Submitted:
21 February 2024
Posted:
22 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, R.S.; Robbins, J.W. Post Placement and Restoration of Endodontically Treated Teeth: A Literature Review. J. Endod. 2004, 30, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Takahashi, Y.; Hirai, M.; Iwami, Y.; Imazato, S.; Ebisu, S. Effect of Endodontic Irrigation on Bonding of Resin Cement to Radicular Dentin. Eur. J. Oral Sci. 2005, 113, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Salameh, Z.; Sorrentino, R.; Papacchini, F.; Ounsi, H.F.; Tashkandi, E.; Goracci, C.; Ferrari, M. Fracture Resistance and Failure Patterns of Endodontically Treated Mandibular Molars Restored Using Resin Composite with or without Translucent Glass Fiber Posts. J. Endod. 2006, 32, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.P.O.; de Melo Alencar, C.; Zambon, M.; de Andrade, M.F.; Fernández, E.; Kuga, M.C. Etch-and-Rinse versus Self-Etch Strategy of a Universal Adhesive in Different Application Methods at the Bonding Interface of Fiber Post Cementation. J. Esthet. Restor. Dent. Off. Publ. Am. Acad. Esthet. Dent. Al 2023, 35, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mannocci, F.; Vichi, A.; Cagidiaco, M.C.; Mjör, I.A. Bonding to Root Canal: Structural Characteristics of the Substrate. Am. J. Dent. 2000, 13, 255–260. [Google Scholar] [PubMed]
- Varela, S.G.; Rábade, L.B.; Lombardero, P.R.; Sixto, J.M.L.; Bahillo, J.D.G.; Park, S.A. In Vitro Study of Endodontic Post Cementation Protocols That Use Resin Cements. J. Prosthet. Dent. 2003, 89, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Geerts, S.; Bolette, A.; Seidel, L.; Guéders, A. An in Vitro Evaluation of Leakage of Two Etch and Rinse and Two Self-Etch Adhesives after Thermocycling. Int. J. Dent. 2012, 2012, 852841. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Luque, I.; Hass, V.; Reis, A.; Loguercio, A.D.; Bombarda, N.H.C. Immediate Bonding Properties of Universal Adhesives to Dentine. J. Dent. 2013, 41, 404–411. [Google Scholar] [CrossRef]
- Mortazavi, V.; Khademi, A.; Khosravi, K.; Fathi, M.; Ebrahimi-Chaharom, M.; Shahnaseri, S.; Khalighinejad, N.; Badrian, H. Effect of MTAD on the Shear Bond Strength of Self-Etch Adhesives to Dentin. Dent. Res. J. 2012, 9, 24–30. [Google Scholar] [CrossRef]
- Brackett, W.W.; Tay, F.R.; Brackett, M.G.; Dib, A.; Sword, R.J.; Pashley, D.H. The Effect of Chlorhexidine on Dentin Hybrid Layers in Vivo. Oper. Dent. 2007, 32, 107–111. [Google Scholar] [CrossRef]
- Dionysopoulos, D. Effect of Digluconate Chlorhexidine on Bond Strength between Dental Adhesive Systems and Dentin: A Systematic Review. J. Conserv. Dent. JCD 2016, 19, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Jung, H.-S.; Kim, H.-J.; Jang, J.-H.; Kim, D.-S.; Choi, K.-K.; Kim, S.-Y. Effect of Zinc on the Collagen Degradation in Acid-Etched Dentin. J. Dent. Sci. 2018, 13, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental Adhesion Review: Aging and Stability of the Bonded Interface. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Al-Ammar, A.; Drummond, J.L.; Bedran-Russo, A.K. The Use of Collagen Cross-Linking Agents to Enhance Dentin Bond Strength. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 419–424. [Google Scholar] [CrossRef]
- Gilberto Henostroza Haro. Adhesión En Odontología Conservadora; 2a; Ripano Editorial Médica: Spain, 2010; ISBN 13 978-84-937238-7-3. [Google Scholar]
- Mao, H.; Chen, Y.; Yip, K.H.-K.; Smales, R.J. Effect of Three Radicular Dentine Treatments and Two Luting Cements on the Regional Bond Strength of Quartz Fibre Posts. Clin. Oral Investig. 2011, 15, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Akman, M.; Eldeniz, A.U.; Ince, S.; Guneser, M.B. Push-out Bond Strength of a New Post System after Various Post Space Treatments. Dent. Mater. J. 2016, 35, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Josic, U.; Mazzitelli, C.; Maravic, T.; Comba, A.; Cadenaro, M.; Radovic, I.; Sebold, M.; Turco, G.; Breschi, L.; Mazzoni, A. The Effect of Carbodiimide on Push-out Bond Strength of Fiber Posts and Endogenous Enzymatic Activity. BMC Oral Health 2023, 23, 399. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Erhardt, M.C.G.; Pimenta, L. a. F.; Osorio, E.; Toledano, M. EDTA Treatment Improves Resin-Dentin Bonds’ Resistance to Degradation. J. Dent. Res. 2005, 84, 736–740. [Google Scholar] [CrossRef]
- Olszowski, S.; Mak, P.; Olszowska, E.; Marcinkiewicz, J. Collagen Type II Modification by Hypochlorite. Acta Biochim. Pol. 2003, 50, 471–479. [Google Scholar] [CrossRef]
- Kasraei, S.; Azarsina, M.; Khamverdi, Z. Effect of Ethylene Diamine Tetra Acetic Acid and Sodium Hypochlorite Solution Conditioning on Microtensile Bond Strength of One-Step Self-Etch Adhesives. J. Conserv. Dent. JCD 2013, 16, 243–246. [Google Scholar] [CrossRef]
- Kim, J.; Uchiyama, T.; Carrilho, M.; Agee, K.A.; Mazzoni, A.; Breschi, L.; Carvalho, R.M.; Tjäderhane, L.; Looney, S.; Wimmer, C.; et al. Chlorhexidine Binding to Mineralized versus Demineralized Dentin Powder. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2010, 26, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.S.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.R.; Carvalho, R.M.; Tay, F.R.; et al. Optimizing Dentin Bond Durability: Control of Collagen Degradation by Matrix Metalloproteinases and Cysteine Cathepsins. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2013, 29, 116–135. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Hu, Y.; Yao, S.; Wang, Y.; Lin, X. Effect of Matrix Metalloproteinase 8 Inhibitor on Resin-Dentin Bonds. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Juan Augusto Fernández Tarazona; Zady Jackeline Torres Rivera Influence of the Application of Chlorhexidine at 2% in the Adhesive Protocol on the Tensile Bond Strength of Glass Fiber Post. Int. J. Dent. Res. 2018, 3, 55–58.
