1. Introduction
In order to guarantee food safety, it is important to monitor the presence of harmful substances throughout the food production chain. With this strategy various compounds have been identified in food and feed in the past years that could represent a potential threat to human health [1-4]. One group of compounds that represents continues health concern are the chlorinated dioxins and dioxin-like polychlorinated biphenyls (dl-PCBs). For this reason these compounds are regulated in feed and food, in Europe since 2011 (EC/277/2012 and EU/2023/915). Dioxins is a collective name for polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs). Dioxins can be formed as by-products from natural and industrial combustion processes and do not have an industrial application [5, 6]. They are very persistent and lipophilic and as a result are present in the environment worldwide, i.e. from fish and animals to soil and ball clay.
While dioxins and dioxin-like PCBs consist of many congeners, i.e. 75 PCDDs, 135 PCDFs and 209 PCBs, only those that have chlorines in the lateral positions (2,3,7,8 for PCDDs/Fs and 3,4 and/or 5 for PCBs) are considered toxic. For these toxic congeners the World Health Organization (WHO) provided toxic equivalency factors (TEF) [
7]. TEF values were established for 7 PCDDs, 10 PCDFs and 12 dioxin-like PCBs (dl-PCBs), relative to the most potent dioxin, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). TCDD is assigned with a TEF value of 1. The toxicity-weighted concentration of mixtures of PCDDs, PCDFs, and PCBs is expressed as TEQ (toxic equivalents). In order to calculate the TEQ value, the concentration of each congener should be multiplied by their TEF values and summed. The equivalent of the TEQ value in Gas Chromatography coupled to high resolution mass spectrometry (GC-HRMS) data is the BEQ (bioanalytical equivalents) value in DR CALUX (Dioxin Responsive Chemical Activated Luciferase gene Expression). Usually the TEQ and BEQ values of a sample lie close together because the TEF values, established by the WHO, are similar to the responses in the DR CALUX and result in an added response in the DR CALUX.
The toxicity-weighted concentration of mixtures of PCDDs, PCDFs, and PCBs is expressed as TEQ (toxic equivalents). In order to calculate the TEQ value, the concentration of each congener should be multiplied by their TEF values and summed. The equivalent of the TEQ value in GC-HRMS data is the BEQ value in DR-CALUX. Usually the TEQ and BEQ values of a sample lie close together because the TEF values, established by the WHO, are similar to the responses in the DR-CALUX and result in an added response in the DR-CALUX [
8]. In case a sample has a BEQ value in the DR-CALUX that substantially higher than the measured TEQ value from the GC-HRMS data, this indicates that another persistent organic pollutant is present in the sample, with the same toxic mode of action as the PCDD/Fs and dl-PCBs.
In European legislation both action levels as well as maximum levels were set for the total sum (ng TEQ/g) of dioxins and dl-PCBs for food and feed items. The purpose of action levels, which are lower than the maximum levels, is to possibly identify the source of contamination in order to extract this source from the food chain to actively direct towards lower levels of dioxins and dl-PCBs in primary food and feed products and thereby lowering human exposure (EU/2023/915). In order to fulfil the legislation, i.e. guarantee products with safe levels of regulated harmful compounds and to check on the possible presence of new emerging harmful compounds (potential risks), a samples are firstly screened with a cell based bioassay. Toxicity from dioxins and dioxin-like compounds are mainly initiated via the interaction with the Aryl hydrocarbon Receptor (AhR) in cells [
9]. The DR CALUX bioassay used as initial screening in our laboratory is such an AhR bioassay. The outcomes of this screening assay are relevant and worldwide fully accepted by regulatory institutions. Samples eliciting a bioassay response indicating levels above the action limit (suspect) for dioxins and PCBs, are confirmed by GC-HRMS analysis. The confirmation analyses by GC-HRMS has a targeted scope focused on the congeners with established TEF values. Hence the GC-HRMS targets 17 PCDDs/Fs and 12 dioxin-like PCBs, in accordance to Regulation (EU) 2017/644 for food and Regulation (EC) No 152/2009 for feed. Other chlorinated congeners are routinely not taken into account due to low or absence of TEFs. Also, other halogenated congeners, e.g. brominated, mixed chlorinated/brominated or iodized, are routinely also not taken into account when performing GC-HRMS confirmation analysis. Thus the most potent 17 PCDDs/Fs and 12 dioxin-like PCBs have been highly regulated and monitored over the years [10-12], while their analogue compounds, got far less attention and are overseen. These compounds are consequently neither regulated nor monitored [
13]. Although an attempt has been made to establish TEF values for the brominated dioxins and dl-PBBs, an expert panel from the World Health Organization (WHO) and United Nations Environment Programme (UNEP), concluded that due to the lack of mammalian
in vitro and
in vivo data, the TEFs of the chlorinated analogues should be used for human risk assessment for these brominated compounds [
14]. Relative potency (REP) values have been determined regarding the AhR activation by experiments with both the rat cell based (DR CALUX) and the human cell based (DRhuman CALUX) bioassay [
15], and indicated similar TEF values for these brominated compounds as their chlorinated counterparts which is in line with the expert opinion. Although not regulated, the brominated dioxins have similar toxicological effects as their chlorinated analogues [
16] [
17].
