Submitted:
21 February 2024
Posted:
21 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
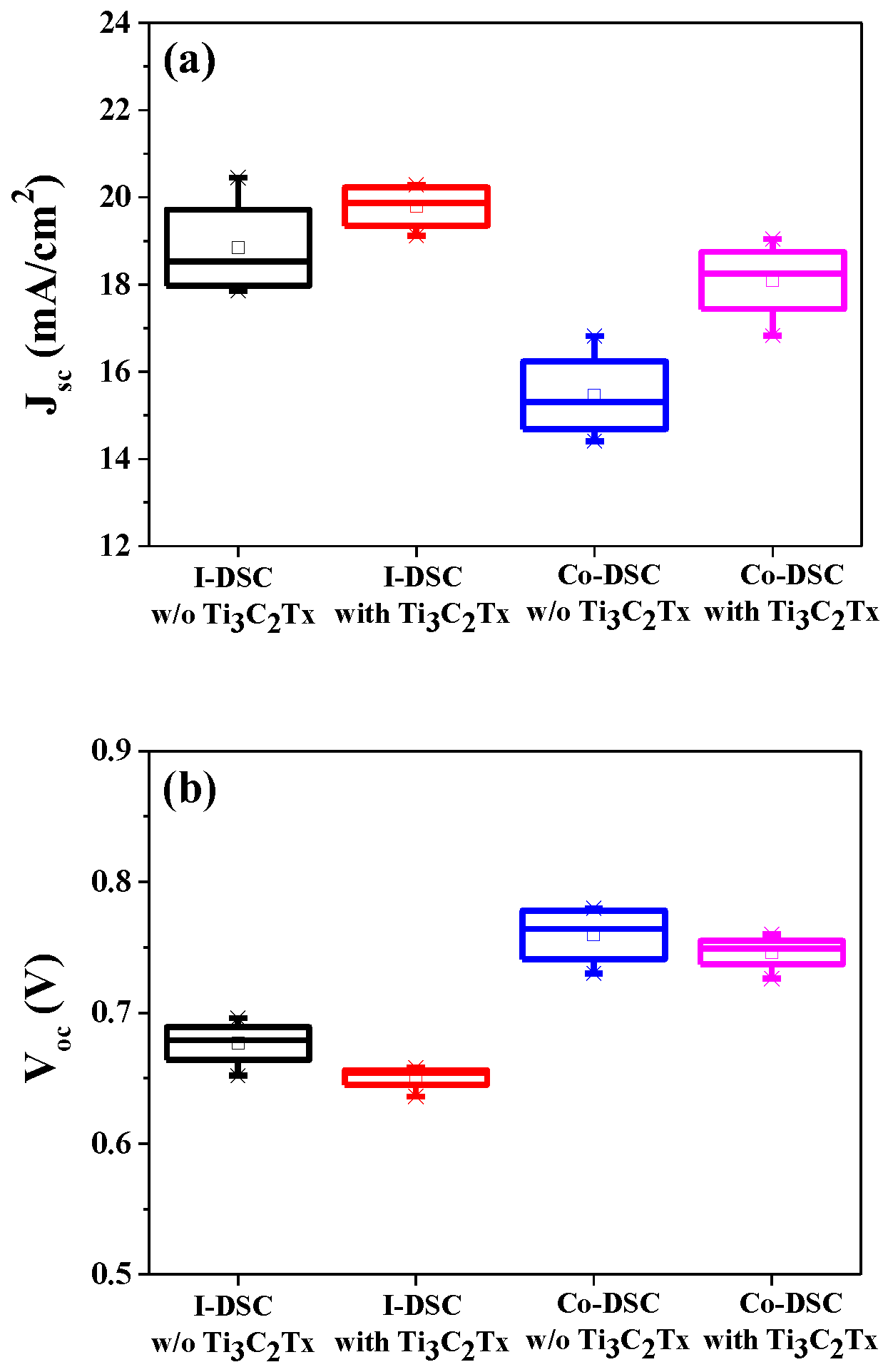
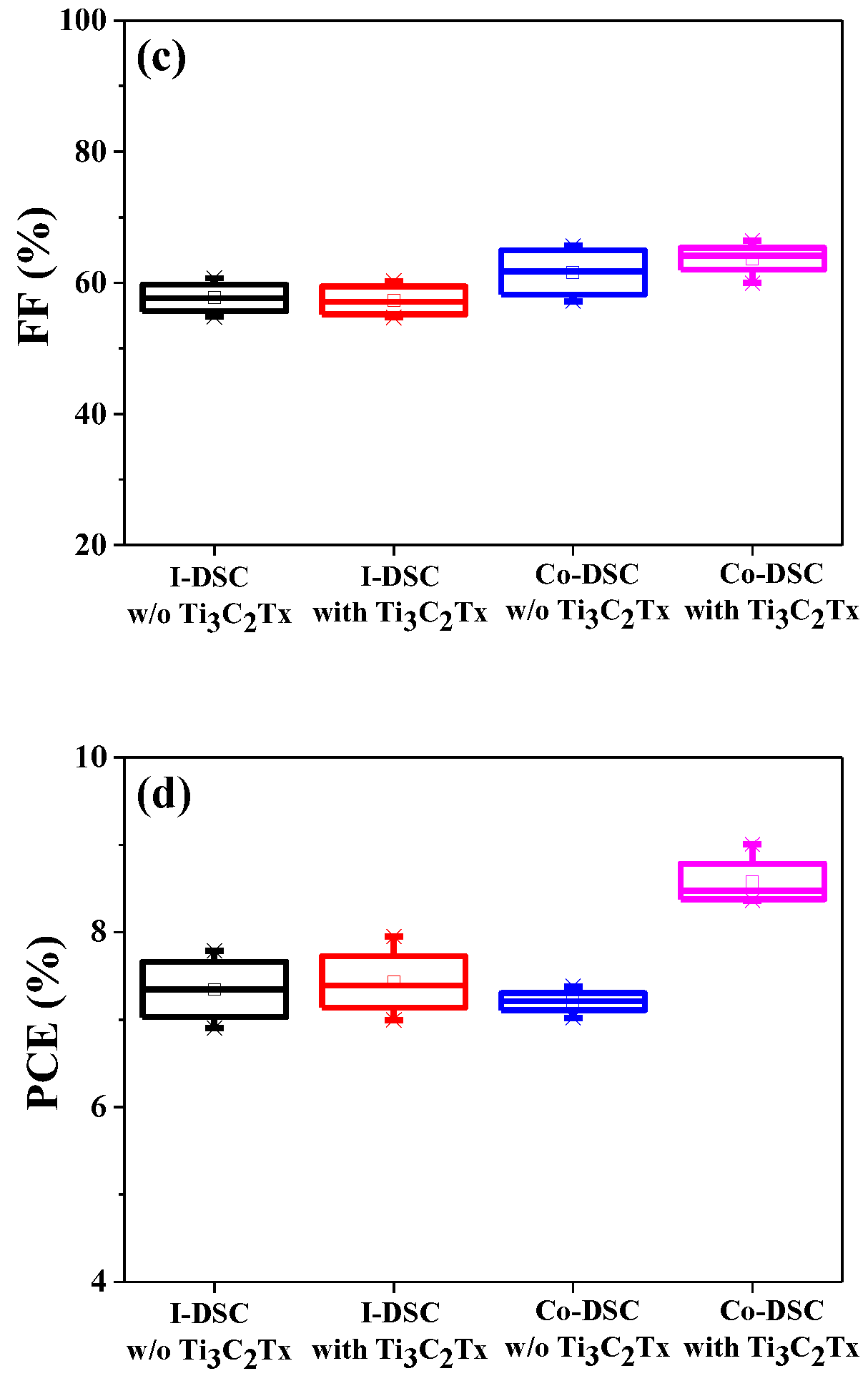
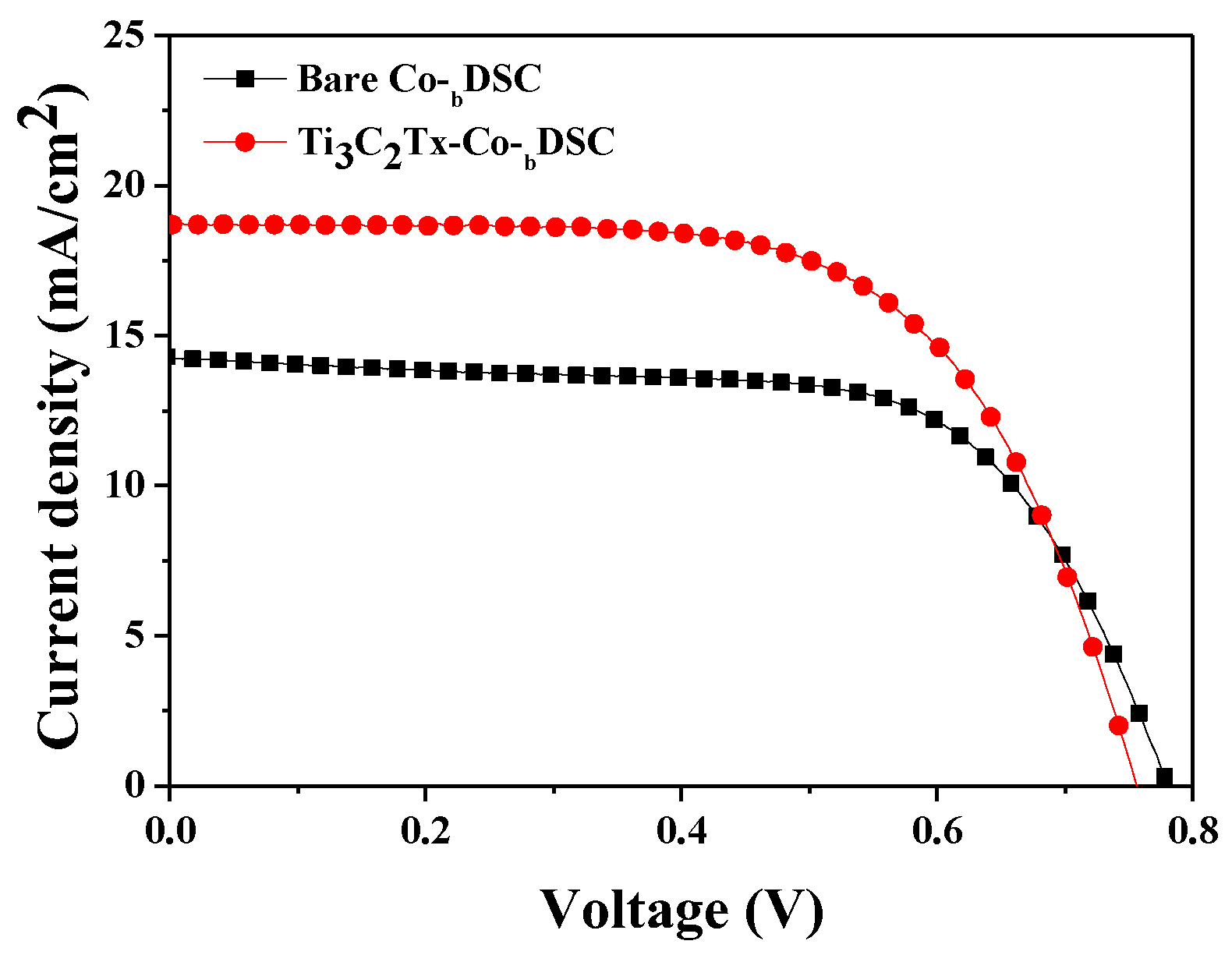
2.1. Photovoltaic Performance of DSCs Based on Ti3C2Tx-Incorporated Co3+/I− Redox Mediators
2.2. Effects of Ti3C2Tx Incorporation on the Jsc of Co-DSCs
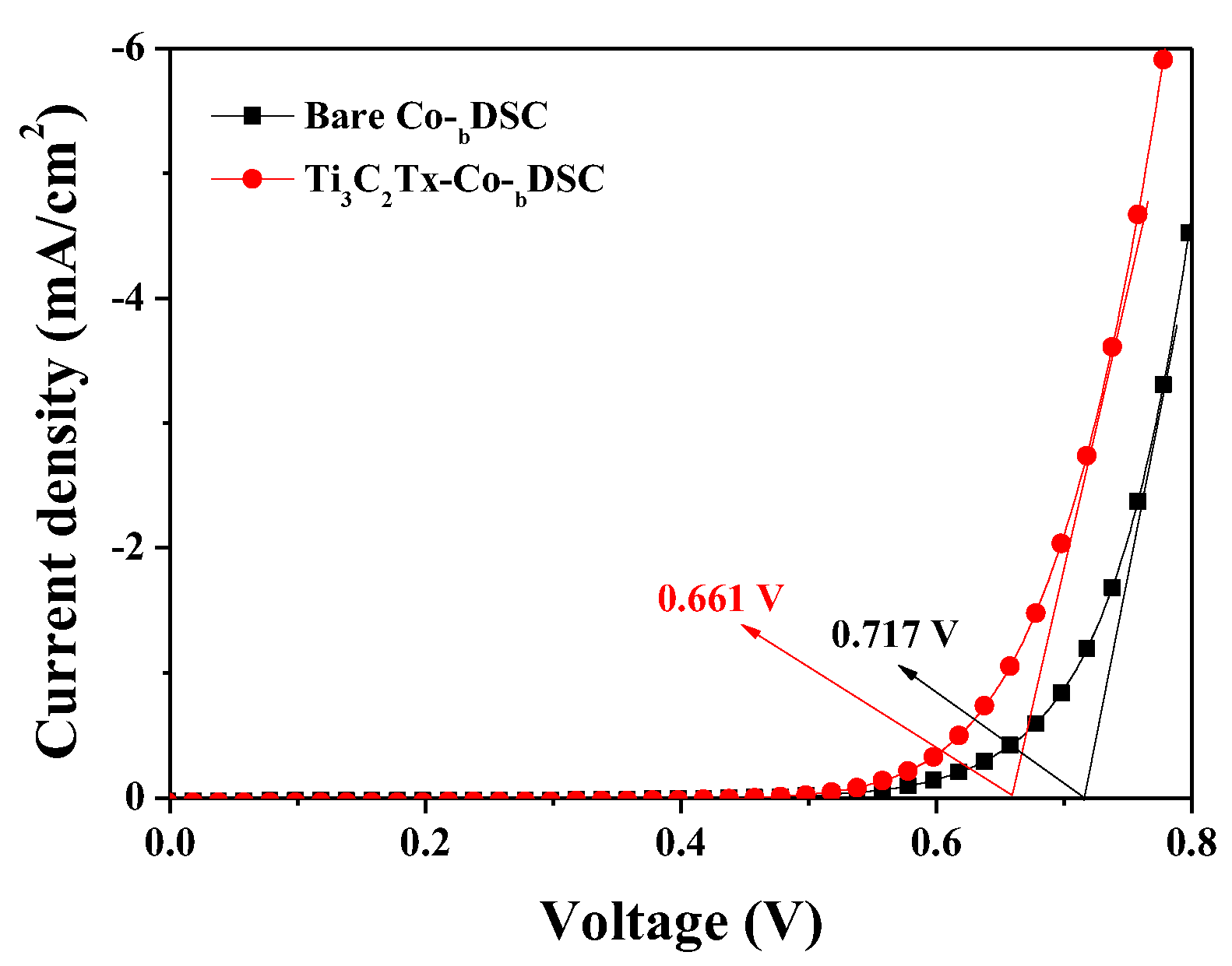
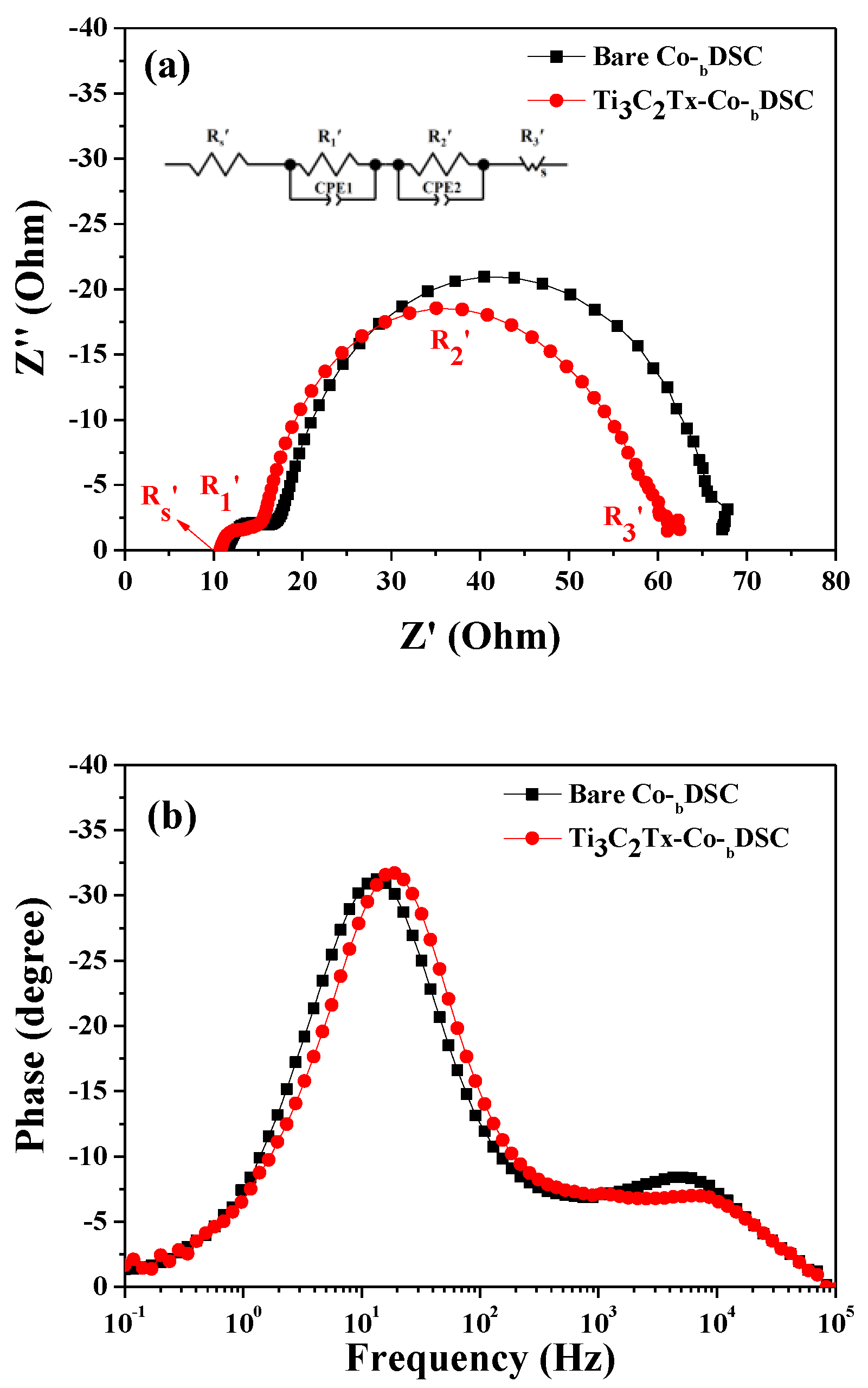
2.3. Effects of Ti3C2Tx Incorporation on the Voc of Co-DSCs
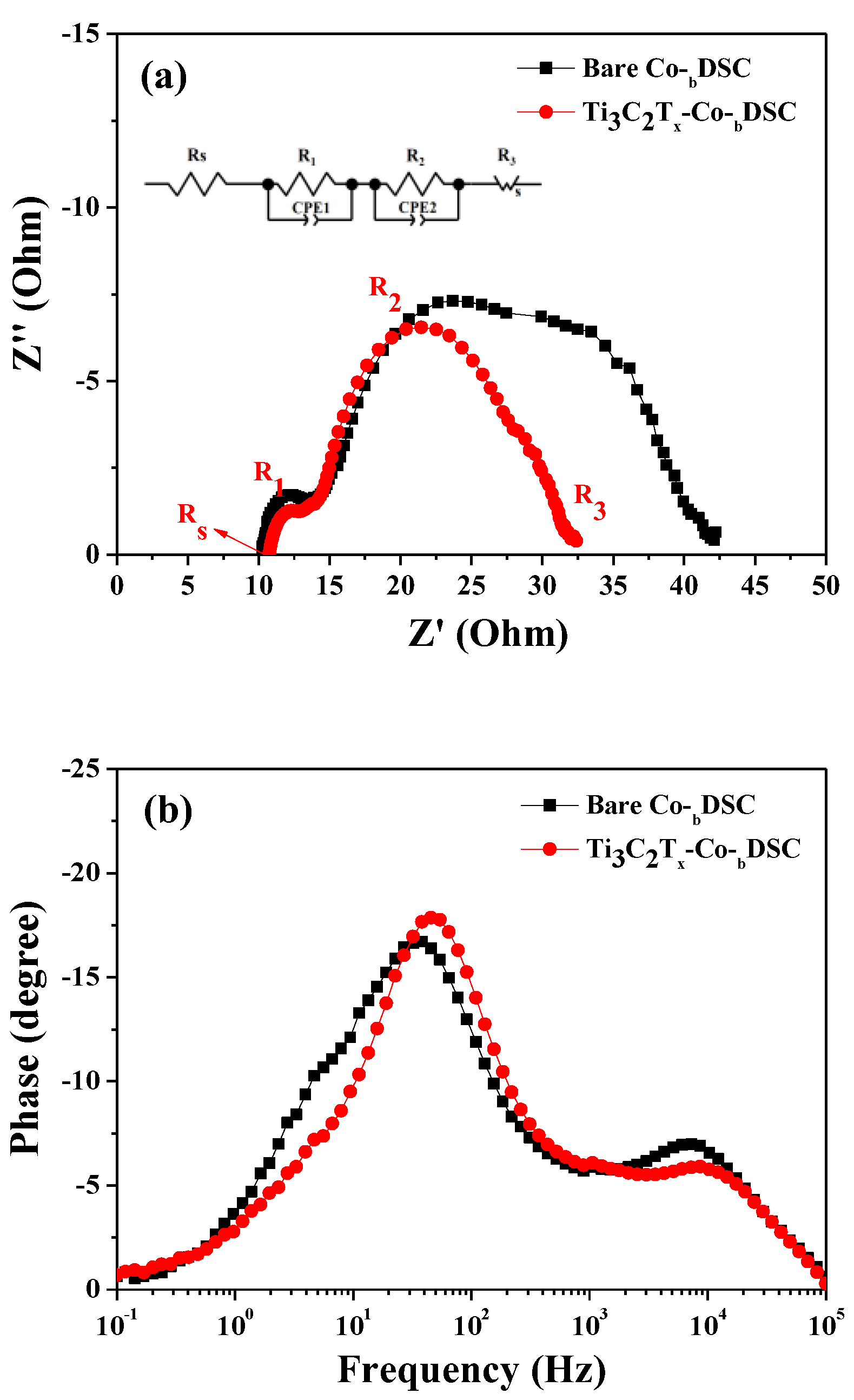
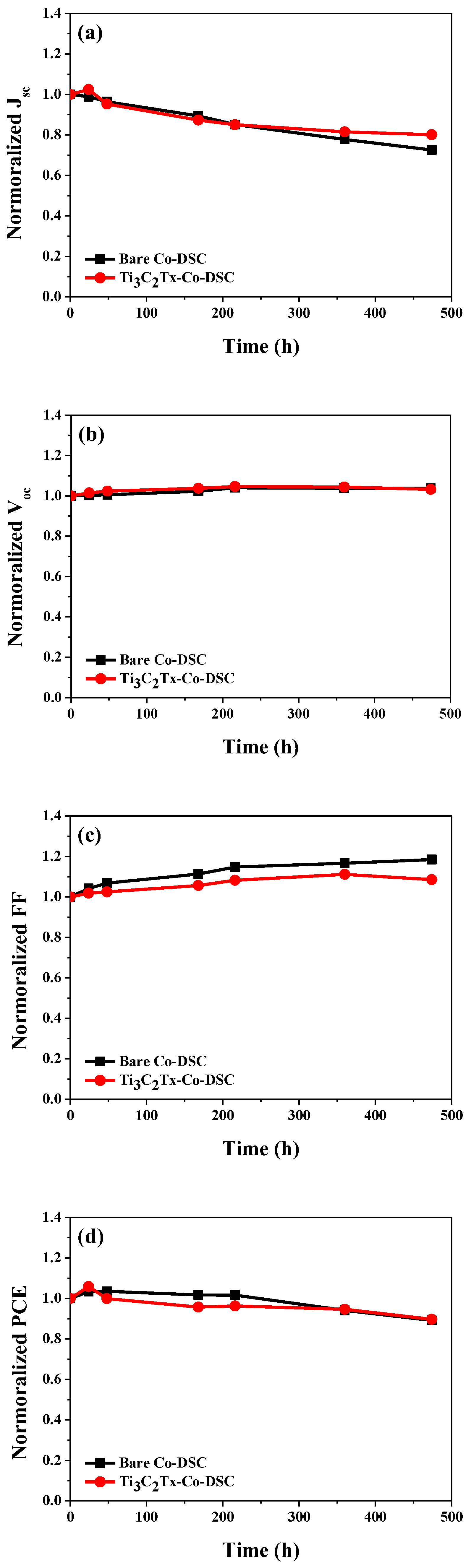
2.4. Long Long-Term Stability of the Bare- and Ti3C2Tx-Co-bDSCs
3. Materials and Methods
3.1. Materials
3.2. Preparation of Co3+/I−-Based Liquid Electrolytes with or without Ti3C2Tx
3.3. Fabrication of DSCs
3.4. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Papadopoulou, K.A.; Chroneos, A.; Parfitt, D.; and Christopoulos, S.-R.G. A perspective on MXenes: their synthesis, properties, and recent applications. J. Appl. Phys. 2020, 128, 170902. [Google Scholar] [CrossRef]
- Jun, B.-M.; Kim, S.; Heo, J.; Park, C.M.; Her, N.; Jang, M.; Huang, Y.; Han, J.; Yoon, Y. Review of MXenes as new nanomaterials for energy storage/delivery and selected environmental applications. Nano Res. 2019, 12, 471–487. [Google Scholar] [CrossRef]
- Jimmy, J.; Kandasubramanian, B. MXene functionalized polymer composites: synthesis and applications. Eur. Polym. J. 2020, 122, 109367. [Google Scholar] [CrossRef]