- Bitter, K.; Gläser, C.; Neumann, K.; Blunck, U.; Frankenberger, R. Analysis of Resin-Dentin Interface Morphology and Bond Strength Evaluation of Core Materials for One Stage Post-Endodontic Restorations. PloS One 2014, 9, e86294. [Google Scholar] [CrossRef] [PubMed]
- Alaghemand, H.; Mirzae, M.; Ahmadi, E.; Saidi, A. Effect of Different Post-Space Pretreatments on Fiber Post Bonding to Root Dentine. Dent. Res. J. 2013, 10, 545–552. [Google Scholar]
- Mendoza, D.B.; Eakle, W.S. Retention of Posts Cemented with Various Dentinal Bonding Cements. J. Prosthet. Dent. 1994, 72, 591–594. [Google Scholar] [CrossRef]
- Machado, M.B.M.; Alves Morgan, L.F.D.S.; Gomes, G.M.; Vasconcellos, W.A.; Cardoso, F.P.; Albuquerque, R. de C. Effects of Immediate and Delayed Intraradicular Preparation on Bond Strength of Fiber Posts. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2015, 26, 244–247. [Google Scholar] [CrossRef]
- Ertas, H.; Ok, E.; Uysal, B.; Arslan, H. Effects of Different Irrigating Solutions and Disinfection Methods on Push-out Bond Strengths of Fiber Posts. Acta Odontol. Scand. 2014, 72, 783–787. [Google Scholar] [CrossRef]
- Mohsen, C.A. Evaluation of Push-out Bond Strength of Surface Treatments of Two Esthetic Posts. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2012, 23, 596–602. [Google Scholar] [CrossRef]
- Shori, D.; Pandey, S.; Kubde, R.; Rathod, Y.; Atara, R.; Rathi, S. To Evaluate and Compare the Effect of Different Post Surface Treatments on the Tensile Bond Strength between Fiber Posts and Composite Resin. J. Int. Oral Health JIOH 2013, 5, 27–32. [Google Scholar]
- Prasansuttiporn, T.; Nakajima, M.; Foxton, R.M.; Tagami, J. Scrubbing Effect of Self-Etching Adhesives on Bond Strength to NaOCl-Treated Dentin. J. Adhes. Dent. 2012, 14, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Thitthaweerat, S.; Nakajima, M.; Foxton, R.M.; Tagami, J. Effect of Solvent Evaporation Strategies on Regional Bond Strength of One-Step Self-Etch Adhesives to Root Canal Dentine. Int. Endod. J. 2013, 46, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Hamouda, I.M.; Ghazy, M.H.; Abo-Madina, M.M. Immediate and Delayed Micro-Tensile Bond Strength of Different Luting Resin Cements to Different Regional Dentin. J. Biomed. Res. 2013, 27, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.; Pujar, M.; Patil, C. Evaluation of Push-out Bond Strength of Two Fiber-Reinforced Composite Posts Systems Using Two Luting Cements in Vitro. J. Conserv. Dent. JCD 2013, 16, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.S.; Swartz, M.L.; Moore, B.K.; Rhodes, B.F. Influence of Selected Variables on Adhesion Testing. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1992, 8, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Goracci, C.; Grandini, S.; Bossù, M.; Bertelli, E.; Ferrari, M. Laboratory Assessment of the Retentive Potential of Adhesive Posts: A Review. J. Dent. 2007, 35, 827–835. [Google Scholar] [CrossRef]
