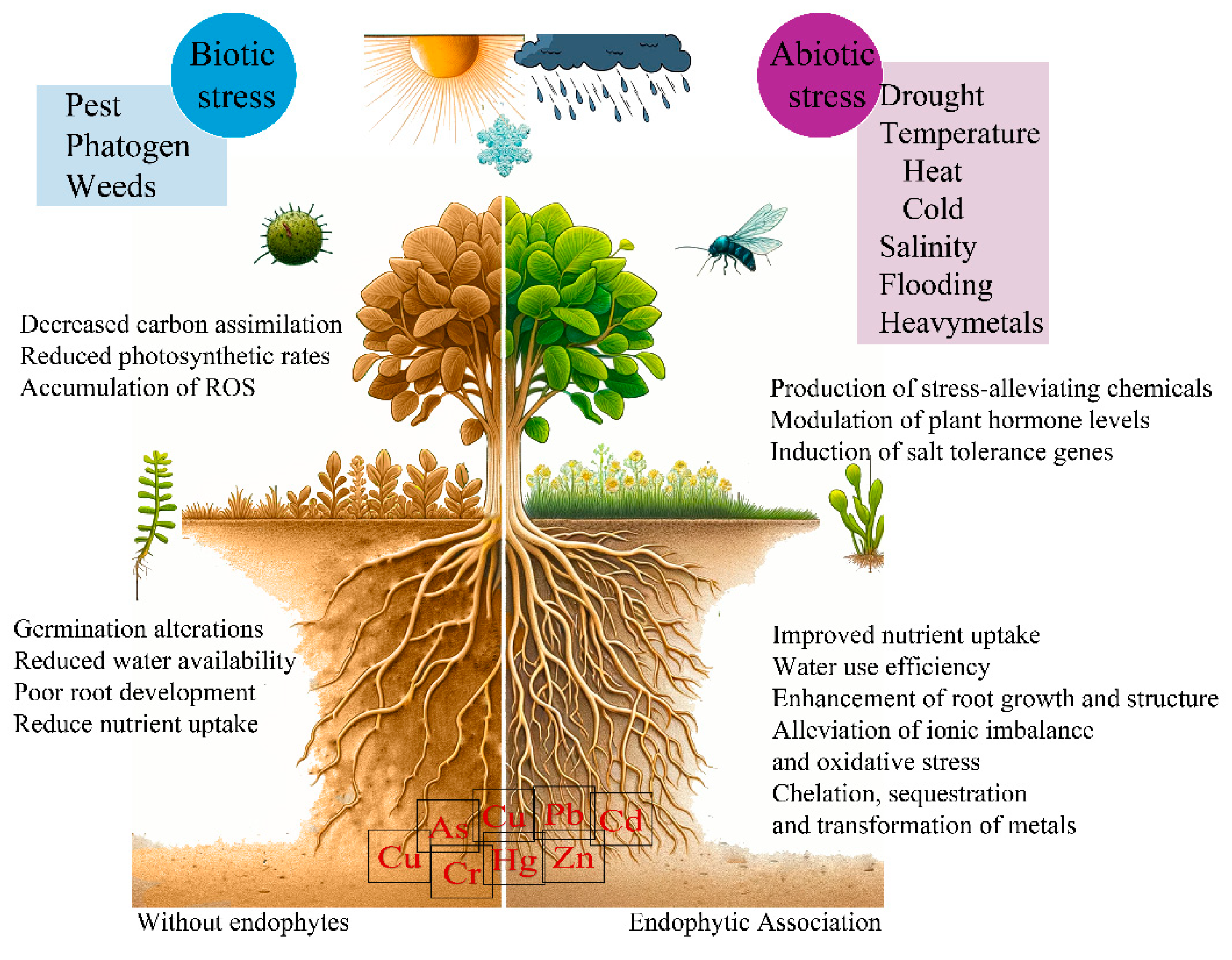
1. Endophytic Fungi in Perspective
Endophytic fungi (EF) are a group of microscopical fungi that live inside the tissues of plants without causing any apparent harm to the host. They have gained significant attention in recent years due to their potential ecological, agricultural, and pharmaceutical applications [1]. EM are a diverse group and can be found in various plant species worldwide. They have been found in almost every plant genus, from grasses to trees and from tropical rainforests to arid deserts [2].
The study of EM sheds light on the complex interactions between plants, fungi, and the environment. They play a significant role in nutrient cycling, carbon sequestration, and the overall health of ecosystems. In many cases, EM form mutualistic relationships with their host plants. They can provide benefits to the host, such as enhanced growth, and improved tolerance to environmental stress factors like drought or salinity. Besides, EM can help protect plants from herbivores and pathogens by increasing plant resistance or by producing toxins that deter herbivores, while others compete with or inhibit plant pathogens [3,4].
