Preprint
Article
The Impact of C-3 Side Chain Modifications on Kynurenic Acid: A Behavioral Analysis of Its Analogs on Motor Domain
Altmetrics
Downloads
157
Views
129
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
19 February 2024
Posted:
20 February 2024
You are already at the latest version
Alerts
Abstract
The central nervous system (CNS) is the final frontier in drug delivery because of the blood-brain barrier (BBB), which poses significant barriers to the access of most drugs to their targets. Kynurenic acid (KYNA), a tryptophan (Trp) metabolite, plays an important role in behavioral functions and abnormal KYNA levels have been observed in neuropsychiatric conditions. The current challenge lies in delivering KYNA to the CNS owing to its polar side chain. Recently, C-3 side chain modified KYNA analogs have been shown to cross the BBB; however, it is unclear whether they retain the biological functions of the parent molecule. This study examined the impact of KYNA analogs, specifically SZR-72, SZR-104, and the newly developed SZRG-21, on behavior. The analogs were administered intracerebroventricularly (i.c.v.), and their effects on the motor domain were compared with those of KYNA. Specifically, open field (OF) and rotarod (RR) tests were employed to assess motor activity and skills. SZR-104 increased horizontal exploratory activity in the OF test at a dose of 0.04 μmol/4 μL, while SZR-72 decreased vertical activity at doses of 0.04 and 0.1 μmol/4 μL. In the RR test, however, neither KYNA nor its analogs showed any significant differences in motor skills at either dose. Side chain modification affects affective motor performance and exploratory behavior, as the results show for the first time. In this study, we showed that KYNA analogs alter emotional components such as motor-associated curiosity and emotions. Consequently, drug design necessitates the development of precise strategies to traverse the BBB while paying close attention to modifications in their effects on behavior.
Keywords:
Subject: Medicine and Pharmacology - Medicine and Pharmacology
1. Introduction
The central nervous system (CNS), which comprises the brain and spinal cord, regulates vital processes, including cognition, motion, and emotion [1,2,3]. Neurological conditions such as Alzheimer's disease (AD), Parkinson's disease (PD), epilepsy, stroke, brain tumors, and psychiatric conditions including major depressive disorders and schizophrenia are examples of disorders that can affect the CNS [4,5,6]. To treat these conditions, medications must reach the cells and tissues of interest in the CNS. However, this is challenging because the blood–brain barrier (BBB) protects the CNS [7,8,9]. In addition to endothelial cells that are interconnected via junctional proteins, the BBB comprises structurally and functionally supporting cells including astrocytes, pericytes, and microglia. The BBB is a natural defense mechanism that prevents toxins, pathogens, and foreign substances from entering the brain where they can potentially cause harm [10,11,12]. However, it also restricts the delivery of most therapeutic agents because only small, lipophilic, and uncharged molecules can passively diffuse across the BBB [13,14,15]. Consequently, because it reduces the bioavailability and efficacy of numerous drugs, the BBB is a significant barrier to drug delivery to the brain.
The BBB restricts the passage of highly polar molecules, such as sugars, amino acids, peptides, and nucleosides, isolating the brain from many essential compounds [16,17,18,19]. Various modifications of their side chains have been explored to facilitate the penetration of these impermeable molecules [16,20,21,22]. One strategy involves the use of hydrocarbon "staples" to link the side chains of polar molecules, and the other is the use of N-methyl phenylalanine-rich peptides, which have been investigated as highly versatile BBB shuttles [23,24,25]. These modifications aim to enhance the ability of highly polar molecules to traverse the BBB, thereby enabling their access to the CNS for potential therapeutic and research applications. Modifying the polarity of molecules by altering their side chains can be an effective approach for enhancing their BBB permeability and improving their CNS efficacy [20,26,27,28].
Tryptophan (Trp) is an essential amino acid involved in various biological processes, such as the synthesis of protein, neurohormone such as serotonin and melatonin, and various indole derivatives [29,30,31,32,33]. Over 90% of Trp in the body is metabolized through the kynurenine (KYN) pathway, which generates several bioactive metabolites that have diverse effects on the central nervous and immune systems [34,35,36,37]. Dysregulation of the KYN metabolism has been implicated in various neuropsychiatric disorders such as depression, schizophrenia, AD, and PD [38,39,40,41,42,43]. The KYN degradation take place through two main branches of metabolism: the neurotoxic and neuroprotective branches [44,45,46]. The balance between the neurotoxic and neuroprotective metabolic branches is crucial for maintaining homeostasis and function of the CNS [47,48,49]. However, this balance can be disturbed by various factors, resulting in the accumulation of neurotoxic KYNs and depletion of neuroprotective KYNs in the CNS [50,51,52]. This imbalance can cause neuronal damage, synaptic dysfunction, and cognitive impairment and contribute to the pathogenesis of neuropsychiatric disorders [53,54,55]. Therefore, KYN metabolism is a potential therapeutic target for neuropsychiatric disorders (Figure 1) [56,57,58].
Kynurenic acid (KYNA) is metabolized by the Trp-KYN metabolic pathway and functions as a neuroprotective metabolite [59,60,61]. Its antioxidant properties and antagonistic activity against ionotropic glutamate receptors, including N-methyl-D-aspartate (NMDA) receptors, are responsible for its neuroprotective effects [62,63,64]. KYNA has been implicated in various neuropsychiatric and neurodegenerative disorders, and KYNA levels in the brain and body are influenced by factors such as inflammation, stress, aging, and genetic variation [62,65,66,67,68,69]. Furthermore, recent studies have shown promising connections between KYNA and emotional learning, shedding light on its potential role in modulating affective motor function and emotional responses [70,71,72,73,74,75,76,77]. The neural substrates involved in emotional learning, particularly KYNA, suggest a plausible impact on the limbic system, including structures such as the amygdala and prefrontal cortex, which are known to be involved in emotional regulation and associative learning [74,78,79,80,81,82,83,84,85,86]. For example, it has been shown that KYNA and its synthetic analogs, such as SZR-72 and SZR-104, possess the ability not only to influence motor domains of behavior but also to potentially modulate emotional responses [87]. Therefore, KYNA appears to be a potential drug candidate for the treatment of neuropsychiatric disorders because it can regulate the balance between neurotoxicity and neuroprotection [70,88,89,90,91,92,93,94,95,96].
However, more research is needed to evaluate its safety and efficacy and to consider its interactions with other metabolic pathways of Trp. KYNA binds to the receptor strychnine-insensitive glycine-binding site of NMDA receptor [97,98]. At millimolar concentrations, KYNA inhibits the postsynaptic ionotropic glutamate receptor, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, kainate receptor, and the interaction with the G protein-coupled receptor 35 [99,100]. NMDA receptors are present in the mammalian brain in the post- and extrasynaptic membranes of glutamatergic neurons (Figure 2) [101,102]. We understand the various NMDA receptor subtypes associated with the gamma-aminobutyric acidergic and dopaminergic systems, as well as their functions [103,104,105,106].
Synthetic analogs of KYNA investigated in this study include N-(2-(dimethylamino)ethyl)-4-oxo-1,4-dihydroquinoline-2-carboxamide (SZR-72) as a KYNA amide derivative, N-(2-(dimethylamino)ethyl)-3-(morpholinomethyl)-4-oxo-1,4-dihydroquinoline-2-carboxamide (SZR-104) as an aminoalkylated amide derivative with the new function at C–3 position, and N-(2-(dimethylamino)ethyl)-3-methyl-4-oxo-1,4-dihydroquinoline-2-carboxamide (SZRG-21) that contains an alkyl group in C–3 position as a transitional derivative between SZR-72 and SZR-104 (Figure 3). They are able to mimic the pharmacological actions of KYNA, including the antagonistic effects on glutamate receptors [107]. They can also affect the morphology and function of microglia, which are brain immune cells, as well as the expression and methylation of histone H3 a protein that controls gene transcription [107,108]. These analogs may have potential therapeutic applications for neuroinflammatory and neurodegenerative disorders.
Given their ability to mimic KYNA's pharmacological actions of KYNA, particularly its antagonistic effects on glutamate receptors, these analogs have the potential to alter the delicate balance within neural circuits implicated in conditions such as schizophrenia, bipolar disorder, and major depressive disorder, all of which are associated with glutamatergic dysregulation [89,109,110,111]. Moreover, the influence of these analogs on microglial morphology and function, along with their impact on histone H3 expression and methylation, suggests their broader implications in neuroinflammatory processes mediated by brain-heart interactions [73,112,113]. By modulating microglial behavior and epigenetic regulation, these analogs may exert neuroprotective effects, potentially attenuating the progression of debilitating disorders [114,115,116,117]. The multifaceted actions of these analogs on both the immune response and epigenetic regulation highlight their promise as a novel class of compounds for addressing the complex pathophysiological mechanisms underlying various neuropsychiatric and neurologic conditions [118,119,120].
The main objective of this study was to investigate the effects of KYNA and its analogs on the motor domain of behavior in mice. This study aimed to synthesize KYNA analogs, SZR-72, SZR-104, and SZRG-21, with different side chain modifications that may affect their permeability to the BBB and administer them intracerebroventricularly (i.c.v.) at two different doses (0.04 and 0.1 mol/4 L) to mice (Figure 3), in order to get the most objective and immediate feedback about the effects of analog molecules in vivo on individual brain regions and thus on memory and motor functions. The study also aimed to measure the exploratory and affective motor function and motor skills of the mice using the OF and RR tests and compare the behavioral outcomes of KYNA and its analogs. The study further opens avenues to analyze the possible mechanisms underlying their effects on the motor domain of behavior and evaluate their potential as novel therapeutic agents for neuropsychiatric disorders involving motor impairments.
2. Results
2.2. Behavioral Tests
2.2.1. Pilot Study
The 1-methyl-8-azabicyclo[3.2.1]octane (Dizocilpine aka. MK-801) molecule (Figure 4) in lower dose, 0.04 μmol/4 μL caused ataxia symptoms in mice. However, KYNA did not cause any side effects at this dose (Figure 5).
Figure 5.
Effects of MK-801 on pilot stereotype/ataxia tests. MK-801 caused massive stereotypes and ataxia symptoms in mice after i.c.v. injection. (A) Stereotype symptoms score of MK-801 treatment, 0.04 μmol/4 μL MK-801 vs. control (Ctrl) group, 0.1 μmol/4 μL MK-801 vs. Ctrl group (P=0.017). The statistical analysis was Kruskal-Wallis Non-parametric test (P<0.05) (B) Ataxia symptoms score of MK-801 treatment, 0.04 μmol/4 μL MK-801 vs. Ctrl group, 0.1 μmol/4 μL MK-801 vs. Ctrl group (P=0.001). The statistical analysis was Kruskal-Wallis Non-parametric test (P <0.05). We show the number of animals and the score category. *: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=5, N(0.04 μmol/4 μL MK-801)=5, and N(0.1 μmol/4 μL MK-801)=5.
Figure 5.
Effects of MK-801 on pilot stereotype/ataxia tests. MK-801 caused massive stereotypes and ataxia symptoms in mice after i.c.v. injection. (A) Stereotype symptoms score of MK-801 treatment, 0.04 μmol/4 μL MK-801 vs. control (Ctrl) group, 0.1 μmol/4 μL MK-801 vs. Ctrl group (P=0.017). The statistical analysis was Kruskal-Wallis Non-parametric test (P<0.05) (B) Ataxia symptoms score of MK-801 treatment, 0.04 μmol/4 μL MK-801 vs. Ctrl group, 0.1 μmol/4 μL MK-801 vs. Ctrl group (P=0.001). The statistical analysis was Kruskal-Wallis Non-parametric test (P <0.05). We show the number of animals and the score category. *: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=5, N(0.04 μmol/4 μL MK-801)=5, and N(0.1 μmol/4 μL MK-801)=5.

On the other hand, when the animals were treated with the higher dose, 0.1 μmol/4 μL, the ataxia and stereotype scores were higher in the MK-801 treated group than in the lower dose group. We observed that the 0.1 μmol/4 μL dose induced ataxia symptoms in the KYNA group (Figure 6).
2.2.2. Stereotype/Ataxia Test
In our previous experiments, we observed that MK-801 had a more pronounced effect at both doses, whereas the effect of KYNA has more pronounced at higher doses. Therefore, we sought to investigate the effects of KYNA and its analog molecules at both doses in further experiments. Specifically, we treated the right lateral brain ventricles of mice with KYNA, SZR-72, SZR-104, and SZRG-21 at 0.04 and 0.1 μmol/4 μL. We did not observe a significant difference in stereotype between the treated groups at the 0.04 μmol/4 μL dose (Figure 7), as this dose did not cause ataxia. However, at the 0.1 μmol/4 μL dose, we did observe a significantly higher ataxia score in the KYNA group (P=0.004) than in the Control, SZR-72, and SZRG-21 groups (Figure 8B), but we did not observe a significant difference in stereotype between the treated groups (Figure 8A).
2.2.3. Open Field (OF) Test
Ten minutes after that we treated i.c.v. the animals with both doses (0.04 and 0.1 μmol/4 μL) of KYNA and its analog molecules and after the stereotype/ataxia behavior test was monitoring, was observed the spontaneous locomotor and exploration activities of mice. The mice were inserted into the center of the open-field box, and their behavior was measured within 15 min. The results of experiment were significant difference in the horizontal motion (ambulation distance) between the 0.04 μmol/ 4 μL dose of KYNA and its analogs treated groups. The ambulation distance was significantly higher in the mice treated with SZR-104 than in the control (P=0.01), KYNA (P=0.001), and SZR-72 (P=0.026) groups (Figure 9A). The vertical motion (rearing count) was significantly different between the SZR-72 and control (P=0.019), SZR-104 (P=0.015), and SZRG-21 (P=0.02) groups (Figure 9B).
When we treated the animals with a 0.1 μmol/4 μL dose of KYNA and analog molecules, there was no significant difference in the horizontal motion (ambulation distance) between the treated groups (Figure 10A). However, in the vertical motion (rearing count), there was a significant difference (P=0.04) between the 0.1 μmol/4 μL dose of SZR-72 and the Control group (Figure 10B).
2.2.4. Rotarod (RR) Test
Based on data from previous experiments, it was confirmed that KYNA did not accumulate in the extracellular space, it eluted rapidly [121], and the KYNA concentration in the mouse serum and CNS samples decrease after 30-40 minutes [122]. Therefore, we were interested in the effect of KYNA on motor coordination and balance in mice 25-30 minutes after i.c.v. treatment. During our investigations, we found that the 0.04 and 0.1 mol/4 L dose of KYNA and its analogs after i.c.v. injection did not significantly affect the locomotion skills of the mice (Figure 11 and Figure 12).
3. Discussion
The CNS plays a critical role in regulating essential functions such as cognition, motion, and emotion [123,124,125,126]. However, they are susceptible to various disorders that impair their function [4,5,6,127,128,129,130,131]. One of the major challenges in treating these disorders is the BBB, a semi-permeable membrane that protects the CNS from harmful substances but also limits the delivery of therapeutic agents to the brain [15,132,133,134]. The BBB is composed of various cells, junctions, and transporters that regulate the transport of molecules between the blood and brain [135,136,137,138]. Strategies have been developed to overcome this barrier by modifying the polarity of highly polar molecules such as sugars, amino acids, peptides, and nucleosides, which are essential for the CNS but cannot cross the BBB [139,140,141]. These modifications enhance the BBB permeability of these molecules, enabling their access to the CNS for potential therapeutic and research applications [20,142,143,144].
Trp is metabolized into neurotoxic and neuroprotective KYNs [44,45,46]. The balance of these KYNs affects the CNS and the immune system, and its dysregulation is linked to neuropsychiatric disorders [38,39,40,145,146,147]. Neurotoxic and neuroprotective branches of KYN, the equilibrium of which is critical for maintaining homeostasis and function in the CNS, have been implicated in its degradation [47,48,49,148,149]. On the contrary, KYN metabolites demonstrate an extensive array of bioactive characteristics, including but not limited to immunomodulating, oxidant, antioxidant, anti-inflammatory, and neurotoxin action [150,151,152]. The specific effects of these metabolites are contingent upon their concentration and the cellular milieu [35,153,154]. Furthermore, the metabolic system operates within intricate positive and negative feedback loops [66,155,156,157]. Furthermore, a critical aspect is the absence of consensus concerning the functionalities of KYN metabolites.
KYNA is a neuroprotective KYN that possess antioxidant property, antagonizes glutamate receptors, and modulates immunity and digestion [158,159,160]. KYNA is involved in neurological disorders, affected by various factors, and is a potential drug candidate for neuropsychiatric conditions [91,161,162,163,164]. The KYNA analog SZR-72 has been demonstrated to attenuate the severity of acute necrotizing pancreatitis in experimental settings, regulate body weight and home-cage activity in mice, and inhibit nitroglycerol-induced enhancement in c-Fos immunoreactivity within the rat caudal trigeminal nucleus [165,166,167]. Its effects are comparable to those of KYNA. Similarly, SZR-104 has been shown to modulate the immune systems, and inhibit glutamate receptors, with effects similar to KYNA [168]. In animal models, SZR-104 has been found to inhibit epileptiform seizures [169]. Furthermore, SZR-104 alters the intracellular distribution and methylation patterns of histone H3, a protein that regulates gene expression [108]. These findings indicate the potential therapeutic applications of these compounds in neurological and psychiatric disorders. However, further studies are required to evaluate its safety and efficacy. SZRG-21, the C-3 alkyl group-transitional derivative of SZR-72 and SZR-104, is a recently synthesized analog whose biological functions have yet to be investigated.
Firstly, the pilot study showed that the effects of KYNA and MK-801, two NMDA receptor antagonists, on mice behavior. MK-801 caused more ataxia and stereotype than KYNA at both doses (0.04 and 0.1 μmol/4 μL). KYNA causes ataxia at a higher dose. Our previous study showed that KYNA elicits antidepressant-like effects and improve learning and memory [163,170]. We investigated the effects of KYNA and its analogs (SZR-72, SZR-104, and SZRG-21) on spontaneous locomotor and exploratory activities of mice after i.c.v. injections. The horizontal and vertical motions of the mice were measured in an OF box for 15 min. The lower dose (0.04 μmol/4 μL) of SZR-104 increased the horizontal motion, while the lower dose of SZR-72 decreased the vertical motion, compared to the control and other groups. The higher dose (0.1 μmol/4 μL) of SZR-72 decreased the vertical motion compared to the control group. The other groups did not show any significant differences at the higher doses. It was also observed that KYNA and its analogs did not affect the motor coordination and balance of mice 25-30 min after i.c.v. injections.