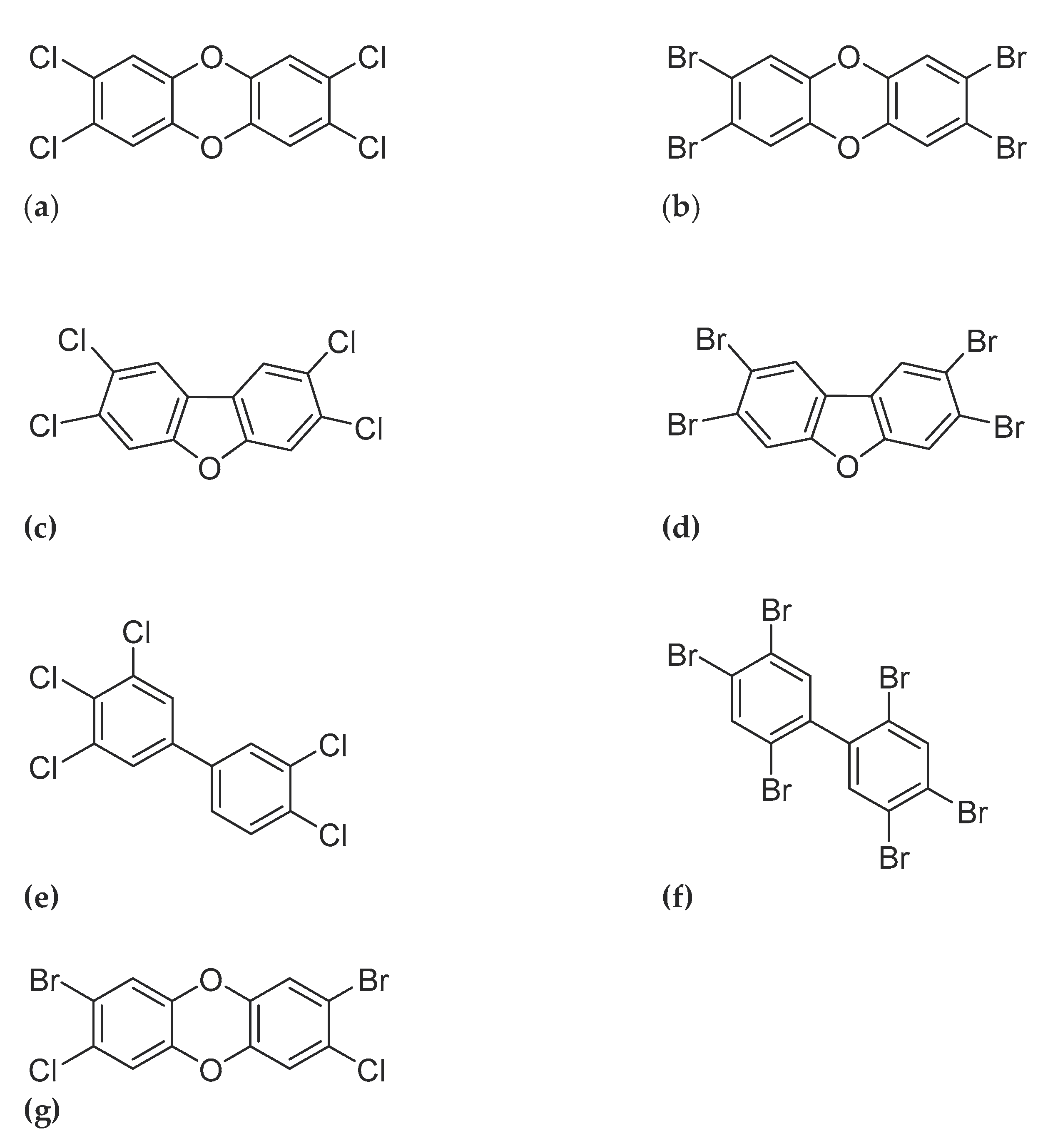
Similar to the chlorinated dioxins, brominated dioxins can be distinguished into two groups: the polybrominated dibenzo-p-dioxins (PBDDs) and polybrominated dibenzofurans (PBDFs) (
Figure 1). Brominated dioxins originate, as by-products from the brominated flame retardant industry for instance from the production of polybrominated diphenyl ether (PBDEs). Mixtures of PBDEs contain significant amounts of PBDD/Fs, especially PBDFs [
18]. Moreover, during the recycling processes new PBDD/Fs can be formed [
19]. There is only a limited amount of studies describing the occurrence of brominated dioxins in food and feed [20-24]. Even though, brominated dioxins are found in food commodities such as fish, shellfish, river fish, marine deep sea, salmon, cod liver, meat, animal fat (bovine, ovine, porcine), eggs and poultry, milk and dairy products, fresh vegetables, fruits and nuts, cooking oils, and even bread [
25]. The WHO estimated that the dietary intake of PCDD/Fs accounts for approximately 90% of the total human exposure to PCDD/Fs and PBDD/Fs [
26].
In poultry egg, the main PBDD/Fs found were 2,3,7,8-TBDF, 1,2,3/4,7,8-PeBDF, 1,2,3,4,7,8-HxBDF and 1,2,3,4,6,7,8-HpBDF and to a lesser extent 1,2,3,(4/6),7,8-HxBDD and 1,2,3,7,8,9-HxBDD. [
27] [
28]. Interestingly, in the eggs of the cormorant only non-2,3,7,8- substituted PBDD/Fs congeners were detected [
29]. In food PBDFs occur more often than PBDDs contrarily to the congener pattern of PCDD/Fs, where dioxins occur more often than furans [
25]. Brominated dioxins have also been found in the feed additive choline chloride [
30]. In this case the feed additive was screened with the DR CALUX and resulted in a suspect result without the presence of chlorinated dioxins. Investigation at that time resulted in the discovery of tetra and penta brominated dioxins and furans with 2,3,7,8-TBDF being the most prominent congener present. The levels of 2,3,7,8-TBDF (up to 2.3 pg/g) in choline chloride were so low and since less than 1% choline chloride is added to an animal feed it was concluded that this low concentration would not result in detectable levels in poultry eggs or meat.
Overall, PBDD/Fs are sporadically monitored compared to PCDD/Fs. Hence a limited amount of data is available for this group of compounds, and therefore human exposure is underestimated [
25].
In the present study, egg and poultry meat were investigated in more detail as suspect DR CALUX outcomes were obtained. Additionally, broiler feed, feed additives, bedding material and seaweed were investigated as possible sources of the bioactivity. The feed additives choline chloride and l-lysine are known to be used extensively in poultry feed [31, 32]. Lysine is a limiting amino acid for poultry [
33]. It is naturally present in soybean, but is added as feed additive for economic reasons. The feed additive form mostly used is l-lysine HCl produced by fermentation by
Corynebacterium glutamicum. Choline chloride is also being added to poultry feed as a supplement in order to increase egg production and broiler fattening [
34]. In addition, seaweed was investigated as seaweed is another protein source in animal feed. Finally, the bedding material was investigated. This material is a specific product used for poultry housing in order to circumvent growth of
Salmonella and demonstrated before to be contaminated with all kinds of dioxin like AhR-agonists (results not shown). Overall, it is of utmost importance to survey and monitor all dioxin-like compounds more intensively in food and feed and additional screening with an AhR bioassay besides targeted analyses showed its importance to get a view on the actual exposure to dioxin-like compounds.
2. Materials and Methods
2.1. Samples
The poultry eggs, broiler meat, l-lysine (7 samples), choline chloride (2 samples), broiler feed (1 sample), bedding material (1 sample) and seaweed (1 sample) were sampled by the Netherlands Food and Consumer Product Safety Authority between 2019 and 2021 within national monitoring plans. Eggs were collected from free-range and deep litter farming.
2.2. Chemicals
Analytical standards and other chemicals used for the chlorinated dioxin analysis by GC-HRMS were as described previously by Ten Dam et al. [
35]. For the brominated dioxins, the analytical standards as well as the
13C-labeled PBDD/Fs and PCDD/Fs were obtained from Cerilliant (Round Rock, TX, USA) via LGC standards. These were: 1,3,7-TriBDF - 2,3,7,8-TBDF - 1,2,3,7,8-PeBDF - 2,3,4,7,8-PeBDF - 1,2,3,4,7,8-HxBDF - 1,2,3,4,6,7,8-HpBDF – OBDF - 1,3,7-TriBDD - 2,3,7,8-TBDD - 1,2,3,7,8-PeBDD -1,2,3,4,7,8HxBDD - 1,2,3,6,7,8-HxBDD - 1,2,3,7,8,9-HxBDD - 1,2,3,4,6,7,8-HpBDD and OBDD.
2.3. Sample preparation
As dioxins are lipophilic they accumulate in fat. Therefore fat was extracted from egg yolk and broiler meat according to Smedes [
36]. In short, sodium sulfate was added to the homogenate of egg yolk, pentane was added and finally this mixture was filtered over sodiumsulfate and the filtrate was evaporated under vacuum. To extract fat from broiler fat, sodiumchloride, iso-propanol and cyclohexane were added. This mixture was first ground using an ultra-turrax, and subsequently centrifuged and filtered over a sodiumsulfate filter and finally the cyclohexane was evaporated. Regarding the broiler feed and seaweed, samples were first ground using an ultra turrax and then extracted using an accelerated solvent extractor (ASE) (Dionex™ ASE™ 350) with hexane/acetone (1:1).
