Submitted:
21 February 2024
Posted:
22 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
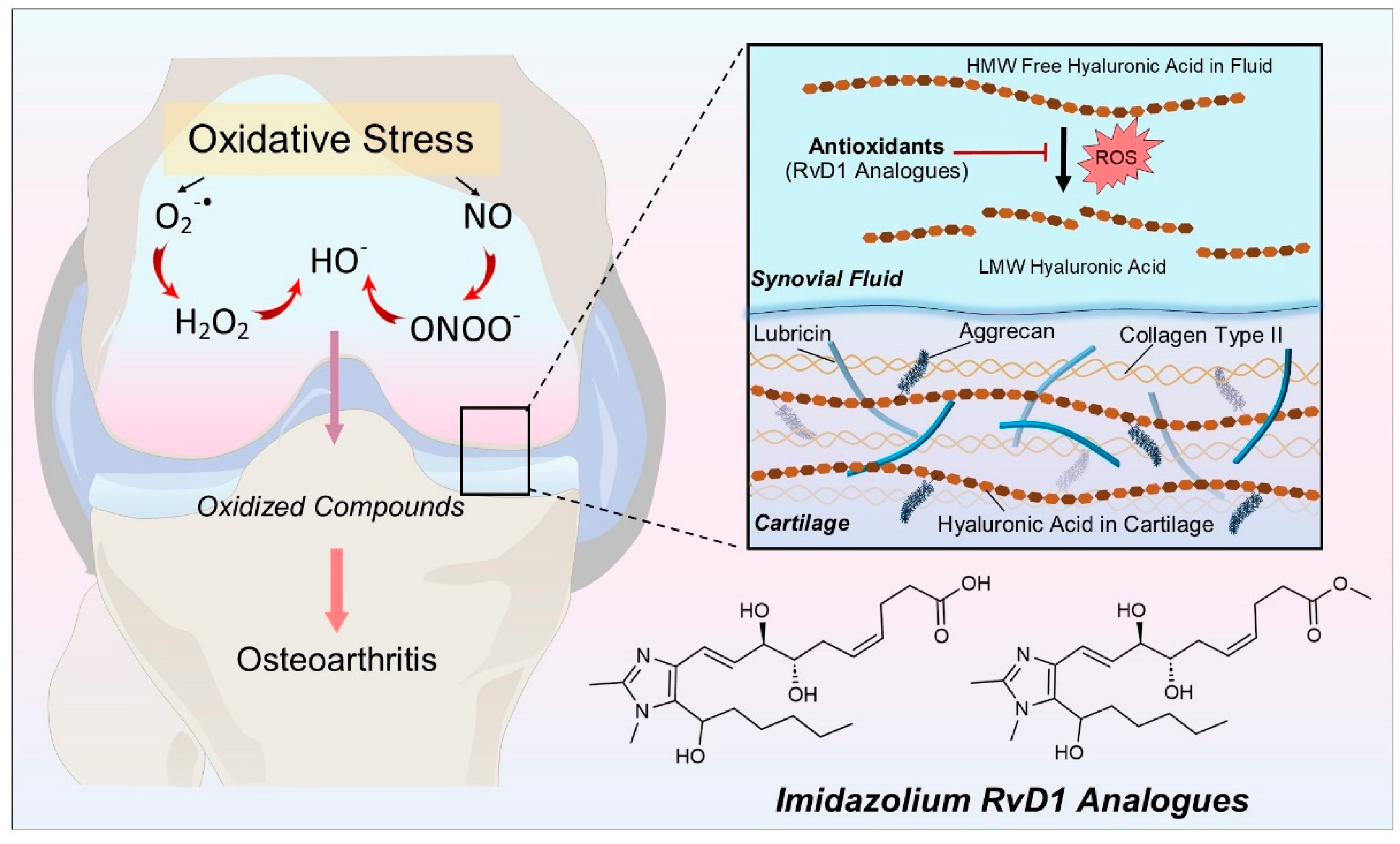
2. Materials and Methods
2.1. Materials
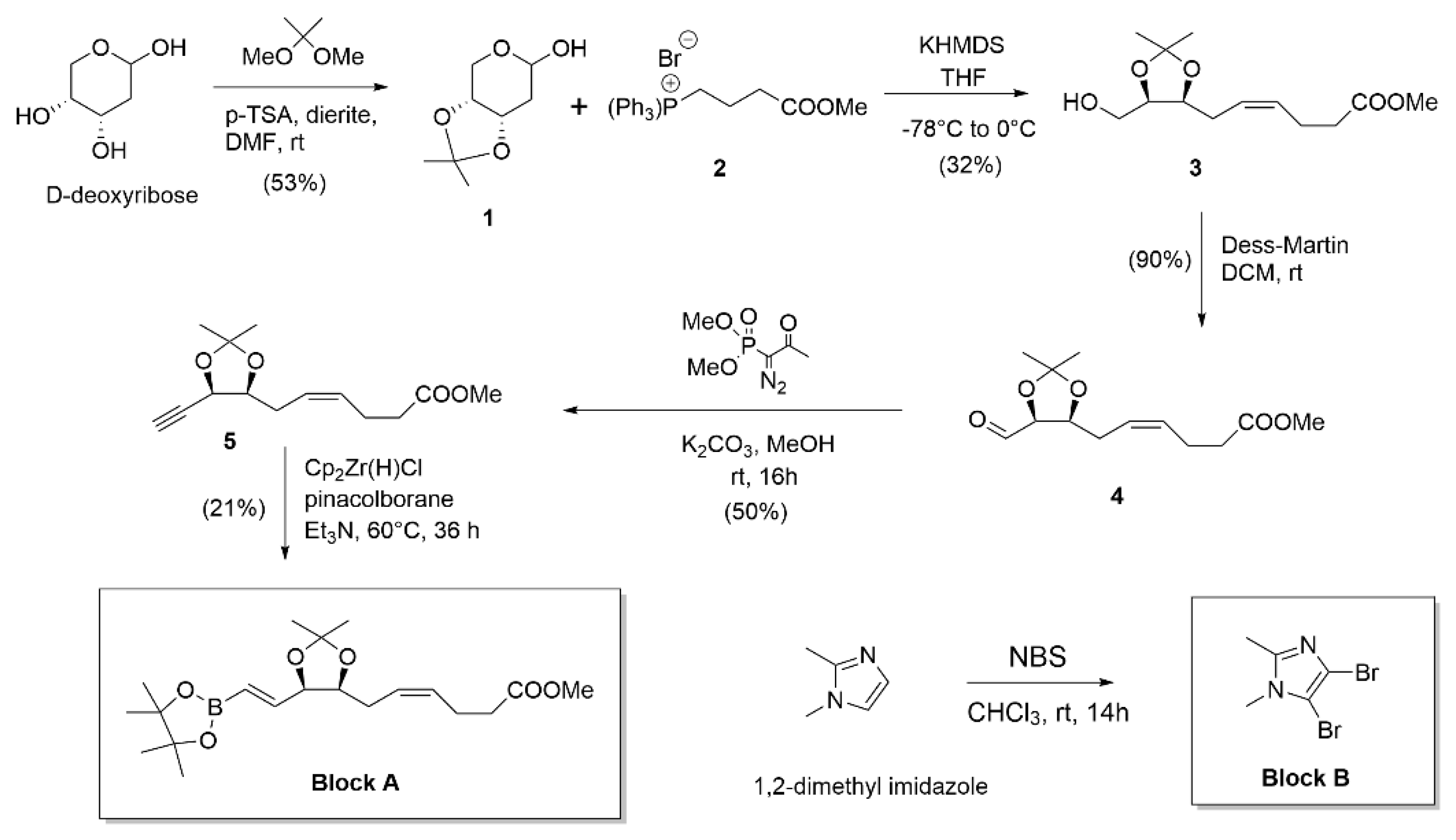
2.2. Analogues synthesis
2.2.1. Block A synthesis
2.2.2. Block B synthesis
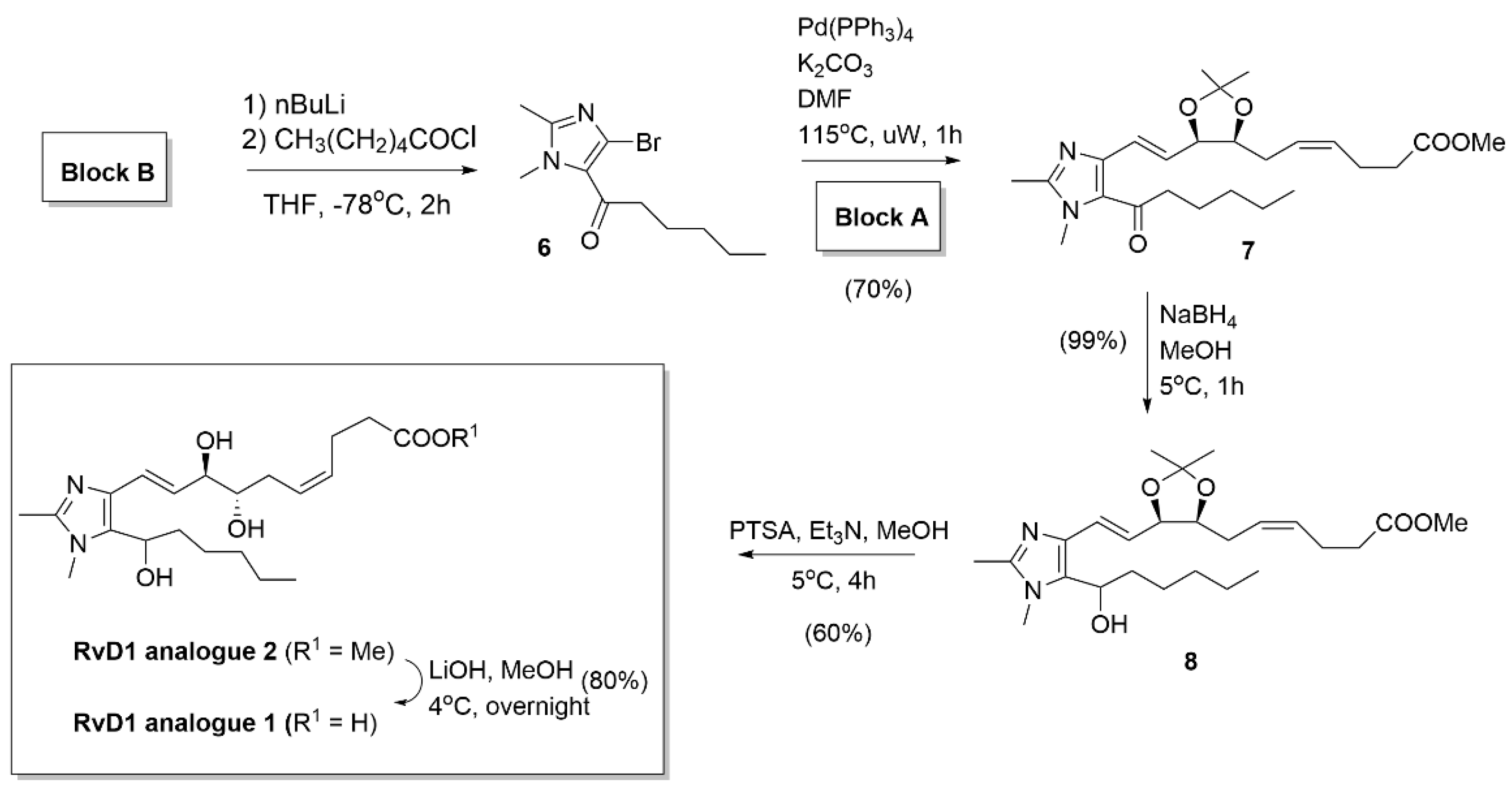
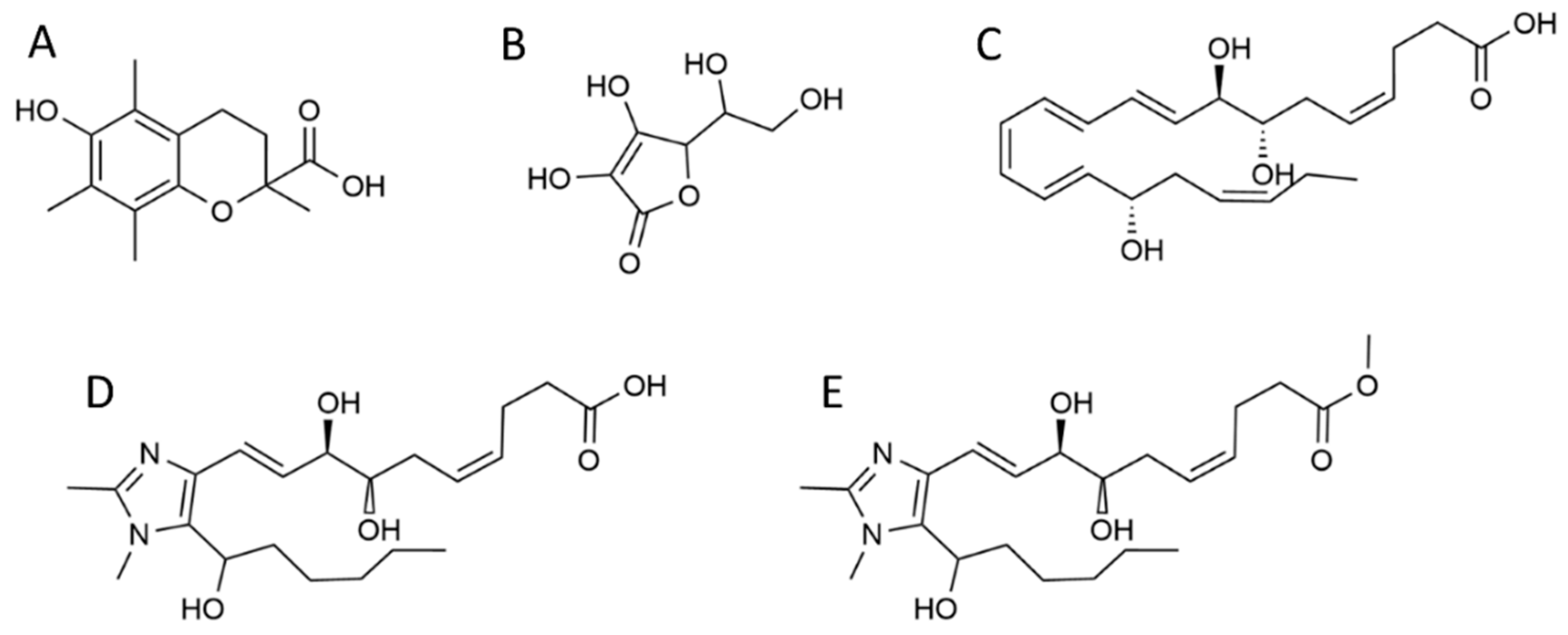
2.2.3. RvD1 analogues 1 and 2 syntheses
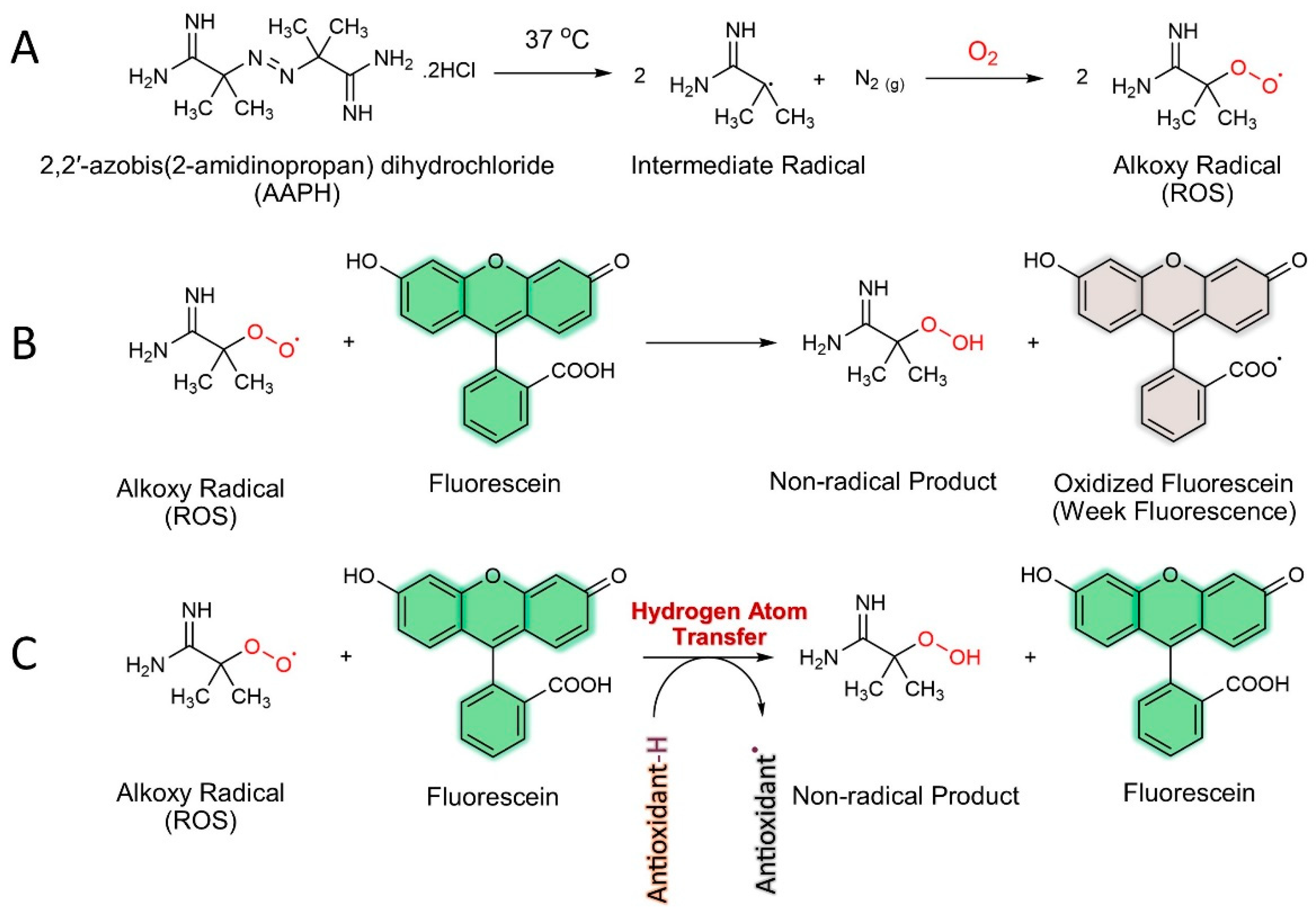
2.3. Antioxidant Activity Assays
2.3.1. Oxygen Radical Absorbance Capacity (ORAC) Assay
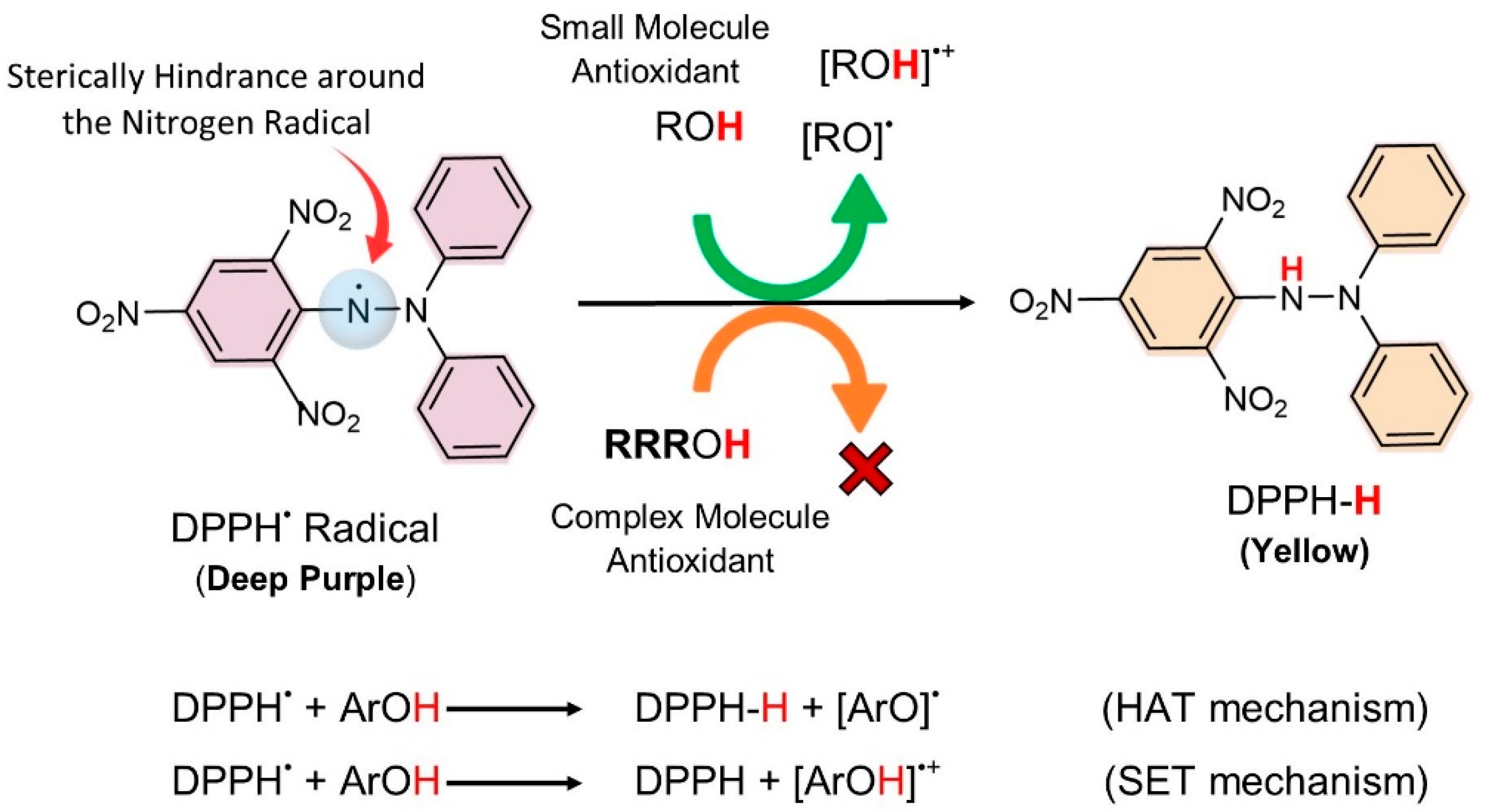
2.3.2. DPPH Assay
2.3.3. FRAP Assay
2.3.4. Hydroxyl Radical Scavenging (HRS)
2.4. Hyaluronic Acid Degradation Study
2.5. Hyaluronic Acid Analysis by Gel Permeation Chromatography
2.6. Computational Studies
3. Results and Discussion
3.1. Antioxidants and Radical Scavenging Activities of Resolvin Analogues
3.1.1. ORAC Assay
| Antioxidant | Analogue 1 | Analogue 2 | RvD1 | Ascorbic acid | Trolox |
| TE Value | 2.17 | 1.87 | 1.60 | 0.40 | 1 |
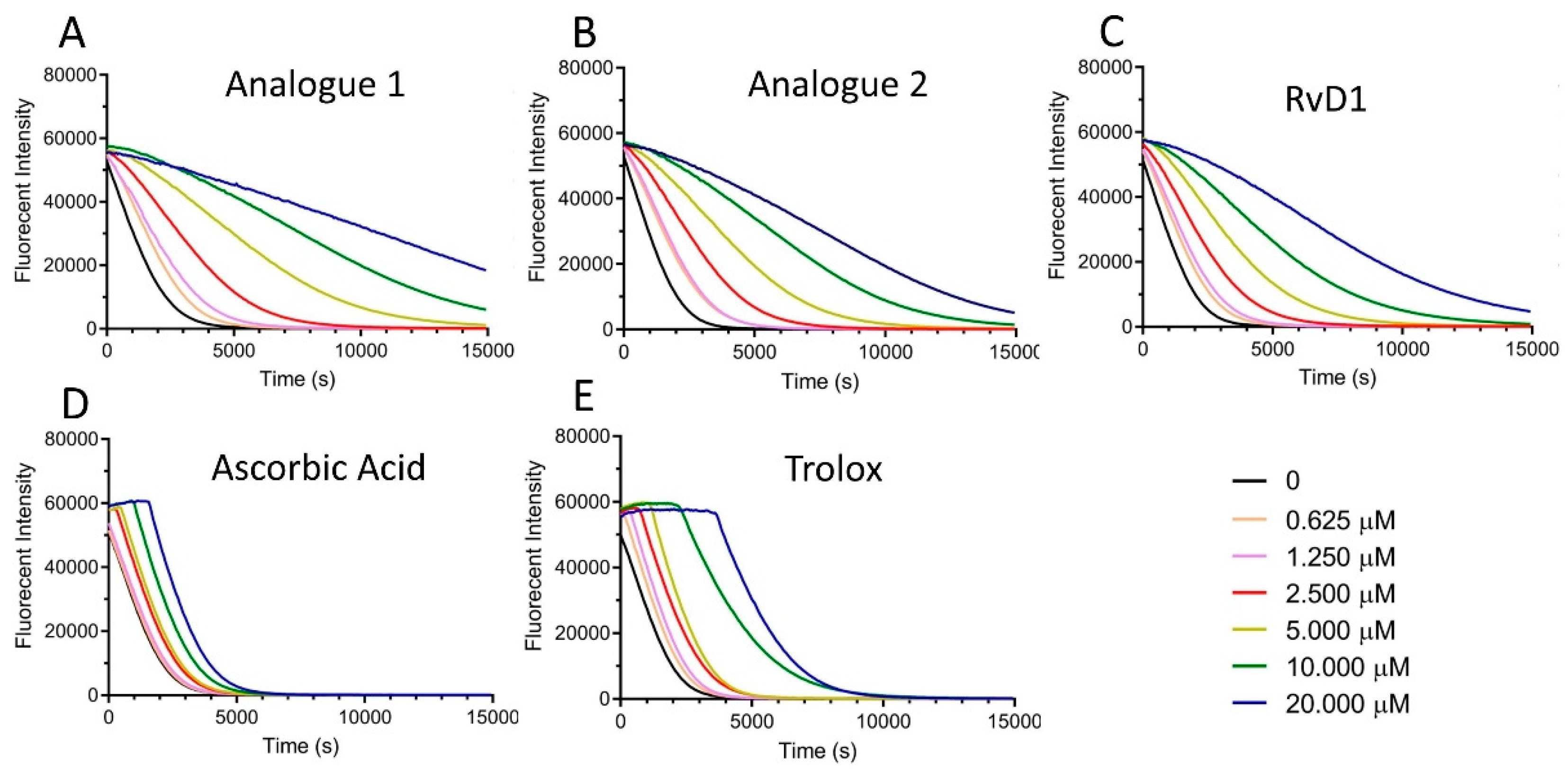
3.1.2. DPPH Assay
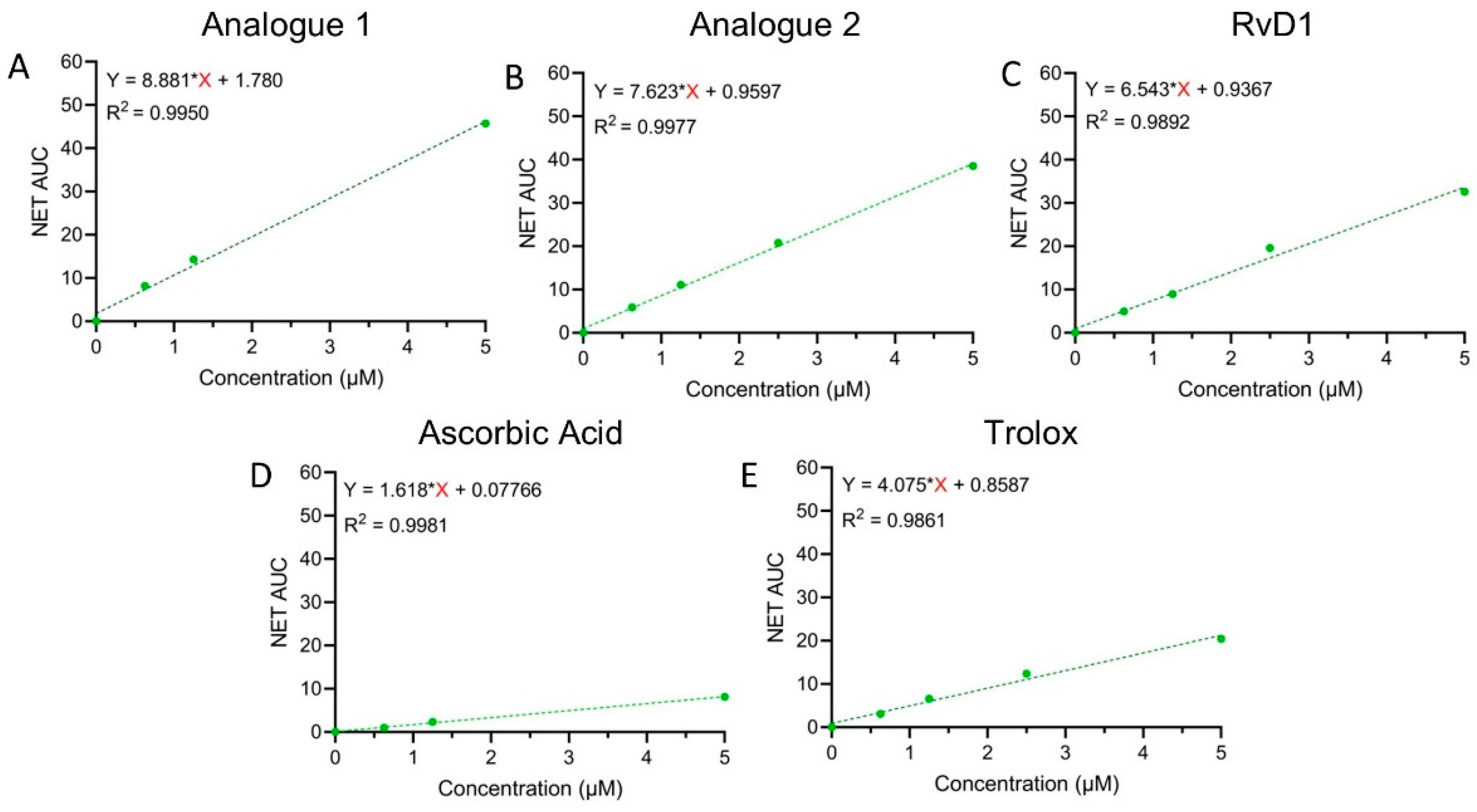
3.1.3. FRAP Assay
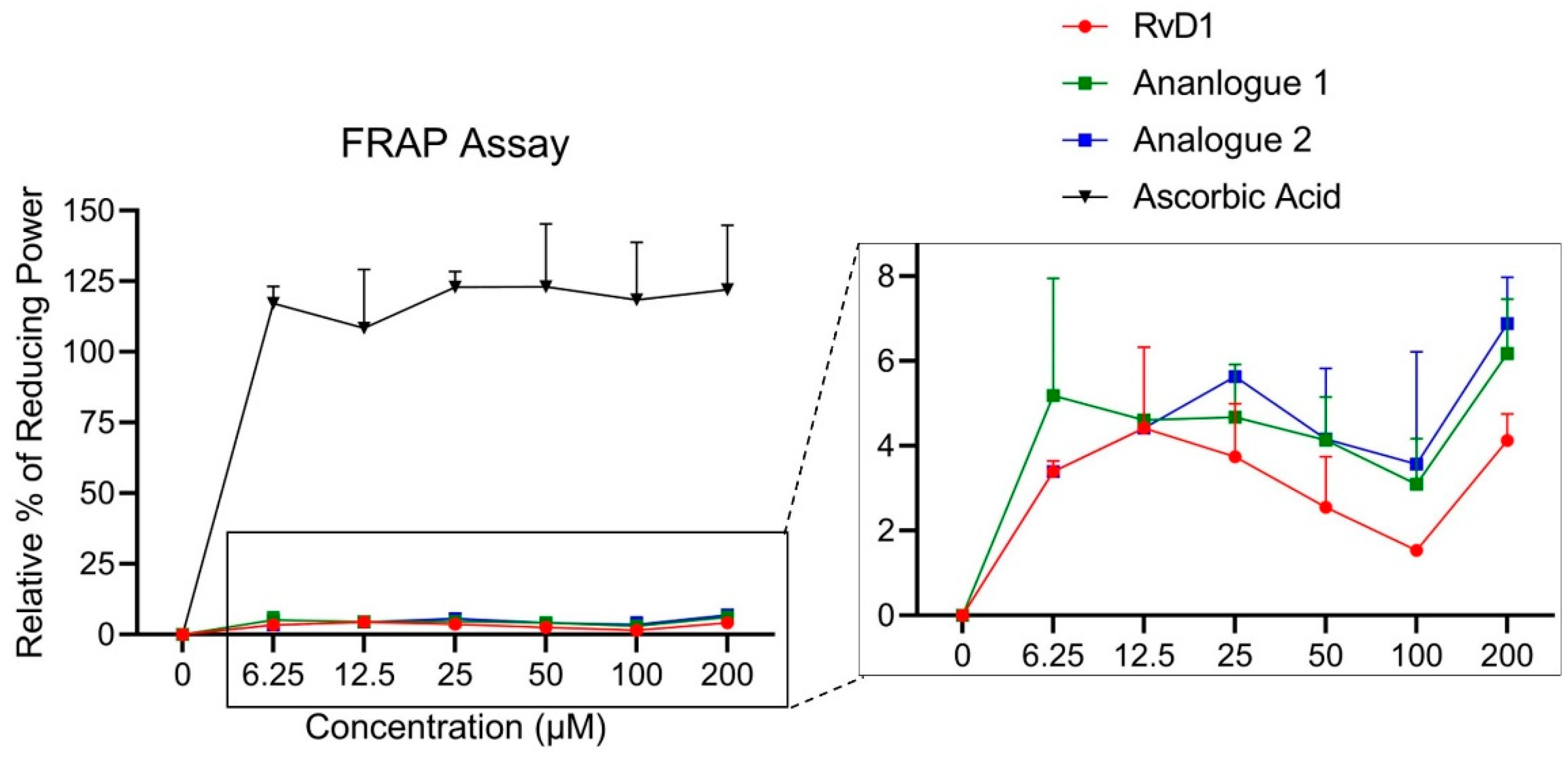
3.1.4. Hydroxyl Radical Scavenging (HRS)
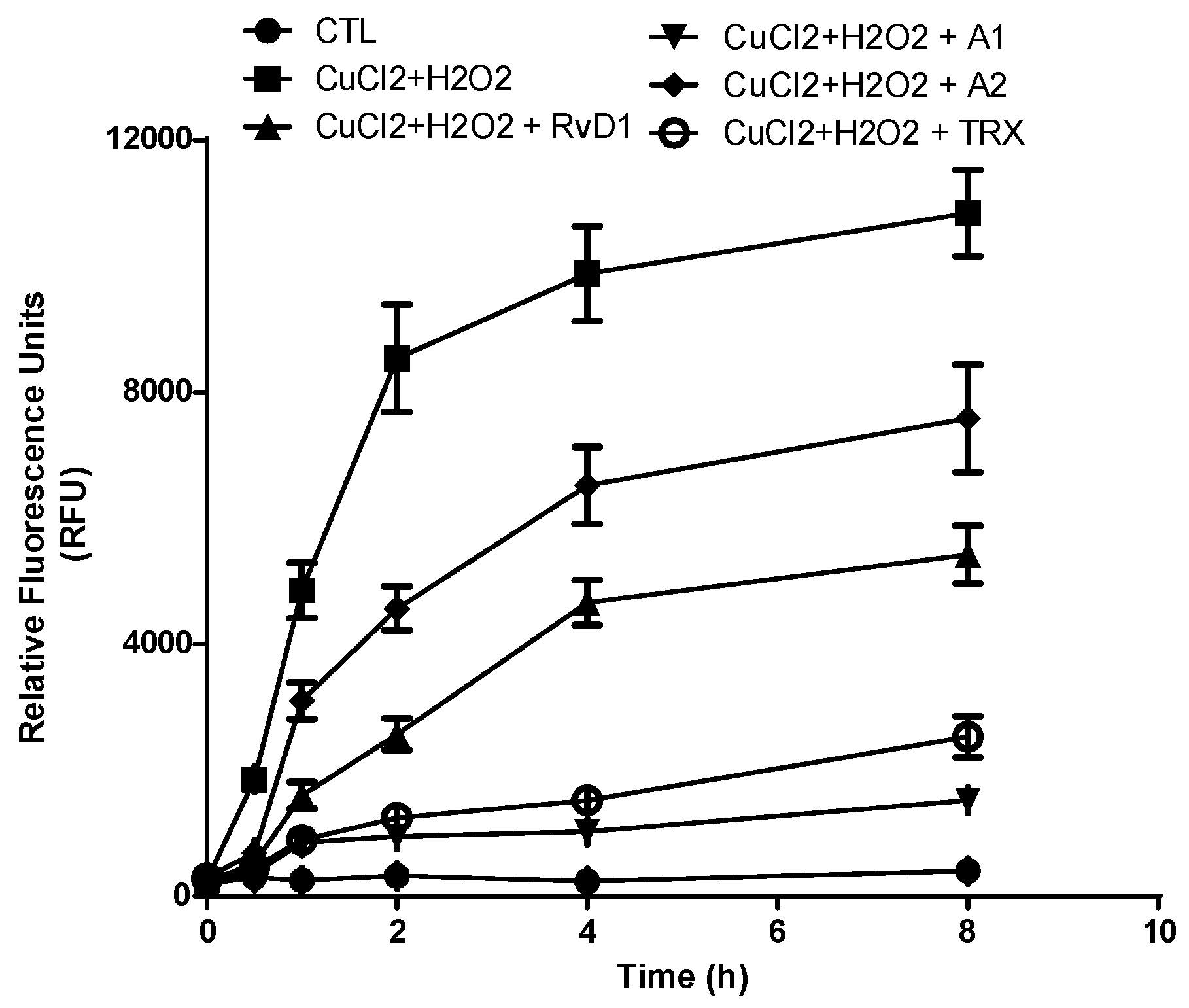
3.2. Protection of Hyaluronic Acid against ROS
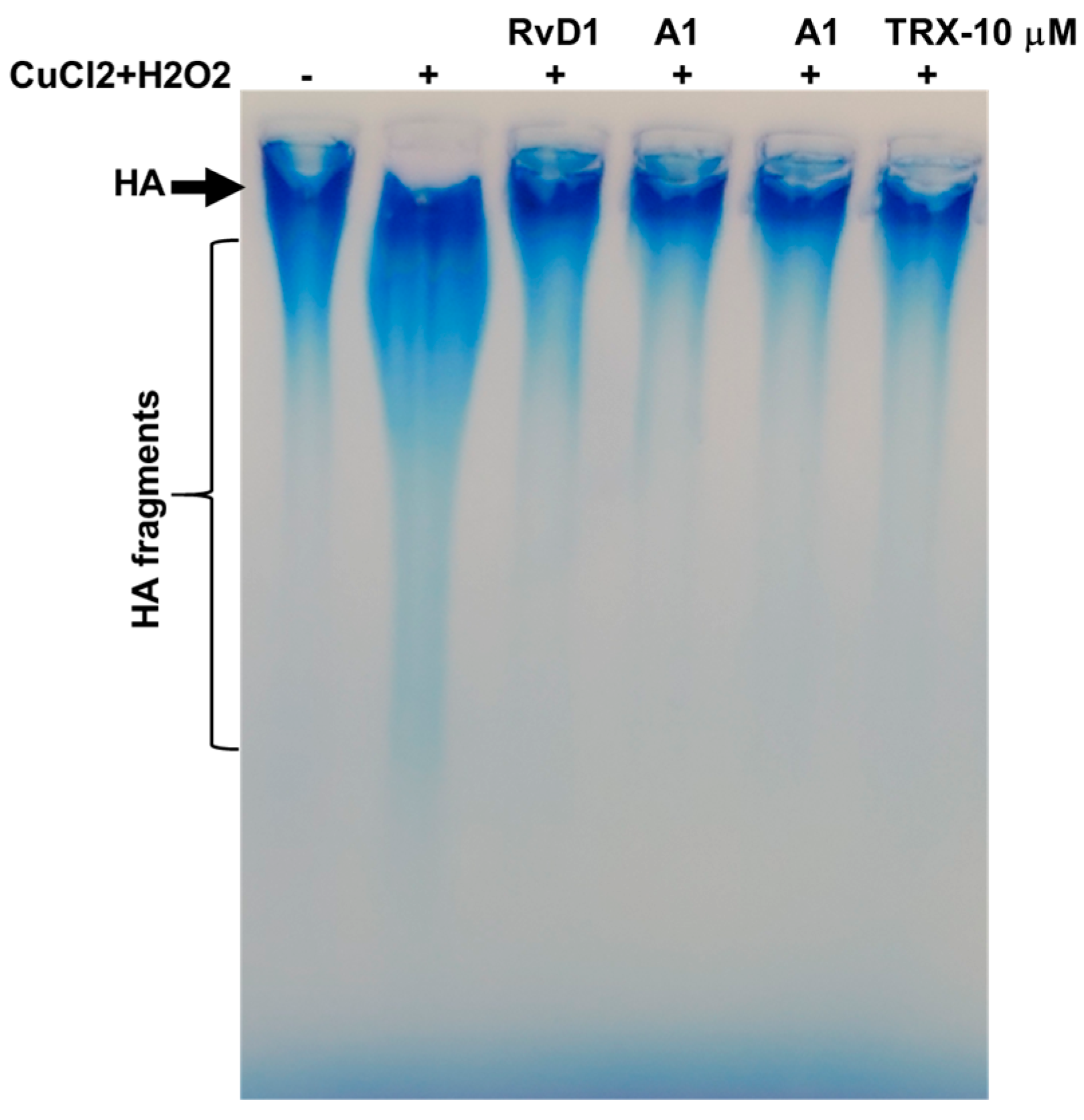
3.2.1. Agarose Gel Electrophoresis
3.2.2. Gel Permeation Chromatography
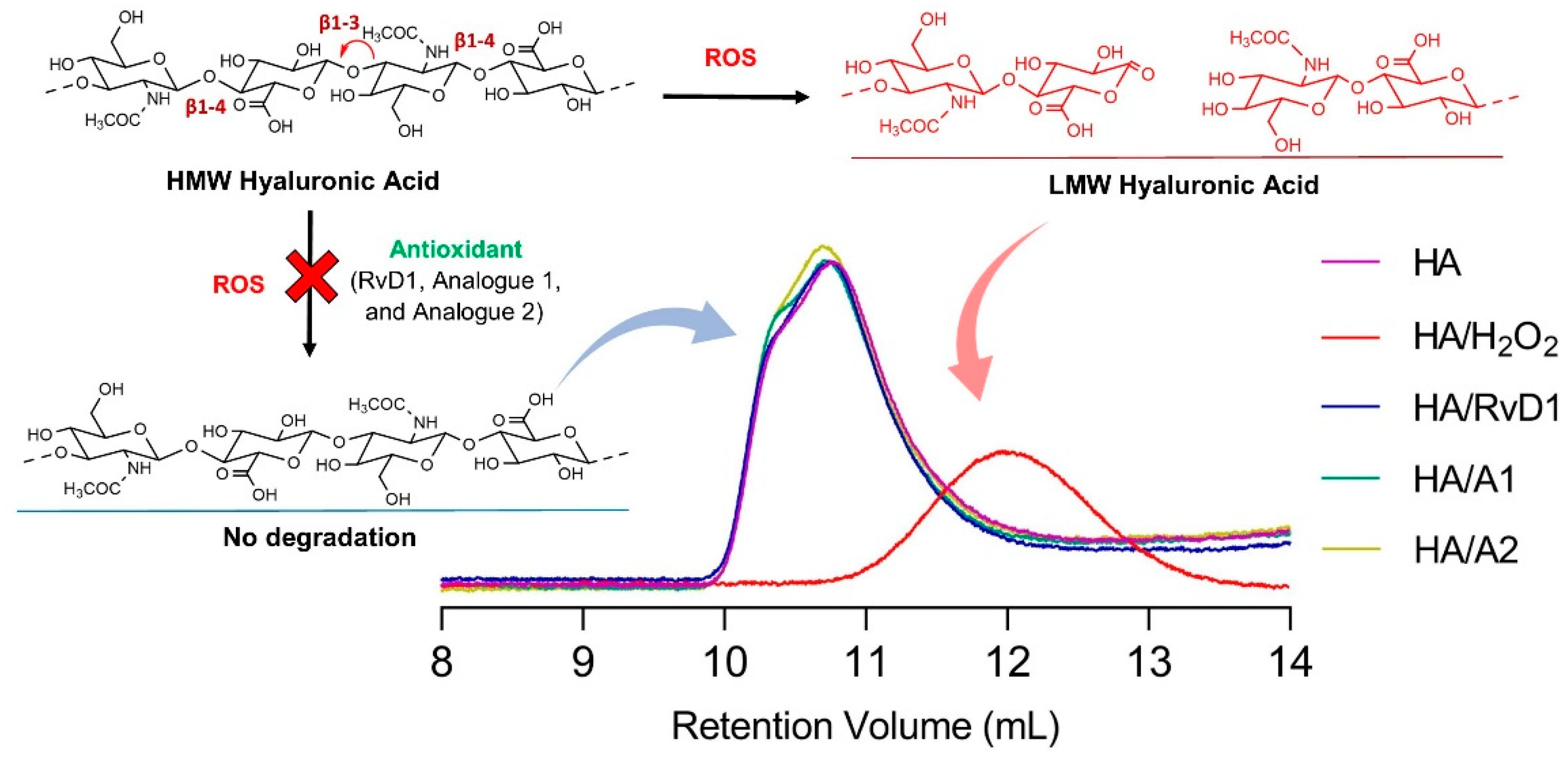
| Sample | Vp1 (mL) |
Mw (Da) |
Mn (Da) |
Mw/Mn2 (-) |
IV3 (mg/mL) |
| Hyaluronic Acid | 10.76 | 1,160,000 | 1,052,000 | 1.103 | 13.5227 |
| Hyaluronic Acid/H2O2 | 11.97 | 217,211 | 131,579 | 1.651 | 4.7605 |
| Hyaluronic Acid/H2O2 + RvD1 | 10.75 | 1,113,000 | 1,031,000 | 1.079 | 13.9877 |
| Hyaluronic Acid/H2O2 + A1 | 10.71 | 1,126,000 | 1,043,000 | 1.079 | 13.9109 |
