Submitted:
20 February 2024
Posted:
21 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant materials and growing conditions
2.2. Cabbage plants, and leaf development in planta and histology studies.
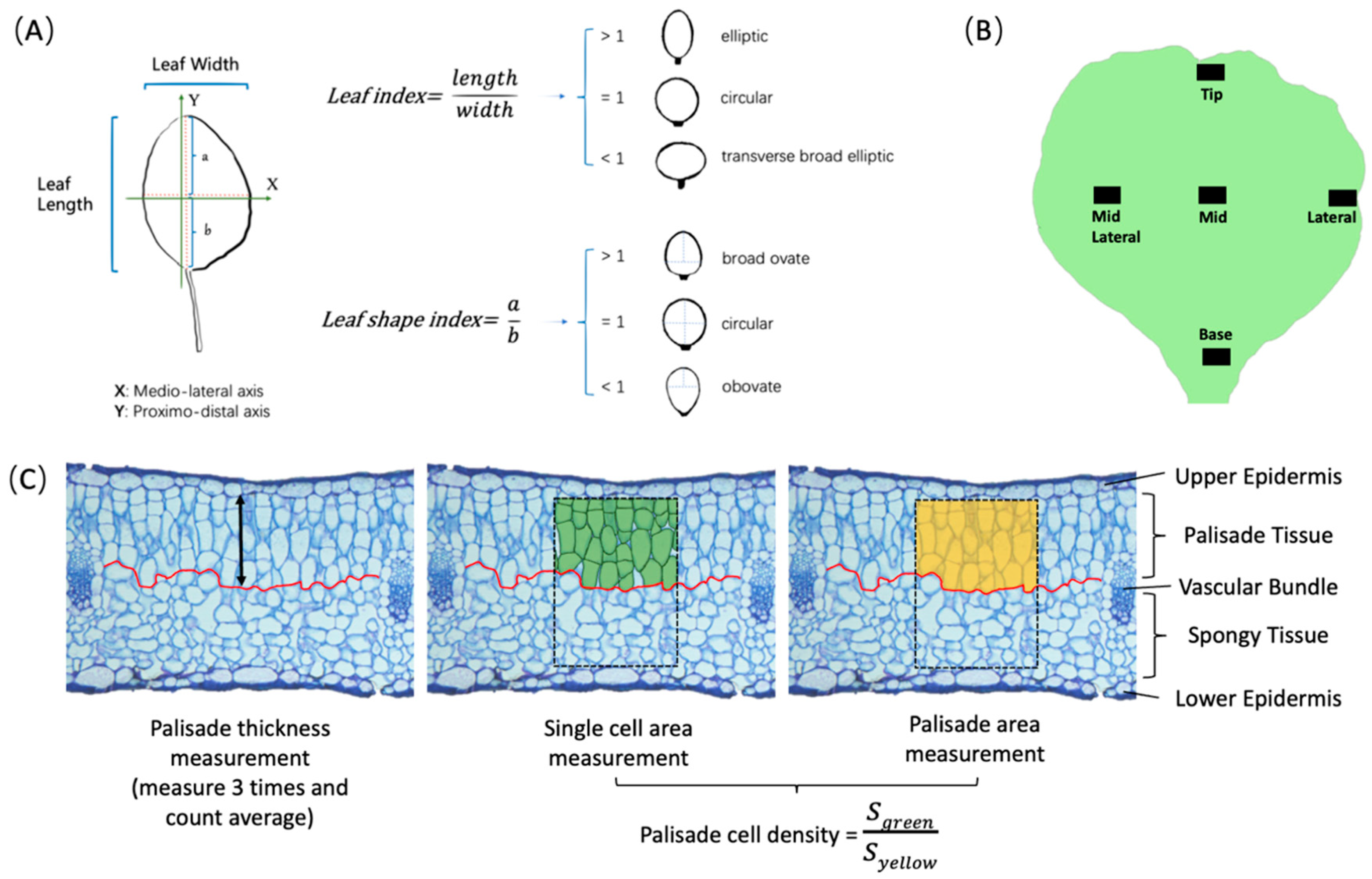
2.3. Leaf morphology studies
3. Results
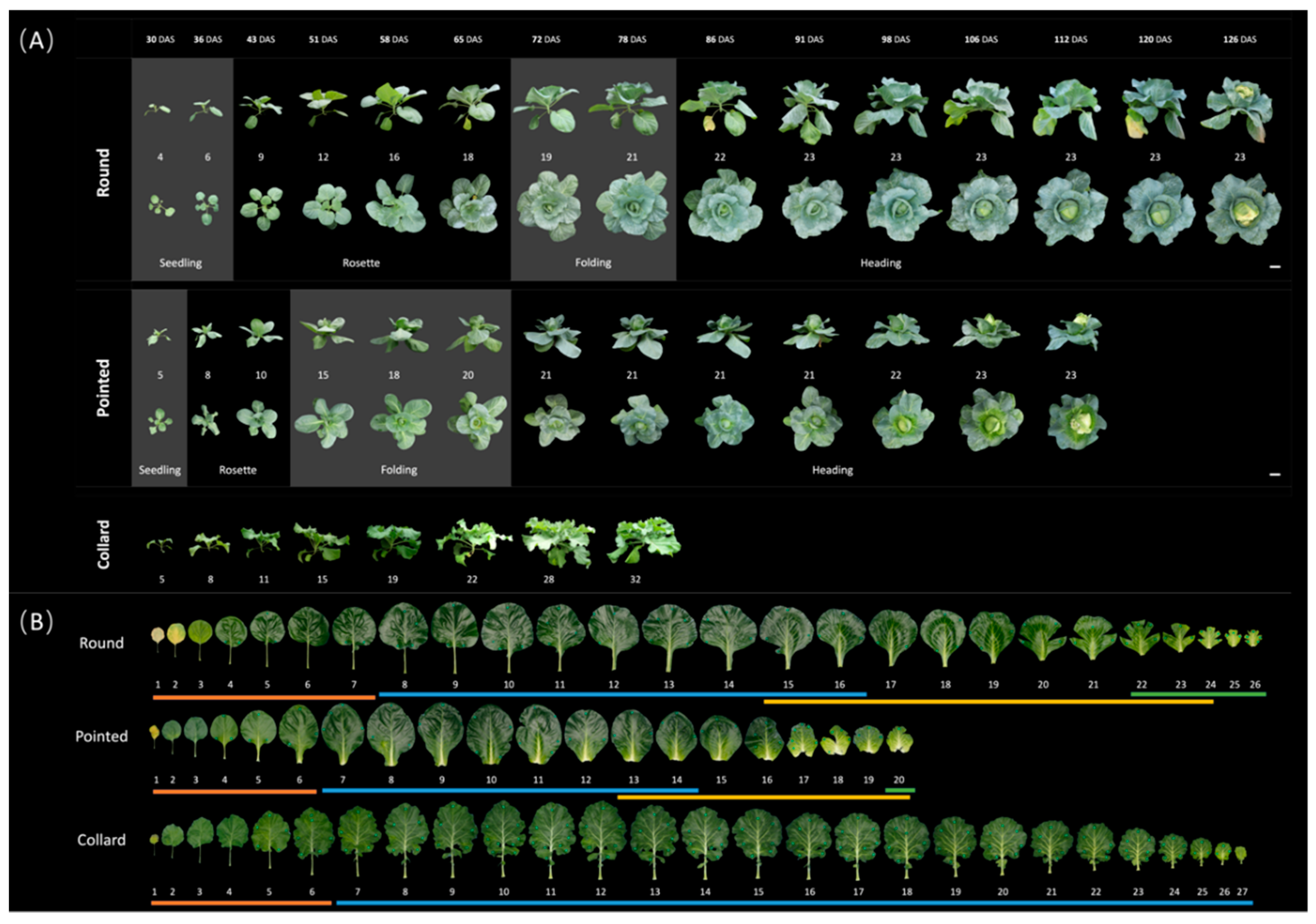
3.1. Whole plant development
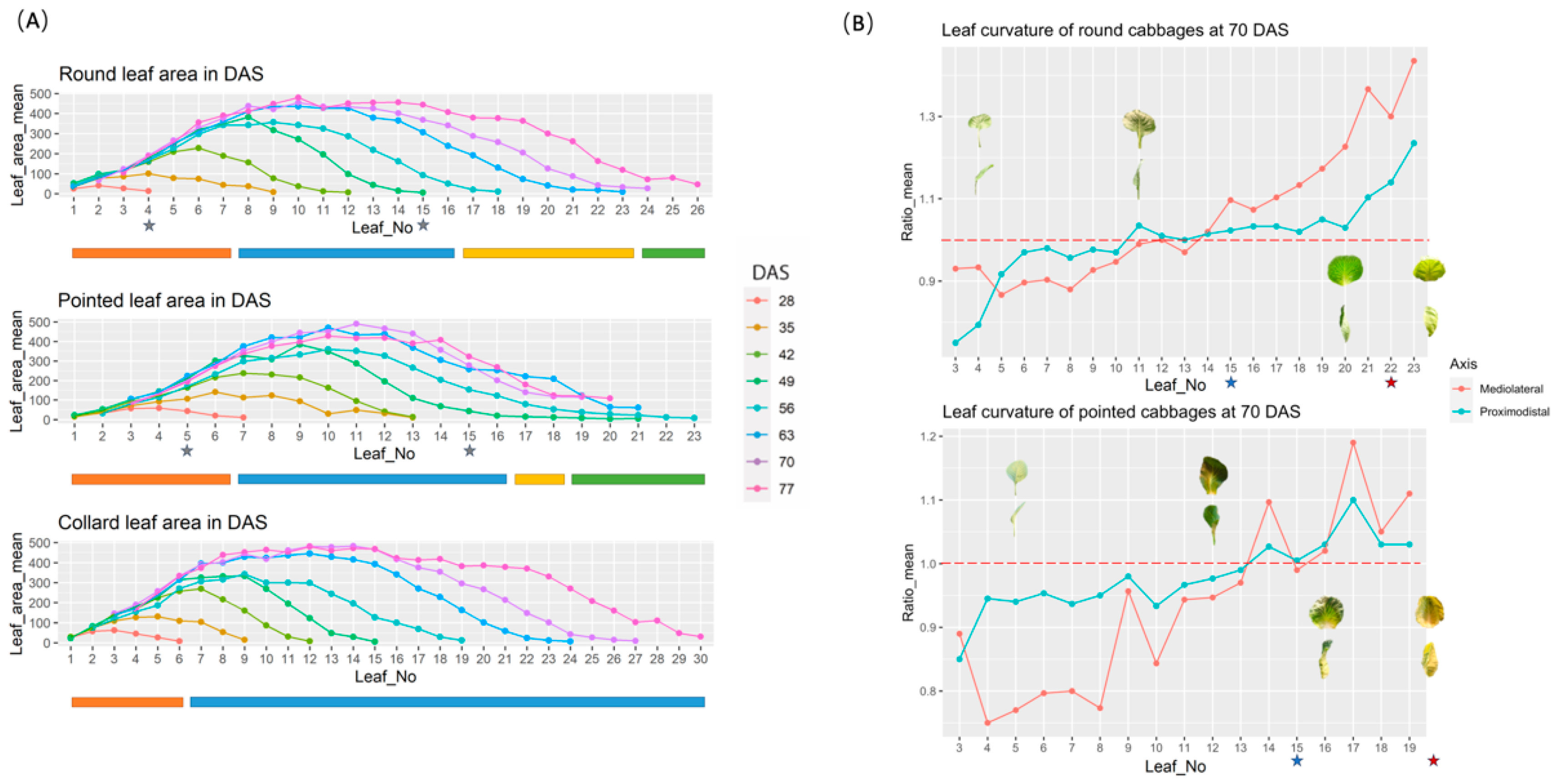
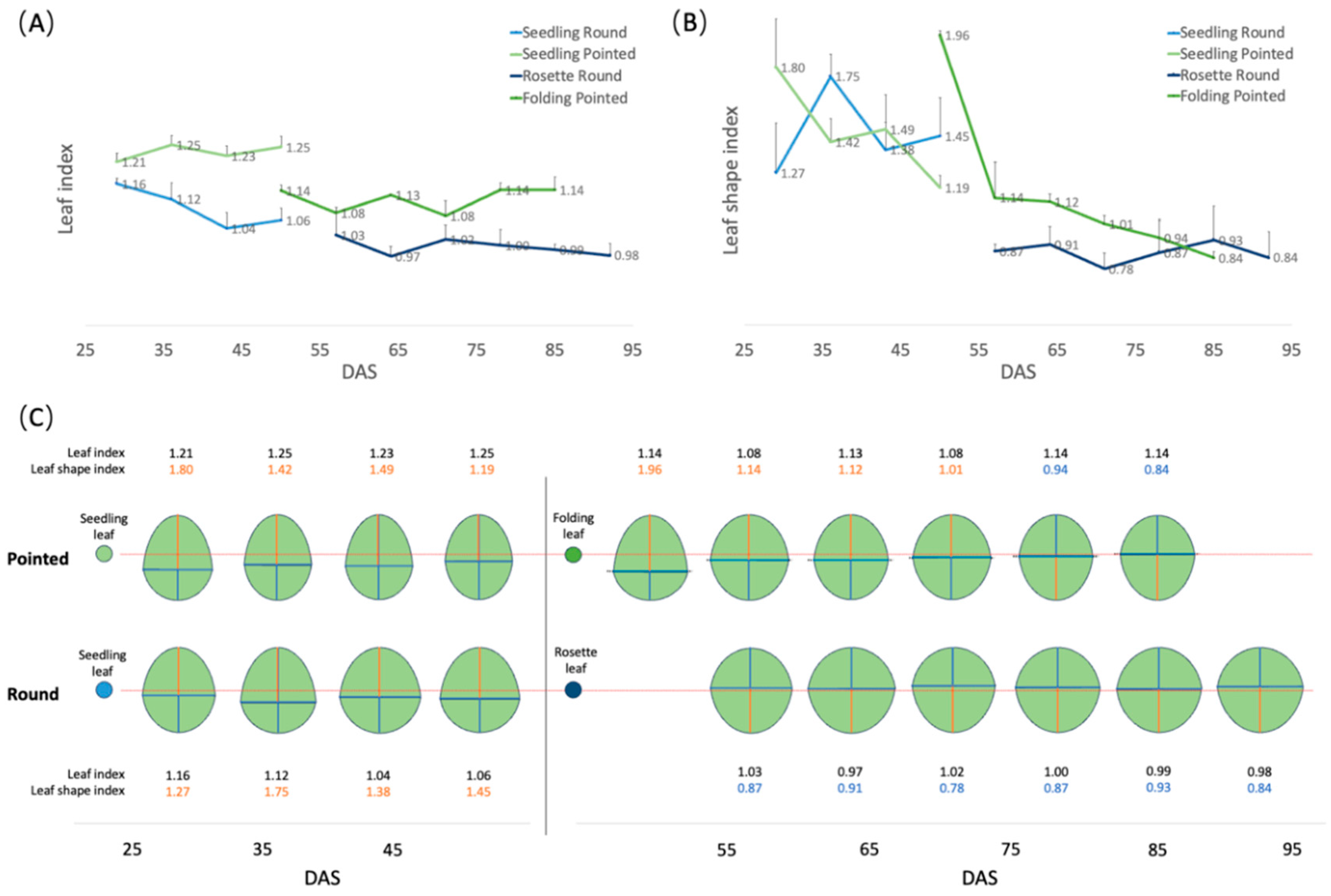
3.2. Changes in leaf morphology during plant development
3.3. Defining the developmental stages of cabbage.
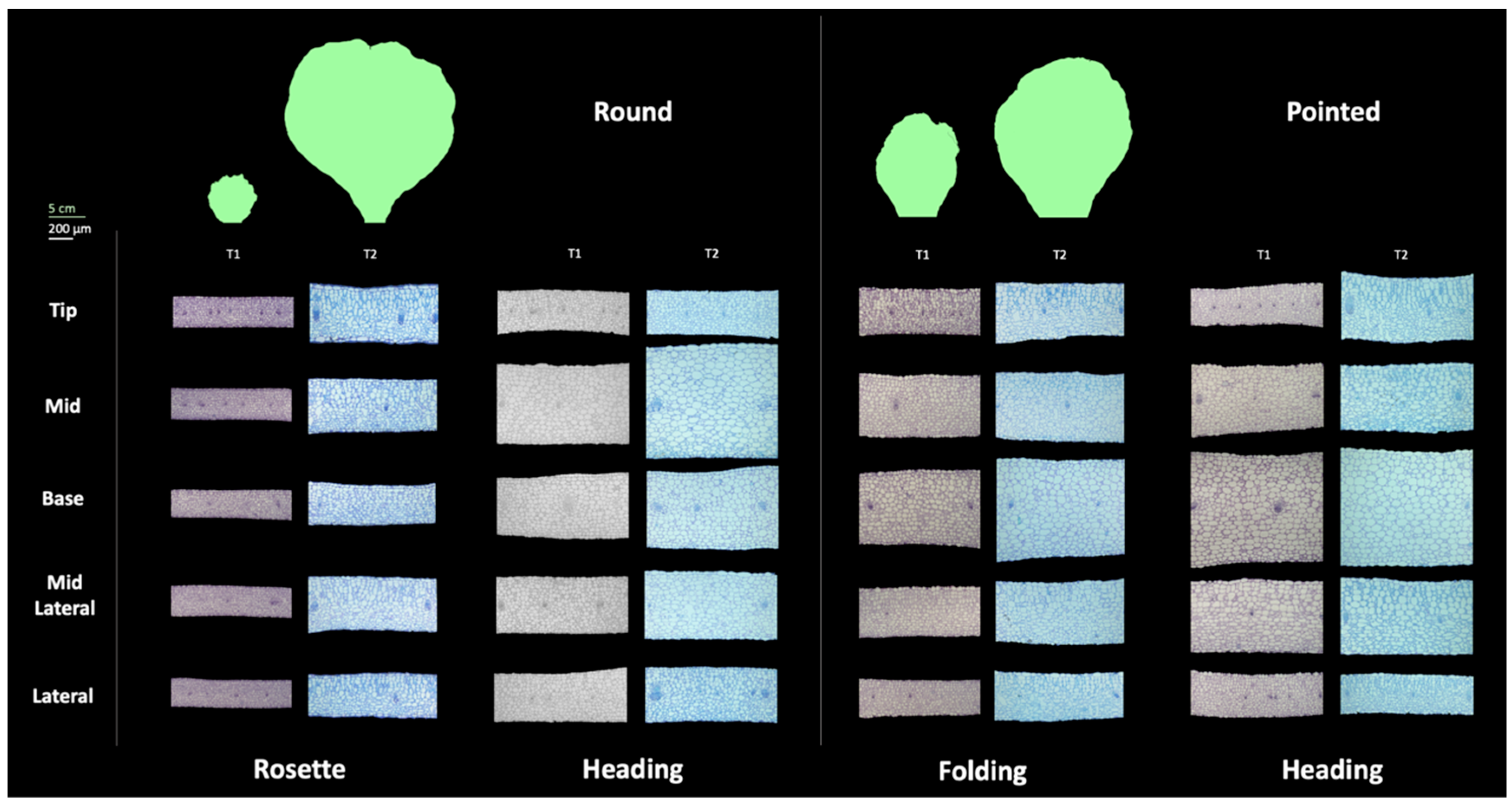
