Submitted:
27 February 2024
Posted:
28 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Brief History of Anaerobic Digestion Technology
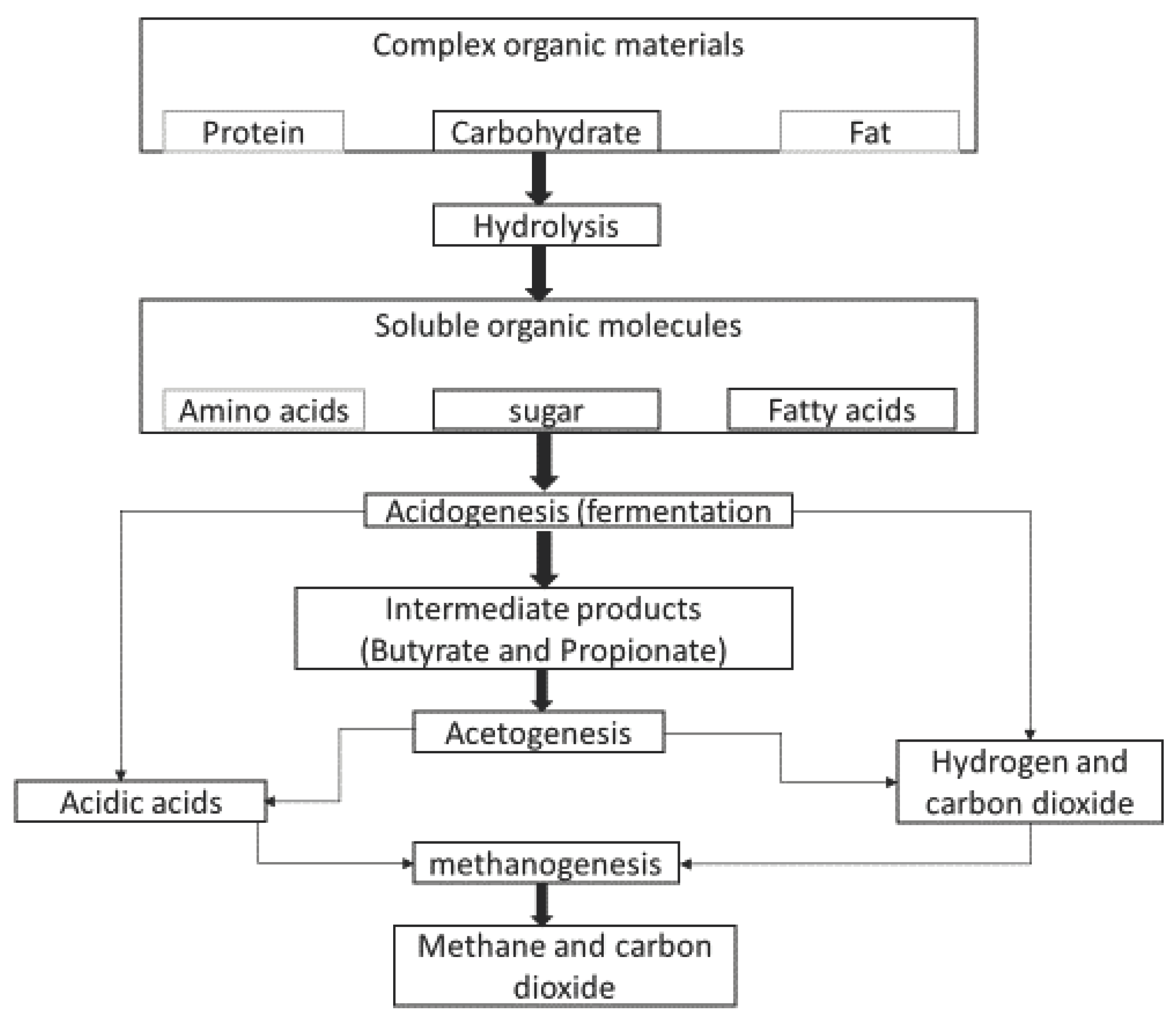
1.2. Anaerobic Digestion’s Biochemical Reactions
1.3. Inhibitory Factors for Anaerobic Digestion
1.4. Mechanical Pre-Treatment of Lignocellulose Biomass for Methane Yield
2. Materials and Methods
2.1. Characterisation Methods for Maximizing Methane Yield
| Lignocellulose Biomass |
Cellulose (%) | Hemicellulose (%) |
Lignin (%) |
|---|---|---|---|
| Corn stover | 37-42 | 20-28 | 18-22 |
| Sugarcane bagasse | 26-50 | 24-34 | 10-26 |
| Wheat straw | 31-44 | 22-24 | 16-24 |
| Hardwood stems | 40-45 | 18-40 | 18-28 |
| Softwood Stems | 34-50 | 21-35 | 28-35 |
| Rice straw |
32-41 | 15-24 | 10-18 |
| Barley straw | 33-40 | 20-35 | 8-17 |
| Switch grass | 33-46 | 22-32 | 12-23 |
| Energy crops | 43-45 | 24-31 | 19-12 |
| Grasses (average)a | 25-40 | 25-50 | 10-30 |
| Manure solid fibres Municipal organic waste |
8-27 21-64 |
12-22 5-22 |
2-13 3-28 |
2.1.1. Particle Size
2.1.2. Clearance
2.1.3. Pre-Treatment Temperature
2.1.4. Pre-Treatment Time
2.1.5. Drainability
2.1.6. Viscosity
2.1.7. Pre-treatment pH
2.2. Anaerobic Digestion’s Process Stability Parameters for Maximizing Methane Yield
2.2.1. Solid Organic Substrate
2.2.2. Anaerobic Digestion Process
2.2.3. Co-Digestion
2.2.4. Inoculum Characteristics
2.2.5. Feedstock-to Inoculum Ratio
2.2.6. pH
2.2.7. Temperature
2.2.8. Mixing
2.2.9. Hydraulic Retention Time
2.2.10. Gas Measurement
2.2.11. Reactor Volume
2.2.12. Headspace Gas and Volume
3. Conclusion
Funding
Conflicts of Interest
References
- Abbassi-Guendouz, A., D. Brockmann, E. Trably, C. Dumas, J.-P. Delgenès, J.-P. Steyer and R. Escudié (2012). "Total solids content drives high solid anaerobic digestion via mass transfer limitation." Bioresource technology 111: 55-61. [CrossRef]
- Abdelgadir, A., X. Chen, J. Liu, X. Xie, J. Zhang, K. Zhang, H. Wang and N. Liu (2014). "Characteristics, process parameters, and inner components of anaerobic bioreactors." BioMed research international 2014. [CrossRef]
- Aboderheeba, A. K., F. A. Alfarjani and A. G. Olabi (2015). "Beating treatment to enhance digestibility of fresh grass." International Journal of Global Warming 7(1): 48-61. [CrossRef]
- Ahn, J. and C. Forster (2002). "The effect of temperature variations on the performance of mesophilic and thermophilic anaerobic filters treating a simulated papermill wastewater." Process Biochemistry 37(6): 589-594. [CrossRef]
- Ajeej, A., J. V. Thanikal, C. Narayanan and r. S. Kumar (2015). "An oveview of bio augmentation of methane by anaerobic co-digestionof municipal sludge along with microalgae and waste paper " 2015 50: 270-276. [CrossRef]