- Goracci, C.; Tavares, A.U.; Fabianelli, A.; Monticelli, F.; Raffaelli, O.; Cardoso, P.C.; Tay, F.; Ferrari, M. The Adhesion between Fiber Posts and Root Canal Walls: Comparison between Microtensile and Push-out Bond Strength Measurements. Eur. J. Oral Sci. 2004, 112, 353–361. [Google Scholar] [CrossRef]
- Cekic-Nagas, I.; Ergun, G.; Nagas, E.; Tezvergil, A.; Vallittu, P.K.; Lassila, L.V.J. Comparison between Regional Micropush-out and Microtensile Bond Strength of Resin Composite to Dentin. Acta Odontol. Scand. 2008, 66, 73–81. [Google Scholar] [CrossRef]
- Albaladejo, A. Método de Preparación Del Espécimen Para Evaluar La Micromorfología de La Interfase Adhesiva Resina-Dentina Con Un Microscopio Electrónico de Barrido. Av. En Odontoestomatol. 2007, 23, 197–206. [Google Scholar]
- Tay, F.R.; Pashley, D.H. Monoblocks in Root Canals: A Hypothetical or a Tangible Goal. J. Endod. 2007, 33, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Carvalho, C.A.; Goracci, C.; Antoniolli, F.; Mazzoni, A.; Mazzotti, G.; Cadenaro, M.; Breschi, L. Influence of Luting Material Filler Content on Post Cementation. J. Dent. Res. 2009, 88, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Marchesi, G.; Cadenaro, M.; Mazzotti, G.; Di Lenarda, R.; Ferrari, M.; Breschi, L. Push-out Stress for Fibre Posts Luted Using Different Adhesive Strategies. Eur. J. Oral Sci. 2009, 117, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Zicari, F.; Couthino, E.; De Munck, J.; Poitevin, A.; Scotti, R.; Naert, I.; Van Meerbeek, B. Bonding Effectiveness and Sealing Ability of Fiber-Post Bonding. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2008, 24, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Radovic, I.; Mazzitelli, C.; Chieffi, N.; Ferrari, M. Evaluation of the Adhesion of Fiber Posts Cemented Using Different Adhesive Approaches. Eur. J. Oral Sci. 2008, 116, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J.; Gomes, G.; Augusto, V. The Effect of Dowel Space on the Bond Strengths of Fiber Posts. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2007, 16, 154–164. [Google Scholar] [CrossRef]
- Sterzenbach, G.; Karajouli, G.; Naumann, M.; Peroz, I.; Bitter, K. Fiber Post Placement with Core Build-up Materials or Resin Cements-an Evaluation of Different Adhesive Approaches. Acta Odontol. Scand. 2012, 70, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Boschian Pest, L.; Cavalli, G.; Bertani, P.; Gagliani, M. Adhesive Post-Endodontic Restorations with Fiber Posts: Push-out Tests and SEM Observations. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2002, 18, 596–602. [Google Scholar] [CrossRef]