3.4. Histology of leaf development
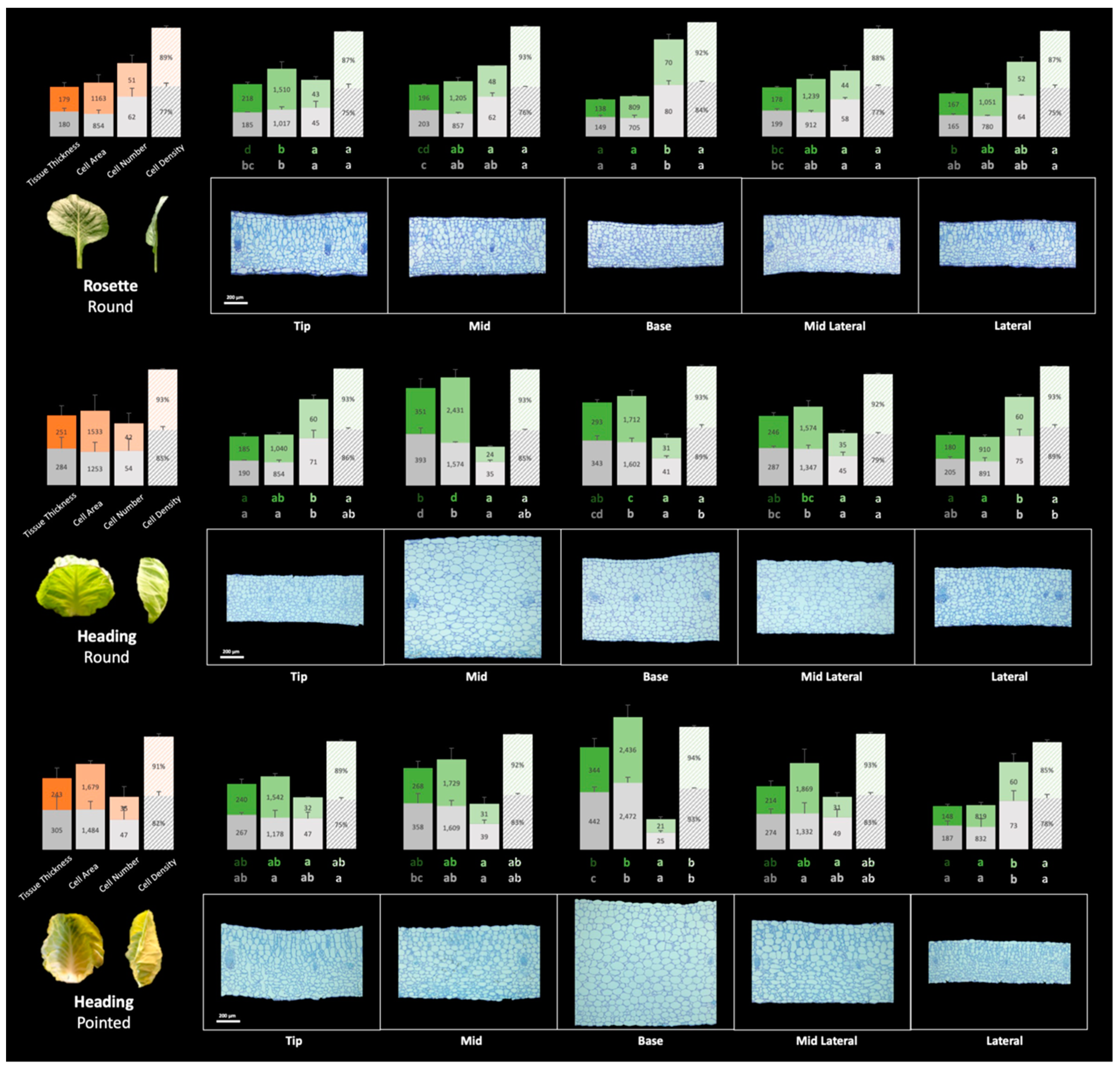
3.4.1. Difference among leaf positions
3.4.2. Difference between adaxial and abaxial sides
3.4.2.1. Tissue Thickness
3.4.2.2. Average Single Cell Area and Cell Density
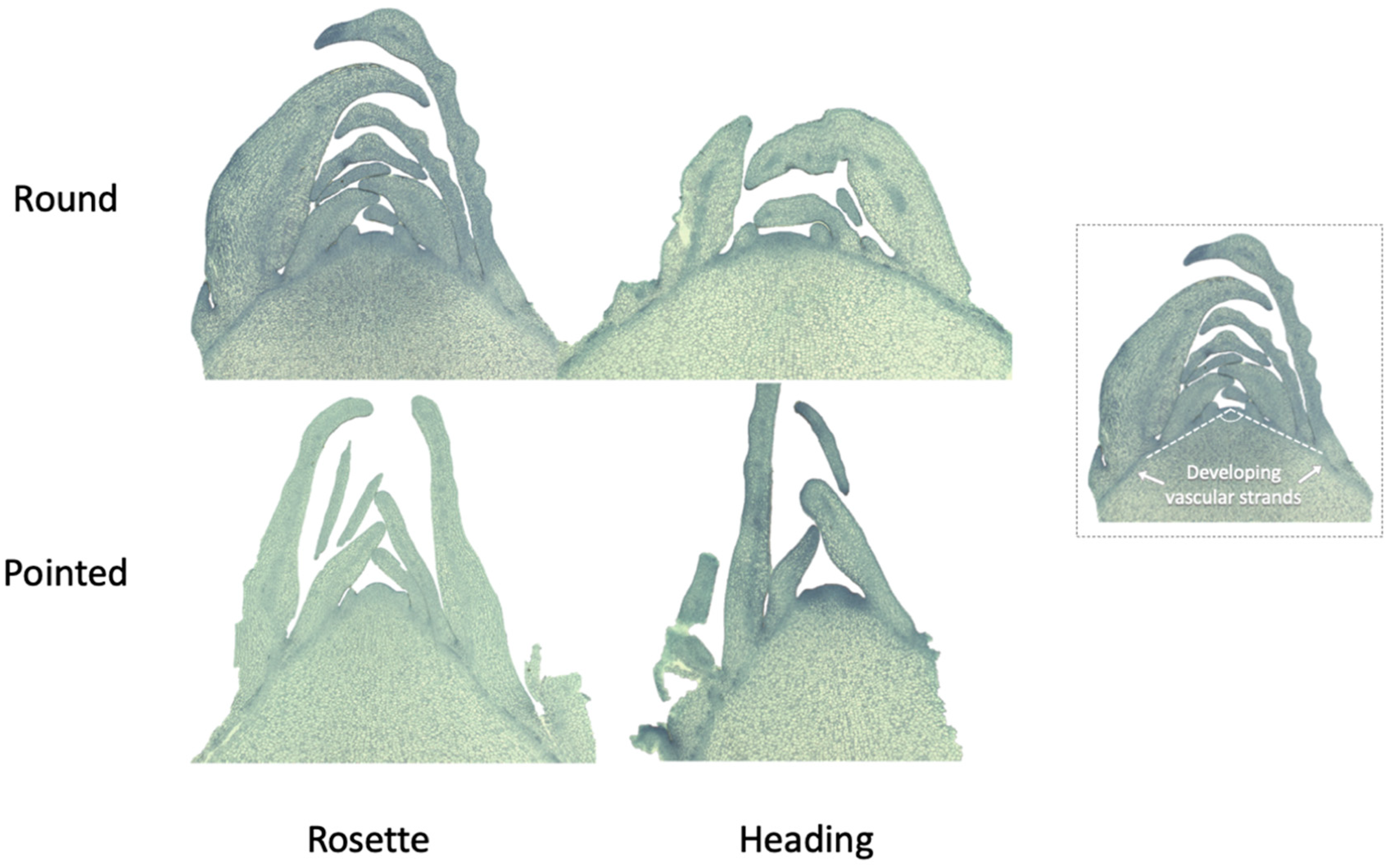
3.4.3. Shoot apical meristem
4. Discussion
4.1. Characterization and timing of developmental stages
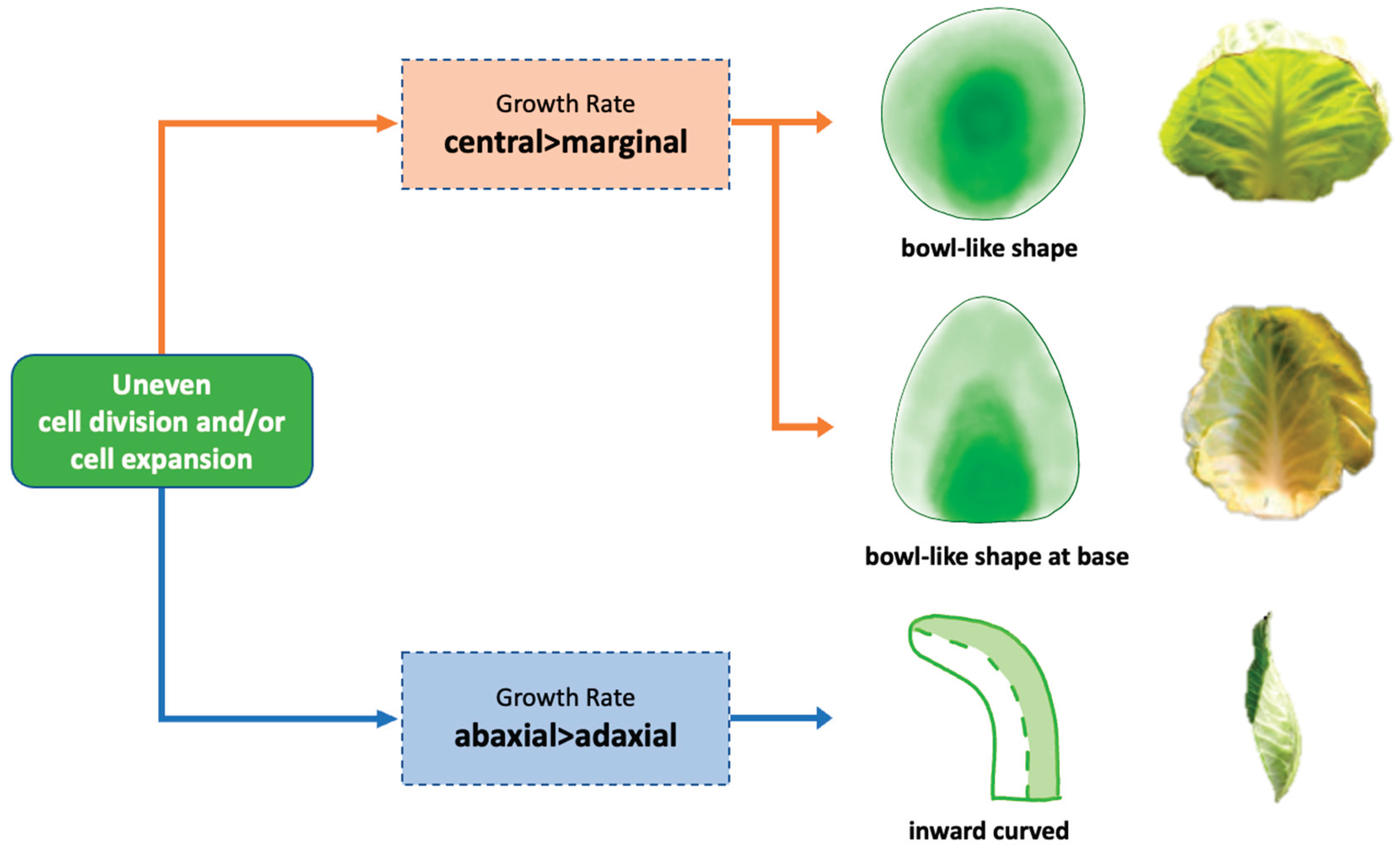
4.2. Differential growth across the leaf blade and between the mesophyll cell layers are associated with leaf curvature
