, 113382. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; De Melo Carrasco, L.D. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.X.; Wang, L.; Du, L.; Guo, S.R.; Wang, X.Q.; Tang, T.T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr. Polym. 2010, 81, 275–283. [Google Scholar] [CrossRef]
- Tan, H.; Peng, Z.; Li, Q.; Xu, X.; Guo, S.; Tang, T. The use of quaternised chitosan-loaded PMMA to inhibit biofilm formation and downregulate the virulence-associated gene expression of antibiotic-resistant staphylococcus. Biomaterials 2012, 33, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ao, H.; Ma, R.; Tang, T. Quaternised chitosan-loaded polymethylmethacrylate bone cement: biomechanical and histological evaluations. J. Orthop. Translat. 2013, 1, 57–66. [Google Scholar] [CrossRef]
- Deb, S.; Doiron, R.; Disilvio, L.; Punyani, S.; Singh, H. PMMA bone cement containing a quaternary amine comonomer with potential antibacterial properties. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85, 130–139. [Google Scholar] [CrossRef]
- Abid, C.K.; Jain, S.; Jackeray, R.; Chattopadhyay, S.; Singh, H. Formulation and characterization of antimicrobial quaternary ammonium dendrimer in poly(methyl methcarylate) bone cement. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 521–530. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, F.; He, J. Synthesis of imidazolium-containing mono-methacrylates as polymerizable antibacterial agents for acrylic bone cements. J. Mech. Behav. Biomed. Mater. 2017, 74, 176–182. [Google Scholar] [CrossRef]
- Wang, H.; Maeda, T.; Miyazaki, T. Preparation of bioactive and antibacterial PMMA-based bone cement by modification with quaternary ammonium and alkoxysilane. J. Biomater. Appl. 2021, 36, 311–320. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Alford, M.A.; Haney, E.F. Antibiofilm activity of host defence peptides: complexity provides opportunities. Nat. Rev. Microbiol. 2021, 19, 786–797. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Horvat Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M.; et al. Antimicrobial Peptides—Mechanisms of Action, Antimicrobial Effects and Clinical Applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M. A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Faber, C.; Stallmann, H.P.; Lyaruu, D.M.; de Blieck, J.M.; Bervoets, T.J.; van Nieuw Amerongen, A.; Wuisman, P.I. Release of antimicrobial peptide Dhvar-5 from polymethylmethacrylate beads. J. Antimicrob. Chemother. 2003, 51, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Faber, C.; Hoogendoorn, R.J.; Lyaruu, D.M.; Stallmann, H.P.; van Marle, J.; van Nieuw Amerongen, A.; Smit, T.H.; Wuisman, P.I.; Skeletal Tissue Engineering Group Amsterdam. The effect of the antimicrobial peptide, Dhvar-5, on gentamicin release from a polymethyl methacrylate bone cement. Biomaterials 2005, 26, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Stallmann, H.P.; Faber, C.; Slotema, E.T.; Lyaruu, D.M.; Bronckers, A.L.; Amerongen, A.V.; Wuisman, P.I. Continuous-release or burst-release of the antimicrobial peptide human lactoferrin 1-11 (hLF1-11) from calcium phosphate bone substitutes. J. Antimicrob. Chemother. 2003, 52, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Stallmann, H.P.; Faber, C.; Bronckers, A.L.; Nieuw Amerongen, A.V.; Wuisman, P.I. Osteomyelitis prevention in rabbits using antimicrobial peptide hLF1-11- or gentamicin-containing calcium phosphate cement. J. Antimicrob. Chemother. 2004, 54, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Faber, C.; Stallmann, H.P.; Lyaruu, D.M.; Joosten, U.; von Eiff, C.; van Nieuw Amerongen, A.; Wuisman, P.I. Comparable efficacies of the antimicrobial peptide human lactoferrin 1-11 and gentamicin in a chronic methicillin-resistant Staphylococcus aureus osteomyelitis model. Antimicrob. Agents Chemother. 2005, 49, 2438–2444. [Google Scholar] [CrossRef] [PubMed]
- Stallmann, H.P.; de Roo, R.; Faber, C.; Amerongen, A.V.; Wuisman, P.I. In vivo release of the antimicrobial peptide hLF1-11 from calcium phosphate cement. J. Orthop. Res. 2008, 26, 531–538. [Google Scholar] [CrossRef]
