Submitted:
22 February 2024
Posted:
22 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. HuNV GII.4 and HAV virus stock preparation
2.2. Virus stock inoculation of groundwater sample
2.3. Cl2 treatment of HuNV GII.4 and HAV in groundwater
2.4. Propidium monoazide (PMA) staining
2.5. Extraction of Viral RNA
2.6. Quantitative analysis of HuNV GII.4 and HAV by qPCR
2.7. Determination of decimal reduction times (D-values) by linear modeling
- N0 = Initial HuNV GII.4 and HAV titer (log copies/μL)
- N = HuNV GII.4 and HAV titer after Cl2 treatment (log copies/μL)
- t = Cl2 concentraion (ppm)
- k = reduction rate constant
2.8. Statistical analysis
3. Results
3.1. Effects of Cl2 on HuNV GII.4 in PMA-untreated and PMA-treated groundwater samples
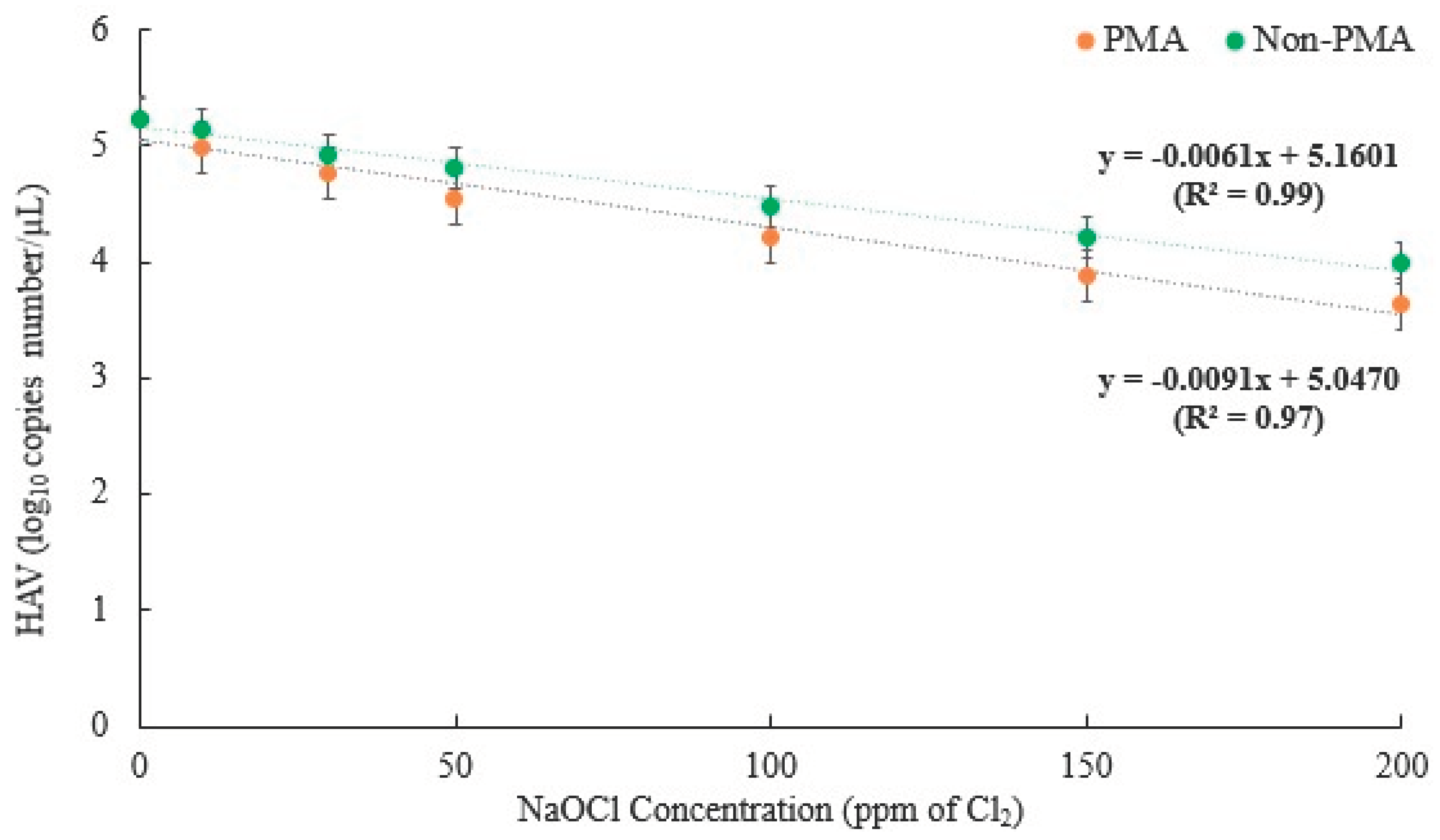
3.2. Effects of Cl2 on HAV in PMA-untreated and PMA-treated groundwater samples
3.3. D-values of HuNV GII.4 and HAV with PMA reduction by first-order kinetics model
4. Discussion
5. Conclusions
Acknowledgments
References
- Struckmeier, W.; Rubin, Y.; Jones, J.A.A. Groundwater - Reservoir for a Thirsty Planet?: Earth Sciences for Society ; a Prospectus for a Key Theme of the International Year of Planet Earth. IUGS, Norway. 2005, 16 p.