Studying the effects of MK-801, KYNA, and KYNA analogs on mice behavior presents several challenges and requires specific knowledge and technology. The research revealed that MK-801 caused more ataxia and stereotypes than KYNA at both doses, whereas KYNA caused ataxia at a higher dose. Additionally, the study tested KYNA analogs and found that they did not cause significant behavioral changes. To achieve these results, researchers need a deeper understanding of NMDA receptor antagonists, mice behavior, and the specific effects of KYNA and its analogs. The technology required for this study includes precise dosing and administration methods for i.c.v. injections, as well as behavioral testing equipment to measure ataxia, stereotype, and spontaneous locomotor and exploration activities in mice [171,172,173]. Additional research is warranted in this field, as these results supplement current knowledge that specific dosages of KYNA enhance cognition and memory in addition to its antidepressant-like properties and pain modulation [163,170,174]. Additionally, KYNA analogs have exhibited promising results in animal models of various neurological and psychiatric disorders, although their mechanisms of action, pharmacokinetics, and safety warrant further investigation [165,166,167,168,175]. Therefore, KYNA analogs represent a novel class of drugs with the potential for future clinical applications.
Preclinical research, including in vitro and in vivo studies, can provide invaluable data, which are not feasible to investigate in humans [176,177,178,179,180,181,182,183,184,185,186,187,188,189,190]. This study showed that incorporation of the C-3 side chain elicits subtle differences in curiosity and emotion in animal models of motor function. Ongoing clinical studies have advanced our understanding of the behavioral domains in neuropsychiatric conditions and have sought their potential management [184,191,192,193,194,195,196,197,198,199]. Furthermore, computational strategies accelerate the advancement in comprehending their pathology as well as developing novel strategies for the management of neurological and psychiatric disorders, including neurotropic computer-assisted drug design [200,201,202,203,204,205,206,207,208]. Integrating these interdisciplinary approaches further adds impetus and optimizes strategies, including drug development research, leading to the testing and assessment of potential lead compounds. These outcomes enable researchers to evaluate the effects of novel interventional approaches such as drug-assisted brain stimulation [106,209,210,211,212,213,214]. These methods demonstrate promise for the development of new and more effective treatments, including novel drugs [215]. Furthermore, advanced imaging techniques have played a significant role in brain research [216,217,218]. Neuroimaging studies have uncovered structural and functional brain changes that are associated with neuropsychiatric disorders and therapeutics [219,220,221,222]. These imaging techniques can aid in identifying unique clinical cases and provide valuable insights into the pathophysiology of these disorders and novel therapeutic strategies [214,223,224,225]. Further research is needed to determine the optimal concentration for neuroprotection and the threshold for neurotoxicity. The findings of this study suggest that KYNA analogs represent a new class of drugs with potential clinical applications in neurological and psychiatric disorders.
4. Materials and Methods
4.1. Materials
We utilized a solution containing 0.9% saline (B Braun Melsungen AG, Hessen, Germany) in a 4 μL volume, as well as MK-801 (Sigma-Aldrich Ltd., Budapest, Hungary) and KYNA (Sigma-Aldrich Ltd., Budapest, Hungary) molecules in a pilot study. In our experiment, we employed 0.9% saline and KYNA (Sigma-Aldrich Ltd., Budapest, Hungary) in a 0.1 μmol/4 μL dose, as well as KYNA analogs (SZR-72, SZR-104, SZRG-21) in equal doses to KYNA. The new analogs were synthesized at the Faculty of Pharmacy, Institute of Pharmaceutical Chemistry, University of Szeged using the procedures described in Section 4.9. Fresh solutions of MK-801, KYNA, and its analogs were prepared by dissolving them in 0.9% aqueous saline and adjusting the pH to 7.4.
4.2. Kynurenic Acid Analogs Synthesis
The synthesis of KYNA analogs SZR-72 and SZR-104 was carried out in accordance with previously reported methods [175]. The synthesis of compound SZRG-21 commenced with aniline 1 (1.00 g, 10.70 mmol) which was reacted with diethyl oxalpropionate (4 mL, ρ = 1.073 g/mL, 21.2 mmol) in ethanol (20 mL) under reflux conditions. The progress of the reaction was monitored by TLC (eluent = n-hexane:ethyl acetate, 4:1), and once the reaction was complete, it was cooled to room temperature. Fewer side products were observed compared to the reaction run in acetic acid, which is commonly used as a solvent in the literature for synthesizing this compound [226,227,228,229]. After evaporation of the ethanol under reduced pressure, column chromatography was employed for purification using n-hexane:ethyl acetate (4:1) as the eluent. Diethyl 2-methyl-3-(phenylamino)maleate (2) was obtained as a yellow oil, yield = 0.81 g (65 %; Figure 13).
1H (500 MHz, DMSO), δ(ppm): 1.06 (3H, t, J = 7.2 Hz); 1.25 (3H, t, J = 6.9 Hz); 1.77 (3 H, s); 4.12-4.21 (4H, m); 6.99 (2H, d, J = 7.2 Hz); 7.07 (1H, t, J = 7.5 Hz); 7.30 (t, 2H, J = 7.6 Hz, 1H); 10.01 (1H, s), 13C (125 MHz, DMSO), δ(ppm): 13.6, 13.9, 14.7, 60.4, 62.2, 95.9, 120.7, 124.2, 129.8, 140.5, 146.9, 164.7, 169.9 (Figure S1 and S2).
The solvents used during the ring closure process were modified from those reported in the literature, including PPA, mineral oil, and diphenyl ether; instead, 1,2-dichlorobenzene (DCB, 20 mL) was employed as the solvent. The reaction was carried out at reflux temperature for a total of 24 h, resulting in almost complete conversion of the starting material to compound 3, with only minor side products evident (as determined by TLC with the eluent DCM:MeOH 19:1). After the removal of the solvent under reduced pressure, crystallization was induced using Et2O (3 mL), yielding a beige crystal with a mass of 0.64 g (95 %; Figure 13).
The amidation process was carried out under neat conditions, starting with 3 (0.60 g, 2.59 mmol) and employing an excess of N1,N1-dimethylethane-1,2-diamine at room temperature. The progress of the reaction was assessed by TLC (eluent: DCM:MeOH 19:1), and upon completion, 5 mL of DCM was added. The resulting precipitate was filtered and washed with diethyl ether in two separate instances (2 × 10 mL). The final product (4) was a white crystal, yield = 0.59 g (83 %; Figure 13), and exhibiting a melting point of 192-194 °C.
1H (500 MHz, DMSO), δ(ppm): 1.99 (3H, s); 2.20 (6H, s); 2.43 (2H, t, J = 6.5 Hz); 3.33-3.43 (2H, m); 7.29 (1H, t, J = 7.5 Hz); 7.57-7.65 (2H, m); 8.08 (1H, d, J = 7.6 Hz); 8.72-8.79 (1H, m); 13C (125 MHz, DMSO), δ(ppm): 11.7, 37.7, 45.7, 58.3, 113.5, 118.7, 123.3, 123.8, 125.4, 132.0, 139.6, 143.6, 163.9, 187.7 (Figure S3, S4).
SZRG-21 was prepared by initiating from 4 (0.55 g, 2.01 mmol) in Et2O (10 mL). HCl/EtOH (22%) was then slowly added until the pH reached 1-2. The resulting crystals were filtered and washed with Et2O (2 × 10 ml). The yield of SZRG-21 was 0.59 g (94 %). During melting point determination SZRG-21 decomposed over 300 °C (Figure 13).
1H (500 MHz, DMSO), δ(ppm): 2.00 (3H, s); 2.85 (6H, d, J = 4.8 Hz); 3.31 (2H, q, J = 6.1 Hz); 3.66 (2H, q, J = 5.9 Hz), 7.32 (1H, t, J = 7.6 Hz); 7.66 (1H, t, J = 7.2 Hz); 7.77 (1H, d, J = 8.1 Hz); 8.11 (1H, d, J = 7.8 Hz); 9.18 (1H, t, J = 5.5 Hz); 10.43 (1H, brs); 12.45 (1H, brs); 13C (125 MHz, DMSO), δ(ppm): 11.6, 34.8, 42.8, 55.7, 113.9, 118.8, 123.6, 123.7, 125.3, 132.2, 139.5, 142.8, 164.3 (Figure S5, S6).
4.3. Animals
The study utilized male C57BL6/J mice (Mus musculus, Charles River Laboratories, Germany) that weighed between 25-30 grams. These animals were 10-12 weeks old and housed in cages containing a maximum of five mice per cage. The mice were kept under standard laboratory conditions, including access to tap water and regular mouse chow, and were maintained on a 12-hour light-dark cycle at a temperature of 24±1 °C and humidity of 50±10%. The animals were handled in accordance with the Regulations of the Faculty of Medicine, University of Szeged, Ethical Committee for the Protection of Animals in Research. This study was approved by the Ethical Committee for the Protection of Animals in Research at the University of Szeged (XVII/275/2023) and the Hungarian Health Committee (40/2013 (II.14.)), and the European Community Council Directive (2010/63/EU).
4.4. Surgery
Mice were anesthetized with 4% chloral hydrate (Sigma-Aldrich Ltd., Budapest, Hungary) at a dose of 0.4 g/kg body weight. A polyethylene cannula (Fisher Scientific, Intramedic Clay Adams polyethylene tube, Budapest Hungary) was inserted into the right lateral brain ventricle and fixed to the skull using cyanoacrylate (Ferrobond, Budapest Hungary). The stereotaxic coordinates were set at anterior-posterior 0.2 mm, medial-lateral 1.09 mm to the bregma, with the cannula extending 2.3 mm deep into the skull surface. After a recovery period of five days, the mice were used for the experiments. On the 8th day following surgery, KYNA or its analogs or saline (4 µL) were injected into the right lateral brain ventricle of the mice using an infusion pump (KD Scientific, Holliston, Massachusetts, USA) at a rate of 8 µL/min. The correct location of the inner i.c.v. cannula was confirmed by injecting 1% methylene blue solution after the experiment [230].
4.5. Behavioral Tests
The tests were carried out at the same time of the day to minimize variations in the diurnal rhythm of the animals. Prior to assessing each animal, the equipment was thoroughly cleaned with 70% alcohol to remove lingering scents [231,232].
4.5.1. Pilot Study
To determine the most effective dose of KYNA that did not cause severe side effects, we compared KYNA with an NMDA receptor antagonist molecule, MK-801. We treated the animals with 0.9 % of saline in 4 μL volumen, MK-801 in 0.04 and 0.1 μmol/4 μL dose and KYNA in equal dose of MK-801 (Figure 4). These molecules were administered to mice in the right lateral brain ventricle. We used five mice per group and had 5 groups during the pilot experiment. We observed the effect of MK-801 and KYNA in the Stereotype/Ataxia behavior test after i.c.v. injections. The duration of the observation was 10 min.
4.5.2. Stereotype Behavior and Ataxia
Behavioral changes, including ataxia and stereotyped behavior, were observed and recorded 10 min after treatment administration. The mice were then monitored for an additional 10 min, during which their behavior was rated using the scale described by Sturgeon et al. (1979) [233] and Contreras (1990) [234]. The scale measures five categories of stereotyped behavior, including sniffing, grooming, and rearing behavior, as well as reciprocal forepaw treading or undirected head movement, backward walking, head weaving, circling behavior, continuous head weaving, circling, or backward walking, and dyskinetic extensions or flexion of the limbs, head, and neck, or weaving greater than four. For ataxia, the scale measures awkward and jerky movements, stumbling or awkward posture, falling, inability to move beyond a small area or support weight on the stomach or haunches, and inability to move, except for twitching movements.
4.5.3. Open Field (OF) Test
Spontaneous locomotor and exploratory activities were assessed using an automated tracking system within the activity chamber. The chamber was linked to a computer that recorded the exploration and locomotor activity of the subjects [235,236,237]. Each animal was placed individually at the center of a black box measuring 60 × 60 × 70 cm, which was equipped with automated infrared photocells for precise measurements. The box platform is divided into nine equal squares. The animals were allowed to move freely for 15 min and divided into three distinct sessions. The movement signals were analyzed using Conducta 1.0. We quantified ambulation distance and duration of rearing, which serve as indicators of horizontal and vertical movement, spontaneous locomotor activity, and exploratory behavior.
4.5.4. Rotarod (RR) Test
The rotarod test was used to evaluate motor coordination and balance. The rod, which rotated about its longitudinal axis, was positioned horizontally, and the animals were required to walk forward to maintain equilibrium and prevent falling off. The time taken for the mice to fall from the rotating rod was measured using an infrared sensor and the latency to fall was automatically scored [238,239]. Motor coordination was assessed by comparing the latency to fall between the treatment groups, whereas motor learning was evaluated by comparing the first trial to subsequent trials after training, which demonstrated an increase in latency to fall over time. Prior to training, each mouse was acclimatized to the device for one hour at rest. On the first and second days, the animals were trained at a constant speed of 5 revolutions per minute (rpm) on a rotating rod, with three trials lasting five minutes each, and 15-minute intervals between trials. On the third day, 25 min after intraventricular (i.c.v.) injection, the latency at which each mouse fell off the rod in standard mode (5 rpm within 5 min) was recorded.
4.6. Statistical Analysis
In our study, we utilized Microsoft SPSS software (version 2.0) for the statistical analysis of our data. To evaluate our results, we employed a One-way ANOVA test that was adjusted using both the LSD and Bonferroni post hoc tests, as well as the Kruskal-Wallis non-parametric test.
5. Conclusion
The BBB hinders drug delivery to the CNS, where many neuropsychiatric disorders arise. KYNA, a Trp metabolite, regulates CNS functions such as cognition, mood, and motor activity. Abnormal KYNA levels are linked to disorders, such as schizophrenia, depression, and AD. However, KYNA cannot cross the BBB owing to its polarity. In this study, we prepared three KYNA analogs with different side chains, SZRG-21, SZR-72, and SZR-104, to improve their lipophilicity and BBB permeability. We tested their effects on mice behavior using the OF and RR tests. We found that SZR-104 increased horizontal exploration, whereas SZR-72 decreased the vertical motion. Neither KYNA nor its analogs affected motor skills. These results show that side-chain modification alters KYNA’s behavioral effects of KYNA and its interactions with its receptors in the CNS. Our study offers new insights into the pharmacological properties of KYNA-based drugs for neuropsychiatric disorders and their pharmacological properties. We also emphasize the need for further research on KYNA and its analogs in the CNS.
Author Contributions
Conceptualization, I.S., and L.V.; methodology, D.M. and B.L.; software, D.M. and B.L.; validation, D.M., B.L., and I.S. ; formal analysis, D.M. and B.L.; investigation, D.M. and B.L.; resources, D.M. and B.L.; data curation, D.M. and B.L.; writing—original draft preparation, D.M., B.L., and M.T.; writing—review and editing, D.M., B.L., I.S., L.V., and M.T.; visualization, D.M. and B.L.; supervision, I.S., L.V., and M.T.; project administration, I.S. and L.V.; funding acquisition, I.S., L.V, and M.T.
Funding
This work was supported by the National Research, Development, and Innovation Office—NKFIH K138125, SZTE SZAOK-KKA No:2022/5S729, and the HUN-REN Hungarian Research Network. The authors’ thanks are due to the Ministry of Human Capacities, Hungary grant, TKP-2021-EGA-32. The work was also supported by the ÚNKP-22-4-SZTE-166 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund.
Institutional Review Board Statement
The animal study protocol was approved by the Ethical Committee for the Protection of Animals in Research of the University of Szeged (Szeged, Hungary), which specifically approved this study (XI/275/2023) and the protocol for animal care approved both by the Hungarian Health Committee (40/2013 (II.14.)) and by the European Communities Council Directive (2010/63/EU).
Acknowledgments
We would like to express our sincere gratitude to the late Prof. Ferenc Fülöp, a pharmaceutical chemist who supported this line of research. His vision, expertise, and mentorship were invaluable for the successful completion of this work. We deeply regret that he could not see the final outcome of his efforts. He will be sorely missed by all.
Conflicts of Interest
The authors declare that they have no conflict of interest and have received no payment in preparation of their manuscript.
Abbreviations
| AA | anthranilic acid |
| Acetyl-CoA | acetyl coenzyme A |
| ACMS | 2-amino-3-carboxymuconate semialdehyde |
| ACMSD | 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AMS | 2-aminomuconic-6-semialdehyde |
| AMSD | 2-aminomuconate semialdehyde dehydrogenase |
| AD | Alzheimer's disease |
| BBB | blood–brain barrier |
| CA | cinnabarinic acid |
| GPR35 | G-protein-coupled receptor 35 |
| CNS | central nervous system |
| 5-HT | serotonin |
| IDOs | indoleamine 2,3-dioxygenases |
| 3-HAA | 3-hydroxyanthranilic acid |
| 3-HK | 3-hydroxy-L-kynurenine |
| 3-HAO | 3-hydroxyanthranilate oxidase |
| KAT | kynurenine aminotransferase |
| KFA | kynurenine formamidase |
| KI | knock-in |
| KMO | kynurenine 3-monooxygenase |
| KO | knockout |
| KYN | kynurenine |
| KYNA | kynurenic acid |
| KYNU | kynureninase |
| MK-801 | 1-methyl-8-azabicyclo[3.2.1]octane |
| NADH | nicotinamide adenine dinucleotide + hydrogen |
| N-fKYN | N-formyl-kynurenine |
| NMDA | N-methyl-D-aspartic acid |
| PC | picolinic acid |
| PD | Parkinson's disease |
| PLP | pyridoxal 5’-phosphate |
| QPRT | quinolinate phosphoribosyltransferase |
| QUIN | quinolinic acid |
| SZR72 | diethyl 2-methyl-3-(phenylamino)maleate |
| SZR-104 | N-(2-(dimethylamino)ethyl)-4-oxo-1,4-dihydroquinazoline-2-carboxamide |
| SZRG-21 | N-(2-(dimethylamino)ethyl)-4-oxo-1,4-dihydroquinazoline-2-carboxamide hydrochloride |
| TDO | tryptophan 2,3-dioxygenase |
| Trp | Tryptophan |
| XA | xanthurenic acid |
References
- Derryberry, D.; Tucker, D.M. Neural mechanisms of emotion. J Consult Clin Psychol 1992, 60, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L. A Network Model of the Emotional Brain. Trends Cogn Sci 2017, 21, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Di Rienzo, F.; El Hoyek, N.; Guillot, A. Autonomic nervous system correlates in movement observation and motor imagery. Front Hum Neurosci 2013, 7, 415. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Körtési, T.; Szok, D.; Tajti, J.; Vécsei, L. From CGRP to PACAP, VIP, and Beyond: Unraveling the Next Chapters in Migraine Treatment. Cells 2023, 12, 2649. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Szok, D.; Csáti, A.; Szabó, Á.; Tanaka, M.; Vécsei, L. Exploring novel therapeutic targets in the common pathogenic factors in migraine and neuropathic pain. International Journal of Molecular Sciences 2023, 24, 4114. [Google Scholar] [CrossRef]
- Zigmond, M.J.; Wiley, C.A.; Chesselet, M.-F. Neurobiology of brain disorders: biological basis of neurological and psychiatric disorders; Academic press: 2022.