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nawaz, H., M.A. Shad, and A. Rauf, Optimization of extraction yield and antioxidant properties of Brassica oleracea Convar Capitata Var L. leaf extracts. Food chemistry, 2018. 242: p. 182-187.
- Guo, X., et al., Series-Spatial Transcriptome Profiling of Leafy Head Reveals the Key Transition Leaves for Head Formation in Chinese Cabbage. Front Plant Sci, 2021. 12: p. 787826.
- He, Y.K., et al., Leafy head formation of the progenies of transgenic plants of Chinese cabbage with exogenous auxin genes. Cell research, 2000. 10(2): p. 151-160.
- Sun, X., et al., Genome-wide transcriptome analysis reveals molecular pathways involved in leafy head formation of Chinese cabbage (Brassica rapa). Horticulture research, 2019. 6.
- Mao, Y., et al., MicroRNA319a-targeted Brassica rapa ssp. pekinensis TCP genes modulate head shape in chinese cabbage by differential cell division arrest in leaf regions. Plant physiology, 2014. 164(2): p. 710-720.
- Wang, Y., et al., BrpSPL9 (Brassica rapa ssp. pekinensis SPL9) controls the earliness of heading time in Chinese cabbage. Plant Biotechnol J, 2014. 12(3): p. 312-21.