- Saeed, M.A.; Shahzad, A.; Rasool, K.; Mateen, F.; Oh, J.-M.; Shim, J.W. 2D MXene: a potential candidate for photovoltaic cells? a critical review. Adv. Sci. 2022, 9, 2104743. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, Y.; Yao, X.; Wang, Y.; Jia, L.; Liu, Q.; Li, J.; Li, Y.; He, D. MXenes for solar cells. Nano-Micro Lett. 2021, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Khaledialidusti, R.; Malaki, M.; Zhang, H. MXene-based materials for solar cell applications. Nanomaterials 2021, 11, 3170. [Google Scholar] [CrossRef] [PubMed]
- Qamar, S.; Fatima, K.; Ullah, N.; Akhter, Z.; Waseem, A.; Sultan, M. Recent progress in use of MXene in perovskite solar cells: for interfacial modification, work-function tuning, and additive engineering. Nanoscale 2022, 14, 13018–13039. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.; Qin, T. Efficient wide-spectrum dye-sensitized solar cell by plasmonic TiN@Ni-MXene as electrocatalyst. Ceram. Int. 2022, 48, 12635–12640. [Google Scholar] [CrossRef]
- Ma, J.-Y.; Sun, M.; Zhu, Y.-A.; Zhou, H.; Wu, K.; Xiao, J.; Wu, M. Highly effective 2D layer structured titanium carbide electrode for dye-sensitized and perovskite solar cells. ChemElectroChem 2020, 7, 1149–1154. [Google Scholar] [CrossRef]
- Nagalingam, S.P.; Grace, A.N. Poly(3,4-ethylenedioxythiophene) decorated MXene as an alternative counter electrode for dye-sensitized solar cells. Mater. Today Chem. 2022, 26, 101113. [Google Scholar] [CrossRef]