- Bors, L.A.; Erdő, F. Overcoming the blood–brain barrier. challenges and tricks for CNS drug delivery. Scientia Pharmaceutica 2019, 87, 6. [Google Scholar] [CrossRef]
- Abbott, N.J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. Journal of inherited metabolic disease 2013, 36, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Barchet, T.M.; Amiji, M.M. Challenges and opportunities in CNS delivery of therapeutics for neurodegenerative diseases. Expert opinion on drug delivery 2009, 6, 211–225. [Google Scholar] [CrossRef]
- Alahmari, A. Blood-brain barrier overview: structural and functional correlation. Neural Plasticity 2021, 2021. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Frontiers in cellular neuroscience 2021, 15, 661838. [Google Scholar] [CrossRef]
- Zinserling, V. Defense Mechanisms and Local Immunity of the Brain. In Infectious Lesions of the Central Nervous System; Springer: 2022; pp. 5–24.
- Fong, C.W. Permeability of the blood–brain barrier: molecular mechanism of transport of drugs and physiologically important compounds. The Journal of membrane biology 2015, 248, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Seelig, A. The role of size and charge for blood–brain barrier permeation of drugs and fatty acids. Journal of molecular neuroscience 2007, 33, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Begley, D.J. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther 2004, 104, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.; Zlokovic, B.V. The blood-brain barrier, amino acids and peptides; Springer Science & Business Media: 2012.
- Oldendorf, W.H. The blood-brain barrier. Experimental eye research 1977, 25, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug delivery 2019, 26, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M. Molecular anatomy of the brain endothelial barrier: an overview of the distributional features. Curr Med Chem 2007, 14, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Witt, K.A.; Gillespie, T.J.; Huber, J.D.; Egleton, R.D.; Davis, T.P. Peptide drug modifications to enhance bioavailability and blood-brain barrier permeability. Peptides 2001, 22, 2329–2343. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Wang, Y.; Chen, Y.; Xing, S.; Liao, Q.; Chen, Y.; Li, Q.; Li, W.; Sun, H. Strategies for structural modification of small molecules to improve blood–brain barrier penetration: a recent perspective. Journal of Medicinal Chemistry 2021, 64, 13152–13173. [Google Scholar] [CrossRef]
- Chung, S.; Yi, Y.; Ullah, I.; Chung, K.; Park, S.; Lim, J.; Kim, C.; Pyun, S.-H.; Kim, M.; Kim, D. Systemic Treatment with Fas-Blocking Peptide Attenuates Apoptosis in Brain Ischemia. International Journal of Molecular Sciences 2024, 25, 661. [Google Scholar] [CrossRef]
- Peraro, L.; Kritzer, J.A. Emerging methods and design principles for cell-penetrant peptides. Angewandte Chemie International Edition 2018, 57, 11868–11881. [Google Scholar] [CrossRef]
- Malakoutikhah, M.; Prades, R.; Teixido, M.; Giralt, E. N-methyl phenylalanine-rich peptides as highly versatile blood− brain barrier shuttles. Journal of medicinal chemistry 2010, 53, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Navarro, M.; Teixido, M.; Giralt, E. Jumping hurdles: peptides able to overcome biological barriers. Accounts of chemical research 2017, 50, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.S.; Ghorpade, A.; Labhasetwar, V. Targeting anti-HIV drugs to the CNS. Expert opinion on drug delivery 2009, 6, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.; Rosch, J.; Putnam, D. Concepts, technologies, and practices for drug delivery past the blood–brain barrier to the central nervous system. Journal of Controlled Release 2016, 240, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Morofuji, Y.; Nakagawa, S. Drug development for central nervous system diseases using in vitro blood-brain barrier models and drug repositioning. Current pharmaceutical design 2020, 26, 1466–1485. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Török, N.; Vécsei, L. Are 5-HT1 receptor agonists effective anti-migraine drugs? Expert Opinion on Pharmacotherapy 2021, 22, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.; Hernández-Ruiz, J. Melatonin: synthesis from tryptophan and its role in higher plant. In Amino acids in higher plants; CAB International Wallingford UK: 2015; pp. 390–435.
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Frontiers in cellular and infection microbiology 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. The uniqueness of tryptophan in biology: properties, metabolism, interactions and localization in proteins. International journal of molecular sciences 2020, 21, 8776. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, F.; Ducrocq, C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. Journal of pineal research 2008, 45, 235–246. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.-H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci (Landmark Ed) 2015, 20, 1116–1143. [Google Scholar] [PubMed]
- Badawy, A.A.-B. Kynurenine pathway and human systems. Experimental Gerontology 2020, 129, 110770. [Google Scholar] [CrossRef] [PubMed]
- Ruddick, J.P.; Evans, A.K.; Nutt, D.J.; Lightman, S.L.; Rook, G.A.; Lowry, C.A. Tryptophan metabolism in the central nervous system: medical implications. Expert reviews in molecular medicine 2006, 8, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Dezsi, L.; Tuka, B.; Martos, D.; Vecsei, L. Alzheimer’s disease, astrocytes and kynurenines. Current Alzheimer Research 2015, 12, 462–480. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial impairment: A common motif in neuropsychiatric presentation? The link to the tryptophan–kynurenine metabolic system. Cells 2022, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Muneer, A. Kynurenine pathway of tryptophan metabolism in neuropsychiatric disorders: pathophysiologic and therapeutic considerations. Clinical Psychopharmacology and Neuroscience 2020, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Kindler, J.; Lim, C.K.; Weickert, C.S.; Boerrigter, D.; Galletly, C.; Liu, D.; Jacobs, K.R.; Balzan, R.; Bruggemann, J.; O’Donnell, M. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Molecular psychiatry 2020, 25, 2860–2872. [Google Scholar] [CrossRef] [PubMed]
- Iwaoka, K.; Otsuka, C.; Maeda, T.; Yamahara, K.; Kato, K.; Takahashi, K.; Takahashi, K.; Terayama, Y. Impaired metabolism of kynurenine and its metabolites in CSF of parkinson’s disease. Neuroscience Letters 2020, 714, 134576. [Google Scholar] [CrossRef] [PubMed]
- Baran, H.; Jellinger, K.; Deecke, L. Kynurenine metabolism in Alzheimer's disease. Journal of neural transmission 1999, 106, 165–181. [Google Scholar] [CrossRef]
- Tan, L.; Yu, J.-T.; Tan, L. The kynurenine pathway in neurodegenerative diseases: mechanistic and therapeutic considerations. Journal of the neurological sciences 2012, 323, 1–8. [Google Scholar] [CrossRef]
- Urbańska, E.M.; Chmiel-Perzyńska, I.; Perzyński, A.; Derkacz, M.; Owe-Larsson, B. Endogenous kynurenic acid and neurotoxicity. Handbook of neurotoxicity 2021, 1–31. [Google Scholar]
- Sharma, R.; Razdan, K.; Bansal, Y.; Kuhad, A. Rollercoaster ride of kynurenines: Steering the wheel towards neuroprotection in Alzheimer’s disease. Expert Opinion on Therapeutic Targets 2018, 22, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Advances in Clinical and Experimental Medicine 2021, 30, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Polyák, H.; Galla, Z.; Nánási, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabó, Á.; Klivényi, P.; Tanaka, M. The tryptophan-kynurenine metabolic system is suppressed in cuprizone-induced model of demyelination simulating progressive multiple sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of kynurenine pathway in oxidative stress during neurodegenerative disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Barone, P. The ‘Yin’and the ‘Yang’of the kynurenine pathway: excitotoxicity and neuroprotection imbalance in stress-induced disorders. Behavioural pharmacology 2019, 30, 163–186. [Google Scholar] [CrossRef]
- Savitz, J.; Drevets, W.C.; Smith, C.M.; Victor, T.A.; Wurfel, B.E.; Bellgowan, P.S.; Bodurka, J.; Teague, T.K.; Dantzer, R. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 2015, 40, 463–471. [Google Scholar] [CrossRef]
- Maddison, D.C.; Giorgini, F. The kynurenine pathway and neurodegenerative disease. In Proceedings of the Seminars in cell & developmental biology; 2015; pp. 134–141. [Google Scholar]
- Pérez-De La Cruz, V.; Königsberg, M.; Santamaría, A. Kynurenine pathway and disease: an overview. CNS Neurol Disord Drug Targets 2007, 6, 398–410. [Google Scholar] [PubMed]
- Stone, T.W.; Darlington, L.G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. British journal of pharmacology 2013, 169, 1211–1227. [Google Scholar] [CrossRef]
- Tao, X.; Yan, M.; Wang, L.; Zhou, Y.; Wang, Z.; Xia, T.; Liu, X.; Pan, R.; Chang, Q. Homeostasis imbalance of microglia and astrocytes leads to alteration in the metabolites of the kynurenine pathway in LPS-induced depressive-like mice. International journal of molecular sciences 2020, 21, 1460. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Sorgdrager, F.J.H.; Naudé, P.J.W.; Kema, I.P.; Nollen, E.A.; Deyn, P.P. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front Immunol 2019, 10, 2565. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci Ther 2022, 28, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Tutakhail, A.; Boulet, L.; Khabil, S.; Nazari, Q.A.; Hamid, H.; Coudoré, F. Neuropathology of kynurenine pathway of tryptophan metabolism. Current pharmacology reports 2020, 6, 8–23. [Google Scholar] [CrossRef]
- Gong, X.; Chang, R.; Zou, J.; Tan, S.; Huang, Z. The role and mechanism of tryptophan–kynurenine metabolic pathway in depression. Reviews in the Neurosciences 2023, 34, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Deora, G.S.; Kantham, S.; Chan, S.; Dighe, S.N.; Veliyath, S.K.; McColl, G.; Parat, M.O.; McGeary, R.P.; Ross, B.P. Multifunctional Analogs of Kynurenic Acid for the Treatment of Alzheimer's Disease: Synthesis, Pharmacology, and Molecular Modeling Studies. ACS Chem Neurosci 2017, 8, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Bratek-Gerej, E.; Ziembowicz, A.; Godlewski, J.; Salinska, E. The mechanism of the neuroprotective effect of kynurenic acid in the experimental model of neonatal hypoxia–ischemia: The link to oxidative stress. Antioxidants 2021, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the etiological links behind neurodegenerative diseases: Inflammatory cytokines and bioactive kynurenines. International Journal of Molecular Sciences 2020, 21, 2431. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune influencers in action: metabolites and enzymes of the tryptophan-kynurenine metabolic pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Török, N.; Török, R.; Molnár, K.; Szolnoki, Z.; Somogyvári, F.; Boda, K.; Tanaka, M.; Klivényi, P.; Vécsei, L. Single Nucleotide Polymorphisms of Indoleamine 2, 3-Dioxygenase 1 Influenced the Age Onset of Parkinson's Disease. Frontiers in Bioscience-Landmark 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vecsei, L. Monitoring the redox status in multiple sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.F. Genetic and hormonal regulation of tryptophan–kynurenine metabolism: implications for vascular cognitive impairment, major depressive disorder, and aging. Annals of the New York Academy of Sciences 2007, 1122, 35–49. [Google Scholar] [CrossRef]
- Battaglia, M.R.; Di Fazio, C.; Battaglia, S. Activated Tryptophan-Kynurenine metabolic system in the human brain is associated with learned fear. Frontiers in Molecular Neuroscience 2023, 16, 1217090. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Vitale, F.; Battaglia, S.; de Vega, M.; Avenanti, A. Task-related modulation of motor response to emotional bodies: A TMS motor-evoked potential study. Cortex 2024, 171, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Tortora, F.; Hadipour, A.L.; Battaglia, S.; Falzone, A.; Avenanti, A.; Vicario, C.M. The role of Serotonin in fear learning and memory: a systematic review of human studies. Brain Sciences 2023, 13, 1197. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Heart's tale of trauma: Fear-conditioned heart rate changes in post-traumatic stress disorder. Acta Psychiatr Scand 2023, 148, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Talarowska, M.; Galecki, P. Cognition and emotions in recurrent depressive disorders-the role of inflammation and the kynurenine pathway. Current Pharmaceutical Design 2016, 22, 955–962. [Google Scholar] [CrossRef]
- Ren, W.H.; Guo, J.D.; Cao, H.; Wang, H.; Wang, P.F.; Sha, H.; Ji, R.R.; Zhao, Z.Q.; Zhang, Y.Q. Is endogenous d-serine in the rostral anterior cingulate cortex necessary for pain-related negative affect? Journal of neurochemistry 2006, 96, 1636–1647. [Google Scholar] [CrossRef]
- Javelle, F. Impulsivity and its biomarkers: A focus on the tryptophan pathways and the moderating effects of physical exercise. 2021.
- Lasoń, W.; Budziszewska, B.; Basta-Kaim, A.; Kubera, M.; Maes, M. New trends in the neurobiology and pharmacology of affective disorders. Pharmacological Reports 2013, 65, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Molecular Psychiatry 2022, 27, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Garofalo, S.; di Pellegrino, G.; Starita, F. Revaluing the role of vmPFC in the acquisition of Pavlovian threat conditioning in humans. Journal of Neuroscience 2020, 40, 8491–8500. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Battaglia, S.; Garofalo, S.; Tortora, F.; Avenanti, A.; di Pellegrino, G. State-dependent TMS over prefrontal cortex disrupts fear-memory reconsolidation and prevents the return of fear. Current Biology 2020, 30, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Samavati, R.; Zádor, F.; Szűcs, E.; Tuka, B.; Martos, D.; Veres, G.; Gáspár, R.; Mándity, I.M.; Fülöp, F.; Vécsei, L. Kynurenic acid and its analogue can alter the opioid receptor G-protein signaling after acute treatment via NMDA receptor in rat cortex and striatum. Journal of the Neurological Sciences 2017, 376, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Morales-Puerto, N.; Gimenez-Gomez, P.; Perez-Hernandez, M.; Abuin-Martinez, C.; de Biedma-Elduayen, L.G.; Vidal, R.; Gutiérrez-López, M.D.; O'Shea, E.; Colado, M.I. Addiction and the kynurenine pathway: A new dancing couple? Pharmacology & therapeutics 2021, 223, 107807. [Google Scholar]
- Klausing, A.D. Effects of Acute Stress on Discriminative Fear Conditioning: A Key Role of Kynurenic Acid in the Medial Prefrontal Cortex. University of Maryland, Baltimore, 2020.
