1. Introduction
Marine microorganisms have developed defense strategies that include the production of different metabolites and enzymes with important applications for humans [
1]. These low molecular weight compounds are produced by living organisms to perform biological functions for their survival and as well as in defense [
2]. Exploration of marine microbiota is recent, but already shows great promise in both medical and biotechnology fields [
3]. Since the oceans cover most of the Earth’s surface, they host a substantial portion of the biodiversity, which lives under distinct and varied conditions and has evolved through a long period of metabolic adaptations. Exploration of the biodiversity and metabolic chemodiversity of the oceans has resulted in the discovery of thousands of structurally unique bioactive marine natural products [
4]. Considering that oceans encompass typical microbial abundances of 10
6 microorganisms per mL in seawater and 10
9 per mL in ocean-bottom sediments [
5], the exploration of marine microorganisms as sources of bioactive marine organisms has led to the discovery of promising new drug candidates [
1,
6]. A review by Newman and Craig (2020) [
7] showed that about 56% of 1881 therapeutic products approved by the US FDA between 1981 and 2019 had natural prototypes, especially obtained from marine microorganisms.
Chagas disease is a parasitic infection considered neglected by the World Health Organization (WHO). This condition affects over eight million people worldwide, mainly in Latin America. It is caused by the protozoan parasite
Trypanosoma cruzi and transmitted mostly by the insect
Triatoma sp or by oral contamination of food. In non-endemic countries, it is present due to immigration and disseminated by contaminated food and organ transplantation [
8,
9]. The parasite can be identified in two clinically relevant forms: trypomastigotes, which are found in blood and extracellular compartments and are considered the infective form, and amastigotes, which are found in the affected tissues during latent and chronic disease and are the intracellular replicative forms. The current treatment employs only two highly toxic drugs, benznidazole or nifurtimox. Both drugs are associated with severe adverse effects, but only benznidazole is still approved in Brazil. A large multicentric study confirmed the low efficacy of benznidazole to eliminate the parasites in chronic patients [
10], emphasizing the urgent need for safer and more effective treatments for Chagas disease.
The marine environment has been a source of more than 20,000 inspirational natural products discovered over the past 50 years. In view of the huge chemodiversity present in microorganisms living in the oceans, numerous biologically active compounds have been described [
11]. For example, small molecules isolated from the marine bacterium
Bacillus pumilus, present in the black coral
Antipathes sp. of the Pacific coast of Panama have shown potent activity against
Trypanosoma cruzi with IC
50 values from 19 to 27 μM [
12]. Penidigiamycin-A, an antiprotozoal compound isolated from the marine bacterium
Paenibacillus sp. DE2SH, showed anti-
Leishmania (IC
50 7 µM), anti-
Trypanosoma brucei (IC
50 0.78 µM) and anti-
Plasmodium falciparum (IC
50 9.1 µM) activity [
13]. The Blue Amazon is the Brazilian oceanic area of the Atlantic that comprises 3.6 million square kilometers and presents a huge biodiversity of potential pharmaceutical candidates to fight Neglected Tropical Diseases (NTDs). In this study, we examined the antiparasitic potential of bacterial metabolites isolated from three corals and sediments collected from the North coast of São Paulo, Brazil (
Figure 1, orange arrow).
3. Discussion
Chagas disease is a serious public health problem in 21 developing countries. In Brazil, only one drug is approved for the treatment, with a limited efficacy and high toxicity. In 2015, Morillo and co-workers published the most complete multicentric and randomized clinical study with benznidazole, involving 2,854 patients with Chagas cardiomyopathy [
10]. The results showed that the therapy significantly reduced parasitemia, however, showed no efficacy to reduce the cardiac damage over the 5-year follow-up of the study [
10]. In the literature there is a consensus about the urgent need for new therapeutic candidates for Chagas disease.
Considering the huge chemodiversity found in marine microorganisms, we investigated for the first time the anti-Trypanosoma cruzi potential of metabolites produced by bacteria that had been previously isolated from invertebrates and sediments from Sao Paulo north coast in Brazil. Among the most active extracts, it was observed that metabolites of the bacterium Vibrio harveyi were amongst the most potent.
This strain was isolated from four sources, one from the coral
Mussismilia hispida and two from the sediments from the São Sebastião Channel and the Buzios Island at 35- and 13-meters m depth. Members of the
Vibrionaceae family are freely in tropical marine waters or in the microbiota of marine animals [
15]. The genus
Vibrio was the most common one found in this study, covering 7 of the 11 identified strains and it has been recognized as part of the microbiome of several invertebrates. It is a holobiont that participates in nitrogen fixation, food resource, chitin decomposition, and the production of antimicrobial metabolites ([
16,
17]).
The Vibrionaceae family has yielded 93 metabolites with different biological activities, including antifungal, antibacterial and anticancer. Most have been isolated from three species,
namely V. parahaemolyticus, V. anguillarum, and V. vulnificus. However, only one compound, prodigiosin, presented antiprotozoal activity against
Plasmodium spp. [
18]
To our knowledge, our study demonstrated for the first time, the anti-trypanosomal activity of metabolites from
Vibrio spp. It was noteworthy that the same species, but collected in different locations, resulted in different potencies against the parasites. This was verified with
V. harveyi, which resulted in metabolites with IC
50 values against
T. cruzi ranging from 8 to 51 µg/mL. These differences in the metabolic profile of genetically similar strains have been reported for
Streptomyces griseus, the most prolific genus in the production of bioactive compounds [
19]. Species closely associated by 16S rRNA maintain a common set of chemical compounds. However, despite the identical genetic sequences of this gene, a noticeable difference was found in the accessory set of accumulation of metabolites, which are unique for each bacterium [
19]. Sottorff and colleagues suggested that, based on phylogenetic proximity and the similarity of metabolites, both strains of
Streptomyces spp. had a common origin that underwent posterior specialization as a function of their habitat [
19]. In our studies, the
Vibrio spp. strains were isolated from completely different environments, which included deep sediments (close to the coast), sediments from shallow waters of an island (approximately 20 miles from the coast) and other three coral species. It is possible that the different microbiomes may have contributed to the process of specialization of their metabolites.
Another potent extract with anti
-T. cruzi activity was produced from
Shewanella pneumatophori, isolated from marine sediments of the Buzios island. This bacterium belongs to a genus generally found in extreme temperatures and has shown antimicrobial [
20] and antifungal activities [
21,
22,
23]. Our study demonstrated, for the first time in the literature, the anti-
T. cruzi activity of metabolites of this genus.
The genus
Bacillus has already shown promising activities in the literature, ranging from antimicrobial activities e.g.
B. subtilis [
24], to antiparasitic activities e.g. B. pulmilus against
Trypanosoma cruzi [
12]. In our study,
Bacillus megaterium, isolated from deep sediments of the São Sebastião Canal at 35 meters depth, showed anti-
T. cruzi activity, but presented the lowest potency among all tested extracts. Generally found in terrestrial environments, this species has the capacity to solubilize natural phosphates in soil and are of great interest for the industry of bioproducts [
25,
26]. The organisms have also shown antimicrobial and antifungal activities, with potential use against dermatophytosis and in aquaculture for biocontrol of
Aspergillus flavus [
27,
28,
29].
Our study is also the first to describe the anti
-T. cruzi potential of
Halomonas aquamarina metabolites. The extract of
H. aquamarina showed high potency activity against
T. cruzi, amongst all the tested bacteria. The
Halomonas genus has a wide geographical distribution and promising antimicrobial and antitumor biological activities [
30,
31].