2.4. Sample clean-up and routine DR CALUX screening
To fat aliquots of 2 g, 5 mL sulfuric acid was added, carefully mixed by rolling and left overnight in the fume hood. Next, samples were extracted twice with 10 mL hexane/diethyl ether (97:3 v/v %). The collected fraction (20 mL) was evaporated to about 2 mL using a vacuum evaporation system (Savant SPD 2010, Speed Vac Concentrator) with temperature set at 45ºC, ramp at 3, vacuum pressure at level 30 and a runtime of 30 minutes. Further extraction and clean-up of this 2 mL fraction on an acid-silica column was performed as described previously [
37]. In short, moulds of cotton (treated to complete dryness at 160º C) were pushed down to the tips of glass columns held in place with a clamp on a ring stand. Ten grams of acid silica was weighed into each glass column followed by 2 grams of Na2SO4 (treated at 125ºC). Each column was conditioned with 20 mL hexane/diethyl ether (97:3 v/v %) and 50 mL vials were placed beneath the columns to collect each sample extract, i.e. the 2 mL extract was pipetted on the columns and the 50 mL vial was rinsed twice with 2 mL hexane/diethyl ether (97:3 v/v %), which were also brought on the column. Further extraction was performed by adding 20 mL hexane/diethyl ether (97:3 v/v %) and subsequently an extra 10 mL of the same eluent, making a total volume of about 36 mL. Regarding feed and feed additives, aliquots of 5 g were treated with 20 mL methanol/water (85/15 v/v %). Next, samples were extracted twice with 20 mL hexane/diethyl ether (97:3 v/v %). The collected fraction (40 mL) was evaporated to about 2 mL using the vacuum evaporation system with temperature set at 45ºC, ramp at 3, vacuum pressure at level 30 and a runtime of 45 minutes. Further extraction and clean-up of this 2 mL fraction on an acid-silica column was performed as described above for the fat extracts, resulting in a final total volume of about 36 mL for these samples. . The 36 mL sample extracts coming from the acid-silica columns were evaporated to about 1 mL using the vacuum evaporation system with temperature set at 45ºC, ramp at 3, vacuum pressure at level 30 and a runtime of 45 minutes. Afterwards, the 1 mL was withdrawn from the vial, rinsed twice with 2 mL hexane/diethyl ether (97:3 v/v %) and transferred to a 6 mL borosilicate tube which already contained 20 µL DMSO (as a keeper). For the fat extracts, the solution was mixed before evaporation in the vacuum system with the same program but reduced runtime and 1 mL of culture medium (AMEM supplemented with 10 % FBS and 0.5 % penicillin/streptomycin) was added, resulting in a final DMSO concentration of approximately 2 %. For the extracts prepared from feed and feed additives, the solution was mixed before evaporation in the vacuum system with the same program but reduced runtime and an extra 20 µL of DMSO was added before 2 mL of culture medium was added, resulting in a final DMSO concentration of approximately 2 %.
The DR CALUX bioassay was performed as described previously by Hoogenboom et al.[
8]. In short, recombinant rat hepatoma cells (H4IIE-luc) were grown at 37ºC (5 % CO2) and 100 % relative humidity in culture medium. For analysis of the fat samples, 100 µL portions of a cell suspension (about 40.000 cells/well) were seeded in 96-well plates (Corning) and grown for 24 hrs before adding 100 µL of each extract in culture medium in triplicate (final volume 200 µL, final concentration DMSO approximately 1 %). For analysis of the feed and feed additive samples, 250 µL portions of a cell suspension (about 40.000 cells/well) were seeded in 48-well plates (Corning) and grown for 24 hrs before adding 250 µL of each extract in culture medium in triplicate (final volume 500 µL, final concentration DMSO approximately 1 %). TCDD standards diluted in culture medium (final concentration DMSO 1%) were included as positive controls as well as reference butter fat samples (containing 0.59 – 1.01 – 2.07 – 3.07 – 6.19 – 10.20 – 17.90 and 35.80 pg TEQ/g fat) and reference poultry feed samples (containing 0.02 – 0.29 – 0.48 – 0.70 – 1.57 and 3.35 pg TEQ/g product).
The luciferase concentration was subsequently measured 24 hrs after exposure. For this, the medium was removed, and the cell monolayers were washed with 200 µL phosphate-buffered saline (PBS) (Oxoid). Cells were lysed using 20 µL cell culture lysis reagent (Promega) and incubated for 25 minutes at room temperature. Luciferase activity of the cell lysate was measured with a CLARIOstar microplate reader (BMG Labtech) from Isogen Life Science BV (Utrecht, The Netherlands) which automatically added 100 µL assay mixture (substrate) containing 20 mM tricine, 1.07 mM (MgCO3)4 Mg (OH)2.5H2O, 2.67 mM MgSO4.7H2O, 0.1 mM EDTA, 33.3 mM DTT, 261 µM Coenzyme A, 470 µM luciferin, and 530 µM ATP at a pH of 7.8.
Dose-response curves obtained with TCDD were fitted using a user-defined exponential equation y=a0/(1+(x/a1)^a2) with SlideWrite Plus v.6.1 (Advanced graphics software, USA). Where a0 is the maximum response, a1 is the concentration showing a half-maximal response (EC50) and a2 is the coefficient for the steepness of the curve. Graphs were plotted with GraphPad Prism 5. Dose-response curves obtained with the refence fat samples and the refence feed samples were fitted using y= a0+a1*exp(-x/a2). Using the fitted dose-reponse curves obtained with the refence fat samples and the refence feed samples, the BEQ levels in the samples were calculated.
2.5. Sample clean-up for GC-HRMS and for additional screening with the DR CALUX bioassay
In order to have enough sample material for multiple analyses, two pool samples were prepared for both poultry egg and broiler fat. One pool contained the fat of samples that had given a non-suspicious DR CALUX result (< 1 pg BEQ/g fat) and the other pool contained the fat of samples that had given a high response in the DR CALUX (> 6.2 pg BEQ/g fat) and contained no elevated levels of PCDD/F or dlPCBs according to GC-HRMS analysis. Both “blank” pools and both “contaminated” pools were tested again in the DR CALUX, confirming that the “blank” pools contained no elevated levels of AhR-agonists (< 1 pg BEQ/g fat) and that the “contaminated” pools contained levels of AhR-agonists exceeding 10 pg BEQ/g (close to the predicted BEQ level calculated from the individual samples). For GC-HRMS analysis, bedding material, broiler feed, seaweed, and the feed additives l-lysine and choline chloride were extracted using an accelerated solvent extractor (ASE) (Dionex™ ASE™ 350) with hexane/acetone (1:1).
