Submitted:
20 March 2024
Posted:
21 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. The Study Area
2.2. Plant and Water Samples
2.3. Analytical Method
3. Results and Discussion
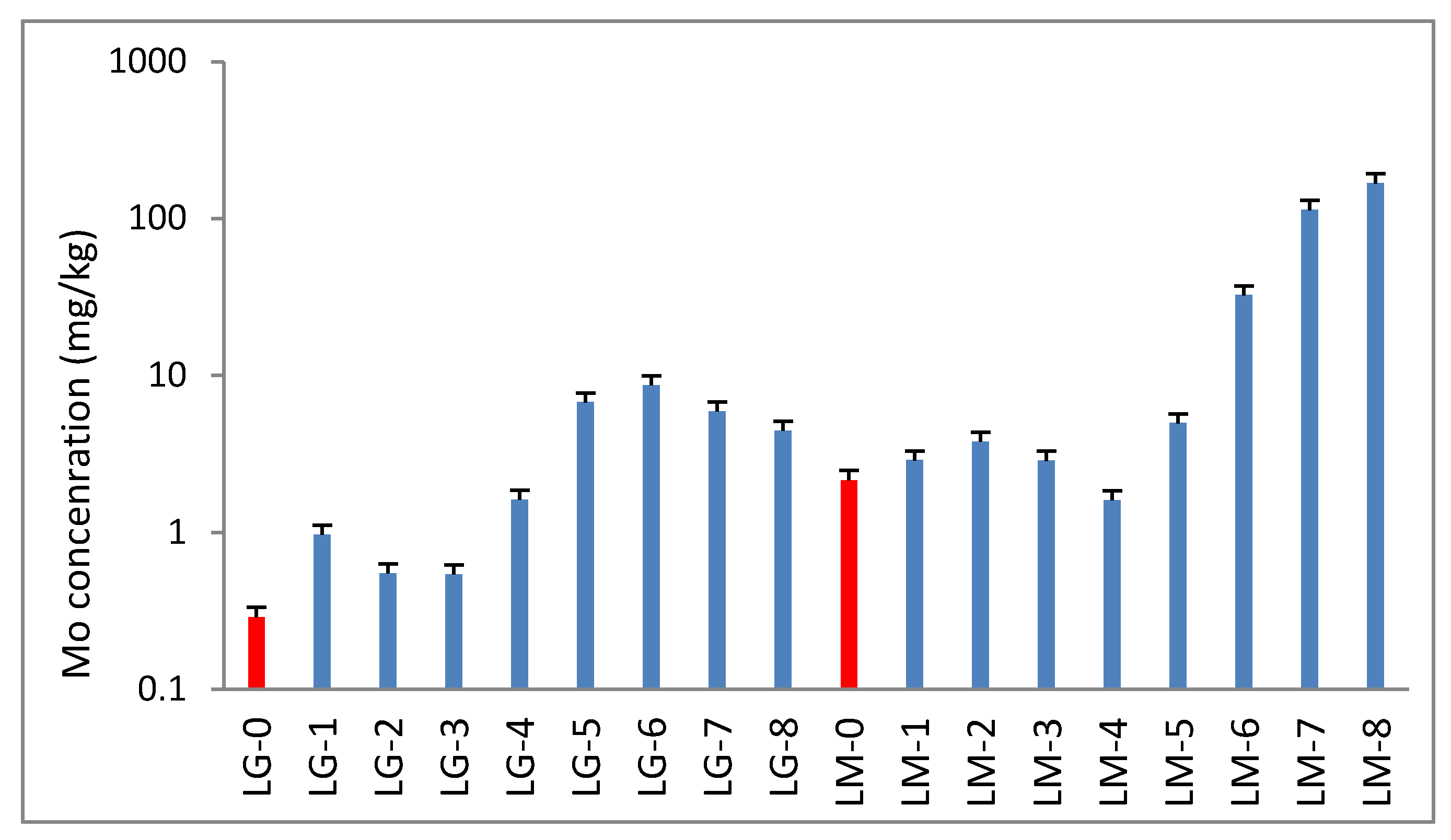
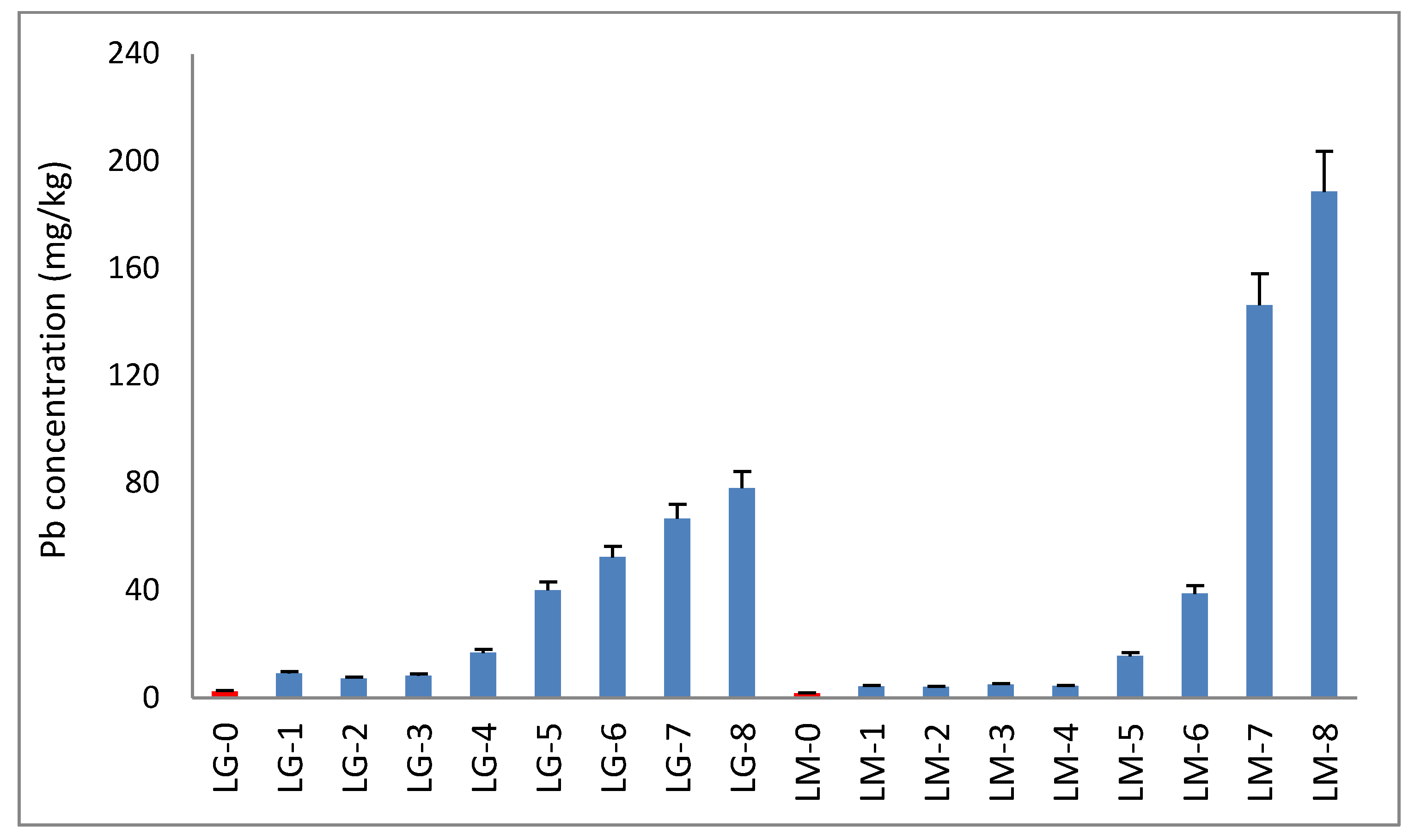
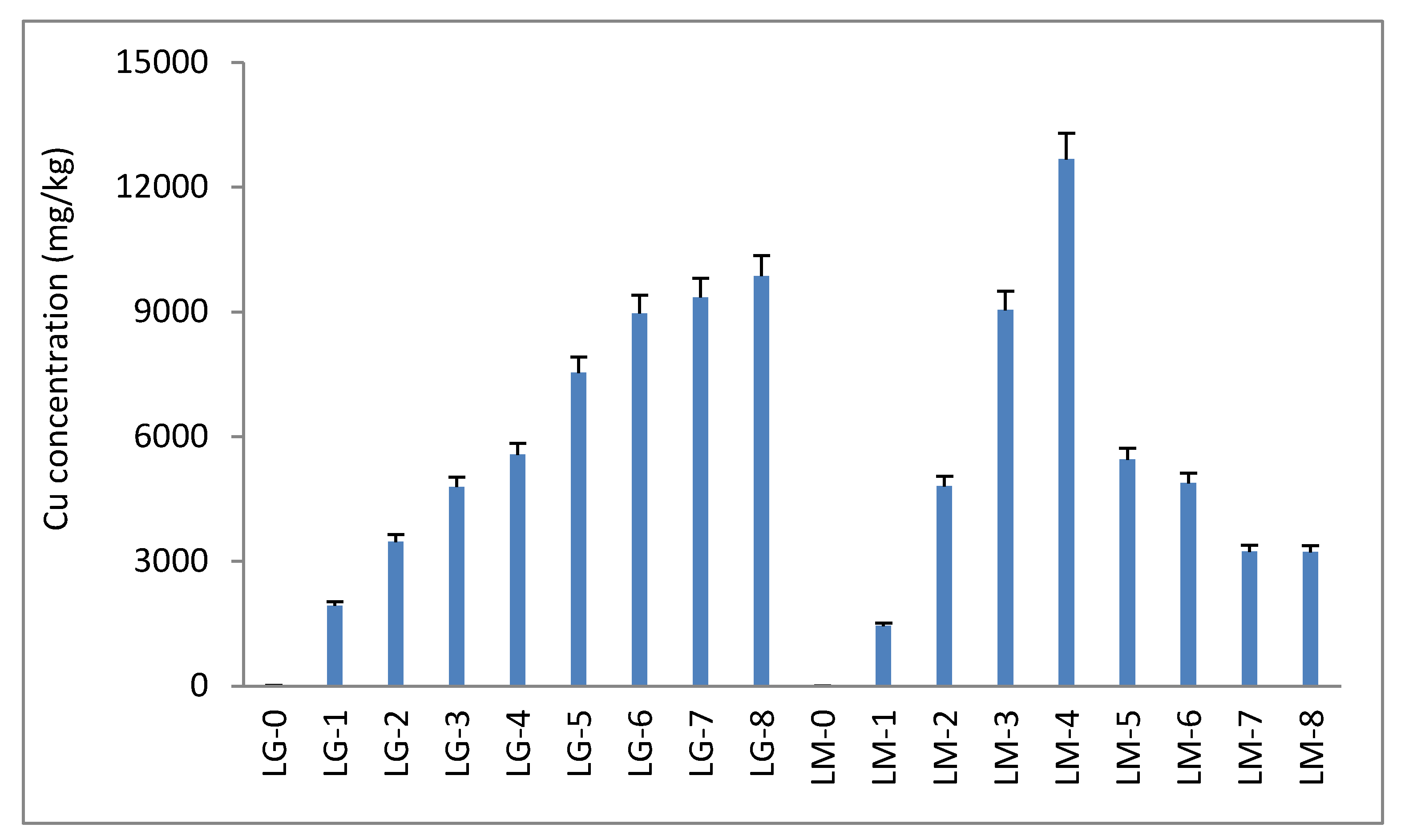
3.1. Mo, Pb, and Cu in Acidic Mining Water
3.2. Lemna gibba (LG) and Lemna minor (LM)
Conclusion
Acknowledgements
Conflicts of Interest
References
- Yamaguchi, K.; Tomiyama, S.; Igarashi, T.; Yamagata, S.; Ebato, M.; Sakoda, M. Effects of Backfilling Excavated Underground Space on Reducing Acid Mine Drainage in an Abandoned Mine. Minerals, 2020, 10, 777. [Google Scholar] [CrossRef]
- Gault, A.G.; Cooke, D.R.; Townsend, A.T.; Charnock, J.M.; Polya, D.A. Mechanisms of arsenic attenuation in acid mine drainage from Mount Bischoff, western Tasmania. Sci. Total Environ. 2005, 345, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Cesar Minga, J.; Elorza, F.J.; Rodriguez, R.; Iglesias, A.; Esenarro, D. Assessment of Water Resources Pollution Associated with Mining Activities in the Parac Subbasin of the Rimac River. Water 2023, 15, 965. [Google Scholar] [CrossRef]
- Kilic, A.D.; Cakmak, B. Elemental analysis of ignimbirite by Raman spectroscopy methods and instrumental neutron activation analysis. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2021, 251. [Google Scholar]
- Lim, H.S.; Lee, J.S.; Chon, H.T.; Sager, M. Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J. Geochem. Explor. 2008, 96, 223–230. [Google Scholar] [CrossRef]
- Tong, L.; Fan, R.; Yang, S.; Li, C. Development and status of the treatment technology for acid mine drainage. Mining, Metall. & Expl. 2021, 38, 315–327. [Google Scholar]
- Hierro, A.; Olías, M.; Cánovas, C.R.; Martín, J.E.; Bolivar, J.P. Trace metal partitioning over a tidal cycle in an estuary affected by acid mine drainage (Tinto estuary, SW Spain). Sci. Total Environ. 2014, 497–498, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Skierszkan, E.K.; Mayer, K.U.; Weis, D.; Beckie, R.D. Molybdenum and zinc stable isotope variation in mining waste rock drainage and waste rock at the Antamina mine, Peru. Sci. Total Environ. 2016, 550, 103–113. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, F.; Zhang, Y.; Ma, S.; Jia, X.; Meng, W. How sulfate-rich mine drainage affected aquatic ecosystem degradation in northeastern China, and potential ecological risk. Sci. Total Environ. 2017, 609, 1093–1102. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opisod, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Igarashi, T.; Herrera, P.S.; Uchiyama, H.; Miyamae, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The two-step neutralization ferrite-formation process for sustainable acid mine drainage treatment: Removal of copper, zinc and arsenic, and the influence of coexisting ions on ferritization. Sci. Total Environ. 2020, 715, 136877. [Google Scholar] [CrossRef] [PubMed]
- Migaszewski, Z.M.; Gałuszka, A.; Dołęgowska, S. Arsenic in the Wiśniówka acid mine drainage area (south-central Poland)—Mineralogy, hydrogeochemistry, remediation. Chem. Geol. 2018, 493, 491–503. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Veerawattananun, S.; Ito, M.; Hiroyoshi, N.; Igarashi, T. Pyrite oxidation in the presence of hematite and alumina: I. Batch leaching experiments and kinetic modeling calculations. Sci. Total Env. 2017, 580, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Tabelin, C.B.; Veerawattananun, S.; Ito, M.; Hiroyoshi, N.; Igarashi, T. Pyrite oxidation in the presence of hematite and alumina: II. Effects on the cathodic and anodic half-cell reactions. Sci. Total Environ. 2017, 581–582, 126–135. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1995; pp. 690–691. [Google Scholar]
- Pan, Y.; Chen, M.; Wang, X.; Chen, Y.; Dong, K. Ecological Risk Assessment and Source Analysis of Heavy Metals in the Soils of a Lead-Zinc Mining Watershed Area. Water 2023, 15, 113. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metals toxicity and the environment. Molecular, clinical and environmental toxicity 2012, 101, 133–164. [Google Scholar]
- Gergen, I.; Harmanescu, M. Application of principal component analysis in the pollution assessment with heavy metals of vegetable food chain in the old mining areas. Chem. Cent. J. 2012, 6, 56. [Google Scholar] [CrossRef]
- Rai, S.; Gupta, S.; Mittal, P.C. Dietary Intakes and Health Risk of Toxic and Essential Heavy Metals through the Food Chain in Agricultural, Industrial, and Coal Mining Areas of Northern India. Human and Ecological Risk Ass 2015, 21, 913–933. [Google Scholar] [CrossRef]