- Alfarjani, F. (2012). Design and Optimization of Process Parameters in Bio-Gas Production Systems, Dublin City University.
- Alp, Ö. (2009). Further treatment of digested blackwater for extraction of valuable components, Technische Universität Hamburg.
- Anbalagan A, Schwede S, Lindberg CF and E. Nehrenheim (2016). "Influence of hydraulic retention time on indigenous microalgae and activated sludge process." Water research: 277-284. [CrossRef]
- Astals, S., V. Nolla-Ardèvol and J. Mata-Alvarez (2012). "Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: Biogas and digestate." Bioresource Technology 110: 63-70. [CrossRef]
- Biermann, C. J. (1996). Handbook of pulping and papermaking, Academic press.
- Biswas, R., H. Uellendahl and B. K. Ahring (2015). "Wet explosion: a universal and efficient pretreatment process for lignocellulosic biorefineries." BioEnergy Research 8(3): 1101-1116. [CrossRef]
- Bonfiglio, F., V. Curbelo, E. Santana and J. Doldánd (2013). "Influence of water conductivity on the drainability of Eucalyptus bleached Kraft pulp.".
- Botheju, D., B. Lie and R. Bakke (2009). "Oxygen effects in anaerobic digestion.". [CrossRef]
- Boulanger, A., E. Pinet, M. Bouix, T. Bouchez and A. A. Mansour (2012). "Effect of inoculum to substrate ratio (I/S) on municipal solid waste anaerobic degradation kinetics and potential." Waste management 32(12): 2258-2265. [CrossRef]
- Braguglia, C., G. mininni, M. Tomei and E. Rolle (2006). "Effect of feed/inoculum ratio on anaerobic digestion of sonicated sludge " Water Science and Technology: 77-84. [CrossRef]
- Brown, D., J. Shi and Y. Li (2012). "Comparison of solid-state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production." Bioresource technology 124: 379-386. [CrossRef]
- Cai, J., Y. He, X. Yu, S. W. Banks, Y. Yang, X. Zhang, Y. Yu, R. Liu and A. V. Bridgwater (2017). "Review of physicochemical properties and analytical characterization of lignocellulosic biomass." Renewable and Sustainable Energy Reviews 76: 309-322. [CrossRef]
- Chae, K., A. Jang, S. Yim and I. S. Kim (2008). "The effects of digestion temperature and temperature shock on the biogas yields from the mesophilic anaerobic digestion of swine manure." Bioresource technology 99(1): 1-6. [CrossRef]
- Chen, Y., J. J. Cheng and K. S. Creamer (2008). "Inhibition of anaerobic digestion process: a review." Bioresource technology 99(10): 4044-4064. [CrossRef]
- Chynoweth, D., C. Turick, J. Owens, D. E. Jerger and M. Peck (1993). "Biochemical methane potential of biomass and waste feedstocks." Biomass and bioenergy 5(1): 95-111. [CrossRef]
- Chynoweth, D. P. (1987). "Anaerobic digestion of biomass.".