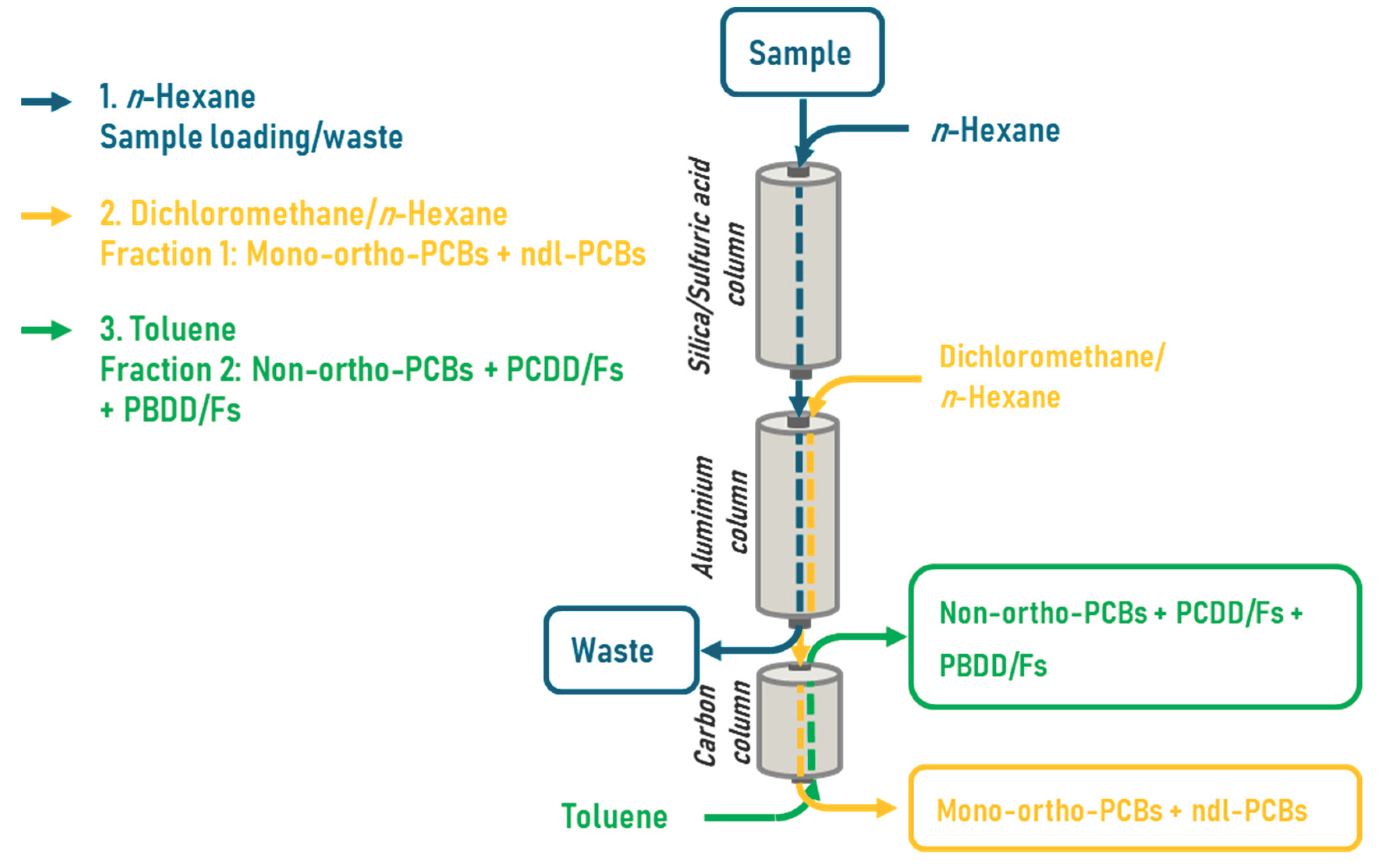
From the 4 pool samples duplicates were prepared, one for the DR CALUX and one for the GC-HRMS analysis. For GC-HRMS analyses, 13C-labeled PBDD/Fs and PCDD/Fs were added to the fat, but not to the ones for DR CALUX analysis. For clean-up of the fat, a DEXTech Plus automated system (LCTech, Obertaufkirchen, Germany) was used (
Figure 2). Therefore, 1 g fat was dissolved in 30 mL hexane and applied onto the DEXTech equipped with 3 columns, i.e. an acidified silica, an alumina, and a carbon column. After loading the sample on the silica column this column and the alumina column are washed with hexane. Next the alumina and carbon columns were eluted with 60 mL hexane/dichloromethane (1:1,v/v) which allows the elution of mono-ortho-PCBs and ndl-PCBs, fraction 1. Then the PCDD/Fs, PBDDs/Fs, PXDD/Fs and dl-PCB’s were eluted via a backflush with 25 mL toluene (1:1,v/v) fraction 2. The volume of the final extract was reduced to 0.5 mL using an automated evaporation system. Fraction 2 was then further evaporated to a volume of 50 μl.
The elution times for each step used was longer than with the conventional clean-up used for PCDD/Fs, respectively 25 min instead of 10 min.
The obtained extract fractions without labelled internal standard were analysed with the DR CALUX bioassay and the fractions with 13C-labelled internal standard with the GC-HRMS.
2.6. GC-HRMS analysis
Analysis of PCDDs/Fs, PBDDs/Fs and dioxin-like PCBs was performed as described previously by Ten Dam et al. [
35]. Although the PBDDs/Fs were analysed in a separate run and analyzed with a different GC-column. In short, extracts including the 13C-labelled compounds were analysed on a Waters autospec high resolution mass spectrometer (Manchester, UK) coupled to an Agilent 6890 gas chromatograph (Santa Clara, SC, USA), a combi PAL autosampler from CTC (Zwingen, Switzerland) and a CIS-4 programmed temperature vaporisation injector (PTV) from Gerstel (Mülheim an der Ruhr, Germany). CO
2 cryogenic cooling was used and the mass spectrometer was operated in electron impact ionization mode, using selected-ion monitoring (SIM) at a resolution of R = 10,000 (at 10% valley). A large volume injector (LVI) was used to inject 100 μL of the extract containing PCDD/Fs and non-ortho-PCBs on the GC while 2 μL of the extract was injected in splitless mode for the mono-ortho-PCBs and ndl-PCBs Measurements for the substance groups were carried out on different GC columns. Dioxin-like PCB’s and PCDD/Fs on a DB5 MS ( 60 m × 0.25 mm × 0.25 μm) and PBDD/Fs on a DB-5 ms (5% phenyl methylpolysiloxane, 15 m × 0.2 mm × 0.1μm). All extracts were measured in SIM mode using quantifier and at least one qualifier ion for the determination of native and labelled congeners.
Table 1 and
Table 2 show qualifiers and quantifiers that are used for the PBDD/Fs and the PXDD/Fs measurements).
The results were corrected for recovery using the 13C-labelled internal standards and the performance was evaluated through two in-house reference samples of butter fat spiked at 0.5 and 1 pg TEQ/g fat PCDD/Fs+PCBs.