- Matochek, M.H.M.; Tomaz, P.L.S.; Oliveira, T. de S.; Polassi, M.R.; Alonso, R.C.B.; Scremin, F.M.; Sauro, S.; Marcucci, M.C.; D’Alpino, P.H.P. Influence of a Propolis-Based Irrigant Solution on Gap Formation and Bond Strength of Posts Bonded to Root Canal Dentin Using Different Resin Cements. Dent. Mater. J. 2020, 39, 490–499. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Inokoshi, S.; Braem, M.; Lambrechts, P.; Vanherle, G. Morphological Aspects of the Resin-Dentin Interdiffusion Zone with Different Dentin Adhesive Systems. J. Dent. Res. 1992, 71, 1530–1540. [Google Scholar] [CrossRef]
- Nadler, A.-M.-O.; da Silva, E.-J.; Lins-Filho, P.-C.; Dias, M.-F.; Guimarães, R.-P.; da Silva, C.-H.-V.; Silva, S.-D.S.; Gomes, A.-S.-L. Influence of Different Adhesion Strategies on Glass Fiber Post Retention. J. Clin. Exp. Dent. 2023, 15, e649–e657. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore Memorial Lecture. Adhesion to Enamel and Dentin: Current Status and Future Challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar] [PubMed]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative Study on Adhesive Performance of Functional Monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Peumans, M.; Munck, J.; Van Landuyt, K.; Lambrechts, P.; Van Meerbeek, B. Three-Year Clinical Effectiveness of a Two-Step Self-Etch Adhesive in Cervical Lesions. Eur. J. Oral Sci. 2005, 113, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Fukegawa, D.; Hayakawa, S.; Yoshida, Y.; Suzuki, K.; Osaka, A.; Van Meerbeek, B. Chemical Interaction of Phosphoric Acid Ester with Hydroxyapatite. J. Dent. Res. 2006, 85, 941–944. [Google Scholar] [CrossRef]
- Nagakane, K.; Yoshida, Y.; Hirata, I.; Fukuda, R.; Nakayama, Y.; Shirai, K.; Ogawa, T.; Suzuki, K.; Van Meerbeek, B.; Okazaki, M. Analysis of Chemical Interaction of 4-MET with Hydroxyapatite Using XPS. Dent. Mater. J. 2006, 25, 645–649. [Google Scholar] [CrossRef]
- Ernst, C.-P. Options for Dentin Bonding. J. Esthet. Restor. Dent. Off. Publ. Am. Acad. Esthet. Dent. Al 2006, 18, 61–67. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Peumans, M.; Poitevin, A.; Mine, A.; Van Ende, A.; Neves, A.; De Munck, J. Relationship between Bond-Strength Tests and Clinical Outcomes. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2010, 26, e100-121. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the Art of Self-Etch Adhesives. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Inoue, S.; Koshiro, K.; Yoshida, Y.; De Munck, J.; Nagakane, K.; Suzuki, K.; Sano, H.; Van Meerbeek, B. Hydrolytic Stability of Self-Etch Adhesives Bonded to Dentin. J. Dent. Res. 2005, 84, 1160–1164. [Google Scholar] [CrossRef]