- Cordoba, V., M. Fernandez and E. Santalla (2016). "The effect of different inoculums on anaerobic digestion of swine wastewater." Journal of environmental chemical engineering 4(1): 115-122. [CrossRef]
- Cristina Gonzalez-Fernandez and P. A. Garcia-Encina (2008). "Impact of substrate to inoculum ratio in anaerobic digestion of swine slurry.". [CrossRef]
- Dai, X., Y. Hua, L. Dai and C. Cai (2019). "Particle size reduction of rice straw enhances methane production under anaerobic digestion." Bioresource technology 293: 122043. [CrossRef]
- Darwin, J. J. Cheng, J. Gontupil and Z. Liu (2016). "Influence of total solid concentration for methane production of cocoa husk co-digested with digested swine manure." International Journal of Environment and Waste Management 17(1): 71-90. [CrossRef]
- Dasari, R. K. and R. E. Berson (2007). The effect of particle size on hydrolysis reaction rates and rheological properties in cellulosic slurries. Applied biochemistry and biotecnology, Springer: 289-299.
- Dechrugsa, S., D. Kantachote and S. Chaiprapat (2013). "Effect of inoculum to substrate ratio, substrate mix ratio and inoculum source on batch co-digestion of grass and pig manure " Bioresource Technology 146: 101-108. [CrossRef]
- Digestion, T. O. I. P. o. A. (2020). "Funding ".
- Du, L., J. Liang, P. Yang, W. Gao and K. Zhang (2014). "Influence of total solid content on anaerobic digestion of swine manure and kinetic analysis." Transactions of the Chinese Society of Agricultural Engineering 30(24): 246-251.
- Esposito, G., L. Frunzo, F. Liotta, A. Panico and F. Pirozzi (2012). "Bio-methane potential tests to measure the biogas production from the digestion and co-digestion of complex organic substrates." The Open Environmental Engineering Journal 5(1). [CrossRef]
- Expert, W.-C. E. R. E. "ON-Farm Anaerobic Digestion Loan Fund.".
- Fagbohungbe, M. o., B. M. J. Herbert, H. Li, L. Ricketts and K. T. Semple (2014). "The effect of substrate to inoculum ratios on the anaerobic digestion of human faecal material " Energy Technology & Innovation 121-129. [CrossRef]
- Fatma, S., A. Hameed, M. Noman, T. Ahmed, M. Shahid, M. Tariq, I. Sohail and R. Tabassum (2018). "Lignocellulosic biomass: A sustainable bioenergy source for the future." Protein and peptide letters 25(2): 148-163. [CrossRef]
- Fernández-Rodríguez, J., M. Pérez and L. Romero (2015). "Temperature-phased anaerobic digestion of Industrial Organic Fraction of Municipal Solid Waste: A batch study." Chemical Engineering Journal 270: 597-604. [CrossRef]
- Fernández, J., M. Pérez and L. I. Romero (2008). "Effect of substrate concentration on dry mesophilic anaerobic digestion of organic fraction of municipal solid waste (OFMSW)." Bioresource technology 99(14): 6075-6080. [CrossRef]
- Forster-Carneiro, T., M. Pérez and L. Romero (2008). "Influence of total solid and inoculum contents on performance of anaerobic reactors treating food waste." Bioresource technology 99(15): 6994-7002. [CrossRef]
- Forster-Carneiro, T., M. Pérez, L. Romero and D. Sales (2007). "Dry-thermophilic anaerobic digestion of organic fraction of the municipal solid waste: focusing on the inoculum sources." Bioresource technology 98(17): 3195-3203. [CrossRef]
- Gallegos, D., H. Wedwitschka, L. Moeller, A. Zehnsdorf and W. Stinner (2017). "Effect of particle size reduction and ensiling fermentation on biogas formation and silage quality of wheat straw." Bioresource technology 245: 216-224. [CrossRef]
- Ganidi, N., S. Tyrrel and E. Cartmell (2009). "Anaerobic digestion foaming causes–a review." Bioresource technology 100(23): 5546-5554. [CrossRef]
- Gao, W., K. Leung, W. Qin and B. Liao (2011). "Effects of temperature and temperature shock on the performance and microbial community structure of a submerged anaerobic membrane bioreactor." Bioresource technology 102(19): 8733-8740. [CrossRef]
- Gunaseelan, V. N. (1995). "Effect of inoculum/substrate ratio and pretreatments on methane yield from Parthenium." Biomass and Bioenergy 8(1): 39-44. [CrossRef]
- Güngör-Demirci, G. and G. N. Demirer (2004). "Effect of initial COD concentration, nutrient addition, temperature and microbial acclimation on anaerobic treatability of broiler and cattle manure." Bioresource technology 93(2): 109-117. [CrossRef]
- Guo, S., H. Zhan, C. Zhang, S. Fu, A. Heijnesson-Hultén, J. Basta and T. Greschik (2009). "PULP AND FIBER CHARACTERIZATION OF WHEAT STRAW AND EUCALUPTUS PULPS-A." BioResources 4(3).
