1. Introduction
Gibberellin, a pivotal phytohormone that interacts synergistically with other plant hormones, functions as a central regulator of diverse developmental processes within plants. Gibberellic acid (GA), a specific form of gibberellin, plays a critical role in promoting parthenocarpy and has garnered extensive attention for application in the cultivation of seedless fruits, augmentation of berry dimensions, and elongation of rachis across a wide spectrum of grape cultivars [
1,
2,
3,
4]. GA
3, in conjunction with auxin, plays a pivotal role in stimulating cell division and expansion. This interaction regulates fruit development and subsequent enlargement following fertilization. Recently, the consumption of fruits has increased owing to their high internal and external appearance quality. This trend is associated with economic growth and changing consumer preferences. To meet market demands, producing grapes with standardized cluster length and uniform and large berry size has become crucial. Consequently, gibberellin finds widespread application in production.
Presently, a number of studies have demonstrated the efficacy of gibberellin as an effective agent in enhancing the elongation of grape inflorescence. Sun [
5] reported that the application of gibberellin to Cabernet Franc grape led to a proportional increase in peduncle length with an increase in gibberellin concentration. Similarly, Wang et al.[
6] observed that grape varieties such as Midknight Beauty, Zaoheibao, and Summer Black exhibited elongated fruit pedicels following gibberellin treatments at a concentration of 10 mg/kg. Yang et al.[
7] revealed that the application of exogenous GA
3 not only inhibited the synthesis of endogenous gibberellin but also orchestrated the modulation of gibberellin signal transduction, thereby promoting inflorescence elongation.
Prior research has highlighted the utilization of GA
3 as a viable strategy in grape cultivation, particularly for seed management. Notably, the application of GA
3 solution at a concentration of 100 mg L
−1 prior to full bloom has demonstrated its potential to produce seedless cultivars and prompt seed abortion in seeded cultivars [
1]. Han and Lee [
8] observed that GA
3 exhibited efficacy in not only fostering fruit enlargement but also augmenting cluster length, cluster weight, and berry weight. Korkutal et al.[
4]further substantiated the multifaceted impacts of gibberellins, revealing their role in promoting stem elongation, triggering flowering induction, stimulating pollen tube growth, yielding seedless fruits, and increasing the size of seedless berries. Zhao[
9] expounded upon the benefits of employing GA
3 and streptomycin before the full-bloom stage, coupled with GA
3 application alone post this stage. This approach was found to notably increase berry size of “Shenxiangwuhe” and “12-17,” concurrently facilitating seedlessness in the latter. For the grape variety “Zhuosexiang,” the combined administration of GA
3 and forchlorfenuron (CPPU) before full bloom, followed by GA
3 application post full bloom, exhibited comparable benefits, yielding a remarkable seedless rate of 71%. Notably, GA
3 application emerged as the most effective approach in enhancing single berry weight and seedless rate, as underscored by Zhao et al.[
9].
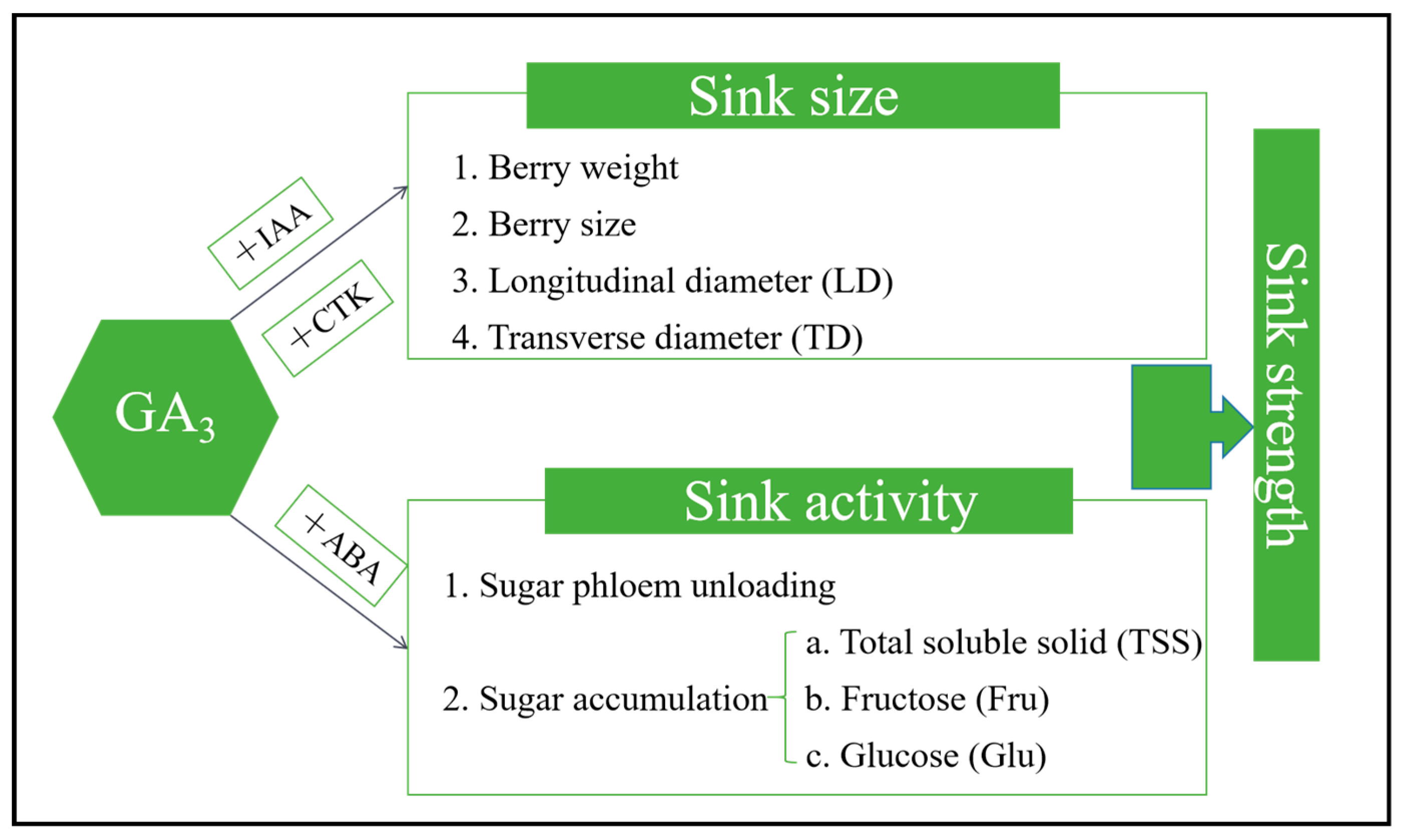
Sink strength, which has been elucidated as the competitive capacity of an organ to efficiently intake photoassimilates, can be considered as a product of two critical components: sink size and sink activity [
10]. Sink size pertains to the physical limitation represented by the total biomass of the sink organ. Notably, the quantification of cells, both in terms of their number and size in the sink organ, can serve as a suitable metric for evaluating sink size. Sink activity is regarded as the physiological limitation that governs the import of assimilates into a sink organ. It is determined by three pivotal physiological processes. First, it involves the unloading of assimilates from the phloem, followed by the subsequent transport of sugars beyond the phloem, leading to their absorption by the sink organ. Second, the sink organ’s own respiratory consumption contributes to sink activity. Third, the accumulation of carbohydrates within sink organs also influences sink activity. Prior research has indicated that phytohormones can modulate sink strength. Notably, hormones such as GA
3, cytokinin (CTK), abscisic acid (ABA), and auxin have been recognized as participants in this regulatory process [
11,
12]. However, the underlying mechanism through which GA specifically influences sink strength in perennial fruit crops, including grape, remains elusive.