- Meier, T.B.; Drevets, W.C.; Wurfel, B.E.; Ford, B.N.; Morris, H.M.; Victor, T.A.; Bodurka, J.; Teague, T.K.; Dantzer, R.; Savitz, J. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain, behavior, and immunity 2016, 53, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, V.; Costafreda, S.G.; Rimmer, R.M.; Rasenick, M.M.; Marangell, L.B.; Fu, C.H. Brain-derived neurotrophic factor association with amygdala response in major depressive disorder. Journal of affective disorders 2020, 267, 103–106. [Google Scholar] [CrossRef]
- Vecchiarelli, H.A.; Gandhi, C.P.; Hill, M.N. Acute psychological stress modulates the expression of enzymes involved in the kynurenine pathway throughout corticolimbic circuits in adult male rats. Neural Plasticity 2016, 2016. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Lőrinczi, B.; Szatmári, I.; Fülöp, F.; Vécsei, L. Antidepressant-like Effects of Kynurenic Acid Analogues. In Proceedings of the Presented at the 1st International Electronic Conference on Biomedicine; 2021; p. 26. [Google Scholar]
- Battaglia, S.; Orsolini, S.; Borgomaneri, S.; Barbieri, R.; Diciotti, S.; di Pellegrino, G. Characterizing cardiac autonomic dynamics of fear learning in humans. Psychophysiology 2022, 59, e14122. [Google Scholar] [CrossRef]
- Battaglia, S.; Di Fazio, C.; Vicario, C.M.; Avenanti, A. Neuropharmacological modulation of N-methyl-D-aspartate, noradrenaline and endocannabinoid receptors in fear extinction learning: Synaptic transmission and plasticity. International Journal of Molecular Sciences 2023, 24, 5926. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S. Neurobiological advances of learned fear in humans. Advances in Clinical and Experimental Medicine 2022, 31, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are kynurenines accomplices or principal villains in dementia? Maintenance of kynurenine metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.Y.; Lovejoy, D.B.; Guillemin, G.J.; Kozak, R.; Stone, T.W.; Koola, M.M. Galantamine-memantine combination and kynurenine pathway enzyme inhibitors in the treatment of neuropsychiatric disorders. Complex psychiatry 2021, 7, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Geng, X. Research progress on the kynurenine pathway in the prevention and treatment of Parkinson’s disease. Journal of Enzyme Inhibition and Medicinal Chemistry 2023, 38, 2225800. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, M.; Chen, X.; Zhang, R.; Le, A.; Hong, M.; Zhang, Y.; Jia, L.; Zang, W.; Jiang, C. Tryptophan Metabolism in Central Nervous System Diseases: Pathophysiology and Potential Therapeutic Strategies. Aging and Disease 2023, 14, 858. [Google Scholar] [CrossRef]
- Sheibani, M.; Shayan, M.; Khalilzadeh, M.; Soltani, Z.E.; Jafari-Sabet, M.; Ghasemi, M.; Dehpour, A.R. Kynurenine pathway and its role in neurologic, psychiatric, and inflammatory bowel diseases. Molecular Biology Reports 2023, 50, 10409–10425. [Google Scholar] [CrossRef]
- S Jayawickrama, G.; R Sadig, R.; Sun, G.; Nematollahi, A.; A Nadvi, N.; R Hanrahan, J.; D Gorrell, M.; Bret Church, W. Kynurenine aminotransferases and the prospects of inhibitors for the treatment of schizophrenia. Current Medicinal Chemistry 2015, 22, 2902–2918. [Google Scholar] [CrossRef]
- Kloog, Y.; Lamdani-Itkin, H.; Sokolovsky, M. The glycine site of the N-methyl-D-aspartate receptor channel: differences between the binding of HA-966 and of 7-chlorokynurenic acid. J Neurochem 1990, 54, 1576–1583. [Google Scholar] [CrossRef]
- Mok, M.S.; Fricker, A.-C.; Weil, A.; Kew, J.N. Electrophysiological characterisation of the actions of kynurenic acid at ligand-gated ion channels. Neuropharmacology 2009, 57, 242–249. [Google Scholar]
- Prescott, C.; Weeks, A.M.; Staley, K.J.; Partin, K.M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci Lett 2006, 402, 108–112. [Google Scholar] [CrossRef]
- Rózsa, E.; Robotka, H.; Vécsei, L.; Toldi, J. The Janus-face kynurenic acid. J Neural Transm (Vienna) 2008, 115, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Gladding, C.M.; Raymond, L.A. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol Cell Neurosci 2011, 48, 308–320. [Google Scholar] [CrossRef]
- Petralia, R.S. Distribution of extrasynaptic NMDA receptors on neurons. The Scientific World Journal 2012, 2012. [Google Scholar] [CrossRef]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.C.; Zarate Jr, C.A. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biological psychiatry 2017, 81, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Pribiag, H.; Jefferson, S.J.; Shorey, M.; Fuchs, T.; Stellwagen, D.; Luscher, B. Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biological psychiatry 2016, 80, 457–468. [Google Scholar] [CrossRef]
- Kasper, J.M.; McCue, D.L.; Milton, A.J.; Szwed, A.; Sampson, C.M.; Huang, M.; Carlton, S.; Meltzer, H.Y.; Cunningham, K.A.; Hommel, J.D. Gamma-Aminobutyric Acidergic Projections From the Dorsal Raphe to the Nucleus Accumbens Are Regulated by Neuromedin U. Biol Psychiatry 2016, 80, 878–887. [Google Scholar] [CrossRef]
- Adeel, M.; Chen, C.-C.; Lin, B.-S.; Chen, H.-C.; Liou, J.-C.; Li, Y.-T.; Peng, C.-W. Safety of Special Waveform of Transcranial Electrical Stimulation (TES): In Vivo Assessment. International journal of molecular sciences 2022, 23, 6850. [Google Scholar] [CrossRef]
- Poles, M.Z.; Nászai, A.; Gulácsi, L.; Czakó, B.L.; Gál, K.G.; Glenz, R.J.; Dookhun, D.; Rutai, A.; Tallósy, S.P.; Szabó, A.; et al. Kynurenic Acid and Its Synthetic Derivatives Protect Against Sepsis-Associated Neutrophil Activation and Brain Mitochondrial Dysfunction in Rats. Front Immunol 2021, 12, 717157. [Google Scholar] [CrossRef] [PubMed]
- Szabo, M.; Lajkó, N.; Dulka, K.; Szatmári, I.; Fülöp, F.; Mihály, A.; Vécsei, L.; Gulya, K. Kynurenic Acid and Its Analog SZR104 Exhibit Strong Antiinflammatory Effects and Alter the Intracellular Distribution and Methylation Patterns of H3 Histones in Immunochallenged Microglia-Enriched Cultures of Newborn Rat Brains. International Journal of Molecular Sciences 2022, 23, 1079. [Google Scholar] [CrossRef]
- Gregorio, F.; Battaglia, S. Advances in EEG-based functional connectivity approaches to the study of the central nervous system in health and disease. Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University.
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies. Molecular psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef]
- Erhardt, S.; Schwieler, L.; Imbeault, S.; Engberg, G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 2017, 112, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Fear-induced bradycardia in mental disorders: Foundations, current advances, future perspectives. Neurosci Biobehav Rev 2023, 149, 105163. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Thayer, J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci 2022, 45, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Serio, G.; Scarpazza, C.; D'Ausilio, A.; Borgomaneri, S. Frozen in (e) motion: How reactive motor inhibition is influenced by the emotional content of stimuli in healthy and psychiatric populations. Behaviour Research and Therapy 2021, 146, 103963. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neuroscience & Biobehavioral Reviews 2021, 127, 334–352. [Google Scholar]
- Borgomaneri, S.; Battaglia, S.; Avenanti, A.; di Pellegrino, G. Don't hurt me no more: State-dependent transcranial magnetic stimulation for the treatment of specific phobia. Journal of affective disorders 2021, 286, 78–79. [Google Scholar] [CrossRef]
- Almeida, P.G.; Nani, J.V.; Oses, J.P.; Brietzke, E.; Hayashi, M.A. Neuroinflammation and glial cell activation in mental disorders. Brain, Behavior, & Immunity-Health 2020, 2, 100034. [Google Scholar]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. Stopping in (e) motion: Reactive action inhibition when facing valence-independent emotional stimuli. Frontiers in Behavioral Neuroscience 2022, 16, 998714. [Google Scholar] [CrossRef]
- Di Gregorio, F.; La Porta, F.; Petrone, V.; Battaglia, S.; Orlandi, S.; Ippolito, G.; Romei, V.; Piperno, R.; Lullini, G. Accuracy of EEG biomarkers in the detection of clinical outcome in disorders of consciousness after severe acquired brain injury: preliminary results of a pilot study using a machine learning approach. Biomedicines 2022, 10, 1897. [Google Scholar] [CrossRef]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. The influence of vicarious fear-learning in “infecting” reactive action inhibition. Frontiers in Behavioral Neuroscience 2022, 16, 946263. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 2012, 13, 465–477. [Google Scholar] [CrossRef]
- nrn3257 [pii].
- Veres, G.; Fejes-Szabo, A.; Zadori, D.; Nagy-Grocz, G.; Laszlo, A.M.; Bajtai, A.; Mandity, I.; Szentirmai, M.; Bohar, Z.; Laborc, K.; et al. A comparative assessment of two kynurenic acid analogs in the formalin model of trigeminal activation: a behavioral, immunohistochemical and pharmacokinetic study. J Neural Transm (Vienna) 2017, 124, 99–112. [Google Scholar] [CrossRef] [PubMed]
- 10.1007/s00702-016-1615-5 [pii].
- Tanaka, M.; Chen, C. Towards a mechanistic understanding of depression, anxiety, and their comorbidity: perspectives from cognitive neuroscience. Frontiers in behavioral neuroscience 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- Quadt, L.; Critchley, H.; Nagai, Y. Cognition, emotion, and the central autonomic network. Auton Neurosci 2022, 238, 102948. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.D.; Todd, R.M. The self-regulating brain: Cortical-subcortical feedback and the development of intelligent action. Cognitive Development 2007, 22, 406–430. [Google Scholar] [CrossRef]
- Maiese, M. Embodiment, emotion, and cognition; Springer: 2010.
- Salim, S. Oxidative Stress and the Central Nervous System. J Pharmacol Exp Ther 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br J Pharmacol 2006, 147 Suppl 1, S232–240. [Google Scholar] [CrossRef]
- Critchley, H.D.; Eccles, J.; Garfinkel, S.N. Interaction between cognition, emotion, and the autonomic nervous system. In Handbook of clinical neurology; Elsevier: 2013; Volume 117, pp. 59–77.
- Šumec, R.; Rektorová, I.; Jech, R.; Menšíková, K.; Roth, J.; Růžička, E.; Sochorová, D.; Dušek, L.; Kaňovský, P.; Rektor, I. Motion and emotion: anxiety–axial connections in Parkinson’s disease. Journal of neural transmission 2017, 124, 369–377. [Google Scholar] [CrossRef]
- Jawer, M.A. Sensitive soul: The unseen role of emotion in extraordinary states; Simon and Schuster: 2020.
- Bellettato, C.M.; Scarpa, M. Possible strategies to cross the blood-brain barrier. Ital J Pediatr 2018, 44, 131. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Drug delivery systems, CNS protection, and the blood brain barrier. BioMed research international 2014, 2014. [Google Scholar] [CrossRef]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiology of disease 2010, 37, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Gawdi, R.; Shumway, K.R.; Emmady, P.D. Physiology, Blood Brain Barrier. StatPearls.
- Copyright © 2023, StatPearls Publishing LLC.: Treasure Island (FL), 2023.
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Brain endothelial cell-cell junctions: how to "open" the blood brain barrier. Curr Neuropharmacol 2008, 6, 179–192. [Google Scholar] [CrossRef]
- Sanchez-Covarrubias, L.; Slosky, L.M.; Thompson, B.J.; Davis, T.P.; Ronaldson, P.T. Transporters at CNS barrier sites: obstacles or opportunities for drug delivery? Current pharmaceutical design 2014, 20, 1422–1449. [Google Scholar] [CrossRef]
- De Boer, A.; Van Der Sandt, I.; Gaillard, P. The role of drug transporters at the blood-brain barrier. Annual review of pharmacology and toxicology 2003, 43, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Neuhaus, W.; Dandekar, T.; Förster, C. Analysing molecular polar surface descriptors to predict blood-brain barrier permeation. Int J Comput Biol Drug Des 2013, 6, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Betz, A.L.; Firth, J.A.; Goldstein, G.W. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain research 1980, 192, 17–28. [Google Scholar] [CrossRef]
- Janicka, M.; Sztanke, M.; Sztanke, K. Predicting the blood-brain barrier permeability of new drug-like compounds via HPLC with various stationary phases. Molecules 2020, 25, 487. [Google Scholar] [CrossRef]
- Sánchez-Dengra, B.; González-Álvarez, I.; Bermejo, M.; González-Álvarez, M. Access to the CNS: Strategies to overcome the BBB. International Journal of Pharmaceutics 2023, 122759. [Google Scholar] [CrossRef]
- Liu, H.-M.; Liu, X.-F.; Yao, J.-L.; Wang, C.-L.; Yu, Y.; Wang, R. Utilization of combined chemical modifications to enhance the blood-brain barrier permeability and pharmacological activity of endomorphin-1. Journal of Pharmacology and Experimental Therapeutics 2006, 319, 308–316. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, L.; Deng, G.; Liu, J.; Chen, Q.; Chen, Z. Systemic delivery to central nervous system by engineered PLGA nanoparticles. American journal of translational research 2016, 8, 749. [Google Scholar] [PubMed]
- Li, D.; Yu, S.; Long, Y.; Shi, A.; Deng, J.; Ma, Y.; Wen, J.; Li, X.; Liu, S.; Zhang, Y.; et al. Tryptophan metabolism: Mechanism-oriented therapy for neurological and psychiatric disorders. Front Immunol 2022, 13, 985378. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.M. Kynurenines: from the perspective of major psychiatric disorders. The FEBS journal 2012, 279, 1375–1385. [Google Scholar] [CrossRef]
- Müller, N.; Myint, A.-M.; Schwarz, M.J. The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders-relation to drug treatment. Dialogues in clinical neuroscience 2009, 11, 319–332. [Google Scholar] [CrossRef]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O'Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.; Outeiro, T.F.; Scrutton, N.S.; Giorgini, F. The causative role and therapeutic potential of the kynurenine pathway in neurodegenerative disease. Journal of Molecular Medicine 2013, 91, 705–713. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front Immunol 2017, 8, 1957. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Singh, T.G.; Prabhakar, N.K.; Mannan, A. Kynurenine Metabolism and Alzheimer's Disease: The Potential Targets and Approaches. Neurochem Res 2022, 47, 1459–1476. [Google Scholar] [CrossRef]
- Martin, K.S.; Azzolini, M.; Ruas, J.L. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. American Journal of Physiology-Cell Physiology.
- González Esquivel, D.; Ramírez-Ortega, D.; Pineda, B.; Castro, N.; Ríos, C.; Pérez de la Cruz, V. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology 2017, 112, 331–345. [Google Scholar] [CrossRef]
- Debnath, N.; Kumar, R.; Kumar, A.; Mehta, P.K.; Yadav, A.K. Gut-microbiota derived bioactive metabolites and their functions in host physiology. Biotechnology and Genetic Engineering Reviews 2021, 37, 105–153. [Google Scholar] [CrossRef]
- Joisten, N.; Ruas, J.L.; Braidy, N.; Guillemin, G.J.; Zimmer, P. The kynurenine pathway in chronic diseases: a compensatory mechanism or a driving force? Trends Mol Med 2021, 27, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guillemin, G.J. Kynurenine pathway metabolites in humans: disease and healthy states. International journal of tryptophan research 2009, 2, IJTR. S2097. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, tryptophan metabolism, and kynurenine pathway: a complex interconnected loop influencing human health status. International journal of tryptophan research 2019, 12, 1178646919852996. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sun-Waterhouse, D.; Cui, C. The therapeutic potential of diet on immune-related diseases: based on the regulation on tryptophan metabolism. Crit Rev Food Sci Nutr 2022, 62, 8793–8811. [Google Scholar] [CrossRef] [PubMed]
- Günther, J.; Fallarino, F.; Fuchs, D.; Wirthgen, E. Immunomodulatory roles of tryptophan metabolites in inflammation and cancer. Frontiers in Immunology, 2020; 11, 1497. [Google Scholar]
- Turski, M.P.; Turska, M.; Paluszkiewicz, P.; Parada-Turska, J.; Oxenkrug, G.F. Kynurenic acid in the digestive system–-new facts, new challenges. International Journal of Tryptophan Research 2013, 6, IJTR. S12536. [Google Scholar] [CrossRef]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for peripheral biomarkers in neurodegenerative diseases: the tryptophan-kynurenine metabolic pathway. International journal of molecular sciences 2020, 21, 9338. [Google Scholar] [CrossRef]
- Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between existential phenomenological psychotherapy and neurological sciences in mood and anxiety disorders. Biomedicines 2021, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacological Reports 2020, 72, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R. The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol 2004, 4, 12–17. [Google Scholar] [CrossRef]
- Balla, Z.; Kormányos, E.S.; Kui, B.; Bálint, E.R.; Fűr, G.; Orján, E.M.; Iványi, B.; Vécsei, L.; Fülöp, F.; Varga, G.; et al. Kynurenic Acid and Its Analogue SZR-72 Ameliorate the Severity of Experimental Acute Necrotizing Pancreatitis. Front Immunol 2021, 12, 702764. [Google Scholar] [CrossRef]
- Gellért, L.; Varga, D.; Ruszka, M.; Toldi, J.; Farkas, T.; Szatmári, I.; Fülöp, F.; Vécsei, L.; Kis, Z. Behavioural studies with a newly developed neuroprotective KYNA-amide. J Neural Transm (Vienna) 2012, 119, 165–172. [Google Scholar] [CrossRef]
- Vámos, E.; Párdutz, A.; Varga, H.; Bohár, Z.; Tajti, J.; Fülöp, F.; Toldi, J.; Vécsei, L. l-kynurenine combined with probenecid and the novel synthetic kynurenic acid derivative attenuate nitroglycerin-induced nNOS in the rat caudal trigeminal nucleus. Neuropharmacology 2009, 57, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Mándi, Y.; Endrész, V.; Mosolygó, T.; Burián, K.; Lantos, I.; Fülöp, F.; Szatmári, I.; Lőrinczi, B.; Balog, A.; Vécsei, L. The Opposite Effects of Kynurenic Acid and Different Kynurenic Acid Analogs on Tumor Necrosis Factor-α (TNF-α) Production and Tumor Necrosis Factor-Stimulated Gene-6 (TSG-6) Expression. Front Immunol 2019, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Demeter, I.; Nagy, K.; Gellért, L.; Vécsei, L.; Fülöp, F.; Toldi, J. A novel kynurenic acid analog (SZR104) inhibits pentylenetetrazole-induced epileptiform seizures. An electrophysiological study: special issue related to kynurenine. Journal of Neural Transmission 2012, 119, 151–154. [Google Scholar] [CrossRef]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory enhancement with kynurenic acid and its mechanisms in neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef]
- Benson, H.A.; Roberts, M.S. Challenges and Innovations of Controlled Drug Delivery. Fundamentals of Drug Delivery 2021, 1–14. [Google Scholar]
- McCarson, K.E. Strategies for behaviorally phenotyping the transgenic mouse. Transgenic Mouse: Methods and Protocols.