| Hyaluronic Acid/H2O2 + A2 | 10.70 | 1,112,000 | 1,026,000 | 1.084 | 13.6087 |
3.3. Computational Study
3.3.1. Geometry Optimization of samples
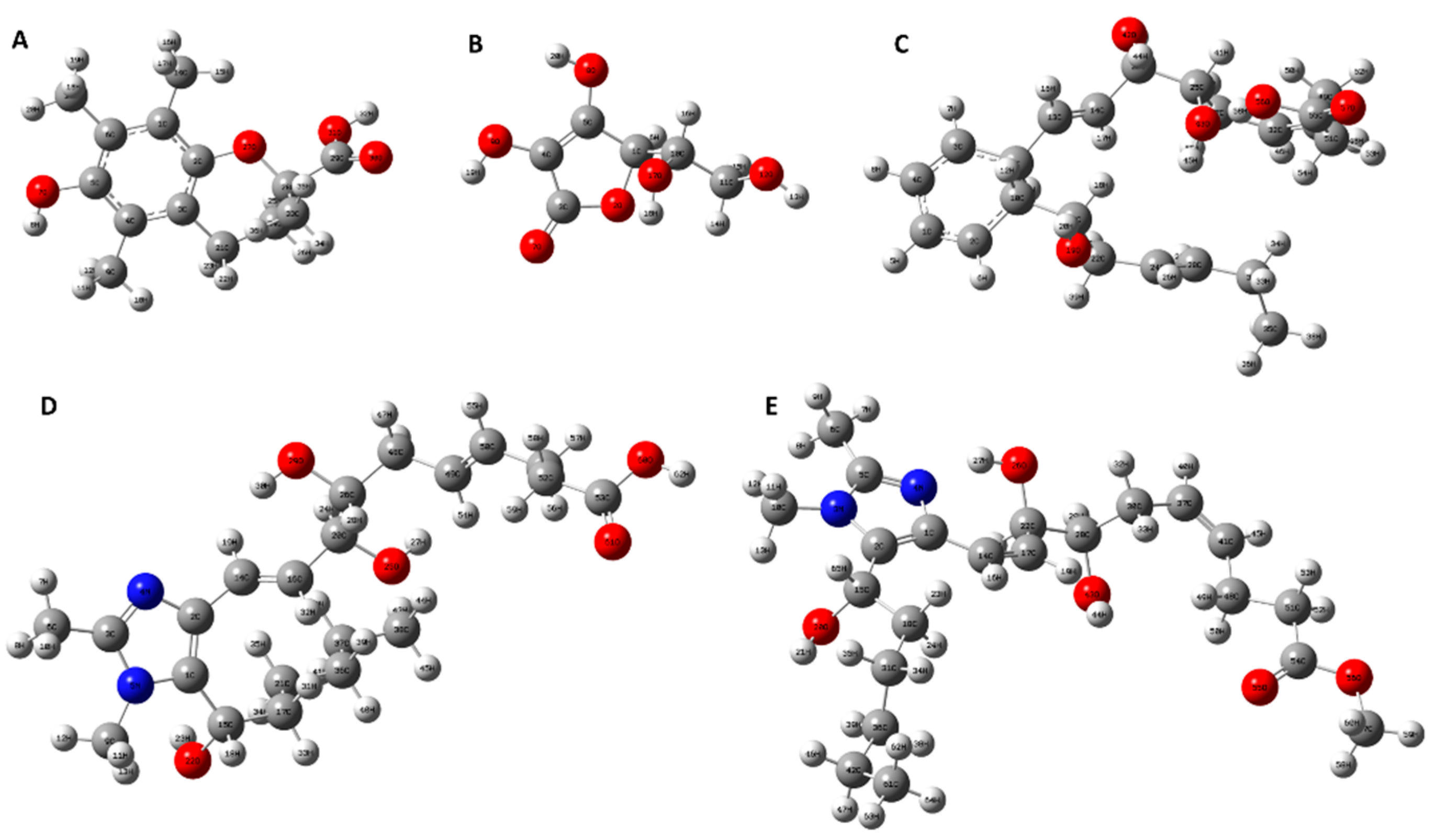
| Antioxidants | Bond |
Bond Length (Å)* |
| Trolox | O7-H8 | 0.9741 |
| O31-H32 | 0.9821 | |
| Ascorbic acid | O8-H20 | 0.9791 |
| O9-H19 | 0.9797 | |
| O12-H13 | 0.9772 | |
| O17-H18 | 0.9797 | |
| RvD1 | O19-H20 | 0.9786 |
| O42-H44 | 0.9821 | |
| O43-H45 | 0.9824 | |
| O56-H58 | 0.9996 | |
| Analogue 1 | O22-H23 | 0.9795 |
| O25-H27 | 0.9780 | |
| O29-H30 | 0.9808 | |
| O60-H62 | 0.9821 | |
| Analogue 2 | O20-H21 | 0.9795 |
| O26-H27 | 0.9981 | |
| O43-H44 | 0.9793 |
3.3.2. Bond Dissociation Enthalpy
| Antioxidants | Bond | BDE |
| Trolox | O7-H8 | 280.9 |
| O31-H32 | 346.9 | |
| Ascorbic acid | O8-H20 | 290.3 |
| O9-H19 | 293.9 | |
| O12-H13 | 383.6 | |
| O17-H18 | 380.5 | |
| RvD1 | O19-H20 | 471.4 |
| O42-H44 | 421.6 | |
| O43-H45 | 385.4 | |
| O56-H58 | 415.0 | |
| Analogue 1 | O22-H23 | 375.4 |
| O25-H27 | 380.5 | |
| O29-H30 | 401.9 | |
| O60-H62 | 411.2 | |
| Analogue 2 | O20-H21 | 386.2 |
| O26-H27 | 403.0 | |
| O43-H44 | 378.5 |
3.3.3. Ionization Potential
| Antioxidants | IP |
| Trolox | 778.9 |
| Ascorbic Acid | 932.2 |
| RvD1 | 875.3 |
| Analogue 1 | 825.6 |
| Analogue 2 | 806.1 |
3.3.4. Frontier Molecular Orbitals
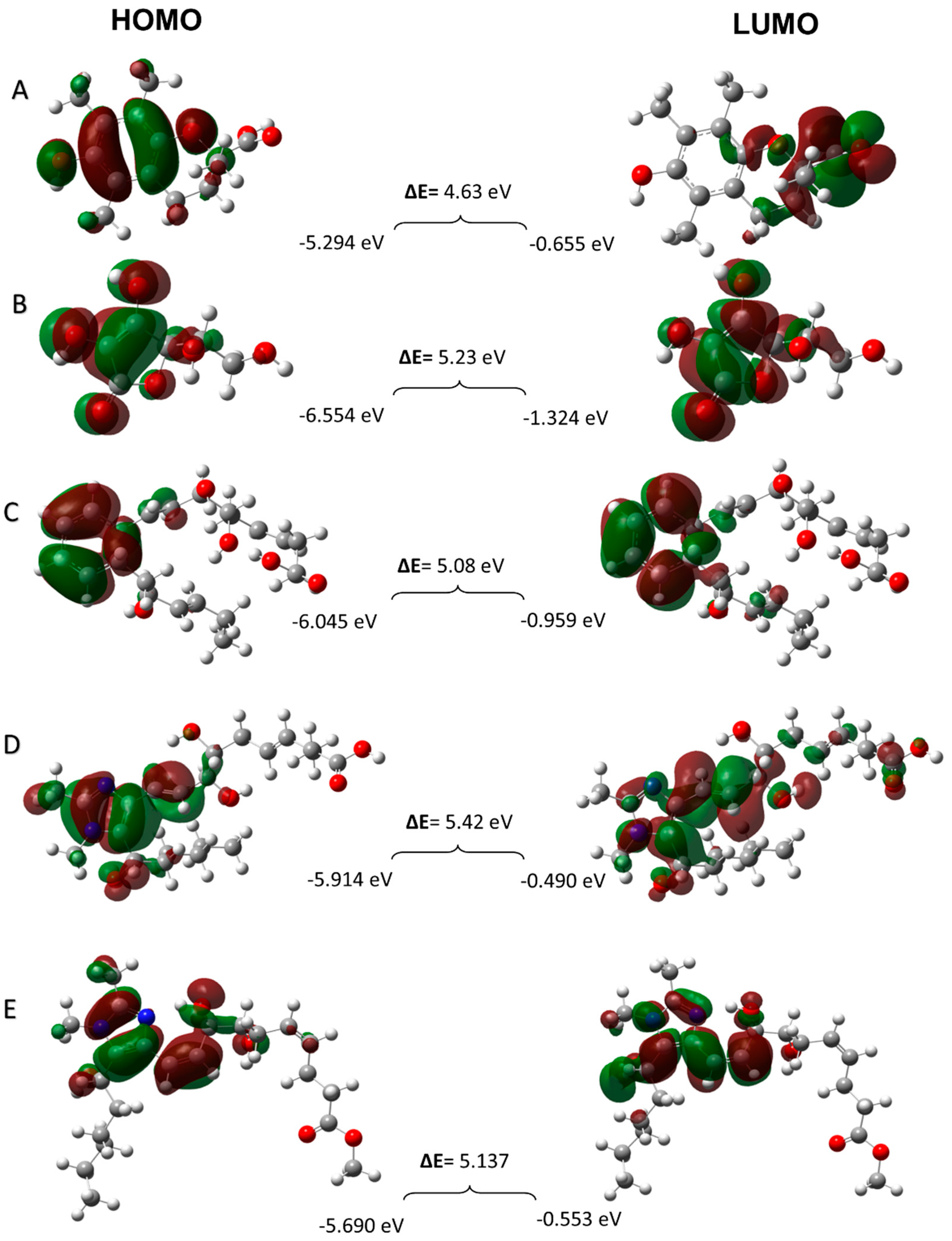
3.3.5. Quantum chemical parameters
| Hardness (eV) |
Softness (eV) |
Electronegativity (eV) |
Electrophilicity (eV) |
Chemical Potential (eV) |
| Antioxidants | EHOMO | ELUMO | η | σ | χ | ꞷ | µ |
| Trolox | -5.294 | -0.655 | 2.31 | 0.432 | 2.974 | 1.914 | -2.974 |
| Ascorbic acid | -6.554 | -1.324 | 2.61 | 0.383 | 3.938 | 2.970 | -3.938 |
| RvD1 | -6.045 | -0.959 | 2.54 | 0.393 | 3.500 | 2.411 | -3.500 |
| Analogue 1 | -5.914 | -0.490 | 2.71 | 0.369 | 3.201 | 1.890 | -3.201 |
| Analogue 2 | -5.690 | -0.553 | 2.56 | 0.390 | 3.121 | 1.902 | -3.121 |