Shine Muscat is a prominent table grape variety extensively cultivated in China. This variety is widely known for its excellent characteristics such as large size, sweet taste, good storage capacity, and aromatic qualities; however, certain issues, such as the occurrence of seeded fruit, bitter seeds and pericarp, and tightly packed bunches, remain persistent. GA is widely employed to cultivate larger, seedless berries and enhance overall fruit quality. Previous investigations have demonstrated that GA can induce inflorescence elongation, foster the development of seedless fruits, and augment the size of seedless berries in different grape varieties. Our previous research has also indicated that GA influences the aromatic components in Shine Muscat berries [
13]. However, a research gap remains, particularly in understanding the interrelationship among the contents of soluble sugars, organic acids, and endogenous hormones and sink strength in Shine Muscat grapes subjected to GA
3 treatments. Therefore, the present study aimed to investigate how GA
3 influences the contents of soluble sugars and organic acids, preliminarily explore the interrelationship among these components, and clarify the mechanism underlying the fruit sink strength modulation by GA
3. Furthermore, this research seeks to contribute substantial data to support the cultivation of table grapes and the utilization of GA in China.
2. Materials and Methods
2.1. Field conditions and materials
The experiment was conducted at the Jinniushan vineyard in Tai’an, Shandong, China (36.127°N, 117.004°E). For the study, Shine Muscat (
Vitis labruscana) grapes, which were self-rooted and planted in 2012, were used. The planting arrangement involved a plant density of 3.0 per hectare (with a spacing of 1.0 m between plants and 3.0 m between rows) within a rain shelter cultivation system. For the experimentation, a total of 30 healthy and well-developed vines were meticulously selected, with each treatment group comprising three replicates. On each vine, four shoots were trained, and for each treatment, a single shoot was allocated. The selected shoots were pruned to retain one basal cluster with one inflorescence. Approximately one week before flowering, the inflorescence was pruned, leaving a uniform 5 cm tip length to ensure synchronized flowering across treatments. The vines were subjected to GA
3 (ProGibb 40, Valent BioScience, Walnut Creek, CA, USA) treatment at varying concentrations and during distinct periods. The experimentation comprised four distinct treatment groups, including three GA treatment groups and one control (CK) group, as detailed in
Table 1.
Note:
1. Rachis elongation treatment: Twenty days prior to anthesis, inflorescences were immersed in a solution containing 5 mg/L GA3.
2. Seedlessness treatment: A day after full bloom, inflorescences were immersed in a solution containing 25 mg/L GA3.
3. Expansion treatment: Within two weeks after bloom withering, inflorescences were immersed in a solution containing 30 mg/L GA3.
4. CK treatment: Inflorescences were treated with water.
2.2. Tissue collection
The cluster length of 30 berries for each treatment was assessed at specific time intervals: 0 h, 12 h, 24 h, 3 d, and 7 d after the rachis elongation treatment. Subsequently, for each treatment, 120 grape seeds were randomly chosen from 30 berries on a regular basis at 7:00 am every 20 days [20, 40, 60, 80, and 100 days after treatment (DAT)] post expansion treatment. The samples were segregated into two groups. The first group samples, comprising 60 seeds from each treatment, were collected and transported in an ice box to the laboratory. The seeds were then analyzed for berry weight, longitudinal diameter (LD), transverse diameter (TD), total soluble solids (TSS), and titratable acidity (TA). The second group samples, comprising 60 seeds from each treatment, were frozen in liquid nitrogen and immediately stored in a refrigerator at −80 ℃. These samples were intended for the assessment of soluble sugars (glucose, fructose, and sucrose) and organic acids (tartaric acid, citric acid, and malic acid) in the grape berries. Moreover, samples comprising 30 seeds with consistent fruit diameter, obtained from 30 berries of each treatment, were collected at specific intervals: 0, 1, 2, 4, 8, 24, and 48 h post expansion treatment. Following liquid nitrogen treatment, these samples were enveloped in tinfoil, refrigerated, and subsequently brought back to the laboratory. The samples were then stored in a low-temperature refrigerator (−80 ℃) for the determination of indole-3-acetic acid (IAA), CTK, GA, and ABA contents in grape berries within one month.