Halomonas is a common genus of halophilic bacteria, which are inhabitants of hypersaline environments. These extremophiles have special abilities to produce extremozymes and other bioactive molecules as potential antibiotics [
32].
Another potent microbial extract against
T. cruzi was obtained from
Mesoflavibacter zeaxanthinifaciens, a bacterium isolated from marine sediments of Buzios island. This is an aerobic Gram-negative, rod-shaped, halo- and mesophilic bacterium, first isolated from a seawater sample collected from the Pacific coastline of Japan [
14]. Considering the antiparasitic potential of these metabolites, we conducted an OSMAC (One-Strain Many Compounds) study, a promising approach to activate cryptic genes and enhance the production of bioactive compounds. Using solid and liquid mediums for cultivation, our results showed that the cultivation in liquid medium with rotation, resulted in a higher mass of microbial metabolites. However, the potency of these anti-
T. cruzi metabolites decreased 8-fold when compared to those produced by bacteria in solid medium (Marine Agar, Difco). English and co-workers used a similar OSMAC approach to test the ability of
Streptomyces sp. to produce antibiotics against
S. aureus. No antimicrobial activity was detected in metabolites isolated from bacteria cultured in liquid medium, but only in agar medium [
33]. Similarly, Guo and co-workers also observed that the marine fungi Penicillium sp. F23-2 showed different chemical compounds when cultivated in solid medium, leading to the isolation of five new analogues of ambuic acid (penicyclones A-E), which antibacterial activity [
34].
Using solid-phase extraction, we conducted a fractionation of the crude EtOAc extract from
Mesoflavibacter zeaxanthinifaciens, resulting in FII, a fraction with trypanocidal effect, as confirmed by the lack of mitochondrial activity, detected by the resazurin assay. The studies with the intracellular amastigotes revealed a potent activity of FII against the clinically relevant form of the parasite. Despite the importance of the trypomastigotes during the acute phase of the disease, the amastigotes are the major parasitic form living inside the cells, contributing to the prevalence of the disease for decades [
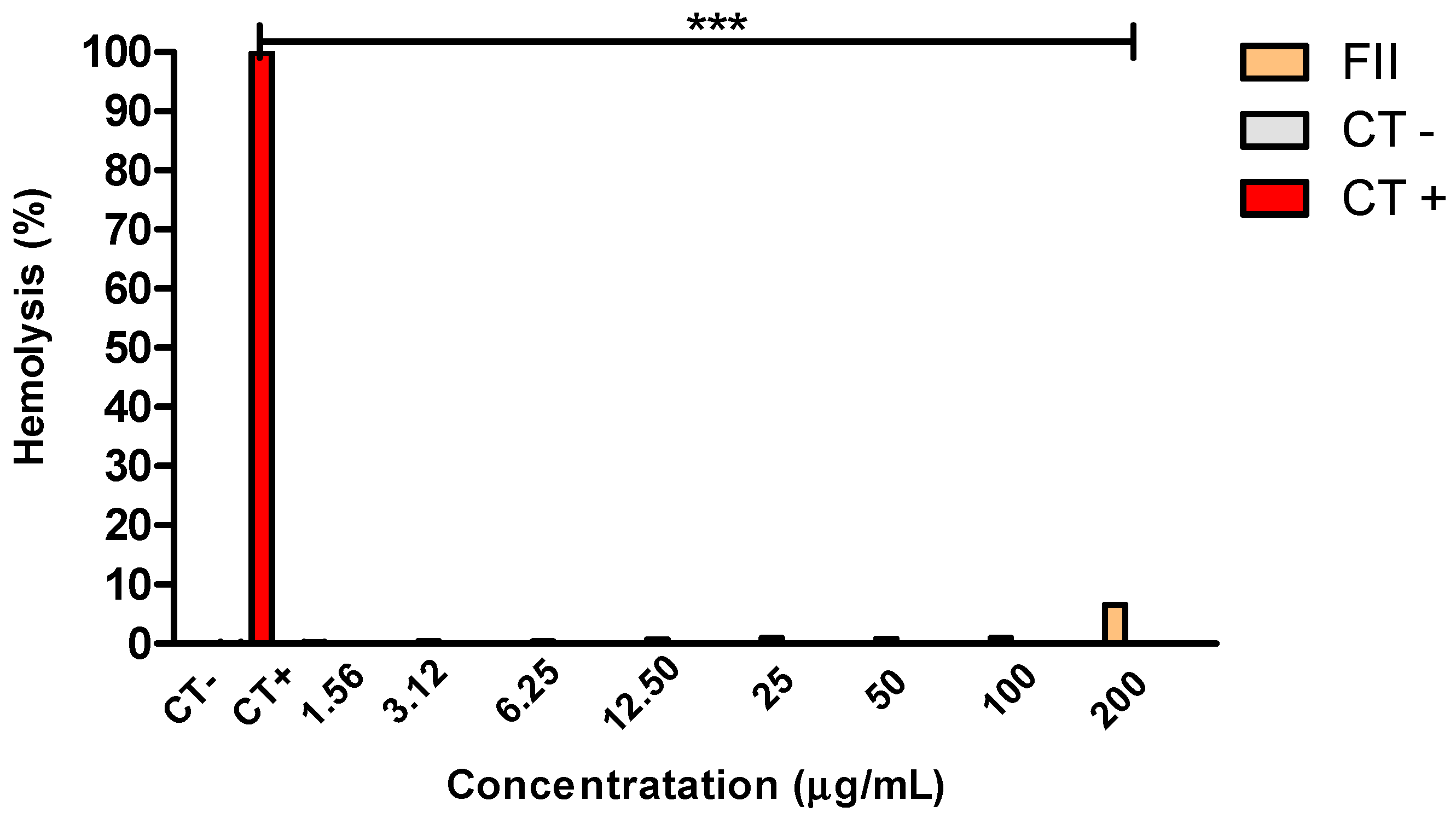
35]. It was noteworthy that FII eliminated both forms of the parasite without affecting the mammalian cells (up to a tested concentration of 200 μg/mL), as demonstrated by the lack of cytotoxicity against fibroblasts. Additionally, FII was also evaluated in erythrocytes for cytotoxicity, but resulted in a low hemolysis at the highest tested concentration. These studies confirmed that FII presented selective compounds against
