Submitted:
21 February 2024
Posted:
22 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
Materials and Methods
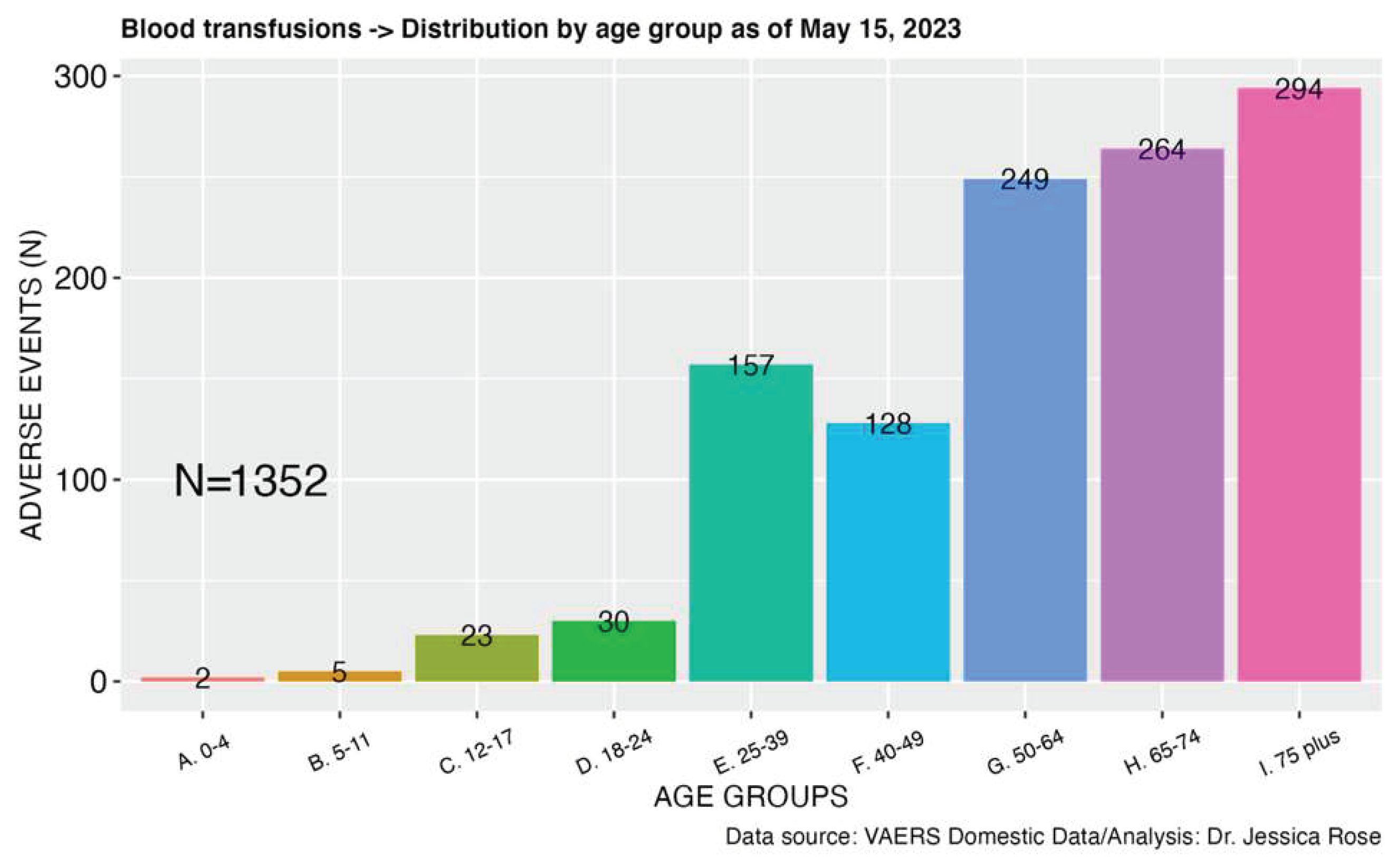
- Firstly, the persistence of vaccine mRNA/adenoviral DNA lipid nanoparticles and their products (ie spike protein) for long periods following vaccinations lends plausibility to this mechanism of harm. This review of the literature evidence establishes biological plausibility. As vaccine particles and altered blood parameters are found months after inujection, these may potentially be passed onto a blood donation recipient.
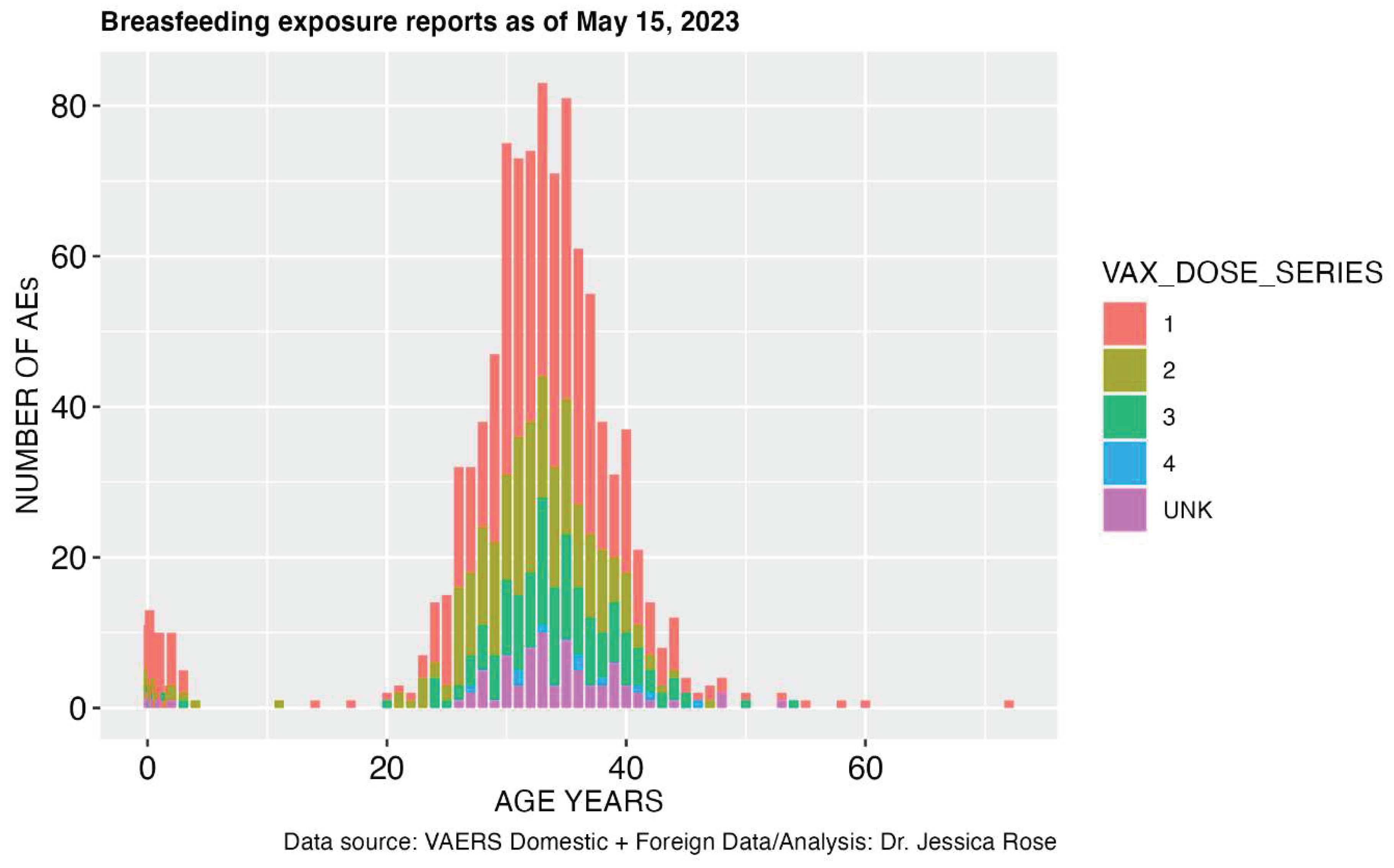
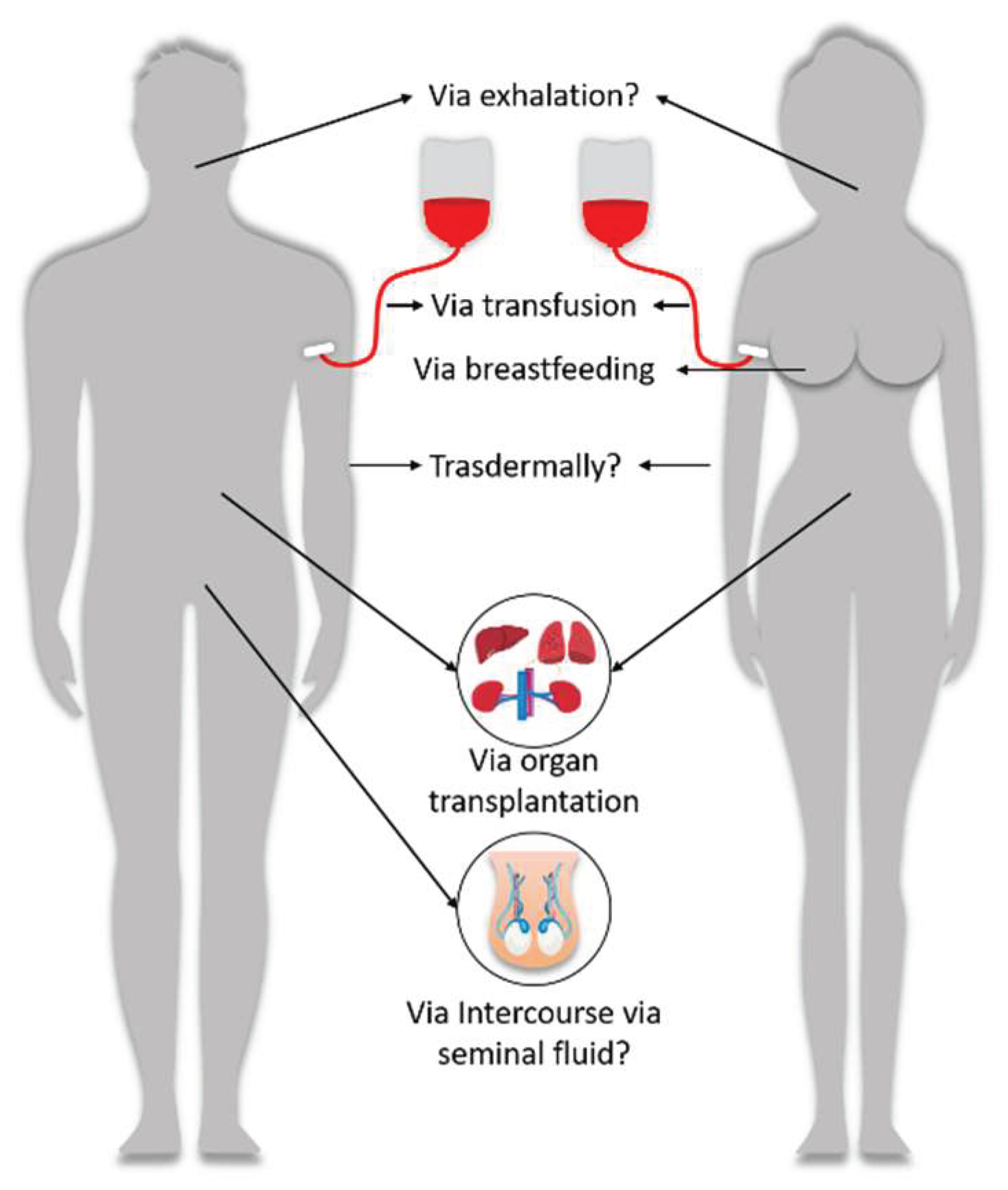
- Secondly, the case report literature demonstrates many circulatory disorders manifesting in differed blood characteristics in cases of the primary recipient of the injection, as well as adverse events following exposure to the bodily fluids of vaccinees. The modalities of transmission for which there is a pharmacovigilance signal are blood transfusion and breastfeeding. These establish a pharmacovigilance signal from exposure to vaccinees blood (in the case of blood donation) and breastmilk, in the case of breastfeeding.