2.3. Measurements of berry growth
The weight of individual fruits was determined using an electronic balance, with an accuracy of 0.01 g. For each analysis, a total of 30 berries were randomly selected from 20 to 30 clusters and each of the three replications. LD and TD of the fruits were measured using a vernier caliper. TSS was assessed by utilizing an aliquot of grapevine juice with the aid of a digital refractometer (Automatic Refractometer SMART-1, Atago, Tokyo, Japan), which is denoted as Brix. Total acidity was quantified through an acid-base titration method, employing 0.1 mol L−1 NaOH to achieve a pH of 8.1. The values are expressed as grams of citric acid per kilogram of fresh weight.
2.4. High-performance liquid chromatography analysis of glucose, fructose, and sucrose
Sugars were extracted following the procedure: for each treatment, 1 g of homogenized grape berry material was accurately weighed and then diluted to 10.0 mL using ultrapure water (Millipore, Bedford, MA, USA). The solution was subsequently incubated for 20 min in a water bath set at 35 °C. Afterward, the supernatant was subjected to centrifugation at 21,000×g for 10 min at room temperature (BHG-Hermle Z 365, Wehingen, Germany). The extraction process was repeated three times, and the resulting supernatants were pooled. The liquid supernatant underwent filtration through a 0.22-μm, 13-mm diameter syringe filter (Shanghai Xingya Purification Material Factory, Shanghai, China). Subsequently, the filtered solution was employed for the analysis of glucose, sucrose, and fructose contents. High-performance liquid chromatography (HPLC; Waters, Milford, MA, USA) was employed for the analysis. The separation conditions used for the soluble sugar analysis were as follows: detector, differential refractive index detector (RID); column, YMC- Pack Polyamine II (4.6 mm × 250 mm); phase, acetonitrile/water = 75:25 (v/v); flow rate, 0.8 mL/min; injection amount, 10 μL; column temperature, 40 °C; analysis time, 20 min. The eluted peaks were detected utilizing an RID-10A differential refractive index detector (Shimadzu Co., Ltd., Kyoto, Japan). The quantification of glucose, fructose, and sucrose was conducted using standard curves of authentic compounds. Each treatment was replicated three times.
2.5. High-performance liquid chromatography analysis of tartaric acid, citric acid, and malic acid
Organic acid analyses were conducted utilizing a Waters series 515 chromatography unit, which was equipped with two 515 pumps and a 2487 dual UV detector (Waters Alliance 2695 HPLC) operating at a wavelength of 210 nm. The separation conditions used for organic acid analysis were as follows: column, Thermo Hypersil COLD aQ (4.6 mm × 250 mm, 5 μm); phase, 10 mmol/L NH4H2PO4 (pH = 2.3)/methanol = 98/2 (v/v); flow rate, 0.8 mL/min; injection amount, 10 μL; column temperature, 25 °C; analysis time, 20 min. Quantification of tartaric acid, citric acid, and malic acid was performed using standard curves of authentic compounds. The analysis encompassed extracts from three replicate tissue samples.
2.6. Extraction and determination of GA3, IAA, CTK, and ABA
HPLC (Nexera LC-30A, Shimadzu Co., Ltd.) was employed for analysis of GA3, IAA, CTK, and ABA. The standard hormone sample utilized was a product of Sigma Company. The chromatographic conditions were as follows: mobile phase, methanol: 0.8% glacial acetic acid solution = 55:45 (v/v); flow rate, 0.8 mL/min; injection amount, 10 μL; column temperature, 30 ℃; detection wavelength, 254 nm. The analysis comprised extracts from three replicate tissue samples.
2.7. Analysis of sugar phloem unloading
The sugar phloem unloading was assessed using the fruit cup method. During the softening stage (August 19) and ripening stage (September 28), one sunward-facing grain in grape berries was randomly selected for each treatment. A cross of about 1 cm was delicately marked at the grape’s umbilical region using a scalpel. This process ensured that the flesh remained unharmed and the grape flesh tissue was not cut. Subsequently, the peel was gently separated from the pedicel using tweezers and carefully cut with anatomical scissors. The prepared Mes buffer, comprising 5 mmol/L Mes (2-(n-morph) ethanesulfonic acid), 2 mol/L CaCl2, 100 mmol/L mannitol (D-mannitol), and 0.2% (w/v) polyvinylpyrrolidone (PVPP), was then used to rinse the peel. The peeled grape fruit was meticulously placed into a 20 mm infusion tube filled with Mes buffer. The opening was sealed with a film, and the fruit cup was secured on the cluster. The outer layer of the fruit cup was covered with tin foil to prevent temperature fluctuations from affecting the test results and extending the “berry cup’s” longevity. Sampling times were designated as 9:30–10:00, 11:30–12:00, 13:30–14:00, 15:30–16:00, and 17:30–18:00, during which the replaced buffer was discharged from the cup. Each time, the buffer liquid from the fruit cup was collected, and it was filled using a syringe. The collected samples were immediately frozen using liquid nitrogen and stored at −80 °C for subsequent analysis of glucose, fructose, and sucrose.
2.8. Statistical analysis
Data analysis was performed using Microsoft Excel 2010 and SPSS 26.0. Graphical representations were created using Prism 9. Pearson correlation analysis was conducted using R statistical software.
