1. Introduction
Microbial communities of industrial societies have been altered gradually and enriched in antibiotic-resistant taxa, which may produce pro-inflammatory responses and gut dysbiosis. Schaan et al. (2021) recently characterised the gut microbiome of several populations living with different lifestyles in the Brazilian Amazon. The authors demonstrated the potential urbanisation in the gut microbiome of rural Amazonian communities and proposed a "tropical urban gut microbiome" as a specific term to better characterise it [
1]. Therefore, the current microbiome profile among traditional populations relates to urban daily life [
1]. For instance, the colonising
S. aureus in the gut represents this latter pattern.
S. aureus is a potentially dangerous pathogen that can originate several diseases, ranging from impetigo to life-threatening conditions such as pneumonia and endocarditis [
2]. The success of
S. aureus as a widespread human pathogen is also a result of its competence to colonise the upper respiratory tract and other tissues, which operate as reservoirs for infection, such as the skin, throat, axillae, vagina, and gut. Regarding the last niche, studies have shown that intestine-colonising
S. aureus can persist following the cessation of antibiotic treatment and form a notable reservoir for nosocomial spread. From an individual perspective, carrying
S. aureus in the gut represents a risk factor for potentially threatening disease development, such as staphylococcal enterocolitis (SEC) [
3,
4,
5,
6].
Initial studies on the intestinal carriage of
S. aureus [
7] were followed by countless efforts to understand and manage Clostridioides difficile infection (CDI). Indeed, SEC also represents a minor portion of case reports and observational studies on staphylococcal infections in the scientific literature [
8,
9]. Here, we present an atypical clinical course of SEC, diagnosed after an unscheduled consultation.
2. Case Presentation
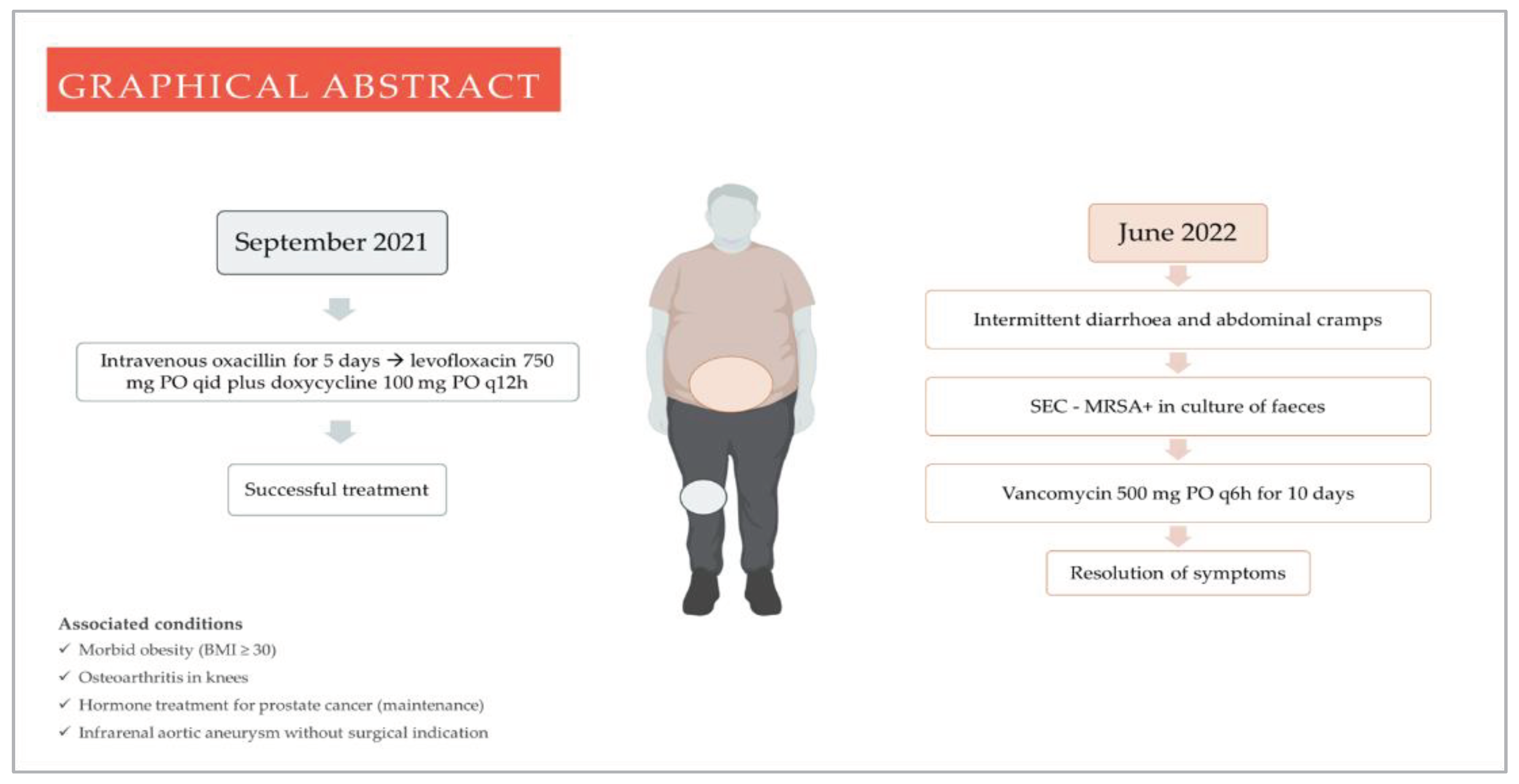
A male patient, in the order of 70 years old, with prostate cancer, obesity (body mass index > 30 kg/m2), osteoarthritis in knees, and recent history (eight months) of a methicillin-susceptible Staphylococcus aureus (MSSA) septic arthritis in the right knee (following an intra-articular steroid injection) presented at a medical consultation to perform a preoperative evaluation before an aortic aneurysm's repair and to fulfil the follow-up of the joint infection.
During the anamnesis, the patient revealed intermittent diarrhoea beginning two months ago, lasting from three to four days, followed by spontaneous remission for approximately seven days, besides hypogastric abdominal colic, evacuation urgency, and at least three daily diarrheic episodes of aqueous content. His general examination was unremarkable. The mental status was preserved, with a blood pressure of 120/80 mmHg, a heart rate of 80/min, an axillar temperature of 36.6 °C, and a respiratory frequency of 17/min. He denied other symptoms and any current diarrhoeic disease among their relatives and friends, besides the absence of gastrointestinal disorders in the family history.