- Lastly, recipients of organ transplant from donors deceased due to Vaccine Induced Thrombosis and Thrombocytopenia (VITT), encountered blood clotting and thrombotic events, suggesting a possible danger for organ donation, as well as blood transfusion. National monitoring for adverse events following organ transplantation also showed an increased rate of adverse events in temporal relationship to mass vaccination, but others show no increase.
2. Results
2.1. Mechanisms of Harm
2.2. Pharmacovigilance
2.2.1. Case Reports of Blood Manifestations
2.2.2. Blood Transfusions
2.2.3. Breastfeeding and Maternal Exposure
2.2.4. Other routes of exposure
2.3. Organ Transplant Safety
3. Discussion
Conclusion
Ethics approval and informed consent
Consent for Publication
Data Availability
Acknowledgements
Competing Interests
Abbreviations
| AE: adverse events |
| CDC: Centers for Disease Control (USA) |
| HLA: human leukocyte antigen |
| IFR: Infection Fatality Rate |
| VAERS: Vaccine adverse event reporting system |
| VITT: vaccine-induced thrombosis and thrombocytopenia |
| WHO: World Health Organization |
References
- Lei Y, Zhang J, Schiavon CR, et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circulation Research. 2021;128(9):1323-1326. [CrossRef]
- Stenmark KR, Frid MG, Gerasimovskaya E, et al. Mechanisms of SARS-CoV-2-induced lung vascular disease: potential role of complement. Pulm Circ. 2021;11(2):20458940211015799. [CrossRef]
- Aid M, Busman-Sahay K, Vidal SJ, et al. Vascular Disease and Thrombosis in SARS-CoV-2-Infected Rhesus Macaques. Cell. 2020;183(5):1354-1366.e13. [CrossRef]
- Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389-391. [CrossRef]
- Elsoukkary SS, Mostyka M, Dillard A, et al. Autopsy Findings in 32 Patients with COVID-19: A Single-Institution Experience. Pathobiology. 2021;88(1):56-68. [CrossRef]
- Yao XH, Luo T, Shi Y, et al. A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res. 2021;31(8):836-846. [CrossRef]
- Hanley B, Lucas SB, Youd E, Swift B, Osborn M. Autopsy in suspected COVID-19 cases. Journal of Clinical Pathology. 2020;73(5):239-242. [CrossRef]
- Stillfried S von, Bülow RD, Röhrig R, et al. First report from the German COVID-19 autopsy registry. The Lancet Regional Health – Europe. 2022;15. [CrossRef]
- Ducloyer M, Gaborit B, Toquet C, et al. Complete post-mortem data in a fatal case of COVID-19: clinical, radiological and pathological correlations. Int J Legal Med. 2020;134(6):2209-2214. [CrossRef]
- Ghasemiyeh P, Mohammadi-Samani S, Firouzabadi N, Dehshahri A, Vazin A. A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. International Immunopharmacology. 2021;100:108162. [CrossRef]
- Francis AI, Ghany S, Gilkes T, Umakanthan S. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgraduate Medical Journal. 2022;98(1159):389-394. [CrossRef]
- Tobaiqy M, Elkout H, MacLure K. Analysis of Thrombotic Adverse Reactions of COVID-19 AstraZeneca Vaccine Reported to EudraVigilance Database. Vaccines. 2021;9(4):393. [CrossRef]
- Wise J. Covid-19: Rare immune response may cause clots after AstraZeneca vaccine, say researchers. BMJ. 2021;373:n954. [CrossRef]
- Geeraerts T, Montastruc F, Bonneville F, Mémier V, Raposo N. Oxford-AstraZeneca COVID-19 vaccine-induced cerebral venous thrombosis and thrombocytopaenia: A missed opportunity for a rapid return of experience. Anaesth Crit Care Pain Med. 2021;40(4):100889. [CrossRef]
- WHO statement on AstraZeneca COVID-19 vaccine safety signals. Accessed December 7, 2022. https://www.who.int/news/item/17-03-2021-who-statement-on-astrazeneca-covid-19-vaccine-safety-signals.
- Shay DK. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine — United States, March–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70. [CrossRef]
- Mahase E. Covid-19: Unusual blood clots are “very rare side effect” of Janssen vaccine, says EMA. BMJ. 2021;373:n1046. [CrossRef]
- Malik B, Kalantary A, Rikabi K, Kunadi A. Pulmonary embolism, transient ischaemic attack and thrombocytopenia after the Johnson & Johnson COVID-19 vaccine. BMJ Case Reports CP. 2021;14(7):e243975. [CrossRef]
- Mahase E. Covid-19: US suspends Johnson and Johnson vaccine rollout over blood clots. BMJ. 2021;373:n970. [CrossRef]
- Tobaiqy M, MacLure K, Elkout H, Stewart D. Thrombotic Adverse Events Reported for Moderna, Pfizer and Oxford-AstraZeneca COVID-19 Vaccines: Comparison of Occurrence and Clinical Outcomes in the EudraVigilance Database. Vaccines. 2021;9(11):1326. [CrossRef]
- Vaccination against covid-19. Accessed October 1, 2022. https://www.sst.dk/en/english/corona-eng/vaccination-against-covid-19.
- Alawed AA. Evaluation of Platelet and D.dimer among covid-19 Vaccinated Individuals in Shandi Town. Thesis. Elfatih Mohammed Abdallah; 2022. Accessed December 4, 2022. http://localhost:8080/xmlui/handle/123456789/1306.
- Mahmoud MAK, Khudhair N. Comparison of Complete Blood Counts between four Groups: a COVID-19 Patient, a Healthy and Healthy Vaccine Recipient, and Patient Vaccinated Recipients in Anbar Province. HIV Nursing. 2022;22(2):1988-1994.
- Ltd TIS. JPAC - Transfusion Guidelines. Accessed December 7, 2022. https://transfusionguidelines.org.uk/.
- Info about COVID-19 vaccines and blood donation. Accessed December 7, 2022. https://www.blood.ca/en/covid19/vaccines-and-blood-donation.
- EBMT COVID Vaccine Information.; 2022. https://www.ebmt.org/sites/default/files/2022-01/COVID%20vaccines%20version%208.3%20-%202022-01-03.pdf.
- Avolio E, Carrabba M, Milligan R, et al. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease. Clin Sci (Lond). 2021;135(24):2667-2689. [CrossRef]
- Trougakos IP, Terpos E, Alexopoulos H, et al. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol Med. 2022;28(7):542-554. [CrossRef]
- Cosentino M, Marino F. Understanding the Pharmacology of COVID-19 mRNA Vaccines: Playing Dice with the Spike? International Journal of Molecular Sciences. 2022;23(18):10881. [CrossRef]
- Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. New England Journal of Medicine. 2021;384(20):1964-1965. [CrossRef]
- Vogel G, Kupferschmidt K. New problems erode confidence in AstraZeneca’s vaccine. Science. 2021;371(6536):1294-1295. [CrossRef]
- See I, Su JR, Lale A, et al. US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448-2456. [CrossRef]
- Karlstad Ø, Hovi P, Husby A, et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiology. 2022;7(6):600-612. [CrossRef]
- Hsieh YL, Rak S, SteelFisher GK, Bauhoff S. Effect of the suspension of the J&J COVID-19 vaccine on vaccine hesitancy in the United States. Vaccine. 2022;40(3):424-427. [CrossRef]
- Paterlini M. Covid-19: Sweden, Norway, and Finland suspend use of Moderna vaccine in young people “as a precaution.” BMJ. 2021;375:n2477. [CrossRef]
- De Michele M, Kahan J, Berto I, et al. Cerebrovascular Complications of COVID-19 and COVID-19 Vaccination. Circ Res. 2022;130(8):1187-1203. [CrossRef]
- McGonagle D, De Marco G, Bridgewood C. Mechanisms of Immunothrombosis in Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Compared to Natural SARS-CoV-2 Infection. Journal of Autoimmunity. 2021;121:102662. [CrossRef]
- Lindsay KE, Bhosle SM, Zurla C, et al. Visualization of early events in mRNA vaccine delivery in non-human primates via PET–CT and near-infrared imaging. Nat Biomed Eng. 2019;3(5):371-380. [CrossRef]
- Spike Protein Behavior. Accessed October 1, 2022. https://www.science.org/content/blog-post/spike-protein-behavior.
- Röltgen K, Nielsen SCA, Silva O, et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell. 2022;185(6):1025-1040.e14. [CrossRef]
- Bansal S, Perincheri S, Fleming T, et al. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. The Journal of Immunology. 2021;207(10):2405-2410. [CrossRef]
- Jaramillo C. Red Cross Accepts Blood Donations From People Vaccinated Against COVID-19. FactCheck.org. Published April 27, 2022. Accessed December 11, 2022. https://www.factcheck.org/2022/04/scicheck-red-cross-accepts-and-uses-blood-donations-from-people-vaccinated-against-covid-19/.
- Ogata AF, Cheng CA, Desjardins M, et al. Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients. Clinical Infectious Diseases. 2022;74(4):715-718. [CrossRef]
- Montague SJ, Smith CW, Lodwick CS, et al. Anti-platelet factor 4 immunoglobulin G levels in vaccine-induced immune thrombocytopenia and thrombosis: Persistent positivity through 7 months. Res Pract Thromb Haemost. 2022;6(3):e12707. [CrossRef]
- Sørvoll IH, Horvei KD, Ernstsen SL, et al. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J Thromb Haemost. 2021;19(7):1813-1818. [CrossRef]
- Panagiota V, Dobbelstein C, Werwitzke S, et al. Long-Term Outcomes after Vaccine-Induced Thrombotic Thrombocytopenia. Viruses. 2022;14(8):1702. [CrossRef]
- Al-Samkari H, Leaf RK, Goodarzi K. Transient Thrombocytopenia With Glycoprotein-Specific Platelet Autoantibodies After Ad26.COV2.S Vaccination: A Case Report. Ann Intern Med. 2021;174(11):1632-1633. [CrossRef]
- Vayne C, Rollin J, Gruel Y, et al. PF4 Immunoassays in Vaccine-Induced Thrombotic Thrombocytopenia. New England Journal of Medicine. 2021;385(4):376-378. [CrossRef]
- Schönborn L, Thiele T, Kaderali L, et al. Most anti-PF4 antibodies in vaccine-induced immune thrombotic thrombocytopenia are transient. Blood. 2022;139(12):1903-1907. [CrossRef]
- Gerotziafas GT, Elalamy I, Lecrubier C, et al. The role of platelet factor 4 in platelet aggregation induced by the antibodies implicated in heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2001;12(7):511-520. [CrossRef]
- Warkentin TE. Platelet-activating anti-PF4 disorders: An overview. Semin Hematol. 2022;59(2):59-71. [CrossRef]
- Vaccine Adverse Event Reporting System (VAERS). Accessed December 7, 2022. https://vaers.hhs.gov/.
- CDC. Enroll in v-safe after vaccination health checker. Centers for Disease Control and Prevention. Published November 14, 2022. Accessed December 7, 2022. https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/v-safe/index.html.
- Coronavirus vaccine - summary of Yellow Card reporting. GOV.UK. Accessed December 7, 2022. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting.