3. Results
3.1. Effects of GA on physicochemical characteristics of grape berries
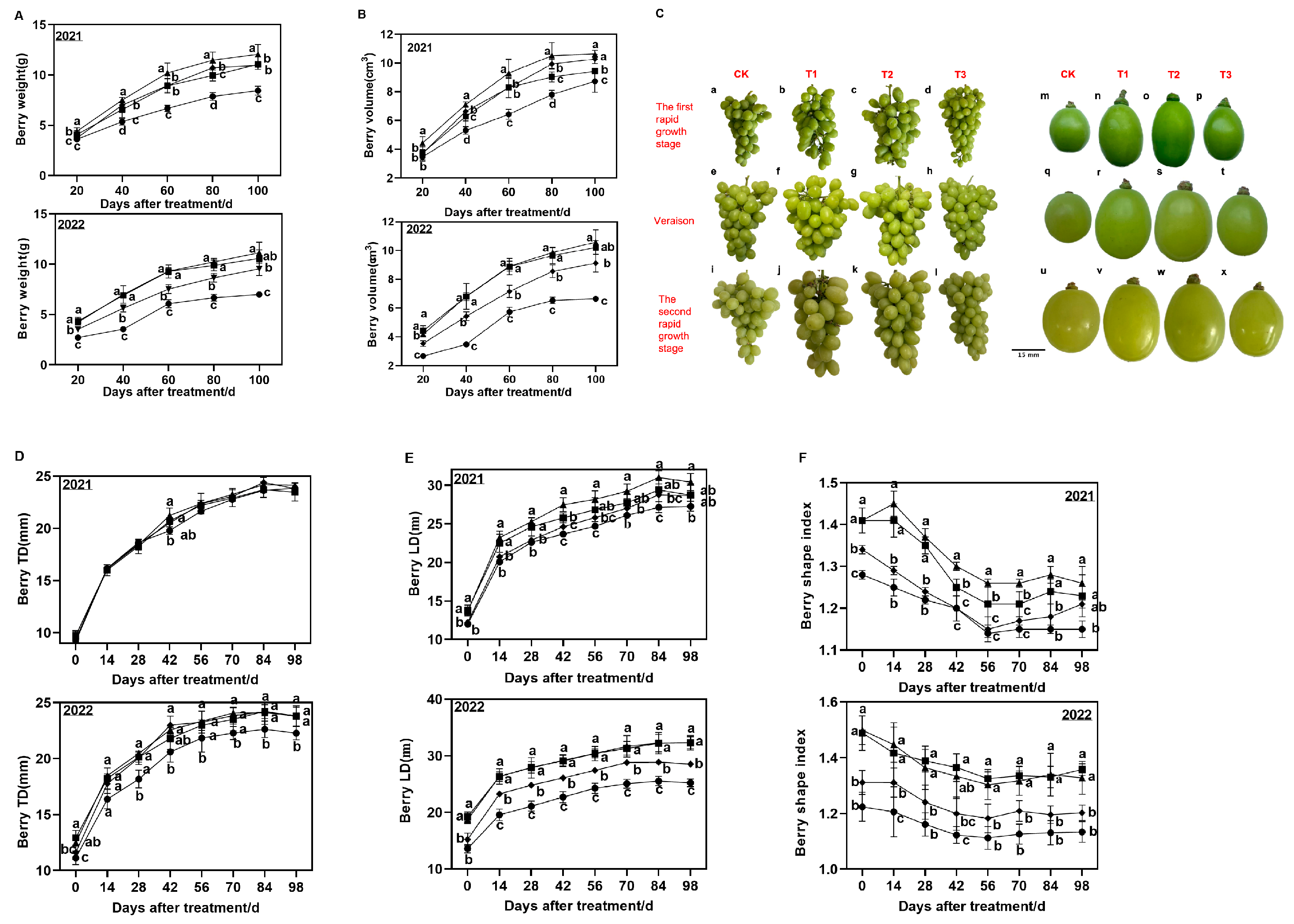
To investigate the effects of GA on Shine Muscat grapes, various key attributes of grape berries during development were evaluated in both GA-treated and CK groups. These parameters included berry weight, berry size, LD, TD, vertical/horizontal ratio (a measure of fruit shape index), TA (g/L), and TSS (Brix) of the berries. As illustrated in
Figure 1A, the trend of changes in berry weight was analogous between GA-treated groups and the CK group. Notably, at each developmental stage, significantly higher berry weights were observed under T1, T2, and T3 treatments than those under the CK treatment. Furthermore, berry weights were substantially higher under T1 and T2 treatments than those under the T3 treatment. At 20–100 DAT, the berry size exhibited a comparable trend to the berry weight for both GA-treated groups and the CK group. The berry weight and volume were more rapidly increased under the T2 treatment than those under any other treatment (as shown in
Figure 1A, B). Specifically, the berry weights were 11.3 g and 12.05 g in 2022 and 2021, respectively, marking a remarkable 52.2% (2022) and 42.8% (2021) increase, respectively, over those in the CK group at 100 DAT (
Figure 1A). Similarly, the berry volumes were 10.59 cm
3 and 10.63 cm
3 in 2022 and 2021, respectively, representing a substantial 21.8% (2021) and 59.2% (2022) increase, respectively, than those in the CK group at 100 DAT (
Figure 1B). Moreover, the berry size was significantly higher in T1, T2, and T3 treatment groups than in the CK group (
Figure 1C). Moreover, no significant increase in TD was observed between GA-treated groups and the CK group (
Figure 1D). In contrast, LD was substantially higher in the three GA-treated groups than in the CK group at 20–100 DAT (
Figure 1E). However, similar LD values were observed for T1 and T2 treatment groups. The fruit shape index was evidently higher in T1 and T2 treatment groups than in the other groups, with that in the CK group being the lowest. At 21–70 DAT, a slight increase in fruit shape index in the Tl group was evident compared to that in the T2 group (
Figure 1F).