- Nagpal, R.; Manuja, N.; Tyagi, S.P.; Singh, U.P. In Vitro Bonding Effectiveness of Self-Etch Adhesives with Different Application Techniques: A Microleakage and Scanning Electron Microscopic Study. J. Conserv. Dent. JCD 2011, 14, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Volpato, C.A.M. Current Perspectives on Dental Adhesion: (3) Adhesion to Intraradicular Dentin: Concepts and Applications. Jpn. Dent. Sci. Rev. 2020, 56, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Ciucchi, B.; Sano, H.; Carvalho, R.M.; Russell, C.M. Bond Strength versus Dentine Structure: A Modelling Approach. Arch. Oral Biol. 1995, 40, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Eick, J.D.; Gwinnett, A.J.; Pashley, D.H.; Robinson, S.J. Current Concepts on Adhesion to Dentin. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 1997, 8, 306–335. [Google Scholar] [CrossRef] [PubMed]
- Chersoni, S.; Suppa, P.; Breschi, L.; Ferrari, M.; Tay, F.R.; Pashley, D.H.; Prati, C. Water Movement in the Hybrid Layer after Different Dentin Treatments. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2004, 20, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, A.; Ramos, J.; Messias, A.; Marques, F.; Caramelo, F.; Mata, A. Microtensile Bond Strength and Micromorphology of Bur-Cut Enamel Using Five Adhesive Systems. J. Adhes. Dent. 2015, 17, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Dhem, A.; Goret-Nicaise, M.; Braem, M.; Lambrechts, P.; VanHerle, G. Comparative SEM and TEM Examination of the Ultrastructure of the Resin-Dentin Interdiffusion Zone. J. Dent. Res. 1993, 72, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Gwinnett, A.J.; Tay, F.R.; Pang, K.M.; Wei, S.H. Quantitative Contribution of the Collagen Network in Dentin Hybridization. Am. J. Dent. 1996, 9, 140–144. [Google Scholar]
- Nakabayashi, N.; Saimi, Y. Bonding to Intact Dentin. J. Dent. Res. 1996, 75, 1706–1715. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Conn, L.J.; Duke, E.S.; Eick, J.D.; Robinson, S.J.; Guerrero, D. Correlative Transmission Electron Microscopy Examination of Nondemineralized and Demineralized Resin-Dentin Interfaces Formed by Two Dentin Adhesive Systems. J. Dent. Res. 1996, 75, 879–888. [Google Scholar] [CrossRef]