- EMA. EudraVigilance system overview. European Medicines Agency. Published September 17, 2018. Accessed December 7, 2022. https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance/eudravigilance-system-overview.
- Centre UM. VigiBase Services. Accessed December 7, 2022. https://who-umc.org/vigibase/vigibase-services/.
- Ceacareanu AC, Wintrob ZAP. Summary of COVID-19 Vaccine-Related Reports in the Vaccine Adverse Event Reporting System. J Res Pharm Pract. 2021;10(3):107-113. [CrossRef]
- Parmar K, Subramanyam S, Del Rio-Pertuz G, Sethi P, Argueta-Sosa E. Cardiac Adverse Events after Vaccination—A Systematic Review. Vaccines. 2022;10(5):700. [CrossRef]
- Jeet Kaur R, Dutta S, Charan J, et al. Cardiovascular Adverse Events Reported from COVID-19 Vaccines: A Study Based on WHO Database. Int J Gen Med. 2021;14:3909-3927. [CrossRef]
- Hajjo R, Sabbah DA, Bardaweel SK, Tropsha A. Shedding the Light on Post-Vaccine Myocarditis and Pericarditis in COVID-19 and Non-COVID-19 Vaccine Recipients. Vaccines. 2021;9(10):1186. [CrossRef]
- Welsh KJ, Baumblatt J, Chege W, Goud R, Nair N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2021;39(25):3329-3332. [CrossRef]
- Yan MM, Zhao H, Li ZR, et al. Serious adverse reaction associated with the COVID-19 vaccines of BNT162b2, Ad26.COV2.S, and mRNA-1273: Gaining insight through the VAERS. Front Pharmacol. 2022;13:921760. [CrossRef]
- Abrams CS, Barnes GD. SARS-CoV-2 Vaccination-Induced Thrombotic Thrombocytopenia: A Rare But Serious Immunologic Complication. Annual Review of Medicine. 2023;74(1):null. [CrossRef]
- Al-Maqbali JS, Rasbi SA, Kashoub MS, et al. A 59-Year-Old Woman with Extensive Deep Vein Thrombosis and Pulmonary Thromboembolism 7 Days Following a First Dose of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine. Am J Case Rep. 2021;22:e932946-1-e932946-4. [CrossRef]
- Wiest NE, Johns GS, Edwards E. A Case of Acute Pulmonary Embolus after mRNA SARS-CoV-2 Immunization. Vaccines. 2021;9(8):903. [CrossRef]
- Fazio S, Vaccariello M, Affuso F. A Case of Adverse Reaction to Booster Dose of COVID-19 Vaccination: Could D-Dimer Elevation Suggest Increased Clotting Risk? Health. 2022;14(2):204-208. [CrossRef]
- Malayala SV, Papudesi BN, Sharma R, Vusqa UT, Raza A. A Case of Idiopathic Thrombocytopenic Purpura After Booster Dose of BNT162b2 (Pfizer-Biontech) COVID-19 Vaccine. Cureus. 2021;13(10). [CrossRef]
- van Dijk MMH, Veldman HD, Aarts F, Barten DG, van den Bergh JP, Dielis AWJH. A case of unusual mild clinical presentation of COVID-19 vaccine-induced immune thrombotic thrombocytopenia with splanchnic vein thrombosis. Ann Hepatol. 2022;27(1):100590. [CrossRef]
- Lee CSM, Liang HPH, Connor DE, et al. A novel flow cytometry procoagulant assay for diagnosis of vaccine-induced immune thrombotic thrombocytopenia. Blood Adv. 2022;6(11):3494-3506. [CrossRef]
- Agbariah N, Bütler VA, Wieland A, Andina N, Hammann F, Kremer Hovinga JA. Acquired immune-mediated thrombotic thrombocytopenic pur-pura (iTTP) following mRNA-based COVID-19 vaccination (BNT162b2). Swiss Medical Weekly. Published online 2021:20S-20S.
- Ruhe J, Schnetzke U, Kentouche K, et al. Acquired thrombotic thrombocytopenic purpura after first vaccination dose of BNT162b2 mRNA COVID-19 vaccine. Ann Hematol. 2022;101(3):717-719. [CrossRef]
- Yoshida K, Sakaki A, Matsuyama Y, et al. Acquired Thrombotic Thrombocytopenic Purpura Following BNT162b2 mRNA Coronavirus Disease Vaccination in a Japanese Patient. Internal Medicine. 2022;61(3):407-412. [CrossRef]
- Ben Saida I, Maatouk I, Toumi R, et al. Acquired Thrombotic Thrombocytopenic Purpura Following Inactivated COVID-19 Vaccines: Two Case Reports and a Short Literature Review. Vaccines (Basel). 2022;10(7):1012. [CrossRef]
- McFadyen JD, Sharma P, Moon MJ, et al. Activation of circulating platelets in vaccine-induced thrombotic thrombocytopenia and its reversal by intravenous immunoglobulin. British Journal of Haematology. 2022;196(1):234-237. [CrossRef]
- Sung PS, Oh JS, Choi J. Acute Budd-Chiari syndrome with thrombotic thrombocytopenia after BNT162b2 mRNA vaccination. Liver Int. 2022;42(6):1447-1448. [CrossRef]
- Tajstra M, Jaroszewicz J, Gąsior M. Acute Coronary Tree Thrombosis After Vaccination for COVID-19. JACC: Cardiovascular Interventions. 2021;14(9):e103-e104. [CrossRef]
- Walter U, Fuchs M, Grossmann A, et al. Adenovirus-Vectored COVID-19 Vaccine–Induced Immune Thrombosis of Carotid Artery: A Case Report. Neurology. 2021;97(15):716-719. [CrossRef]
- Kolahchi Z, Khanmirzaei M, Mowla A. Acute ischemic stroke and vaccine-induced immune thrombotic thrombocytopenia post COVID-19 vaccination; a systematic review. J Neurol Sci. 2022;439:120327. [CrossRef]
- Costentin G, Ozkul-Wermester O, Triquenot A, et al. Acute Ischemic Stroke Revealing ChAdOx1 nCov-19 Vaccine-Induced Immune Thrombotic Thrombocytopenia: Impact on Recanalization Strategy. J Stroke Cerebrovasc Dis. 2021;30(9):105942. [CrossRef]
- Chen PW, Tsai ZY, Chao TH, Li YH, Hou CJY, Liu PY. Addressing Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT) Following COVID-19 Vaccination: A Mini-Review of Practical Strategies. Acta Cardiol Sin. 2021;37(4):355-364. [CrossRef]
- Lioudaki S, Kontopodis N, Pontikoglou C, et al. Multiple Sites of Arterial Thrombosis in A 35-Year Old Patient after ChAdOx1 (AstraZeneca) Vaccination, Requiring Emergent Femoral and Carotid Surgical Thrombectomy. Annals of Vascular Surgery. 2022;79:438.e1-438.e4. [CrossRef]
- Islam A, Bashir MS, Joyce K, Rashid H, Laher I, Elshazly S. An Update on COVID-19 Vaccine Induced Thrombotic Thrombocytopenia Syndrome and Some Management Recommendations. Molecules. 2021;26(16):5004. [CrossRef]
- Ahmed SH, Shaikh TG, Waseem S, Qadir NA, Yousaf Z, Ullah I. Vaccine-induced thrombotic thrombocytopenia following coronavirus vaccine: A narrative review. Annals of Medicine and Surgery. 2022;73:102988. [CrossRef]
- Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct Immune Globulin for Vaccine-Induced Immune Thrombotic Thrombocytopenia. New England Journal of Medicine. 2021;385(8):720-728. [CrossRef]
- Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021;596(7873):565-569. [CrossRef]
- Reilly-Stitt C, Kitchen S, Jennings I, et al. Anti-PF4 testing for vaccine-induced immune thrombocytopenia and thrombosis and heparin induced thrombocytopenia: Results from a UK National External Quality Assessment Scheme exercise April 2021. Journal of Thrombosis and Haemostasis. 2021;19(9):2263-2267. [CrossRef]
- Marcucci R, Marietta M. Vaccine-induced thrombotic thrombocytopenia: the elusive link between thrombosis and adenovirus-based SARS-CoV-2 vaccines. Intern Emerg Med. 2021;16(5):1113-1119. [CrossRef]
- Mancuso M, Lauretti DL, Cecconi N, et al. Arterial intracranial thrombosis as the first manifestation of vaccine-induced immune thrombotic thrombocytopenia (VITT): a case report. Neurol Sci. 2022;43(3):2085-2089. [CrossRef]
- Sessa A, Gattamorta M, Punginelli M, Maggioni G. Arterial Thrombosis in an Unusual Site (Ulnar Artery) after COVID-19 Vaccination—A Case Report. Clinics and Practice. 2022;12(3):237-242. [CrossRef]
- Chen VM, Curnow JL, Tran HA, Choi PY. Australian and New Zealand approach to diagnosis and management of vaccine-induced immune thrombosis and thrombocytopenia. Medical Journal of Australia. 2021;215(6):245. [CrossRef]
- Rodríguez Y, Rojas M, Beltrán S, et al. Autoimmune and autoinflammatory conditions after COVID-19 vaccination. New case reports and updated literature review. J Autoimmun. 2022;132:102898. [CrossRef]
- Gaignard ME, Lieberherr S, Schoenenberger A, Benz R. Autoimmune Hematologic Disorders in Two Patients After mRNA COVID-19 Vaccine. Hemasphere. 2021;5(8):e618. [CrossRef]
- Elrashdy F, Tambuwala MM, Hassan SkS, et al. Autoimmunity roots of the thrombotic events after COVID-19 vaccination. Autoimmunity Reviews. 2021;20(11):102941. [CrossRef]
- Ryan E, Benjamin D, McDonald I, et al. AZD1222 vaccine-related coagulopathy and thrombocytopenia without thrombosis in a young female. Br J Haematol. 2021;194(3):553-556. [CrossRef]
- Waqar U, Ahmed S, Gardezi SMHA, et al. Thrombosis with Thrombocytopenia Syndrome After Administration of AZD1222 or Ad26.COV2.S Vaccine for COVID-19: A Systematic Review. Clin Appl Thromb Hemost. 2021;27:10760296211068487. [CrossRef]
- Cari L, Fiore P, Naghavi Alhosseini M, Sava G, Nocentini G. Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: An analysis of European data. Journal of Autoimmunity. 2021;122:102685. [CrossRef]
- Ihnatko M, Truchla I, Ihnatková L, Prohászka Z, Lazúrová I. Case Report: A Case of COVID Vaccine-Induced Thrombotic Thrombocytopenia Manifested as Pulmonary Embolism and Hemorrhagia. A First Reported Case From Slovakia. Front Med (Lausanne). 2021;8:789972. [CrossRef]
- Braun T, Viard M, Juenemann M, et al. Case Report: Take a Second Look: Covid-19 Vaccination-Related Cerebral Venous Thrombosis and Thrombotic Thrombocytopenia Syndrome. Frontiers in Neurology. 2021;12. Accessed December 4, 2022. https://www.frontiersin.org/articles/10.3389/fneur.2021.763049.
