Submitted:
22 February 2024
Posted:
23 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
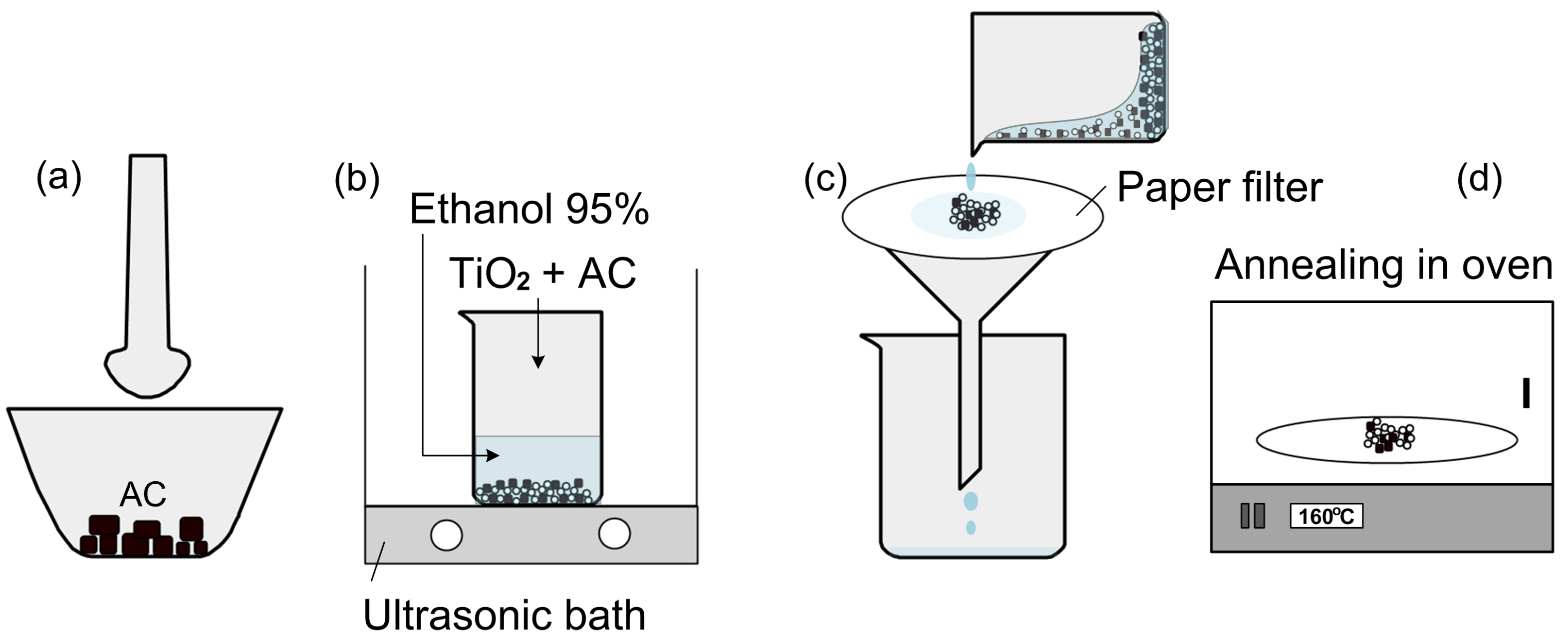
2. Materials and Methods
2.1. Materials
2.2. Photocatalytic Activity in Degradation MB of TiO2/AC
2.3. Characterization Methods
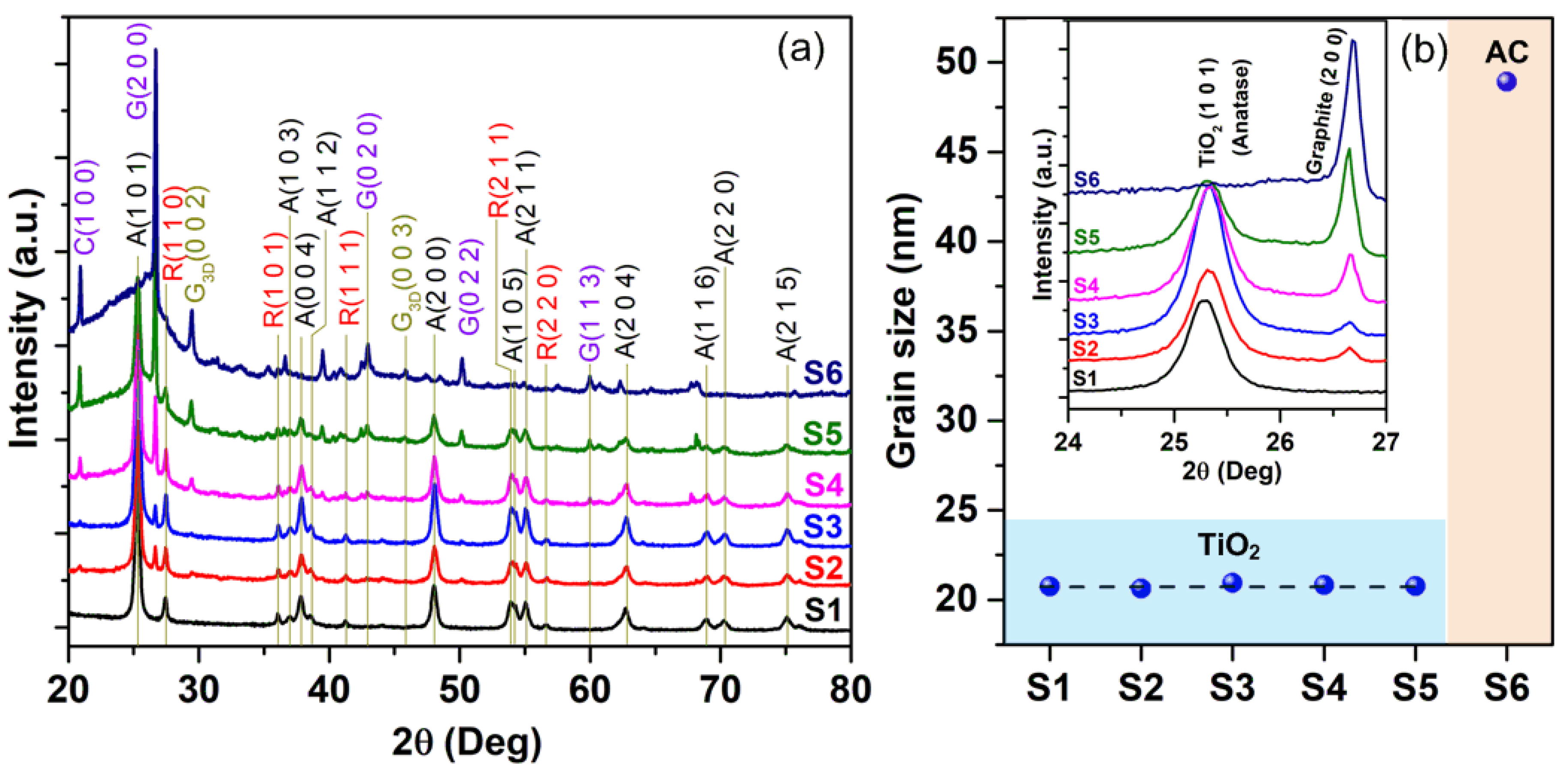
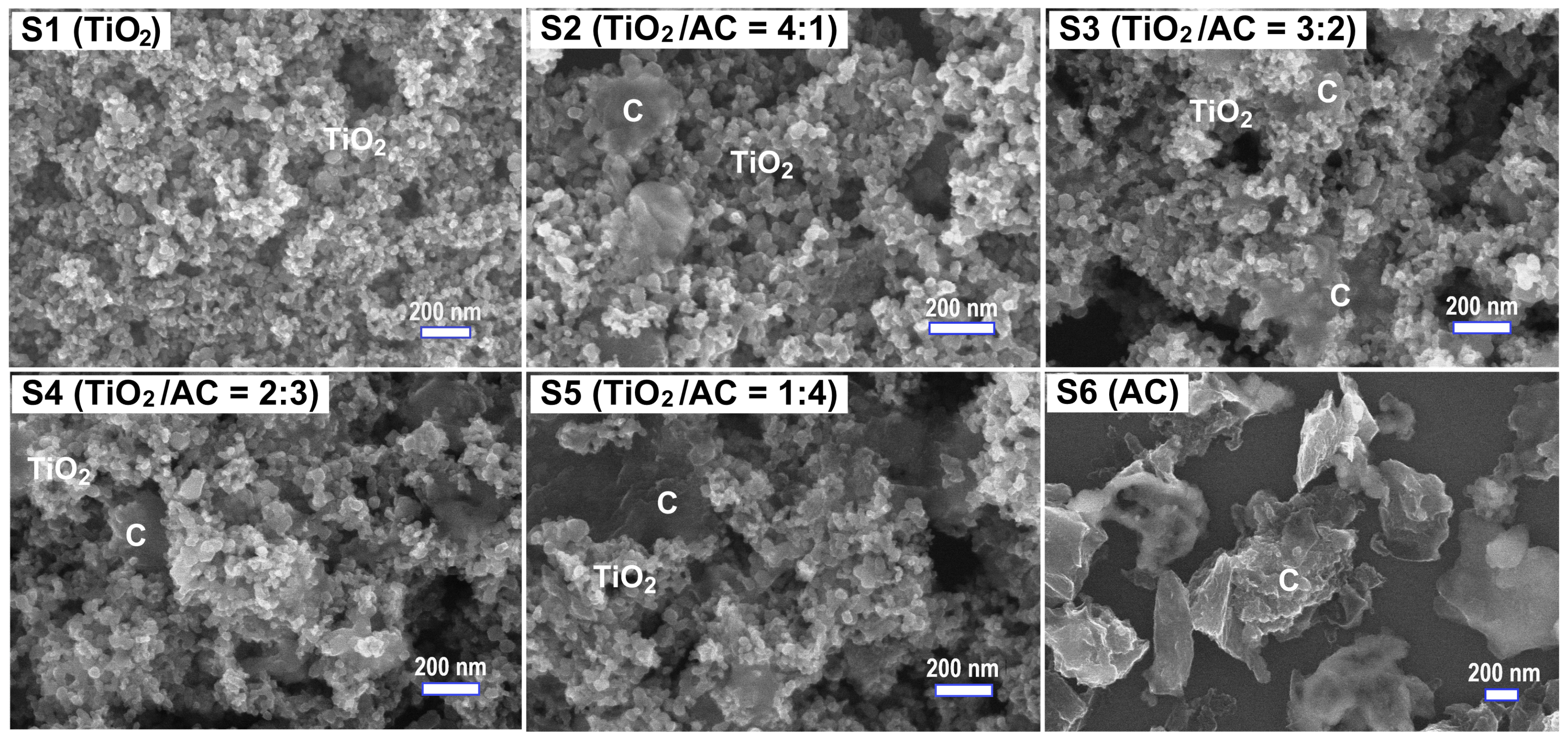
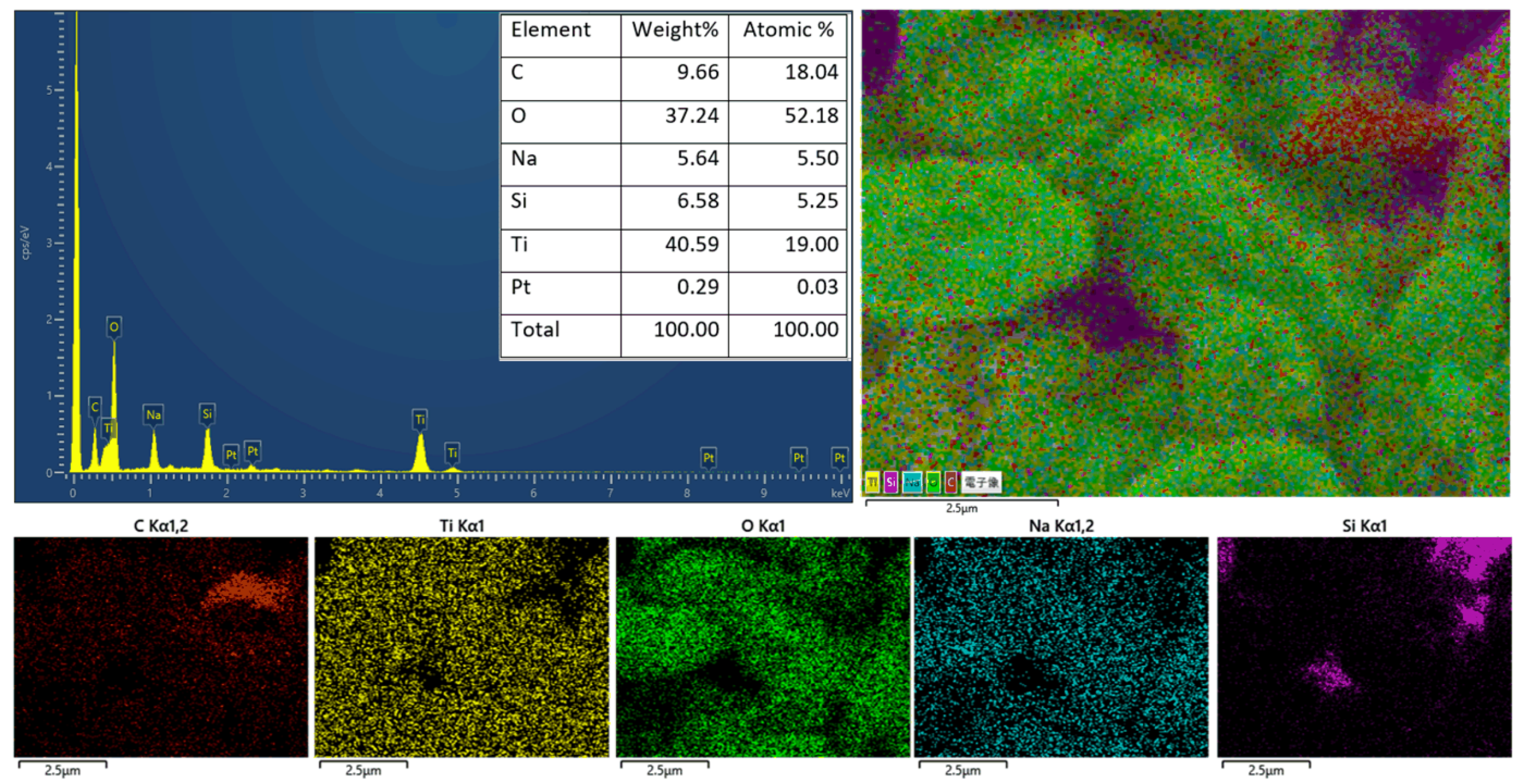
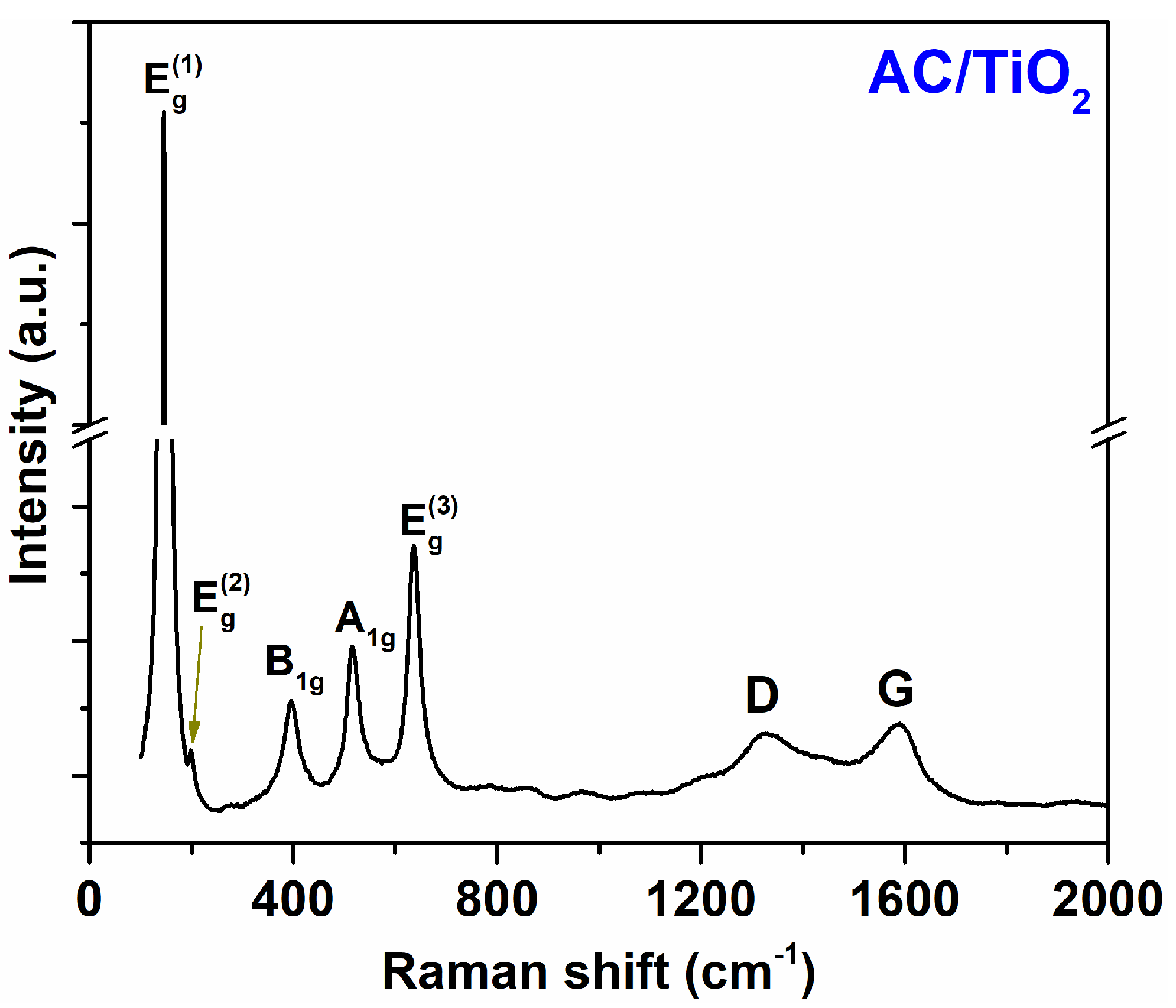
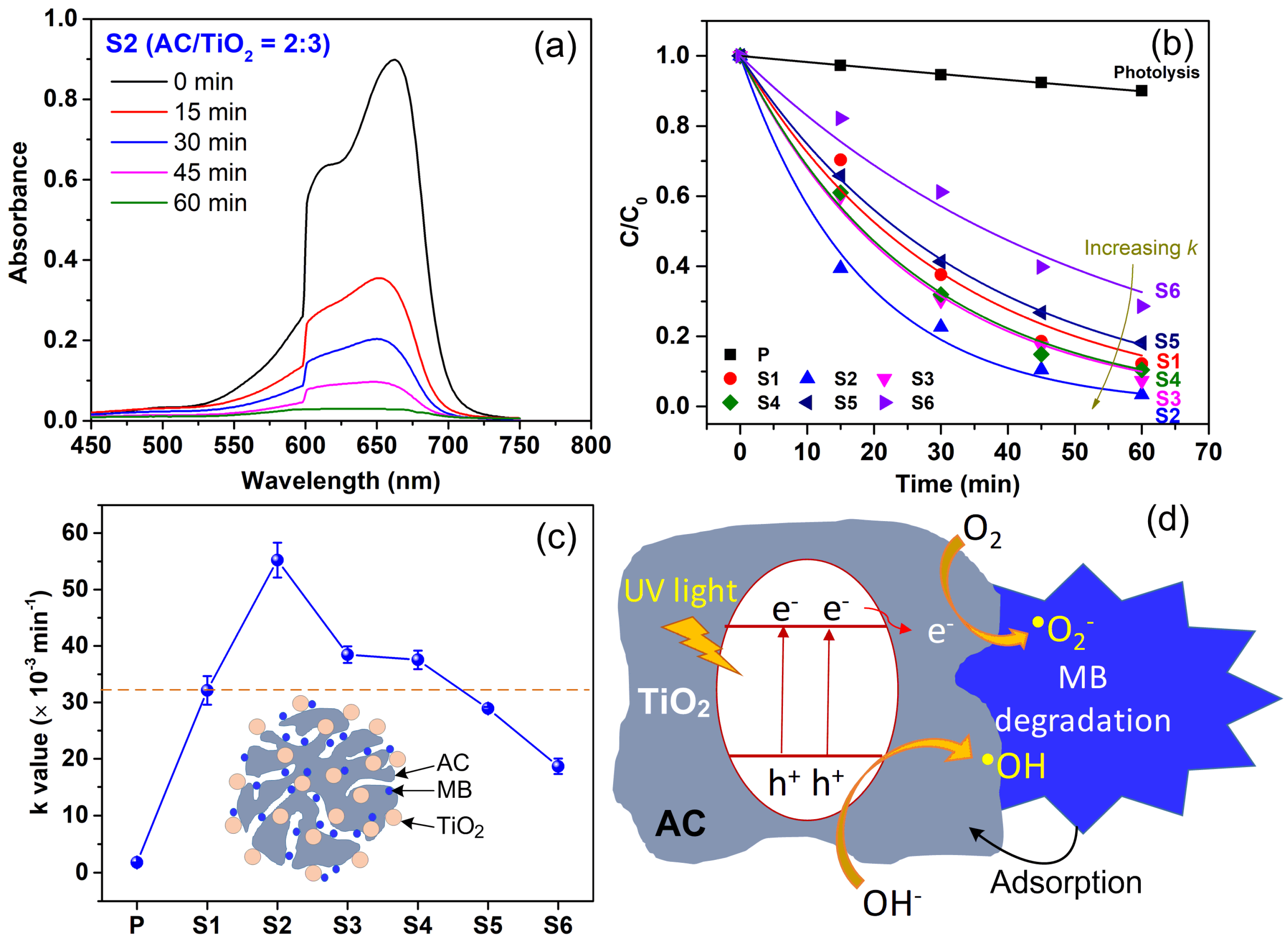
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Science of the Total Environment 2020, 717, 137222. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Applied Catalysis B: Environmental 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Thakur, M.; Pathania, D. Chapter 12 - Environmental fate of organic pollutants and effect on human health. Abatement of Environmental Pollutants: Trend and Strategies. Elsevier 2020, 245-262. [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Mazivila, S.J.; Ricardo, I.A.; Leitão, J.M.M.; Esteves da Silva, J.C.G. A review on