- Hallac, B. B. and A. J. Ragauskas (2011). "Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol." Biofuels, Bioproducts and Biorefining 5(2): 215-225. [CrossRef]
- Hammar, L., M. Backstrom and M. Htun (2000). "Effect of the counterion on the beatability of unbleached kraft pulps." NORDIC PULP & PAPER RESEARCH JOURNAL 15(3): 189-193. [CrossRef]
- Hansen, T. L., J. E. Schmidt, I. Angelidaki, E. Marca, J. la Cour Jansen, H. Mosbæk and T. H. Christensen (2004). "Method for determination of methane potentials of solid organic waste." Waste management 24(4): 393-400. [CrossRef]
- Hashimoto, A. G. (1989). "Effect of inoculum/substrate ratio on methane yield and production rate from straw." Biological wastes 28(4): 247-255. [CrossRef]
- Heo, N. H., S. C. park and p. H. Kang (2006). "Effect of mixture ratio and hydraulic retention time on anaerobic co-digestion of foodwaste and waste activated sludge.".
- Herrmann, C., M. Heiermann, C. Idler and A. Prochnow (2012). "Particle size reduction during harvesting of crop feedstock for biogas production I: effects on ensiling process and methane yields." BioEnergy Research 5(4): 926-936. [CrossRef]
- Hidalgo, D. and J. M. Martín-Marroquín (2014). "Effects of inoculum source and co-digestion strategies on anaerobic digestion of residues generated in the treatment of waste vegetable oils." Journal of environmental management 142: 17-22. [CrossRef]
- Himanshu, H., M. A. Voelklein, J. D. Murphy, J. Grant and P. O'Kiely (2017). "Factors controlling headspace pressure in a manual manometric BMP method can be used to produce a methane output comparable to AMPTS." Bioresource technology 238: 633-642. [CrossRef]
- Hjorth, M., K. Gränitz, A. P. Adamsen and H. B. Møller (2011). "Extrusion as a pretreatment to increase biogas production." Bioresource Technology 102(8): 4989-4994. [CrossRef]
- Hoffmann, R. A., M. L. Garcia, M. Veskivar, K. Karim, M. H. Al-Dahhan and L. T. Angenent (2008). "Effect of shear on performance and microbial ecology of continuously stirred anaerobic digesters treating animal manure." Biotechnology and Bioengineering 100(1): 38-48. [CrossRef]
- Hubbe, M. (2012). "Mini-encyclopedia of papermaking wet-end chemistry." Anti-Foam/Defoamers.[WWW].[Viitattu 10.6. 2012]. Saatavissa: http://www4. ncsu. edu/~ hubbe/DFOM. htm.
- Ibrahem, A., M. Yousef and S. El-Meadawy (1989). "Effect of beating on fibre crystallinity and physical properties of paper sheets." Medical Journal of Islamic World Academy of Sciences 2(4): 295-298.
- Izumi, K., Y.-k. Okishio, N. Nagao, C. Niwa, S. Yamamoto and T. Toda (2010). "Effects of particle size on anaerobic digestion of food waste." International biodeterioration & biodegradation 64(7): 601-608. [CrossRef]
- Jiang, Y., S. Xie, C. Dennehy, P. Lawlor, Z. Hu, G. Wu, X. Zhan and G. Gardiner (2020). "Inactivation of pathogens in anaerobic digestion systems for converting biowastes to bioenergy: A review." Renewable and Sustainable Energy Reviews 120: 109654. [CrossRef]
- Jin, S. and H. Chen (2006). "Superfine grinding of steam-exploded rice straw and its enzymatic hydrolysis." Biochemical Engineering Journal 30(3): 225-230. [CrossRef]
- Kafle, G. K., S. Bhattarai, S. H. Kim and L. Chen (2014). "Effect of feed to microbe ratios on anaerobic digestion of Chinese cabbage waste under mesophilic and thermophilic conditions: biogas potential and kinetic study." Journal of environmental management 133: 293-301. [CrossRef]
- Kang, X., Y. Zhang, B. Song, Y. Sun, L. Li, Y. He, X. Kong, X. Luo and Z. Yuan (2019). "The effect of mechanical pretreatment on the anaerobic digestion of Hybrid Pennisetum." Fuel 252: 469-474. [CrossRef]
- Kaparaju, P., I. Buendia, L. Ellegaard and I. Angelidakia (2008). "Effects of mixing on methane production during thermophilic anaerobic digestion of manure: Lab-scale and pilot-scale studies." Bioresource technology 99(11): 4919-4928. [CrossRef]
- Kato, K. and R. Cameron (1999). "A review of the relationship between thermally-accelerated ageing of paper and hornification." Cellulose 6(1): 23-40. [CrossRef]
- Kato, M., J. Field and G. Lettinga (1997). "Anaerobe tolerance to oxygen and the potentials of anaerobic and aerobic cocultures for wastewater treatment." Brazilian Journal of Chemical Engineering 14(4). [CrossRef]