- Xie, Z.P.; Cui, X.; Zhao, C.J.; Huang, W.H.; Wang, J.Q.; Zhang, C.Q. Antimicrob. Agents Ch. 2013, 57, 3293–3298. [CrossRef]
- Fan, J.B.; Huang, C.; Jiang, L.; Wang, S. Nanoporous microspheres: from controllable synthesis to healthcare applications. J. Mater. Chem. B. 2013, 1, 2222–2235. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Y.; Cong, Z.; Chen, K.; Xiao, X.; Wu, X.; Liu, L.; She, Y.; Liu, S.; Zhou, R.; et al. Peptide Polymer-Doped Cement Acting as an Effective Treatment of MRSA-Infected Chronic Osteomyelitis. Adv. Funct. Mater. 2022, 32, 2107942. [Google Scholar] [CrossRef]
- Melicherčík, P.; Nešuta, O.; Čeřovský, V. Antimicrobial Peptides for Topical Treatment of Osteomyelitis and Implant-Related Infections: Study in the Spongy Bone. Pharmaceuticals (Basel) 2018, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Volejníková, A.; Melicherčík, P.; Nešuta, O.; Vaňková, E.; Bednárová, L.; Rybáček, J.; Čeřovský, V. Antimicrobial peptides prevent bacterial biofilm formation on the surface of polymethylmethacrylate bone cement. J. Med. Microbiol. 2019, 68, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Melicherčík, P.; Kotaška, K.; Jahoda, D.; Landor, I.; Čeřovský, V. Antimicrobial peptide in polymethylmethacrylate bone cement as a prophylaxis of infectious complications in orthopedics-an experiment in a murine model. Folia Microbiol (Praha). 2022, 67, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bai, J.; Tao, H.; Hao, L.; Yin, W.; Ren, X.; Gao, A.; Li, N.; Wang, M.; Fang, S.; et al. Rational integration of defense and repair synergy on PEEK osteoimplants via biomimetic peptide clicking strategy. Bioact. Mater. 2021, 8, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.Y.; Zhao, Y.; Yang, Y.; Wang, W.; Wu, C.; Yang, B.; Zhang, Z.; Zhang, L.; Liu, Y.; et al. pH-Switchable Antimicrobial Nanofiber Networks of Hydrogel Eradicate Biofilm and Rescue Stalled Healing in Chronic Wounds. ACS Nano 2019, 13, 11686–11697. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, J.; Cai, X.; Han, J.; Chen, X.; You, L.; Xiong, C.; Wang, S. pH-Switchable Antimicrobial Supramolecular Hydrogels for Synergistically Eliminating Biofilm and Promoting Wound Healing. ACS Appl. Mater. Interfaces. 2022, 14, 18120–18132. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, M.; Cui, Z.K.; Jia, Y.G.; Liu, S.; Chen, K.F.; Chen, X.H.; Zhang, Y.Q.; Fang, Z.; Chen, Y.H. One-pot quaternization of dual-responsive poly (vinyl alcohol) with AIEgens for pH-switchable imaging and killing of bacteria. Mater. Chem. Front. 2020, 4, 2635–2645. [Google Scholar] [CrossRef]
- Dong, A.; Wang, Y.J.; Gao, Y.; Gao, T.; Gao, G. Chemical Insights into Antibacterial N-Halamines. Chem. Rev. 2017, 117, 4806–4862. [Google Scholar] [CrossRef]
- Bastarrachea, L.J.; McLandsborough, L.A.; Peleg, M.; Goddard, J.M. Antimicrobial N-halamine modified polyethylene: characterization, biocidal efficacy, regeneration, and stability. J. Food Sci. 2014, 79, E887–E897. [Google Scholar] [CrossRef]
- Luo, J.; Porteous, N.; Lin, J.; Sun, Y. Acyclic N-halamine-immobilized polyurethane: Preparation and antimicrobial and biofilm-controlling functions. J. Bioact. Compat. Polym. 2015, 30, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Sun, Y. Polymeric N-halamine latex emulsions for use in antimicrobial paints. ACS Appl. Mater. Interfaces 2009, 1, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, J.; Zou, L.; Luo, S.; Yao, R.; Zheng, B.; Liang, G.; Wu, D.; Li, Y. Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 2021, 12, 3303. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Chu, X.; Li, C.; Zhang, C.; Miao, G.; Li, W.; Peng, F.; Zhao, X.; Li, M. Surface modification of titanium with antibacterial porous N-halamine coating to prevent peri-implant infection. Biomed. Mater. 2022, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Kan, Y.; Xie, Y.; Guo, R.; Li, C.; Asilebieke, A.; Xu, Y.; Chu, J. A review on non-leaching antibacterial bone cement for orthopedic surgery: From past to current insights. AIP Adv. 2023, 13, 105034. [Google Scholar] [CrossRef]