advanced oxidation processes: From classical to new perspectives coupled to two- and multi-way calibration strategies to monitor degradation of contaminants in environmental samples. Trends in Environmental Analytical Chemistry 2019, 24, e00072. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and rutile in evonik aeroxide P25: Heterojunctioned or individual nanoparticles? Catalysis Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Yen, Y.C.; Chen, J.A.; Ou, S.; Chen, Y.S.; Lin, K.J. Plasmon-Enhanced Photocurrent Using Gold Nanoparticles on a Three-Dimensional TiO2 Nanowire-Web Electrode. Scientific Reports 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Xu, Y.-Y.; Fang, H.-B.; Wang, Y.; Tao, X. Au nanoparticle homogeneously decorated C@TiO2 for enhanced visible-light-driven photocatalytic activity. RSC Adv. 2015, 5, 103790–103796. [Google Scholar] [CrossRef]
- Ozawa, K.; Emori, M.; Yamamoto, S.; Yukawa, R.; Yamamoto, S.; Hobara, R.; Fujikawa, K.; Sakama, H.; Matsuda, I. Electron − Hole Recombination Time at TiO2 Single-Crystal Surfaces: Influence of Surface Band Bending. J. Phys. Chem. Lett. 2014, 5, 1953–1957. [Google Scholar] [CrossRef] [PubMed]