- Su PH, Yu YC, Chen WH, et al. Case Report: Vaccine-Induced Immune Thrombotic Thrombocytopenia in a Pancreatic Cancer Patient After Vaccination With Messenger RNA−1273. Front Med (Lausanne). 2021;8:772424. [CrossRef]
- See I, Lale A, Marquez P, et al. Case Series of Thrombosis With Thrombocytopenia Syndrome After COVID-19 Vaccination—United States, December 2020 to August 2021. Ann Intern Med. 2022;175(4):513-522. [CrossRef]
- Siegler JE, Klein P, Yaghi S, et al. Cerebral Vein Thrombosis With Vaccine-Induced Immune Thrombotic Thrombocytopenia. Stroke. 2021;52(9):3045-3053. [CrossRef]
- Adekoya A, Adelekan-Popoola F, Fabamwo A, et al. Chadox1 Ncov-19 Vaccination: A Clinico-Pathological Review of Coagulation Derangement in Four Cases. 2022;9:11-21. [CrossRef]
- Suresh P, Petchey W. ChAdOx1 nCOV-19 vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis (CVST). BMJ Case Reports CP. 2021;14(6):e243931. [CrossRef]
- Greinacher A, Schönborn L, Siegerist F, et al. Pathogenesis of vaccine-induced immune thrombotic thrombocytopenia (VITT). Seminars in Hematology. 2022;59(2):97-107. [CrossRef]
- Sánchez van Kammen M, Aguiar de Sousa D, Poli S, et al. Characteristics and Outcomes of Patients With Cerebral Venous Sinus Thrombosis in SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. JAMA Neurol. 2021;78(11):1314-1323. [CrossRef]
- Lee AYY, Al Moosawi M, Peterson EA, et al. Clinical care pathway for the evaluation of patients with suspected VITT after ChAdOx1 nCoV-19 vaccination. Blood Adv. 2022;6(11):3315-3320. [CrossRef]
- Rizk JG, Gupta A, Sardar P, et al. Clinical Characteristics and Pharmacological Management of COVID-19 Vaccine-Induced Immune Thrombotic Thrombocytopenia With Cerebral Venous Sinus Thrombosis: A Review. JAMA Cardiol. 2021;6(12):1451-1460. [CrossRef]
- Mungmunpuntipantip R, Wiwanitkit V. COVID-19, neurovascular thrombotic problem and short summary on blood coagulation disorder: a brief review. Egypt J Neurol Psychiatr Neurosurg. 2022;58(1):6. [CrossRef]
- Khan Z, Besis G, Candilio L. COVID-19 Vaccine-Induced Thrombotic Thrombocytopaenia With Venous and Arterial Thrombosis: A Case Report. Cureus. 2022;14(8):e28535. [CrossRef]
- Pishko AM, Bussel JB, Cines DB. COVID-19 vaccination and immune thrombocytopenia. Nat Med. 2021;27(7):1145-1146. [CrossRef]
- Carli G, Nichele I, Ruggeri M, Barra S, Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med. 2021;16(3):803-804. [CrossRef]
- Ledford H. COVID vaccines and blood clots: five key questions. Nature. 2021;592(7855):495-496. [CrossRef]
- Greinacher A. COVID vaccine-induced immune thrombotic thrombocytopenia: Rare but relevant. Eur J Intern Med. 2022;105:20-22. [CrossRef]
- Lai CC, Ko WC, Chen CJ, et al. COVID-19 vaccines and thrombosis with thrombocytopenia syndrome. Expert Rev Vaccines. 2021;20(8):1027-1035. [CrossRef]
- Pavord S, Scully M, Hunt BJ, et al. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. New England Journal of Medicine. 2021;385(18):1680-1689. [CrossRef]
- Alam W. COVID-19 vaccine-induced immune thrombotic thrombocytopenia: A review of the potential mechanisms and proposed management. Sci Prog. 2021;104(2):368504211025927. [CrossRef]
- Deucher W, Sukumar S, Cataland SR. Clinical relapse of immune-mediated thrombotic thrombocytopenic purpura following COVID-19 vaccination. Research and Practice in Thrombosis and Haemostasis. 2022;6(1):e12658. [CrossRef]
- Scully M, Singh D, Lown R, et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(23):2202-2211. [CrossRef]
- Hwang J, Han YJ, Yon DK, et al. Clinical significance of hepatosplenic thrombosis in vaccine-induced immune thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. International Journal of Infectious Diseases. 2022;116:114-121. [CrossRef]
- Günther A, Brämer D, Pletz MW, et al. Complicated Long Term Vaccine Induced Thrombotic Immune Thrombocytopenia-A Case Report. Vaccines (Basel). 2021;9(11):1344. [CrossRef]
- Mekheal EM, Millet C, Mekheal N, Ghrewati M, Mechineni A, Maroules M. Coincidental or causal? A case report of acquired thrombotic thrombocytopenic purpura following mRNA-1273 Covid-19 vaccination. Hematology, Transfusion and Cell Therapy. Published online November 28, 2022. [CrossRef]
- Gan G, Liu H, Liang Z, Zhang G, Liu X, Ma L. Vaccine-associated thrombocytopenia. Thromb Res. 2022;220:12-20. [CrossRef]
- Alsmady MM, Al-Qaryouti RA, Sultan NG, Khrais OI, Khrais H. Upper Limb Ischemia Due to Arterial Thrombosis after COVID-19 Vaccination. Case Rep Med. 2022;2022:4819131. [CrossRef]
- Laroche A, Soulet D, Bazin M, et al. Live imaging of platelets and neutrophils during antibody-mediated neurovascular thrombosis. Blood Advances. 2022;6(12):3697-3702. [CrossRef]
- Bandapaati S, Bobba H, Navinan MR. Coeliac artery and splenic artery thrombosis complicated with splenic infarction 7 days following the first dose of Oxford vaccination, causal relationship or coincidence? BMJ Case Reports CP. 2021;14(7):e243799. [CrossRef]
- Chang JC, Hawley HB. Vaccine-Associated Thrombocytopenia and Thrombosis: Venous Endotheliopathy Leading to Venous Combined Micro-Macrothrombosis. Medicina. 2021;57(11):1163. [CrossRef]
- Pavord S, Hunt BJ, Horner D, Bewley S, Karpusheff J. Vaccine induced immune thrombocytopenia and thrombosis: summary of NICE guidance. BMJ. 2021;375:n2195. [CrossRef]
- Yamada S, Asakura H. Coagulopathy and Fibrinolytic Pathophysiology in COVID-19 and SARS-CoV-2 Vaccination. International Journal of Molecular Sciences. 2022;23(6):3338. [CrossRef]
- Abdel-Bakky MS, Amin E, Ewees MG, et al. Coagulation System Activation for Targeting of COVID-19: Insights into Anticoagulants, Vaccine-Loaded Nanoparticles, and Hypercoagulability in COVID-19 Vaccines. Viruses. 2022;14(2):228. [CrossRef]
- Franchini M, Liumbruno GM, Pezzo M. COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): Diagnostic and therapeutic recommendations for a new syndrome. European Journal of Haematology. 2021;107(2):173-180. [CrossRef]
- Thachil J. COVID-19 Vaccine-Induced Immune Thrombosis with Thrombocytopenia (VITT) and the Shades of Grey in Thrombus Formation. Semin Thromb Hemost. 2022;48(1):15-18. [CrossRef]
- Thaler J, Jilma P, Samadi N, et al. Long-term follow-up after successful treatment of vaccine-induced prothrombotic immune thrombocytopenia. Thrombosis Research. 2021;207:126-130. [CrossRef]
- Abrignani MG, Murrone A, De Luca L, et al. COVID-19, Vaccines, and Thrombotic Events: A Narrative Review. J Clin Med. 2022;11(4):948. [CrossRef]
- Danish F i A, Rabani AE, Subhani F e R, Yasmin S, Koul SS. COVID-19: Vaccine-induced immune thrombotic thrombocytopenia. European Journal of Haematology. 2022;109(6):619-632. [CrossRef]
- Napoli R, Visonà E. Deep vein thrombosis and acute hepatitis after ChAdOx1 nCov-19 vaccination in a Charcot-Marie-Tooth patient: a case report. Clin Exp Vaccine Res. 2022;11(3):294-297. [CrossRef]
- Mouta Nunes de Oliveira P, Mendes-de-Almeida DP, Bertollo Gomes Porto V, et al. Vaccine-induced immune thrombotic thrombocytopenia after COVID-19 vaccination: Description of a series of 39 cases in Brazil. Vaccine. 2022;40(33):4788-4795. [CrossRef]
- Herrera-Comoglio R, Lane S. Vaccine-Induced Immune Thrombocytopenia and Thrombosis after the Sputnik V Vaccine. New England Journal of Medicine. 2022;387(15):1431-1432. [CrossRef]
- Dotan A, Shoenfeld Y. Perspectives on vaccine induced thrombotic thrombocytopenia. Journal of Autoimmunity. 2021;121:102663. [CrossRef]
- Lavin M, Elder PT, O’Keeffe D, et al. Vaccine-induced immune thrombotic thrombocytopenia (VITT) – a novel clinico-pathological entity with heterogeneous clinical presentations. British Journal of Haematology. 2021;195(1):76-84. [CrossRef]
- Hrastelj J, Robertson NP. Vaccine-induced immune thrombosis and thrombocytopaenia: incidence, mechanism and treatment. J Neurol. 2021;268(11):4396-4397. [CrossRef]
- De Michele M, Iacobucci M, Chistolini A, et al. Malignant cerebral infarction after ChAdOx1 nCov-19 vaccination: a catastrophic variant of vaccine-induced immune thrombotic thrombocytopenia. Nat Commun. 2021;12(1):4663. [CrossRef]
- Smadja DM, Yue QY, Chocron R, Sanchez O, Louet ALL. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. European Respiratory Journal. 2021;58(1). [CrossRef]
- Atoui A, Jarrah K, Al Mahmasani L, Bou-Fakhredin R, Taher AT. Deep venous thrombosis and pulmonary embolism after COVID-19 mRNA vaccination. Ann Hematol. 2022;101(5):1111-1113. [CrossRef]
- Wiedmann M, Skattør T, Stray-Pedersen A, et al. Vaccine Induced Immune Thrombotic Thrombocytopenia Causing a Severe Form of Cerebral Venous Thrombosis With High Fatality Rate: A Case Series. Frontiers in Neurology. 2021;12. Accessed December 4, 2022. https://www.frontiersin.org/articles/10.3389/fneur.2021.721146.