- Foxton, R.M.; Nakajima, M.; Tagami, J.; Miura, H. Adhesion to Root Canal Dentine Using One and Two-Step Adhesives with Dual-Cure Composite Core Materials. J. Oral Rehabil. 2005, 32, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.S.; Silva-Sousa, Y.T.C.; Sousa-Neto, M.D. Bond Strength of Fiber Posts to Weakened Roots after Resin Restoration with Different Light-Curing Times. J. Endod. 2009, 35, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.C.; Melo, R.M. de; Chaves, C.; Galhano, G.A.P.; Bottino, M.A.; Balducci, I. The Adhesive System and Root Canal Region Do Not Influence the Degree of Conversion of Dual Resin Cement. J. Appl. Oral Sci. Rev. FOB 2010, 18, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Serafino, C.; Gallina, G.; Cumbo, E.; Ferrari, M. Surface Debris of Canal Walls after Post Space Preparation in Endodontically Treated Teeth: A Scanning Electron Microscopic Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Yu, H.; Lin, Q.; Taira, Y.; Cheng, H. Effects of Different Root Canal Obturation Techniques on the Bond Strength of Fiber Post to Intraradicular Dentine. Chin. J. Dent. Res. 2019, 22, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Bouillaguet, S.; Troesch, S.; Wataha, J.C.; Krejci, I.; Meyer, J.M.; Pashley, D.H. Microtensile Bond Strength between Adhesive Cements and Root Canal Dentin. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2003, 19, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Vichi, A.; Grandini, S.; Geppi, S. Influence of Microbrush on Efficacy of Bonding into Root Canals. Am. J. Dent. 2002, 15, 227–231. [Google Scholar] [PubMed]
- Oskoee, S.S.; Bahari, M.; Kimyai, S.; Asgary, S.; Katebi, K. Push-out Bond Strength of Fiber Posts to Intraradicular Dentin Using Multimode Adhesive System. J. Endod. 2016, 42, 1794–1798. [Google Scholar] [CrossRef]
- do Amaral, R.C.; Stanislawczuk, R.; Zander-Grande, C.; Michel, M.D.; Reis, A.; Loguercio, A.D. Active Application Improves the Bonding Performance of Self-Etch Adhesives to Dentin. J. Dent. 2009, 37, 82–90. [Google Scholar] [CrossRef]
- Reis, A.; Pellizzaro, A.; Dal-Bianco, K.; Gones, O.M.; Patzlaff, R.; Loguercio, A.D. Impact of Adhesive Application to Wet and Dry Dentin on Long-Term Resin-Dentin Bond Strengths. Oper. Dent. 2007, 32, 380–387. [Google Scholar] [CrossRef]
- Elnaghy, A.M. Effect of QMix Irrigant on Bond Strength of Glass Fibre Posts to Root Dentine. Int. Endod. J. 2014, 47, 280–289. [Google Scholar] [CrossRef]
- Jessie Reyes-Carmona Irrigation Protocols Effects on Radicular Dentin: Cleaning, Disinfection and Remaining Ultrastructure. ODOVTOS-Int. J. Dent. Sci. 2023, 25, 14–21. [CrossRef]
- Khoroushi, M.; Kachuei, M. Pull-out Bond Strength of a Self-Adhesive Resin Cement to NaOCl-Treated Root Dentin: Effect of Antioxidizing Agents. Restor. Dent. Endod. 2014, 39, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kambara, K.; Nakajima, M.; Hosaka, K.; Takahashi, M.; Thanatvarakorn, O.; Ichinose, S.; Foxton, R.M.; Tagami, J. Effect of Smear Layer Treatment on Dentin Bond of Self-Adhesive Cements. Dent. Mater. J. 2012, 31, 980–987. [Google Scholar] [CrossRef]