- Chen, T.; Tong, G.; Xu, E.; Li, H.; Li, P.; Zhu, Z.; Tang, J.; Qi, Y.; Jiang, Y. Accelerating hole extraction by inserting 2D Ti3C2-MXene interlayer to all inorganic perovskite solar cells with long-term stability. J. Mater. Chem. A 2019, 7, 20597–20603. [Google Scholar] [CrossRef]
- Jin, X.; Yang, L.; Wang, X.-F. Efficient two-dimensional perovskite solar cells realized by incorporation of Ti3C2Tx MXene as nano-dopants. Nano-Micro Lett. 2021, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Agresti, A.; Pazniak, A.; Pescetelli, S.; Di Vito, A.; Rossi, D.; Pecchia, A.; Auf der Maur, M.; Liedl, A.; Larciprete, R.; Kuznetsov, D.V.; Saranin, D.; Di Carlo, A. Titanium-carbide MXenes for work function and interface engineering in perovskite solar cells. Nat. Mater. 2019, 18, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Yu, H. Modifying the nanostructures of PEDOT:PSS/Ti3C2TX composite hole transport layers for highly efficient polymer solar cells. J. Mater. Chem. C 2020, 8, 4169–4180. [Google Scholar] [CrossRef]
- Hou, C.; Yu, H.; Huang, C. Solution-processable Ti3C2Tx nanosheets as an efficient hole transport layer for high-performance and stable polymer solar cells. J. Mater. Chem. C 2019, 7, 11549–11558. [Google Scholar] [CrossRef]