- Bérezné A, Bougon D, Blanc-Jouvan F, et al. Deterioration of vaccine-induced immune thrombotic thrombocytopenia treated by heparin and platelet transfusion: Insight from functional cytometry and serotonin release assay. Research and Practice in Thrombosis and Haemostasis. 2021;5(6):e12572. [CrossRef]
- Furie KL, Cushman M, Elkind MSV, Lyden PD, Saposnik G, null null. Diagnosis and Management of Cerebral Venous Sinus Thrombosis With Vaccine-Induced Immune Thrombotic Thrombocytopenia. Stroke. 2021;52(7):2478-2482. [CrossRef]
- Pai M, Grill A, Ivers N, et al. Vaccine Induced Prothrombotic Immune Thrombocytopenia (VIPIT) Following AstraZeneca COVID-19 Vaccination. Ontario COVID-19 Science Advisory Table; 2021. [CrossRef]
- Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hamostaseologie. 2021;41(3):184-189. [CrossRef]
- Long B, Bridwell R, Gottlieb M. Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines. The American Journal of Emergency Medicine. 2021;49:58-61. [CrossRef]
- Ceschia N, Scheggi V, Gori AM, et al. Diffuse prothrombotic syndrome after ChAdOx1 nCoV-19 vaccine administration: a case report. J Med Case Reports. 2021;15(1):496. [CrossRef]
- Klok FA, Pai M, Huisman MV, Makris M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022;9(1):e73-e80. [CrossRef]
- Pai M. Epidemiology of VITT. Seminars in Hematology. 2022;59(2):72-75. [CrossRef]
- Brazete C, Aguiar A, Furtado I, Duarte R. Thrombotic events and COVID-19 vaccines. The International Journal of Tuberculosis and Lung Disease. 2021;25(9):701-707. [CrossRef]
- Berlot G, Tomasini A, La Fata C, Pintacuda S, Rigutti S, Falanga A. Widespread Arterial Thrombosis after ChAdOx1 nCov-19 Vaccination. Case Reports in Critical Care. 2022;2022:e6804456. [CrossRef]
- Taylor P, Allen L, Shrikrishnapalasuriyar N, Stechman M, Rees A. Vaccine-induced thrombosis and thrombocytopenia with bilateral adrenal haemorrhage. Clin Endocrinol (Oxf). 2022;97(1):26-27. [CrossRef]
- Makris M, Pavord S, Lester W, Scully M, Hunt B. Vaccine-induced Immune Thrombocytopenia and Thrombosis (VITT). Res Pract Thromb Haemost. 2021;5(5):e12529. [CrossRef]
- Guditi S, Setty G, Verma M, et al. Vaccine-Induced Thrombotic Thrombocytopenia Due to Coronavirus Disease 2019 Vaccine From a Deceased Donor: A Case Report. Transplant Proc. 2022;54(6):1534-1538. [CrossRef]
- Salih F, Schönborn L, Kohler S, et al. Vaccine-Induced Thrombocytopenia with Severe Headache. New England Journal of Medicine. 2021;385(22):2103-2105. [CrossRef]
- Lai CMB, Lee AYY, Parkin SBI. Vaccine-induced prothrombotic immune thrombocytopenia without thrombosis may not require immune modulatory therapy: A case report. Research and Practice in Thrombosis and Haemostasis. 2022;6(4):e12716. [CrossRef]
- Al Rawahi B, BaTaher H, Jaffer Z, Al-Balushi A, Al-Mazrouqi A, Al-Balushi N. Vaccine-induced immune thrombotic thrombocytopenia following AstraZeneca (ChAdOx1 nCOV19) vaccine–A case report. Research and Practice in Thrombosis and Haemostasis. 2021;5(6):e12578. [CrossRef]
- Dalan R, Boehm BO. Thrombosis post COVID-19 vaccinations: Potential link to ACE pathways. Thrombosis Research. 2021;206:137-138. [CrossRef]
- Sachs UJ, Cooper N, Czwalinna A, et al. PF4-Dependent Immunoassays in Patients with Vaccine-Induced Immune Thrombotic Thrombocytopenia: Results of an Interlaboratory Comparison. Thromb Haemost. 2021;121(12):1622-1627. [CrossRef]
- Curcio R, Gandolfo V, Alcidi R, et al. Vaccine-induced massive pulmonary embolism and thrombocytopenia following a single dose of Janssen Ad26.COV2.S vaccination. International Journal of Infectious Diseases. 2022;116:154-156. [CrossRef]
- Aladdin Y, Algahtani H, Shirah B. Vaccine-Induced Immune Thrombotic Thrombocytopenia with Disseminated Intravascular Coagulation and Death following the ChAdOx1 nCoV-19 Vaccine. Journal of Stroke and Cerebrovascular Diseases. 2021;30(9):105938. [CrossRef]
- Charidimou A, Samudrala S, Cervantes-Arslanian AM, Sloan JM, Dasenbrock HH, Daneshmand A. Vaccine-Induced Immune Thrombotic Thrombocytopenia with Concurrent Arterial and Venous Thrombi Following Ad26.COV2.S Vaccination. Journal of Stroke and Cerebrovascular Diseases. 2021;30(12). [CrossRef]
- Connors JM, Iba T. Vaccine-induced immune thrombotic thrombocytopenia and patients with cancer. Thrombosis Research. 2022;213:S77-S83. [CrossRef]
- Sharifian-Dorche M, Bahmanyar M, Sharifian-Dorche A, Mohammadi P, Nomovi M, Mowla A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. Journal of the Neurological Sciences. 2021;428:117607. [CrossRef]
- Mohseni Afshar Z, Babazadeh A, Janbakhsh A, et al. Vaccine-induced immune thrombotic thrombocytopenia after vaccination against Covid-19: A clinical dilemma for clinicians and patients. Reviews in Medical Virology. 2022;32(2):e2273. [CrossRef]
- Warkentin TE, Pai M. The Epidemiology of Thrombosis With Thrombocytopenia Syndrome: Analogies With Heparin-Induced Thrombocytopenia. Ann Intern Med. 2022;175(4):604-605. [CrossRef]
- Kuter DJ. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. British Journal of Haematology. 2021;195(3):365-370. [CrossRef]
- Graça LL, Amaral MJ, Serôdio M, Costa B. Extensive thrombosis after COVID-19 vaccine: cause or coincidence? BMJ Case Reports CP. 2021;14(8):e244878. [CrossRef]
- Gresele P, Marietta M, Ageno W, et al. Management of cerebral and splanchnic vein thrombosis associated with thrombocytopenia in subjects previously vaccinated with Vaxzevria (AstraZeneca): a position statement from the Italian Society for the Study of Haemostasis and Thrombosis (SISET). Blood Transfus. 2021;19(4):281-283. [CrossRef]
- Toom S, Wolf B, Avula A, Peeke S, Becker K. Familial thrombocytopenia flare-up following the first dose of mRNA-1273 Covid-19 vaccine. Am J Hematol. 2021;96(5):E134-E135. [CrossRef]
- de Bruijn S, Maes MB, De Waele L, Vanhoorelbeke K, Gadisseur A. First report of a de novo iTTP episode associated with an mRNA-based anti-COVID-19 vaccination. Journal of Thrombosis and Haemostasis. 2021;19(8):2014-2018. [CrossRef]
- Lee E, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534-537. [CrossRef]
- Marietta M, Coluccio V, Luppi M. Potential mechanisms of vaccine-induced thrombosis. Eur J Intern Med. 2022;105:1-7. [CrossRef]
- Anderson A, Seddon M, Shahzad K, Lunevicius R. Post-COVID-19 vaccination occurrence of splenic infarction due to arterial thrombosis. BMJ Case Reports CP. 2021;14(12):e243846. [CrossRef]
- Sangli S, Virani A, Cheronis N, et al. Thrombosis With Thrombocytopenia After the Messenger RNA–1273 Vaccine. Ann Intern Med. 2021;174(10):1480-1482. [CrossRef]
- Kenda J, Lovrič D, Škerget M, Milivojević N. Treatment of ChAdOx1 nCoV-19 Vaccine-Induced Immune Thrombotic Thrombocytopenia Related Acute Ischemic Stroke. J Stroke Cerebrovasc Dis. 2021;30(11):106072. [CrossRef]
- Othman M, Baker AT, Gupalo E, et al. To clot or not to clot? Ad is the question—Insights on mechanisms related to vaccine-induced thrombotic thrombocytopenia. Journal of Thrombosis and Haemostasis. 2021;19(11):2845-2856. [CrossRef]
- Hosseinzadeh R, Barary M, Mehdinezhad H, Sio TT, Langer F, Khosravi S. Thrombotic thrombocytopenia After Sinopharm BBIBP-CorV COVID-19 vaccination. Research and Practice in Thrombosis and Haemostasis. 2022;6(4):e12750. [CrossRef]
- Gabarin N, Arnold DM, Nazy I, Warkentin TE. Treatment of vaccine-induced immune thrombotic thrombocytopenia (VITT). Semin Hematol. 2022;59(2):89-96. [CrossRef]
- Rzymski P, Perek B, Flisiak R. Thrombotic Thrombocytopenia after COVID-19 Vaccination: In Search of the Underlying Mechanism. Vaccines. 2021;9(6):559. [CrossRef]
- Stoll SE, Werner P, Wetsch WA, et al. Transjugular intrahepatic portosystemic shunt, local thrombaspiration, and lysis for management of fulminant portomesenteric thrombosis and atraumatic splenic rupture due to vector-vaccine-induced thrombotic thrombocytopenia: a case report. Journal of Medical Case Reports. 2022;16(1):271. [CrossRef]
- Kim AY, Woo W, Yon DK, et al. Thrombosis patterns and clinical outcome of COVID-19 vaccine-induced immune thrombotic thrombocytopenia: A Systematic Review and Meta-Analysis. International Journal of Infectious Diseases. 2022;119:130-139. [CrossRef]
- Iba T, Levy JH. Thrombosis and thrombocytopenia in COVID-19 and after COVID-19 vaccination. Trends in Cardiovascular Medicine. 2022;32(5):249-256. [CrossRef]
- Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. New England Journal of Medicine. 2021;384(22):2124-2130. [CrossRef]
- Nicolai L, Leunig A, Pekayvaz K, et al. Thrombocytopenia and splenic platelet-directed immune responses after IV ChAdOx1 nCov-19 administration. Blood. 2022;140(5):478-490. [CrossRef]
- Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and Intracranial Venous Sinus Thrombosis after “COVID-19 Vaccine AstraZeneca” Exposure. Journal of Clinical Medicine. 2021;10(8):1599. [CrossRef]
- Patriquin CJ, Laroche V, Selby R, et al. Therapeutic Plasma Exchange in Vaccine-Induced Immune Thrombotic Thrombocytopenia. New England Journal of Medicine. 2021;385(9):857-859. [CrossRef]
- Thaler J, Ay C, Gleixner KV, et al. Successful treatment of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT). Journal of Thrombosis and Haemostasis. 2021;19(7):1819-1822. [CrossRef]
- Helms JM, Ansteatt KT, Roberts JC, et al. Severe, Refractory Immune Thrombocytopenia Occurring After SARS-CoV-2 Vaccine. J Blood Med. 2021;12:221-224. [CrossRef]
- Ling VWT, Fan BE, Lau SL, Lee XH, Tan CW, Lee SY. Severe Thrombocytopenia, Thrombosis and Anti-PF4 Antibody after Pfizer-BioNTech COVID-19 mRNA Vaccine Booster—Is It Vaccine-Induced Immune Thrombotic Thrombocytopenia? Vaccines. 2022;10(12):2023. [CrossRef]
- Afshar ZM, Barary M, Babazadeh A, et al. SARS-CoV-2-related and Covid-19 vaccine-induced thromboembolic events: A comparative review. Reviews in Medical Virology. 2022;32(4):e2327. [CrossRef]
- Hsiao PJ, Wu KL, Chen YC, et al. The role of anti-platelet factor 4 antibodies and platelet activation tests in patients with vaccine-induced immune thrombotic thrombocytopenia: Brief report on a comparison of the laboratory diagnosis and literature review. Clin Chim Acta. 2022;529:42-45. [CrossRef]
- Cines DB, Bussel JB. SARS-CoV-2 Vaccine–Induced Immune Thrombotic Thrombocytopenia. New England Journal of Medicine. 2021;384(23):2254-2256. [CrossRef]
- Liu Y, Shao Z, Wang H. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. Thrombosis Research. 2022;209:75-79. [CrossRef]
- Kashir J, Ambia AR, Shafqat A, Sajid MR, AlKattan K, Yaqinuddin A. Scientific premise for the involvement of neutrophil extracellular traps (NETs) in vaccine-induced thrombotic thrombocytopenia (VITT). Journal of Leukocyte Biology. 2022;111(3):725-734. [CrossRef]