- Taniguchi, G.; Nakajima, M.; Hosaka, K.; Iwamoto, N.; Ikeda, M.; Foxton, R.M.; Tagami, J. Improving the Effect of NaOCl Pretreatment on Bonding to Caries-Affected Dentin Using Self-Etch Adhesives. J. Dent. 2009, 37, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Saber, S.-E.D.M.; El-Askary, F.S. The Outcome of Immediate or Delayed Application of a Single-Step Self-Etch Adhesive to Coronal Dentin Following the Application of Different Endodontic Irrigants. Eur. J. Dent. 2009, 3, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Torii, Y.; Hikasa, R.; Iwate, S.; Oyama, F.; Itou, K.; Yoshiyama, M. Effect of EDTA Conditioning on Bond Strength to Bovine Dentin Promoted by Four Current Adhesives. Am. J. Dent. 2003, 16, 395–400. [Google Scholar] [PubMed]
- Rasimick, B.J.; Shah, R.P.; Musikant, B.L.; Deutsch, A.S. Effect of EDTA Conditioning upon the Retention of Fibre Posts Luted with Resin Cements. Int. Endod. J. 2008, 41, 1101–1106. [Google Scholar] [CrossRef]
- Taşman, F.; Cehreli, Z.C.; Oğan, C.; Etikan, I. Surface Tension of Root Canal Irrigants. J. Endod. 2000, 26, 586–587. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Qian, W.; Gao, Y. Irrigation in Endodontics. Dent. Clin. North Am. 2010, 54, 291–312. [Google Scholar] [CrossRef]
- Perdigao, J.; Denehy, G.E.; Swift, E.J. Effects of Chlorhexidine on Dentin Surfaces and Shear Bond Strengths. Am. J. Dent. 1994, 7, 81–84. [Google Scholar] [PubMed]
- el-Housseiny, A.A.; Jamjoum, H. The Effect of Caries Detector Dyes and a Cavity Cleansing Agent on Composite Resin Bonding to Enamel and Dentin. J. Clin. Pediatr. Dent. 2000, 25, 57–63. [Google Scholar] [CrossRef] [PubMed]
- de Castro, F.L.A.; de Andrade, M.F.; Duarte Júnior, S.L.L.; Vaz, L.G.; Ahid, F.J.M. Effect of 2% Chlorhexidine on Microtensile Bond Strength of Composite to Dentin. J. Adhes. Dent. 2003, 5, 129–138. [Google Scholar] [PubMed]
- Say, E.C.; Nakajima, M.; Senawongse, P.; Soyman, M.; Ozer, F.; Tagami, J. Bonding to Sound vs Caries-Affected Dentin Using Photo- and Dual-Cure Adhesives. Oper. Dent. 2005, 30, 90–98. [Google Scholar] [PubMed]
- Hashem, A.A.R.; Ghoneim, A.G.; Lutfy, R.A.; Fouda, M.Y. The Effect of Different Irrigating Solutions on Bond Strength of Two Root Canal-Filling Systems. J. Endod. 2009, 35, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, R.M.; Lassila, L.V.J.; Salo, V.; Vallittu, P.K.; Tjäderhane, L. Effect of Chlorhexidine on Initial Adhesion of Fiber-Reinforced Post to Root Canal. J. Dent. 2010, 38, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, A.; Ari, H.; Güngüneş, H.; Belli, S. Effect of Medications for Root Canal Treatment on Bonding to Root Canal Dentin. J. Endod. 2004, 30, 113–116. [Google Scholar] [CrossRef]