- Sukoff Rizzo, S.J.; Crawley, J.N. Behavioral phenotyping assays for genetic mouse models of neurodevelopmental, neurodegenerative, and psychiatric disorders. Annual Review of Animal Biosciences 2017, 5, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, Á.; Vécsei, L. Co-players in chronic pain: neuroinflammation and the tryptophan-kynurenine metabolic pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef]
- Molnár, K.; Lőrinczi, B.; Fazakas, C.; Szatmári, I.; Fülöp, F.; Kmetykó, N.; Berkecz, R.; Ilisz, I.; Krizbai, I.A.; Wilhelm, I. Szr-104, a novel kynurenic acid analogue with high permeability through the blood–brain barrier. Pharmaceutics 2021, 13, 61. [Google Scholar] [CrossRef]
- Castillo-Mariqueo, L.; Giménez-Llort, L. Impact of Behavioral Assessment and Re-Test as Functional Trainings That Modify Survival, Anxiety and Functional Profile (Physical Endurance and Motor Learning) of Old Male and Female 3xTg-AD Mice and NTg Mice with Normal Aging. Biomedicines 2022, 10, 973. [Google Scholar] [CrossRef]
- Lee, E.C.; Hong, D.-Y.; Lee, D.-H.; Park, S.-W.; Lee, J.Y.; Jeong, J.H.; Kim, E.-Y.; Chung, H.-M.; Hong, K.-S.; Park, S.-P. Inflammation and rho-associated protein kinase-induced brain changes in vascular dementia. Biomedicines 2022, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Belozertseva, I.V.; Tamkovich, N.V.; Baranov, K.O.; Kudryavtseva, N.N. Chronic Lithium Treatment affects anxious behaviors and theExpression of serotonergic genes in Midbrain Raphe nuclei of defeated male mice. Biomedicines 2021, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Vila-Merkle, H.; González-Martínez, A.; Campos-Jiménez, R.; Martínez-Ricós, J.; Teruel-Martí, V.; Blasco-Serra, A.; Lloret, A.; Celada, P.; Cervera-Ferri, A. The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala–Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders. Biomedicines 2021, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Santana-Santana, M.; Bayascas, J.-R.; Giménez-Llort, L. Fine-Tuning the PI3K/Akt Signaling Pathway Intensity by Sex and Genotype-Load: Sex-Dependent Homozygotic Threshold for Somatic Growth but Feminization of Anxious Phenotype in Middle-Aged PDK1 K465E Knock-In and Heterozygous Mice. Biomedicines 2021, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Muntsant, A.; Giménez-Llort, L. Genotype Load Modulates Amyloid Burden and Anxiety-Like Patterns in Male 3xTg-AD Survivors despite Similar Neuro-Immunoendocrine, Synaptic and Cognitive Impairments. Biomedicines 2021, 9, 715. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Marin-Pardo, D.; Marazuela, P.; Hernández-Guillamón, M. Survival Bias and Crosstalk between Chronological and Behavioral Age: Age-and Genotype-Sensitivity Tests Define Behavioral Signatures in Middle-Aged, Old, and Long-Lived Mice with Normal and AD-Associated Aging. Biomedicines 2021, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Samra, A.I.; Kamel, A.S.; Abdallah, D.M.; El Fattah, M.A.A.; Ahmed, K.A.; El-Abhar, H.S. Preclinical Evidence for the Role of the Yin/Yang Angiotensin System Components in Autism Spectrum Disorder: A Therapeutic Target of Astaxanthin. Biomedicines 2023, 11, 3156. [Google Scholar] [CrossRef] [PubMed]
- Canfora, F.; Ottaviani, G.; Calabria, E.; Pecoraro, G.; Leuci, S.; Coppola, N.; Sansone, M.; Rupel, K.; Biasotto, M.; Di Lenarda, R. Advancements in Understanding and Classifying Chronic Orofacial Pain: Key Insights from Biopsychosocial Models and International Classifications (ICHD-3, ICD-11, ICOP). Biomedicines 2023, 11, 3266. [Google Scholar] [CrossRef]
- Cruz-Martínez, Y.; Aguilar-Ponce, L.; Romo-Araiza, A.; Chávez-Guerra, A.; Martiñón, S.; Ibarra-García, A.P.; Arias-Santiago, S.; Gálvez-Susano, V.; Ibarra, A. Supplementation with a Symbiotic Induced Neuroprotection and Improved Memory in Rats with Ischemic Stroke. Biomedicines 2024, 12, 209. [Google Scholar] [CrossRef]
- Baliellas, D.E.; Barros, M.P.; Vardaris, C.V.; Guariroba, M.; Poppe, S.C.; Martins, M.F.; Pereira, Á.A.; Bondan, E.F. Propentofylline Improves Thiol-Based Antioxidant Defenses and Limits Lipid Peroxidation following Gliotoxic Injury in the Rat Brainstem. Biomedicines 2023, 11, 1652. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaokun, O.O. The Beneficial Role of Apigenin against Cognitive and Neurobehavioural Dysfunction: A Systematic Review of Preclinical Investigations. Biomedicines 2024, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, G.; Yi, J.; Huang, Y. Intraoperative Hypothermia Induces Vascular Dysfunction in the CA1 Region of Rat Hippocampus. Brain Sciences 2022, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wei, S.; Low, S.W.; Poore, C.P.; Lee, A.T.; Nilius, B.; Liao, P. TRPM4 Blocking Antibody Protects Cerebral Vasculature in Delayed Stroke Reperfusion. Biomedicines 2023, 11. [Google Scholar] [CrossRef]
- Salafutdinov, II; Gatina, D.Z.; Markelova, M.I.; Garanina, E.E.; Malanin, S.Y.; Gazizov, I.M.; Izmailov, A.A.; Rizvanov, A.A.; Islamov, R.R.; Palotás, A.; Safiullov, Z.Z. A Biosafety Study of Human Umbilical Cord Blood Mononuclear Cells Transduced with Adenoviral Vector Carrying Human Vascular Endothelial Growth Factor cDNA In Vitro. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, A.J.; Finner-Prével, M.; Sudar, F.P.; Langer, F.H.; Keskin, F.; Gebel, A.; Zweerings, J.; Mathiak, K. The interplay between vitamin D, exposure of anticholinergic antipsychotics and cognition in schizophrenia. Biomedicines 2022, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Simonato, M.; Dall’Acqua, S.; Zilli, C.; Sut, S.; Tenconi, R.; Gallo, N.; Sfriso, P.; Sartori, L.; Cavallin, F.; Fiocco, U. Tryptophan metabolites, cytokines, and fatty acid binding protein 2 in myalgic encephalomyelitis/chronic fatigue syndrome. Biomedicines 2021, 9, 1724. [Google Scholar] [CrossRef]
- Panov, G.; Dyulgerova, S.; Panova, P. Cognition in Patients with Schizophrenia: Interplay between Working Memory, Disorganized Symptoms, Dissociation, and the Onset and Duration of Psychosis, as Well as Resistance to Treatment. Biomedicines 2023, 11, 3114. [Google Scholar] [CrossRef]
- de Oliveira, M.; Santinelli, F.B.; Lisboa-Filho, P.N.; Barbieri, F.A. The blood concentration of metallic nanoparticles is related to cognitive performance in people with multiple sclerosis: An exploratory analysis. Biomedicines 2023, 11, 1819. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Biomarkers of Neurodegeneration in Post-Traumatic Stress Disorder: An Integrative Review. Biomedicines 2023, 11, 1465. [Google Scholar] [CrossRef]
- Stojsavljević, A.; Lakićević, N.; Pavlović, S. Mercury and Autism Spectrum Disorder: Exploring the Link through Comprehensive Review and Meta-Analysis. Biomedicines 2023, 11, 3344. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Kasselimis, D.; Laskaris, N.; Potagas, C. Unraveling the Thread of Aphasia Rehabilitation: A Translational Cognitive Perspective. Biomedicines 2023, 11, 2856. [Google Scholar] [CrossRef]
- Zakia, H.; Iskandar, S. Case report: Depressive disorder with peripartum onset camouflages suspected intracranial tuberculoma. Frontiers in Psychiatry 2022, 13, 932635. [Google Scholar] [CrossRef]
- Liu, M.; Xie, X.; Xie, J.; Tian, S.; Du, X.; Feng, H.; Zhang, H. Early-onset Alzheimer’s disease with depression as the first symptom: a case report with literature review. Frontiers in Psychiatry 2023, 14, 1192562. [Google Scholar] [CrossRef]
- Komatsu, H.; Watanabe, E.; Fukuchi, M. Psychiatric neural networks and precision therapeutics by machine learning. Biomedicines 2021, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.M.; Ahmad, F.; Bhat, A.; Aein, Q.-u.; Ahmad, A.; Reshi, A.A.; Kaul, R.-u.-R. Unraveling Down Syndrome: From Genetic Anomaly to Artificial Intelligence-Enhanced Diagnosis. Biomedicines 2023, 11, 3284. [Google Scholar] [CrossRef] [PubMed]
- Ikonnikova, A.; Anisimova, A.; Galkin, S.; Gunchenko, A.; Abdukhalikova, Z.; Filippova, M.; Surzhikov, S.; Selyaeva, L.; Shershov, V.; Zasedatelev, A. Genetic Association Study and Machine Learning to Investigate Differences in Platelet Reactivity in Patients with Acute Ischemic Stroke Treated with Aspirin. Biomedicines 2022, 10, 2564. [Google Scholar] [CrossRef] [PubMed]
- Rassler, B.; Blinowska, K.; Kaminski, M.; Pfurtscheller, G. Analysis of Respiratory Sinus Arrhythmia and Directed Information Flow between Brain and Body Indicate Different Management Strategies of fMRI-Related Anxiety. Biomedicines 2023, 11, 1028. [Google Scholar] [CrossRef]
- Wasko, M.J.; Pellegrene, K.A.; Madura, J.D.; Surratt, C.K. A role for fragment-based drug design in developing novel lead compounds for central nervous system targets. Frontiers in neurology 2015, 6, 197. [Google Scholar] [CrossRef]
- Klon, A.E. Computational models for central nervous system penetration. Current Computer-Aided Drug Design 2009, 5, 71–89. [Google Scholar] [CrossRef]
- Ghose, A.K.; Herbertz, T.; Hudkins, R.L.; Dorsey, B.D.; Mallamo, J.P. Knowledge-based, central nervous system (CNS) lead selection and lead optimization for CNS drug discovery. ACS chemical neuroscience 2012, 3, 50–68. [Google Scholar] [CrossRef]
- Koszła, O.; Stępnicki, P.; Zięba, A.; Grudzińska, A.; Matosiuk, D.; Kaczor, A.A. Current Approaches and Tools Used in Drug Development against Parkinson's Disease. Biomolecules 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nisha, C.M.; Silakari, C.; Sharma, I.; Anusha, K.; Gupta, N.; Nair, P.; Tripathi, T.; Kumar, A. Current and novel therapeutic molecules and targets in Alzheimer's disease. J Formos Med Assoc 2016, 115, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.; Kouzani, A.Z.; Berk, M.; Tye, S.J.; Rusheen, A.E.; Blaha, C.D.; Bennet, K.E.; Lee, K.H.; Shin, H.; Kim, J.H. Deep brain stimulation for addictive disorders—where are we now? Neurotherapeutics 2022, 19, 1193–1215. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, L.L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study. Biomedicines 2023, 11, 2219. [Google Scholar] [CrossRef]
- Senevirathne, D.K.L.; Mahboob, A.; Zhai, K.; Paul, P.; Kammen, A.; Lee, D.J.; Yousef, M.S.; Chaari, A. Deep Brain Stimulation beyond the Clinic: Navigating the Future of Parkinson’s and Alzheimer’s Disease Therapy. Cells 2023, 12, 1478. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Wang, W.-L.; Shieh, Y.-H.; Peng, H.-Y.; Ho, C.-S.; Tsai, H.-C. Case Report: Low-Frequency Repetitive Transcranial Magnetic Stimulation to Dorsolateral Prefrontal Cortex and Auditory Cortex in a Patient With Tinnitus and Depression. Frontiers in Psychiatry 2022, 13, 847618. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, J.; Wieczorek, T.; Fila-Witecka, K.; Smarzewska, K.; Weiser, A.; Piotrowski, P.; Tabakow, P. Various neuromodulation methods including Deep Brain Stimulation of the medial forebrain bundle combined with psychopharmacotherapy of treatment-resistant depression—Case report. Frontiers in Psychiatry 2023, 13, 3014. [Google Scholar] [CrossRef] [PubMed]
- Schindler, E.A.D. Psychedelics in the Treatment of Headache and Chronic Pain Disorders. Curr Top Behav Neurosci 2022, 56, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Tao, Q.; Niu, X.; Zhang, M.; Gao, X.; Yang, Z.; Yu, M.; Wang, W.; Han, S.; Cheng, J. Meta-analysis of structural and functional brain abnormalities in cocaine addiction. Frontiers in Psychiatry 2022, 13, 927075. [Google Scholar] [CrossRef]
- Borbély, K.; Emri, M.; Kenessey, I.; Tóth, M.; Singer, J.; Barsi, P.; Vajda, Z.; Pál, E.; Tóth, Z.; Beyer, T. Pet/Mri in the presurgical evaluation of patients with epilepsy: a concordance analysis. Biomedicines 2022, 10, 949. [Google Scholar] [CrossRef]
- Okanda Nyatega, C.; Qiang, L.; Jajere Adamu, M.; Bello Kawuwa, H. Altered striatal functional connectivity and structural dysconnectivity in individuals with bipolar disorder: a resting state magnetic resonance imaging study. Frontiers in Psychiatry 2022, 13, 1054380. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, Y.; Hong, Y.; Huo, J.; Chang, T.; Wang, H.; Huang, Y.; Li, W.; Zhang, Y. Altered brain activities in mesocorticolimbic pathway in primary dysmenorrhea patients of long-term menstrual pain. Frontiers in Neuroscience 2023, 17, 1098573. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, B.; Wang, H.; Zeng, Y.; Xin, J.; Li, X. The non-linear correlation between the volume of cerebral white matter lesions and incidence of bipolar disorder: A secondary analysis of data from a cross-sectional study. Frontiers in Psychiatry 2023, 14, 1149663. [Google Scholar] [CrossRef]
- Adamu, M.J.; Qiang, L.; Nyatega, C.O.; Younis, A.; Kawuwa, H.B.; Jabire, A.H.; Saminu, S. Unraveling the pathophysiology of schizophrenia: insights from structural magnetic resonance imaging studies. Frontiers in Psychiatry 2023, 14, 1188603. [Google Scholar] [CrossRef]
- Nyatega, C.O.; Qiang, L.; Adamu, M.J.; Kawuwa, H.B. Gray matter, white matter and cerebrospinal fluid abnormalities in Parkinson’s disease: A voxel-based morphometry study. Frontiers in Psychiatry 2022, 13, 1027907. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Kim, S.-H.; Han, C.; Jeong, H.-G.; Lee, M.-S.; Kim, J. Antidepressant-induced mania in panic disorder: a single-case study of clinical and functional connectivity characteristics. Frontiers in Psychiatry 2023, 14, 1205126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, R.; DeSouza, J.F.; Shen, Y.; Zhang, H.; Zhu, C.; Huang, P.; Wang, C. Differential responses from the left postcentral gyrus, right middle frontal gyrus, and precuneus to meal ingestion in patients with functional dyspepsia. Frontiers in Psychiatry 2023, 14, 1184797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cao, Y.; Deng, G.; Fang, J.; Qiu, C. Transient splenial lesion syndrome in bipolar-II disorder: a case report highlighting reversible brain changes during hypomanic episodes. Frontiers in Psychiatry 2023, 14. [Google Scholar] [CrossRef]
- Forrest, M.P.; Parnell, E.; Penzes, P. Dendritic structural plasticity and neuropsychiatric disease. Nat Rev Neurosci 2018, 19, 215–234. [Google Scholar] [CrossRef]
- Salvaggio, F.; Hodgkinson, J.T.; Carro, L.; Geddis, S.M.; Galloway, W.R.; Welch, M.; Spring, D.R. The synthesis of quinolone natural products from pseudonocardia sp. 2016.
- Piochon, M.; Coulon, P.M.; Caulet, A.; Groleau, M.-C.; Déziel, E.; Gauthier, C. Synthesis and antimicrobial activity of Burkholderia-related 4-hydroxy-3-methyl-2-alkenylquinolines (HMAQs) and their N-oxide counterparts. Journal of Natural Products 2020, 83, 2145–2154. [Google Scholar] [CrossRef]
- Nam, D.H.; Lee, K.S.; Kim, S.H.; Kim, S.M.; Jung, S.Y.; Chung, S.H.; Kim, H.J.; Kim, N.D.; Jin, C.; Lee, Y.S. Design and synthesis of 4-quinolinone 2-carboxamides as calpain inhibitors. Bioorganic & medicinal chemistry letters 2008, 18, 205–209. [Google Scholar]
- Vyas, V.K.; Variya, B.; Ghate, M.D. Design, synthesis and pharmacological evaluation of novel substituted quinoline-2-carboxamide derivatives as human dihydroorotate dehydrogenase (hDHODH) inhibitors and anticancer agents. European Journal of Medicinal Chemistry 2014, 82, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jung, J.S.; Moon, Y.S.; Song, D.K. Central or peripheral norepinephrine depletion enhances MK-801-induced plasma corticosterone level in mice. Prog Neuropsychopharmacol Biol Psychiatry 2009, 33, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L.; Giménez-Llort, L. Emerging translational research in neurological and psychiatric diseases: from in vitro to in vivo models. 2023; 24, 15739. [Google Scholar]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Preclinical modeling in depression and anxiety: Current challenges and future research directions. Adv. Clin. Exp. Med 2023, 32, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, R.D.; Fessler, R.G.; Meltzer, H.Y. Behavioral rating scales for assessing phencyclidine-induced locomotor activity, stereotyped behavior and ataxia in rats. Eur J Pharmacol 1979, 59, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Contreras, P.C. D-serine antagonized phencyclidine- and MK-801-induced stereotyped behavior and ataxia. Neuropharmacology 1990, 29, 291–293. [Google Scholar] [CrossRef]
- Lim, C.J.M.; Platt, B.; Janhunen, S.K.; Riedel, G. Comparison of automated video tracking systems in the open field test: ANY-Maze versus EthoVision XT. J Neurosci Methods 2023, 397, 109940. [Google Scholar] [CrossRef] [PubMed]
- Stanford, S.C. The Open Field Test: reinventing the wheel. J Psychopharmacol 2007, 21, 134–135. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The Open-Field Test: a critical review. Psychol Bull 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Pritchett, K.; Mulder, G.B. The rotarod. Contemp Top Lab Anim Sci 2003, 42, 49. [Google Scholar]
- Osmon, K.J.; Vyas, M.; Woodley, E.; Thompson, P.; Walia, J.S. Battery of Behavioral Tests Assessing General Locomotion, Muscular Strength, and Coordination in Mice. J Vis Exp 2018. [Google Scholar] [CrossRef]
Figure 1.