- Sasmaz, A.; Kılıç, A.D.; Akgül, B.; Sasmaz, B. A spectral approach on mineralogy and geochemistry of garnet skarns in Arc-Type granitoids. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2023, 286. [Google Scholar] [CrossRef]
- Mendel, R.R. , Biology of the molybdenum cofactor. J. Exp. Bot. 2007, 2289–2296. [Google Scholar] [CrossRef]
- Schwarz, G. , Mendel, R.R., Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Halmagyi, A.; Keul, M.; Dobrotă, C.; Fodorpataki, L.; Pintea, A.; Mocan, A.; Pop, V.; Coste, A. Impact of Arieş River Contaminants on Algae and Plants. Toxics, 2023, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Washington, 2011; p. 412. [Google Scholar]
- WHO. Guidelines for drinking-water quality.; Geneva, Switzerland, 2011. [Google Scholar]
- Smedley, P.L.; Kinniburgh, D.G. Molybdenum in natural waters: A review of occurrence, distributions and controls. Applied Geochemistry, 2017, 84, 387–432. [Google Scholar] [CrossRef]
- US EPA. United States Environmental Protection Agency. Ambient Water Quality Criteria for Silver; US EPA: Washington, DC, USA, 2000. [Google Scholar]
- WHO. World Health Organization Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2006; p. 553. [Google Scholar]
- Goswami, C.; Majumder, A. Potential of Lemna minor in Ni and Cr removal from aqueous solution. Pollution 2015, 1, 373–385. [Google Scholar]
- Goswami, C.; Majumder, A.; Misra, A.K.; Bandyopadhyay, K. Arsenic uptake by Lemna minor in hydroponic system. Int J Phyto, 2014, 16, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, F.K.; Khalil, W.K.B.; Bassem, S.M.; Kumar, V.; Parisi, C.; Inglese, S.; Temraz, T.A.; Nassar, H.F.; Guerriero, G. The Duckweed, Lemna minor Modulates Heavy Metal-Induced Oxidative Stress in the Nile Tilapia, Oreochromis niloticus. Water 2020, 12, 2983. [Google Scholar] [CrossRef]
- Haffner, O.; Kučera, E.; Drahoš, P.; Cigánek, J.; Kozáková, A.; Urminská, B. Lemna minor Bioassay Evaluation Using Computer Image Analysis. Water 2020, 12, 2207. [Google Scholar] [CrossRef]
- Sasmaz, M.; Topal, E.I.A.; Obek, E.; Sasmaz, A. The potential of Lemna gibba L. and Lemna minor L. to remove Cu, Pb, Zn, and As in gallery water in a mining area in Keban, Turkey. J. Environ. Man. 2015, 163, 246–253. [Google Scholar] [CrossRef]