T. cruzi, suggesting a promising biological activity for drug discovery exploration.
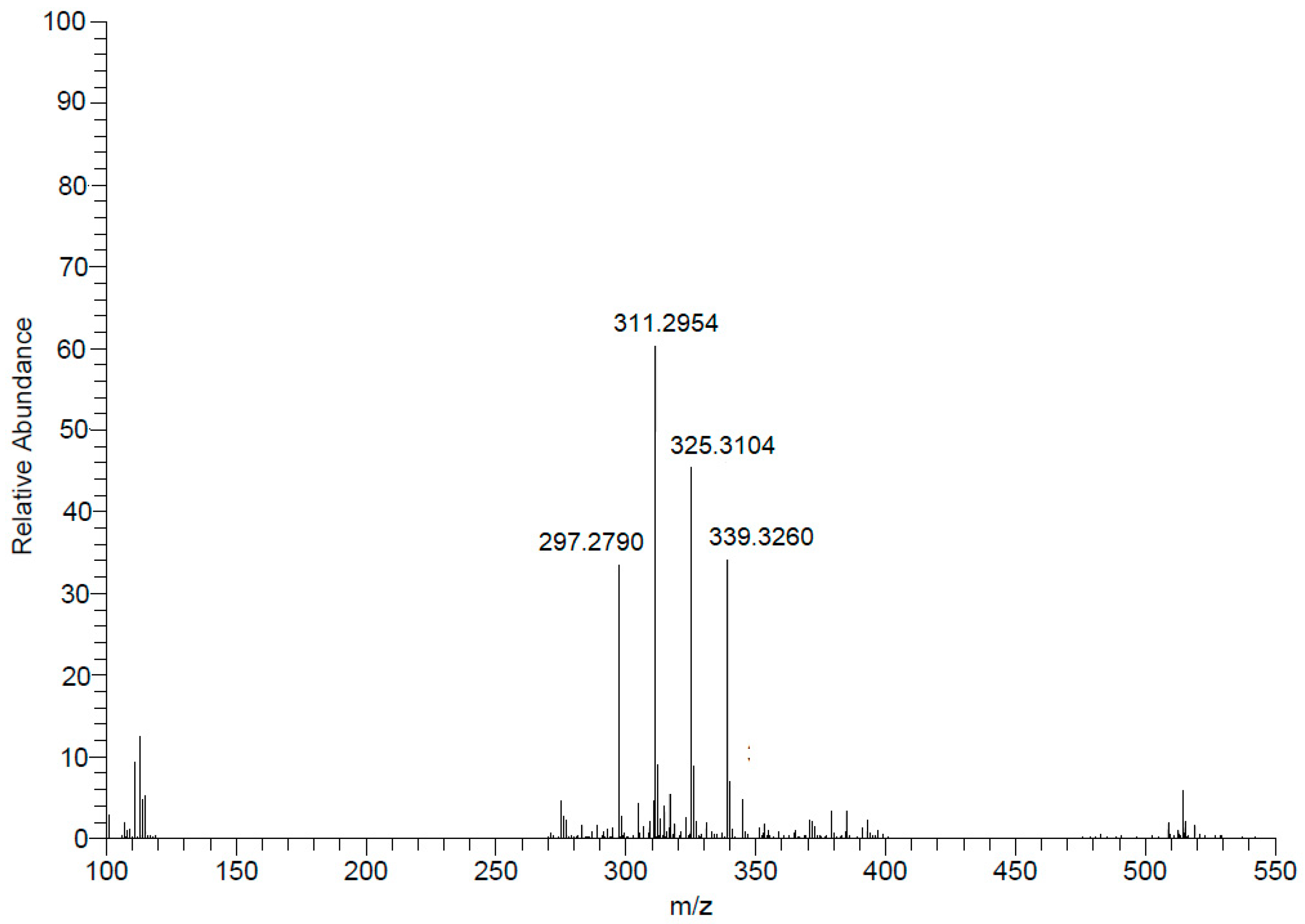
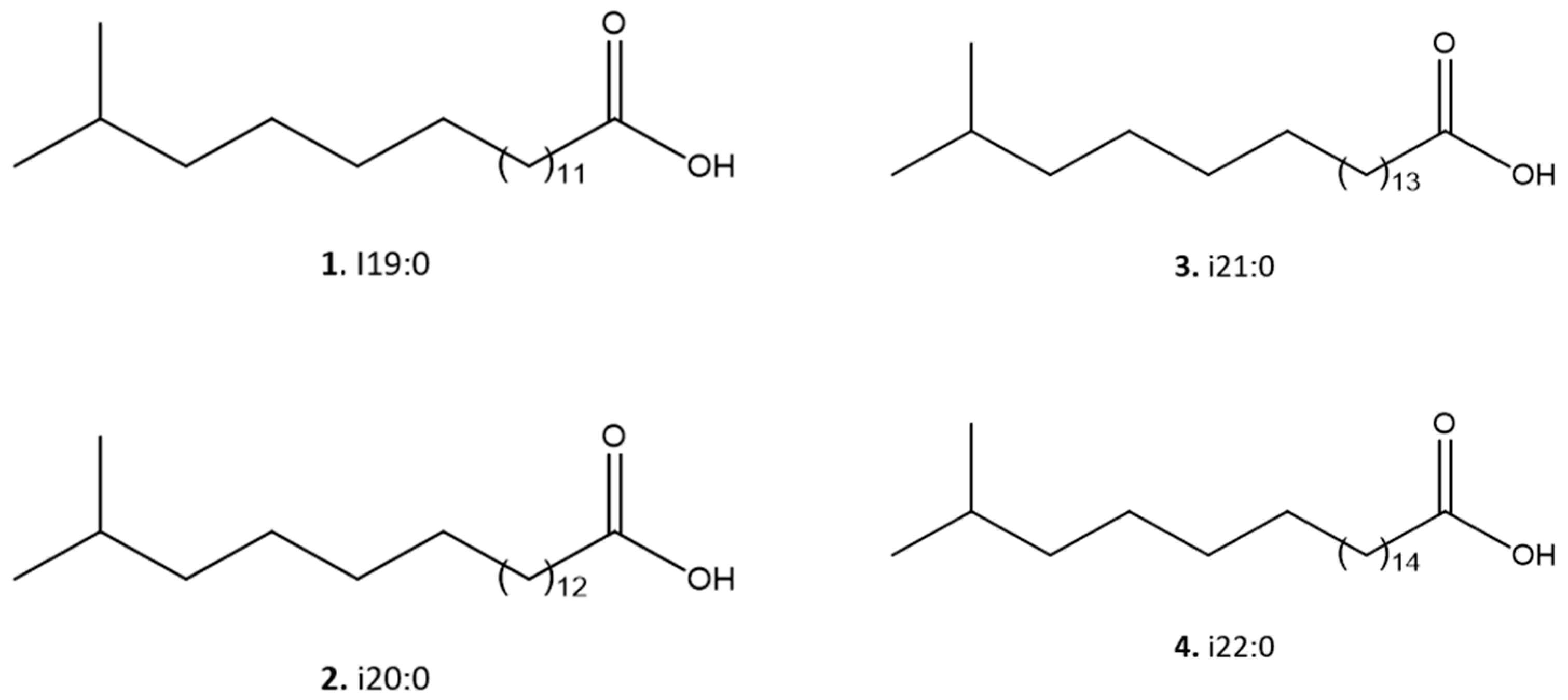
Using
1H NMR and ESI-HRMS analysis, four iso fatty acids (1: C
19H
37O
2, 2: C
20H
39O
2, 3: C
21H
41O
2, and 4: C
22H
43O
2) were identified as main compounds in FII. Our study corroborated the known predominance of branched saturated fatty acids, in addition to branched monounsaturated and branched hydroxyacids in specimens of the family Flavobacteriaceae [
14,
36]. In the literature, studies about the antiparasitic activity of saturated fatty acids are limited. Londero and co-workers showed acetylenic fatty acids can change the membrane potential and kill
T. cruzi [
37]. Other fatty acids, with antimalarial, antimycobacterial and anfungal properties have been reported. They might be able to cause pores in the bacterial membrane and alter cell permeability, activating signaling pathways that lead to the microorganism to death [
38].