3.3.6. Molecular electrostatic potential (MEP)
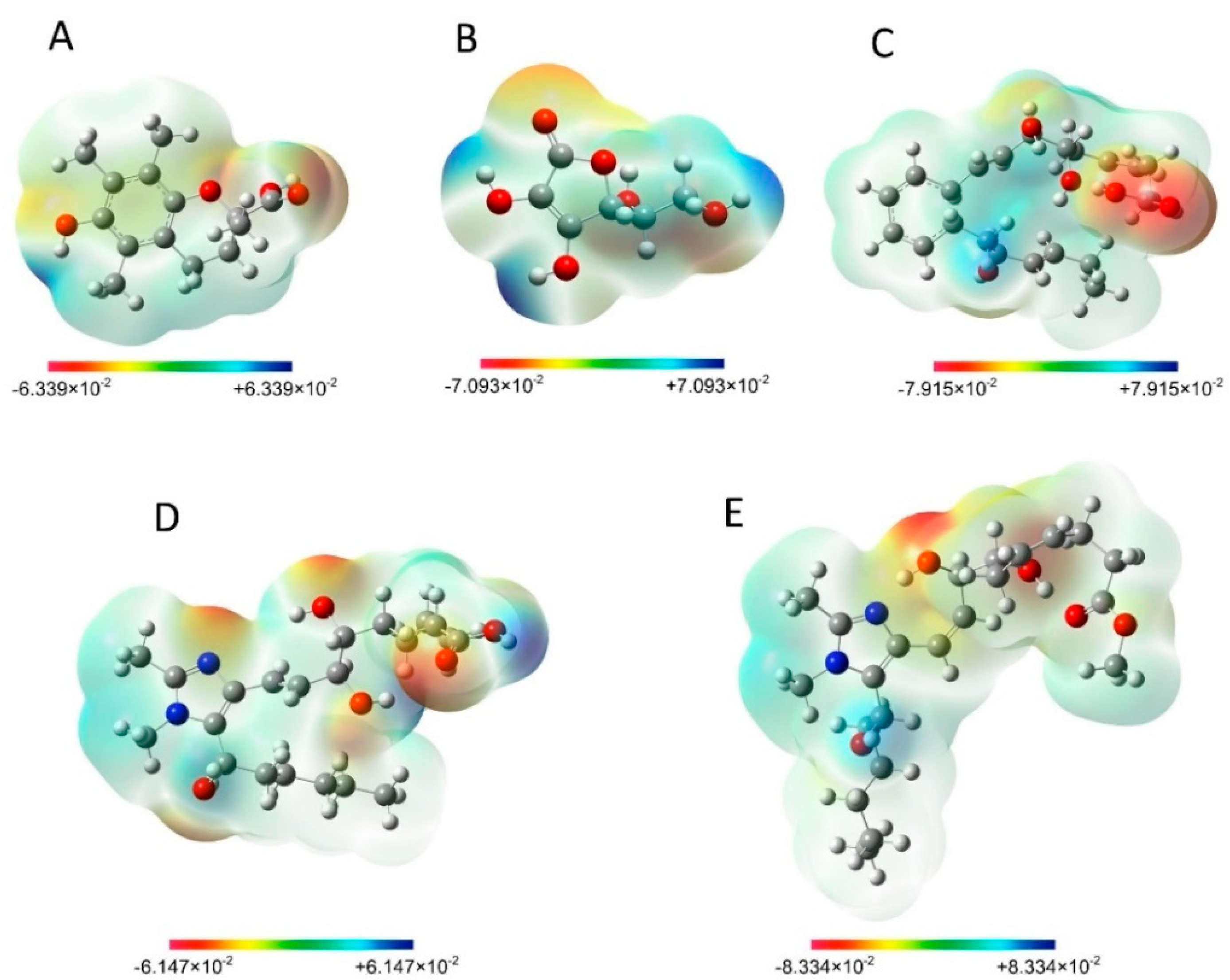
3.3.7. Mulliken Charge Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Nicola, V. Degenerative osteoarthritis a reversible chronic disease. Regenerative Therapy 2020, 15, 149–160. [Google Scholar] [CrossRef]
- Rahimi, M.; Charmi, G.; Matyjaszewski, K.; Banquy, X.; Pietrasik, J. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomaterialia 2021, 123, 31–50. [Google Scholar] [CrossRef]
- McClurg, O.; Tinson, R.; Troeberg, L. Targeting Cartilage Degradation in Osteoarthritis. Pharmaceuticals 2021, 14. [Google Scholar] [CrossRef]
- Mehana, E.-S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sciences 2019, 234, 116786. [Google Scholar] [CrossRef]
- Panahi, Y.; Alishiri, G.H.; Parvin, S.; Sahebkar, A. Mitigation of Systemic Oxidative Stress by Curcuminoids in Osteoarthritis: Results of a Randomized Controlled Trial. Journal of Dietary Supplements 2016, 13, 209–220. [Google Scholar] [CrossRef]
- Shi, Q.; Abusarah, J.; Zaouter, C.; Moldovan, F.; Fernandes, J.C.; Fahmi, H.; Benderdour, M. New evidence implicating 4-hydroxynonenal in the pathogenesis of osteoarthritis in vivo. Arthritis Rheumatol 2014, 66, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Morquette, B.; Shi, Q.; Lavigne, P.; Ranger, P.; Fernandes, J.C.; Benderdour, M. Production of lipid peroxidation products in osteoarthritic tissues: New evidence linking 4-hydroxynonenal to cartilage degradation. Arthritis & Rheumatism 2006, 54, 271–281. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Invited review: oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicologic pathology 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Wang, Y.; Zhang, Y.; Xu, N. Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. International Journal of Biological Macromolecules 2018, 115, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Tudorachi, N.B.; Totu, E.E.; Fifere, A.; Ardeleanu, V.; Mocanu, V.; Mircea, C.; Isildak, I.; Smilkov, K.; Cărăuşu, E.M. The Implication of Reactive Oxygen Species and Antioxidants in Knee Osteoarthritis. Antioxidants 2021, 10. [Google Scholar] [CrossRef]
- Henrotin, Y.; Mobasheri, A. Curcumin And Curcuminoids For The Treatment Of Osteoarthritis: Proof Of Concept. Journal of Tropical Medicinal Plants 2009, 10. [Google Scholar]
- Berdiaki, A.; Neagu, M.; Spyridaki, I.; Kuskov, A.; Perez, S.; Nikitovic, D. Hyaluronan and Reactive Oxygen Species Signaling—Novel Cues from the Matrix? Antioxidants 2023, 12–824. [Google Scholar] [CrossRef]
- Žádníková, P.; Šínová, R.; Pavlík, V.; Šimek, M.; Šafránková, B.; Hermannová, M.; Nešporová, K.; Velebný, V. The Degradation of Hyaluronan in the Skin. Biomolecules 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11. [Google Scholar] [CrossRef]
- Schiller, J.; Volpi, N.; Hrabárová, E.; Šoltés, L. Hyaluronic acid: a natural biopolymer. Biopolymers: biomedical and environmental applications 2011, 70. [Google Scholar]
- Soltés, L.; Kogan, G.; Stankovska, M.; Mendichi, R.; Rychlý, J.; Schiller, J.; Gemeiner, P. Degradation of high-molar-mass hyaluronan and characterization of fragments. Biomacromolecules 2007, 8, 2697–2705. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.; Baron, H.; Baerer, C.; Bodere, C.; Conrozier, T. Predictors for patient satisfaction of a single intra-articular injection of crosslinked hyaluronic acid combined with mannitol (HANOX-M-XL) in patients with temporomandibular joint osteoarthritis. Results of a prospective open-label pilot study (HAPPYMINI-ARTEMIS trial). BMC Musculoskeletal Disorders 2022, 23, 392. [Google Scholar] [CrossRef]