Tryptophan-kynurenine metabolic pathways and consequences of its imbalance. AA: anthranilic acid; ACMSD: 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase; 3-HAA: 3-hydroxyanthranilic acid; 3-HAO: 3-hydroxyanthranilate oxidase; 3-HK: 3-hydroxy-L-kynurenine; IDOs: indoleamine 2,3-dioxygenases; KATs: kynurenine aminotransferases; KMO: kynurenine 3-monooxygenase; KYNA: kynurenic acid; KYNU: kynureninase; L-KYN: L-kynurenine; NADH: nicotinamide adenine dinucleotide + hydrogen; N-fKYN: N-formyl-kynurenine; PA: picolinic acid; QPRT: quinolinate phosphoribosyltransferase; QUIN: quinolinic acid; TDO: tryptophan 2,3-dioxygenase; Trp: tryptophan; and XA: xanthurenic acid; IAld: Indole-3-Aldehyde; IPA: Indole-3-Propionic Acid.
Figure 1.
Tryptophan-kynurenine metabolic pathways and consequences of its imbalance. AA: anthranilic acid; ACMSD: 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase; 3-HAA: 3-hydroxyanthranilic acid; 3-HAO: 3-hydroxyanthranilate oxidase; 3-HK: 3-hydroxy-L-kynurenine; IDOs: indoleamine 2,3-dioxygenases; KATs: kynurenine aminotransferases; KMO: kynurenine 3-monooxygenase; KYNA: kynurenic acid; KYNU: kynureninase; L-KYN: L-kynurenine; NADH: nicotinamide adenine dinucleotide + hydrogen; N-fKYN: N-formyl-kynurenine; PA: picolinic acid; QPRT: quinolinate phosphoribosyltransferase; QUIN: quinolinic acid; TDO: tryptophan 2,3-dioxygenase; Trp: tryptophan; and XA: xanthurenic acid; IAld: Indole-3-Aldehyde; IPA: Indole-3-Propionic Acid.
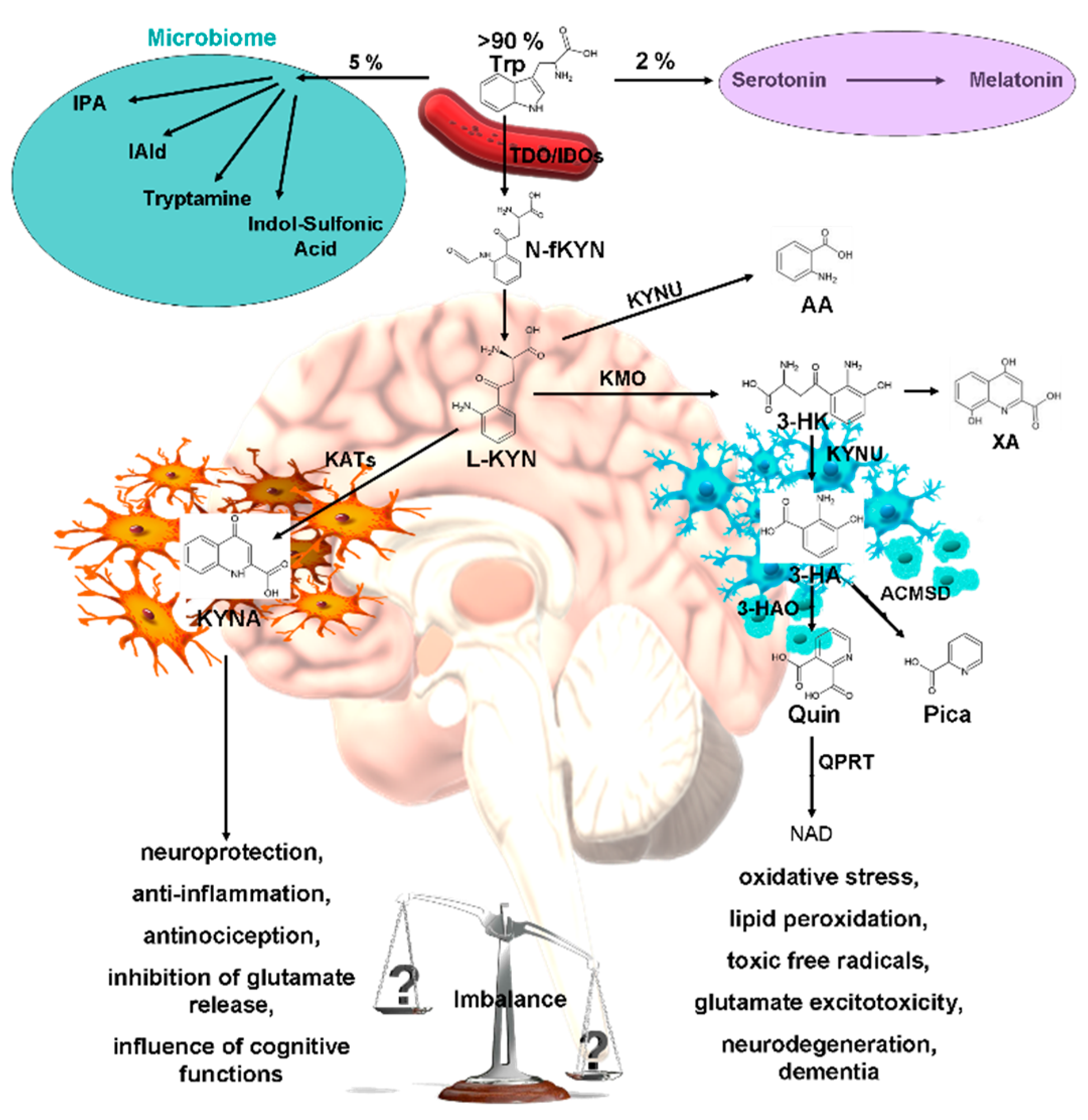
Figure 2.
N-methyl-D-aspartic acid (NMDA) receptor complex physiological function in post- and extra-synaptic membranes of glutamatergic neurons and their function under the antagonistic effects of MK-801, kynurenic acid (KYNA), and KYNA analogs. NMDA receptors are made of four subunits: GLUNR1 and GLUNR3, which bind L-glycine and D-serine, and GLUNR2A and GLUNR2B, which bind glutamate. GLUNR2A supports cell survival, while GLUNR2B triggers cell death by allowing Ca2+ and Na+ influx. These subunits form a cationic channel that opens when L-glycine, D-serine, and glutamate bind simultaneously and Mg2+ is remove. NMDA receptor agonists with GLUNR2A may have therapeutic benefits. Created with BioRender.com.
Figure 2.
N-methyl-D-aspartic acid (NMDA) receptor complex physiological function in post- and extra-synaptic membranes of glutamatergic neurons and their function under the antagonistic effects of MK-801, kynurenic acid (KYNA), and KYNA analogs. NMDA receptors are made of four subunits: GLUNR1 and GLUNR3, which bind L-glycine and D-serine, and GLUNR2A and GLUNR2B, which bind glutamate. GLUNR2A supports cell survival, while GLUNR2B triggers cell death by allowing Ca2+ and Na+ influx. These subunits form a cationic channel that opens when L-glycine, D-serine, and glutamate bind simultaneously and Mg2+ is remove. NMDA receptor agonists with GLUNR2A may have therapeutic benefits. Created with BioRender.com.
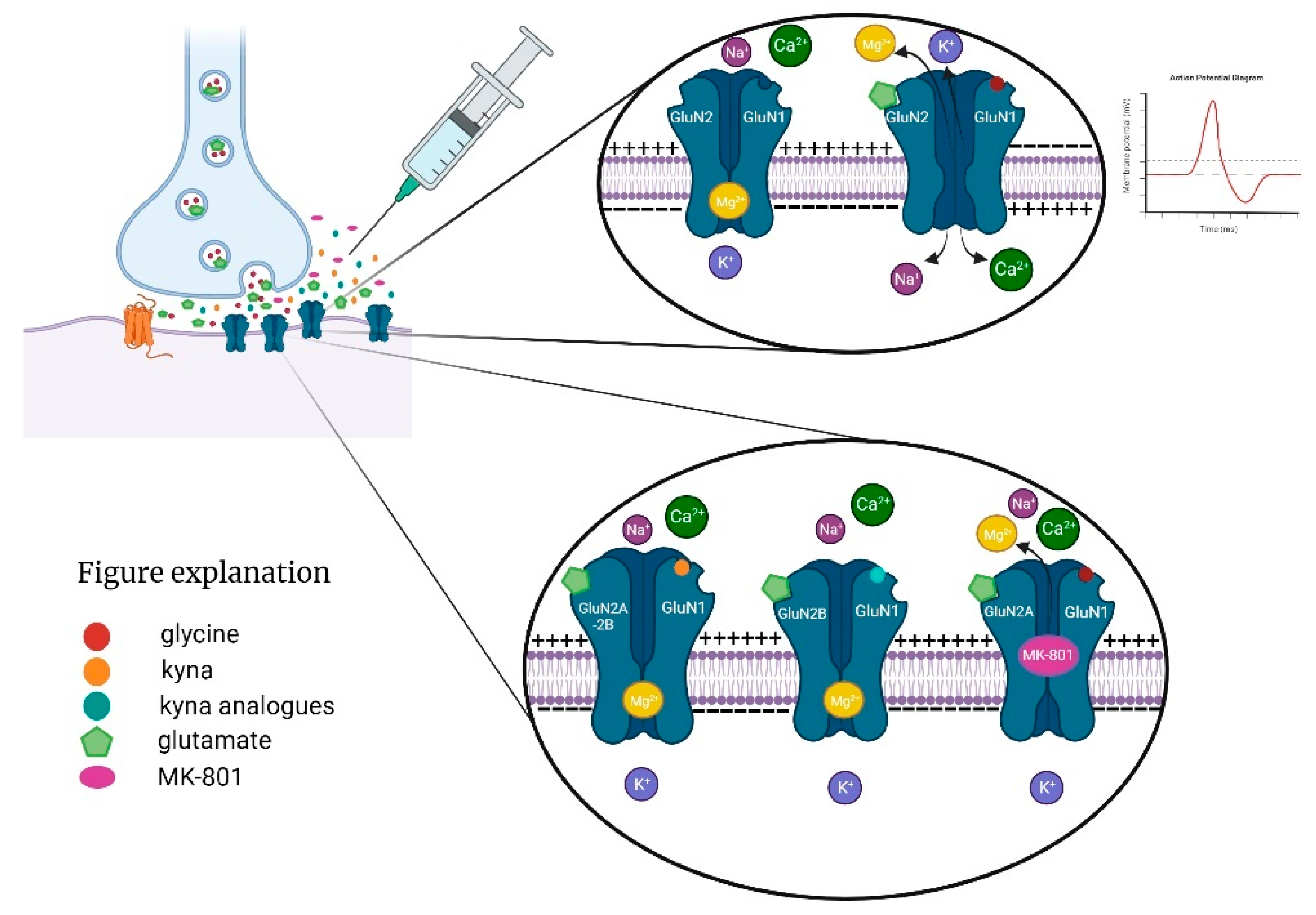
Figure 3.
Chemical structures and highlighted differences of kynurenic acid and its analogs. SZR-72: N-(2-(dimethylamino)ethyl)-4-oxo-1,4-dihydroquinoline-2-carboxamide hydrochloride; SZR-104: N-(2-(dimethylamino)ethyl)-3-(morpholinomethyl)-4-oxo-1,4-dihydroquinoline-2-carboxamide; and SZRG-21: N-(2-(dimethylamino)ethyl)-3-methyl-4-oxo-1,4-dihydroquinoline-2-carboxamide hydrochloride.
Figure 3.
Chemical structures and highlighted differences of kynurenic acid and its analogs. SZR-72: N-(2-(dimethylamino)ethyl)-4-oxo-1,4-dihydroquinoline-2-carboxamide hydrochloride; SZR-104: N-(2-(dimethylamino)ethyl)-3-(morpholinomethyl)-4-oxo-1,4-dihydroquinoline-2-carboxamide; and SZRG-21: N-(2-(dimethylamino)ethyl)-3-methyl-4-oxo-1,4-dihydroquinoline-2-carboxamide hydrochloride.
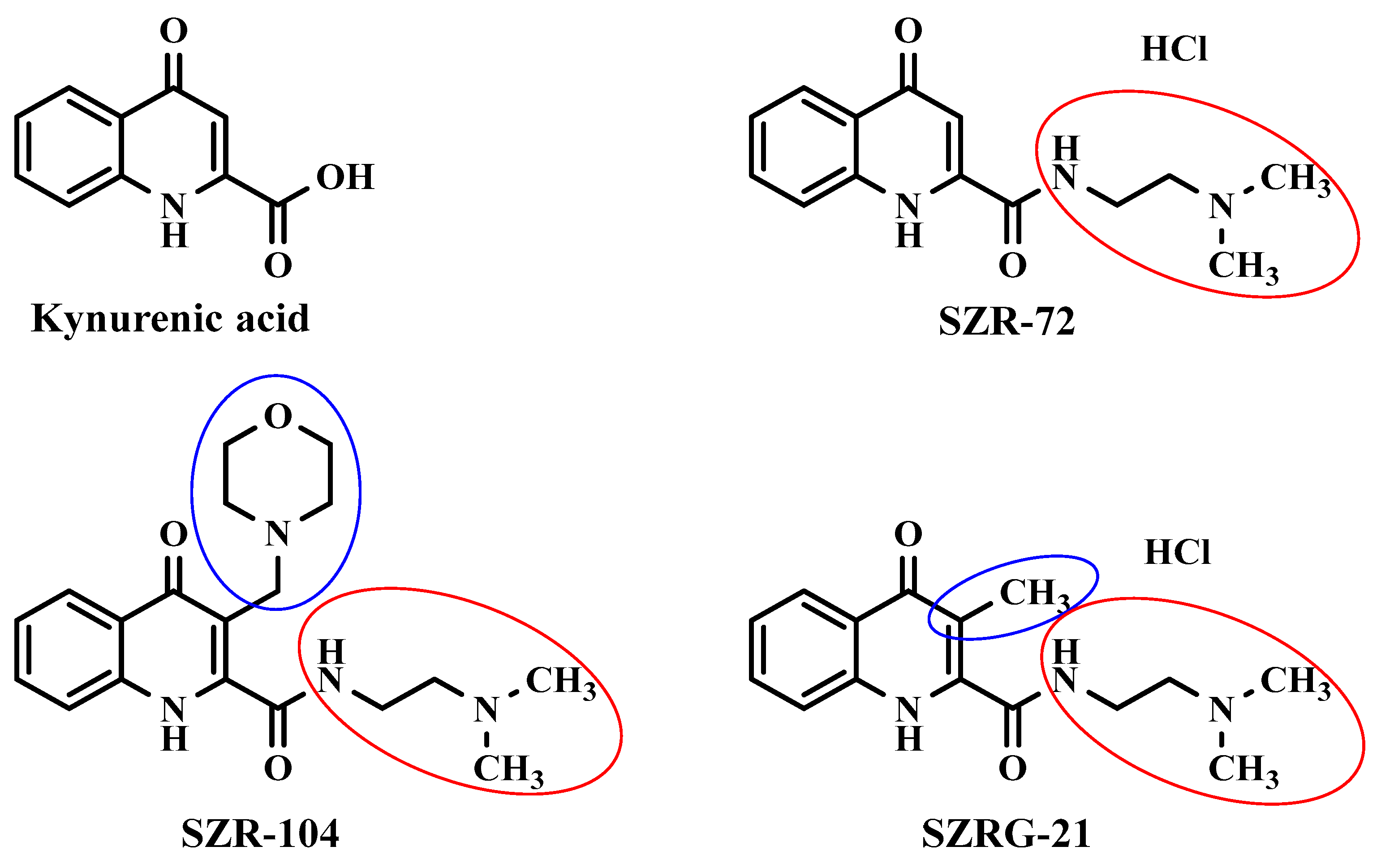
Figure 4.
Chemical Structure of MK-801.
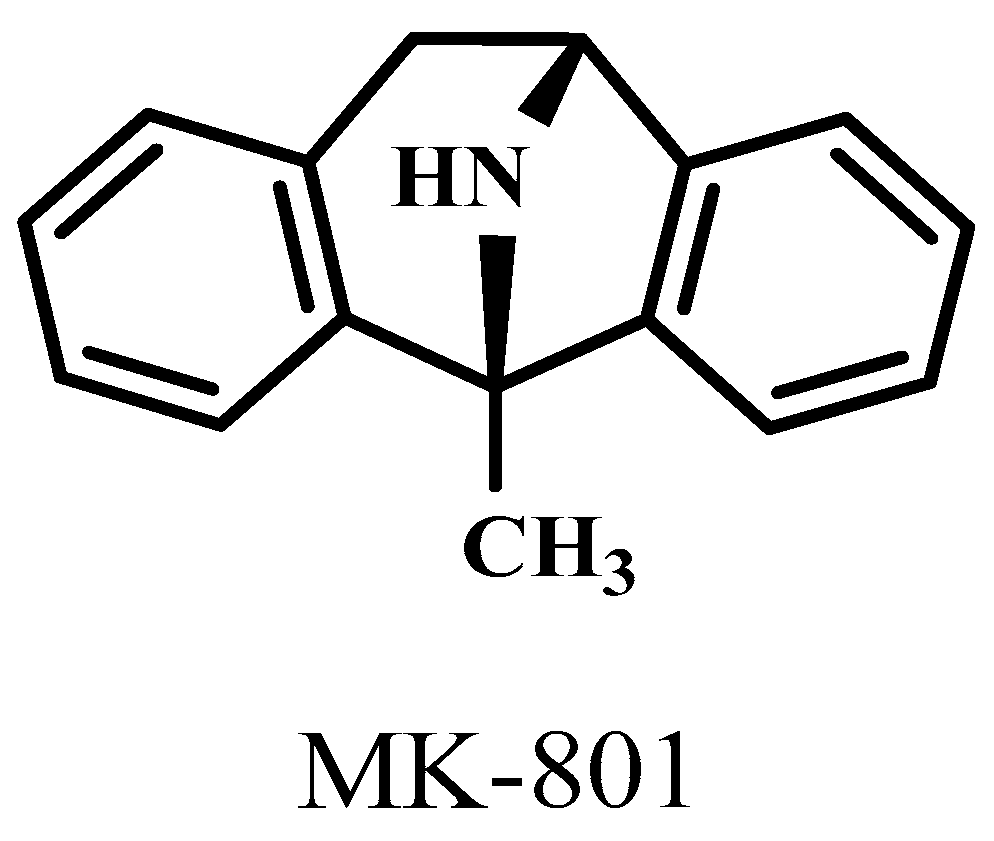
Figure 6.