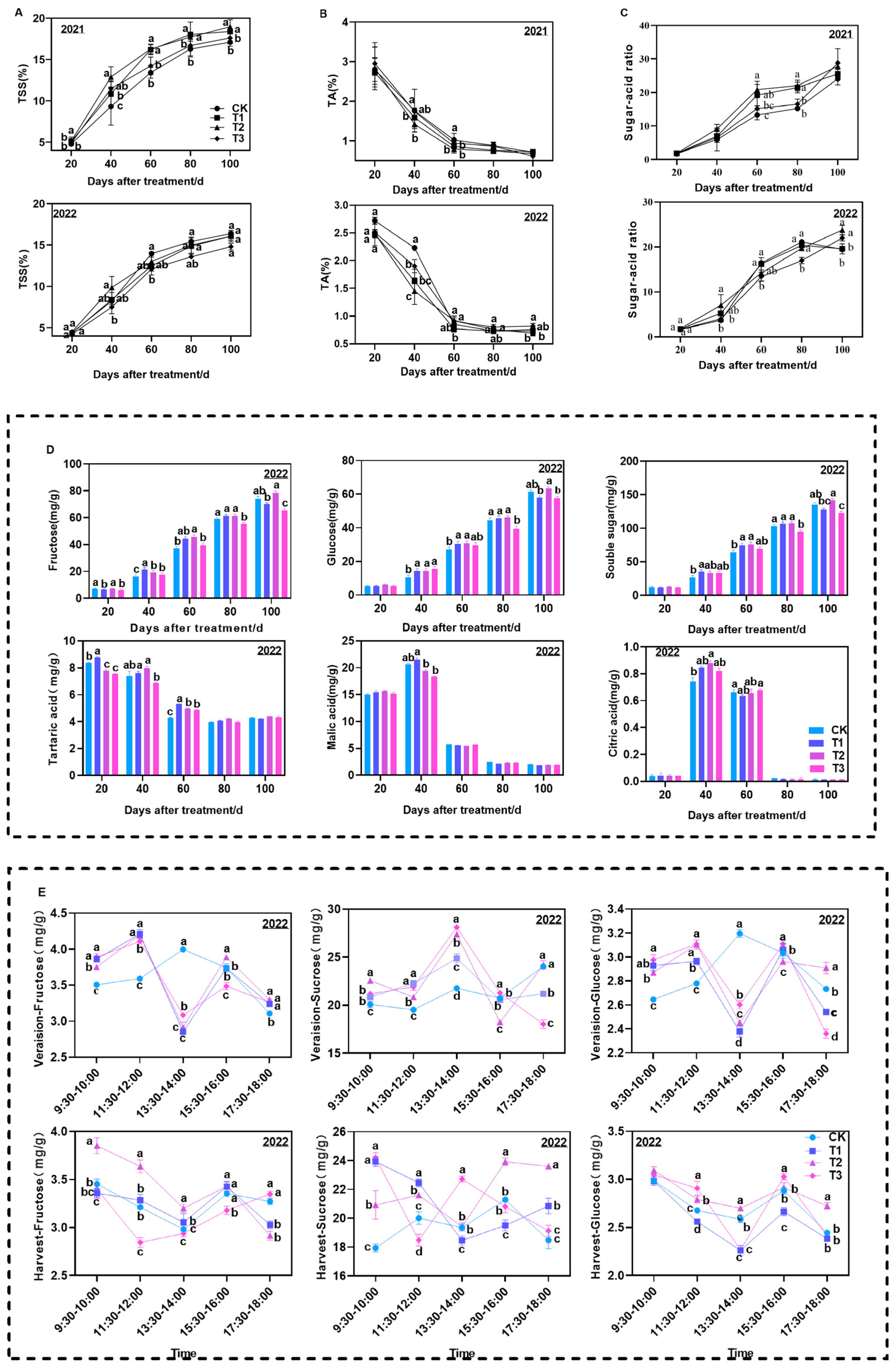
The TSS content in grape berries is illustrated in
Figure 2A. It displayed an increasing trend as the fruits ripened. In 2021, at 20 DAT, remarkably higher TSS contents were observed in GA-treated groups than those in the CK group. At 40 DAT, the TSS content demonstrated the following trend: T2 > T3 > T1 > CK. At 60, 80, and 100 DAT, significantly higher TSS contents were observed under T1 and T2 treatments than those under T3 and CK treatments. Moreover, in 2022, TSS contents increased across all treatments as the fruits matured. At 60 DAT, a significant difference in TSS content was observed between the T3 and CK groups. However, at 20, 40, 80, and 100 DAT, no significant difference was observed between GA-treated groups and the CK group.
An evident declining trend in TA during fruit development was observed, particularly at 20–60 DAT (
Figure 2B). In 2021, no significant differences were observed among the treatments at 20, 80, and 100 DAT. However, at 40 DAT, CK and T3 groups exhibited higher TA compared to T1 and T2 groups. At 60 DAT, the CK group displayed the highest TA among the treatments. In 2022, no significant differences in TA were recorded among the treatments at 20 DAT. At 40 DAT, the overall trend observed was as follows: CK > T3 > T1 > T2, with TA in the CK group being significantly higher than that in GA-treated groups. At 100 DAT, a significant difference in TA was observed under the T3 and T1 (or T2) treatments.
The TSS/TA ratio demonstrated a consistent increasing trend during the course of fruit development. In 2021, varying GA treatments yielded minimal influence on the TSS/TA ratio across different time periods. However, at 100 DAT, the TSS/TA ratio in CK, T1, and T2 groups was significantly higher than that in the T3 group; however, no significant differences were observed among CK, T1, and T2 groups (
Figure 2C).
3.2. Effect of GA on soluble sugar content in grape berries
As illustrated in
Figure 2D, the soluble sugar composition of grape berries primarily consists of glucose and fructose. Notably, the sucrose content was negligible. The glucose and fructose contents within all four groups exhibited a consistent increasing trend; however, throughout the shelf life, the fructose content consistently surpassed that of glucose. The increase in total sugar content was significantly higher at 40–60 DAT than during the maturation stage (80–100 DAT). Prior to reaching 60 DAT, the total sugar content in T1 and T2 treatment groups was significantly higher than that in the CK group. Moreover, the total sugar content in the T3 group was the lowest at 80 DAT among all four treatment groups. During the ripening stage, the T2 group exhibited the highest total sugar content among all four treatment groups.