- Gwack, J.; Lee, K.C.; Lee, H.J.; Kwak, W.S.; Lee, D.W.; Choi, Y.H.; Kim, J.S.; Kang, Y.A. Trends in water- and foodborne disease outbreaks in Korea, 2007–2009. Osong. Pub. Health Res. Perspect. 2010, 1, 50–54. [CrossRef]
- Lee, M.J.; Kim, W.H.; Cho, H.G.; Lee, S.S. Epidemiological study of ground waterborne norovirus GI.3-associated gastroenteritis outbreaks in Gyeonggi province of South Korea in May 2011. J. Bacteriol. Virol. 2012, 42, 232–241. [CrossRef]
- Wallender, E.K.; Ailes, E.C.; Yoder, J.S.; Roberts, V.A.; Brunkard. J.M. Contributing factors to disease outbreaks associated with untreated groundwater. Groundwater. 2014, 52, 886–897. [CrossRef]
- Kauppinen, A.; Pitkanen, T.; Miettinen, I.T. Persistent norovirus contamination of groundwater supplies in two waterborne outbreaks. Food Environ. Virol. 2018, 10, 39–50. [CrossRef]
- Ferguson, C.; Husman, A.M. de R.; Altavilla, N.; Deere, D.; Ashbolt, N. Fate and transport of surface water pathogens in watersheds. Crit. Rev. Environ. Sci. Technol. 2003, 33, 299–361. [CrossRef]
- Rhee, C.H.; Kim, S.; Kang, Y.E.; Han, B.; Swo, S-J.; Kim, Y.W.; Her, M.; Jeong, W. Virucidal efficacy of acidic electrolyzed water (AEW) against African swine fever virus and avian influenza virus. J Vet. Med. Sci. 2021, 83, 201–207. [CrossRef]
- Korea Centers for Disease Control and Prevention (KCDC) Management guidelines for water & foodborne diseases [Internet] [cited 2020 Sep 1]. 2020. Available from: https://www.kdca.go.kr/upload_comm/syview/doc.html?fn=158209329137500.pdf&rs=/upload_comm/docu/0019/ (Accessed 3 Jun 2023).
- Matthews, J.E.; Dickey, B.W.; Miller, R.D.; Felzer, J.R.; Dawson, B.P.; Lee, A.S.; Rocks, J.J.; Kiel, J.; Montes, J. S.; Moe, C.L.; Eisenberg, J.N.S.; Leon, J.S. The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiol Infect. 2012, 140, 1161–1172. [CrossRef]
- Lodder, W.J.; De Roda, A.M. Husman, Presence of noroviruses and other enteric viruses in sewage and surface waters in The Netherlands. Appl. Environ. Microbiol. 2005, 71, 1453–1461. [CrossRef]
- Cristina, J.; Costa-Mattioli, M. Genetic variability and molecular evolution of hepatitis a virus. Virus Res. 2007, 127, 151–157. [CrossRef]
- Ryu, S.; Won, S.A.; Uh, J.; Song, J.Y. Hepatitis A virus infection from a contaminated tap of ground water facility in a neighborhood park, Republic of Korea. Infect Chemother. 2019, 51, 62–66. [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Guidance Documents of Food poisoning. 2022. Available online: http://various.foodsafetykorea.go.kr/fsd/#/ext/Document/FC (accessed on 15 June 2023).