- Santos, J.N.; Carrilho, M.R. de O.; De Goes, M.F.; Zaia, A.A.; Gomes, B.P.F. de A.; Souza-Filho, F.J. de; Ferraz, C.C.R. Effect of Chemical Irrigants on the Bond Strength of a Self-Etching Adhesive to Pulp Chamber Dentin. J. Endod. 2006, 32, 1088–1090. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the Art Etch-and-Rinse Adhesives. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef]
- Wang, L.; Pinto, T.A.; Silva, L.M.; Araújo, D.F.G.; Martins, L.M.; Hannas, A.R.; Pedreira, A.P.R.V.; Francisconi, P. a. S.; Honório, H.M. Effect of 2% Chlorhexidine Digluconate on Bond Strength of a Glass-Fibre Post to Root Dentine. Int. Endod. J. 2013, 46, 847–854. [Google Scholar] [CrossRef]
- Haragushiku, G.A.; Back, E.D.E.E.; Tomazinho, P.H.; Baratto Filho, F.; Furuse, A.Y. Influence of Antimicrobial Solutions in the Decontamination and Adhesion of Glass-Fiber Posts to Root Canals. J. Appl. Oral Sci. Rev. FOB 2015, 23, 436–441. [Google Scholar] [CrossRef]
- Sanabe, M.E.; Costa, C.A.; Hebling, J. Exposed Collagen in Aged Resin-Dentin Bonds Produced on Sound and Caries-Affected Dentin in the Presence of Chlorhexidine. J. Adhes. Dent. 2011, 13, 117–124. [Google Scholar] [CrossRef]

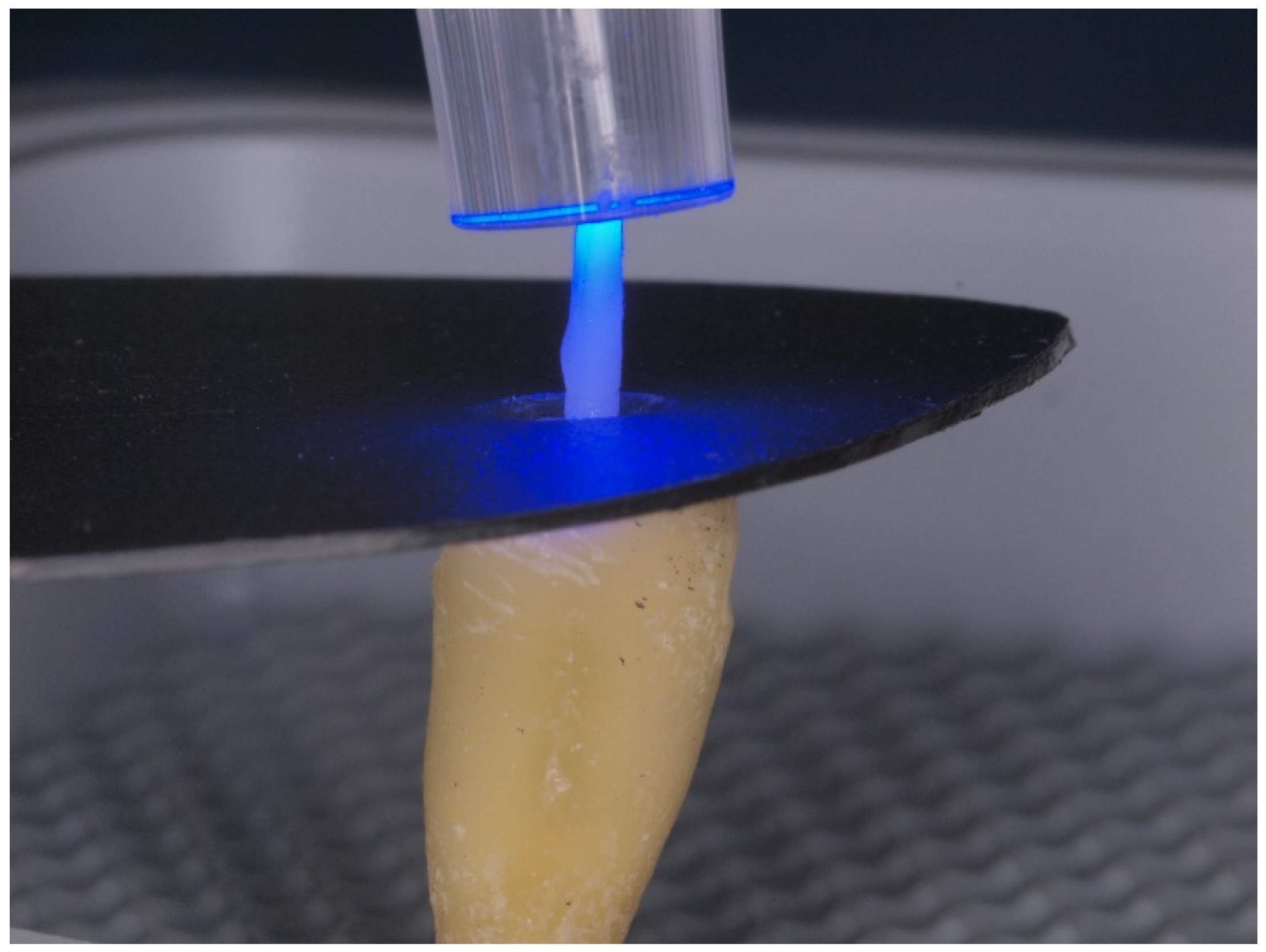
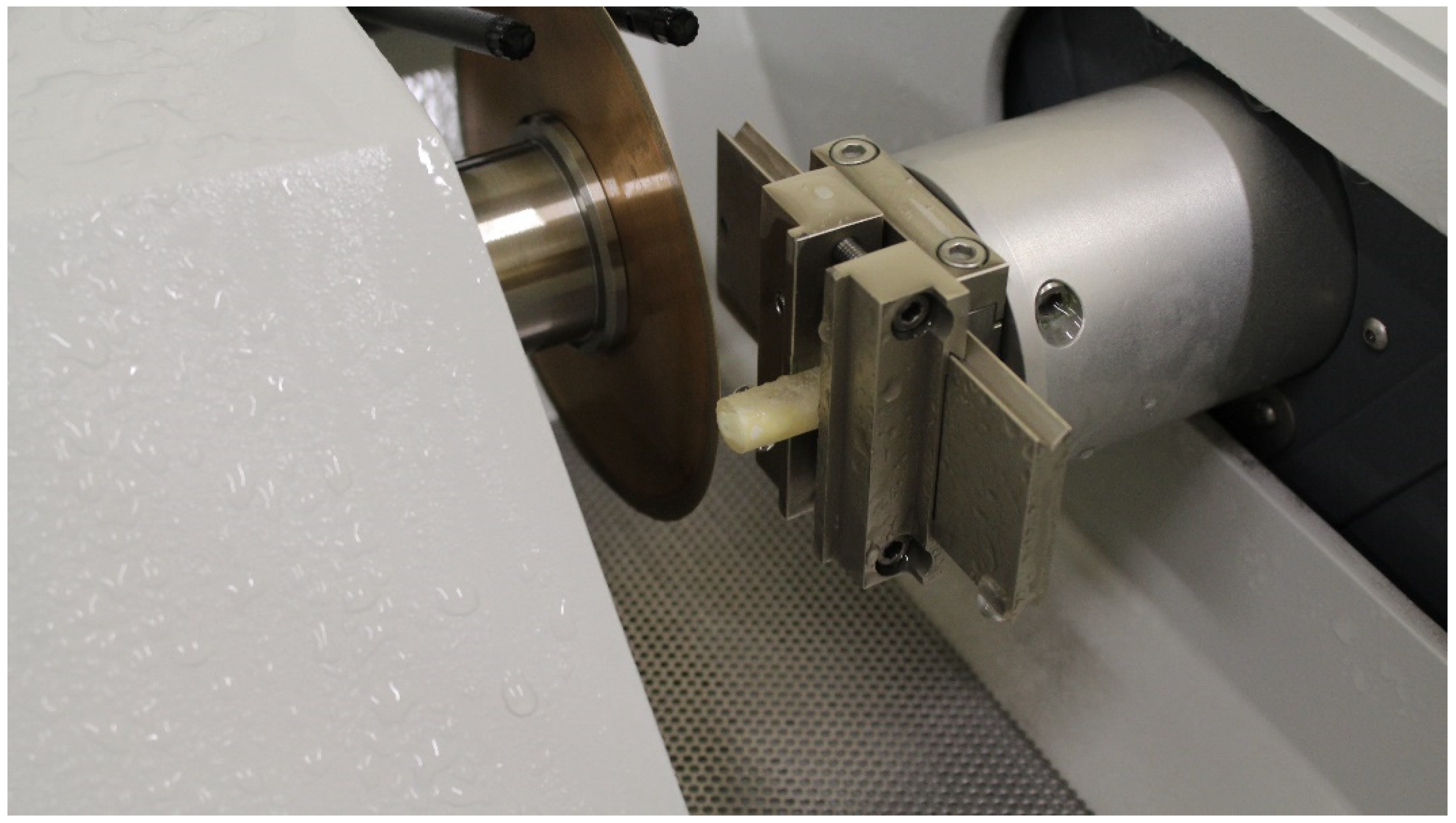
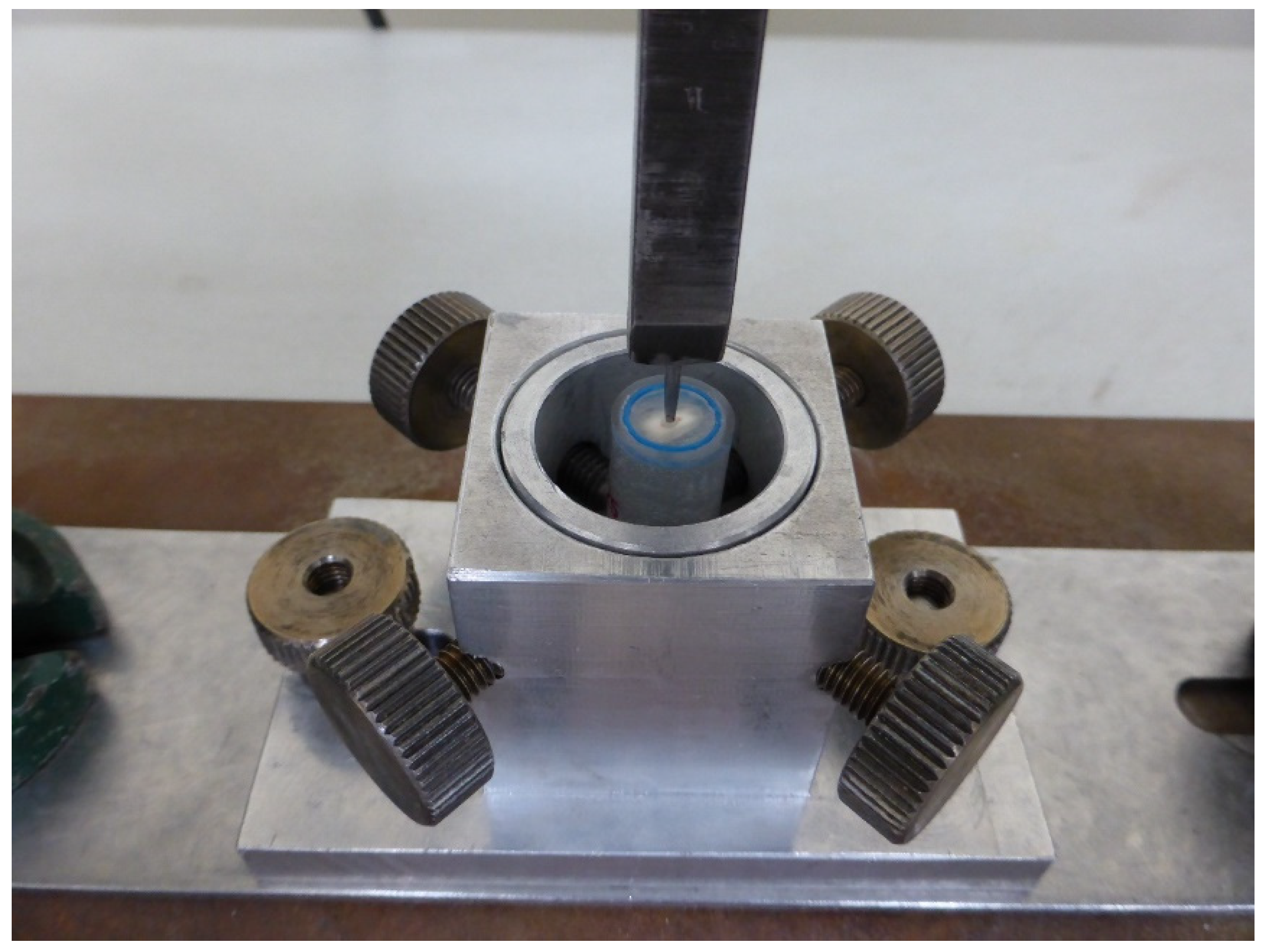
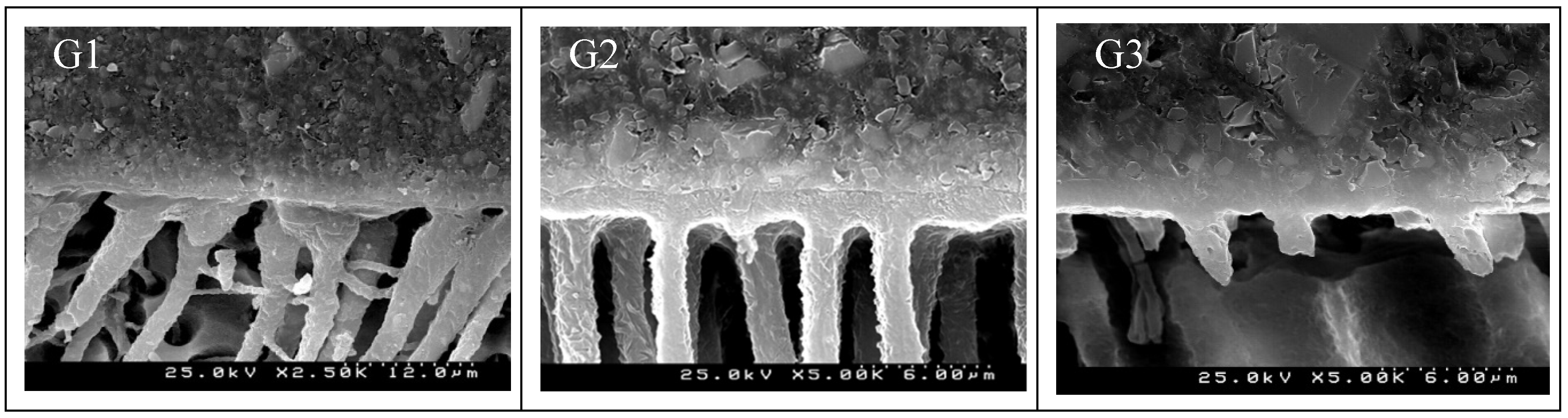
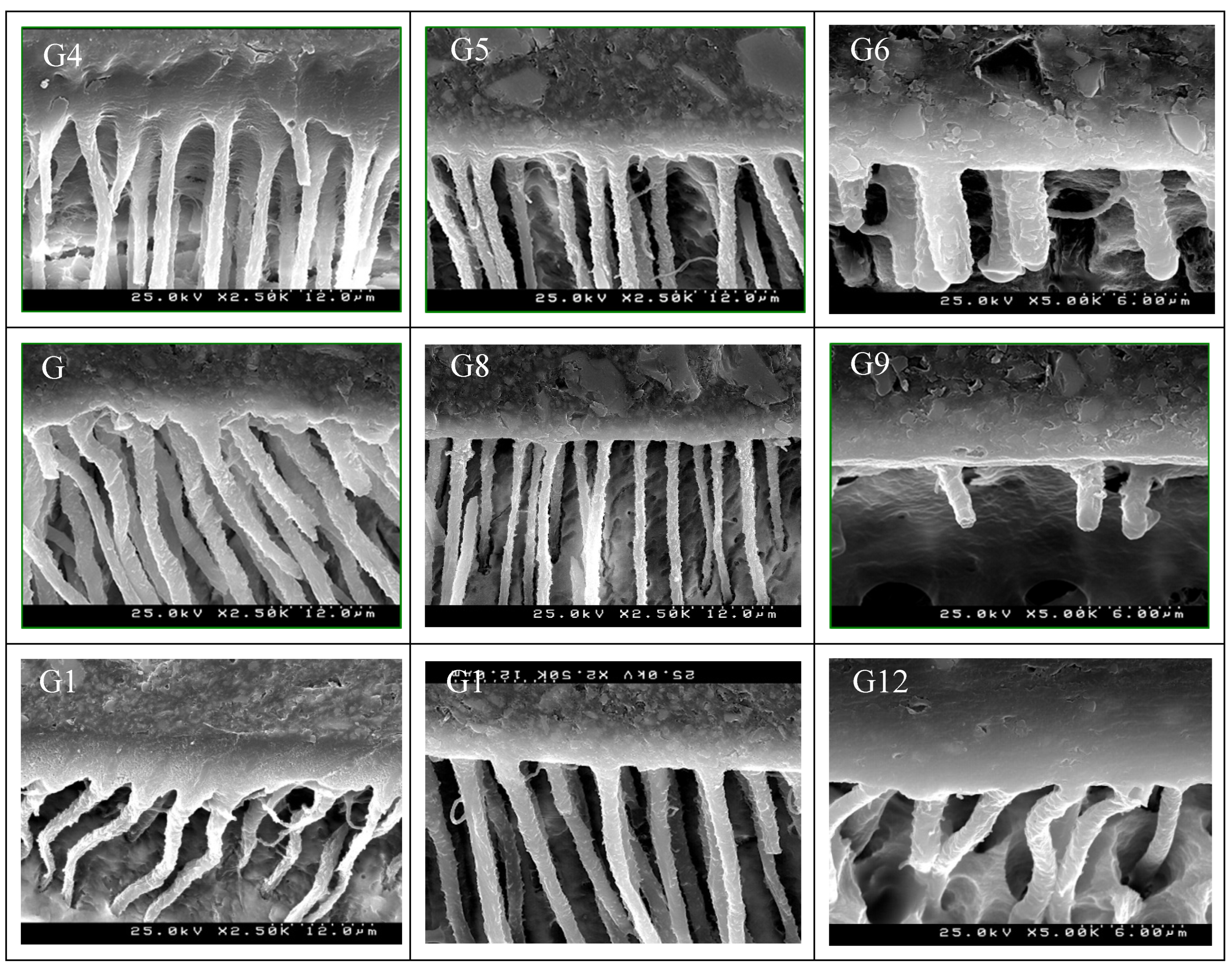
| Ortophosforic acid 37% PARABOND A+B PARACORE |
Acondicionador Non Rinse PARABOND A+B PARACORE |
CLEARFIL UNIVERSAL CLEARFIL DC CORE PLUS |
|
|---|---|---|---|
| DISTILLED WATER (control) | G1 | G2 | G3 |
| EDTA 17% | G4 | G5 | G6 |
| NaOCl5% | G7 | G8 | G9 |
| CHX 2% | G10 | G11 | G12 |
|
Etch&Rinse adhesive Orthofosforic acid 37% Parabond A+B Paracore |
Two steps self-etch adhesive ParaBond® Non-Rinse Conditioner Parabond A+B Paracore |
Universal adhesive Clearfil Universal Clearfil DC Core Plus |
Total | |
| Distilled Water (control) | G1= 16,760aa±4,43 | G2= 16,261aa±5,88 | G3= 17,067abc±4,33 | 16,696ac |
| EDTA 17% | G4= 17,069aa±5,03 | G5= 14,951aa±5,14 | G6= 16,663aac±4,84 | 16,228ab |
| NaOCl 5% | G7= 14,606aa±3,81 | G8= 14,095aa±4,64 | G9= 12,919a±3,79 | 13,873 |
| CHX 2% | G10= 17,304aa±5,95 | G11= 16,479aa±3,04 | G12=16,604aab±4,58 | 16,796bc |
| Total | 16,435a | 15,447a | 15,813a | 15,898a |
| Coronal | Media | Apical | |
| (MPa) | 16,198 | 16,333 | 15,163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