- Alemán-Báez, J., et al., Genetic dissection of morphological variation in rosette leaves and leafy heads in cabbage (Brassica oleracea var. capitata). Theoretical and Applied Genetics, 2022. 135(10): p. 3611-3628.
- Zhang, K., et al., A cluster of transcripts identifies a transition stage initiating leafy head growth in heading morphotypes of Brassica. The Plant Journal, 2022. 110(3): p. 688-706.
- Yue, X., et al., The Adaxial/Abaxial Patterning of Auxin and Auxin Gene in Leaf Veins Functions in Leafy Head Formation of Chinese Cabbage. Frontiers in Plant Science, 2022. 13: p. 918112.
- Oguchi, R., et al., Leaf anatomy and function. The leaf: a platform for performing photosynthesis, 2018: p. 97-139.
- Nath, U., et al., Genetic control of surface curvature. Science, 2003. 299(5611): p. 1404-1407.
- Cheng, F., et al., Subgenome parallel selection is associated with morphotype diversification and convergent crop domestication in Brassica rapa and Brassica oleracea. Nature genetics, 2016. 48(10): p. 1218-1224.
- Liang, J., et al., Genetic variation and divergence of genes involved in leaf adaxial-abaxial polarity establishment in Brassica rapa. Frontiers in Plant Science, 2016. 7: p. 94.
- Moon, J. and S. Hake, How a leaf gets its shape. Current opinion in plant biology, 2011. 14(1): p. 24-30.
- Ren, W., et al., BcpLH organizes a specific subset of microRNAs to form a leafy head in Chinese cabbage (Brassica rapa ssp. pekinensis). Horticulture research, 2020. 7.
- Han, M.-H., et al., The Arabidopsis double-stranded RNA-binding protein HYL1 plays a role in microRNA-mediated gene regulation. Proceedings of the National Academy of Sciences, 2004. 101(4): p. 1093-1098.