Of note, the septic arthritis was managed with oxacillin 2 g IV four times a day (q6h) for five days, followed by doxycycline 100 mg by oral intake (PO) twice daily (q12h) and levofloxacin 750 mg PO once daily. However, after the patient reported a previously diagnosed aortic aneurysm, we kept doxycycline as monotherapy for six weeks, as fluoroquinolones are a risk factor for aortic disease.
2.1. Investigations
Given the previous antimicrobial use and the biased clinical appraisal (as an infectologist performed this), the medical hypotheses of CDI and SEC added the spotlight. Furthermore, the intermittent pattern of diarrhoea was a clue to target SEC since, in several reported cases of SEC, there is an association between toxic shock syndrome toxin-1 (TSST-1) and staphylococcal enterotoxins or even the isolated identification of TSST-1. The latter is involved in the pathogenesis of staphylococcal toxic shock syndrome (TSS), whose recurrence has been reported in previous studies [
10,
11,
12]. Therefore, even in the absence of criteria for TSS - probable and definitive - the presented case's recurrent nature weighed on the investigation's continuance. Thus, we required laboratory tests to investigate both pathogens on faeces. It is important to emphasise that the absence of additional laboratory tests after the initial evaluation was based on the rapid exam results and the high suspicion of antibiotic-associated diarrhoea, even in an atypical clinical setting. The CDI test was negative, but the faecal culture was positive for MRSA.
Table 1 shows the antimicrobial sensitivity test of MRSA.
2.2. Treatment
Then, we prescribed vancomycin at 500 gm PO q6h for ten days and asked him to alarm us if an out-of-context doctor prescribed a new antibiotic. Concerning vancomycin usage, the prescription followed the existing case reports and literature review studies since the therapeutic approach lacks randomised clinical trials or guidelines to support decisions. [
14,
15,
16]. From the third day on vancomycin (the patient had diarrhoea at that time), there was an improvement in abdominal cramps and frequency of bowel movements, and the stools became pasty. The patient achieved complete remission, which improved his mood and allowed him to leave the house more often, no longer attached to the fear of the cramps that had plagued him for weeks. In addition, he was able to repair the aortic aneurysm thirty days after finishing the treatment of SEC. After surgery, monthly consultations followed. There was no recurrence at the end of 6 months, arbitrarily defined follow-up time (
Figure 1).