- Kalaska B, Miklosz J, Swieton J, Jakimczuk A, Pawlak D, Mogielnicki A. The effect of ChAdOx1 nCov-19 vaccine on arterial thrombosis development and platelet aggregation in female rats. Vaccine. 2022;40(13):1996-2002. [CrossRef]
- Pavenski K. Relapse of Immune Thrombotic Thrombocytopenic Purpura Following Vaccination with COVID19 mRNA Vaccine. TH Open. 2021;05(3):e335-e337. [CrossRef]
- Rock G, Weber V, Stegmayr B. Therapeutic plasma exchange (TPE) as a plausible rescue therapy in severe vaccine-induced immune thrombotic thrombocytopenia. Transfusion and Apheresis Science. 2021;60(4):103174. [CrossRef]
- Okada Y, Sakai R, Sato-Fitoussi M, et al. Potential Triggers for Thrombocytopenia and/or Hemorrhage by the BNT162b2 Vaccine, Pfizer-BioNTech. Front Med (Lausanne). 2021;8:751598. [CrossRef]
- Chong KM, Yang CY, Lin CC, Lien WC. Severe immune thrombocytopenia following COVID-19 vaccination (Moderna) and immune checkpoint inhibitor. The American Journal of Emergency Medicine. 2022;56:395.e1-395.e3. [CrossRef]
- Schettle S, Frantz R, Stulak J, Villavicencio M, Rosenbaum A. HeartWare Thrombosis After mRNA COVID-19 Vaccination. Mayo Clinic Proceedings. 2022;97(7):1399-1401. [CrossRef]
- Jacob C, Rani KA, Holton PJ, et al. Malignant middle cerebral artery syndrome with thrombotic thrombocytopenia following vaccination against SARS-CoV-2. Journal of the Intensive Care Society. 2022;23(4):479-484. [CrossRef]
- Cliff-Patel N, Moncrieff L, Ziauddin V. Renal Vein Thrombosis and Pulmonary Embolism Secondary to Vaccine-induced Thrombotic Thrombocytopenia (VITT). Eur J Case Rep Intern Med. 2021;8(6):002692. [CrossRef]
- Melas N. “Portal vein thrombosis occurring after the first dose of mRNA SARS-CoV-2 vaccine in a patient with antiphospholipid syndrome.” Thrombosis Update. 2021;5:100069. [CrossRef]
- Marchandot B, Carmona A, Trimaille A, Curtiaud A, Morel O. Procoagulant microparticles: a possible link between vaccine-induced immune thrombocytopenia (VITT) and cerebral sinus venous thrombosis. J Thromb Thrombolysis. 2021;52(3):689-691. [CrossRef]
- Favaloro EJ, Pasalic L, Lippi G. Review and evolution of guidelines for diagnosis of COVID-19 vaccine induced thrombotic thrombocytopenia (VITT). Clinical Chemistry and Laboratory Medicine (CCLM). 2022;60(1):7-17. [CrossRef]
- Franceschi AM, Petrover DR, McMahon TM, et al. Retrospective review COVID-19 vaccine induced thrombotic thrombocytopenia and cerebral venous thrombosis-what can we learn from the immune response. Clin Imaging. 2022;90:63-70. [CrossRef]
- Lindhoff-Last E, Schoenborn L, Piorkowski M, et al. Heterogeneity of Vaccine-Induced Immune Thrombotic Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination and Safety of Second Vaccination with BNT162b2. Thromb Haemost. 2022;122(2):304-307. [CrossRef]
- Sissa C, Al-Khaffaf A, Frattini F, et al. Relapse of thrombotic thrombocytopenic purpura after COVID-19 vaccine. Transfusion and Apheresis Science. 2021;60(4). [CrossRef]
- Leung HHL, Perdomo J, Ahmadi Z, et al. NETosis and thrombosis in vaccine-induced immune thrombotic thrombocytopenia. Nat Commun. 2022;13(1):5206. [CrossRef]
- Wang CA, Yeh JS, Hong CY. Repeated Coronary Artery Thrombosis after mRNA-1273 COVID-19 Vaccination. Acta Cardiol Sin. 2022;38(6):793-795. [CrossRef]
- Refractory vaccine-induced immune thrombotic thrombocytopenia (VITT) managed with delayed therapeutic plasma exchange (TPE). [CrossRef]
- Iba T, Levy JH, Warkentin TE. Recognizing Vaccine-Induced Immune Thrombotic Thrombocytopenia. Crit Care Med. 2022;50(1):e80-e86. [CrossRef]
- Julian JA, Mathern DR, Fernando D. Idiopathic Thrombocytopenic Purpura and the Moderna Covid-19 Vaccine. Annals of Emergency Medicine. 2021;77(6):654-656. [CrossRef]
- Paul M, Abraham L, Sophy M, Varghese D, Thomas J. Idiopathic Thrombotic Microangiopathy with ChAdOx1 nCov-19 Vaccination in a Middle Aged Male: A Clinical Challenge in the Covid Era. Published online July 25, 2021:431.
- Pasin F, Calabrese A, Pelagatti L. Immune thrombocytopenia following COVID-19 mRNA vaccine: casuality or causality? Intern Emerg Med. 2022;17(1):295-297. [CrossRef]
- Choi PYI, Hsu D, Tran HA, et al. Immune thrombocytopenia following vaccination during the COVID-19 pandemic. Haematologica. 2021;107(5):1193-1196. [CrossRef]
- Tarawneh O, Tarawneh H. Immune thrombocytopenia in a 22-year-old post Covid-19 vaccine. Am J Hematol. 2021;96(5):E133-E134. [CrossRef]
- Condorelli A, Markovic U, Sciortino R, Di Giorgio MA, Nicolosi D, Giuffrida G. Immune Thrombocytopenic Purpura Cases Following COVID-19 Vaccination. Mediterr J Hematol Infect Dis. 2021;13(1):e2021047. [CrossRef]
- Kelton JG, Arnold DM, Nazy I. Lessons from vaccine-induced immune thrombotic thrombocytopenia. Nat Rev Immunol. 2021;21(12):753-755. [CrossRef]
- Favaloro EJ. Laboratory testing for suspected COVID-19 vaccine–induced (immune) thrombotic thrombocytopenia. International Journal of Laboratory Hematology. 2021;43(4):559-570. [CrossRef]
- Thiele T, Weisser K, Schönborn L, et al. Laboratory confirmed vaccine-induced immune thrombotic thrombocytopenia: Retrospective analysis of reported cases after vaccination with ChAdOx-1 nCoV-19 in Germany. Lancet Reg Health Eur. 2022;12:100270. [CrossRef]
- Al-Ahmad M, Al Rasheed M, Altourah L, Rodriguez-Bouza T, Shalaby N. Isolated thrombosis after COVID-19 vaccination: case series. Int J Hematol. 2022;115(2):153-157. [CrossRef]
- Malayala SV, Mohan G, Vasireddy D, Atluri P. Purpuric Rash and Thrombocytopenia After the mRNA-1273 (Moderna) COVID-19 Vaccine. Cureus. 2021;13(3). [CrossRef]
- Mendes-de-Almeida DP, Martins-Gonçalves R, Morato-Santos R, et al. Intracerebral hemorrhage associated with vaccine-induced thrombotic thrombocytopenia following ChAdOx1 nCOVID-19 vaccine in a pregnant woman. Haematologica. 2021;106(11):3025-3028. [CrossRef]
- Passariello M, Vetrei C, Amato F, De Lorenzo C. Interactions of Spike-RBD of SARS-CoV-2 and Platelet Factor 4: New Insights in the Etiopathogenesis of Thrombosis. Int J Mol Sci. 2021;22(16):8562. [CrossRef]
- Greinacher A, Selleng K, Palankar R, et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138(22):2256-2268. [CrossRef]
- Zheng X, Gao F, Wang L, Meng Y, Ageno W, Qi X. Incidence and outcomes of splanchnic vein thrombosis after diagnosis of COVID-19 or COVID-19 vaccination: a systematic review and meta-analysis. J Thromb Thrombolysis. Published online November 19, 2022:1-14. [CrossRef]
- Dix C, McFadyen J, Huang A, Chunilal S, Chen V, Tran H. Understanding vaccine-induced thrombotic thrombocytopenia (VITT). Internal Medicine Journal. 2022;52(5):717-723. [CrossRef]
- Tsilingiris D, Vallianou NG, Karampela Ι, Dalamaga Μ. Vaccine induced thrombotic thrombocytopenia: The shady chapter of a success story. Metabolism Open. 2021;11:100101. [CrossRef]
- Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. Journal of Thrombosis and Haemostasis. 2021;19(7):1771-1775. [CrossRef]
- Al-Mayhani T, Saber S, Stubbs MJ, et al. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J Neurol Neurosurg Psychiatry. 2021;92(11):1247-1248. [CrossRef]
- Cascio Rizzo A, Giussani G, Agostoni EC. Ischemic Stroke and Vaccine-Induced Immune Thrombotic Thrombocytopenia following COVID-19 Vaccine: A Case Report with Systematic Review of the Literature. Cerebrovasc Dis. Published online May 5, 2022:1-13. [CrossRef]
- Ferro JM, Sousa DA de, Coutinho JM, Martinelli I. European stroke organization interim expert opinion on cerebral venous thrombosis occurring after SARS-CoV-2 vaccination. European Stroke Journal. 2021;6(3):CXVI-CXXI. [CrossRef]
- Mereuta OM, Rossi R, Douglas A, et al. Characterization of the ‘White’ Appearing Clots that Cause Acute Ischemic Stroke. Journal of Stroke and Cerebrovascular Diseases. 2021;30(12):106127. [CrossRef]
- Rahmig J, Altarsha E, Siepmann T, Barlinn K. Acute Ischemic Stroke in the Context of SARS-CoV-2 Vaccination: A Systematic Review. Neuropsychiatr Dis Treat. 2022;18:1907-1916. [CrossRef]
- Saleh M, Zimmermann J, Lehnen NC, Pötzsch B, Weller JM. Late-Onset Vaccine-Induced Immune Thombotic Thrombocytopenia (VITT) with Cerebral Venous Sinus Thrombosis. J Stroke Cerebrovasc Dis. 2022;31(4):106311. [CrossRef]
- Stefanou MI, Palaiodimou L, Sousa DA de, et al. Acute Arterial Ischemic Stroke Following COVID-19 Vaccination: A Systematic Review and Meta-analysis. Neurology. 2022;99(14):e1465-e1474. [CrossRef]
- Wills A, Swallow G, Kirkman MA, Rajan K, Subramanian G. Arterial and venous thrombotic stroke after ChAdOx1 nCoV-19 vaccine. Clin Med (Lond). 2022;22(2):184-186. [CrossRef]
- van de Munckhof A, Lindgren E, Kleinig TJ, et al. Outcomes of Cerebral Venous Thrombosis due to Vaccine-Induced Immune Thrombotic Thrombocytopenia After the Acute Phase. Stroke. 2022;53(10):3206-3210. [CrossRef]
- Alonso Castillo R, Martínez Castrillo JC. Neurological manifestations associated with COVID-19 vaccine. Neurologia (Engl Ed). Published online October 23, 2022:S2173-5808(22)00141-9. [CrossRef]
- Mohseni Afshar Z, Sharma A, Babazadeh A, et al. A review of the potential neurological adverse events of COVID-19 vaccines. Acta Neurol Belg. Published online November 16, 2022:1-36. [CrossRef]
- Dutta S, Kaur R, Charan J, et al. Analysis of Neurological Adverse Events Reported in VigiBase From COVID-19 Vaccines. Cureus. 2022;14(1):e21376. [CrossRef]
- Mirandola L, Arena G, Pagliaro M, et al. Massive cerebral venous sinus thrombosis in vaccine-induced immune thrombotic thrombocytopenia after ChAdOx1 nCoV-19 serum: case report of a successful multidisciplinary approach. Neurol Sci. 2022;43(3):1499-1502. [CrossRef]
- Sriwastava S, Sharma K, Khalid SH, et al. COVID-19 Vaccination and Neurological Manifestations: A Review of Case Reports and Case Series. Brain Sci. 2022;12(3):407. [CrossRef]
- Dutta A, Ghosh R, Bhattacharya D, et al. Anti-PF4 antibody negative cerebral venous sinus thrombosis without thrombocytopenia following immunization with COVID-19 vaccine in an elderly non-comorbid Indian male, managed with conventional heparin-warfarin based anticoagulation. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2021;15(4):102184. [CrossRef]
- Park J, Park MS, Kim HJ, Song TJ. Association of Cerebral Venous Thrombosis with mRNA COVID-19 Vaccines: A Disproportionality Analysis of the World Health Organization Pharmacovigilance Database. Vaccines. 2022;10(5):799. [CrossRef]
- Taquet M, Husain M, Geddes JR, Luciano S, Harrison PJ. Cerebral venous thrombosis: a retrospective cohort study of 513,284 confirmed COVID-19 cases and a comparison with 489,871 people receiving a COVID-19 mRNA vaccine. :23.