- Ko, J. K., Y. Um, Y.-C. Park, J.-H. Seo and K. H. Kim (2015). "Compounds inhibiting the bioconversion of hydrothermally pretreated lignocellulose." Applied microbiology and biotechnology 99(10): 4201-4212. [CrossRef]
- Koch, K., Y. B. Fernández and J. E. Drewes (2015). "Influence of headspace flushing on methane production in Biochemical Methane Potential (BMP) tests." Bioresource technology 186: 173-178. [CrossRef]
- Kowalczyk, A., E. Harnisch, S. Schwede, M. Gerber and R. Span (2013). "Different mixing modes for biogas plants using energy crops." Applied energy 112: 465-472. [CrossRef]
- Krause, M. J., G. W. Chickering, T. G. Townsend and P. Pullammanappallil (2018). "Effects of temperature and particle size on the biochemical methane potential of municipal solid waste components." Waste management 71: 25-30. [CrossRef]
- Kumari, D. and R. Singh (2018). "Pretreatment of lignocellulosic wastes for biofuel production: a critical review." Renewable and Sustainable Energy Reviews 90: 877-891. [CrossRef]
- Labatut, R. A., L. T. Angenent and N. R. Scott (2014). "Conventional mesophilic vs. thermophilic anaerobic digestion: A trade-off between performance and stability?" Water research 53: 249-258. [CrossRef]
- Li, W., M. A. H. Siddhu, F. R. Amin, Y. He, R. Zhang, G. Liu and C. Chen (2018). "Methane production through anaerobic co-digestion of sheep dung and waste paper." Energy Conversion and Management 156: 279-287. [CrossRef]
- Li, Y., Y. Chen and J. Wu (2019). "Enhancement of methane production in anaerobic digestion process: A review." Applied energy 240: 120-137. [CrossRef]
- Li, Y., S. Y. Park and J. Zhu (2011). "Solid-state anaerobic digestion for methane production from organic waste." Renewable and sustainable energy reviews 15(1): 821-826. [CrossRef]
- Li, Y., R. Zhang, C. Chen, G. Liu, Y. He and X. Liu (2013). "Biogas production from co-digestion of corn stover and chicken manure under anaerobic wet, hemi-solid, and solid state conditions." Bioresource technology 149: 406-412. [CrossRef]
- Lin, Q., J. De Vrieze, J. Li and X. Li (2016). "Temperature affects microbial abundance, activity and interactions in anaerobic digestion." Bioresource Technology 209: 228-236. [CrossRef]
- Lindmark, J., N. Leksell, A. Schnürer and E. Thorin (2012). "Effects of mechanical pre-treatment on the biogas yield from ley crop silage." Applied Energy 97: 498-502. [CrossRef]
- Lindmark, J., E. Thorin, R. B. Fdhila and E. Dahlquist (2014). "Effects of mixing on the result of anaerobic digestion." Renewable and Sustainable Energy Reviews 40: 1030-1047. [CrossRef]
- Liotta, F., G. d’Antonio, G. Esposito, M. Fabbricino, L. Frunzo, E. D. Van Hullebusch, P. N. Lens and F. Pirozzi (2014). "Effect of moisture on disintegration kinetics during anaerobic digestion of complex organic substrates." Waste Management & Research 32(1): 40-48. [CrossRef]
- Liu, G., R. Zhang, H. M. El-Mashad and R. Dong (2009). "Effect of feed to inoculum ratios on biogas yields of food and green wastes." Bioresource technology 100(21): 5103-5108. [CrossRef]
- Mansouri, S., R. Khiari, N. Bendouissa, S. Saadallah, F. Mhenni and E. Mauret (2012). "Chemical composition and pulp characterization of Tunisian vine stems." Industrial Crops and Products 36(1): 22-27. [CrossRef]
- Menardo, S., G. Airoldi and P. Balsari (2012). "The effect of particle size and thermal pre-treatment on the methane yield of four agricultural by-products." Bioresource technology 104: 708-714. [CrossRef]
- Menardo, S., F. Gioelli and P. Balsari (2011). "The methane yield of digestate: effect of organic loading rate, hydraulic retention time, and plant feeding." Bioresource technology 102(3): 2348-2351. [CrossRef]
- Meng, L., L. Xie, C. T. Kinh, T. Suenaga, T. Hori, S. Riya, A. Terada and M. Hosomi (2018). "Influence of feedstock-to-inoculum ratio on performance and microbial community succession during solid-state thermophilic anaerobic co-digestion of pig urine and rice straw." Bioresource technology 252: 127-133. [CrossRef]
- Mohlin, U. (2002). "Industrial refining of unbleached kraft pulps—The effect of pH and refining intensity." Sweden: STFI AB, Swedish Pulp and Paper Research Institute.