Effect of kynurenic acid (KYNA) on pilot stereotype/ataxia test. KYNA caused stereotype and ataxia symptoms at the higher dose after i.c.v. injection. (A) Stereotype symptoms score of KYNA treatment, 0.1 μmol/4 μL KYNA vs. control (Ctrl) group, 0.1 μmol/4 μL KYNA vs. 0.04 μmol/4 μL KYNA group (P=0.004). The statistical analysis was Kruskal-Wallis Non-parametric test (P<0.05). (B) Ataxia symptoms score of KYNA treatment, 0.1 μmol/4 μL KYNA vs. Ctrl group, 0.1 μmol/4 μL KYNA vs. 0.04 μmol/4 μL KYNA group (P=0.001). The statistical analysis was Kruskal-Wallis Non-parametric Test (P <0.05). We show the number of animals and the score category, *: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=5, N(0.04 μmol/4 μL KYNA)=5, and N(0.1 μmol/4 μL KYNA)=5.
Figure 6.
Effect of kynurenic acid (KYNA) on pilot stereotype/ataxia test. KYNA caused stereotype and ataxia symptoms at the higher dose after i.c.v. injection. (A) Stereotype symptoms score of KYNA treatment, 0.1 μmol/4 μL KYNA vs. control (Ctrl) group, 0.1 μmol/4 μL KYNA vs. 0.04 μmol/4 μL KYNA group (P=0.004). The statistical analysis was Kruskal-Wallis Non-parametric test (P<0.05). (B) Ataxia symptoms score of KYNA treatment, 0.1 μmol/4 μL KYNA vs. Ctrl group, 0.1 μmol/4 μL KYNA vs. 0.04 μmol/4 μL KYNA group (P=0.001). The statistical analysis was Kruskal-Wallis Non-parametric Test (P <0.05). We show the number of animals and the score category, *: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=5, N(0.04 μmol/4 μL KYNA)=5, and N(0.1 μmol/4 μL KYNA)=5.
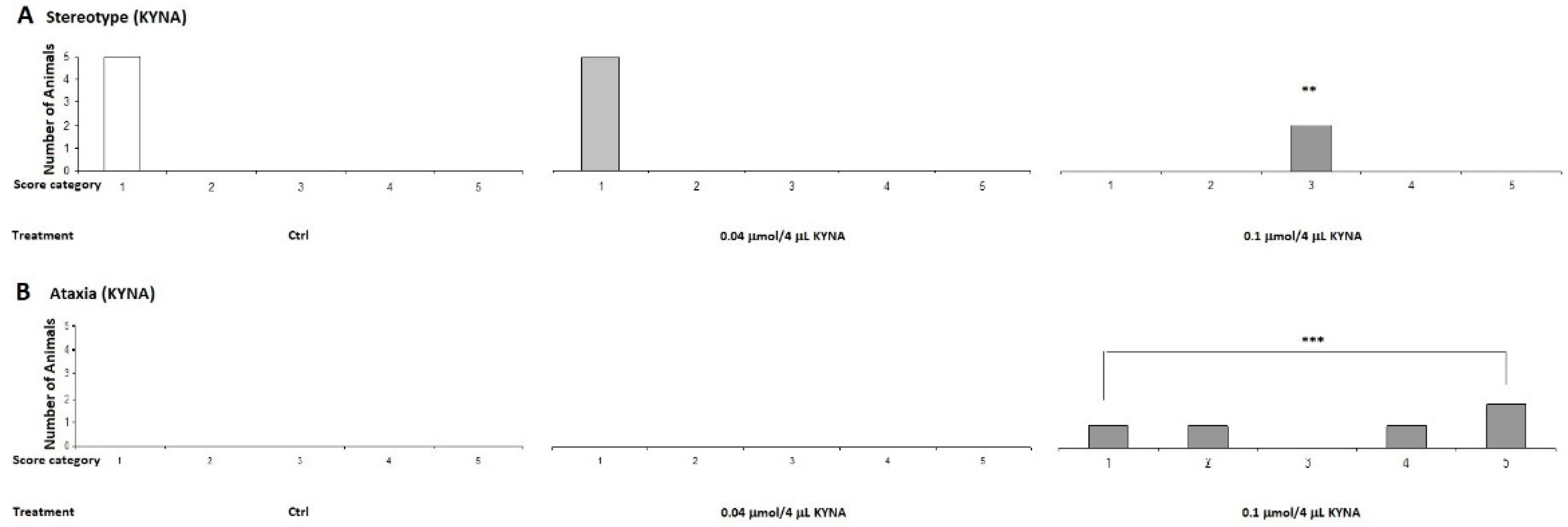
Figure 7.
Effect of 0.04 μmol/4 μL doses of KYNA and its analogs in stereotype and ataxia tests. Stereotype symptom scores after treatment were not significantly different between treatment groups. The statistical analysis was One-way Anova with Bonferroni Post Hoc Test (P<0.05). We show the number of animals and the score category, *: 0.05: **: 0.01; ***: 0.001, N(Ctrl)=10, N(0.04 μmol/4 μL KYNA)=10, N(0.04 μmol/4 μL SZR-72)=10, N(0.04 μmol/4 μL SZR-104)=10, and N(0.04 μmol/4 vL SZRG-21)=10.
Figure 7.
Effect of 0.04 μmol/4 μL doses of KYNA and its analogs in stereotype and ataxia tests. Stereotype symptom scores after treatment were not significantly different between treatment groups. The statistical analysis was One-way Anova with Bonferroni Post Hoc Test (P<0.05). We show the number of animals and the score category, *: 0.05: **: 0.01; ***: 0.001, N(Ctrl)=10, N(0.04 μmol/4 μL KYNA)=10, N(0.04 μmol/4 μL SZR-72)=10, N(0.04 μmol/4 μL SZR-104)=10, and N(0.04 μmol/4 vL SZRG-21)=10.
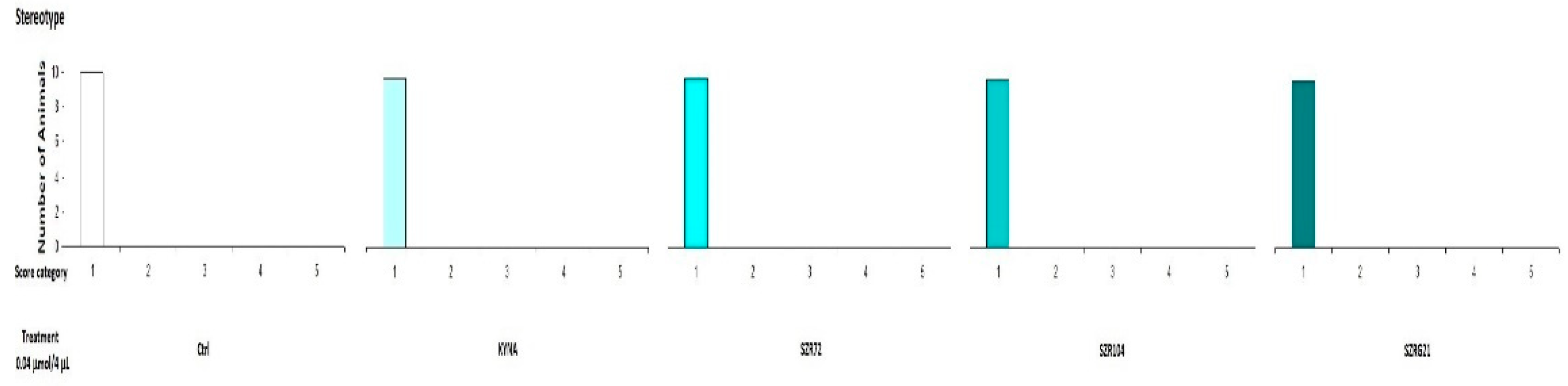
Figure 8.
Effect of 0.1 μmol/4 μL doses of KYNA and its analogs in stereotype and ataxia tests. (A) Stereotype symptom scores after treatment were not significantly different between treatment groups. The statistical analysis was Kruskal-Wallis Non-parametric Test (P<0.05). (B) Ataxia symptoms score after the treatment, 0.1 μmol/4 μL KYNA vs. Ctrl, SZR-72 and SZRG-21 groups (P=0.004). The statistical analysis was Kruskal-Wallis Non-parametric Test (P <0.05). ). We show the number of animals and the score category, *: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=10, N(0.1 μmol/4 μL KYNA)=10, N(0.1 μmol/4 μL SZR-72)=10, N(0.1 μmol/4 μL SZR-104)=10, and N(0.1 μmol/4 μL SZRG-21)=10.
Figure 8.
Effect of 0.1 μmol/4 μL doses of KYNA and its analogs in stereotype and ataxia tests. (A) Stereotype symptom scores after treatment were not significantly different between treatment groups. The statistical analysis was Kruskal-Wallis Non-parametric Test (P<0.05). (B) Ataxia symptoms score after the treatment, 0.1 μmol/4 μL KYNA vs. Ctrl, SZR-72 and SZRG-21 groups (P=0.004). The statistical analysis was Kruskal-Wallis Non-parametric Test (P <0.05). ). We show the number of animals and the score category, *: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=10, N(0.1 μmol/4 μL KYNA)=10, N(0.1 μmol/4 μL SZR-72)=10, N(0.1 μmol/4 μL SZR-104)=10, and N(0.1 μmol/4 μL SZRG-21)=10.
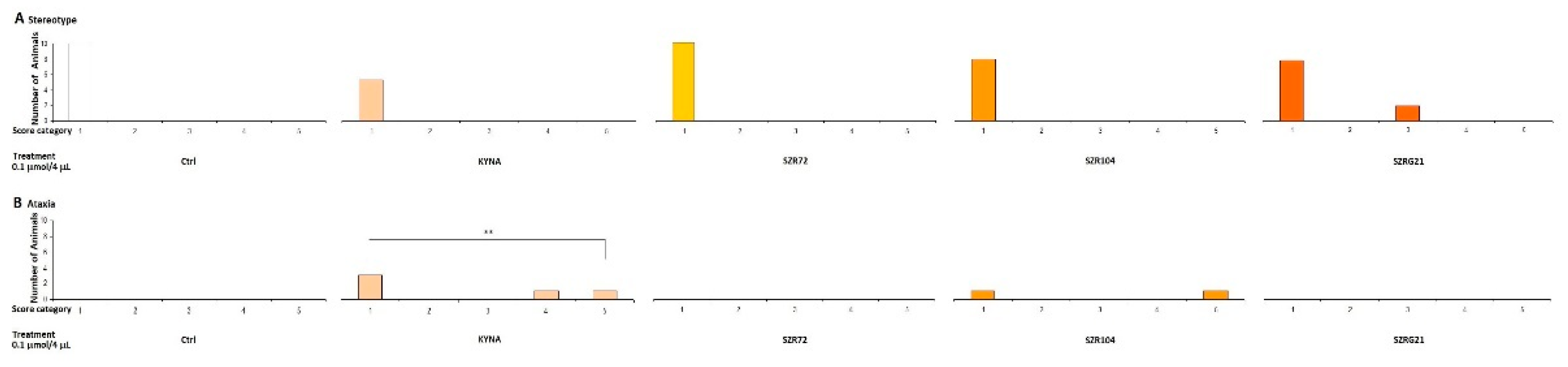
Figure 9.
Open field behavior test after treatment with KYNA and its analogs in 0.04 μmol/4 μL doses. (A) Ambulation distance, we found significant difference in behavior the SZR-104 treated group between the Control (Ctrl) (P=0.01), the KYNA (P=0.001) and the SZR-72 (P=0.026) group, The statistical analysis was One-way ANOVA with LSD Post Hoc Test (P<0.05). (B) Rearing count, we found significant difference between the 0.04 μmol SZR-72 vs. Ctrl group (P=0.019), vs. SZR-104 group (P=0.015) and vs. SZRG-21 group (P=0.02). Statistical analysis was performed using one-way ANOVA with LSD post-hoc test (P <0.05). We show the data mean ± SEM, *: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=10, N(0.04 μmol/4 μL KYNA)=10, N(0.04 μmol/4 μL SZR-72)=10, N(0.04 μmol/4 μL SZR-104)=10, and N(0.04 μmol/4 μL SZRG-21)=10.
Figure 9.
Open field behavior test after treatment with KYNA and its analogs in 0.04 μmol/4 μL doses. (A) Ambulation distance, we found significant difference in behavior the SZR-104 treated group between the Control (Ctrl) (P=0.01), the KYNA (P=0.001) and the SZR-72 (P=0.026) group, The statistical analysis was One-way ANOVA with LSD Post Hoc Test (P<0.05). (B) Rearing count, we found significant difference between the 0.04 μmol SZR-72 vs. Ctrl group (P=0.019), vs. SZR-104 group (P=0.015) and vs. SZRG-21 group (P=0.02). Statistical analysis was performed using one-way ANOVA with LSD post-hoc test (P <0.05). We show the data mean ± SEM, *: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=10, N(0.04 μmol/4 μL KYNA)=10, N(0.04 μmol/4 μL SZR-72)=10, N(0.04 μmol/4 μL SZR-104)=10, and N(0.04 μmol/4 μL SZRG-21)=10.
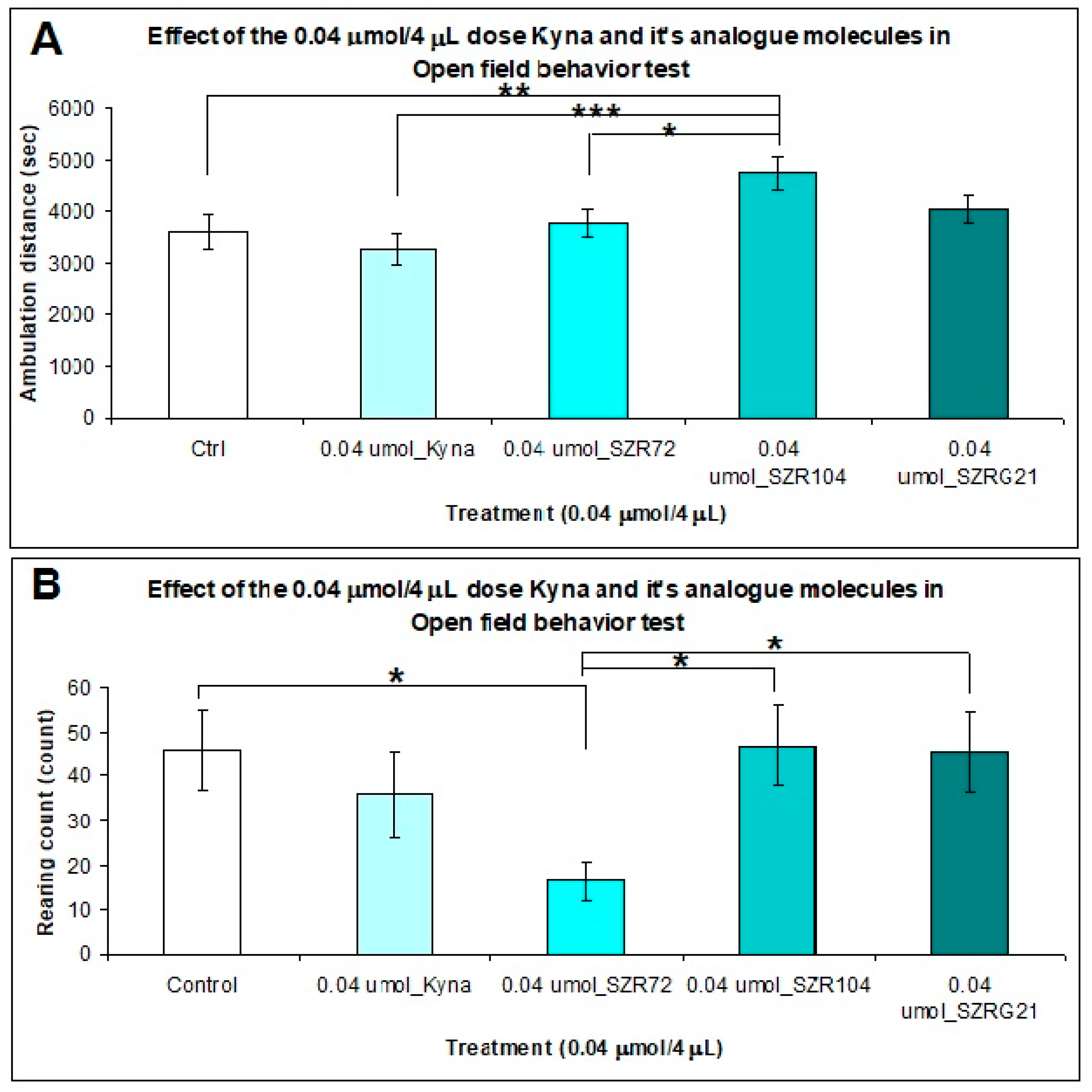
Figure 10.
Open field (OF) test after treatment with KYNA and its analogs in 0.1 μmol/4 μL doses. (A) Ambulation distance, we did not find significant differences in behavior between the treated groups or the control (Ctrl) group; the statistical analysis was One-way ANOVA with LSD Post Hoc Test (P<0.05). (B) Rearing count, we found a significant difference only between the 0.1 μmol SZR-72 vs. Ctrl group (P=0.04), and the statistical analysis was One-way ANOVA with LSD Post Hoc Test (P <0.05). We show the data mean ± SEM, **: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=10, N(0.1 μmol/4 μL KYNA)=10, N(0.1 μmol/4 μL SZR-72)=10, N(0.1 μmol/4 μL SZR-104)=10, and N(0.1 μmol/4 μL SZRG-21)=10.
Figure 10.