- Gong, J.; Sumathya, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-sensitized solar cells: fundamentals and current status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G. Electrolytes in dye-sensitized solar cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef]
- Iftikhar, H.; Sonai, G.G.; Hashmi, S.G.; Nogueira, A.F.; Lund, P.D. Progress on electrolytes development in dye-sensitized solar cells. Materials 2019, 12, 1998. [Google Scholar] [CrossRef]
- Wen, J.; Liu, Y.; Li, T.; Liu, C.; Wang, T.; Liu, Y.; Zhou, Y.; Li, G.; Sun, Z. Low cost and strongly adsorbed melamine formaldehyde sponge electrolyte for nontraditional quasi-solid dye-sensitized solar cells. ACS Appl. Energy Mater. 2023, 6, 4952–4960. [Google Scholar] [CrossRef]
- Wen, J.; Sun, Z.; Qiao, Y.; Zhou, Y.; Liu, Y.; Zhang, Q.; Liu, Y.; Jiao, S. Ti3C2 MXene-reduced graphene oxide composite polymer-based printable electrolyte for quasi-solid-State dye-sensitized solar cells. ACS Appl. Energy Mater. 2022, 5, 3329–3338. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, Y.; Cho, Y.; Ahn, K.-S.; Han, Y.S. Novel heterologous binary redox mediator based on an ionic liquid and cobalt complex for efficient organic-solvent-free dye-sensitized solar cells. J. Ind. Eng. Chem. 2022, 115, 263–271. [Google Scholar] [CrossRef]