Considering the promising results of fraction II of
Mesoflavibacter zeaxanthinifaciens EtOAc extract against
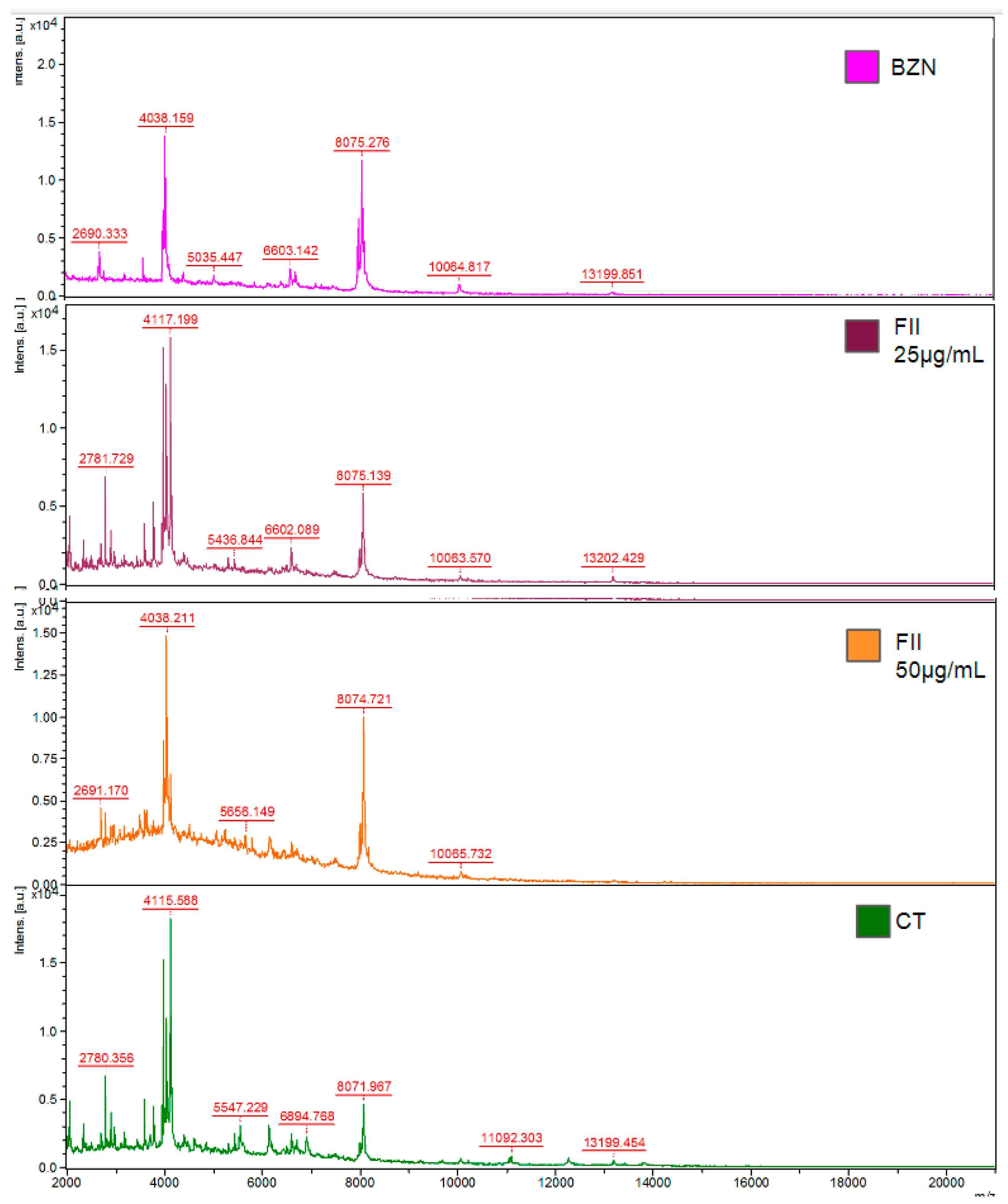
T. cruzi, we investigated the mechanism of action in the parasite. Using MALDI-ToF/MS, our studies demonstrated that FII induced significant dose-dependent alterations of the protein profile of trypomastigotes after treatment, reducing the intensities of protein signals when compared to untreated parasites, similarly to the standard drug benznidazole. This effect has also been shown for 6-brome-2’-de-N-methylaplysinopsin, a marine alkaloid isolated from the coral
Tubastrea tagusensis with potent activity against
T. cruzi [
39].
The
T. cruzi plasma membrane acts as a physical barrier between the external environment and the inner cell organelles, allowing the change of molecules, maintenance of the cell potential, and plays a vital role in the shape of the cell [
40]. In our study, the plasma membrane of trypomastigotes was rapidly permeabilized in the presence of fraction II and this was accompanied by the loss of the elongated shape of trypomastigotes to an amastigote-like form. It is known that permeabilization of plasma membrane can contribute to the leakage of intracellular components leading to the death of the cell. For example, amphotericin B, an antifungal approved drug used for the treatment of Leishmaniasis, alters the permeability of the parasite, causing pores in the membrane of the parasite [
41]. It is possible that a similar mechanism occurs in
T. cruzi treated with FII.
4. Materials and Methods
4.1. Mice
BALB/c mice were obtained from the animal breeding facility at the Instituto Adolfo Lutz, Brazil. The animals were maintained in sterilized cages under a controlled environment and received water and food ad libitum. All procedures were approved by the Animal Care and Use Committee from Instituto Adolfo Lutz – Secretary of Health of Sao Paulo State (Project number CEUA 05/2018) in agreement with the Guide for the Care and Use of Laboratory Animals from the National Academy of Sciences.
4.2. Parasites and mammalian cell maintenance
Macrophages were collected from the peritoneal cavity of BALB/c mice by washing the cavities with RPMI-1640 medium (Sigma-Aldrich, USA) supplemented with 10% (v/v) fetal calf serum (Sigma-Aldrich, USA) and were maintained at 37ºC in a 5% (v/v) CO2 humidified incubator. Trypomastigotes of T. cruzi (Y strain) were maintained in Rhesus monkey kidney cells (LLC-MK2 - ATCC CCL 7), cultivated in RPMI-1640 medium supplemented with 2% (v/v) fetal calf serum at 37ºC in a 5% (v/v) CO2 humidified incubator. The murine conjunctival cells (NCTC clone 929, ATCC) were maintained in RPMI-1640 supplemented with 10% (v/v) FBS at 37ºC in a 5% (v/v) CO2 humidified incubator.
4.3. Analytical methods
1H NMR spectra (500 MHz) were recorded on a Varian INOVA 500 spectrometer using CD3OD (Sigma-Aldrich) as the solvent and internal standard. ESI-HRMS spectra were obtained with electrospray ionization in the positive ion mode on a Bruker Daltonics MicroTOF QII spectrometer. The MALDI-TOF/MS data was analyzed on a Bruker MicroFlex spectrometer at a 20 kV accelerating voltage, in the positive mode (500 laser shots). The signals were collected in a range between m/z 2,.000 -20,.000 with the AutoXecute tool (Bruker-Daltonics).
4.4. Collection of marine corals and sediments
The collections were made by scuba diving in the São Sebastião region at CEBIMar-USP at depths ranging from 5 to 35 meters. The marine invertebrates were identified by Dr. Álvaro E. Migotto (ICMBio MMA 10186-2). The sea sediments were collected with the help of a dredge in the São Sebastião Channel (at 35 meters depth) (S 23°49’40.7" W045°24’44.7"), as well as on Buzios Island, by scuba diving (13 meters deep) (23°48’05.2"; W 45°08’;32.2").
4.5. Isolation and cultivation of microorganisms
After collection, the samples were immediately processed at the Centro de Biologia Marinha (CEBIMar), Universidade de São Paulo, under sterile conditions and the contents of the corals and sediment samples were inoculated on to 90x15 mm Petri dishes in a BOD incubator (SolidSteel, Brazil) at 25ºC, using Marine in an agar medium rich in nutrients and specific for heterotrophic marine bacteria (Marine Agar (Difco, USA). From this material, several isolates were obtained. The isolates were stored in a liquid medium specific for heterotrophic marine bacteria (Marine Broth, Difco, USA) containing 15% (v/v) DMSO (Merck, USA) at -85°C. Gram staining of bacterial isolates was done and the bacteria morphology was observed [
42].