- Chu, J.; Li, C.; Guo, J.; Xu, Y.; Fu, Y. Preparation of new bio-based antibacterial acrylic bone cement via modification with a biofunctional monomer of nitrofurfuryl methacrylate. Polym. Chem. 2022, 13, 4675–4683. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, Y.; Kan, Y.; Li, H.; Guo, R.; Han, L.; Bu, W.; Chu, J. Comparison of antibacterial activity and biocompatibility of non-leaching nitrofuran bone cement loaded with vancomycin, gentamicin, and tigecycline. J. Orthop. Surg. Res. 2023, 18, 569. [Google Scholar] [CrossRef]
- Sun, X.; Qian, Z.; Luo, L.; Yuan, Q.; Guo, X.; Tao, L.; Wei, Y.; Wang, X. Antibacterial Adhesion of Poly(methyl methacrylate) Modified by Borneol Acrylate. ACS Appl. Mater. Interfaces 2016, 8, 28522–28528. [Google Scholar] [CrossRef]
- Wen, X.; Almousa, R.; Anderson, G.G.; Xie, D. Developing a novel antibacterial dental resin composite with improved properties. J. Compos. Mater. 2019, 53, 3085–3092. [Google Scholar] [CrossRef]
- Chen, Y.; Caneli, G.; Xie, D. A PMMA bone cement with improved antibacterial function and flexural strength. J. Biomater. Sci. Polym. Ed. 2022, 33, 1398–1414. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, F.; Yu, B.; He, J. Preparation of antibacterial acrylic bone cement with methacrylate derived from benzothiazole. J. Mech. Behav. Biomed. Mater. 2021, 117, 104403. [Google Scholar] [CrossRef] [PubMed]
- Wright, Z.M.; Pandit, A.M.; Holt, B.D.; Sydlik, S.A. Therapeutic Methacrylic Comonomers for Covalently Controlled Release from Mechanically Robust Bone Cement: Kinetics and Structure–Function Relationships. Macromolecules 2019, 52, 3775–3786. [Google Scholar] [CrossRef]
- Weng, Y.; Howard, L.; Guo, X.; Chong, V.J.; Gregory, R.L.; Xie, D. A novel antibacterial resin composite for improved dental restoratives. J. Mater. Sci. Mater. Med. 2012, 23, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Ghalanbor, Z.; Körber, M.; Bodmeier, R. Improved lysozyme stability and release properties of poly(lactide-co-glycolide) implants prepared by hot-melt extrusion. Pharm. Res. 2010, 27, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Vesna Milacic, V.M.; Schwendeman, S.P. Lysozyme release and polymer erosion behavior of injectable implants prepared from PLGA-PEG block copolymers and PLGA/PLGA-PEG blends. Pharm. Res. 2014, 31, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.T.; Sok, M.C.P.; Martin, K.E.; Kalelkar, P.P.; Caplin, J.D.; Botchwey, E.A.; García, A.J. Lysostaphin and BMP-2 co-delivery reduces S. aureus infection and regenerates critical-sized segmental bone defects. Sci. Adv. 2019, 5, eaaw1228. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, S.; Lei, M.; Cai, Y.; Wang, H.; Sun, J.; Cui, J.; Liu, C.; Qu, X. A suture-free, shape self-adaptive and bioactive PEG-Lysozyme implant for Corneal stroma defect repair and rapid vision restoration. Bioact. Mater. 2023, 29, 1–15. [Google Scholar] [CrossRef]
- Buchholz, H.W.; Engelbrecht, H. Depot effects of various antibiotics with Palacos resins. Chirurg 1970, 11, 511–515. [Google Scholar]
- Kang, M.S.; Lee, N.H.; Singh, R.K.; Mandakhbayar, N.; Perez, R.A.; Lee, J.H.; Kim, H.W. Nanocements produced from mesoporous bioactive glass nanoparticles. Biomaterials 2018, 162, 183–199. [Google Scholar] [CrossRef]
- Al Thaher, Y.; Khalil, R.; Abdelghany, S.; Salem, M.S. Antimicrobial PMMA Bone Cement Containing Long Releasing Multi-Walled Carbon Nanotubes. Nanomaterials (Basel) 2022, 12, 1381. [Google Scholar] [CrossRef]
- Kim, D.A.; Lee, J.H.; Jun, S.K.; Kim, H.W.; Eltohamy, M.; Lee, H.H. Sol-gel-derived bioactive glass nanoparticle-incorporated glass ionomer cement with or without chitosan for enhanced mechanical and biomineralization properties. Dent. Mater. 2017, 33, 805–817. [Google Scholar] [CrossRef]
- Yang, L.; Yergeshov, A.A.; Al-Thaher, Y.; Avdokushina, S.; Statsenko, E.; Abdullin, T.I.; Prokopovich, P. Nanocomposite orthopaedic bone cement combining long-acting dual antimicrobial drugs. Biomater. Adv. 2023, 153, 213538. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).