- Noh, J.H.; Jeon, N.J.; Choi, Y.C.; Nazeeruddin, M.K.; Grätzel, M.; Seok, S.I. Nanostructured TiO2/CH3NH3PbI3 heterojunction solar cells employing spiro-OMeTAD/Co-complex as hole-transporting material. J. Mater. Chem. A 2013, 1, 11842–11847. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Shi, C.; Yan, F. Highly efficient dye-sensitized solar cells based on low concentration organic thiolate/disulfide redox couples. RSC Adv. 2016, 6, 70460–70467. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, H.; Chen, X.; Yan, F. Imidazolium functionalized cobalt tris(bipyridyl) complex redox shuttles for high efficiency ionic liquid electrolyte dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 11933–11941. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Q.; He, B.; Lin, L.; Yu, L. Platinum-free binary Co-Ni alloy counter electrodes for efficient dye-sensitized solar cells. Angew. Chem. 2014, 126, 10975–10979. [Google Scholar] [CrossRef]
- Diamant, Y.; Chen, S.G.; Melamed, O.; Zaban, A. Core-shell nanoporous electrode for dye sensitized solar cells: the effect of the SrTiO3 shell on the electronic properties of the TiO2 core. J. Phys. Chem. B 2003, 107, 1977–1981. [Google Scholar] [CrossRef]
- Baek, G.W.; Kim, Y.-J.; Jung, K.-H.; Han, Y.S. Enhancement of solar cell performance through the formation of a surface dipole on polyacrylonitrile-treated TiO2 photoelectrodes. J. Ind. Eng. Chem. 2019, 73, 260–267. [Google Scholar] [CrossRef]
- Shin, S.; Kim, J.; Kwon, S.-J.; Ryu, K.H.; Choi, B.; Han, Y.S. Enhancement of photovoltaic performance of solvent-free dye-sensitized solar cells with doped poly(3-hexylthiophene). J. Ind. Eng. Chem. 2023, 123, 428–435. [Google Scholar] [CrossRef]