The fructose and glucose contents within each treatment group gradually increased throughout the shelf life of the fruit. Notably, the changes in glucose and fructose contents in fruits at 80 DAT closely paralleled those observed in the total sugar content. Upon attaining complete maturation, the contents of fructose and glucose in the T2 treatment group exceeded those observed in other treatment groups (glucose: 78.66 ± 1.30 mg/g; fructose: 63.49 ± 1.03 mg/g). Notably, among all groups, the lowest glucose (65.36 ± 1.40 mg/g) and fructose (57.58 ± 1.01 mg/g) contents were observed in the T3 treatment group.
3.3. Effect of GA on organic acid content in grape berries
As illustrated in
Figure 2D, the primary organic acids present in grape berries include tartaric acid, malic acid, and citric acid, with citric acid content being relatively minimal. During the period of shelf life, we noted a distinctive V-shaped trend in the tartaric acid content, which peaked at 20 DAT and plummeted at 80 DAT across all treatments. Prior to 60 DAT, significant differences were observed in the tartaric content among all four treatments; however, these differences were not evident at 100 DAT. At 100 DAT, we observed the lowest and highest tartaric acid contents under T1 (4.31 ± 0.08 mg/g) and T2 (4.38 ± 0.02 mg/g) treatments, respectively.
The malic acid content in grape berries initially increased and then decreased over the course of shelf life. Notably, a significant difference among the four treatments was evident at 40 DAT alone. During the maturation phase, the lowest malic acid content was observed under the T1 treatment (1.83 mg/g), which was 8.74% lower than that observed under the CK treatment.
The change trends in citric acid content paralleled those of malic acid content. At 40 DAT, the citric acid content in all groups reached its peak during the shelf life. Notably, significant differences in citric acid content were observed across all groups at 40 and 60 DAT. At 100 DAT, the lowest citric acid content was noted under the T2 treatment (0.012 mg/g), which was 8.33% lower than those observed under the T1 and T3 treatments.
3.4. Analysis of sugar unloading in phloem
Our results demonstrated that the primary sugars in grape berries were fructose and glucose, with relatively minimal sucrose content at the veraison stage. Notably, the fructose content exceeded that of glucose. As depicted in
Figure 2E, the changes in fructose and glucose unloading in the phloem exhibited a double-peak curve at five distinct time points following GA treatment. In the morning, sugar unloading increased rapidly, reaching its peak at 12:00, closely aligned with the increase in photosynthetic rate. Subsequently, a sharp decline was observed at 14:00. Furthermore, a resurgence in sugar unloading was observed with an increase in photosynthetic rate, reaching a minor peak at 16:00, followed by a gradual decline at sunset. Notably, the morning unloading volume was approximately 1.1 times that of the afternoon unloading. However, the changes in fructose and glucose unloading in the phloem exhibited a unimodal curve at the five time points under the CK treatment. In the morning, sugar unloading increased rapidly, reaching its peak at 14:00, followed by a rapid decrement.
The maximum fructose unloading (3.89 mg/g) was observed between 9:30 and 10:00 in the T3 treatment fruits, significantly surpassing that observed in the CK treatment fruits (3.51 mg/g). For the T1 treatment, the peak fructose unloading (4.21 mg/g) occurred between 11:30 and 12:00. Similarly, the peak fructose unloading for the CK treatment reached 4.00 mg/g at 13:30–14:00 and that for the T2 treatment reached 3.89 mg/g and 3.30 mg/g at 15:30–16:00 and 17:30–18:00, respectively.
Furthermore, the peak glucose unloading for the T3 treatment occurred at 9:30–10:00 and 11:30–12:00, measuring 2.98 mg/g and 3.11 mg/g, respectively. For the CK treatment, the highest glucose unloading occurred at 13:30–14:00, following a pattern similar to that of fructose. Notably, the T3 treatment exhibited the highest glucose unloading (3.11 mg/g) at 15:30–16:00. In addition, at 17:30–18:00, glucose unloading was significantly higher in the T2 group than that observed in other groups. Overall, these findings indicate that the highest sugar (fructose+glucose) unloading (32.25 mg/g) was observed in the T2 treatment group during the veraison stage.
Similarly, in vivo analysis of sugar unloading at the maturation stage resembled that at the veraison stage. The primary sugars in grape berries were fructose and glucose, with relatively minimal amounts of sucrose owing to incomplete transformation. As illustrated in
Figure 2E, the changes in fructose and glucose unloading in the phloem exhibited a unimodal curve at each time point during the maturation stage. Sugar unloading reached its peak at 10:00 in the morning, gradually declining to a minimum at 14:00. Subsequently, sugar unloading increased again with an increase in photosynthetic rate, achieving a minor peak at 16:00 and finally decreasing at sunset. The unloading value observed in the morning was 1 fold higher than that observed in the afternoon. Among the treatments, the T2 treatment demonstrated the highest fructose unloading at the first four time points, while the highest unloading under the T3 treatment was observed at 17:30–18:00. Moreover, the maximum glucose unloading under the T2 treatment was evident at 9:30–10:00 (3.09 mg/g), 13:30–14:00 (2.92 mg/g), and 17:30–18:00 (2.72 mg/g). The T3 treatment group exhibited the highest glucose unloading at 11:30–12:00 (2.91 mg/g) and 15:30–16:00 (3.03 mg/g).
Based on the sugar unloading data at various time points for each treatment, the T2 treatment displayed the highest sugar unloading (fructose+glucose) at the maturation stage, with a value of 31.26 mg/g.