- Montanes, R., R. Solera and M. Perez (2015). "Anaerobic co-digestion of sewage sludge and sugar beet pulp lixiviation in batch reactor: Effect of temperature.." Bioresource Technology 180: 177-184. [CrossRef]
- Montañés, R., R. Solera and M. Pérez (2015). "Anaerobic co-digestion of sewage sludge and sugar beet pulp lixiviation in batch reactors: Effect of temperature." Bioresource technology 180: 177-184. [CrossRef]
- Mshandete, A., L. Björnsson, A. K. Kivaisi, M. S. Rubindamayugi and B. Mattiasson (2006). "Effect of particle size on biogas yield from sisal fibre waste." Renewable energy 31(14): 2385-2392. [CrossRef]
- Mshandete, A., A. Kivaisi, M. Rubindamayugi and B. Mattiasson (2004). "Anaerobic batch co-digestion of sisal pulp and fish wastes." Bioresource technology 95(1): 19-24. [CrossRef]
- Nayono, S. E. (2010). Anaerobic digestion of organic solid waste for energy production, KIT scientific Publishing.
- Neves, L., R. Oliveira and M. Alves (2004). "Influence of inoculum activity on the bio-methanization of a kitchen waste under different waste/inoculum ratios." Process Biochemistry 39(12): 2019-2024. [CrossRef]
- Ohemeng-Ntiamoah, J. and T. Datta (2018). "Evaluating analytical methods for the characterization of lipids, proteins and carbohydrates in organic substrates for anaerobic co-digestion." Bioresource technology 247: 697-704. [CrossRef]
- Ohemeng-Ntiamoah, J. and T. Datta (2019). "Perspectives on variabilities in biomethane potential test parameters and outcomes: A review of studies published between 2007 and 2018." Science of the Total Environment. [CrossRef]
- Pakarinen, O., A. Lehtomaki and J. Rintala (2007). "Batch dark fermentative hydrogen production from grass silage: The effectof inoculum, pH, temperature and VS ratio." International Journal of Hydrogen Energy 33(2): 594-601. [CrossRef]
- Palmowski, L. and J. Muller (1999). "Influence of comminution of biogenic materials on their bioavailability." Muell Abfall 31(6): 368-372.
- Palmowski, L. and J. Müller (2000). "Influence of the size reduction of organic waste on their anaerobic digestion." Water science and technology 41(3): 155. [CrossRef]
- Panigrahi, S. and B. K. Dubey (2019). "A critical review on operating parameters and strategies to improve the biogas yield from anaerobic digestion of organic fraction of municipal solid waste." Renewable Energy 143: 779-797. [CrossRef]
- Pap, B., Á. Györkei, I. Z. Boboescu, I. K. Nagy, T. Bíró, É. Kondorosi and G. Maróti (2015). "Temperature-dependent transformation of biogas-producing microbial communities points to the increased importance of hydrogenotrophic methanogenesis under thermophilic operation." Bioresource Technology 177: 375-380. [CrossRef]
- Park, K. and H. Lee (2020). "Effects of nitrogen gas flushing in comparison with argon on rumen fermentation characteristics in in vitro studies." Journal of Animal Science and Technology 62(1): 52. [CrossRef]
- parra-Orobio, B. A., A. Donoso-Bravo, J. c. Ruiz-Sanchez, K. J. Valencia-Molina and P. Torres-Lozada (2018). "Effect of inoculum on anaerobic digestion of food waste accounting for concentration of waste elements." Waste Management 71: 342-349. [CrossRef]
- Pavi, S., L. E. Kramer, L. P. Gomes and L. A. S. Miranda (2017). "Biogas production from co-digestion of organic fraction of municipal solid waste and fruit and vegetable waste." Bioresource technology 228: 362-367. [CrossRef]
- Pellera, F.-M. and E. Gidarakos (2016). "Effect of substrate to inoculum ratio and inoculum type on the biochemical methane potential of solid agroindustrial waste." Journal of environmental chemical engineering 4(3): 3217-3229. [CrossRef]
- Pereira, S. C., L. Maehara, C. M. Machado and C. S. Farinas (2016). "Physical–chemical–morphological characterization of the whole sugarcane lignocellulosic biomass used for 2G ethanol production by spectroscopy and microscopy techniques." Renewable Energy 87: 607-617. [CrossRef]
- Pommier, S., A. M. Llamas and X. Lefebvre (2010). "Analysis of the outcome of shredding pretreatment on the anaerobic biodegradability of paper and cardboard materials." Bioresource technology 101(2): 463-468. [CrossRef]
- Raposo, F., C. Banks, I. Siegert, S. Heaven and R. Borja (2006). "Influence of inoculum to substrate ratio on the biochemical methane potential of maize in batch tests." Process Biochemistry 41(6): 1444-1450. [CrossRef]
- Raposo, F., R. Borja, M. Martín, A. Martín, M. De la Rubia and B. Rincón (2009). "Influence of inoculum–substrate ratio on the anaerobic digestion of sunflower oil cake in batch mode: process stability and kinetic evaluation." Chemical Engineering Journal 149(1-3): 70-77. [CrossRef]
- Raposo, F., M. De la Rubia, V. Fernández-Cegrí and R. Borja (2012). "Anaerobic digestion of solid organic substrates in batch mode: an overview relating to methane yields and experimental procedures." Renewable and Sustainable Energy Reviews 16(1): 861-877.