- Watson, D.F.; Meyer, G.J. Cation effects in nanocrystalline solar cells. Coord. Chem. Rev. 2004, 248, 1391–1406. [Google Scholar] [CrossRef]
- Lemos, H.G.; Ronchi, R.M.; Portugal, G.R.; Rossato, J.H.H.; Selopal, G.S.; Barba, D.; Venancio, E.C.; Rosei, F.; Arantes, J.T.; Santos, S.F. Efficient Ti3C2Tx MXene/TiO2 hybrid photoanodes for dye-sensitized solar cells. ACS Appl. Energy Mater. 2022, 5, 15928–15938. [Google Scholar] [CrossRef]
- Peng, T.; Shi, W.; Wu, S.; Ying, Z.; Ri, J.H. Sea urchin-like TiO2 microspheres as scattering layer of nanosized TiO2 film-based dye-sensitized solar cell with enhanced conversion efficiency. Mater. Chem. Phys. 2015, 164, 238–245. [Google Scholar] [CrossRef]
- Zaban, A.; Greenshtein, M.; Bisquert, J. Determination of the Electron lifetime in nanocrystalline dye solar cells by open-circuit voltage decay measurements. ChemPhysChem 2003, 4, 859–864. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.H.; Kim, D.-H.; Han, Y.S. Effects of a dianion compound as a surface modifier on the back reaction of photogenerated electrons in TiO2-based solar cells. Arabian J. Chem. 2020, 13, 2340–2348. [Google Scholar] [CrossRef]