3. Discussion
Most cases of SEC present acutely with non-specific symptoms such as fever, vomiting and abdominal pain, plus large-volume, "cholera-like" diarrhoea [
10,
11,
12]. When measured, stool volumes can be several litres daily, with over eight litres/day recorded in one study [
9]. Fever is a regular sign (may be absent), and the average body temperature before diarrhoea has already been described. However, this form of clinical presentation has not been reported in the literature, which can lead clinicians to consider other disorders, such as inflammatory bowel disease and eosinophilic and drug-induced colitis [
14,
15,
16]. Therefore, the reasons for the described clinical course remain to be understood. The presence of the TSST-1 and the absence of enough neutralising antibodies to staphylococcal toxins may explain the intermittent character since toxin-1 suppresses humoral immunity [
17]. In addition, men harbour more effective immune responses to staphylococcal superantigens, up to the majority, than women. However, a study in distinct populations identified increasing levels of neutralising antibodies in more than 80% of young women [
12].
SEC is a condition that has become almost forgotten and is rarely investigated by clinicians routinely, even in the face of a classic clinical picture. This disorder is usually associated with prior antibiotic therapy, particularly with fluoroquinolones (FQ), recent abdominal surgery, and compromised immune conditions [
12,
17]. The patient had two facilitators: prior FQ therapy and the immune dysfunction caused by cancer. It is possible that the patient was an intestinal carrier of
S.
aureus, either before or after septic arthritis. Concerning the isolation of MRSA in faeces (and not MSSA, the phenotype identified during osteoarticular infection), the most likely hypothesis would be the previous use of an FQ, a significant risk factor for MRSA colonisation and infection [
18,
19,
20].
4. Conclusions
Reintroducing SEC on the agenda is critical. The need for novel observational studies to better characterise the clinical aspects of SEC, including mortality, is urgent. In addition, microbiological information and whole genome sequence can also identify the most common MRSA lineages and their virulence factors.
Author Contributions
Conceptualisation, Rodrigo Cuiabano Paes Leme and Fábio Aguiar-Alves; methodology, Rodrigo Cuiabano Paes Leme and Renata Freire Alves Pereira; software, Felipe Ramos Pinheiro; validation, Fábio Aguiar-Alves and Renata Freire Alves Pereira; formal analysis, Rodrigo Cuiabano Paes Leme and Fábio Aguiar-Alves; investigation, Rodrigo Cuiabano Paes Leme and Renata Freire Alves Pereira; resources, Felipe Ramos Pinheiro; data curation, Felipe Ramos Pinheiro and Rodrigo Cuiabano Paes Leme; writing—original draft preparation, Rodrigo Cuiabano Paes Leme; writing—review and editing, Renata Freire Alves Pereira and Felipe Ramos Pinheiro; visualisation, Rodrigo Cuiabano Paes Leme and Fábio Aguiar-Alves; supervision, Fábio Aguiar-Alves; project administration, Rodrigo Cuiabano Paes Leme. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study since it aims for educational purposes rather than research and because data were collected as part of routine care. The demographic, physical, or illustrative information minimises patient identification risk.
Informed Consent Statement
Written informed consent to publish this paper has been obtained from the patient.
Data Availability Statement
This study did not produce data that should be linked or registered in scientific repositories.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schaan, A.P.; Sarquis, D.; Cavalcante, G.C.; Magalhães, L.; Sacuena, E.R.P.; Costa, J.; Fonseca, D.; Mello, V.J.; Guerreiro, J.F.; Ribeiro-dos-Santos, Â. The Structure of Brazilian Amazonian Gut Microbiomes in the Process of Urbanisation. NPJ Biofilms and Microbiomes 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus Aureus: Molecular Characterisation, Evolution, and Epidemiology. Clinical microbiology reviews 2018, 31. [Google Scholar] [CrossRef] [PubMed]