Recent research has focused on bioprospecting EM for novel compounds and bioactive substances. These microorganisms represent a valuable resource for discovering new drugs, agricultural solutions, and biotechnological products. EM are known to produce a wide range of bioactive specialized metabolites. These compounds have attracted attention for their potential use in pharmaceuticals, agriculture, and industry [5]. While the potential of EM is exciting, there are challenges in harnessing their benefits. Research continues into understanding their biology and ecology, the specific mechanisms of their interactions with plants, and how to effectively utilize them in agriculture, biotechnology, and medicine.
2. Mechanisms of Endophytic Fungi Colonization of Plant Tissues
Given that the definitive characteristic of EM is the colonization of plant tissues, it is imperative to delve deeper into this aspect. EM colonize plant tissues through many complex mechanisms. Each colonization mechanism can vary depending on the specific fungal strain and the plant host, but there are some general processes involved in the colonization of plant tissues. At the beginning of the plant-fungal interaction, the EM typically enter the plant host through natural openings such as stomata, wounds, or root tips. Further, some fungi may produce specialized structures or adhesive molecules to attach to the plants surface [6].
The way by which EM localize and approach the host plant seems to be mediated by chemotaxis, nevertheless further research is required. Some EM can actively move toward chemical cues released by the host plant, guiding them toward potential entry points [7]. Once attached to the plant’s surface, EM employ various strategies to penetrate the plant tissue. These can involve the secretion of enzymes, such as cellulases or pectinases, to break down the plant cell wall and gain entry [1,8]. Then, inside the plant tissue, EM can grow both intercellularly (between plant cells) and intracellularly (inside plant cells) [8]. Fungi that grow intracellularly may form specialized structures called hyphal coils or microsclerotia [9].
Successful endophytes often have mechanisms to evade or suppress the plant immune response. They can secrete molecules that inhibit plant defense mechanisms, making them less likely to be recognized as pathogens. Some EM produce bioactive specialized metabolites, which can further contribute to their successful colonization of plant tissues [3,10].
EM can persist within the plant for extended periods, potentially throughout the plant’s life cycle. They may spread throughout the plant and colonize various tissues, including seeds, leaves, stems, and roots [11]. Then, EM can be transmitted vertically (from parent to offspring) and horizontally (between plants) through various means, including seeds, root-to-root contact, and aerial spore dispersal [12,13].
3. Endophytic Fungi—Host Plant Interactions
The EM-host plant interactions can be as specific as the host species and the endophyte strain. Among these, the mutualistic relationship is interesting due to the various benefits to the plants. As part of these benefits, EM can help plants acquire essential nutrients, such as phosphorus and nitrogen. Endofungi can access nutrients from soil that may be otherwise unavailable to the plant and transfer these nutrients to the host [14]. Besides, some EM produce specialized metabolites that have antifungal or antibacterial properties. This can help protect the host plant from pathogen attacks. For example, EM in grasses can produce alkaloids that deter herbivores [15]. Further, EM can also contribute to the drought tolerance of host plants. EF help plants by improving water and nutrient uptake, which is especially valuable in arid or water-stressed environments [16]. EM can also interact with the plant through chemical signaling using sophisticated molecular mechanisms by releasing molecules that modulate plant growth and development, contributing to their mutualistic effects [8].
Not all EM-plant interactions are mutually beneficial. Some endophytes can become pathogenic under certain conditions, causing harm to their host plants [4]. This duality makes the study of these interactions even more complex. Beyond the relationship between EM and host plant, this phenomenon has ecological implications since EM play a role in plant community dynamics and ecosystem diversity. Endofungi can influence the composition of plant communities and the overall structure of ecosystems.
4. Endophytic Fungi and Host Plant Fitness
The relationship between EM and host plant fitness is a subject of great interest in ecology and plant biology. The fitness of a host plant refers to its ability to survive, reproduce, and pass on its genetic material to the next generation [17,18]. EM can have both positive and negative impacts on host plant fitness, depending on various factors [19].
Beneficially, EM can enhance the uptake of essential nutrients, such as nitrogen and phosphorus, from the soil. This increased nutrient availability can lead to improved plant growth, which, in turn, can enhance the fitness of the plant by increasing its reproductive potential [3]. Also, endofungi can help host plants tolerate environmental stress factors such as drought, high salinity, or extreme temperatures. This stress tolerance can enable the plant to survive and reproduce under adverse conditions, thereby enhancing its fitness [20]. Furthermore, some EM produce specialized metabolites that deter herbivores and protect the plant from pathogens. So, the reduction of herbivory and pathogen damage can lead to higher plant fitness by reducing the loss of resources and high-value tissues such as the reproductive structures [21]. Besides, endofungi can help plants compete with neighboring vegetation for resources. This competitive advantage can also lead to increased fitness by allowing the plant to access more resources and grow more vigorously [11].