- Shourong, Z.; Qingguo, H.; Jun, Z.; Bingkun, W. A study on dye photoremoval in TiO2 suspension solution. Journal of Photochemistry and Photobiology A: Chemistry 1997, 108, 235–238. [Google Scholar] [CrossRef]
- Leyva, E.; Montalvo, C.; Moctezuma, E.; Leyva, S. Photocatalytic degradation of pyridine in water solution using ZnO as an alternative catalyst to TiO2. Journal of Ceramic Processing Research 2008, 9, 455–462. [Google Scholar]
- Hu, L.; Flanders, P.M.; Miller, P.L.; Strathmann, T.J. Oxidation of Sulfamethoxazole and Related Antimicrobial Agents by TiO2 Photocatalysis. Water Research 2007, 41, 2612–2626. [Google Scholar] [CrossRef]
- Hapeshi, E.; Achilleos, A.; Vasquez, M.I.; Michael, C.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Kassinos, D. Drugs degrading photocatalytically: Kinetics and mechanisms of ofloxacin and atenolol removal on titania suspensions. Water Research 2010, 44, 1737–1746. [Google Scholar] [CrossRef]
- Westerhoff, P.; Song, G.; Hristovski, K.; Kiser, M.A. Occurrence and removal of titanium at full scale wastewater treatment plants: implications for TiO2 nanomaterials. J. Environ. Monit. 2011, 13, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-Y.; Hsu, H.-L.; Leu, J. TiO2 Nanowires on Anodic TiO2 Nanotube Arrays (TNWs/TNAs): Formation Mechanism and Photocatalytic Performance. Journal of The Electrochemical Society 2012, 159, H722–H727. [Google Scholar] [CrossRef]
- Do, T.C.M.V.; Nguyen, D.Q.; Nguyen, K.T.; Le, P.H. TiO2 and Au-TiO2 Nanomaterials for Rapid Photocatalytic Degradation of Antibiotic Residues in Aquaculture Wastewater. Materials 2019, 12, 2434. [Google Scholar] [CrossRef] [PubMed]
- Uyen, N.N.; Tuyen, L.T.C.; Hieu, L.T.; Nguyen, T.T.T.; Thao, H.P.; Do, T.C.MV.; Nguyen, K.T.; Hang, N.T.N.; Jian, S.-R.; Tu, L.A.; Le, P.H.; Luo, C.W. TiO2 Nanowires on TiO2 Nanotubes Arrays (TNWs/TNAs) Decorated with Au Nanoparticles and Au Nanorods for Efficient Photoelectrochemical Water Splitting and Photocatalytic Degradation of Methylene Blue. Coatings, 2022, 12, 1957. [CrossRef]