2.7. Determination of the relative potency (REP) of 2,3,7,8-TBDF in the DR CALUX bioassay
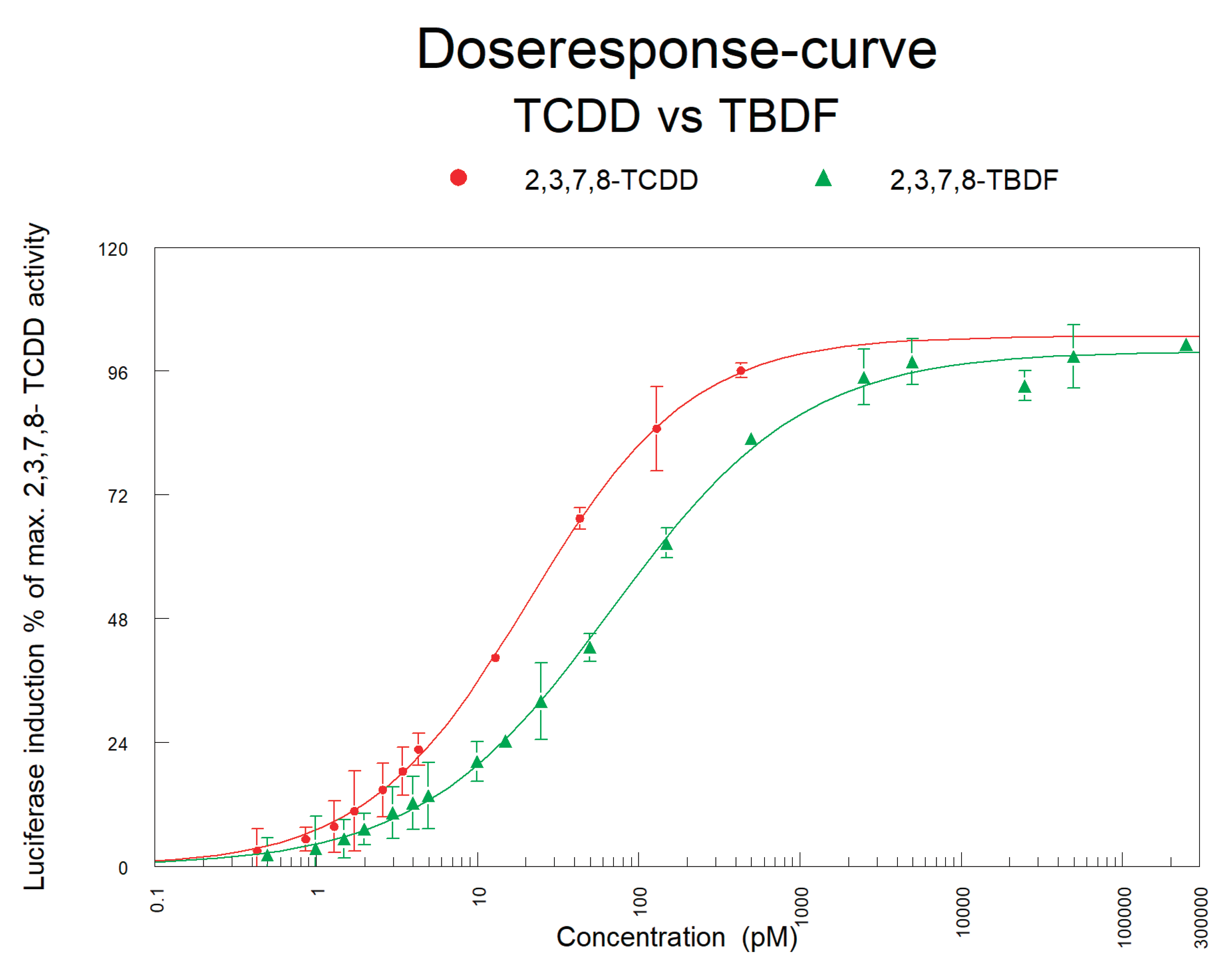
To establish the relative potency of 2,3,7,8-TBDF in the DR CALUX bioassay, a standard of 2,3,7,8-TBDF was prepared in DMSO and the concentration was checked with GC-HRMS. Then serial dilutions of this standard were made in DMSO and each standard concentration was tested in threefold in the DR CALUX bioassay. The dose response of 2,3,7,8-TBDF was compared to that of TCDD, and after fitting, the EC50 value obtained for 2,3,7,8-TBDF was compared to that of TCDD and used to calculate a TEF for 2,3,7,8-TBDF.
2.8. Feed-food converter
To estimate the transfer of PBDD/Fs from feed to egg, a transfer model was used developed by the National Institute for Public Health and the Environment (RIVM, Bilthoven, The Netherlands) and Wageningen Food Safety Research (WFSR, Wageningen, The Netherlands) [
38]
The kinetics of the brominated dioxins might differ from the chlorinated analogues. Therefore, it is not certain if the uptake of the brominated dioxin is similar to that of its chlorinated analogue TCDD, but a great difference between 2,3,7,8-TBDF and 2,3,7,8-TCDD is rather unlikely. Thus when using the transfer kinetics of TCDD for 2,3,7,8-TBDF, the kinetic model will give an indication of the amounts of 2,3,7,8-TBDF to be found in the egg. Broiler is not included in the feed-food converter.
4. Conclusions
The present study shows that the DR CALUX indicated several samples as suspect, while they were compliant according to GC-HRMS analysis for PCDD/F and dl-PCBs. Upon further investigation it turned out that egg and broiler were heavily contaminated with 2,3,7,8-TBDF and that the source was probably a contaminated poultry feed. From this it can be concluded that by including bioassays in sample analysis offers several advantages over using targeted MS methods only. As in general, effect based bioassays will detect both known and unknown bioactive compounds. The DR CALUX bioassay detects all active AhR agonists present in a sample extract. This includes the known PCDD/Fs and dlPCBs, but also the PBDD/Fs, dl-PBBs, PAHs, halogenated PAHs, and PCNs {Zhou, 2021 #92). Bioassays like the DR CALUX thus have the potential to detect new and emerging risks.
In addition, all feed additives contained PBDDs/Fs, which is rather alarming and more research is needed on how these brominated dioxins can end up in these feed additives. Both PBDD/Fs as well as PXDD/Fs are poorly monitored in agricultural products throughout Europe. Only in some extreme cases and often only by laboratories using effect based screening assays, such as in the present case, are these compounds detected. Therefore, it is advised to include the use of bioassays in monitoring programs or to include both PBDD/FS and PXDD/Fs in the applied GC-HRMS analyses.
Figure 1.
Molecular structures of representatives of the groups PCDD/Fs, PBDD/Fs, dioxin like-PCB’s and mixed- PXDD/Fs: (a) 2,3,7,8-tetrachloro-dibenzo-p-dioxin (b) 2,3,7,8-tetrabromo-dibenzo-p-dioxin (c) 2,3,7,8,-tetrachloro-dibenzo-furan (d) 2,3,7,8,-tetrabromo-dibenzo-furan (e) 3,3’,5,5’-pentachlorobiphenyl (PCB-126) (f) 3,3’,5,5’-pentabromobiphenyl (PBB-126) (g) 2,8-dibromo-3,7-dichlorodibenzo-p-dioxin.
Figure 1.
Molecular structures of representatives of the groups PCDD/Fs, PBDD/Fs, dioxin like-PCB’s and mixed- PXDD/Fs: (a) 2,3,7,8-tetrachloro-dibenzo-p-dioxin (b) 2,3,7,8-tetrabromo-dibenzo-p-dioxin (c) 2,3,7,8,-tetrachloro-dibenzo-furan (d) 2,3,7,8,-tetrabromo-dibenzo-furan (e) 3,3’,5,5’-pentachlorobiphenyl (PCB-126) (f) 3,3’,5,5’-pentabromobiphenyl (PBB-126) (g) 2,8-dibromo-3,7-dichlorodibenzo-p-dioxin.
Figure 2.
A schematic overview of the clean-up of a fat sample to extract the mono-ortho and ndl-PCBs (fraction 1) and the PCDD/Fs, PBBD/Fs, no-PCB’s and PXDD/Fs (fraction 2) using an automated DEXTech Plus system.