- Kim, K.S.; Song, H.; Nam, S.H.; Kim, S.-M.; Jeong, H.; Kim, W.B.; Jung, G.Y. Fabrication of an efficient light-scattering functionalized photoanode using periodically aligned ZnO hemisphere crystals for dye-sensitized solar cells. Adv. Mater. 2012, 24, 792–798. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, B.; Qiu, L.; Cao, H.; Li, Q.; Chen, X.; Yan, F. Efficient light-scattering functionalized TiO2 photoanodes modified with cyanobiphenyl-based benzimidazole for dye-sensitized solar cells with additive-free electrolytes. J. Mater. Chem. 2012, 22, 18380–18386. [Google Scholar] [CrossRef]
- Tian, H.; Hu, L.; Zhang, C.; Liu, W.; Huang, Y.; Mo, L.; Guo, L.; Sheng, J.; Dai, S. Retarded charge recombination in dye-sensitized nitrogen-doped TiO2 solar cells. J. Phys. Chem. C 2010, 114, 1627–1632. [Google Scholar] [CrossRef]
- Park, K.-H.; Dhayal, M. High efficiency solar cell based on dye sensitized plasma treated nano-structured TiO2 films. Electrochem. Commun. 2009, 11, 75–79. [Google Scholar] [CrossRef]
- Mandal, D.; Hamann, T.W. Charge distribution in nanostructured TiO2 photoanode determined by quantitative analysis of the band edge unpinning. ACS Appl. Mater. Interfaces 2016, 8, 419–424. [Google Scholar] [CrossRef]
| Device | Measurement condition | Redox mediator |
Jsc (mA/cm2) |
Voc (V) |
FF (%) |
PCE (%) |
Ref. | |
| Quasi-solid-state DSC |
AM 1.5 | MF-sponge-based I−/I3− | Without Ti3C2Tx | 14.979 ± 0.175 |
0.778 ± 0.004 |
65.3 ± 0.3 |
7.610 ± 0.106 |
[21] |
| With Ti3C2Tx | 15.085 ± 0.188 |
0.781 ± 0.003 |
66.4 ± 0.6 |
7.822 ± 0.092 |
[21] | |||
| 1000 lux | MF-sponge-based I−/I3− | Without Ti3C2Tx | 0.177 ± 0.001 |