- Fan BE, Shen JY, Lim XR, et al. Cerebral venous thrombosis post BNT162b2 mRNA SARS-CoV-2 vaccination: A black swan event. Am J Hematol. 2021;96(9):E357-E361. [CrossRef]
- Franchini M, Testa S, Pezzo M, et al. Cerebral venous thrombosis and thrombocytopenia post-COVID-19 vaccination. Thrombosis Research. 2021;202:182-183. [CrossRef]
- de Gregorio C, Colarusso L, Calcaterra G, et al. Cerebral Venous Sinus Thrombosis following COVID-19 Vaccination: Analysis of 552 Worldwide Cases. Vaccines. 2022;10(2):232. [CrossRef]
- Omidian N, Mohammadi P, Sadeghalvad M, Mohammadi-Motlagh HR. Cerebral microvascular complications associated with SARS-CoV-2 infection: How did it occur and how should it be treated? Biomed Pharmacother. 2022;154:113534. [CrossRef]
- De Michele M, Iacobucci M, Nicolini E, et al. Malignant Cerebral Infarction, Systemic Venous Thrombosis and Thrombocytopenia after ChAdOx1 nCov Vaccination: A Possible Catastrophic Variant of Vaccine Induced Thrombotic Thrombocytopenia. In Review; 2021. [CrossRef]
- Schulz JB, Berlit P, Diener HC, et al. COVID-19 Vaccine-Associated Cerebral Venous Thrombosis in Germany. Annals of Neurology. 2021;90(4):627-639. [CrossRef]
- Fadul A, Abdalla Elm, Abdelmahmuod E, et al. COVID-19 Vaccine-Induced Cerebral Sinus Thrombosis: Coincidence vs. Cause? Cureus. 2022;14(6):e26436. [CrossRef]
- Bs T. Acute Hemoglobin Decline with Signs of Hemolysis in Chronically Transfused Beta-thalassemia Patient Post Pfizer-BioNTech COVID-19 (BNT162b2) Vaccine: A Case Report. :4.
- Kumar TA, Sunka S. A Rare Case of M-RNA Vaccine (COVID-19) Induced Autoimmune Hemolytic Anaemia. Indian Journal of Critical Care Medicine. Published online 2022:S13-S13.
- Delaporta P, Lampropoulou E, Moschoviti A, et al. Adverse Events Following COVID-19 Vaccination in Transfusion-Dependent -Thalassemia Patients. Blood. 2021;138:2015. [CrossRef]
- Ferrer F, Roldão M, Figueiredo C, Lopes K. Atypical Hemolytic Uremic Syndrome after ChAdOx1 nCoV-19 Vaccination in a Patient with Homozygous CFHR3/CFHR1 Gene Deletion. NEF. 2022;146(2):185-189. [CrossRef]
- Fattizzo B, Barcellini W. Autoimmune hemolytic anemia: causes and consequences. Expert Review of Clinical Immunology. 2022;18(7):731-745. [CrossRef]
- Rico AC, Ferreirol AG, Zorilla SR, Gonzalez AM. Drug-induced autoimmune hemolytic anemia, as an adverse effect to a vaccine against COVID-19, description of 1 case and review of the literature. Galicia Clinica. Published online 2021:218-219.
- Kamura Y, Sakamoto T, Yokoyama Y, et al. Hemolysis induced by SARS-CoV-2 mRNA vaccination in patients with paroxysmal nocturnal hemoglobinuria. Int J Hematol. 2022;116(1):55-59. [CrossRef]
- Al-kuraishy HM, Al-Gareeb AI, Kaushik A, Kujawska M, Batiha GES. Hemolytic anemia in COVID-19. Ann Hematol. 2022;101(9):1887-1895. [CrossRef]
- Pérez-Lamas L, Moreno-Jiménez G, Tenorio-Núñez MC, et al. Hemolytic crisis due to Covid-19 vaccination in a woman with cold agglutinin disease. Am J Hematol. 2021;96(8):E288-E291. [CrossRef]
- Shakoor MT, Birkenbach MP, Lynch M. ANCA-Associated Vasculitis Following Pfizer-BioNTech COVID-19 Vaccine. American Journal of Kidney Diseases. 2021;78(4):611-613. [CrossRef]
- Mücke VT, Knop V, Mücke MM, Ochsendorf F, Zeuzem S. First description of immune complex vasculitis after COVID-19 vaccination with BNT162b2: a case report. BMC Infect Dis. 2021;21(1):958. [CrossRef]
- Chen CY, Chen TT, Hsieh CY, Lien MY, Yeh SP, Chen CC. Case reports of management of aplastic anemia after COVID-19 vaccination: a single institute experience in Taiwan. Int J Hematol. Published online September 4, 2022. [CrossRef]
- Suzuki Y, Shiba T. Chronic cold agglutinin disease after a third COVID-19 mRNA vaccination. Int J Hematol. Published online October 29, 2022. [CrossRef]
- Camacho-Domínguez L, Rodríguez Y, Polo F, et al. COVID-19 vaccine and autoimmunity. A new case of autoimmune hepatitis and review of the literature. Journal of Translational Autoimmunity. 2022;5:100140. [CrossRef]
- Mörz M. A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Vaccines. 2022;10(10):1651. [CrossRef]
- Gill JR, Tashjian R, Duncanson E. Autopsy Histopathologic Cardiac Findings in 2 Adolescents Following the Second COVID-19 Vaccine Dose. Archives of Pathology & Laboratory Medicine. 2022;146(8):925-929. [CrossRef]
- Number of blood transfusions U.S. 2019. Statista. Accessed May 25, 2023. https://www.statista.com/statistics/1204079/number-blood-tranfusions-us/.
- Rose J. Critical Appraisal of VAERS Pharmacovigilance: Is the U.S. Vaccine Adverse Events Reporting System (VAERS) a Functioning Pharmacovigilance System? Published online April 21, 2023.
- United States Population (2023) - Worldometer. Accessed May 26, 2023. https://www.worldometers.info/world-population/us-population/.
- Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus Pandemic (COVID-19). Our World in Data. Published online March 5, 2020. Accessed July 25, 2022. https://ourworldindata.org/covid-deaths.
- Hanna N, Heffes-Doon A, Lin X, et al. Detection of Messenger RNA COVID-19 Vaccines in Human Breast Milk. JAMA Pediatrics. Published online September 26, 2022. [CrossRef]
- CDC. COVID Data Tracker. Centers for Disease Control and Prevention. Published March 28, 2020. Accessed May 26, 2023. https://covid.cdc.gov/covid-data-tracker.
- NVSS - Birth Data. Published May 3, 2023. Accessed May 26, 2023. https://www.cdc.gov/nchs/nvss/births.htm.
- 2022 Breastfeeding Report Card. Centers for Disease Control and Prevention. Published April 13, 2023. Accessed May 26, 2023. https://www.cdc.gov/breastfeeding/data/reportcard.htm.
- Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33(36):4398-4405. [CrossRef]
- Ioannidis JPA. Infection fatality rate of COVID-19 inferred from seroprevalence data. Bull World Health Organ. 2021;99(1):19-33F. [CrossRef]
- Abas AH, Marfuah S, Idroes R, et al. Can the SARS-CoV-2 Omicron Variant Confer Natural Immunity against COVID-19? Molecules. 2022;27(7):2221. [CrossRef]
- Young BE, Seppo AE, Diaz N, et al. Association of Human Milk Antibody Induction, Persistence, and Neutralizing Capacity With SARS-CoV-2 Infection vs mRNA Vaccination. JAMA Pediatrics. 2022;176(2):159-168. [CrossRef]
- Narayanaswamy V, Pentecost BT, Schoen CN, et al. Neutralizing Antibodies and Cytokines in Breast Milk After Coronavirus Disease 2019 (COVID-19) mRNA Vaccination. Obstet Gynecol. 2022;139(2):181-191. [CrossRef]
- Schenk-Braat EAM, van Mierlo MMKB, Wagemaker G, Bangma CH, Kaptein LCM. An inventory of shedding data from clinical gene therapy trials. The Journal of Gene Medicine. 2007;9(10):910-921. [CrossRef]
- Mendonça SA, Lorincz R, Boucher P, Curiel DT. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. npj Vaccines. 2021;6(1):1-14. [CrossRef]
- Kedl RM, Hsieh EWY, Morrison TE, et al. Evidence for Aerosol Transfer of SARS-CoV-2–Specific Humoral Immunity. Immunohorizons. 2023;7(5):307-309. [CrossRef]
- Purpura LJ, Alukal J, Chong AM, et al. SARS-CoV-2 RNA Shedding in Semen and Oligozoospermia of Patient with Severe Coronavirus Disease 11 Weeks after Infection. Emerg Infect Dis. 2022;28(1):196-200. [CrossRef]
- Lestari SW, Restiansyah G, Yunihastuti E, Pratama G. Comparison of sperm parameters and DNA fragmentation index between infertile men with infection and vaccines of COVID-19. Asian Journal of Andrology.:10.4103/aja202310. [CrossRef]
- Dong Y, Li X, Li Z, et al. Effects of inactivated SARS-CoV-2 vaccination on male fertility: A retrospective cohort study. Journal of Medical Virology. 2023;95(1):e28329. [CrossRef]
- Olana S, Mazzilli R, Salerno G, et al. 4BNT162b2 mRNA COVID-19 vaccine and semen: What do we know? Andrology. 2022;10(6):1023-1029. [CrossRef]
- Ma YC, Cheng C, Yuan C, Xiang LY, Wen J, Jin X. The effect of COVID-19 vaccines on sperm parameters: a systematic review and meta-analysis. Asian Journal of Andrology. 2023;25(4):468. [CrossRef]
- Banoun H. Current state of knowledge on the excretion of mRNA and spike produced by anti-COVID-19 mRNA vaccines; possibility of contamination of the entourage of those vaccinated by these products. Infectious Diseases Research. 2022;2022;3(4):22. [CrossRef]
- Craddock V, Mahajan A, Spikes L, et al. Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19. Journal of Medical Virology. 2023;95(2):e28568. [CrossRef]
- Dobhal G, Datta A, Ayupova D, Teesdale-Spittle P, Goreham RV. Isolation, characterisation and detection of breath-derived extracellular vesicles. Sci Rep. 2020;10(1):17381. [CrossRef]
- Sinha A, Yadav AK, Chakraborty S, et al. Exosome-enclosed microRNAs in exhaled breath hold potential for biomarker discovery in patients with pulmonary diseases. Journal of Allergy and Clinical Immunology. 2013;132(1):219-222.e7. [CrossRef]
- Lucchetti D, Santini G, Perelli L, et al. Detection and characterisation of extracellular vesicles in exhaled breath condensate and sputum of COPD and severe asthma patients. European Respiratory Journal. 2021;58(2). [CrossRef]
- Parry PI, Lefringhausen A, Turni C, et al. ‘Spikeopathy’: COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines. 2023;11(8):2287. [CrossRef]
- Yamamoto M, Kase M, Sano H, Kamijima R, Sano S. Persistent varicella zoster virus infection following mRNA COVID-19 vaccination was associated with the presence of encoded spike protein in the lesion. Journal of Cutaneous Immunology and Allergy. 2023;6(1):18-23. [CrossRef]
- Yonker LM, Swank Z, Bartsch YC, et al. Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis. Circulation. 2023;147(11):867-876. [CrossRef]
- Castruita JAS, Schneider UV, Mollerup S, et al. SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination. APMIS. 2023;131(3):128-132. [CrossRef]
- Duquesnoy RJ. Are We Ready for Epitope-Based HLA Matching in Clinical Organ Transplantation? Transplantation. 2017;101(8):1755-1765. [CrossRef]
- Aita KSU, Monte SJH, Silva AS, et al. Time is life: EpAssistant - a new tool for the automatic identification of anti-HLA antibody epitope specificity in transplant programs. Transplant Immunology. 2018;51:1-5. [CrossRef]
- Opelz G, Wujciak T, Döhler B, Scherer S, Mytilineos J. HLA compatibility and organ transplant survival. Collaborative Transplant Study. Rev Immunogenet. 1999;1(3):334-342.