From a negative impact on plant fitness, some EM can turn pathogenic under certain conditions, causing harm to the host plant. Pathogenic endophytes can lead to decreased plant survival by causing diseases, reducing growth, or even killing the plant [22]. On the other hand, the resources invested by host in maintaining endophytic associations might be diverted from other critical plant functions, which can affect overall fitness. This is particularly relevant when endophytes do not provide substantial benefits [23]. While the advantages of a mutualistic plant-endophytic fungus symbiosis have been extensively documented, the factors and mechanisms related to non-beneficial relationship, the balance of endophytic/pathogenic behavior, and the associated cost-benefit of EM infection need more research. By understanding the molecular mechanisms, the genetic cues, and the physiological traits related to the process by which some EM become pathogenic and vice versa new insights for biotechnology and agroecology can be discovered.
5. The Impact of Endophytic Fungi on Crops Abiotic-Stress Adaptation
Among all benefits associated with EM-plant interaction (
Figure 1), the potential to enhance crop adaptation to various abiotic stresses including drought tolerance, salinity resistance, and temperature and pH stress is of particular interest due to its implications in sustainable agriculture, food sovereignty, eradication of hunger and malnutrition, and care of the environment. Strategies involving the use of specific EM or manipulating their interactions with host plants through inoculation or genetic engineering hold promise in developing stress-tolerant crop varieties. However, practical application in agriculture requires a deeper understanding of the mechanisms involved, optimal conditions for fungal-plant interactions, and potential impacts on the environment and ecosystem. In the following lines are described some of the benefits provided by EM to the abiotic-stress tolerance of crops.
5.1. Role of Endophytic Fungi in Salt Stress Aminoration
Among all abiotic stresses, soil salinization is the most harmful. Soil salinization includes saline and alkaline soil exposure, both defined as high salt concentration. Salinization is commonly linked to high sodium cation (Na+) concentration, and high pH, often due to elevated alkali concentration [24]. Otherwise, soil is classified as saline if its osmotic pressure is more than 0.2 MPa and its electrical conductivity is greater than 4 ds/m, corresponding to around 40 mM NaCl [25]. Soil salinization can occur due to both natural factors and human activities, leading to the accumulation of soluble salts in soil layers. The presence of dissolved ions in water can have a direct impact on the growth of crops, for example the cations attached to soil particles can influence soil structure, indirectly affecting crop productivity. According to a Food and Agriculture Organization (FAO) report from the year 2000, the global extent of salt-affected soils, which includes both saline and sodic soils, was estimated to cover an area of 831 million hectares. Experts estimate that by 2050, soil salinity will have damaged at least 50 % of the world’s farmland [26]. In addition to this, climate change increases concerns related to soil salinization. As stated by predictions of primary soil salinization under a climate change, the following regions are considered hotspots for soil salinization: South America, Southern and Western Australia, Mexico, Outwest United States, and South Africa [27]. Soil salinity has several negative impacts on crop productivity, leading to low economic returns and soil erosion. Salinity affects various aspects of plant development, including germination, vegetative growth, and reproductive development. It imposes ion toxicity, osmotic stress, nutrient deficiency, and oxidative stress on plants, limiting water uptake from the soil. Salinity also disrupts the nutrient balance and uptake in plants. It adversely affects photosynthesis, reduces leaf area and chlorophyll content, and inhibits reproductive development. Salinity can also hinder seedling growth, enzyme activity, and DNA, RNA and protein synthesis. Overall, soil salinity has detrimental effects on plant growth and development, leading to reduced crop productivity [26,28].
The severity of the impact depends on the duration and intensity of salt stress, as well as the sensitivity of the plant species or variety. To mitigate the negative effects of salt stress on plant growth and yield, strategies such as the use of salt-tolerant crop varieties, soil amendments, and the application of beneficial microorganisms like fungal endophytes are being explored.
Fungal endophytes play a crucial role in enhancing salt tolerance in plants. They can withstand high salt concentrations and are able to colonize the roots of plants. When inoculated on salt-sensitive plants, fungal endophytes have been shown to promote germination and improve biomass-related parameters under salt-stress conditions. They achieve this by increasing host plant biomass and nutrient acquisition rate, altering root architecture, carrying out osmotic adjustment, and alleviating oxidative stress in the host cells. Overall, fungal endophytes contribute to the salt stress tolerance of plants by enhancing their growth and mitigating the negative effects of salt on plant development [29].
For example, fungal endophytes
Ulocladium sp.,
Fusarium avenaceum,
Chaetomium sp., and
F. tricinctum have been found to assist plants in coping with salt stress. These endophytes were isolated from plants in the Northwestern Himalayan region and have shown the ability to impart salinity stress tolerance in mung bean plants (Vigna radiata L. Wilczek).
F. avenaceum was found superior in inducing salinity stress tolerance and increasing yield in mung bean plants. These endophytes help their host plants survive under salt stress conditions by reducing the deleterious effects of salt stress through various mechanisms such as scavenging reactive oxygen species (ROS), maintaining ion homeostasis, and accumulating organic solutes, sugars, proteins, and lipids [30].
Table 1 describes some plant-endofungi interactions with a positive impact on plant fitness.
5.2. Endophytic Fungi Increse Crops Tolerance to Temperature Stress
As mentioned, the lack of plant adaptation to abiotic stress could result in crop losses and soil deterioration. The use of fungal endophytes to increase yield production and plant stress tolerance could be an eco-friendly alternative for this purpose. EM can mitigate plant abiotic stress by their ability to colonize plant tissues, secondary metabolite synthesis, and the induction of the plant’s systemic defense [31].
Heat stress is defined as the increase of ambient temperature up to 10-15ºC. The level of damage in plant tissues caused by heat stress involves physiological and cellular changes affecting the stability of proteins and cellular components [32]. Under heat stress an intricate biochemical and molecular response is activated, that involves a sequence of processes from signal perception up to the expression of heat shock proteins (HSP), reactive oxygen species (ROS) and antioxidant enzymes [33,34]. Some biochemical responses involved a disruption of photosystems, rubisco heat-degradation activation and chlorophyll reduction [35]. A common response during drought and heat stress is the upregulation of the transcription factor APETALA 2 (AP2)/Ethylene-responsive factor (ERF) [36]. Likewise, it has been observed that heat stress can generate transcriptional memory after its induction, at least in the ascorbate peroxidase 2 (APX2) gene and the histone H3K4 hypermethylation to produce a chromatin modification [37]. Plants can naturally face heat stress by a network pulse model that provokes a signal cascade allowing the stress tolerance and acclimatization [38]. Further, some authors suggest that plants can ameliorate heat stress by the overexpression of rubisco activase (Rca) to offset the heat-induced rubisco inhibition [38].
The use of fungal endophytes for heat stress tolerance has been evaluated in different crops, for example, a study in soybean and sunflower using Aspergillus flavus as endophyte found that this symbiosis mitigates heat stress and significantly enhanced the plant synthesis of proline, abscisic acid, and phenolic compounds in comparison with controls at 40ºC [40]. Similar results were observed in the same plants seedlings inoculated with Rhizopus oryzae and exposed to temperatures of 25°C and 40°C, both species showed high levels of ascorbic acid oxidase (AAO), catalase (CAT), proline, phenolics, flavonoids, sugars, proteins and lipids, in this study also was detected that the endofungus increase the chlorophyll content, shoot and root lengths, and biomass as compared to control plants [41]. The accumulation of specialized metabolites and the enzymatic activity upregulation of enzymes involved in oxidative stress metabolism is a feature observed when plants growth under heat stress, then endophytes could stimulate this beneficial process as has been describe in cucumber inoculated by Thermomyces sp. and sunflower and soybean inoculated by Asperguillus niger [41,42]. Furthermore, in geothermal soil simulation experiments tomato plants and panic grass were evaluated in the presence of Curvularia protuberata, improving the level of drought and heat tolerance [43].
Table 1.
Positive interactions between fungal endophytes and their host plants in response to some abiotic stress.
Table 1.
Positive interactions between fungal endophytes and their host plants in response to some abiotic stress.
| Symbiotic relationship (Endofungus/Plant) |
Benefit for plant host |
Reference |
|
Piriformospora indica / Barley |
Enhanced growth and yield under salt stress. Improved efficiency of water use and nutrient uptake. Increased antioxidant capacity. |
[44] |
| |
| |
|
Trichoderma spp / Maize, tomatoes, cucumbers |
Alleviate drought stress. Enhanced root development, leading to better water and nutrient uptake. |
[45] |
|
Aspergillus ochraceus / Barley |
Enhanced antioxidant capacity under salt stress. Protection against fungal pathogens. Production of IAA. |
[46] |
|
Stemphylium lycopersici / Maize |
Improved chlorophyll a/b ratio. Carotenoids and specialized metabolites production. Enhanced antioxidant and enzymatic activities. Lipid peroxidation reduction. Mitigation of ionic ratio misbalance under salt stress. |
[47] |
|
Penicillium minioluteum / Soybean |
Increased shoot length and biomass production. Major chlorophyll and flavonoid content, leaf area, and nitrogen uptake. Regulation of hormone production under salt stress. |
[48] |
|
Periconia macrospinosa, Neocamarosporium chichastianum, Neocamarosporium goegapense / Cucumber, tomato |
Increased chlorophyll concentration, leaf proline content, and enzymatic activity. |
[49] |
| |
|
|
Temperature stress includes also cold stress. It is known that cold induces a series of morphological and physiological changes in plants affecting the lipid composition, membranes fluidity, differential flow of Ca2+, protein turnover and the induction of oxidative stress [50,51]. Several studies have shown the positive influence of fungal endophytes on the cold stress tolerance. For example, the symbiosis between Arabidopsis and the fungus Piriformospora indica increases the plant expression of C-Repeat Binding Factor (CBFs) and cold-regulated genes (CORs) triggering cold acclimatation [52]. In Hordeum vulgare has also been demonstrated that P. indica acts positively in the nutrient uptake under cold stress [53]. Some studies in banana variety Tianbaojiao (Musa acuminate) at 4°C, root colonized by Piriformospora indica, have demonstrated the reduction of malondialdehyde (MDA) and hydrogen peroxide (H2O2) concentrations and the increase of superoxide dismutase (SOD) and catalase (CAT) activities and the augmentation of soluble sugar (SS) and proline content [54].
Besides, the use of endophytes is not limited to monoculture, in a polyculture study using lettuce, chard and spinach, and a combined inoculum of Penicillium fuscuglaucum and P. glabrum as endophytes, was observed the increase of the plants’ nutritional status, and the augmentation of antioxidant compounds (flavonoids) content, compared with uninoculated plants under temperature stress [55].
5.3. Crops Protection against Oxidative Stress by Endophytic Fungi
As was mentioned before, the interaction between host plant and endophytes gives many benefits for both. This interaction allows plants to colonize and survive in niches where other plants cannot. Most of the hard conditions that the plant must resist include the oxidative stress produced by toxic metals, high salt concentration, acidic pH, etc. [56].
The oxidative stress is related to the production of dangerous oxygen radicals or other molecules capable of removing electrons from the biomolecules [57]. This misbalance in the redox status of biomolecules provokes its quick degradation, affecting dramatically the pathway where the biomolecules are related. Most of the organisms have protection mechanisms against oxidative stress, most of them related to the synthesis of free radical scavengers such as glutathione, flavonoids, and other antioxidant molecules [58].
In a similar way as for the other types of abiotic stress mentioned above, EM can give protection to the plants in high oxidative environments. Among all the sources of oxidative stress that affect plant development, the oxidative effect of soils polluted with toxic metals is of special interest. The pollution with toxic metals (TM) has increased because the industrial activities and mining extraction, producing a high accumulation of minerals, and other chemicals in the environment [59]. Within the problems caused by TM pollution, the impact on crops production is a red flag for hunger and malnutrition eradication. The toxicity and oxidative activity of TM is dependent on its reactivity and oxidation state, which is related to soil pH, and organic matter and ions presence. An alternative to reduce the oxidative stress related to TM is the use of fungal endophytes. Endofungi colonize plant tissues without producing notably symptoms and protect the plant against the oxidative effects of TM [60].
Fungal and bacterial endophytes can protect plants against TM by many vias, one of these mechanisms is mediated by metals flocculating molecules. Endophyte produce exopolysaccharides, lipids, glycolipids, and proteins that can capture and flocculate metal ions. The increased plant tolerance to TM in presence of endofungi is related to metal ions biosorption and immobilization, metal redox status modification, and the production of biopolymers and siderophores which chelate the toxic metal ions, preventing the associated oxidative stress [61]. In addition to these mechanisms, as mentioned before, the endophyte-host plant interaction encourages a better nutrient uptake, promoting a better plant response to oxidative stress. An example of this is the endophyte Sporobolomyces ruberrimus, this basidiomycete protects the host using siderophores, and promotes the development of lateral roots. S. ruberrimus chelates the excess of Fe but not interfere for other micronutrients uptake [62].
Plants modulate their stress response using many mechanisms, being the phytohormone signaling one of the common against abiotic stress. Cytokinin, abscisic acid (ABA), ethylene, inositol phosphate, and NO2 are some examples of these molecules, which regulate the response against ROSs produced during abiotic or biotic stress [58]. The endophytes could reacts promoting or regulating the phytohormones form the host, as it has described for Sporobolomyces rubberriums and the regulation of ethylene related to resist the oxidative stress produce by Fe [62]. Even the endophytes could produce some of the phytohormones or regulate those from the host, the intimate relationship is coordinated to work together [63] increasing of plant levels of antioxidant enzymes or antioxidant molecules. One example is the production of organic acids, as ascorbic acid, as an antioxidative strategy to neutralize heavy metal effect and decrees the ROS effect. Auxins (IAA), cytokinin, ethylene, gibberellin (GA) and ABA are essential for an adaptative response in a oxidate stress related to abiotic factors. Related to this, endophytes produce metabolites that stimulate and regulate the host hormones production, increasing the plant metabolic response against abiotic stress [63].
6. Conclusions
Research is needed on the diversity of endophytes present in various ecosystems, focusing on those that have demonstrated potential to improve the resilience of crops under environmental stresses such as drought, salinity, and nutrient deficiency. It is necessary to consider the unique attributes of endophytes from extreme environments (e.g., deserts, saline soils) and their potential application in the development of stress-resistant crop varieties, as these endophytes are often well adapted to harsh conditions. The potential applications of endophytes in not only improving stress tolerance but also in enhancing overall plant fitness, growth, nutrient uptake, and yield, thereby providing comprehensive benefits to the crops. Agricultural practices can be optimized to harness the full potential of endophytic fungi in improving crop resilience and productivity, especially in the face of increasing environmental challenges and helping hunger eradication.
Author Contributions
All authors contributed equally to writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by TecNM/ITS Irapuato.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jha, P.; Kaur, T.; Chhabra, I.; Panja, A.; Paul, S.; Kumar, V.; Malik, T. Endophytic fungi: Hidden treasure chest of antimicrobial metabolites interrelationship of endophytes and metabolites. Front Microbiol 2023, 14. [CrossRef]
- Wen, J.; Okyere, S. K.; Wang, S.; Wang, J.; Xie, L.; Ran, Y.; Hu, Y. Endophytic fungi: An effective alternative source of plant-derived bioactive compounds for pharmacological studies. J Fungi 2022, 8(2). [CrossRef]
- Bhardwaj, M.; Kailoo, S.; Khan, R. T.; Khan, S. S.; Rasool, S. Harnessing fungal endophytes for natural management: a biocontrol perspective. Front Microbiol 2023, 14. [CrossRef]
- Alam, B.; Lǐ, J.; Gě, Q.; Khan, M. A.; Gōng, J.; Mehmood, S.; et al. Endophytic fungi: From symbiosis to secondary metabolite communications or vice versa? Front Plant Sci 2021, 12. [CrossRef]
- Kumari, P.; Deepa, N.; Trivedi, P. K.; Singh, B. K.; Srivastava, V.; Singh, A. Plants and endophytes interaction: a “secret wedlock” for sustainable biosynthesis of pharmaceutically important secondary metabolites. Microb Cell Fact 2023, 22(1). [CrossRef]
- Mengistu, A. A. Endophytes: colonization, behaviour, and their role in defense mechanism. Internat J Microbiol 2020, 2020. [CrossRef]
- Tsai, A. Y. L.; Oota, M.; Sawa, S. Chemotactic host-finding strategies of plant endoparasites and endophytes. Front Plant Sci 2020, 11. [CrossRef]
- Ji, X.; Xia, Y.; Zhang, H.; Cui, J. L. The microscopic mechanism between endophytic fungi and host plants: From recognition to building stable mutually beneficial relationships. Microbiol Res 2022, 261. [CrossRef]
- Chambers, S. M.; Curlevski, N. J.; Cairney, J. W. Ericoid mycorrhizal fungi are common root inhabitants of non-Ericaceae plants in a south-eastern Australian sclerophyll forest. FEMS Microbiol Ecol 2008, 65(2). [CrossRef]
- Lu, H.; Wei, T.; Lou, H.; Shu, X.; Chen, Q. A critical review on communication mechanism within plant-endophytic fungi interactions to cope with biotic and abiotic stresses. J Fungi 2021, 7(9). [CrossRef]
- Baron, N. C.; Rigobelo, E. C. Endophytic fungi: a tool for plant growth promotion and sustainable agriculture. Mycology 2022, 13(1). [CrossRef]
- Shahzad, R.; Khan, A. L.; Bilal, S.; Asaf, S.; Lee, I. J. What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Front Plant Sci 2018, 9. [CrossRef]
- Grabka, R.; d’Entremont, T. W.; Adams, S. J.; Walker, A. K.; Tanney, J. B.; Abbasi, P. A.; Ali, S. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants 2022, 11(3). [CrossRef]
- Sharma, I.; Raina, A.; Choudhary, M.; Kaul, S.; Dhar, M. K. Fungal endophyte bioinoculants as a green alternative towards sustainable agriculture. Heliyon 2023. [CrossRef]
- Fuchs, B.; Krauss, J. Can Epichloë endophytes enhance direct and indirect plant defence? Fungal Ecol 2019, 38. [CrossRef]
- Javed, J.; Rauf, M.; Arif, M.; Hamayun, M.; Gul, H.; Ud-Din; et al. Endophytic fungal consortia enhance basal drought-tolerance in Moringa oleifera by upregulating the antioxidant enzyme (APX) through Heat shock factors. Antioxidants 2022, 11(9). [CrossRef]
- de la Fuente Cantó, C.; Simonin, M., King, E.; Moulin, L.; Bennett, M. J.; Castrillo, G.; Laplaze, L. An extended root phenotype: the rhizosphere, its formation and impacts on plant fitness. Plant J 2020, 103(3). [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol 2015, 206(4). [CrossRef]
- Shankar Naik, B. Functional roles of fungal endophytes in host fitness during stress conditions. Symbiosis 2019, 79(2). [CrossRef]
- Kamran, M.; Imran, Q. M.; Ahmed, M. B.; Falak, N.; Khatoon, A.; Yun, B. W. Endophyte-mediated stress tolerance in plants: a sustainable strategy to enhance resilience and assist crop improvement. Cells 2022, 11(20). [CrossRef]
- Akram, S.; Ahmed, A.; He, P.; He, P.; Liu, Y.; Wu, Y.; et al. Uniting the Role of Endophytic Fungi against Plant Pathogens and Their Interaction. J Fungi 2023, 9(1). [CrossRef]
- Kogel, K. H.; Franken, P.; Hückelhoven, R. Endophyte or parasite–what decides?. Curr Op Plant Biol 2006, 9(4). [CrossRef]
- Cheplick, G. P. Costs of fungal endophyte infection in Lolium perenne genotypes from Eurasia and North Africa under extreme resource limitation. Environ Exp Bot 2007, 60(2). [CrossRef]
- Daliakopoulos, I. N.; Tsanis, I. K.; Koutroulis, A.; Kourgialas, N. N.; Varouchakis, A. E.; Karatzas, G. P.; Ritsema, C. J. The threat of soil salinity: A European scale review. Sci Total Environ 2016, 573. [CrossRef]
- Bernstein, N. (2019). Chapter 5—Plants and salt: Plant response and adaptations to salinity. En J. Seckbach & P. Rampelotto (Eds.), Model Ecosystems in Extreme Environments (pp. 101-112): Academic Press.
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 2015, 22. [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat Comm 2021, 12. [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12. [CrossRef]
- Manjunatha, N.; Li, H.; Sivasithamparam, K.; Jones, M. G. K.; Edwards, I.; Wylie, S. J.; Agarrwal, R. Fungal endophytes from salt-adapted plants confer salt tolerance and promote growth in wheat (Triticum aestivum L.) at early seedling stage. Microbiol 2022, 168. [CrossRef]
- Pandit, A. G.; Vendan, K. T.; Earanna, N. Fungal Endophyte Mediated Salinity Stress Tolerance in Mung Bean (Vigna radiata L. Wilczek). Mysore J Agric Sci 2023, 57(2).
- Chaudhary, P.; Agri, U.; Chaudhary, A.; Kumar, A.; Kumar, G. Endophytes and their potential in biotic stress management and crop production. Front Microbiol 2023, 13. [CrossRef]
- Guihur, A.; Rebeaud, M. E.; Goloubinoff, P. How do plants feel the heat and survive?. Trends Bioch Sci 2022, 47(10). [CrossRef]
- Songy, A.; Fernandez, O.; Clément, C.; Larignon, P.; Fontaine, F. Grapevine trunk diseases under thermal and water stresses. Planta 2019, 249. [CrossRef]
- Shaffique, S.; Khan, M. A.; Wani, S. H.; Pande, A.; Imran, M.; Kang, S. M.; et al. A review on the role of endophytes and plant growth promoting rhizobacteria in mitigating heat stress in plants. Microor 2022, 10(7). [CrossRef]
- Crafts-Brandner, S. J.; Law, R. D. Effect of heat stress on the inhibition and recovery of the ribulose-1, 5-bisphosphate carboxylase/oxygenase activation state. Planta 2000, 212. [CrossRef]
- Rocheta, M.; Becker, J. D.; Coito, J. L.; Carvalho, L.; Amâncio, S. Heat and water stress induce unique transcriptional signatures of heat-shock proteins and transcription factors in grapevine. Func Integ Gen 2014, 14. [CrossRef]
- Oberkofler, V.; Bäurle, I. Inducible epigenome editing probes for the role of histone H3K4 methylation in Arabidopsis heat stress memory. Plant Physiol 2022, 189(2). [CrossRef]
- Kollist, H.; Zandalinas, S. I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid responses to abiotic stress: priming the landscape for the signal transduction network. Trends Plant Sci 2019, 24(1). [CrossRef]
- Qu, L.; Gu, X.; Li, J.; Guo, J.; Lu, D. Leaf photosynthetic characteristics of waxy maize in response to different degrees of heat stress during grain filling. BMC Plant Biol 2023, 23(1). [CrossRef]
- Hamayun, M.; Hussain, A.; Khan, S. A.; Iqbal, A.; Lee, I. J. An endophytic fungus Aspergillus violaceofuscus can be used as heat stress adaptive tool for Glycine max L. and Helianthus annuus L. J App Bot Food Qual 2020, 93.
- Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S. A.; Lee, I. J. Aspergillus niger boosted heat stress tolerance in sunflower and soybean via regulating their metabolic and antioxidant system. J Plant Interac 2020, 15(1). [CrossRef]
- Ali, A. H.; Abdelrahman, M.; Radwan, U.; El-Zayat, S.; El-Sayed, M. A. Effect of Thermomyces fungal endophyte isolated from extreme hot desert-adapted plant on heat stress tolerance of cucumber. App Soil Ecol 2018, 124. [CrossRef]
- Rodriguez, F.; Arsene-Ploetze, F.; Rist, W.; Rüdiger, S.; Schneider-Mergener, J.; Mayer, M. P.; Bukau, B. Molecular basis for regulation of the heat shock transcription factor σ32 by the DnaK and DnaJ chaperones. Mol Cell 2008, 32(3). [CrossRef]
- Baltruschat, H.; Fodor, J.; Harrach, B. D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K. H.; Schafer, P.; Schwarczinger, I.; Zuccaro, A.; Skoczowski, A. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol 2008, 180. [CrossRef]
- Harman, G. E. Overview of Mechanisms and Uses of Trichoderma spp. Phytopath 2006, 96. [CrossRef]
- Badawy, A. A.; Alotaibi, M. O.; Abdelaziz, A. M.; Osman, M. S.; Khalil, A. M. A.; Saleh, A. M.; Mohammed, A. E.; Hashem, A. H. Enhancement of Seawater Stress Tolerance in Barley by the Endophytic Fungus Aspergillus ochraceus. Metabol 2021, 11. [CrossRef]
- Ali, R.; Gul, H.; Rauf, M.; Arif, M.; Hamayun, M.; Khilji, S. A.; Ud-Din, A.; Sajid, Z. A.; Lee, I. J. Growth-Promoting Endophytic Fungus (Stemphylium lycopersici) Ameliorates Salt Stress Tolerance in Maize by Balancing Ionic and Metabolic Status. Front Plant Sci 2022, 13. [CrossRef]
- Khan, A. L.; Hamayun, M.; Ahmad, N.; Hussain, J.; Kang, S. M.; Kim, Y. H.; Adnan, M.; Tang, D. S.; Waqas, M.; Radhakrishnan, R.; Hwang, Y. H.; Lee, I. J. Salinity stress resistance offered by endophytic fungal interaction between Penicillium minioluteum LHL09 and glycine max. L. J Microbiol Biotechnol 2011, 21. [CrossRef]
- Hosseyni Moghaddam, M. S.; Safaie, N.; Soltani, J.; Hagh-Doust, N. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol Biochem 2021, 160. [CrossRef]
- De Santis, A.; Landi, P.; Genchi, G. Changes of mitochondrial properties in maize seedlings associated with selection for germination at low temperature. Fatty acid composition, cytochrome c oxidase, and adenine nucleotide translocase activities. Plant Physiol 1999, 119(2). [CrossRef]
- Acuña-Rodríguez, I. S.; Newsham, K. K.; Gundel, P. E.; Torres-Díaz, C.; Molina-Montenegro, M. A. Functional roles of microbial symbionts in plant cold tolerance. Ecol Lett 2020, 23(6). [CrossRef]
- Jiang, B.; Shi, Y.; Peng, Y.; Jia, Y.; Yan, Y.; Dong, X. et al. Cold-induced CBF–PIF3 interaction enhances freezing tolerance by stabilizing the phyB thermosensor in Arabidopsis. Mol Plant 2020, 13(6). [CrossRef]
- Murphy, B. R.; Doohan, F. M.; Hodkinson, T. R. Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis 2014, 62. [CrossRef]
- Li, D.; Bodjrenou, D. M.; Zhang, S.; Wang, B.; Pan, H.; Yeh, K. W.; et al. The endophytic fungus Piriformospora indica reprograms banana to cold resistance. Internat J Mol Sci 2021, 22(9). [CrossRef]
- Molina-Montenegro, M. A.; Escobedo, V. M.; Atala, C. Inoculation with extreme endophytes improves performance and nutritional quality in crop species grown under exoplanetary conditions. Front Plant Sci 2023, 14. [CrossRef]
- El-Shafey, N. M.; Marzouk, M. A.; Yasser, M. M.; Shaban, S. A.; Beemster, G. T. S.; Abdelgawad, H. Harnessing endophytic fungi for enhancing growth, tolerance and quality of rose-scented geranium (Pelargonium graveolens (l’hér) thunb.) plants under cadmium stress: A biochemical study. J Fungi 2021, 7(12). [CrossRef]
- Schu, A.; Polle, A.; Institut, F.; I, A.; Baumphysiologie, F.; Universita, G. A.; Schützendübel, A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exper Bot 2002, 53(372). [CrossRef]
- Yadav, S. K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African J of Bot 2010, 76(2). [CrossRef]
- Arcega-Cabrera, F.; Garza-Perez, R.; Noreña-Barroso, E.; Oceguera-Vargas, I. Impacts of geochemical and environmental factors on seasonal variation of heavy metals in a coastal lagoon Yucatan, Mexico. Bull Environ Cont Tox 2015, 94(1). [CrossRef]
- Ignatova, L.; Kistaubayeva, A.; Brazhnikova, Y.; Omirbekova, A.; Mukasheva, T.; Savitskaya, I.; et al. Characterization of cadmium-tolerant endophytic fungi isolated from soybean (Glycine max) and barley (Hordeum vulgare). Heliyon 2021, 7(11). [CrossRef]
- Cao, G. H.; Li, X. G.; Zhang, C. R.; Xiong, Y. R.; Li, X.; Li, T.; He, S.; Cui, Z. G.; Yu, J. Physiological response mechanism of heavy metal-resistant endophytic fungi isolated from the roots of Polygonatum kingianum. Environ Microbiol Reports 2023, 15(6). [CrossRef]
- Domka, A.; Jędrzejczyk, R.; Ważny, R.; Gustab, M.; Kowalski, M.; Nosek, M.; Bizan, J.; Puschenreiter, M.; Vaculίk, M.; Kováč, J.; Rozpądek, P. Endophytic yeast protects plants against metal toxicity by inhibiting plant metal uptake through an ethylene-dependent mechanism. Plant Cell Environ 2023, 46(1). [CrossRef]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S. K.; Sharma, V. K.; et al. The potential application of endophytes in management of stress from drought and salinity in crop plants. Microorganisms 2021, 9(8). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).