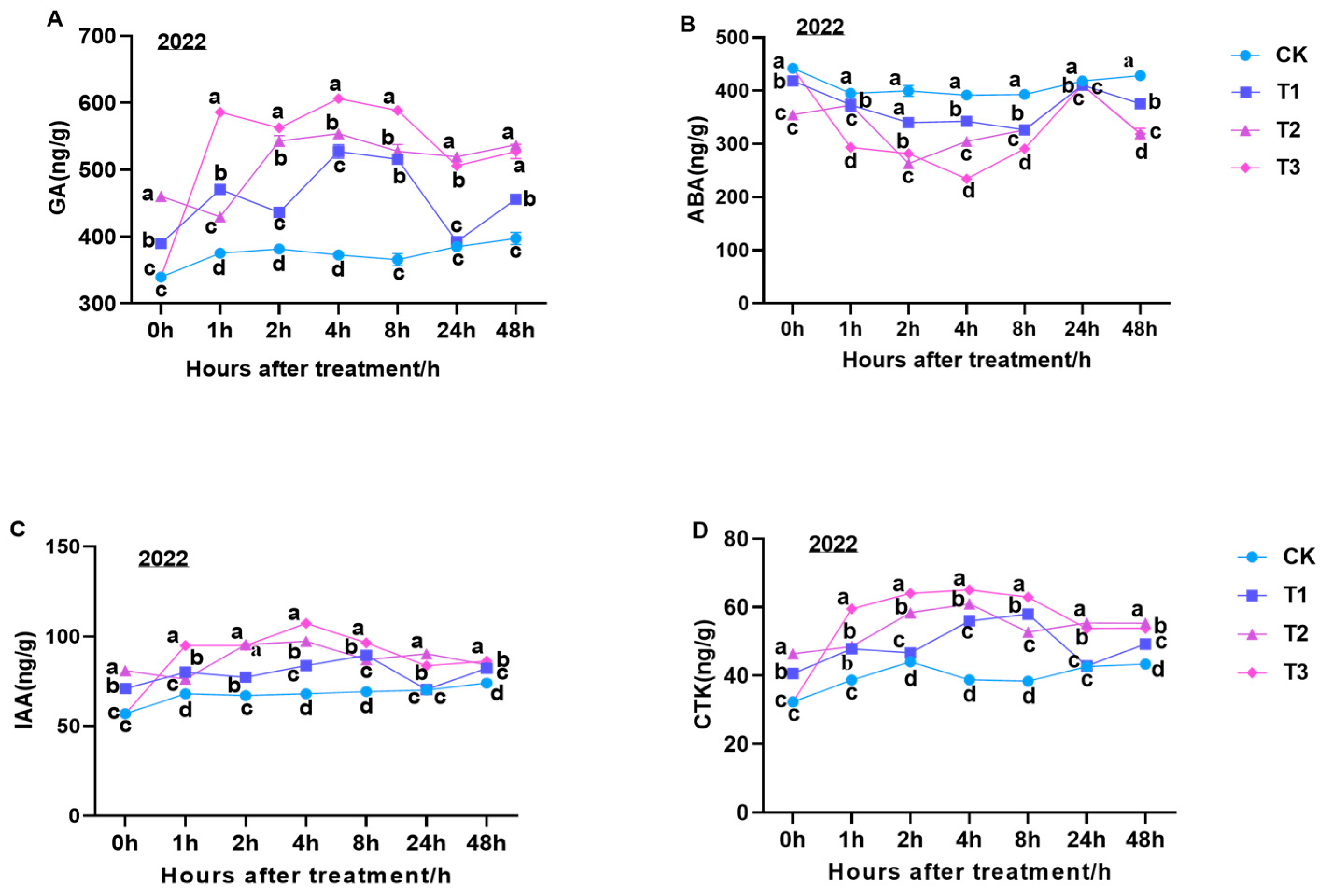
3.5. Effect of GA on endogenous hormone contents in grape berries
Four types of endogenous hormones—GA, ABA, IAA, and CTK—were analyzed. The GA content demonstrated a fluctuating trend at 0–48 h after GA
3 expansion treatment. It reached a peak at 0–4 h, followed by a sharp decline and a rapid increase at 24 h. In contrast, the GA content under the CK treatment exhibited a gradual increase at 48 h. Notably, the overall trend in GA content at 1–24 h was as follows: T3 > T2 > T1 > CK (
Figure 3A). The ABA contents were significantly higher in CK treatment fruits than those in fruits subjected to the GA treatment. The lowest and highest ABA contents in berries were observed at 4 h and 24 h after GA treatment, respectively. At 48 h, the overall trend in ABA content was as follows: CK > T1 > T3 > T2 (
Figure 3B). The IAA content in fruits displayed a trend similar to that observed for GA. The highest IAA content in berries under the T1 treatment was observed at 8 h, and at 48 h, the following overall trend was noted: T3 > T2 > T1 > CK (
Figure 3C). The CTK content in berries increased rapidly at 0–2 h under CK, T2, and T3 treatments, followed by a slower upward trend that gradually decreased at 4 h. Moreover, the CTK content showed an upward trend under the T1 treatment at 0–8 h. At 48 h, the following trend was observed: T2 > T3 > T1 > CK (
Figure 3D).