Figure 2.
A schematic overview of the clean-up of a fat sample to extract the mono-ortho and ndl-PCBs (fraction 1) and the PCDD/Fs, PBBD/Fs, no-PCB’s and PXDD/Fs (fraction 2) using an automated DEXTech Plus system.
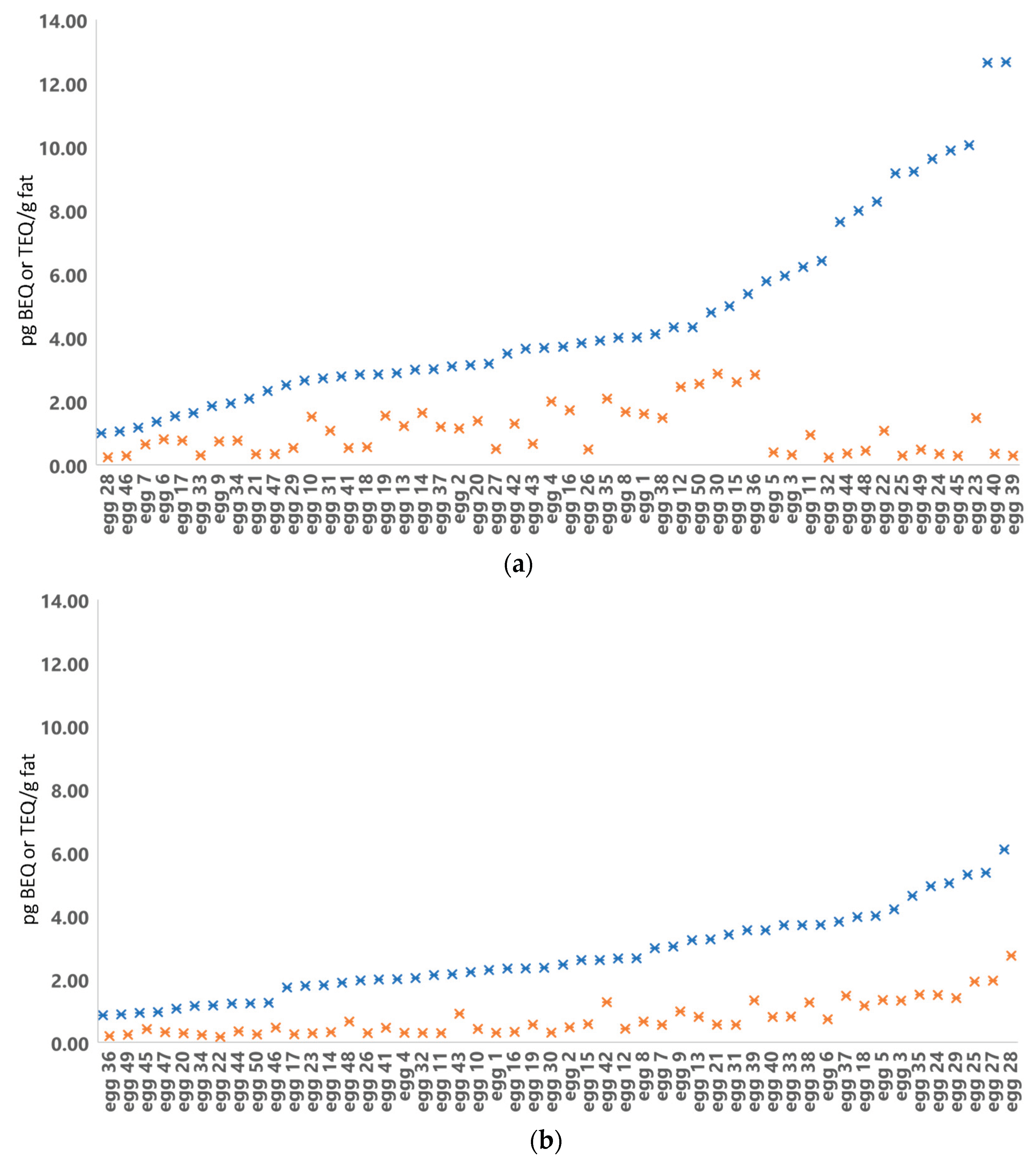
Figure 3.
Egg sample 2019 (figure 3a) and 2020 (figure 3b) results from DR CALUX in pg BEQ/g fat (blue cross) and GC-HRMS in pg WHO(2005)-PCDD/F-PCB-TEQ/g fat (orange cross) analyses. The graph is arranged in increasing DR CALUX results.
Figure 3.
Egg sample 2019 (figure 3a) and 2020 (figure 3b) results from DR CALUX in pg BEQ/g fat (blue cross) and GC-HRMS in pg WHO(2005)-PCDD/F-PCB-TEQ/g fat (orange cross) analyses. The graph is arranged in increasing DR CALUX results.
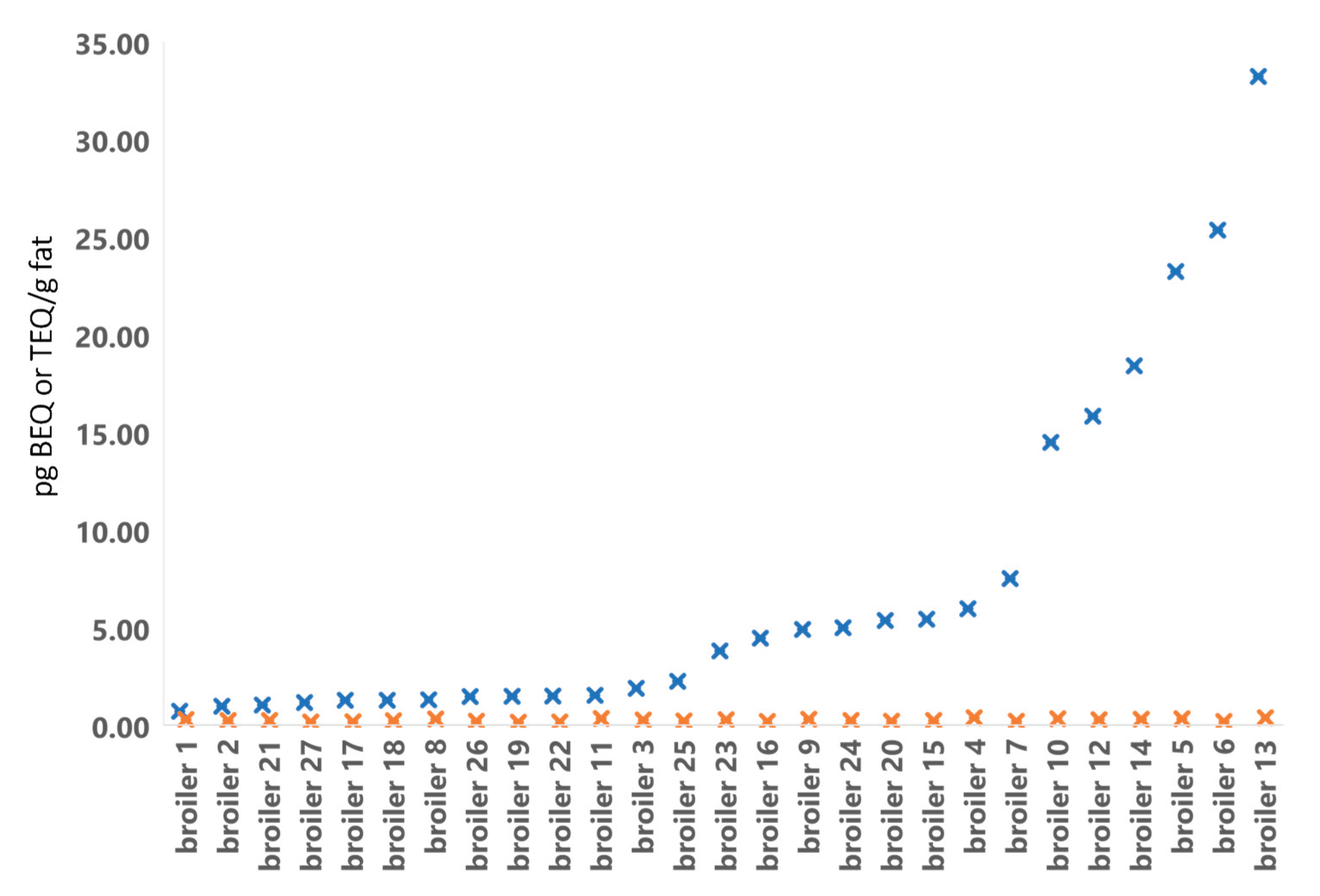
Figure 4.