Open field (OF) test after treatment with KYNA and its analogs in 0.1 μmol/4 μL doses. (A) Ambulation distance, we did not find significant differences in behavior between the treated groups or the control (Ctrl) group; the statistical analysis was One-way ANOVA with LSD Post Hoc Test (P<0.05). (B) Rearing count, we found a significant difference only between the 0.1 μmol SZR-72 vs. Ctrl group (P=0.04), and the statistical analysis was One-way ANOVA with LSD Post Hoc Test (P <0.05). We show the data mean ± SEM, **: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=10, N(0.1 μmol/4 μL KYNA)=10, N(0.1 μmol/4 μL SZR-72)=10, N(0.1 μmol/4 μL SZR-104)=10, and N(0.1 μmol/4 μL SZRG-21)=10.
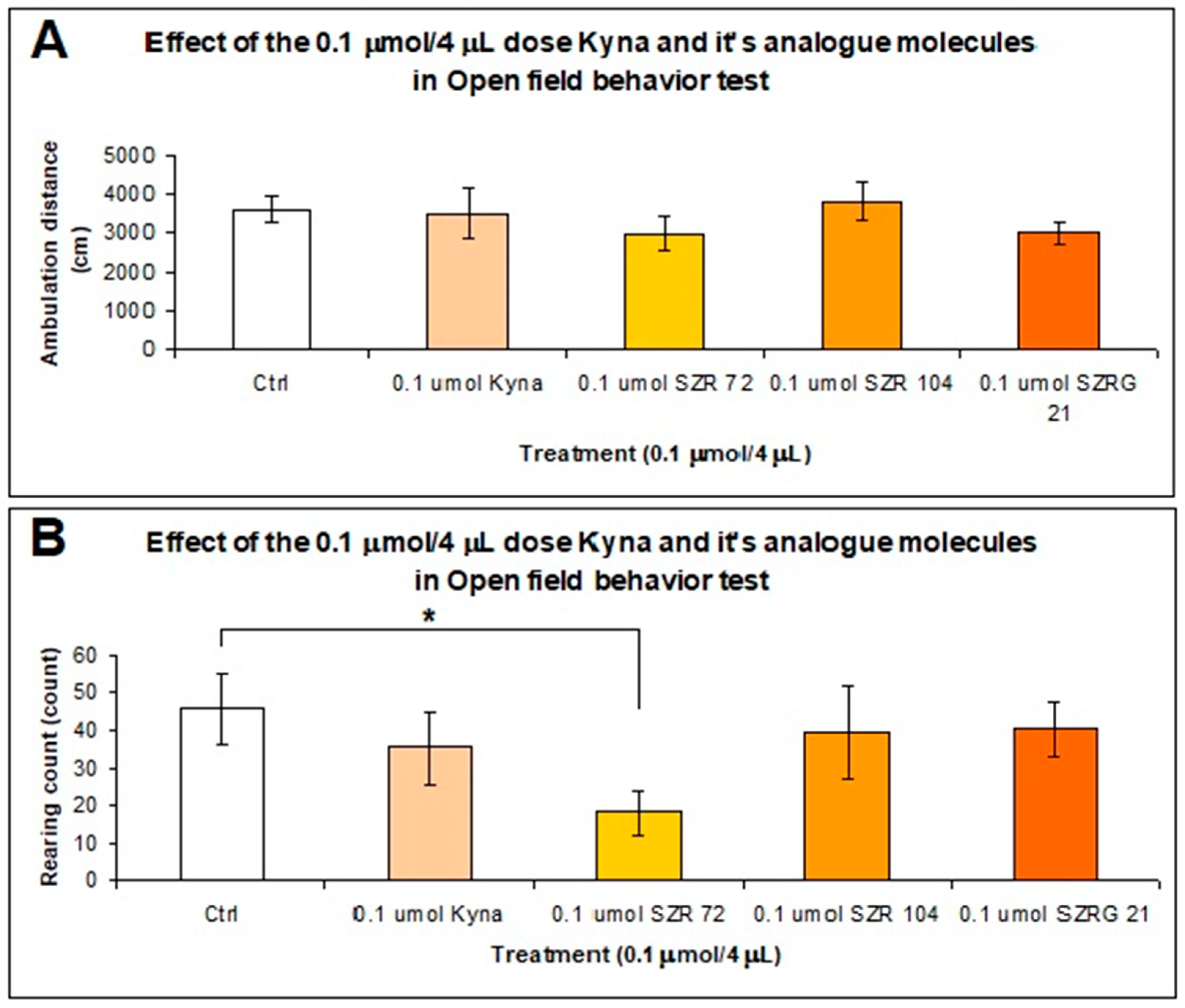
Figure 11.
Rotarod behavior test of mice were treated with 0.04 μmol/4 μL KYNA and its analog molecules. Kruskal-Wallis Non-parametric Test was used for statistical analyses (P <0.05). We show the data median ± SD, *: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=10, N(0.04 μmol/4 μL KYNA)=10, N(0.04 μmol/4 μL SZR-72)=10, N(0.04 μmol/4 μL SZR-104)=10, and N(0.04 μmol/4 μL SZRG-21)=10.
Figure 11.
Rotarod behavior test of mice were treated with 0.04 μmol/4 μL KYNA and its analog molecules. Kruskal-Wallis Non-parametric Test was used for statistical analyses (P <0.05). We show the data median ± SD, *: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=10, N(0.04 μmol/4 μL KYNA)=10, N(0.04 μmol/4 μL SZR-72)=10, N(0.04 μmol/4 μL SZR-104)=10, and N(0.04 μmol/4 μL SZRG-21)=10.
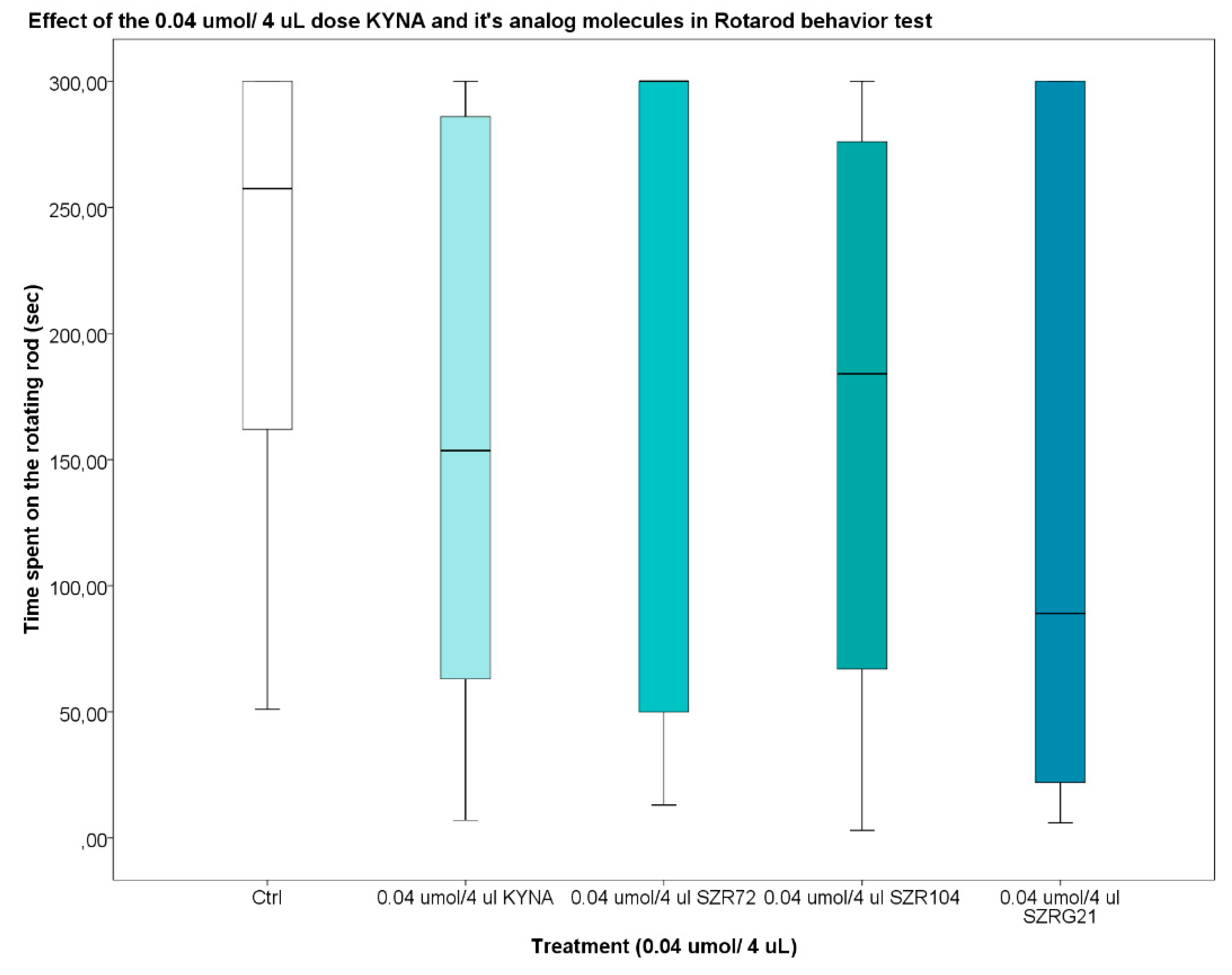
Figure 12.
Rotarod behavior test of mice were treated with 0.1 μmol/4 μL KYNA and its analog molecules. Kruskal-Wallis Non-parametric Test was used for statistical analyses (P <0.05). We show the data median ± SD, *: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=10, N(0.1 μmol/4 μL KYNA)=10, N(0.1 μmol/4 μL SZR-72)=10, N(0.1 μmol/4 μL SZR-104)=10, and N(0.1 μmol/4 μL SZRG-21)=10.
Figure 12.
Rotarod behavior test of mice were treated with 0.1 μmol/4 μL KYNA and its analog molecules. Kruskal-Wallis Non-parametric Test was used for statistical analyses (P <0.05). We show the data median ± SD, *: 0.05; **: 0.01; ***: 0.001, N(Ctrl)=10, N(0.1 μmol/4 μL KYNA)=10, N(0.1 μmol/4 μL SZR-72)=10, N(0.1 μmol/4 μL SZR-104)=10, and N(0.1 μmol/4 μL SZRG-21)=10.
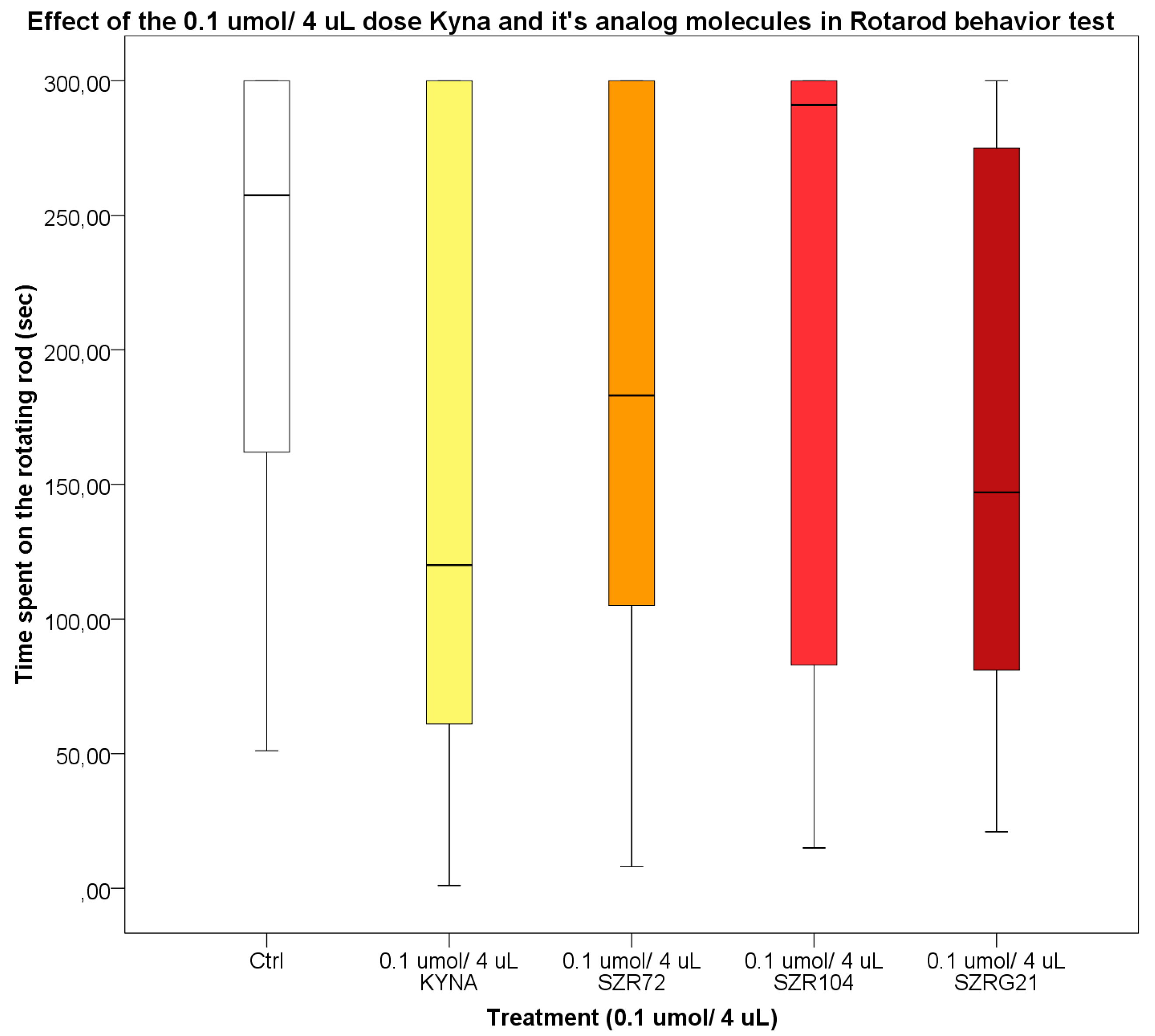
Figure 13.
Synthesis of SZRG-21 (N-(2-(dimethylamino)ethyl)-4-oxo-1,4-dihydroquinazoline-2-carboxamide hydrochloride).
Figure 13.
Synthesis of SZRG-21 (N-(2-(dimethylamino)ethyl)-4-oxo-1,4-dihydroquinazoline-2-carboxamide hydrochloride).
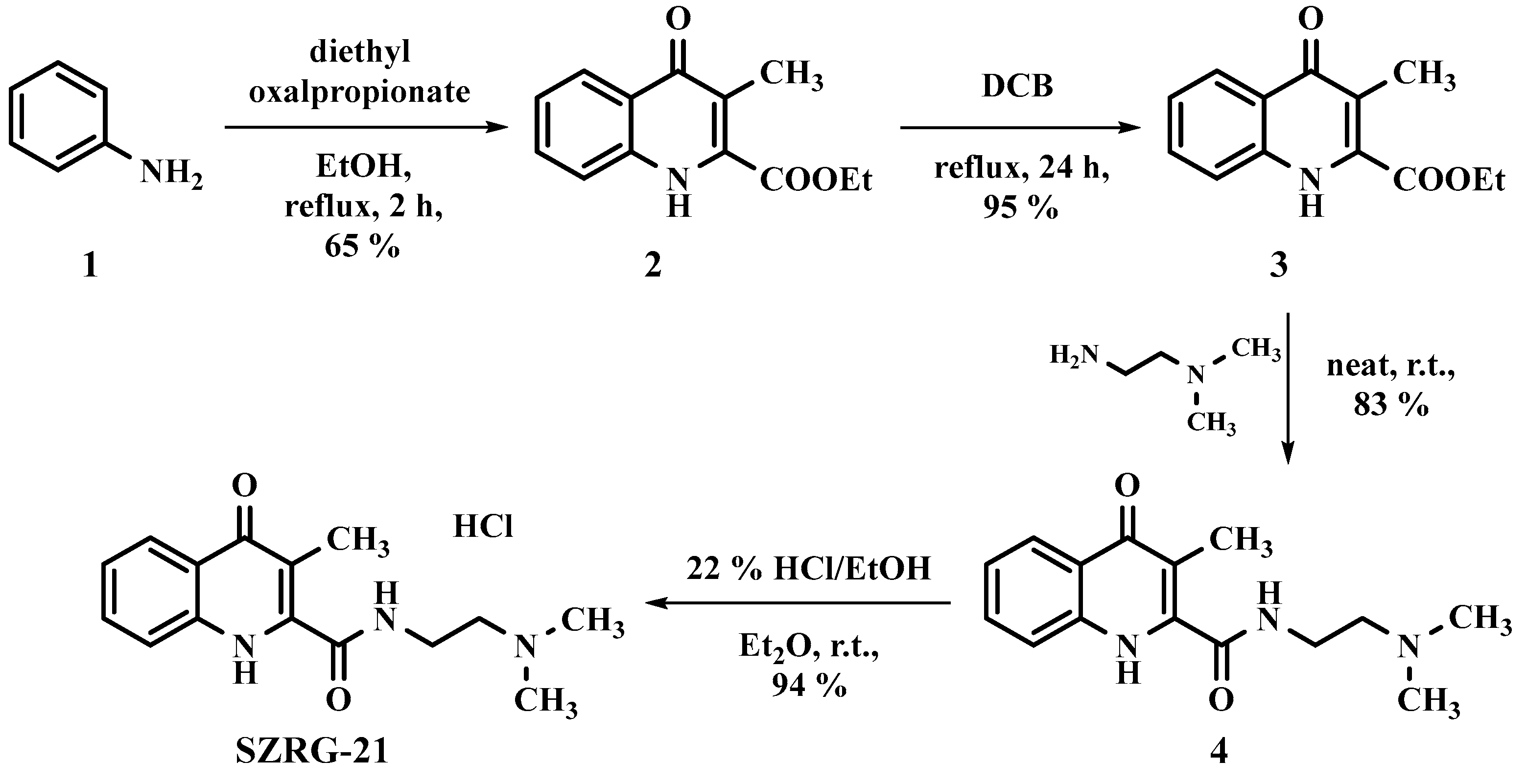
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