- Ministry of Government Legislation (MOLEG). Regulations on water quality standards and inspection for drinking water Article No. 4. 2023. Available online: http://www.law.go.kr/%EB%B2%95%EB%A0%B9/%EB%A8%B9%EB%8A%94%EB%AC%BC%EC%88%98%EC%A7%88%EA%B8%B0%EC%A4%80%EB%B0%8F%EA%B2%80%EC%82%AC%EB%93%B1%EC%97%90%EA%B4%80%ED%95%9C%EA%B7%9C%EC%B9%99 (Accessed 23 June 2023).
- Jeon, E.B.; Choi, M.-S.; Kim, J.Y.; Ha, K.S.; Kwon, J.Y.; Jeong, S.H.; Lee, H.J.; Jung, Y.J.; Ha, J.-H.; Park, S.Y. Characterizing the effects of thermal treatment on human norovirus GII.4 viability using propidium monoazide combined with RT-qPCR and quality assessments in mussels. Food Control. 2019, 109, 106954. [CrossRef]
- Genter, F.; Willetts, J.; Foster, T. Fecal contamination of groundwater self-supply in low- and middle-income countries: Systematic review and meta-analysis. Water Res. 2021, 201, 117350. [CrossRef]
- Han, J.; Zhang, X.; Li, W.; Jiang, J. Low chlorine impurity might be beneficial in chlorine dioxide disinfection. Water Res. 2021, 188, 116520. [CrossRef]
- Rachmadi, A.T.; Kitajima, M.; Kato, T.; Kato, H.; Okabe, S.; Sano, D. Required chlorination doses to fulfill the credit value for disinfection of enteric viruses in water: a critical review. Environ. Sci. Technol. 2020, 54, 2068–2077. [CrossRef]
- U.S.EPA. Contaminant Candidate List (CCL) and Regulatory Determination. 2018. Available online: https//www.epa.gov/ccl/microbial-contaminants-ccl-4 (Accessed 23 March 2023).
- Thurston-Enriquez, J.A.; Haas, C.N.; Jacangelo, J.; Gerba, C.P. Chlorine inactivation of adenovirus type 40 and feline calicivirus. Appl. Environ. Microbiol. 2003, 69, 3979–3985. [CrossRef]
- El-Senousy, W.M.; El-Gamal, M.S.; Mousa, A.A.E.; El-Hawary, S.E.; Kamel, M.M.; Fathi, M.N.; El-Mahdy, E.M. Effect of Chlorine on Noroviruses, Rotaviruses and Hepatitis E Virus in Drinking Water. WASJ. 2014, 32, 2206–2212. [CrossRef]