- Rémond, R., J. Passard and P. Perré (2007). "The effect of temperature and moisture content on the mechanical behaviour of wood: A comprehensive model applied to drying and bending." European Journal of Mechanics-A/Solids 26(3): 558-572. [CrossRef]
- Rodriguez, C., A. Alaswad, Z. El-Hassan and A.-G. Olabi (2017). "Mechanical pretreatment of waste paper for biogas production." Waste Management 68: 157-164. [CrossRef]
- Rodriguez, C., A. Alaswad, Z. El-Hassan and A. G. Olabi (2018). "Waste paper and macroalgae co-digestion effect on methane production." Energy 154: 119-125. [CrossRef]
- Rodriguez, C., Z. El-Hassan, A. G. Olabi and A. Alaswad (2016). Optimization of anaerobic digestion for mechanically pretreated waste paper. 11th Conference on Sustainable Development of Energy, Water and Environment Systems, SDEWES. Faculty of Mechanical Engineering and Naval Architecture, Zagreb, Lisbon.
- Rodriguez, C., Z. El-Hassan, A. G. Olabi and A. Alaswad (2016). Optimization of the anaerobic digestion process of mechanically pretreated algae. 11th Conference on Sustainable Development of Energy, Water and Environment Systems, SDEWES; Faculty of Mechanical Engineering and Naval Architecture: Zagreb, Croatia.
- Saad, S., I. El-Anwar and N. Metwally (1979). "Evaluation of Egyptian Unbleached Kraft Bagasse Pulp-Part I. Effect of Beating on Chemical and Mechanical Properties." Holzforschung-International Journal of the Biology, Chemistry, Physics and Technology of Wood 33(3): 90-92. [CrossRef]
- SAMBUSITI, C. (2013). "Physical, chemical and biological pretreatments to enhance biogas production from lignocellulosic substrates.".
- Schneider, N. and M. Gerber (2014). "Correlation between viscosity, temperature and total solid content of algal biomass." Bioresource technology 170: 293-302. [CrossRef]
- Sharma, S. K., I. Mishra, M. P. Sharma and J. Saini (1988). "Effect of particle size on biogas generation from biomass residues." Biomass 17(4): 251-263. [CrossRef]
- Shen, J., X.-S. Wang, M. Garcia-Perez, D. Mourant, M. J. Rhodes and C.-Z. Li (2009). "Effects of particle size on the fast pyrolysis of oil mallee woody biomass." Fuel 88(10): 1810-1817. [CrossRef]
- Silvester, F. D. (2013). Timber: Its mechanical properties and factors affecting its structural use, Elsevier.
- Singh, V., E. Khullar, B. Dien, K. Rausch and M. Tumbleson (2013). "Effect of particle size on enzymatic hydrolysis of pretreated miscanthus.". [CrossRef]
- Sosnowski, P., A. Wieczorek and S. Ledakowicz (2003). "Anaerobic co-digestion of sewage sludge and organic fraction of municipal solid wastes." Advances in Environmental Research 7(3): 609-616. [CrossRef]
- Stephenson, J. N. (1951). Pulp and Paper Manufacture. Vol. 1: Preparation and Treatment of Wood Pulp, JSTOR.
- Strömberg, S., M. Nistor and J. Liu (2014). "Towards eliminating systematic errors caused by the experimental conditions in Biochemical Methane Potential (BMP) tests." Waste Management 34(11): 1939-1948. [CrossRef]
- Stroot, P. G., K. D. McMahon, R. I. Mackie and L. Raskin (2001). "Anaerobic codigestion of municipal solid waste and biosolids under various mixing conditions—I. Digester performance." Water research 35(7): 1804-1816.
- Suksong, W., C. Mamimin, P. Prasertsan, P. Kongjan and O. Sompong (2019). "Effect of inoculum types and microbial community on thermophilic and mesophilic solid-state anaerobic digestion of empty fruit bunches for biogas production." Industrial Crops and Products 133: 193-202. [CrossRef]
- Tedesco, S. (2013). Mechanical Pre-treatment Assessment of Marine Biomass, Dublin City University. School of Mechanical and Manufacturing Engineering.
- Tedesco, S., K. Benyounis and A. Olabi (2013). "Mechanical pretreatment effects on macroalgae-derived biogas production in co-digestion with sludge in Ireland." Energy 61: 27-33. [CrossRef]
- Tedesco, S. and S. Daniels (2019). "Evaluation of inoculum acclimatation and biochemical seasonal variation for the production of renewable gaseous fuel from biorefined Laminaria sp. waste streams." Renewable Energy 139: 1-8. [CrossRef]
- Tedesco, S., D. Mac Lochlainn and A. G. Olabi (2014). "Particle size reduction optimization of Laminaria spp. biomass for enhanced methane production." Energy 76: 857-862. [CrossRef]
- Teghammar, A., K. Karimi, I. S. Horváth and M. J. Taherzadeh (2012). "Enhanced biogas production from rice straw, triticale straw and softwood spruce by NMMO pretreatment." Biomass and Bioenergy 36: 116-120. [CrossRef]
- Umetsu, K., S. Yamazaki, T. Kishimoto, J. Takahashi, Y. Shibata, C. Zhang, T. Misaki, O. Hamamoto, I. Ihara and M. Komiyama (2006). Anaerobic co-digestion of dairy manure and sugar beets. International Congress Series, Elsevier. [CrossRef]
- Valero, D., J. A. Montes, J. L. Rico and C. Rico (2016). "Influence of headspace pressure on methane production in Biochemical Methane Potential (BMP) tests." Waste management 48: 193-198. [CrossRef]
- Vasco-Correa, J., S. Khanal, A. Manandhar and A. Shah (2018). "Anaerobic digestion for bioenergy production: Global status, environmental and techno-economic implications, and government policies." Bioresource technology 247: 1015-1026. [CrossRef]
- VDI, V. D. I. (2006). "Fermentation of organic materials, Characterisation of Substrate, Sampling, Collection of Material Data, Fermentation Tests." VDI, Gesellschaft, Energietechnik.