0.569 ± 0.007 |
70.3 ± 0.5 |
23.35 ± 0.43 |
[21] | |
| With Ti3C2Tx | 0.196 ± 0.003 |
0.579 ± 0.004 |
71.9 ± 0.4 |
26.92 ± 0.43 |
[21] | |||
| AM 1.5 | PEO/PVDH-HFP-based I−/I3− | Without rGO/Ti3C2Tx | - | - | - | - | - | |
| With rGO/Ti3C2Tx | 15.170 ± 0.203 |
0.783 ± 0.002 |
69.5 ± 0.5 |
8.255 ± 0.109 |
[22] | |||
| 1000 lux | PEO/PVDH-HFP-based I−/I3− | Without Ti3C2Tx | - | - | - | - | - | |
| With rGO | 0.189 ± 0.002 |
0.544 ± 0.002 |
76.1 ± 0.4 |
23.22 ± 0.43 |
[22] | |||
| With rGO/Ti3C2Tx | 0.223 ± 0.001 |
0.561 ± 0.004 |
75.7 ± 0.1 |
29.94 ± 0.49 |
[22] | |||
| Liquid-electrolyte DSC | AM 1.5 | Co3+/ I− (FK209/MPII) | Without Ti3C2Tx | 15.46 ± 1.04 |
0.760 ± 0.023 |
61.33 ± 3.70 |
7.18 ± 0.11 |
This study |
| With Ti3C2Tx | 18.09 ± 0.94 |
0.746 ± 0.014 |
63.66 ± 2.69 |
8.58 ± 0.30 |
This study | |||
| Devices |
Jsc (mA/cm2) |
Voc (V) |
FF (%) |
PCE (%) |
|
| I-DSC | Without Ti3C2Tx | 18.84 ± 1.18 | 0.677 ± 0.018 | 57.70 ± 2.55 | 7.35 ± 0.39 |
| With Ti3C2Tx | 19.95 ± 0.78 | 0.651 ± 0.010 | 57.29 ± 2.59 | 7.43 ± 0.40 | |
| Co-DSC | Without Ti3C2Tx | 15.46 ± 1.04 | 0.760 ± 0.023 | 61.33 ± 3.70 | 7.18 ± 0.11 |
| With Ti3C2Tx | 18.09 ± 0.94 | 0.746 ± 0.014 | 63.66 ± 2.69 | 8.58 ± 0.30 | |
| Best-performing cells |
Jsc (mA/cm2) |
Voc (V) |
FF (%) |
PCE (%) |
| Bare Co-bDSC | 14.41 | 0.780 | 64.66 | 7.27 |
| Ti3C2Tx-Co-bDSC | 18.45 | 0.760 | 64.23 | 9.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