- Cai, C., et al., Evidence for two domestication lineages supporting a middle-eastern origin for Brassica oleracea crops from diversified kale populations. Horticulture research, 2022. 9.
- Schneider, C.A., W.S. Rasband, and K.W. Eliceiri, NIH Image to ImageJ: 25 years of image analysis. Nature methods, 2012. 9(7): p. 671-675.
- Jorge Alemán-Báez, J.F.A.-Z., Johan Bucher, Chengcheng Cai, Roeland E. Voorrips and Guusje Bonnema. Expression changes of miRNA-regulated genes associated with the formation of the leafy head in cabbage (Brassica oleracea var. capitata). Horticultural Plant Journal 2024 (Accepted).
- Sun, X., et al., Genetic analysis of Chinese cabbage reveals correlation between rosette leaf and leafy head variation. Frontiers in Plant Science, 2018. 9: p. 1455.
- Yu, L., et al., HYL1 gene maintains venation and polarity of leaves. Planta, 2005. 221: p. 231-242.
- Ren, W., et al., Association of microRNAs with types of leaf curvature in Brassica rapa. Frontiers in Plant Science, 2018. 9: p. 73.
- Li, X., et al., Large-scale gene expression alterations introduced by structural variation drive morphotype diversification in Brassica oleracea. Nature Genetics, 2024.

| Sowing date | Plants in total | Plants utilized in the experiment | Type of experiment | Experiment | Morphotypes | Material scored | Scoring timepoints (DAS) | Traits scored |
|---|---|---|---|---|---|---|---|---|
| March | 18 (2 cabbage morphotypes * 3 blocks * 3 plants) | 3 plants per morphotype (1 per block) | Non-destructive | Whole plant development | Round- and pointed cabbage | Aerial part of cabbage plants | 30, 36, 43 51, 58, 65, 72, 78, 86, 91, 98, 106, 112, 120 and 126 | Plant growth |
| Leaf development in planta | 4th leaf for round cabbage, 5th leaf for pointed cabbage | 29, 36, 43 and 50 | Leaf blade area, leaf index and leaf shape | |||||
| 15th leaf for round- and pointed cabbage | 50, 57, 64, 71, 78, 85 and 92 | |||||||
| 3 plants per morphotype (1 per block) | Histology of leaves | Round cabbage | Leaves 15th, 15th, 15th | T1 = 57 and T2 = 85 | Leaf tissue from 5 positions (tip, mid, base, mid-lateral, and lateral) | |||
| Leaves 22nd, 22nd, 24th | T1 = 98 and T2 = 126 | |||||||
| Pointed cabbage | Leaves 15th, 15th, 17th | T1 = 57 and T2 = 85 | ||||||
| Leaves 17th, 21st, 22nd | T1 = 71 and T2 = 99 | |||||||
| August | 90 (3 morphotypes * 3 blocks * 10 plants) | 3 plants per timepoint per morphotype (1 per block) | Destructive | Leaf morphology and development | Round- and pointed cabbage and collard | Leaves | 28, 35, 42, 49, 56, 63, 70, and 77 | Total number of leaves and their leaf area |
| Round- and pointed cabbage | Leaf blade | 70 | Leaf curvature | |||||
| Leafy head | - | First heading leaf |
| Round (Excalibur hybrid variety) | Stages | Seedling | Rosette | Folding | Heading |
| DAS | ~10 to ~38 | ~38 to ~60 | ~60 to ~70 (latest 84) | ~70 to cracking | |
| Duration (days) | ~28 | ~22 | ~10 | > 56 | |
| Leaf number | 1st to 7th | 8th to ~15th | ~16th to ~23rd (latest 24th) | ~24th to … | |
| Pointed (Sonsma hybrid variety) | DAS | ~8 to ~33 | ~33 to ~48 | ~48 to ~56 (latest 84) | ~56 to cracking |
| Duration (days) | ~25 | ~15 | ~8 | > 56 | |
| Leaf number | 1st to 6th | 7th to ~14th | ~15th to ~17th (latest 21st) | ~18th to … |
 Rosette Round |
P/S (T1) | P/S (T2) | ∆P/∆S | |
| Tissue Thickness | 1.00 | 1.00 | 1.00 | |
| Single Cell Area | 1.02 | 1.36 | 1.48 | |
| Cell Density | 1.02 | 1.15 | -0.24 | |
 Heading Round |
P/S (T1) | P/S (T2) | ∆P/∆S | |
| Tissue Thickness | 0.88 | 0.86 | 0.79 | |
| Single Cell Area | 1.23 | 1.21 | 1.17 | |
| Cell Density | 1.05 | 1.08 | 0.46 | |
 Heading Pointed |
P/S (T1) | P/S (T2) | ∆P/∆S | |
| Tissue Thickness | 0.79 | 0.79 | 0.74 | |
| Single Cell Area | 1.03 | 1.13 | 1.41 | |
| Cell Density | 1.06 | 1.10 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