- Jampawal, J.; Supothina, S.; Chuaybamroong, P. Solar photocatalytic degradation of carbaryl in water using TiO2-coated filters with different binders and effect of the operating conditions. Environmental Science and Pollution Research 2022, 29, 88027–88040. [Google Scholar] [CrossRef]
- Gloria, D.C.S.; Brito, C.H.V.; Mendonça, T.A.P.; Brazil, T.R.; Domingues, R.A.; Vieira, N.C.S.; Santos, E.B.; Gonçalves, M. Preparation of TiO2/activated carbon nanomaterials with enhanced photocatalytic activity in paracetamol degradation. Materials Chemistry and Physics 2023, 305, 127947. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Lourenço, V.S.; Carvalho, W.A. Removal of phenol in seawater by heterogeneous photocatalysis using activated carbon materials modified with TiO2. Catalysis Today 2022, 389-389, 247–258. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Li, J.; Yin, J. Photocatalytic degradation ofmethyl orange by TiO2-coated activated carbon and kinetic study. Water Research 2006, 40, 1119–1126. [Google Scholar] [CrossRef]
- Ao, W.; Qu, J.; Yu, H.; Liu, Y.; Liu, C.; Fu, J.; Dai, J.; Bi, X.; Yuan, Y.; Jin, Y. TiO2/activated carbon synthesized by microwave-assisted heating for tetracycline photodegradation. Environmental Research 2022, 214, 113837. [Google Scholar] [CrossRef]
- Liu, W.; Wang, B.; Zhang, M. Effect of Process Parameters on the Microstructure and Performance of TiO2-Loaded Activated Carbon. ACS Omega 2021, 6, 35076–35092. [Google Scholar] [CrossRef]
- Arutanti, O.; Sari, A.L.; Kartikowati, C.W.; Sari, A.A.; Arif, A.F. Design and Application of Homogeneous-structured TiO2/Activated Carbon Nanocomposite for Adsorption–Photocatalytic Degradation of MO. Water Air Soil Pollut 2022, 233, 118. [Google Scholar] [CrossRef]
- Xu, W.; Jin, Y.; Ren, Y.; Li, J.; Wei, Z.; Ban, C.; Cai, H.; Chen, M. Synergy mechanism for TiO2 /activated carbon composite material: Photocatalytic degradation of methylene blue solution. Can J Chem Eng 2021, 1–15. [Google Scholar] [CrossRef]