- Pegreffi, F.; Reginster, J.-Y.; Bruyère, O.; Veronese, N. Comment on: The Effect of Intra-articular Hyaluronic Acid Injections and Payer Coverage on Total Knee Arthroplasty Procedures: Evidence From Large US Claims Database. Arthroplasty Today 2023, 21. [Google Scholar] [CrossRef]
- Bruyère, O.; Honvo, G.; Vidovic, E.; Cortet, B. Assessment of the Response Profile to Hyaluronic Acid Plus Sorbitol Injection in Patients with Knee Osteoarthritis: Post-Hoc Analysis of a 6-Month Randomized Controlled Trial. Biomolecules 2021, 11, 1498. [Google Scholar] [CrossRef]
- Rinaudo, M.; Lardy, B.; Grange, L.; Conrozier, T. Effect of Mannitol on Hyaluronic Acid Stability in Two in Vitro Models of Oxidative Stress. Polymers 2014, 6, 1948–1957. [Google Scholar] [CrossRef]
- Mendoza, G.; Alvarez, A.I.; Pulido, M.M.; Molina, A.J.; Merino, G.; Real, R.; Fernandes, P.; Prieto, J.G. Inhibitory effects of different antioxidants on hyaluronan depolymerization. Carbohydr Res 2007, 342, 96–102. [Google Scholar] [CrossRef]
- Mongkhon, J.-M.; Thach, M.; Shi, Q.; Fernandes, J.C.; Fahmi, H.; Benderdour, M. Sorbitol-modified hyaluronic acid reduces oxidative stress, apoptosis and mediators of inflammation and catabolism in human osteoarthritic chondrocytes. Inflammation Research 2014, 63, 691–701. [Google Scholar] [CrossRef]
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The protective role of glutathione in osteoarthritis. Journal of Clinical Orthopaedics and Trauma 2021, 15, 145–151. [Google Scholar] [CrossRef]
- Henrotin, Y.; Kurz, B. Antioxidant to treat osteoarthritis: dream or reality? Current drug targets 2007, 8, 347–357. [Google Scholar] [CrossRef]
- Suantawee, T.; Tantavisut, S.; Adisakwattana, S.; Tanavalee, A.; Yuktanandana, P.; Anomasiri, W.; Deepaisarnsakul, B.; Honsawek, S. Oxidative stress, vitamin e, and antioxidant capacity in knee osteoarthritis. Journal of clinical and diagnostic research: JCDR 2013, 7, 1855–1859. [Google Scholar] [CrossRef]