Results from DR CALUX (blue cross) in pg BEQ/g fat and GC/HRMS (orange cross) in pg WHO(2005)-PCDD/F-PCB-TEQ/g fat analyses of broiler fat from 2019.
Figure 4.
Results from DR CALUX (blue cross) in pg BEQ/g fat and GC/HRMS (orange cross) in pg WHO(2005)-PCDD/F-PCB-TEQ/g fat analyses of broiler fat from 2019.
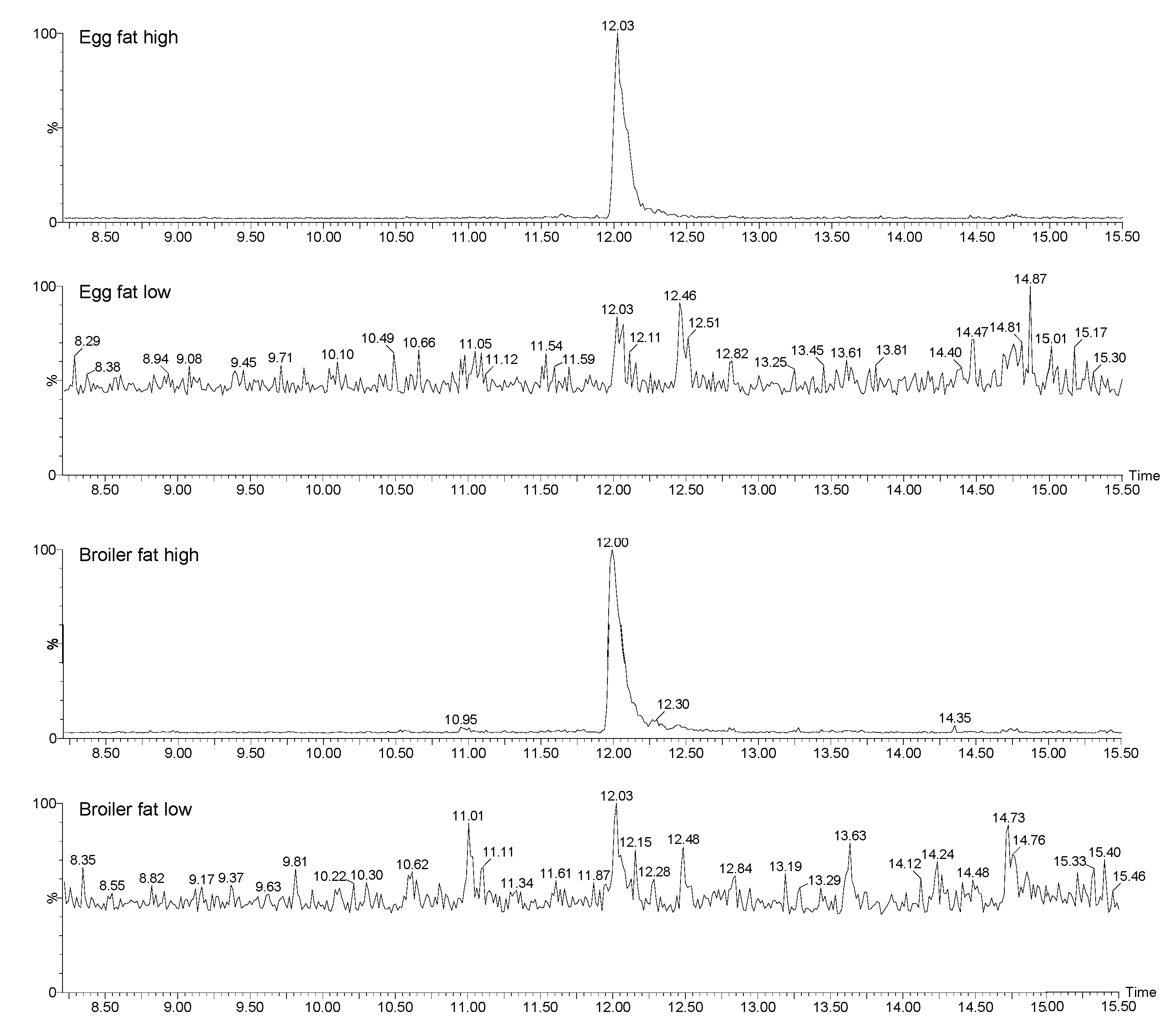
Figure 5.
The GC-HRMS chromatograms of 2,3,7,8-TBDF in broiler and egg fat pool samples which had a high and low response in the DR CALUX.
Figure 5.
The GC-HRMS chromatograms of 2,3,7,8-TBDF in broiler and egg fat pool samples which had a high and low response in the DR CALUX.
Figure 6.
The dose-responses of 2,3,7,8-TCDD and 2,3,7,8-TBDF as obtained in the DR CALUX bioassay.
Figure 6.
The dose-responses of 2,3,7,8-TCDD and 2,3,7,8-TBDF as obtained in the DR CALUX bioassay.
Table 1.
Measured masses (m/z) of the brominated dioxins and furans including the internal standards.
Table 1.