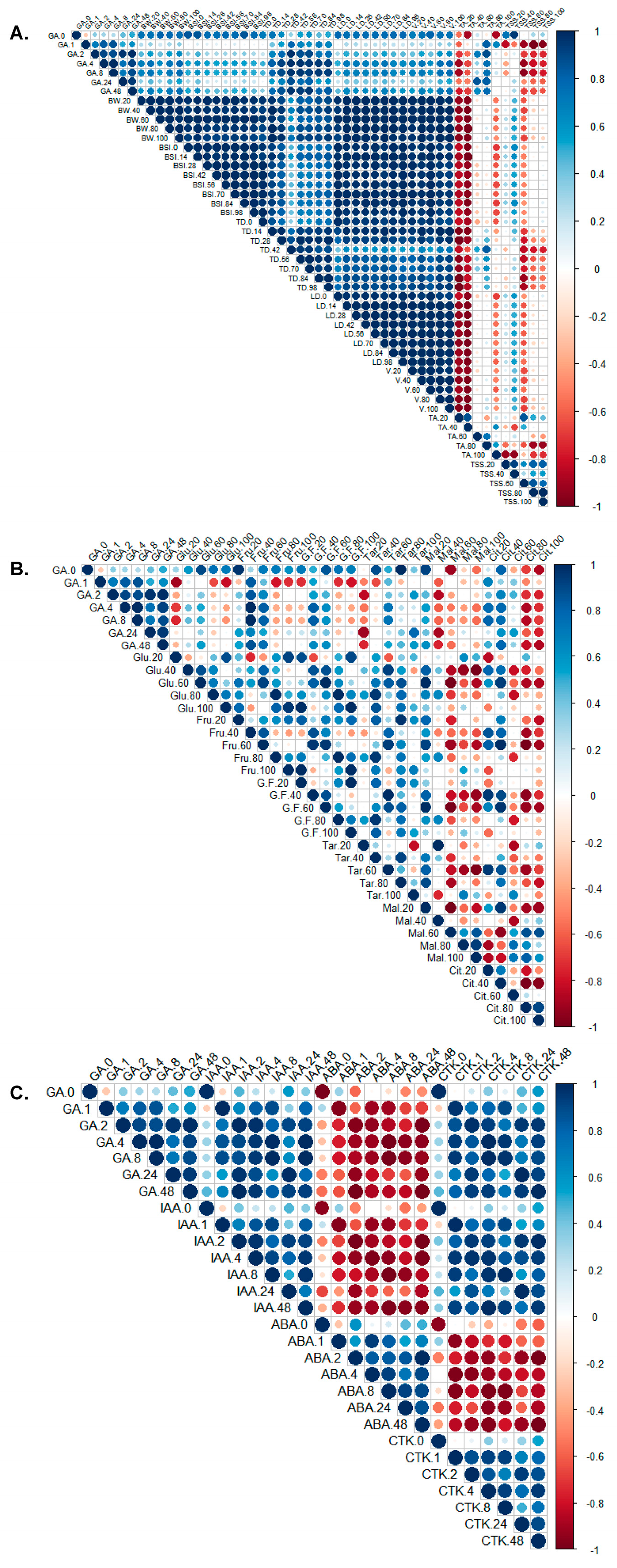
3.6. Correlation analysis
The correlation analysis depicted in
Figure 4 examines the relationships among the contents of endogenous GA, soluble sugars, organic acids, and key parameters, namely, berry weight, berry shape index, TD, LD, volume, TSS, and TA. Through correlation analysis, we observed a significant positive correlation between the GA content and the key parameters, including berry weight, berry shape index, TD, LD, and volume, particularly with TD. Furthermore, a significant negative correlation was noted between GA and TSS contents at 60, 80, and 100 DAT, as well as between the GA content and TA at 20 and 40 DAT. In contrast, a significant positive correlation was observed between the GA content and TA at 60 and 80 DAT (
Figure 4A).
The contents of reducing sugars, glucose, and fructose in fruits displayed a significant positive correlation with GA contents at 40 and 60 DAT (Figure. 4B); however, a significant negative correlation between them was observed at 80 and 100 DAT. Notably, the GA content was significantly negatively correlated with tartaric acid content at 20 DAT, malic acid content at 40, 60, 80, and 100 DAT, and citric acid content at 80 and 100 DAT (Figure. 4B). In contrast, the GA content was significantly positively correlated with tartaric acid content at 60, 80, and 100 DAT, malic acid content at 20 DAT, and citric acid content at 20, 40 and 60 DAT (
Figure 4B).
The heatmap presented in
Figure 4C indicates that IAA and CTK contents were significantly positively correlated with GA
3 contents at 1, 2, 4, 8, 24, and 48 h. Furthermore, the correlation analysis highlighted that ABA contents were significantly negatively correlated with GA
3 contents and IAA and CTK contents at 1, 2, 4, 8, 24, and 48 h. Notably, a significant positive correlation was observed between IAA and CTK contents.
5. Conclusions
Collectively, this study introduced two significant and novel findings that contribute to our understanding of grape physiology and growth regulation: (i) GA3 exerted significant effects on the contents of soluble sugars, organic acids, and endogenous hormones (IAA, CTK, and ABA). The application of GA3 led to enhanced sugar unloading during the softening and ripening stages. (ii) The appropriate application of GA3 plays a crucial role in orchestrating the modulation of sink size and activity, including the enhancement of berry size, the facilitation of sugar phloem unloading, and the accumulation of sugars within sink cells. These insights collectively exert a robust effect on the overall sink strength in grape development. Moreover, these novel findings significantly enhance our comprehension of the complex interplay between GA3 and soluble sugar, organic acid, and endogenous hormone contents. This study presents compelling empirical evidence that contributes to the broader body of knowledge supporting effective strategies for table grape cultivation and the proficient utilization of GA3, ultimately benefiting the grape industry in China.
Author Contributions
Conceptualization, X.J.L., Z.S.X., L.L. and B.L.; methodology, X.J.L., Z.H.C. and Z.S.X.; sample preparation and analysis, Z.H.C.; investigation, X.L.L. and G.W.Y; data curation, Z.H.C. and S.X.L.; writing—original draft preparation, L.L.; writing—review and editing, B.L.; Supervision, X.L.L., Y.S.W. and Z.H.; project administration, B.L.; funding acquisition, Z.H., L.L. and B.L. All authors approved the final manuscript. All authors have read and agreed to the published version of the manuscript.