4.6. Bacteria Identification by Mass Spectrometry (MALDI-ToF/MS)
MALDI-TOF/MS (Matrix Associated Laser Desorption-Ionization - Time of Flight) analysis was used to identify the microorganisms. The samples were extracted in 1.5 mL tubes or directly on the plate. For tube extraction, up to five colonies were suspended in 300 µL of ultrapure water (Milli-Q) and mixed by vortex. Next, 900 µL of EtOH was added, vortex mixed, and centrifuged (Eppendorf, USA) at maximum speed for 1 minute. After elimination of EtOH, 50 µL of 70% (v/v) formic acid and 50 µL of acetonitrile (100%) were added to the pellet. The tube was centrifuged for 2 min and the supernatant was dispensed in duplicates (1 µL) into a 96-well plate for MALDI-TOF/MS and air-dried. The matrix, α-cyano-4-hydroxy-cinnamic acid (HCCA) (Bruker-Daltonics), was prepared at a concentration of 50 mg/mL in 50% (v/v) acetonitrile and 50% (v/v) water with 2.5% (v/v) trifluoroacetic acid and 1 µL of the matrix was added to the dried sample. In the direct plate extraction, a few colonies were transferred to the 96-well plate and covered with the matrix as described above [
43]. A DH5-α protein extract from
Escherichia coli (Bruker-Daltonics) was added to the plate as an external control. The analyses were performed on the MALDI-TOF/MS Microflex mass spectrometer (Bruker-Daltonics) with a nitrogen laser (337 nm) operating in linear mode with delayed extraction (260 ns) at 20 kV accelerating voltage. Each spectrum was automatically collected in positive-ion mode as an average of 500 laser shots (50 laser shots at 10 different point positions). A mass range between 2,000 and 20,000 m/z (mass-to-charge ratio) was selected to collect the signals with the Auto Xecute tool of the flexcontrol acquisition software (Version 2.4; Bruker-Daltonics). Only peaks with a signal-to-noise ratio were considered. The software utilizes a logarithmically transformed similarity score that is computed by evaluating several factors. Log(score) values above 2 indicate species-level identification of bacteria. Scores between 1.7 and 2.0 suggest identification of microorganisms at the genus level. Scores below 1.7 signify that the spectrum cannot be effectively identified using the MALDI Biotyper method ([
43]
4.7. Bacterial identification by sequencing of the partial 16S rRNA gene
Some strains were not identified by MALDI- ToF/MS method and were selected for genetic sequencing. Genomic DNA from the bacterial culture was isolated using the Wizard® Genomic DNA Purification Kit, with some modifications. In this modified protocol we increased the incubation period with Proteinase K (2 hours) and used a higher volume of RNase (10 ul). The partial amplification of the 16S rRNA gene was performed with the GoTaq Master Mix kit (Promega) and universal primers for eubacteria [
44]. The PCR products were purified using the ExoSAP-IT (Thermo Fisher Scientific) and sequencing reactions were prepared using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific). A precipitation reaction with ethanol-sodium acetate was performed and the products were sequenced using the ABI 3730 DNA Analyzer, a 48-capillary analysis system (Applied Biosystems). Sequences were analyzed using BioNumerics v.8.0 software (Applied Maths, BioMerieux) in comparison with the sequences present in the Ezbiocloud and Microbenet databases for species database and also in NCBI (
http://www.ncbi.nlm.nih.gov/) using the Basic Local Alignment Search Tool (BLAST) and the 16S ribosomal RNA sequences database.
4.8. Extraction of metabolites from marine bacteria grown in agar and broth medium
4.8.1. Agar
The bacterial strains were seeded in Petri dishes (140 x 15 mm) and grown for 120 h at 25°C. Colonies were scraped with a cell scraper (Corning) and diluted in ultrapure water (Milli-Q) in a glass vial. After vortex mixing for 2 min and sonication in an ultrasonic bath for 10 min, 200 mL of EtOAc (JT Baker) were added and the solution was transferred to the ultrasonic bath for 40 min. After partitioning, the organic phase was filtered, and the solvent was removed under reduced pressure at 40°C to afford the crude EtOAc extract.
4.8.2. Broth
Colonies grown in Marine Agar (Difco) were transferred to 20 mL of liquid medium Marine Broth and cultured for 24h in a BOD incubator (SolidSteel, Brazil) at 25ºC. The optical density of this culture was measured and adjusted to 0.5 on the McFarland scale, i.e. 1.5x108 CFU/mL. The culture was kept at 25°C in an incubator (SolidSteel, Brazil) with an orbital shaker or static for a total of 120h. After this period, the cultures were transferred to 50 mL plastic conical tubes and centrifuged at 4000g for 20 min. The cell precipitate was separated from the supernatant and concentrated in 100 mL ultrapure water (Milli-Q). After vortex mixing for 2 min and sonication in an ultrasonic bath for 10 min, 100 mL EtOAc was added to this aqueous phase. The extraction procedure was then followed.
4.9. Bio-Guided Fractionation of Mesoflavibacter zeaxanthinifaciens organic extract
Fractionation of EtOAc extract of Mesoflavibacter zeaxanthinifaciens (500 mg) was performed using a solid phase extraction cartridge (SPE Bond Elute C18 - Agilent). The cartridge was activated with MeOH, conditioned with MeOH:H2O (9:1) and after application of the crude EtOAc extract (500 mg), four fractions (F0 – 158,2 mg, FI– 11,9 mg, FII 10,2 and FIII – 161,6 mg) were collected using different mixtures of MeOH:H2O (7:3, 4:6, 9:13:2 and pure MeOH, respectively) as eluents. Fractions were dried over reduced pressure and subjected to evaluation of anti-T. cruzi activity.
4.10. Evaluation of the anti-Trypanosoma cruzi activity
LLC-MK2-derived trypomastigotes were seeded (1 x 106 cells/well) in 96-well plates and incubated with the extracts/compounds serially diluted 2-fold (200 to 1.5 µg/mL), during 24h in RPMI 1640 medium at 37ºC in a 5% (v/v) CO2 humidified incubator. Subsequently, resazurin (0.011% (w/v) in PBS) was added to check for parasite viability for 24h. The optical density was determined in the FilterMax F5 (Molecular Devices) at λ570 nm. Benznidazole was used as the standard drug [
45].
For anti-amastigote assay, mouse peritoneal macrophages (1 x 10
5 cells/well, were seeded in 16-well chamber slide (NUNC, Thermo Fisher Scientific) and infected with trypomastigotes (10:1 parasite:macrophage ratio). After 2h, the extracts / compounds were diluted in different concentrations (200 to 1.5 µg/mL) and incubated with infected macrophages for 48h at 37ºC in a 5% (v/v) CO2 humidified incubator. Finally, slides were fixed with methanol (100%), stained with Giemsa and observed under a light microscope (EVOS M5000, Termo, USA) with digital image acquirement. The 50% inhibitory concentration (IC
50) values were determined by the infection index. Benznidazole was used as standard drug [
39,
45]
4.11. Cytotoxicity against mammalian cells
Fibroblast NCTC cells (clone 929) (6 x 10
4 cells/well) were seeded in 96-well plates and incubated with the extracts / compounds (1.5 to 200 µg/mL) for 48h at 37ºC in a 5% (v/v) CO
2 humidified incubator. The 50% cytotoxic concentration (CC
50) was determined by the MTT colorimetric assay, as described previously [
39].