- Li, J.W.; Xin, Z.T.; Wang, X.W.; Zheng, J.L.; Chao, F.H. Mechanisms of inactivation of hepatitis A virus in water by chlorine dioxide. Water Res. 2004, 38, 1514–1519. [CrossRef]
- Rachmadi, A.T.; Kitajima, M.; Watanabe, K.; Yaegashi, S.; Serrana, J.; Nakamura, A.; Nakagomi, T.; Nakagomi, O.; Katayama, K.; Okabe, S.; Sano, D. Free Chlorine Disinfection as a Selection Pressure on Norovirus. Appl. Environ. Microbiol. 2018, 84, 00244–18. [CrossRef]
- Kitajima, M.; Tohya, Y.; Matsubara, K.; Haramoto, E.; Utagawa, E.; Katayama, H. Chlorine inactivation of human norovirus, murine norovirus and poliovirus in drinking water. Applied Microbiology. 2010, 51, 119–121. [CrossRef]
- Tree, J.A.; Adams, M.R.; Lees, D.N. Disinfection of feline calicivirus (a surrogate for norovirus) in wastewaters. J. Appl. Microbiol. 2005, 98, 155–162. [CrossRef]
- Kim, S.Y.; Ko, G. Using propidium monoazide to distinguish between viable and nonviable bacteria, MS2 and murine norovirus. Lett Appl Microbiol. 2012, 55,182–188. [CrossRef]
- Karim, M.R.; Fout, G.S.; Johnson, C.H.; White, K.M.; Parshionikar, S.U. Propidium monoazide reverse transcriptase PCR and RT-qPCR for detecting infectious enterovirus and norovirus. J Virol Methods. 2015, 219, 51–61. [CrossRef]
- Anfruns-Estrada, E.; Bottaro, M.; Pinto, R.M.; Guix, S.; Bosch, A. Effectiveness of consumers washing with sanitizers to reduce human norovirus on mixed salad. Foods. 2019, 8, 637. [CrossRef]
- Fittipaldi, M.; Rodriguez, N.J.; Codony, F.; Adrados, B.; Peñuela, G.A.; Morató, J. Discrimination of infectious bacteriophage T4 virus by propidium monoazide real time PCR. J. Virol. Methods. 2010, 168, 228–232. [CrossRef]