- Tatar, S.Y.; Obek, E. Potential of Lemna gibba L. and Lemna minor L. for accumulation of Boron from secondary effluents. Ecol. Eng. 2014, 70, 332–336. [Google Scholar] [CrossRef]
- Sood, A.; Uniyal, P.L.; Prasanna, R.; Ahluwalia, A.S. Phytoremediation potential of aquatic macrophyte, Azolla. Ambio 2012, 41, 122–137. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Majumder, A.; Misra, A.M.; Bandyopadhyay, K. Cadmium removal by Lemna minör and Spirodela polyrhiza. Int. J. Phytorem 2014, 16, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Obek, E. Bioaccumulation of heavy metals from the secondary treated municipal waste water by Lemna gibba. Fres. Environ. Bull. 2009, 18, 2159–2164. [Google Scholar]
- Sasmaz, A.; Obek, E. The accumulation of arsenic, uranium, and boron in Lemna gibba L. exposed to secondary effluents. Ecol.Eng. 2009, 35, 1564–1567. [Google Scholar] [CrossRef]
- Gerardo, R.; de Lima, I.P. Monitoring Duckweeds (Lemna minor) in Small Rivers Using Sentinel-2 Satellite Imagery: Application of Vegetation and Water Indices to the Lis River (Portugal). Water 2022, 14, 2284. [Google Scholar] [CrossRef]
- Li, S.X. , Feng-Ying, F.; Yang, H.; Jian-Cong, N. Thorough removal of inorganic and organic mercury from aqueous solutions by adsorption on Lemna minor powder. J. Hazard. Mater. 2011, 186, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Dirilgen, N. Mercury and lead: assessing the toxic effects on growth and metal accumulation by Lemna minor. Ecotoxicol. Environ. Saf. 2011, 74, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Lobotková, M.; Hybská, H.; Samešová, D.; Turˇcániová, E.; Barnová, J.; Rétfalvi, T.; Krakovský, A.; Bad’o, F. Study of the Applicability of the Root Wastewater Treatment Plants with the Possibility of the Water Recirculation in Terms of the Surfactant Content. Water 2022, 14, 2817. [Google Scholar] [CrossRef]
- Khataee, A.; Movafeghi, A.; Torbati, S.; Salehi Lisar, S.; Zarei, M. Phytoremediation potential of duckweed (Lemna minor) in degradation of CI Acid Blue 92: artificial neural network modeling. Ecotox. Env. Saf. 2012, 80, 291–298. [Google Scholar] [CrossRef]