4.12. Hemolytic activity
Whole blood was collected from male BALB/c (25 g) mice after euthanasia. Erythrocytes were collected by centrifugation and used to prepare a 3% (v/v) solution in PBS. In a round-bottomed titration microplate, the fraction obtained from active extract from
Mesoflavibacter zeaxanthinifaciens (FII), was dispensed in serial dilutions of concentrations from 200 to 1.5 µg/mL. Then the suspension of erythrocytes (100 µL/well) was incubated for 2 hours at 24°C and incubated for 2h at 25ºC in a BOD incubator (Solidsteel, Brazil). The supernatant was collected, and the optical density was determined at λ570 nm (FilterMax F5 Multi-Mode Microplate Reader, Molecular Devices). The maximum hemolysis was obtained using hemocytes suspended in ultrapure distilled water pure distilled water (Milli-Q) (positive control) and untreated hemocytes as the negative control [
46].
4.13. Evaluation of T. cruzi trypomastigotes protein profile
Trypomastigotes (1x107 /well) were treated with FII fraction from Mesoflavibacter zeaxanthinifaciens (25 µg/mL and 50 µg/mL) or benznidazole (40 µM) for 24 hours in RPMI medium. After this period, the sample suspensions from the expanded cultures were centrifuged, the supernatant removed, and the precipitate was washed twice in Milli-Q water. The precipitate was resuspended in 300 µL of Milli-Q water before adding 900 µL of 70% EtOH. After further centrifugation, 20 µL of 70% formic acid and 20 µL of acetonitrile were added to the precipitate and the solution was vortexed and centrifuged. Each centrifugation step was performed at 10,000 g for 10 min at room temperature. Untreated parasites were used as control.
The supernatant was dispensed (3 µL) in duplicates into a 96-well steel plate for MALDI-ToF/MS (Bruker-Daltonics) and dried at room temperature. The matrix, α-cyano-4- hydroxy-cinnamic acid (HCCA) (Bruker-Daltonics), was prepared at a concentration of 50 mg/mL in 50% acetonitrile and 50% H2O with 2.5% TFA, and was added (1 µL) on the plate. A DH5-alpha protein extract from
Escherichia coli (Bruker-Daltonics) was added to the plate for external control. The analyses were performed on a Bruker-Daltonics Microflex MALDI-ToF/MS mass spectrometer with a nitrogen laser (337 nm) operating in linear mode with delayed extraction (260 ns) at 20 kV accelerating voltage. Each spectrum was automatically collected in positive ion mode as an average of 500 laser shots (50 laser shots at 10 different point positions). A mass range between 3,000 and 20,000 m/z (mass-to-charge ratio) was selected to collect the signals with the Auto Xecute tool of the FlexControl acquisition software (Version 2.4; Bruker-Daltonics). Only peaks with signal-to-noise ratio were considered [
47,
48].
4.14. Evaluation of Plasma Membrane Permeability
Sytox® Green, a fluorescent nucleic acid marker, impermeable to viable cells, was used to evaluate possible changes in the permeability of the plasma membrane. T. cruzi
trypomastigotes were added to 96-well black microtiter plates (2x106 parasites/well) and incubated with Sytox® Green (1 µM) in HBSS medium supplemented with NaHCO
3 (4.2 mM) and D-glucose (10 mM). Subsequently, a basal reading of the plate was done, and the FII fraction from
Mesoflavibacter zeaxanthinifaciens was added (t=0) at IC
50 value and the plate was incubated at 24ºC for 15 min [
48]. The fluorescence signal was monitored every 20 min for 180 min. Measurements were made in a spectrofluorometer (FilterMax F5 Multi-Mode Microplate Reader) with excitation filters of λ485 nm and emission filters of λ535 nm. The maximum permeabilization was obtained in the presence of 0.5% (v/v) Triton X-100, and untreated parasites were used as negative control (100% viability, integral membrane) [
48]. Digital Images of the trypomastigotes after treatment with FII fraction from
Mesoflavibacter zeaxanthinifaciens were obtained in a digital fluorescence microscope (EVOS M 5000, Thermo, USA) using SYTOX Green [
49].
4.15. Statistical Analysis
IC50 and CC50 values were calculated from sigmoidal dose-response curves. Unless otherwise stated, the data reported were the mean ± standard error of at least two independent experiments performed with duplicate samples. For hemolytic activity, One-way ANOVA with Tukey’s Multiple Comparison test was applied for significance (p value< 0.05) using GraphPad Prism 6.0 software. The samples were tested in duplicate/triplicate and the experiments were repeated at least twice.
Figure 1.
Map comprising the Blue Amazon area of Brazil. Orange arrow indicates the approximate location where samples were collected by scuba diving.
Figure 1.
Map comprising the Blue Amazon area of Brazil. Orange arrow indicates the approximate location where samples were collected by scuba diving.
Figure 2.
Marine invertebrates collected in São Sebastião Channel and Buzios Island, São Paulo, Brazil. TC: Tubastraea coccinea; MH:Mussismilia hispida; MD: Madracis decactis; Photos from Álvaro E. Migotto and Marcelo Visentini Kitahara. Available at The Cifonauta Image Bank (University of São Paulo,
http://cifonauta.cebimar.usp.br/).
Figure 2.
Marine invertebrates collected in São Sebastião Channel and Buzios Island, São Paulo, Brazil. TC: Tubastraea coccinea; MH:Mussismilia hispida; MD: Madracis decactis; Photos from Álvaro E. Migotto and Marcelo Visentini Kitahara. Available at The Cifonauta Image Bank (University of São Paulo,
http://cifonauta.cebimar.usp.br/).
Figure 3.
High resolution mass spectra of the fraction II of Mesoflavibacter zeaxanthinifaciens EtOAc extract (FII) obtained in spectrometer (Bruker-Daltonics MicroTOF QII), using an electrospray ionization source, operating in negative mode via direct injection.
Figure 3.
High resolution mass spectra of the fraction II of Mesoflavibacter zeaxanthinifaciens EtOAc extract (FII) obtained in spectrometer (Bruker-Daltonics MicroTOF QII), using an electrospray ionization source, operating in negative mode via direct injection.
Figure 4.
Molecular structures of the iso chain fatty acids 1 – 4 identified in the bioactive fraction II from Mesoflavibacter zeaxanthinifaciens EtOAc extract.
Figure 4.
Molecular structures of the iso chain fatty acids 1 – 4 identified in the bioactive fraction II from Mesoflavibacter zeaxanthinifaciens EtOAc extract.
Figure 5.
Hemolytic activity of fraction II of Mesoflavibacter zeaxanthinifaciens EtOAc extract (FII) in murine erythrocytes after 2 h incubation. CT- (negative control); CT+ (positive control). The absorbance was measured in a plate spectrophotometer (λ=570 nm) (FilterMax F5, Molecular Devices).***p <0.005.
Figure 5.
Hemolytic activity of fraction II of Mesoflavibacter zeaxanthinifaciens EtOAc extract (FII) in murine erythrocytes after 2 h incubation. CT- (negative control); CT+ (positive control). The absorbance was measured in a plate spectrophotometer (λ=570 nm) (FilterMax F5, Molecular Devices).***p <0.005.
Figure 6.
Evaluation of the protein profile of trypanomastigotes of T. cruzi in the presence of fraction II of Mesoflavibacter zeaxanthinifaciens EtOAc extract (FII) by MALDI-TOF/MS Microflex. Spectrum of mass of trypanomastigotes treated FII (20 and 50 μg/mL), treated with benznidazole (BZN-40 μM) and untreated control (CT). The parasites were treated for 24 h and the protein profile was compared to untreated parasites.
Figure 6.
Evaluation of the protein profile of trypanomastigotes of T. cruzi in the presence of fraction II of Mesoflavibacter zeaxanthinifaciens EtOAc extract (FII) by MALDI-TOF/MS Microflex. Spectrum of mass of trypanomastigotes treated FII (20 and 50 μg/mL), treated with benznidazole (BZN-40 μM) and untreated control (CT). The parasites were treated for 24 h and the protein profile was compared to untreated parasites.