- Lee, C.H. Role of specialized pro-resolving lipid mediators and their receptors in virus infection: a promising therapeutic strategy for SARS-CoV-2 cytokine storm. Archives of Pharmacal Research 2021, 44, 84–98. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Simental-Mendía, L.E.; Barreto, G.E.; Sahebkar, A. Anti-inflammatory effects of resolvins in diabetic nephropathy: Mechanistic pathways. Journal of Cellular Physiology 2019, 234, 14873–14882. [Google Scholar] [CrossRef] [PubMed]
- Molaei, E.; Molaei, A.; Hayes, A.W.; Karimi, G. Resolvin D1, therapeutic target in acute respiratory distress syndrome. European Journal of Pharmacology 2021, 911, 174527. [Google Scholar] [CrossRef] [PubMed]
- Benabdoune, H.; Rondon, E.-P.; Shi, Q.; Fernandes, J.; Ranger, P.; Fahmi, H.; Benderdour, M. The role of resolvin D1 in the regulation of inflammatory and catabolic mediators in osteoarthritis. Inflammation Research 2016, 65, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chemical reviews 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [PubMed]
- Benabdoun, H.A.; Kulbay, M.; Rondon, E.P.; Vallieres, F.; Shi, Q.; Fernandes, J.; Fahmi, H.; Benderdour, M. In vitro and in vivo assessment of the proresolutive and antiresorptive actions of resolvin D1: relevance to arthritis. Arthritis Res Ther 2019, 21, 72. [Google Scholar] [CrossRef]
- Norling, L.V.; Headland, S.E.; Dalli, J.; Arnardottir, H.H.; Haworth, O.; Jones, H.R.; Irimia, D.; Serhan, C.N.; Perretti, M. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI insight 2016, 1. [Google Scholar] [CrossRef]
- Dravid, A.A.; Dhanabalan, K.M.; Naskar, S.; Vashistha, A.; Agarwal, S.; Padhan, B.; Dewani, M.; Agarwal, R. Sustained release resolvin D1 liposomes are effective in the treatment of osteoarthritis in obese mice. Journal of Biomedical Materials Research Part A 2023, 111, 765–777. [Google Scholar] [CrossRef]