- Materazzi, S.; Canepari, S.; Aquili, S. Monitoring heavy metal pollution by aquatic plants. Environ. Sci. Poll. Res. 2012, 19, 3292–3298. [Google Scholar] [CrossRef]
- Ran, N.; Agami, M.; Oron, G. A pilot study of constructed wetlands using duckweed (Lemna gibba L.) for treatment of domestic primary effluent in Israel. Water Res. 2004, 38, 2241–2248. [Google Scholar] [CrossRef]
- León, M.D.C.C.D.; Flores-Alamo, N.; Solache-Ríos, M.J.; De La Rosa-Gómez, I.; Díaz-Campos, G. Lead and Copper Adsorption Behaviour by Lemna gibba: Kinetic and Equilibrium Studies. CLEAN—Soil Air Water 2017, 45, 1600357. [Google Scholar]
- Tang, Y.; Chen, L.; Wei, X.; Yao, Q.; Li, T. Removal of lead ions from aqueous solution by the dried aquatic plant, Lemna perpusilla Torr. J. Hazard. Mater. 2013, 244, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ledezma, J.L.; Uribe-Ramírez, D.; Cristiani-Urbina, E.; Morales-Barrera, L. Biosorptive removal of acid orange 74 dye by HCl-pretreated Lemna sp. PLoS ONE 2020, 15, 0228595. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, N.E.; Walsh, É.; Ahern, R.; Burnell, G.; Kuehnhold, H.; Jansen, M. A. Flow Rate and Water Depth Alters Biomass Production and Phytoremediation Capacity of Lemna minor. Plants, 2021, 11, 2170. [Google Scholar] [CrossRef] [PubMed]
- Erler, A. The hydrothermal alteration around Madenköy-Siirt massive sulphide deposit. METU, Doc. Thesis. 1982, 131 p. (unpublished).
- Sasmaz Kislioglu, M. Removal of Ag, Au, and As from acid mine water using Lemna gibba and Lemna minör: A performance analysis. Water 2023, 15, 1293. [Google Scholar] [CrossRef]
- Davis, P.H. Flora of Turkey and The East Aegean Island; Edinb. Univ. Press, 1984. [Google Scholar]
- Cabrera, L.I.; Salazar, G.A.; Chase, M.W.; Mayo, S.J.; Bogner, J.; Davila, P. Phylogenetic relationships of aroids and duckweeds (Araceae) inferred from coding and noncoding plastid DNA. American J. Botany 2008, 95, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- EFSA, EFSA Panel on Nutrition, Novel Foods and Food Allergens, Scientific Opinion on the safety ofLemna minor and Lemna gibba whole plant material as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2022, 20, 7598–20.
- Dong, D.; Li, H.; Zhang, J.; Sun, L. Removal of heavy metals from mine water by cyanobacterial calcification. Min. Sci. Technol. 2010, 20, 566–570. [Google Scholar] [CrossRef]
- Caussy, D.; Gochfeld, M. Gurzau E. Lessons from case studies of metals: Investigating exposure bioavailability and risk. Ecotox. Environ Safe 2003, 45–51. [Google Scholar] [CrossRef]
- Ning, N.; Liyuan, Y.; Jirui, D.; Xugui, P. Heavy Metal Pollution in Surface Water of Linglong Gold Mining Area, China. Proc. Environ. Sci. 2011, 10, 914–917. [Google Scholar] [CrossRef]
- Reimann, C.; de Caritat, P. Chemical Elements in the Environment. Factsheets for the Geochemist and Environmental Scientist; Springer-Verlag: Berlin, 1998. [Google Scholar]
- Linnik, P.N.; Ignatenko, I.I. Molybdenum in natural surface waters: content and forms of occurrence (a review). Hydrobiol. J. 2015, 51, 80–103. [Google Scholar] [CrossRef]
- Gaillardet, J.; Viers, J.; Dupre, B. Trace elements in river waters. In Treatise on Geochemistry, second ed; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, 2014; pp. 195–235. [Google Scholar]
- Zhao, Z.; Pei, J.; Zhang, X.; Zhou, X. Adsorptive stripping voltammetry determination of molybdenum(VI) in water and soil. Talanta 1990, 37, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, W.; Singh, S.K.; Raghav, S. Dissolved Mo and U in rivers and estuaries of India: implication to geochemistry of redox sensitive elements and their marine budgets. Chem. Geol. 2010, 278, 160–172. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water analyses. Trans. Am. Geophys. Union 1944, 25, 914–923. [Google Scholar]
- Pilon-Smits, E.A. Phytoremediation. Ann. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Tangahu, B.V.; Abdullah, S.R.S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 939161. [Google Scholar] [CrossRef]
- Cenci, R.M. The use of aquatic moss (Fontinalis antipyretica) as monitor of contamination in standing and running waters: limits and advantages, J. Limnol. 2000, 60, 53–61. [Google Scholar] [CrossRef]
- Sasmaz, A. Öbek E. The accumulation of arsenic, uranium, and boron in Lemna gibba L. exposed to secondary effluents, Ecol. Eng. 2009, 35, 1564–1567. [Google Scholar]
- Sasmaz, A.; Obek, E. The accumulation of silver and gold in Lemna gibba exposed to secondary effluents. 2012, 72, 149-152.