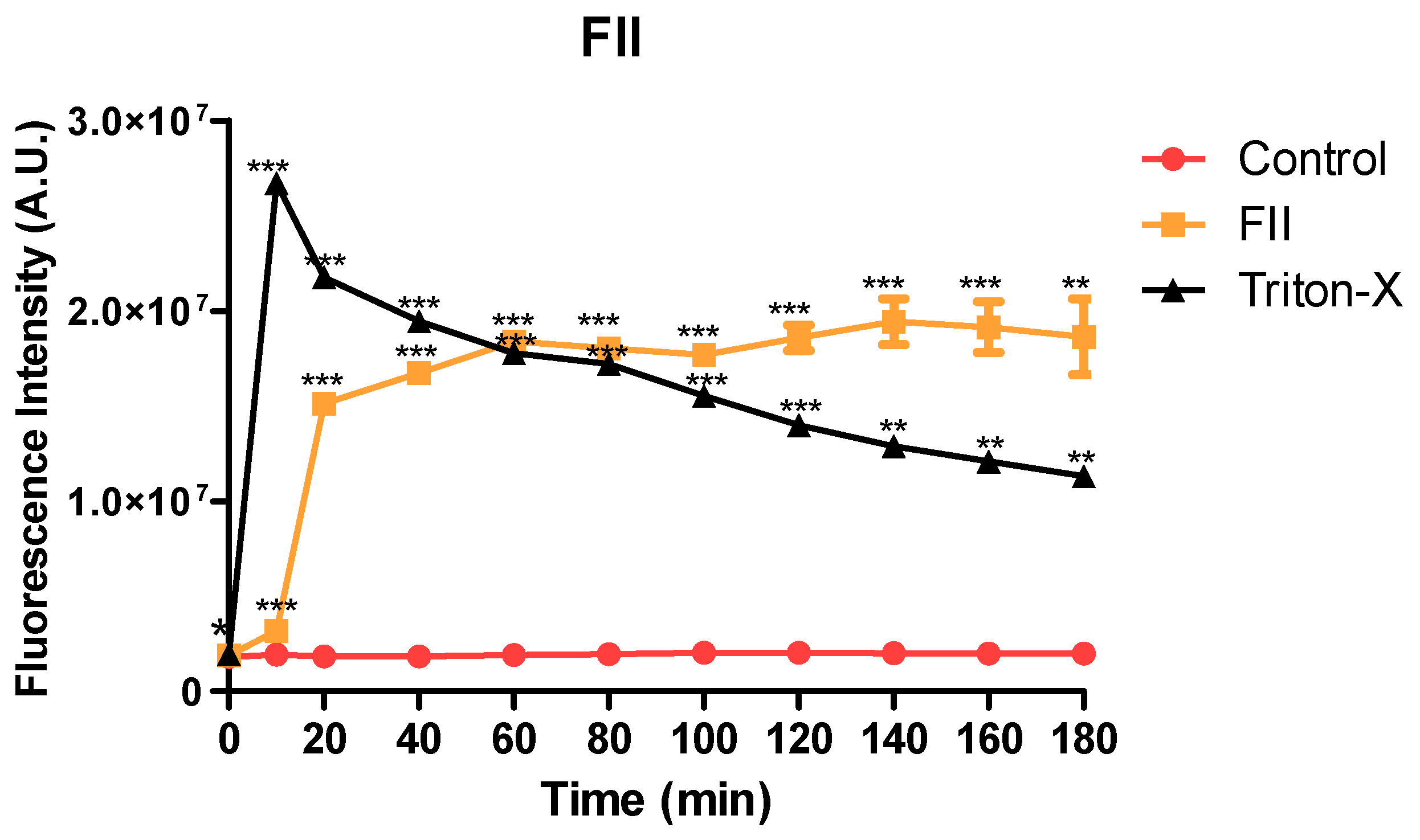
Figure 7.
Evaluation of membrane permeability of trypomastigotes after treatment with fraction II from Mesoflavibacter zeaxanthinifaciens EtOAc extract (FII), using the probe SYTOX Green dye and spectrofluorimeter. Untreated parasites (control group) and treated with 0.5 % Triton X-100 were eavaluated as minimal and maximal permeabilization, respectively. ***P<0.0001; ** p< 0,01.
Figure 7.
Evaluation of membrane permeability of trypomastigotes after treatment with fraction II from Mesoflavibacter zeaxanthinifaciens EtOAc extract (FII), using the probe SYTOX Green dye and spectrofluorimeter. Untreated parasites (control group) and treated with 0.5 % Triton X-100 were eavaluated as minimal and maximal permeabilization, respectively. ***P<0.0001; ** p< 0,01.
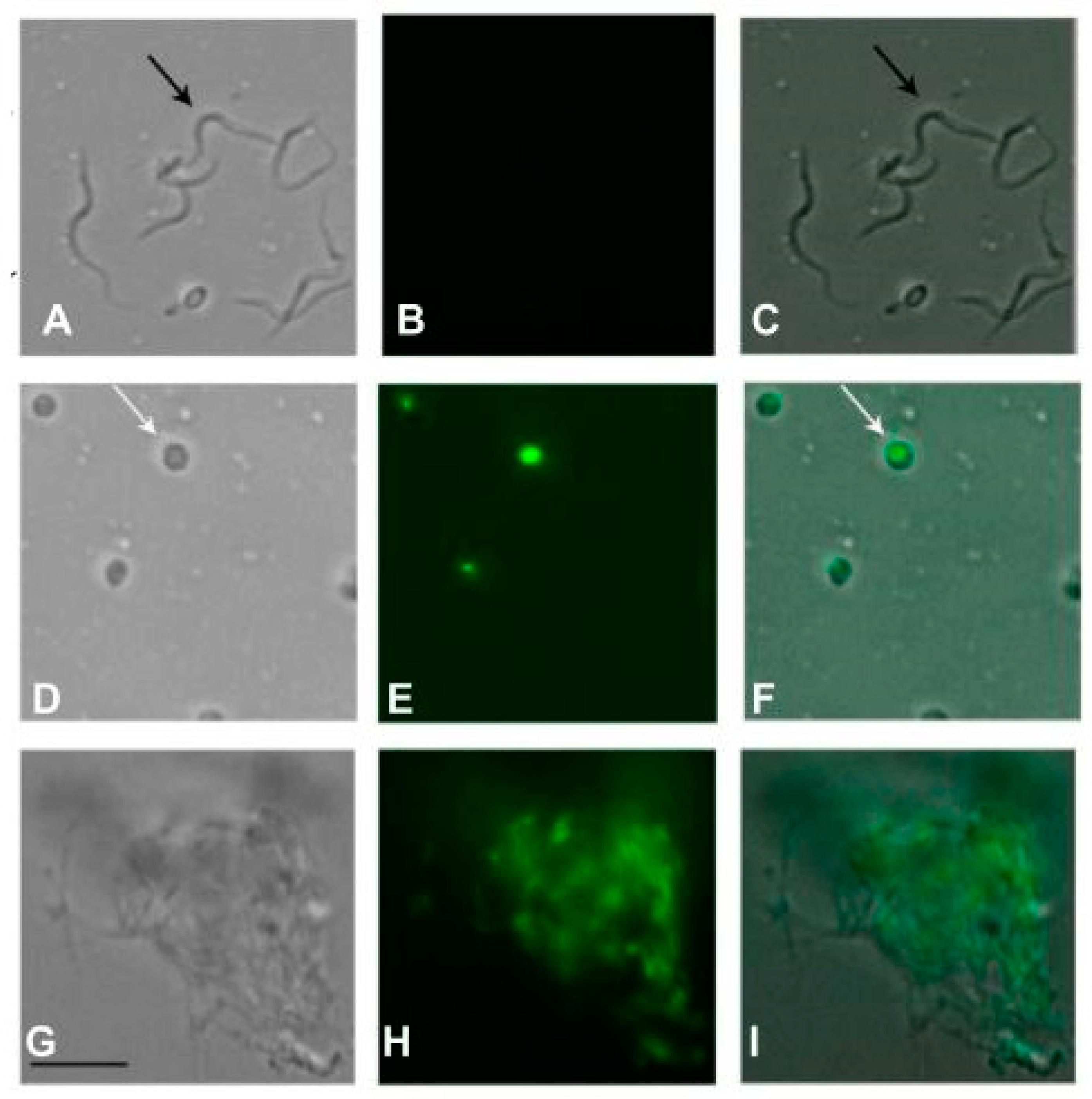
Figure 8.