- Chen, L.; Zhang, H.; Liu, Q.; Pang, X.; Zhao, X.; Yang, H. Sanitising efficacy of lactic acid combined with low-concentration sodium hypochlorite on Listeria innocua in organic broccoli sprouts. Int. J. Food Microbiol. 2019, 295, 41–48. [CrossRef]
- Meister, S.; Verbyla, M. E.; Klinger, M.; Kohn, T. Variability in Disinfection Resistance between Currently Circulating Enterovirus B Serotypes and Strains. Environ. Sci. Technol. 2018, 52, 3696–3705. [CrossRef]
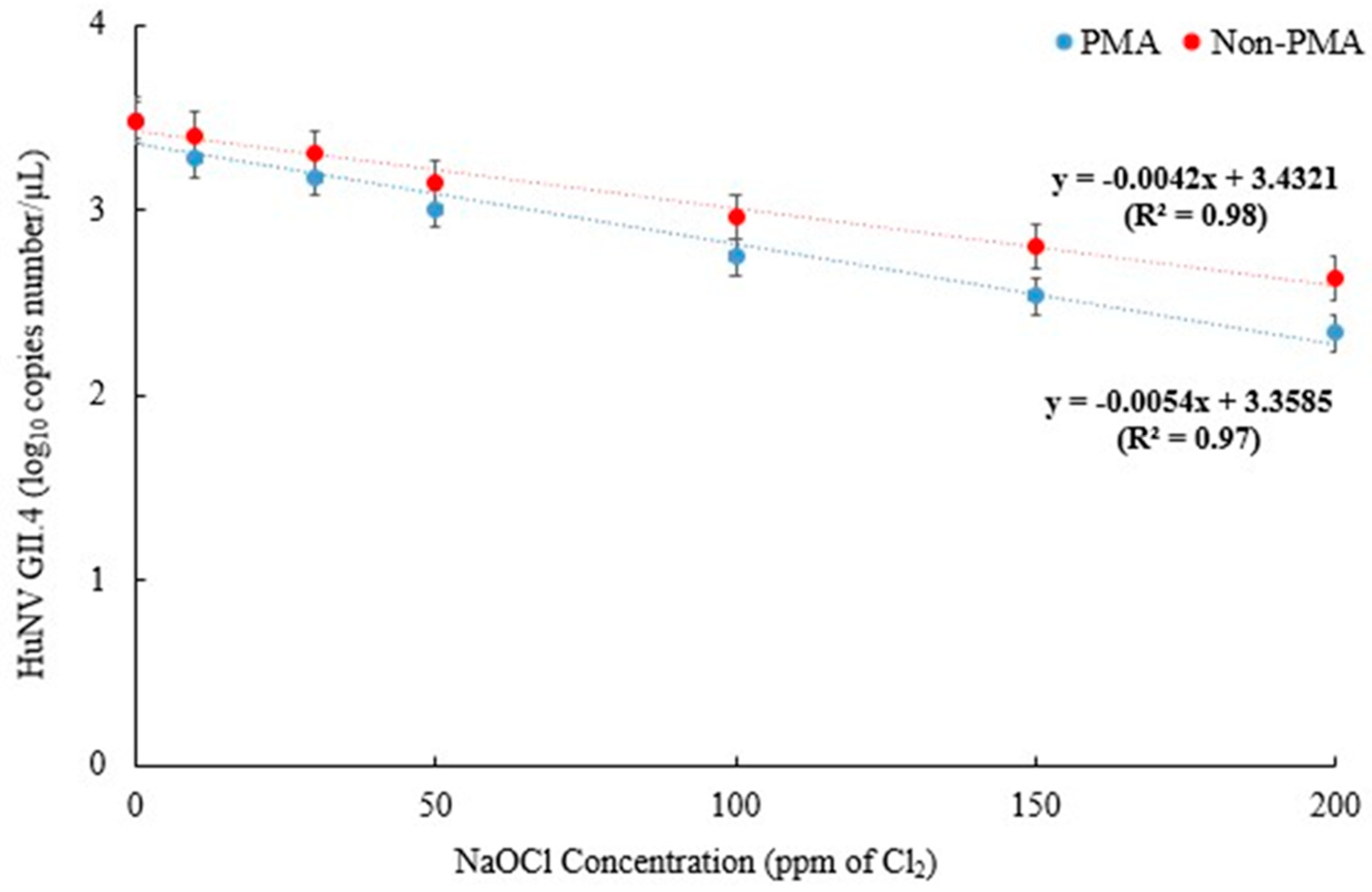
| RT-qPCR | PMA+RT-qPCR | Before/after using PMA to HNV reduction difference (log10 copies number/µL) | |
| log10 copies number/µL | log10 copies number/µL | ||
| Control | 3.49 ± 0.04aA | 3.49 ± 0.04aA | - |
| 10 ppm | 3.41 ± 0.04bA | 3.28 ± 0.03bB | (3.41–3.28) = 0.13 |
| 30 ppm | 3.31 ± 0.03cA | 3.18 ± 0.03cB | (3.31–3.18) = 0.13 |
| 50 ppm | 3.15 ± 0.04dA | 3.01 ± 0.02dB | (3.15–3.01) = 0.14 |
| 100 ppm | 2.97 ± 0.02eA | 2.75 ± 0.04eB | (2.97–2.75) = 0.22 |
| 150 ppm | 2.81 ± 0.05fA | 2.54 ± 0.05fB | (2.81–2.54) = 0.27 |
| 200 ppm | 2.64 ± 0.03gA | 2.34 ± 0.02gB | (2.64–2.34) = 0.30 |
| RT-qPCR | PMA+RT-qPCR | Before/after using PMA to HAV reduction difference (log10 copies number/µL) | |
| log10 copies number/µL | log10 copies number/µL | ||
| Control | 5.22 ± 0.03aA | 5.22 ± 0.03aA | - |
| 10 ppm | 5.15 ± 0.03bA | 4.99 ± 0.04bB | (5.15–4.99) = 0.16 |
| 30 ppm | 4.92 ± 0.04cA | 4.76 ± 0.03cB | (4.92–4.76) = 0.16 |
| 50 ppm | 4.82 ± 0.05dA | 4.55 ± 0.02dB | (4.82–4.55) = 0.27 |
| 100 ppm | 4.48 ± 0.03eA | 4.21 ± 0.04eB | (4.48–4.21) = 0.27 |
| 150 ppm | 4.22 ± 0.03fA | 3.89 ± 0.04fB | (4.22–3.89) = 0.33 |
| 200 ppm | 3.99 ± 0.04gA | 3.64 ± 0.03gB | (3.99–3.64) = 0.35 |
| D-values of NaOCl concentration (ppm of Cl2) | ||
|---|---|---|
| Non-PMA/RT-qPCR | PMA/RT-qPCR | |
| HuNV GII.4 | 214.3 ± 0.05aA | 116.7 ± 0.04bA |
| HAV | 147.5 ± 0.07aB | 98.9 ± 0.06bB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