- Uysal, Y. Removal of chromium ions from wastewater by duckweed, Lemna minor L. by using a pilot system with continuous flow. J Hazard Mater 2013, 263, 486–492. [Google Scholar] [CrossRef]
- Abdallah, M.A. Phytoremediation of heavy metals from aqueous solutions by two aquatic macrophytes, Ceratophyllum demersum and Lemna gibba. Environ. Tech. 2012, 33, 1609–1614. [Google Scholar] [CrossRef]
- Ucuncu, E.; Tunca, E.; Fikirdeşici, S.; Özkan, A.D.; Altindag, A. Phytoremediation of Cu, Cr and Pb mixtures by Lemna minor. Bull Environ. Contam. Toxicol. 2013, 91, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Sasmaz, M.; Obek, E.; Sasmaz, A. The accumulation of La, Ce and Y by Lemna minor and Lemna gibba in the Keban gallery water, Elazig Turkey. Water & Env. J. 2018, 32, 75–83. [Google Scholar]
- Leblebici, Z.; Kar, M.; Yalcin, V. Comparative study of Cd, Pb, and Ni removal potential by Salvinia natans. All. and Lemna minor: interactions with growth parameters. Rom Biotech Lett. 2018, 23, 13235–13248. [Google Scholar]
- Amare, E.; Kebede, F.; Berihu, T.; Mulat, W. Field-based investigation on phytoremediation potentials of Lemna minor and Azolla filiculoides in tropical, semiarid regions: case of Ethiopia. Inter J Phytorem. 2018, 20, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Tatar, S.; Obek, E.; Arslan Topal, E.I.; Topal, M. Uptake of some elements with aquatic plants exposed to the effluent of wastewater treatment plant. Pollut 2019, 5, 377–386. [Google Scholar]


| Parameter | T | pH | EC | HCO3 | NO3- | SO4 | F | Ca | Mg | K | Na | Fe |
| (oC) | (mScm-1) | (mgL-1) | (mgL-1) | (mgL-1) | (mgL-1) | (mgL-1) | (mgL-1) | (mgL-1) | (mgL-1) | (mgL-1) | ||
| Detection Limit |
- | - | - | - | - | - | - | 0,05 | 0,05 | 0,05 | 0,05 | 10 |
| Mining water |
22,6 | 5,76 | 2,55 | 282 | 1,86 | 128 | 0,41 | 482 | 426 | 5,8 | 115 | 118 |
| ±1.6 | ± 0.1 | ± 0.2 | ±16 | ± 0.06 | ±8 | ±0.1 | ±24 | ±18 | ±0.3 | ±6 | ±7 | |
| Parameter | Mn | S | P | B | Zn | Cr | Ni | Co | As | Mo | Pb | Cu |
| (mgL-1) | (mgL-1) | (μgL-1) | (μgL-1) | (μg L-1) | (μgL-1) | (μgL-1) | (μgL-1) | (μgL-1) | (μg L-1) | (μg L-1) | (μg L-1) | |
| Detection Limit |
0,05 | 1 | 10 | 5 | 0,5 | 0,5 | 0,2 | 0,02 | 0,5 | 0,1 | 0,1 | 0,02 |
| Mining water |
6,4 | 670 | 236 | 850 | 2852 | 202 | 965 | 1766 | 193 | 30 | 260 | 15535 |
| ±0.3 | ±28 | ±12 | ±45 | ±84 | ±16 | ± 58 | ±72 | ±12 | ±4 | ±12 | ±322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