Digital fluorescence images (100× magnification) obtained after treatment of trypomastigotes with fraction II of Mesoflavibacter zeaxanthinifaciens EtOAc extract (FII) (D, E, F). Black arrows represent elongated forms of T. cruzi (trypomastigotes) and white arrows represent rounded-forms (amastigote-like forms) after treatment. SYTOX Green dye was used to evaluate the permeabilization effect. Untreated parasites were used as a negative control (A,B,C) and parasites treated with 0.5 % Triton X-100 were used as a positive control for maximal permeabilization (G, H, I). A representative experiment is shown.
Figure 8.
Digital fluorescence images (100× magnification) obtained after treatment of trypomastigotes with fraction II of Mesoflavibacter zeaxanthinifaciens EtOAc extract (FII) (D, E, F). Black arrows represent elongated forms of T. cruzi (trypomastigotes) and white arrows represent rounded-forms (amastigote-like forms) after treatment. SYTOX Green dye was used to evaluate the permeabilization effect. Untreated parasites were used as a negative control (A,B,C) and parasites treated with 0.5 % Triton X-100 were used as a positive control for maximal permeabilization (G, H, I). A representative experiment is shown.
Table 1.
Origin of the microorganisms associated with corals or sediments.
Table 1.
Origin of the microorganisms associated with corals or sediments.
| Acronym |
Origin |
Depth (meters) |
| TC |
Tubastraea coccinea |
5 |
| MH |
Mussismilia hispida |
10 |
| MD |
Madracis decactis |
10 |
| SCSB |
Sediment of the São Sabastião Channel |
35 |
| SIBUZ |
Sediment of Buzios Island |
13 |
Table 2.
Identification and morphology of bacteria isolated from corals and marine sediments according to MALDI-TOF/MS score or sequencing.
Table 2.
Identification and morphology of bacteria isolated from corals and marine sediments according to MALDI-TOF/MS score or sequencing.
| Acronym |
Morphology and Gram |
Identification |
Method |
| TC 2.0.2 |
Bacillus (-) |
Alteromonas macleodi |
S |
| TC 2.2 |
Bacillus (-) |
Vibrio alginolyticus |
MS |
| MH 3.0 |
Bacillus (-) |
Vibrio harveyi |
MS |
| MH 3.3 |
Bacillus (-) |
Vibrio alginolyticus |
MS |
| MD 5.0 |
Coccobacillus (-) |
Vibrio harveyi |
MS |
| SCSB 6.0.2.1 |
Bacillus (+) |
Shewanella pneumatophori |
S |
| SCSB 6.0.2.2 |
Bacillus (+) |
Mesoflavibacter zeaxanthinifaciens |
S |
| SCSB 6.1 |
Bacillus (-) |
Bacillus megaterium |
MS |
| SCSB 6.2 |
Bacillus (-) |
Vibrio harveyi |
MS |
| SIBUZ 7 |
Coccobacillus (-) |
Vibrio harveyi |
MS |
| SIBUZ 7.2.2 |
Coccobacillus (-) |
Halomonas aquamarina |
S |
Table 3.
Identification of marine strains by partial sequencing of the 16S rRNA gene, according to the BLAST 16S rRNA sequences database.
Table 3.
Identification of marine strains by partial sequencing of the 16S rRNA gene, according to the BLAST 16S rRNA sequences database.
| Acronym |
Microorganism |
Identity (%) |
Query Cover (%) |
Acc number |
| TC 2.0.2 |
Alteromonas macleodii |
99.78 |
99 |
OP163900 |
| SCSB 6.0.2.1 |
Shewanella pneumatophori |
99.72 |
99 |
OP163959 |
| SCSB 6.0.2.2 |
Mesoflavibacter zeaxanthinifaciens |
100 |
96 |
OR479885 |
| SIBUZ 7.2.2 |
Halomonas aquamarina |
99.72 |
100 |
OP163958 |
Table 4.
Quantification of the 50% inhibitory concentration (IC50) of microbial metabolites against T. cruzi trypomastigotes, with the respective yields of extracted metabolites.
Table 4.
Quantification of the 50% inhibitory concentration (IC50) of microbial metabolites against T. cruzi trypomastigotes, with the respective yields of extracted metabolites.
| Acronym |
Strain |
Mass of metabolites (mg) |
IC50 ± SD (μg/mL) |
| TC 2.0.2 |
Alteromonas macleodi |
2.6 |
31.2 ± 2.6 |
| TC 2.2 |
Vibrio alginolyticus |
2.1 |
13.9 ± 3.0 |
| MH 3.0 |
Vibrio harveyi |
11.4 |
51.3 ± 1.1 |
| MH 3.3 |
Vibrio alginolyticus |
8.4 |
25.4 ± 1.2 |
| MD 5.0 |
Vibrio harveyi |
3.4 |
18.3 ± 2.6 |
| SCSB 6.0.2.1 |
Shewanella pneumatophori |
5.5 |
15.1 ± 3.3 |
| SCSB 6.0.2.2 |
Mesoflavibacter zeaxanthinifaciens |
4.8 |
17.9 ± 0.7 |
| SCSB 6.1 |
Bacillus megaterium |
2.7 |
59.9 ± 0.1 |
| SCSB 6.2 |
Vibrio harveyi |
3.9 |
32.8 ± 3.9 |
| SIBUZ 7 |
Vibrio harveyi |
8.9 |
8.0 ± 0.7 |
| SIBUZ 7.2.2 |
Halomonas aquamarina |
3.2 |
15.4 ± 0.5 |
Table 5.
Evaluation of the 50% Inhibitory Concentration (IC50) against trypomastigotes and amastigotes of T. cruzi and the 50% Cytotoxic Concentration (CC50) of fraction II from Mesoflavibacter zeaxanthinifaciens EtOAc extract and the positive control benznidazole.
Table 5.
Evaluation of the 50% Inhibitory Concentration (IC50) against trypomastigotes and amastigotes of T. cruzi and the 50% Cytotoxic Concentration (CC50) of fraction II from Mesoflavibacter zeaxanthinifaciens EtOAc extract and the positive control benznidazole.
| Compound |
Trypomastigotes(IC50±SD) |
Amastigotes (IC50±SD) |
Cytotoxicity (CC50±SD) |
| Fraction II |
17.7 ± 3.5 μg/mL |
23.8 ± 2.7 μg/mL |
>200 μg/mL |
| Benznidazole |
3.6 ± 0.9 μg/mL |
1.4 ± 0.5 μg/mL |
49.4 μg/mL |