- Inactivation, E. Resolvin D1 and Its Aspirin-triggered 17R Epimer. THE JOURNAL OF BIOLOGICAL CHEMISTRY 2007, 282, 9323–9334. [Google Scholar] [CrossRef]
- Orr, S.K.; Colas, R.A.; Dalli, J.; Chiang, N.; Serhan, C.N. Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent. Am J Physiol Lung Cell Mol Physiol 2015, 308, L904–L911. [Google Scholar] [CrossRef]
- Benderdour M, Fernandes J, Maltais R, Sanceau JY. Resolvin Analogs Compounds, Methods and Used Thereof. In: WO2023137554A1. vol. WO2023137554A1. Canada; 2023.
- de Gaetano, M.; Butler, E.; Gahan, K.; Zanetti, A.; Marai, M.; Chen, J.; Cacace, A.; Hams, E.; Maingot, C.; McLoughlin, A.; et al. Asymmetric synthesis and biological evaluation of imidazole- and oxazole-containing synthetic lipoxin A4 mimetics (sLXms). European Journal of Medicinal Chemistry 2019, 162, 80–108. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. Journal of Agricultural and Food Chemistry 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, S.; Renn, K. Zweiwertiger Stickstoff: Über das α, α-Diphenyl-β-trinitrophenyl-hydrazyl.(IV. Mitteilung über Amin-Oxydation). Berichte der deutschen chemischen Gesellschaft (A and B Series) 1922, 55, 628–643. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Analytical Biochemistry 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Govindappa, C.M.; Sadananda, T.S.; Chandrappa, C.P. Phytochemical Analysis of Loranthus micranthus Extracts and Their in Vitro Antioxidant and Antibacterial Activities. Journal of Biologically Active Products from Nature 2014, 4, 303–315. [Google Scholar] [CrossRef]
- Chen, H.; Qin, J.; Hu, Y. Efficient Degradation of High-Molecular-Weight Hyaluronic Acid by a Combination of Ultrasound, Hydrogen Peroxide, and Copper Ion. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Yogesh, K.; Jha, S.N.; Ahmad, T. Antioxidant potential of aqueous extract of some food grain powder in meat model system. Journal of Food Science and Technology 2014, 51, 3446–3451. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. Use and Abuse of the DPPH• Radical. Journal of Agricultural and Food Chemistry 2015, 63, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. International Journal of Molecular Sciences 2021, 22. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: a review. Journal of Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A. Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food chemistry 2006, 96, 131–136. [Google Scholar] [CrossRef]
- Baranowska, M.; Suliborska, K.; Chrzanowski, W.; Kusznierewicz, B.; Namieśnik, J.; Bartoszek, A. The relationship between standard reduction potentials of catechins and biological activities involved in redox control. Redox Biology 2018, 17, 355–366. [Google Scholar] [CrossRef]
- Cowman, M.K.; Lee, H.-G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The Content and Size of Hyaluronan in Biological Fluids and Tissues. Frontiers in Immunology 2015, 6. [Google Scholar] [CrossRef]
- Nasidi, I.I.; Kaygili, O.; Majid, A.; Bulut, N.; Alkhedher, M.; ElDin, S.M. Halogen Doping to Control the Band Gap of Ascorbic Acid: A Theoretical Study. ACS omega 2022, 7, 44390–44397. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, L.; Nguyen, T.L.A.; Dao, D.Q. Experimental and theoretical investigation of cyclometalated phenylpyridine iridium(iii) complex based on flavonol and ibuprofen ligands as potent antioxidant. RSC Advances 2019, 9, 17220–17237. [Google Scholar] [CrossRef] [PubMed]
- Farrokhnia, M. Density Functional Theory Studies on the Antioxidant Mechanism and Electronic Properties of Some Bioactive Marine Meroterpenoids: Sargahydroquionic Acid and Sargachromanol. ACS Omega 2020, 5, 20382–20390. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