- Harhay MN, Klassen AC, Zaidi H, et al. Living Organ Donor Perspectives and Sources of Hesitancy about COVID-19 Vaccines. Kidney360. 2021;2(7):1132-1140. [CrossRef]
- Pullen LC. COVID-19 Spurs Transplant Vaccination Policy. American Journal of Transplantation. 2021;21(12):3817-3818. [CrossRef]
- Kates OS, Stohs EJ, Pergam SA, et al. The limits of refusal: An ethical review of solid organ transplantation and vaccine hesitancy. American Journal of Transplantation. 2021;21(8):2637-2645. [CrossRef]
- Hippen BE, Axelrod DA, Maher K, et al. Survey of current transplant center practices regarding COVID-19 vaccine mandates in the United States. American Journal of Transplantation. 2022;22(6):1705-1713. [CrossRef]
- Kute V, Meshram HS, Sharma A, et al. Update on Coronavirus 2019 Vaccine Guidelines for Transplant Recipients. Transplantation Proceedings. 2022;54(6):1399-1404. [CrossRef]
- Kute VB, Asthana S, Gupta S, et al. NOTTO guidelines for vaccine-induced thrombotic thrombocytopenia in organ donation and transplantation. Indian Journal of Transplantation. 2022;16(1):3. [CrossRef]
- Uzun G, Bohnert BN, Althaus K, et al. Organ Donation From a Brain Dead Donor With Vaccine-induced Immune Thrombotic Thrombocytopenia After Ad26.COV2.S: The Risk of Organ Microthrombi. Transplantation. 2022;106(3):e178-e180. [CrossRef]
- Hann A, Hartog H, Nutu A, et al. Liver graft outcomes from donors with vaccine induced thrombosis and thrombocytopenia (VITT): United Kingdom multicenter experience. American Journal of Transplantation. 2022;22(3):996-998. [CrossRef]
- Greenhall GHB, Ushiro-Lumb I, Pavord S, et al. Kidney Transplantation From Deceased Donors With Vaccine-induced Immune Thrombocytopenia and Thrombosis: An Updated Analysis of the UK Experience. Transplantation. 2022;106(9):1824-1830. [CrossRef]
- Greenhall GHB, Ushiro-Lumb I, Pavord S, et al. Organ transplantation from deceased donors with vaccine-induced thrombosis and thrombocytopenia. Am J Transplant. 2021;21(12):4095-4097. [CrossRef]
- Loupy A, Goutaudier V, Jacquelinet C, Kerbaul F. Solid organ procurement and transplantation from deceased donors with vaccine-induced thrombosis and thrombocytopenia. American Journal of Transplantation. 2021;21(12):4098-4101. [CrossRef]
- van Bruchem M, van Rosmalen M, Warmerdam A, et al. Outcome After Organ Transplantation From Brain-dead Donors After a Cerebral Insult Following SARS-CoV-2 Vaccination Within the Eurotransplant Region. Transplantation. 2022;106(1):e100. [CrossRef]
- Centonze L, Lauterio A, De Carlis R, Ferla F, De Carlis L. Successful Liver Transplantation From a Deceased Donor With Vaccine-Induced Thrombotic Thrombocytopenia Causing Cerebral Venous Sinus and Hepatic Veins Thrombosis After ChAdOx1 nCov-19 Vaccination. Transplantation. 2021;105(10):e144. [CrossRef]
- Valsecchi M, Lauterio A, Crocchiolo R, et al. New-Onset Antibodies to Platelet Factor 4 Following Liver Transplantation From a Donor With Vaccine-Induced Thrombotic Thrombocytopenia. Liver Transpl. 2022;28(2):314-316. [CrossRef]
- Jamme M, Elalamy I, d’Izarny Gargas T, et al. Transplantation Outcome in Recipients Engrafted With Organs Recovered From the First French Deceased Donor With a SARS-COV-2 Vaccine-induced Thrombotic Thrombocytopenia. Transplantation. 2021;105(8):e84. [CrossRef]
- Kramer AH, Baht R, Doig CJ. Time trends in organ donation after neurologic determination of death: a cohort study. CMAJ Open. 2017;5(1):E19-E27. [CrossRef]
- Verhoeven CJ, Simon TC, de Jonge J, et al. Liver grafts procured from donors after circulatory death have no increased risk of microthrombi formation. Liver Transplantation. 2016;22(12):1676-1687. [CrossRef]
- Dubbeld J, Hoekstra H, Farid W, et al. Similar liver transplantation survival with selected cardiac death donors and brain death donors. British Journal of Surgery. 2010;97(5):744-753. [CrossRef]
- Grewal HP, Willingham DL, Nguyen J, et al. Liver transplantation using controlled donation after cardiac death donors: An analysis of a large single-center experience. Liver Transplantation. 2009;15(9):1028-1035. [CrossRef]
- Yamamoto S, Wilczek HE, Duraj FF, Groth CG, Ericzon BG. Liver Transplantation with Grafts from Controlled Donors after Cardiac Death: A 20-Year Follow-up at a Single Center. American Journal of Transplantation. 2010;10(3):602-611. [CrossRef]
- Vivalda S, Zhengbin H, Xiong Y, Liu Z, Wang Z, Ye Q. Vascular and Biliary Complications Following Deceased Donor Liver Transplantation: A Meta-analysis. Transplantation Proceedings. 2019;51(3):823-832. [CrossRef]
- Swissmedic 2019 © Copyright. Evaluation of haemovigilance reports in 2021. Accessed December 11, 2022. https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/marktueberwachung/haemovigilance/haemovigilance-publications-events/haemovigilance-report-2021.html.
- Actualité - Rapport d’activité hémovigilance 2021 - ANSM. Accessed December 11, 2022. https://ansm.sante.fr/actualites/rapport-dactivite-hemovigilance-2021.
- Funk M, Heiden M, Müller S, et al. Hämovigilanz-Bericht Des Paul-Ehrlich-Instituts 2020: Auswertung Der Meldungen von Reaktionen Und Zwischenfällen Nach § 63i AMG. Accessed December 11, 2022. www.pei.de/haemovigilanzbericht.
- Martina V. Hämovigilanzbericht 2021.
- Danish Hemovigilance Committee. Blood Donation Adverse Reactions and Events Annual Report 2021. Accessed May 25, 2023. https://dski.dk/wp-content/uploads/2023/03/blood-donation-adverse-reaction-and-events-annual-report-2021.pdf.
- S Narayan (Ed) D Poles et al. The 2021 Annual SHOT Report. Serious Hazards of Transfusion (SHOT) Steering Group; 2022. Accessed December 11, 2022. https://www.shotuk.org/wp-content/uploads/myimages/SHOT-REPORT-2021-FINAL-bookmarked-V3-November.pdf.
- 2020 to 2021 Cells, tissues, and organs surveillance system (CTOSS). Published December 22, 2022. Accessed May 25, 2023. https://www.canada.ca/en/public-health/services/surveillance/blood-safety-contribution-program/cells-tissues-organs-surveillance-system-2020-2021-infographic.html.
- Haemovigilance by JRCS 2021. Japanese Red Cross Accessed May 25, 2023. https://www.jrc.or.jp/mr/english/pdf/Haemovigilance%20by%20JRCS%202021.pdf.
| Condition | Case Reports |
| VITT | 12,14,30,37,44,45(p4),61,63–101,102(p1),103–233 |
| Stroke | 36,78,79,88,98,101,108,146,234–250,251(p284),252–258 |
| Hemolysis | 92,259–267 |
| Vasculitis | 4,268,269 |
| Anemia | 270 |
| Cold agglutinin disease | 271 |
| Hepatitis | 135,272 |
| Study | Donor | Organ | Recipient | Outcome | Thrombotic AE rate [AE rate including microthrombi, organ/graft rejection and Positive anti-PF4] |
| 314 | 50 year female with VITT | Heart | Unknown | No thrombosis or thrombocytopenia Anti-PF4 antibodies negative 3 weeks after transplantation |
0/1 [0/1] |
| 321 | 18 year old brain-dead female who dies from VITT-related intracranial hemorrhage | Liver | 58 year old female | Rapid drop in platelet count from 104x109/liter to 30x109/liter Anti-PF4 IgG strongly positive Grade 3 (severe) thrombocytopenia |
1/1 [1/1] |
| 315 | (n = 8, aged between 22 and 55 years) Died of catastrophic intracerebral hemorrhage or thrombosis, had received the first dose of ChAdOx1 nCOV-19 vaccine 9 to 19 days before hospital admission, and had detectable anti-PF4, low fibrinogen and elevated D-Dimers |
Liver | (n = 9, aged 2–43 years) | Four recipients with positive anti-PF4 antibodies without bleeding or thrombotic complications Two recipients with severe thrombotic events, requiring emergency retransplantation. Anti-PF4 antibodies negative. |
2/9 [6/9] |
| 316 | N=16 Median age 44 75% female |
Kidney Microthrombi observed in 4/11 biopsies |
N=30 Median age 48 47% female |
2 recipients with anti-PF4 antibodies but no clinical disease Major hemorrhagic complications in 3 recipients w/ independent risk factors |
3/30 [5/30] |
| 318 | Male, 41 Female, 69 Male, 67 All deceased from VITT |
Heart Kidney Liver Lungs |
N=9 Median age 58 (40-70) 44% female |
Glomerular microthrombi in 2 kidney recipients Pulmonary embolism in lung recipient No anti-PF4 antibodies observed |
1/9 [3/9] |
| 319 | N=6 Aged 37-72 years 50% female |
Liver Kidney Lung Heart |
N=17 Aged <1 to 77 years 42% female |
Liver cell necrosis and re-transplantation in one recipient Microangiopathy in one kidney recipient Two recipients (11.8%) developed thrombosis-related complications |
2/17 [4/17] |
| 320 | 32-year-old female deceased from VITT-induced stroke | Liver | 69-year-old female | No adverse events, operation successful | 0/1 [0/1] |
| 317 | N=13 Median age 34 (21 to 63) 85% female |
Kidney Liver Heart Lung Pancreas |
N=26 Median age 40 (2 to 63) |
Thrombsis Thromboembolism in 7/26 recipients (3 liver recipients and 4 Kidney/SPK/islet recipients) Graft dysfunction in 4/26 recipients Anti-PF4 antibodies positive in 3/13 (23%) tests with results |
7/26 [10/26] |
| 322 | Female aged 60-69 | Liver & Heart Lungs Right Kidney Left Kidney |
63-year-old male 58-year-old woman 70-year-old man 52-year-old man |
No AEs No AEs Thrombi is pre-implantation biopsy, uneventful transplantation Glomerular inflammation and hemorrhagic suffusion |
0/4 [2/4] |
| Summary | 16/98, 16% [29/98, 30%] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