- Victorin, M. (2016). "Development of characterization methods for lignocellulosic biogas substrates.".
- Wang, B. (2016). "Factors that influence the biochemical methane potential (BMP) test." Lund University.
- Wang, B., S. Strömberg, I. A. Nges, M. Nistor and J. Liu (2016). "Impacts of inoculum pre-treatments on enzyme activity and biochemical methane potential." Journal of bioscience and bioengineering 121(5): 557-560. [CrossRef]
- Wang, G. H., Lei Wang, Xue Jun Tan, Yi Xian Wang and F. Wang (2014). "Two-Phase Mesophilic Anaerobic Co-Digestion of Food Waste and Sewage Sludge: Effect of Hydraulic Retention Time." Advanced Materials Research 852. [CrossRef]
- 789-796.
- Wang, K., J. Yin, D. Shen and N. Li (2014). "Anaerobic digestion of foodwaste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH." Bioresource technology 161: 395-401. [CrossRef]
- Wang, M., A. K. Sahu, B. Rusten and C. Park (2013). "Anaerobic co-digestion of microalgae Chlorella sp. and waste activated sludge." Bioresource technology 142: 585-590. [CrossRef]
- Wang, X., G. Yang, Y. Feng, G. Ren and X. HAn (2012). "Optimising feeding composition and carbon-nitrogen ratiosfor improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw." Bioresource technology 120: 78-83. [CrossRef]
- Wang, X., G. Yang, Y. Feng, G. Ren and X. Han (2012). "Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw." Bioresource technology 120: 78-83. [CrossRef]
- Whitman, W. B., T. L. Bowen and D. R. Boone (2006). "The methanogenic bacteria." Prokaryotes 3(Chapter 9): 165-207.
- Xie, S., R. Wickham and L. D. Nghiem (2017). "Synergistic effect from anaerobic co-digestion of sewage sludge and organic wastes." International Biodeterioration & Biodegradation 116: 191-197. [CrossRef]
- Xu, F., Z.-W. Wang, L. Tang and Y. Li (2014). "A mass diffusion-based interpretation of the effect of total solids content on solid-state anaerobic digestion of cellulosic biomass." Bioresource technology 167: 178-185. [CrossRef]
- Xu, R., K. Zhang, P. Liu, A. Khan, J. Xiong, F. Tian and X. Li (2018). "A critical review on the interaction of substrate nutrient balance and microbial community structure and function in anaerobic co-digestion." Bioresource technology 247: 1119-1127. [CrossRef]
- Yenigün, O. and B. Demirel (2013). "Ammonia inhibition in anaerobic digestion: a review." Process Biochemistry 48(5-6): 901-911. [CrossRef]
- Yu, M., A. Womac, C. Igathinathane, P. Ayers and M. Buschermohle (2006). "Switchgrass ultimate stresses at typical biomass conditions available for processing." Biomass and Bioenergy 30(3): 214-219. [CrossRef]
- Zamri, M., S. Hasmady, A. Akhiar, F. Ideris, A. Shamsuddin, M. Mofijur, I. R. Fattah and T. Mahlia (2021). "A comprehensive review on anaerobic digestion of organic fraction of municipal solid waste." Renewable and Sustainable Energy Reviews 137: 110637. [CrossRef]
- Zeng, S., X. Yuan, X. Shi and Y. Qiu (2010). "Effect of inoculum/substrate ratio on methane yield and orthophosphate release from anaerobic digestion of Microcystis spp." Journal of Hazardous Materials 178(1-3): 89-93. [CrossRef]
- Zhang, C., G. Xiao, L. Peng, H. Su and T. Tan (2013). "The anaerobic co-digestion of food waste and cattle manure." Bioresource technology 129: 170-176. [CrossRef]
- Zheng, Z., J. Liu, X. Yuan, X. Wang, W. Zhu, F. Yang and Z. Cui (2015). "Effect of dairy manure to switchgrass co-digestion ratio on methane production and the bacterial community in batch anaerobic digestion." Applied Energy 151: 249-257. [CrossRef]
- Zhu, J., Y. Zheng, F. Xu and Y. Li (2014). "Solid-state anaerobic co-digestion of hay and soybean processing waste for biogas production." Bioresource technology 154: 240-247. [CrossRef]
- Zhu, Z., M. K. Hsueh and Q. He (2011). "Enhancing biomethanation of municipal waste sludge with grease trap waste as a co-substrate." Renewable Energy 36(6): 1802-1807. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