- Claassen-Weitz, S.; Shittu, A.O.; Ngwarai, M.R.; Thabane, L.; Nicol, M.P.; Kaba, M. Fecal Carriage of Staphylococcus Aureus in the Hospital and Community Setting: A Systematic Review. Frontiers in Microbiology 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Bergevin, M.; Marion, A.; Farber, D.; Golding, G.R.; Lévesque, S. Severe MRSA Enterocolitis Caused by a Strain Harboring Enterotoxins D, G, and I. Emerg Infect Dis 2017, 23, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Otto, M. Probiotics to Prevent Staphylococcus Aureus Disease? Gut Microbes 2020, 11, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Bryan, A.; Atluri, V.; Ma, J.; Durowoju, L.; Bandhlish, A.; Boonyaratanakornkit, J. Fatal Infection with Enterocolitis from Methicillin-Resistant Staphylococcus Aureus and the Continued Value of Culture in the Era of Molecular Diagnostics. Leuk Res Rep 2021, 15, 100254. [Google Scholar] [CrossRef] [PubMed]
- Acton, D.S.; Tempelmans Plat-Sinnige, M.J.; van Wamel, W.; de Groot, N.; van Belkum, A. Intestinal Carriage of Staphylococcus Aureus: How Does Its Frequency Compare with That of Nasal Carriage and What Is Its Clinical Impact? European Journal of Clinical Microbiology & Infectious Diseases 2009, 28, 115. [Google Scholar] [CrossRef]
- Gravet, A.; Rondeau, M.; Harf-Monteil, C.; Grunenberger, F.; Monteil, H.; Scheftel, J.M.; Prévost, G. Predominant Staphylococcus Aureus Isolated from Antibiotic-Associated Diarrhea Is Clinically Relevant and Produces Enterotoxin A and the Bicomponent Toxin LukE-LukD. Journal of Clinical Microbiology 1999, 37, 4012–4019. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Havill, N.L. Nosocomial Antibiotic-Associated Diarrhea Associated with Enterotoxin-Producing Strains of Methicillin-Resistant Staphylococcus Aureus. Official journal of the American College of Gastroenterology | ACG 2005, 100, 1828. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.-M.; Parent, E.M.; Barry, M.; Parsonnet, J. Recurrent Nonmenstrual Toxic Shock Syndrome: Clinical Manifestations, Diagnosis, and Treatment. Clinical Infectious Diseases 2001, 32, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Cone, L.A.; Byrd, R.G.; Schulz, K.; Schlievert, P.M. Recurrent Nonmenstrual Toxic Shock. CLIN INFECT DIS 2002, 34, 289–289. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, W.; Michie, C.; Kenol, B.; van der Bijl, S. Recurrent Menstrual Toxic Shock Syndrome With and Without Tampons in an Adolescent. Pediatric Infectious Disease Journal 2014, 33, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Brazilian Committee on Antimicrobial Susceptibility Testing - BrCAST - Tabelas de pontos de corte para interpretação de CIMs e diâmetros de halos 2020.
- Lin, Z.; Kotler, D.P.; Schlievert, P.M.; Sordillo, E.M. Staphylococcal Enterocolitis: Forgotten but Not Gone? Digestive Diseases and Sciences 2010, 55, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Doi, A.; Fukuchi, T.; Ohji, G.; Shirota, Y.; Sakai, T.; Kagawa, H. A Systematic Review for Pursuing the Presence of Antibiotic-Associated Enterocolitis Caused by Methicillin-Resistant Staphylococcus Aureus. BMC Infectious Diseases 2014, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Gururangan, K.; Holubar, M.K. A Case of Postoperative Methicillin-Resistant Staphylococcus Aureus Enterocolitis in an 81-Year-Old Man and Review of the Literature. American Journal of Case Reports 2020, 21. [Google Scholar] [CrossRef]
- Kotler, D.P.; Sandkovsky, U.; Schlievert, P.M.; Sordillo, E.M. Toxic Shock-Like Syndrome Associated with Staphylococcal Enterocolitis in an HIV-Infected Man. Clinical Infectious Diseases 2007, 44, e121–e123. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, P.; Parienti, J.-J.; Thibon, P.; Ramakers, M.; Daubin, C.; du Cheyron, D.; Lebouvier, G.; Le Coutour, X.; Leclercq, R. ; French Fluoroquinolone Free (3F) Study Group Fluoroquinolone Use and Methicillin-Resistant Staphylococcus Aureus Isolation Rates in Hospitalized Patients: A Quasi Experimental Study. Clin Infect Dis 2006, 42, 778–784. [Google Scholar] [CrossRef]
- LeBlanc, L.; Pépin, J.; Toulouse, K.; Ouellette, M.-F.; Coulombe, M.-A.; Corriveau, M.-P.; Alary, M.-E. Fluoroquinolones and Risk for Methicillin-Resistant Staphylococcus Aureus, Canada. Emerg Infect Dis 2006, 12, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Limoncu, M.H.; Ermertcan, S.; Cetin, C.B.; Cosar, G.; Dinç, G. Emergence of Phenotypic Resistance to Ciprofloxacin and Levofloxacin in Methicillin-Resistant and Methicillin-Sensitive Staphylococcus Aureus Strains. Int J Antimicrob Agents 2003, 21, 420–424. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).