- Asiltürk, M.; Sener, S. TiO2-activated carbon photocatalysts: Preparation, characterization and photocatalytic activities. Chemical Engineering Journal 2012, 180, 354–363. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A. Activated Carbon: Progress and Applications. The Royal Society of Chemistry 2023, 1–450. [Google Scholar] [CrossRef]
- Mountassir, E.M.E.; Daou, C.; Rafqah, S.; Najjar, F.; Anane, H.; Piram, A.; Hamade, A.; Briche, S.; Wong-wah-chung, P. TiO2 and activated carbon of Argania Spinosa tree nutshells composites for the adsorption photocatalysis removal of pharmaceuticals from aqueous solution. Journal of Photochemistry & Photobiology A: Chemistry 2020, 338, 112183. [Google Scholar] [CrossRef]
- Tuyen, L.T.C.; Jian, S.R.; Tien, N.T.; Le, P.H. Nanomechanical and Material Properties of Fluorine-Doped Tin Oxide Thin Films Prepared By Ultrasonic Spray Pyrolysis: Effects of F-doping. Materials 2019, 12, 1665. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman Spectroscopy of Carbon Materials and Their Composites: Graphene, Nanotubes and Fibres. Progress in Materials Science 2023, 135, 101089. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Sowmiya, K.; Pragasan, L.A.; Rajagopal, R.; Sathya, R.; Ragupathy, S.; Krishnakumar, M.; Reddy, V.R.M. Enhanced photocatalytic degradation of organic pollutants by Ag–TiO2 loaded cassava stem activated carbon under sunlight irradiation. Chemosphere 2022, 302, 134844. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; He, X.; Du, J.; Gong, W. Synergistic effect of photocatalysis and adsorption of nano-TiO2 self-assembled onto sulfanyl/activated carbon composite. Environ Sci Pollut Res 2016, 23, 21733–21740. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.L.; Kuznetsov, A.; Araujo, J.R.; Neves, R.S.; Archanjo, B.S.; Canela, M.C.; Marques, M. Optimization of Benzodiazepine Drugs Removal from Water by Heterogeneous Photocatalysis Using TiO2/ Activated Carbon Composite. Water Air Soil Pollut 2019, 230, 141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