Measured masses (m/z) of the brominated dioxins and furans including the internal standards.
| Congener |
Elemental composition |
Qualifier
(m/z) |
Quantifier
(m/z) |
Internal standard |
Qualifier
(m/z) |
| 1,3,7-Tribromodibenzofuran |
C12H5Br3O |
403.788 |
405.785 |
13C-2,3,7,8-TBDF |
491.723 |
| 2,3,7,8-TBDF |
C12H4Br4O |
483.695 |
481.697 |
13C-2,3,7,8-TBDF |
491.723 |
| 1,2,3,7,8-PeBDF |
C12H3Br5O |
561.606 |
563.604 |
13C-1,2,3,7,8-PeBDF |
569.633 |
| 2,3,4,7,8-PeBDF |
C12H3Br5O |
561.606 |
563.604 |
13C-2,3,4,7,8-PeBDF |
569.633 |
| 1,2,3,4,7,8-HxBDF |
C12H2Br6O |
641.514 |
643.512 |
13C-1,2,3,4,7,8-HxBDF |
649.542 |
| 1,2,3,4,6,7,8-HpBDF |
C12H1Br7O |
719.425 |
721.423 |
13C-1,2,3,4,6,7,8-HpBDF |
727.452 |
| OBDF |
C12Br8O |
799.333 |
797.335 |
13C-1,2,3,4,6,7,8-HpBDF |
727.452 |
| 1,3,7-Tribromodibenzo-p-dioxin |
C12H5Br3O2
|
419.782 |
421.780 |
13C-2,3,7,8-TBDD |
507.718 |
| 2,3,7,8-TBDD |
C12H4Br4O2
|
499.690 |
497.692 |
13C-2,3,7,8-TBDD |
507.718 |
| 1,2,3,7,8-PeBDD |
C12H3Br5O2
|
577.600 |
579.598 |
13C-1,2,3,7,8-PeBDD |
585.628 |
| 1,2,3,4,7,8-1,2,3,6,7,8-HxBDD |
C12H2Br6O2
|
657.509 |
655.511 |
13C-1,2,3,7,8,9-HxBDD |
665.537 |
| 1,2,3,7,8,9-HxBDD |
C12H2Br6O2
|
657.509 |
655.511 |
13C-1,2,3,4,7,8-HxBDD |
665.537 |
| 1,2,3,4,6,7,8-HpBDD |
C12H1Br7O2
|
735.419 |
737.417 |
13C-1,2,3,4,6,7,8-HpBDD |
743.447 |
| OBDD |
C12Br8O2
|
815.328 |
813.330 |
13C-OBDD |
823.356 |
Table 2.
Measured masses (m/z) of the mixed dioxins and furans including the internal standards.
Table 2.
Measured masses (m/z) of the mixed dioxins and furans including the internal standards.
| Congener |
Elemental composition |
Qualifier
(m/z) |
Quantifier
(m/z) |
Internal standard |
Qualifier
(m/z) |
| 2,8-dibromo-3,7-dichloro-dibenzo-p-dioxin |
C12H4Br2Cl2O2
|
409.7933 |
411.791 |
13C-2,3,7,8-TBDF |
491.723 |
| 8-bromo-2,3,4-trchlorodibenzofuran |
C12H4BrCl3O |
349.8487 |
351.846 |
13C-2,3,7,8-TBDF |
491.723 |
| 4-bromo-2,4,7,8-tetrachlorodibenzofuran |
C12H3BrCl4O |
383.8096 |
385.8069 |
13C-1,2,3,7,8-PeBDF |
569.633 |
| 2-bromo-3,7,8-trichlorodibenzofuran |
C12H4BrCl3O |
349.8487 |
351.846 |
13C-2,3,4,7,8-PeBDF |
569.633 |
| 2-bromo-1,3,7,8,-tetrachlorodibenzofuran |
C12H3BrCl4O |
383.8096 |
385.8069 |
13C-1,2,3,4,7,8-HxBDF |
649.542 |
| 2,3-dibromo-7,8-dichlorodibenzo-p-dioxin |
C12H4Br2Cl2O2
|
409.7933 |
411.791 |
13C-1,2,3,4,6,7,8-HpBDF |
727.452 |
| 2-bromo-1,3,7,8,-tetrachlorodibenzo-p-dioxin |
C₁₂H₃BrCl₄O₂ |
399.8045 |
401.8019 |
13C-1,2,3,4,6,7,8-HpBDF |
727.452 |
Table 3.
DR CALUX results from 4 pool samples i.e. eggs low, eggs high and broiler low, broiler high.
Table 3.
DR CALUX results from 4 pool samples i.e. eggs low, eggs high and broiler low, broiler high.
| Sample |
DR CALUX result |
Assessment |
| Eggs low |
0.55 pg BEQ/g |
Negative |
| Eggs high |
10.45 pg BEQ/g |
Suspect |
| Broiler low |
0.02 pg BEQ/g |
Negative |
| Broiler high |
18.58 pg BEQ/g |
Suspect |
Table 4.
Results from DR CALUX and GC/HRMS analyses of: pool samples, broiler- and egg fat -, choline chloride, poultry feed and L-lysine. Expressed in bioanalytical equivalent (BEQ), WHO 2005 sum PCDD/F-PCB-TEQ and PBDD/F congener per pg/g fat or product. .
Table 4.
Results from DR CALUX and GC/HRMS analyses of: pool samples, broiler- and egg fat -, choline chloride, poultry feed and L-lysine. Expressed in bioanalytical equivalent (BEQ), WHO 2005 sum PCDD/F-PCB-TEQ and PBDD/F congener per pg/g fat or product. .
| Sample type |
DR CALUX
ng BEQ/kg |
GC/HRMS
PCDD/Fs+PCBs
ng TEQ/kg |
GC/HRMS
PBDD/F
pg/g fat/product |
| |
|
|
2,3,7,8
TBDF |
1,2,3,7,8
PeBDF |
2,3,4,7,8
PeBDF |
1,2,3,4,7,8
HxBDF |
1,2,3,4,6,7,8
HpBDF |
2,3,7,8
TBDD |
1,2,3,7,8,9
HxBDD |
| |
|
|
|
|
|
|
|
|
|
| Broiler fat (pool) high |
19 |
0.03 |
31 |
1 |
1 |
1 |
1 |
1 |
1 |
| Broiler fat (pool) low |
0.02 |
|
|
|
|
|
|
|
|
| Egg fat (pool) high |
10 |
0.16 |
21 |
1 |
1 |
1 |
1 |
1 |
1 |
| Egg fat (pool) low |
0.55 |
|
|
|
|
|
|
|
|
| Choline chloride |
1 |
0.045 |
0.23 |
1 |
1 |
1 |
1 |
1 |
1 |
| Choline chloride |
3 |
0.044 |
3.13 |
0.41 |
1 |
1 |
1 |
1 |
1 |
| Poultry feed |
4 |
0.103 |
3.6 |
1 |
1 |
1 |
1 |
1 |
1 |
| L-lysine |
7 |
0.039 |
1.86 |
1.59 |
1 |
1.04 |
1 |
1 |
1 |
| L-lysine |
15 |
0.449 |
2.39 |
1.71 |
0.26 |
0.25 |
1 |
1 |
1 |
| L-lysine |
16 |
0.155 |
4.92 |
1.47 |
0.26 |
2 |
0.21 |
1 |
0.08 |
| L-lysine |
19 |
0.062 |
5.34 |
2.09 |
0.31 |
1 |
1 |
1 |
1 |
| L-lysine |
22 |
0.248 |
8.33 |
2.53 |
0.27 |
1.11 |
0.15 |
1 |
1 |
| L-lysine |
22 |
0.508 |
8.8 |
3.53 |
0.62 |
0.61 |
1 |
1 |
1 |
| L-lysine |
45 |
0.243 |
22.3 |
0.13 |
1 |
1 |
1 |
0.05 |
1 |
| Bedding material |
750 |
143 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
| Seaweed (me hijiki) |
2.24 |
0.15 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
| Butter fat 0.5 |
0.59 |
0.36 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
| Butter fat 6.0 |
6.19 |
4.5 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |