Submitted:
22 February 2024
Posted:
22 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
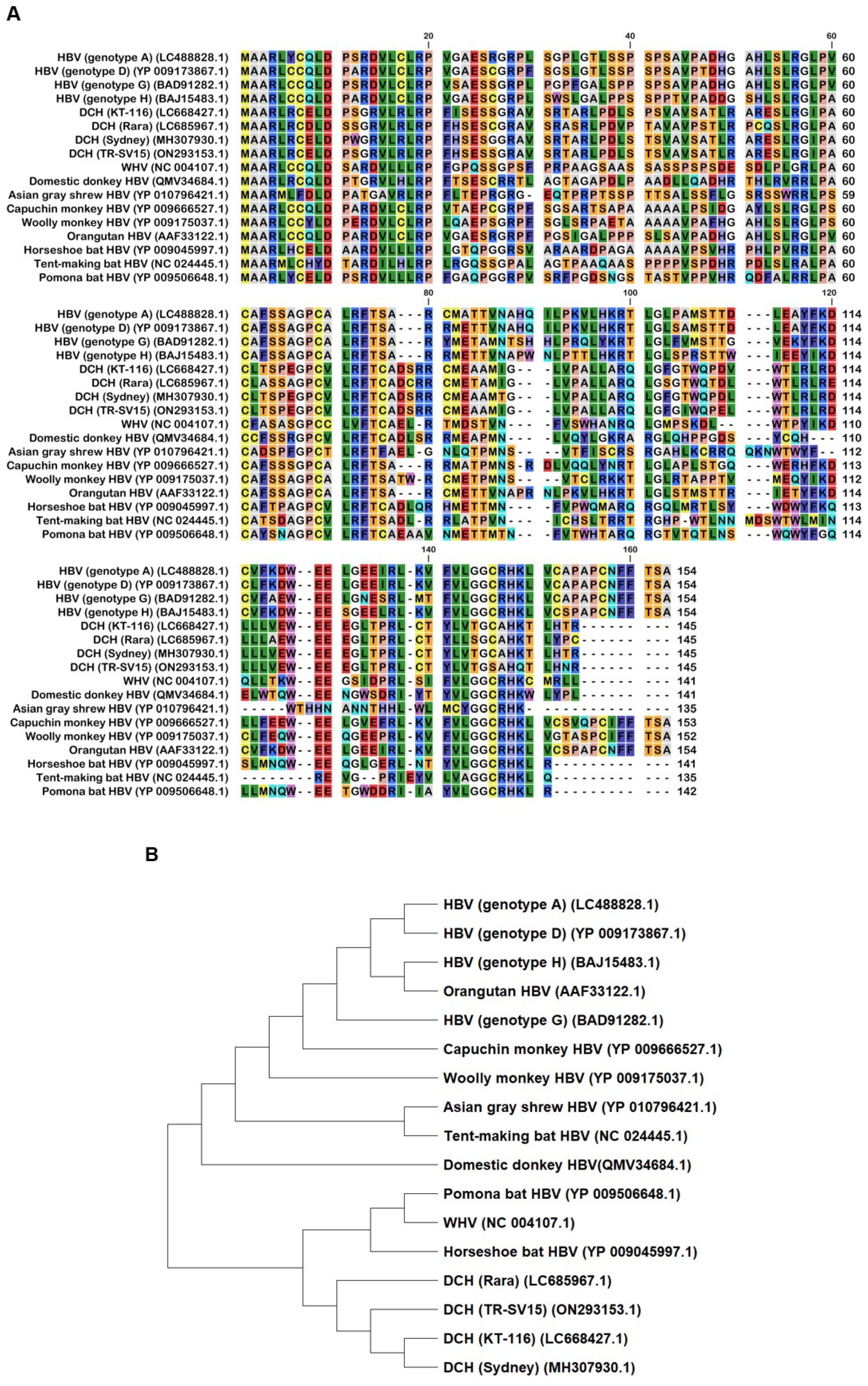
2.1. Genetic characteristics of Orthohepadnavirus X proteins
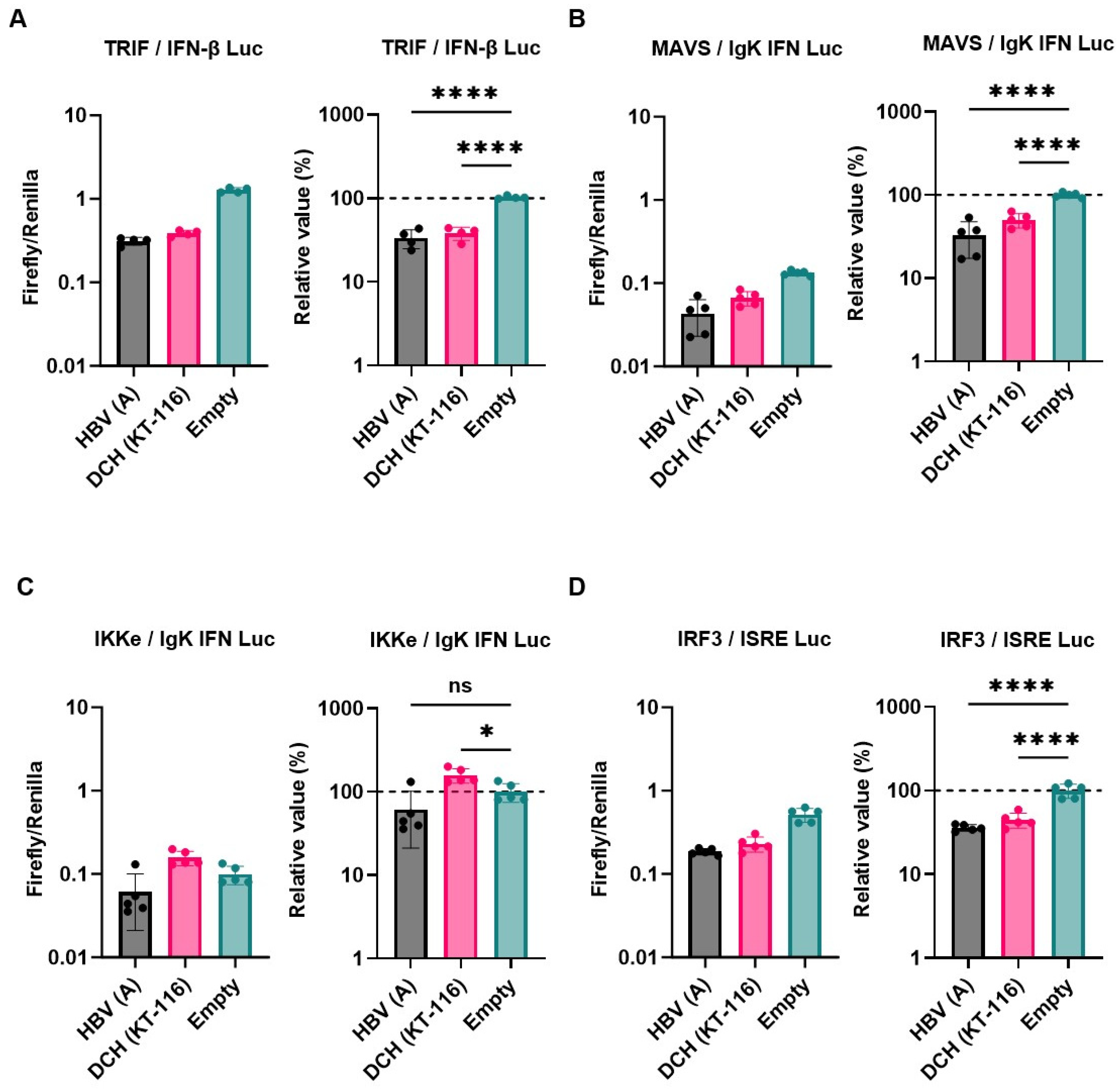
2.2. Both HBV (A) and DCH (KT-116) X proteins inhibited IFN-β signaling mediated by TRIF, MAVS, and IRF3
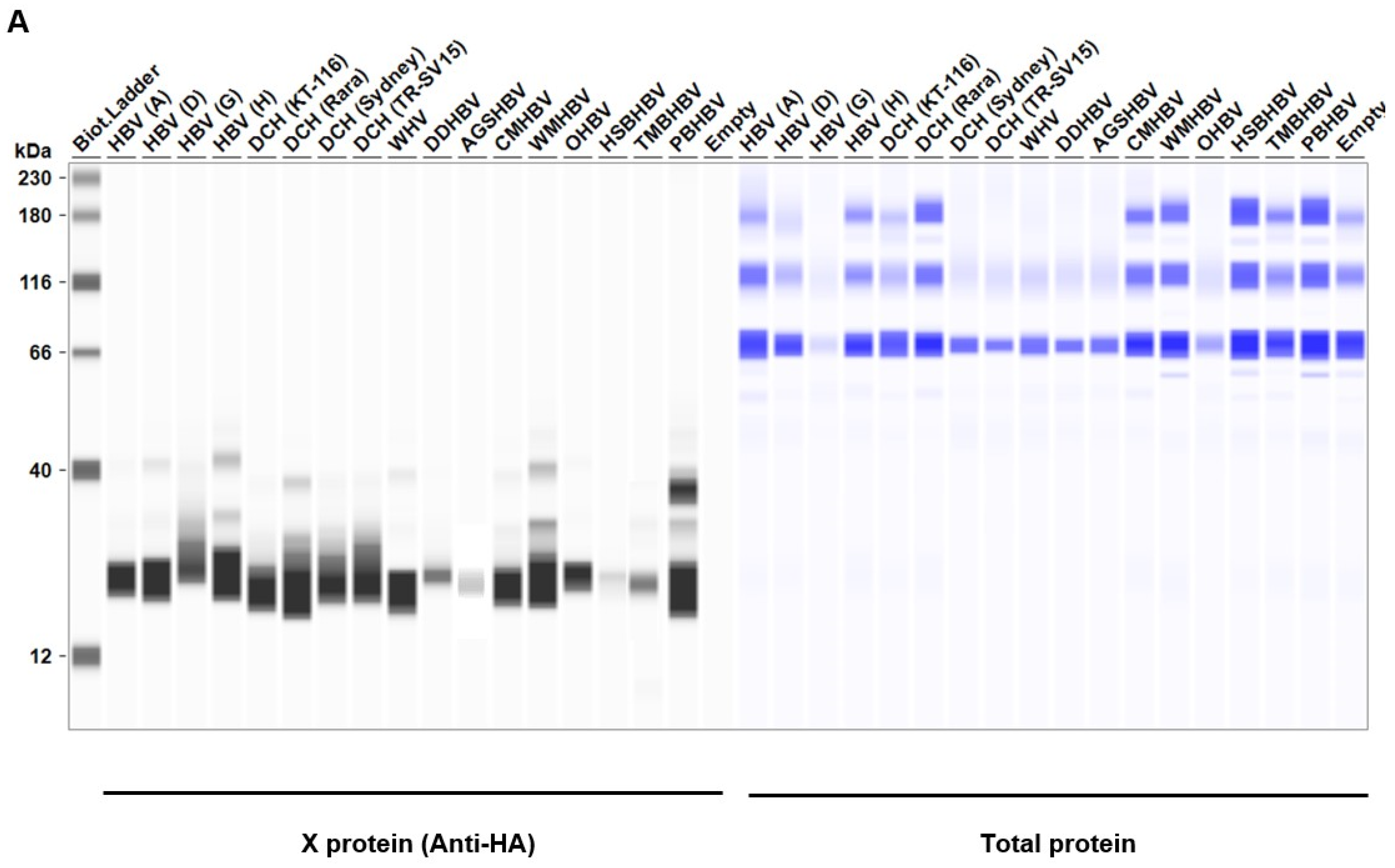
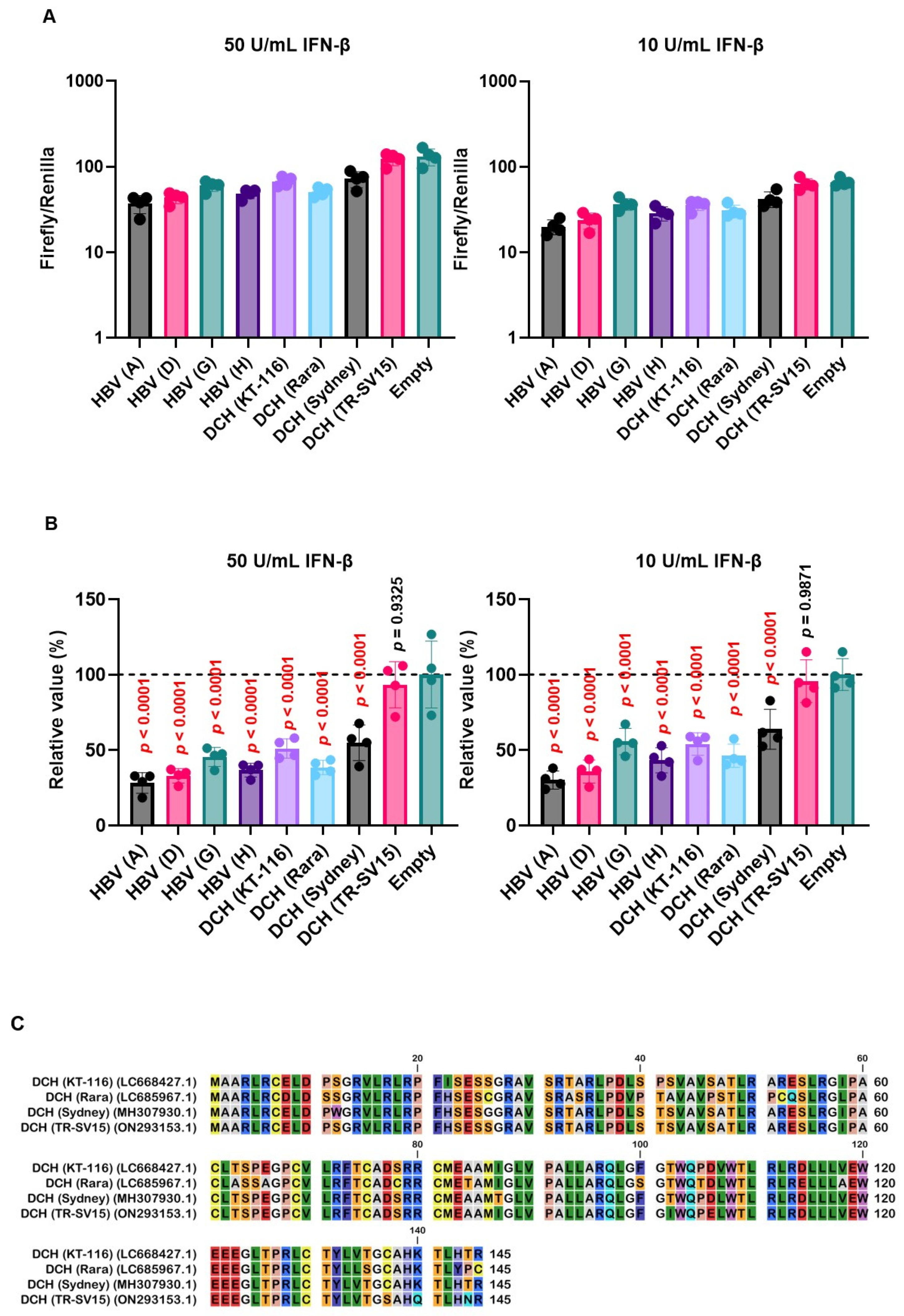
2.3. X proteins derived from a range of Orthohepadnavirus sp. inhibited TRIF-mediated IFN signaling
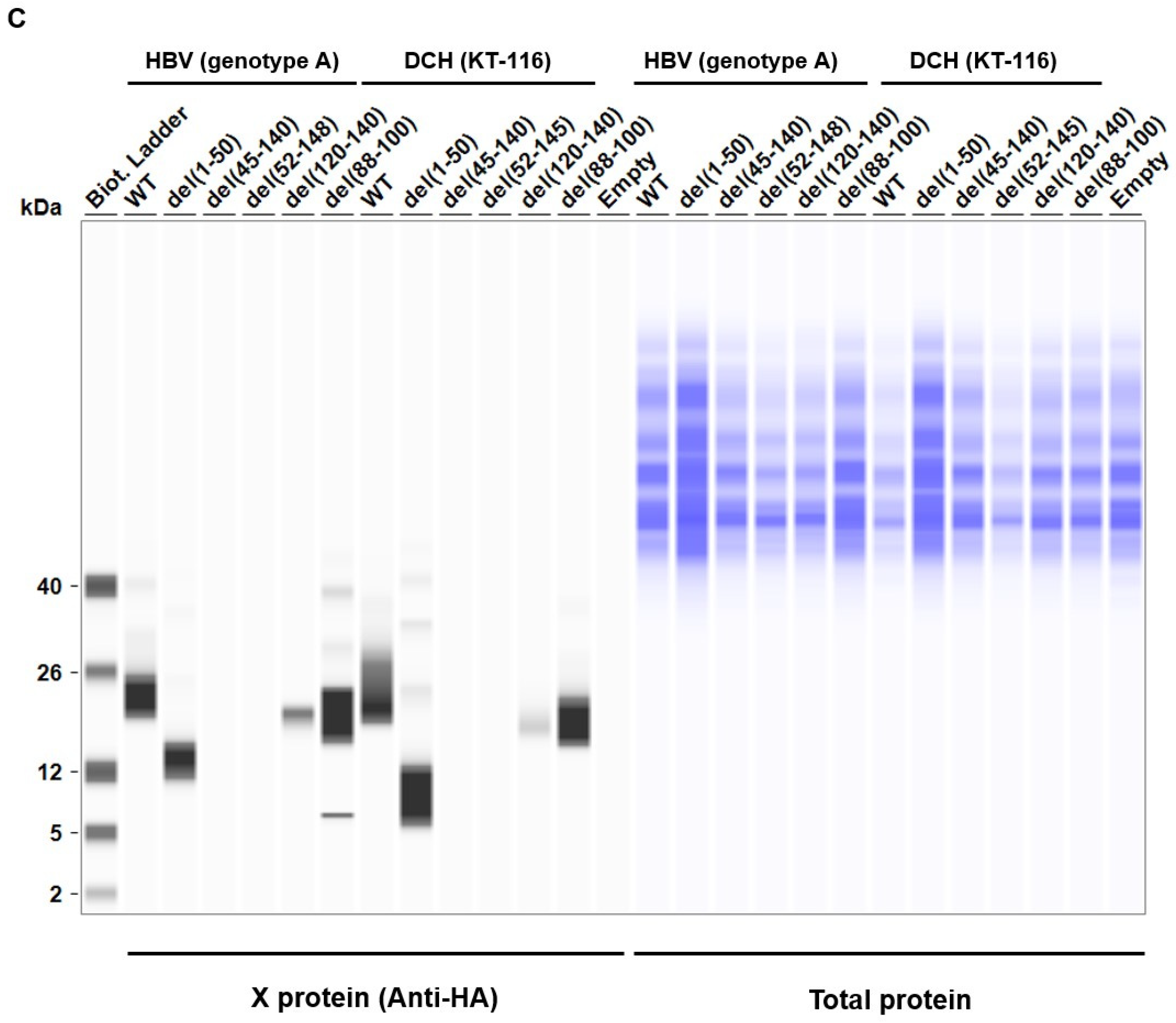
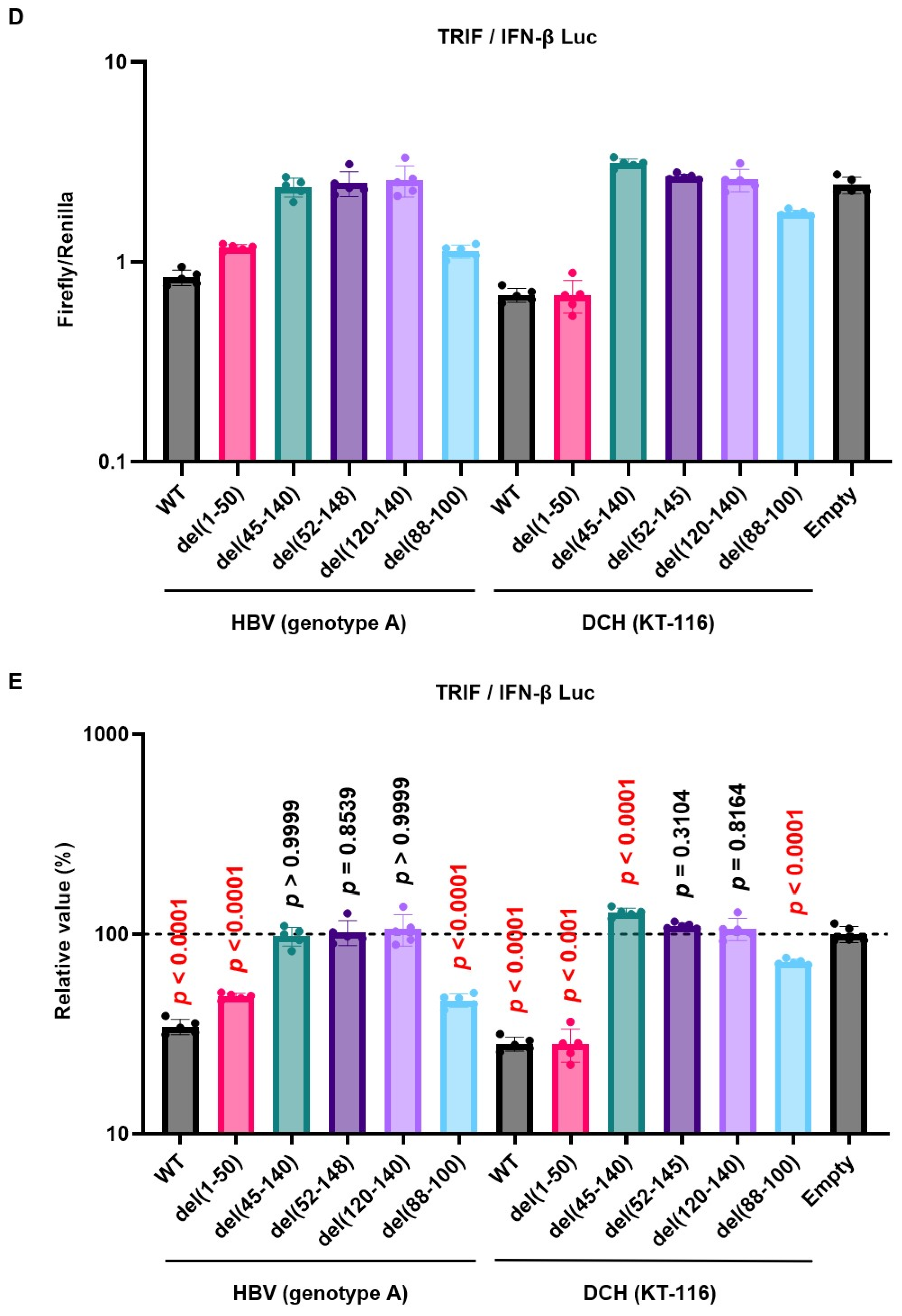
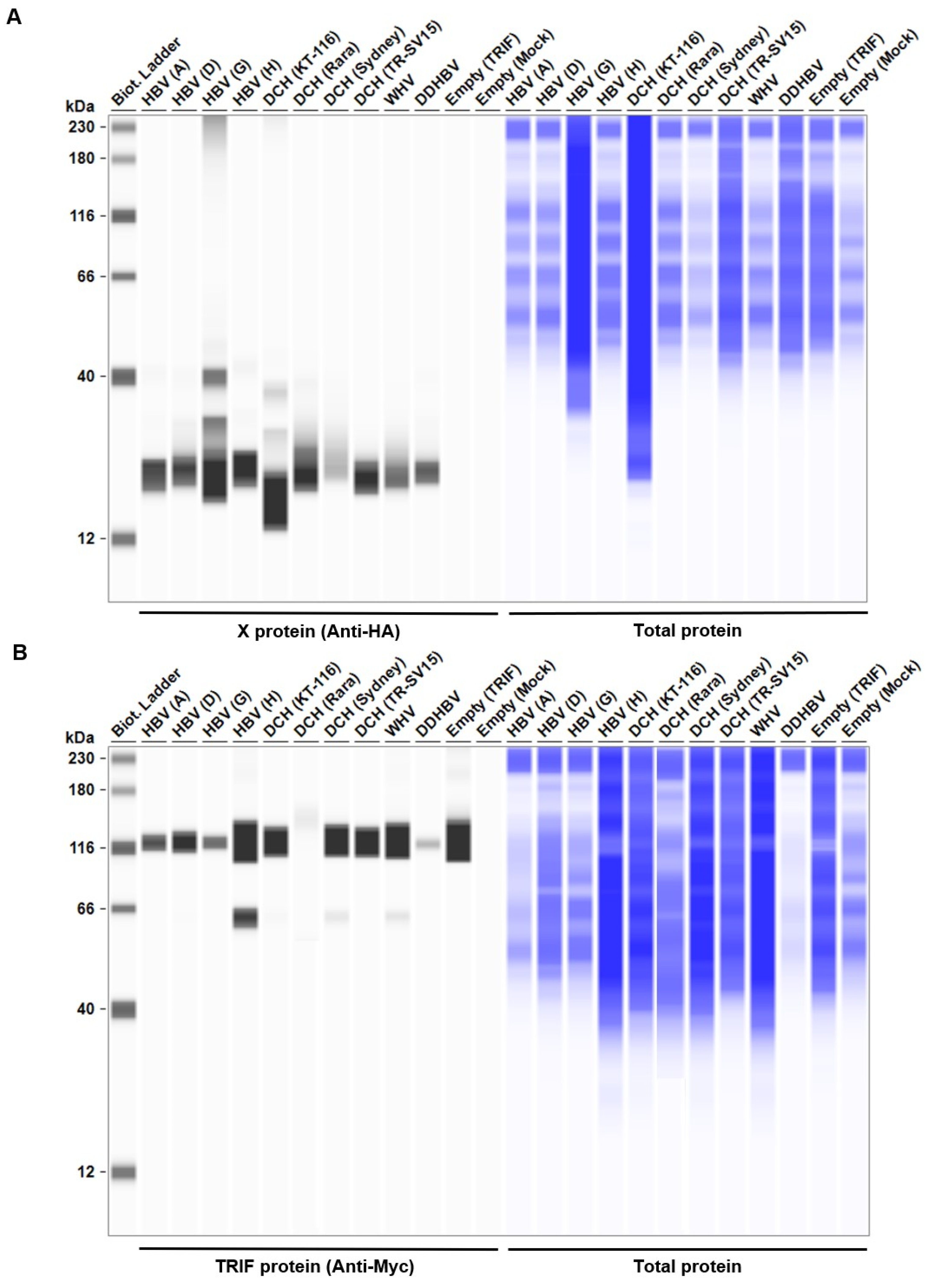
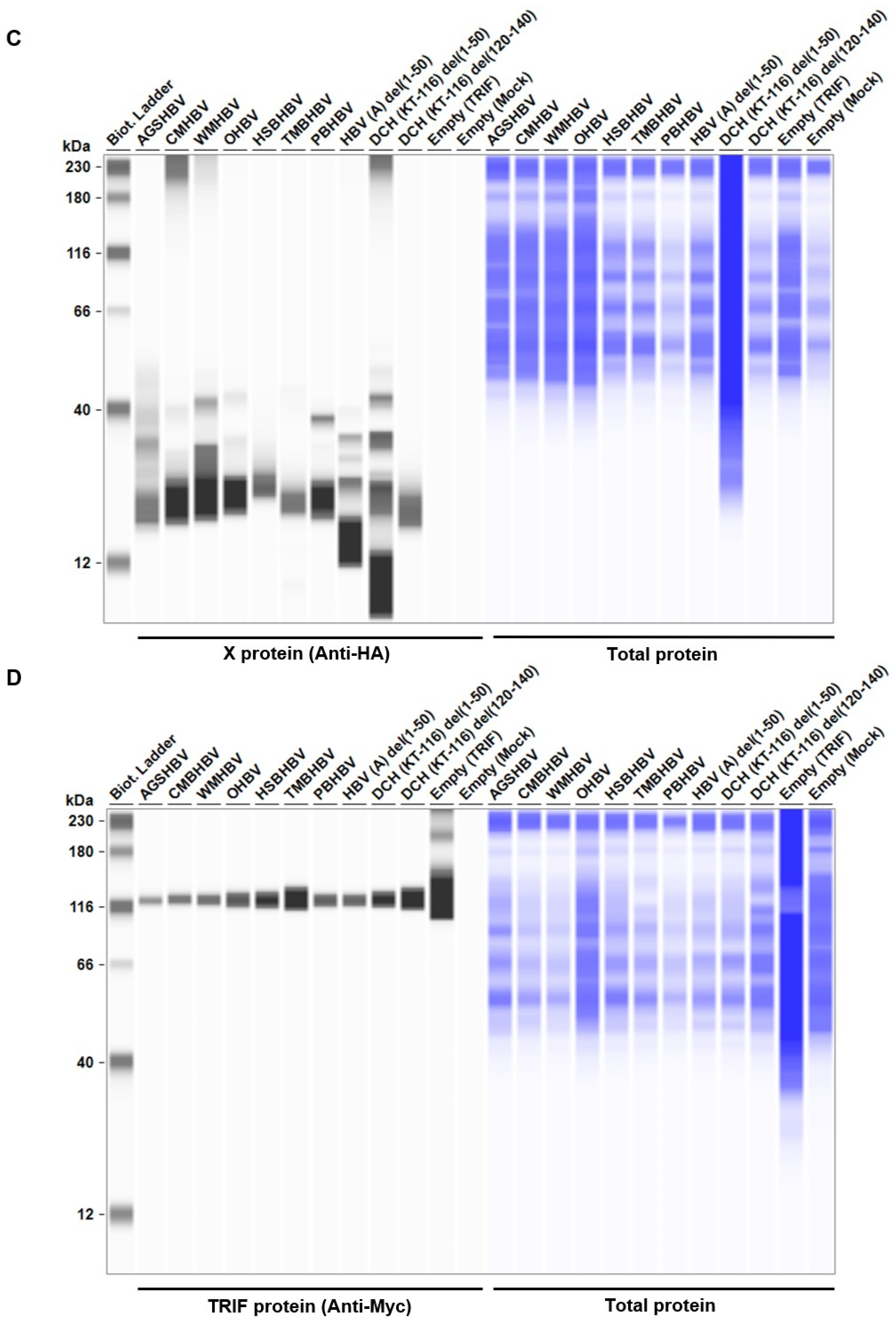
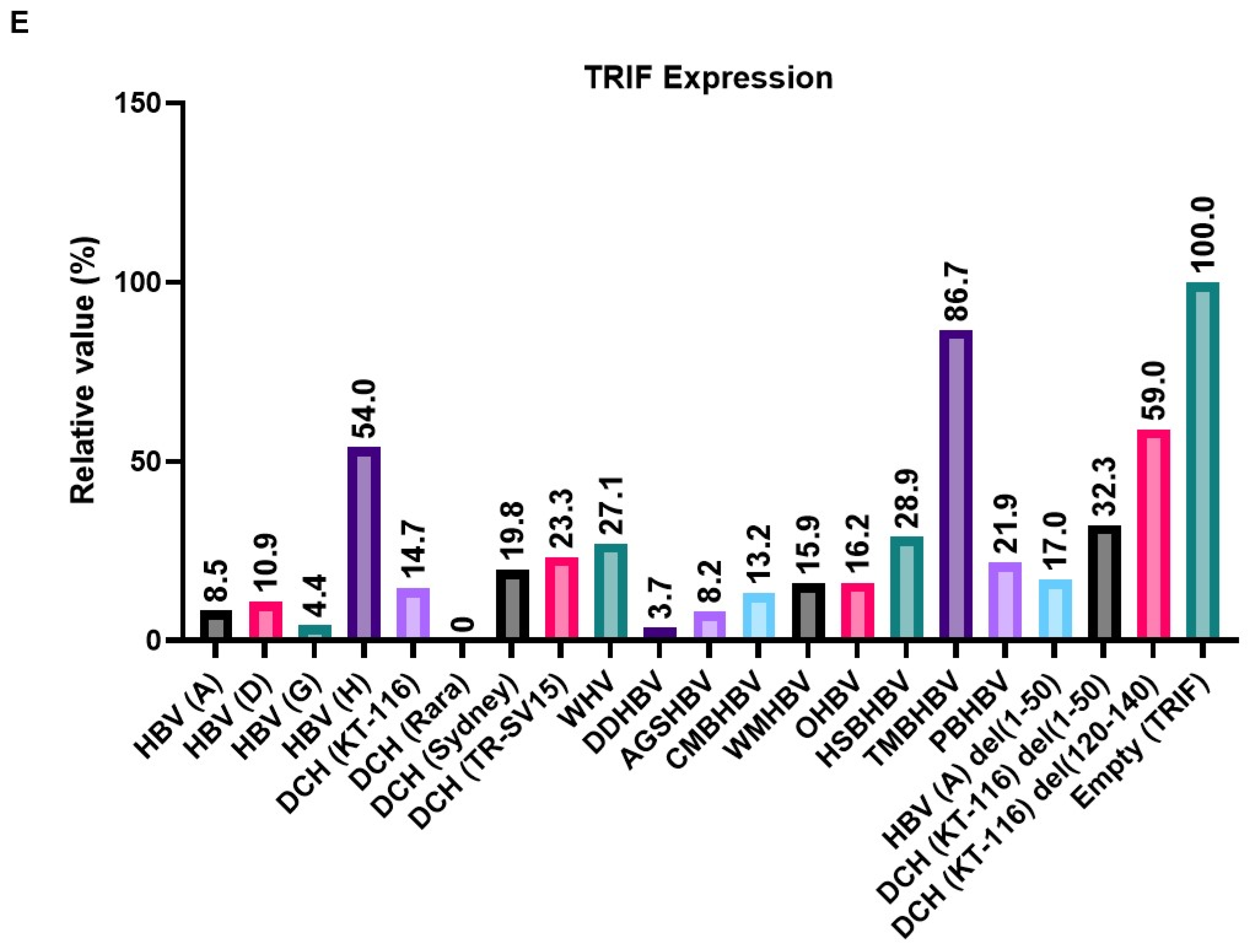
2.4 The C-terminus transactivation domain of X proteins plays an important role in stabilizing protein expression and function
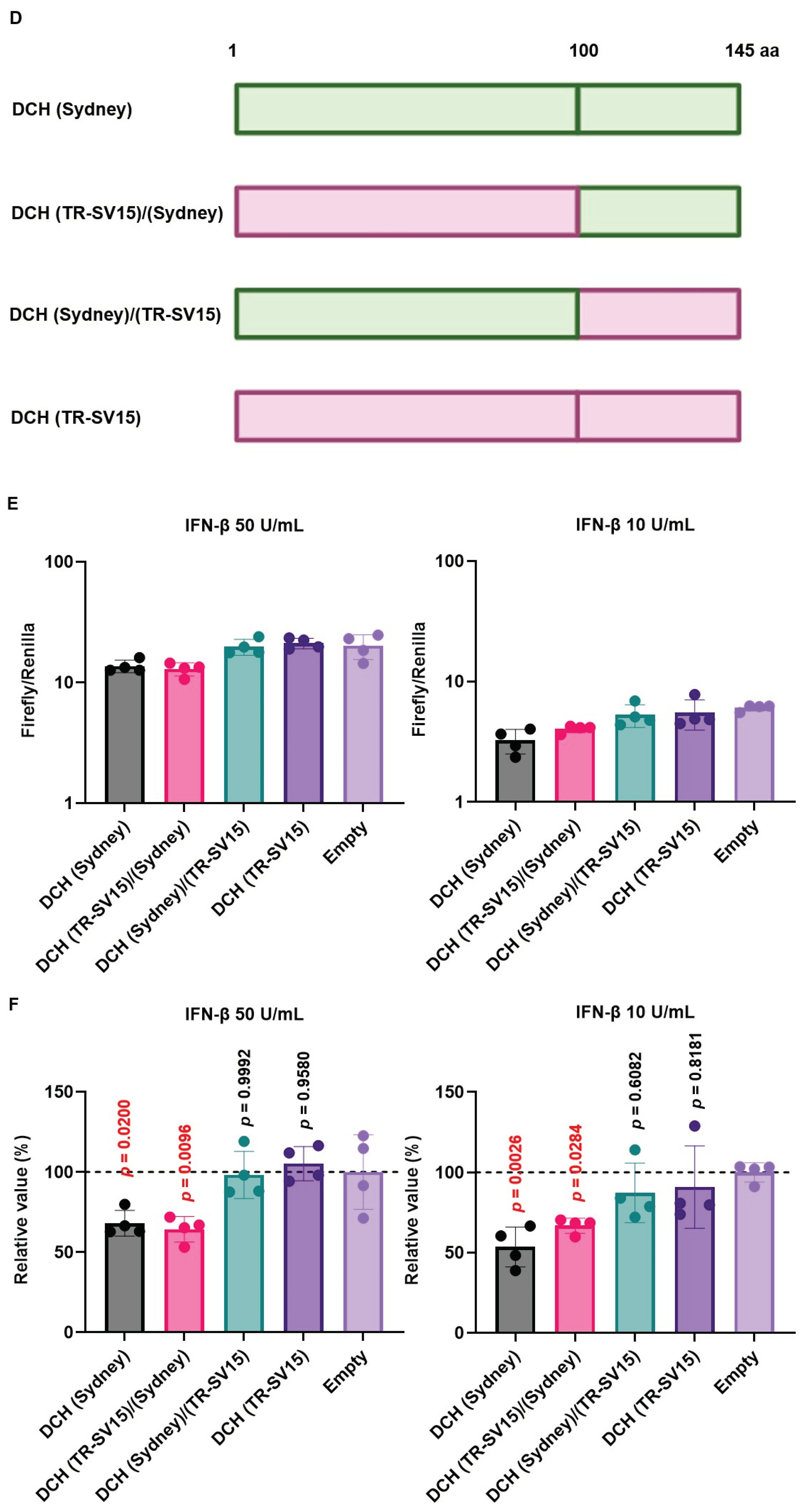
2.6. Variation in the C-terminus transactivation domain of DCH X protein determines the inhibitory effect on ISRE-mediated IFN-β signaling
3. Discussion
4. Materials and Methods
4.1. Plasmids
4.2. Construction of plasmids encoding Orthohepadnavirus X proteins with deletions
4.3. Construction of plasmids encoding Myc-tagged TRIF
4.4. Construction of plasmids encoding chimeric DCH X proteins
4.5. Cell culture
4.6. Generation of 293T-ISRE-luc2 cells
4.7. Western blotting
4.8. Luciferase reporter assay
4.8.1. IFN-β luciferase reporter assay
4.8.2. IgK-IFN and ISRE luciferase reporter assay
4.8.3. IFN-β bioassay in 293T-ISRE-luc2 cells
4.9. TRIF-degradation assay
4.10. Immunofluorescence assay
4.11. Alignment of Orthohepadnavirus protein X and phylogenetic analysis
4.12. Statistical analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization WHO Guidelines on Hepatitis B and C Testing; World Health Organization: Geneva, 2017; ISBN 978-92-4-154998-1.
- Magnius, L.; Mason, W.S.; Taylor, J.; Kann, M.; Glebe, D.; Dény, P.; Sureau, C.; Norder, H. ; ICTV Report Consortium ICTV Virus Taxonomy Profile: Hepadnaviridae. J. Gen. Virol. 2020, 101, 571–572. [Google Scholar] [CrossRef]
- Wieland, S.; Thimme, R.; Purcell, R.H.; Chisari, F.V. Genomic Analysis of the Host Response to Hepatitis B Virus Infection. Proc. Natl. Acad. Sci. 2004, 101, 6669–6674. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, L.G.; Isogawa, M.; Chisari, F.V. Host–Virus Interactions in Hepatitis B Virus Infection. Curr. Opin. Immunol. 2015, 36, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Barber, G.N. STING Signaling and Host Defense against Microbial Infection. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, X.; Wu, J.; Cong, Q.; Chen, X.; Li, T.; Du, F.; Ren, J.; Wu, Y.-T.; Grishin, N.V.; et al. Phosphorylation of Innate Immune Adaptor Proteins MAVS, STING, and TRIF Induces IRF3 Activation. Science 2015, 347, aaa2630. [Google Scholar] [CrossRef]
- Mani, S.K.K.; Andrisani, O. Interferon Signaling during Hepatitis B Virus (HBV) Infection and HBV-Associated Hepatocellular Carcinoma. Cytokine 2019, 124, 154518. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Song, H.; Xu, F.; Cheng, G. When Hepatitis B Virus Meets Interferons. Front. Microbiol. 2018, 9, 1611. [Google Scholar] [CrossRef]
- Wang, H.; Ryu, W.-S. Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion. PLoS Pathog. 2010, 6, e1000986. [Google Scholar] [CrossRef]
- Lang, T.; Lo, C.; Skinner, N.; Locarnini, S.; Visvanathan, K.; Mansell, A. The Hepatitis B e Antigen (HBeAg) Targets and Suppresses Activation of the Toll-like Receptor Signaling Pathway. J. Hepatol. 2011, 55, 762–769. [Google Scholar] [CrossRef]
- Fernández, M.; Quiroga, J.A.; Carreño, V. Hepatitis B Virus Downregulates the Human Interferon-Inducible MxA Promoter through Direct Interaction of Precore/Core Proteins. J. Gen. Virol. 2003, 84, 2073–2082. [Google Scholar] [CrossRef]
- Soussan, P.; Garreau, F.; Zylberberg, H.; Ferray, C.; Brechot, C.; Kremsdorf, D. In Vivo Expression of a New Hepatitis B Virus Protein Encoded by a Spliced RNA. J. Clin. Invest. 2000, 105, 55–60. [Google Scholar] [CrossRef]
- Wei, C.; Ni, C.; Song, T.; Liu, Y.; Yang, X.; Zheng, Z.; Jia, Y.; Yuan, Y.; Guan, K.; Xu, Y.; et al. The Hepatitis B Virus X Protein Disrupts Innate Immunity by Downregulating Mitochondrial Antiviral Signaling Protein. J. Immunol. 2010, 185, 1158–1168. [Google Scholar] [CrossRef]
- Megahed, F.A.K.; Zhou, X.; Sun, P. The Interactions Between HBV and the Innate Immunity of Hepatocytes. Viruses 2020, 12, 285. [Google Scholar] [CrossRef]
- Lucifora, J.; Arzberger, S.; Durantel, D.; Belloni, L.; Strubin, M.; Levrero, M.; Zoulim, F.; Hantz, O.; Protzer, U. Hepatitis B Virus X Protein Is Essential to Initiate and Maintain Virus Replication after Infection. J. Hepatol. 2011, 55, 996–1003. [Google Scholar] [CrossRef]
- Slagle, B.L.; Bouchard, M.J. Role of HBx in Hepatitis B Virus Persistence and Its Therapeutic Implications. Curr. Opin. Virol. 2018, 30, 32–38. [Google Scholar] [CrossRef]
- Sekiba, K.; Otsuka, M.; Funato, K.; Miyakawa, Y.; Tanaka, E.; Seimiya, T.; Yamagami, M.; Tsutsumi, T.; Okushin, K.; Miyakawa, K.; et al. HBx-Induced Degradation of Smc5/6 Complex Impairs Homologous Recombination-Mediated Repair of Damaged DNA. J. Hepatol. 2022, 76, 53–62. [Google Scholar] [CrossRef]
- Murakami, S.; Cheong, J.H.; Kaneko, S. Human Hepatitis Virus X Gene Encodes a Regulatory Domain That Represses Transactivation of X Protein. J. Biol. Chem. 1994, 269, 15118–15123. [Google Scholar] [CrossRef]
- Tang, H.; Oishi, N.; Kaneko, S.; Murakami, S. Molecular Functions and Biological Roles of Hepatitis B Virus x Protein. Cancer Sci. 2006, 97, 977–983. [Google Scholar] [CrossRef]
- Parashar Misra, K.; Mukherji, A.; Kumar, V. The Conserved Amino-Terminal Region (Amino Acids 1–20) of the Hepatitis B Virus X Protein Shows a Transrepression Function. Virus Res. 2004, 105, 157–165. [Google Scholar] [CrossRef]
- Kumar, V.; Jayasuryan, N.; Kumar, R. A Truncated Mutant (Residues 58-140) of the Hepatitis B Virus X Protein Retains Transactivation Function. Proc. Natl. Acad. Sci. 1996, 93, 5647–5652. [Google Scholar] [CrossRef]
- Lizzano, R.A.; Yang, B.; Clippinger, A.J.; Bouchard, M.J. The C-Terminal Region of the Hepatitis B Virus X Protein Is Essential for Its Stability and Function. Virus Res. 2011, 155, 231–239. [Google Scholar] [CrossRef]
- Jiang, J.; Tang, H. Mechanism of Inhibiting Type I Interferon Induction by Hepatitis B Virus X Protein. Protein Cell 2010, 1, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shen, F.; Wang, Y.; Li, Z.; Chen, J.; Yuan, Z. Residues Asn118 and Glu119 of Hepatitis B Virus X Protein Are Critical for HBx-Mediated Inhibition of RIG-I-MAVS Signaling. Virology 2020, 539, 92–103. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, L.; Xie, H.; Zheng, S. Innate Immune Evasion by Hepatitis B Virus-Mediated Downregulation of TRIF. Biochem. Biophys. Res. Commun. 2015, 463, 719–725. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Shi, M.; Barrs, V.; McLuckie, A.; Lindsay, S.; Jameson, B.; Hampson, B.; Holmes, E.; Beatty, J. A Novel Hepadnavirus Identified in an Immunocompromised Domestic Cat in Australia. Viruses 2018, 10, 269. [Google Scholar] [CrossRef]
- Lanave, G.; Capozza, P.; Diakoudi, G.; Catella, C.; Catucci, L.; Ghergo, P.; Stasi, F.; Barrs, V.; Beatty, J.; Decaro, N.; et al. Identification of Hepadnavirus in the Sera of Cats. Sci. Rep. 2019, 9, 10668. [Google Scholar] [CrossRef]
- Piewbang, C.; Wardhani, S.W.; Chaiyasak, S.; Yostawonkul, J.; Chai-in, P.; Boonrungsiman, S.; Kasantikul, T.; Techangamsuwan, S. Insights into the Genetic Diversity, Recombination, and Systemic Infections with Evidence of Intracellular Maturation of Hepadnavirus in Cats. PLOS ONE 2020, 15, e0241212. [Google Scholar] [CrossRef]
- Anpuanandam, K.; Selvarajah, G.T.; Choy, M.M.K.; Ng, S.W.; Kumar, K.; Ali, R.M.; Rajendran, S.K.; Ho, K.L.; Tan, W.S. Molecular Detection and Characterisation of Domestic Cat Hepadnavirus (DCH) from Blood and Liver Tissues of Cats in Malaysia. BMC Vet. Res. 2021, 17, 9. [Google Scholar] [CrossRef]
- Jeanes, E.C.; Wegg, M.L.; Mitchell, J.A.; Priestnall, S.L.; Fleming, L.; Dawson, C. Comparison of the Prevalence of Domestic Cat Hepadnavirus in a Population of Cats with Uveitis and in a Healthy Blood Donor Cat Population in the United Kingdom. Vet. Ophthalmol. 2022, 25, 165–172. [Google Scholar] [CrossRef]
- Takahashi, K.; Kaneko, Y.; Shibanai, A.; Yamamoto, S.; Katagiri, A.; Osuga, T.; Inoue, Y.; Kuroda, K.; Tanabe, M.; Okabayashi, T.; et al. Identification of Domestic Cat Hepadnavirus from a Cat Blood Sample in Japan. J. Vet. Med. Sci. 2022, 84, 648–652. [Google Scholar] [CrossRef]
- Stone, C.; Petch, R.; Gagne, R.B.; Nehring, M.; Tu, T.; Beatty, J.A.; VandeWoude, S. Prevalence and Genomic Sequence Analysis of Domestic Cat Hepadnavirus in the United States. Viruses 2022, 14, 2091. [Google Scholar] [CrossRef]
- Capozza, P.; Carrai, M.; Choi, Y.R.; Tu, T.; Nekouei, O.; Lanave, G.; Martella, V.; Beatty, J.A.; Barrs, V.R. Domestic Cat Hepadnavirus: Molecular Epidemiology and Phylogeny in Cats in Hong Kong. Viruses 2023, 15, 150. [Google Scholar] [CrossRef]
- Silva, B.B.I.; Chen, J.-Y.; Villanueva, B.H.A.; Lu, Z.-Y.; Hsing, H.-Z.; Montecillo, A.D.; Shofa, M.; Minh, H.; Chuang, J.-P.; Huang, H.-Y.; et al. Genetic Diversity of Domestic Cat Hepadnavirus in Southern Taiwan. Viruses 2023, 15, 2128. [Google Scholar] [CrossRef]
- Adıgüzel, E.; Erdem-Şahinkesen, E.; Koç, B.T.; Demirden, C.; Oğuzoğlu, T.Ç. The Detection and Full Genomic Characterization of Domestic Cat Orthohepadnaviruses from Türkiye. Vet. Med. Sci. 2023, 9, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- Piewbang, C.; Dankaona, W.; Poonsin, P.; Yostawonkul, J.; Lacharoje, S.; Sirivisoot, S.; Kasantikul, T.; Tummaruk, P.; Techangamsuwan, S. Domestic Cat Hepadnavirus Associated with Hepatopathy in Cats: A Retrospective Study. J. Vet. Intern. Med. 2022, 36, 1648–1659. [Google Scholar] [CrossRef]
- Pesavento; Jackson; Hampson; Munday; Barrs; Beatty A Novel Hepadnavirus Is Associated with Chronic Hepatitis and Hepatocellular Carcinoma in Cats. Viruses 2019, 11, 969. [CrossRef]
- Shofa, M.; Ohkawa, A.; Kaneko, Y.; Saito, A. Conserved Use of the Sodium/Bile Acid Cotransporter (NTCP) as an Entry Receptor by Hepatitis B Virus and Domestic Cat Hepadnavirus. Antiviral Res. 2023, 217, 105695. [Google Scholar] [CrossRef]
- Luo, M.; Qu, X.; Pan, R.; Zhu, D.; Zhang, Y.; Wu, J.; Pan, Z. The Virus-Induced Signaling Adaptor Molecule Enhances DNA-Raised Immune Protection against H5N1 Influenza Virus Infection in Mice. Vaccine 2011, 29, 2561–2567. [Google Scholar] [CrossRef]
- Lui, W.-Y.; Bharti, A.; Wong, N.-H.M.; Jangra, S.; Botelho, M.G.; Yuen, K.-S.; Jin, D.-Y. Suppression of cGAS- and RIG-I-Mediated Innate Immune Signaling by Epstein-Barr Virus Deubiquitinase BPLF1. PLOS Pathog. 2023, 19, e1011186. [Google Scholar] [CrossRef]
- Han, K.-J.; Yang, Y.; Xu, L.-G.; Shu, H.-B. Analysis of a TIR-Less Splice Variant of TRIF Reveals an Unexpected Mechanism of TLR3-Mediated Signaling. J. Biol. Chem. 2010, 285, 12543–12550. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Xu, F.; Xiao, Q.; Tan, G. Hepatitis B Virus X Protein and Its Host Partners. Cell. Mol. Immunol. 2021, 18, 1345–1346. [Google Scholar] [CrossRef] [PubMed]
- Henkler, F.; Hoare, J.; Waseem, N.; Goldin, R.D.; McGarvey, M.J.; Koshy, R.; King, I.A. Intracellular Localization of the Hepatitis B Virus HBx Protein. J. Gen. Virol. 2001, 82, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Balsano, C.; Avantaggiati, M.L.; Natoli, G.; De Marzio, E.; Will, H.; Perricaudet, M.; Levrero, M. Full-Length and Truncated Versions of the Hepatitis B Virus (HBV) X Protein (pX) Transactivate the cMYC Protooncogene at the Transcriptional Level. Biochem. Biophys. Res. Commun. 1991, 176, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.K.; Kwun, H.J.; Lee, J.-O.; Arora, P.; Jang, K.L. Hepatitis B Virus X Protein Differentially Affects the Ubiquitin-Mediated Proteasomal Degradation of β-Catenin Depending on the Status of Cellular P53. J. Gen. Virol. 2007, 88, 2144–2154. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.-R.; Oh, M.; Koh, S.S.; Malilas, W.; Srisuttee, R.; Jhun, B.H.; Pellegrini, S.; Fuchs, S.Y.; Chung, Y.-H. Hepatitis B Virus X Protein Inhibits Extracellular IFN-α-Mediated Signal Transduction by Downregulation of Type I IFN Receptor. Int. J. Mol. Med. 2012, 29, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Kuipery, A.; Gehring, A.J.; Isogawa, M. Mechanisms of HBV Immune Evasion. Antiviral Res. 2020, 179, 104816. [Google Scholar] [CrossRef]
- Nijhara, R.; Jana, S.S.; Goswami, S.K.; Kumar, V.; Sarkar, D.P. An Internal Segment (Residues 58–119) of the Hepatitis B Virus X Protein Is Sufficient to Activate MAP Kinase Pathways in Mouse Liver. FEBS Lett. 2001, 504, 59–64. [Google Scholar] [CrossRef]
- Ye, J.; Chen, J. Interferon and Hepatitis B: Current and Future Perspectives. Front. Immunol. 2021, 12, 733364. [Google Scholar] [CrossRef]
- Lim, K.-H.; Park, E.-S.; Kim, D.H.; Cho, K.C.; Kim, K.P.; Park, Y.K.; Ahn, S.H.; Park, S.H.; Kim, K.-H.; Kim, C.W.; et al. Suppression of Interferon-Mediated Anti-HBV Response by Single CpG Methylation in the 5′-UTR of TRIM22. Gut 2018, 67, 166–178. [Google Scholar] [CrossRef]
- Gentili, M.; Kowal, J.; Tkach, M.; Satoh, T.; Lahaye, X.; Conrad, C.; Boyron, M.; Lombard, B.; Durand, S.; Kroemer, G.; et al. Transmission of Innate Immune Signaling by Packaging of cGAMP in Viral Particles. Science 2015, 349, 1232–1236. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; McWhirter, S.M.; Faia, K.L.; Rowe, D.C.; Latz, E.; Golenbock, D.T.; Coyle, A.J.; Liao, S.-M.; Maniatis, T. IKKε and TBK1 Are Essential Components of the IRF3 Signaling Pathway. Nat. Immunol. 2003, 4, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Loo, Y.-M.; Horner, S.M.; Gale, M.; Malik, H.S. Convergent Evolution of Escape from Hepaciviral Antagonism in Primates. PLoS Biol. 2012, 10, e1001282. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, J.L.; Denny, E.M.; Baltimore, D. CARD11 Mediates Factor-Specific Activation of NF-κB by the T Cell Receptor Complex. EMBO J. 2002, 21, 5184–5194. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Smith, K.; Hsieh, P.N.; Mburu, Y.K.; Chattopadhyay, S.; Sen, G.C.; Sarkar, S.N. High-Throughput Screening for TLR3–IFN Regulatory Factor 3 Signaling Pathway Modulators Identifies Several Antipsychotic Drugs as TLR Inhibitors. J. Immunol. 2010, 184, 5768–5776. [Google Scholar] [CrossRef]
- Hitoshi, N.; Ken-ichi, Y.; Jun-ichi, M. Efficient Selection for High-Expression Transfectants with a Novel Eukaryotic Vector. Gene 1991, 108, 193–199. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




