1. Introduction
Cancer is a serious public health problem that is increasing worldwide, and its treatment has become an important focus of medicine. Despite the recent development of innovative cancer therapies and improvements in the survival and quality of life of cancer patients, the management of patients with advanced cancer remains a difficult challenge1. In particular, patients with advanced cancers that do not respond adequately to existing therapies, such as surgery, radiation therapy, and chemotherapy have limited treatment options, and often have an unfavorable prognosis2. In addition, these standard therapies are double-edged swords, sometimes resulting in a variety of health problems owing to adverse events. To overcome these difficulties, researchers and clinicians are investigating complementary approaches to conventional therapies.
Malignant tumors produce high levels of lactate, or H+, as a result of enhanced aerobic glycolysis, and the extracellular efflux of H+ leads to acidification of the tumor microenvironment (TME)3. An acidic TME attracts inflammatory cells, leading to chronic inflammation, tumor progression, and drug resistance4. In our clinic, we have implemented "alkalization therapy" aimed at neutralizing the acidic TME, and have achieved some efficacy in various cancers5-8.
In addition, many patients are trying natural products (antioxidants, vitamins, herbs, and other natural remedies) in addition to standard cancer treatments, which have been reported to reduce symptoms, maintain physical fitness, and possibly improve treatment outcomes9. Various natural products have been shown to inhibit the growth, migration, and invasion of cancer cells owing to their anti-inflammatory, antioxidant, and even anticancer properties10. Plants and foods containing these compounds have long been used in traditional medicine in many parts of the world, and are now available as medical supplements and dietary/health food items, and are expected to have various effects. However, the clinical efficacy and function of these natural products remain to be elucidated. In this review, we will provide an overview of our approach to alkalization of the TME and the effects of various natural products on cancer, including a literature review. We also report a cohort of patients with advanced cancer who had metastasis and postoperative recurrence, in whom long-term survival was achieved by alkalization therapy and treatment with natural products, in addition to standard treatments.
2. “Alkalization therapy” for cancer
2.1. Concept of alkalization therapy
"Alkalization therapy" is simply defined as "systematic alkalization of the body using diet and alkalizing agents to increase the pH of the local TME of the tumor". The theory of this therapy is as follows. First, tumor cells in malignant tumors have damaged mitochondria, and consequently use the glycolytic system for cellular metabolism (the Warburg effect)11. This is because malignant tumor cells have a 10- to 40-fold higher rate of glucose uptake than normal cells, and produce lactate 10- to 100-times faster, resulting in a 10- to 100-fold higher rate of lactate production, leading to lactate accumulation outside of the tumor cells11. This acidic environment, or decreased TME pH3, attracts inflammatory cells, leading to chronic inflammation4, and is also associated with distant metastasis12, creating an environment more favorable for cancer survival. Although various other molecular mechanisms have also been focused on as the cause of pH dysregulation in the peritumoral environment13, 14, studies investigating TME alkalization as a possible cancer therapy have already passed the basic research phase, and clinical studies are now being performed.(Studies supporting alkalization therapy).
Hirschhaeuser et al. have shown from various basic research studies that lactic acid produced from tumors is involved in the early growth process of cancer and in blunting the tumor immune response15. Several studies have reported that increasing the pH of the acidic TME can have anticancer effects. Pötzl et al. reported that systemic alkalization by the oral administration of bicarbonate enhances natural killer cell interferon-gamma expression, and significantly slows lymphoma growth in mice16. Another report demonstrated that the oral administration of bicarbonate controls breast cancer invasion in mice17, and inhibits distant metastasis in mouse models of breast and prostate cancer18. Another study reported that the oral ingestion of alkaline water (an alkalizing agent dissolved in water) slowed the growth of malignant melanoma in mice19, and a different study demonstrated that a combination of high-dose proton pump inhibitors (PPIs) and alkaline water together with chemotherapy resulted in increased anticancer effects and extended the life span of dogs and cats with advanced cancer20. Furthermore, some reports have suggested that alkalizing agents, such as bicarbonate, may not only inhibit local invasion and growth by alkalizing the TME, but may also have a synergistic effect with chemotherapy and immunotherapy21, 22.
Regarding the association between diet and cancer, studies have shown that specific foods have different systemic buffering effects23. In addition, Robey et al. have shown that animal protein and salt make the body more acidic, whereas fruits and vegetables make the body more alkaline, suggesting that changes in body pH caused by diet may affect molecular mechanisms at the cellular level24.
There are still only a limited number of studies that have applied the idea of alkalization of the TME as an anticancer treatment in clinical practice. Park et al. estimated the dietary acid load of patients using potential renal acid load (PRAL), and concluded that breast cancer incidence was highest in the group with the highest PRAL, in which patients had a diet that was high in meat consumption and low in fruit and vegetable intake, and hence a diet with limited meat and high fruit and vegetable intake may reduce breast cancer incidence25.
In practice, few studies have used diet and alkalizing agents as anticancer therapies, but as a palliative treatment, studies have shown that treatment with dimethyl sulfoxide and sodium bicarbonate infusion lead to substantial improvements in clinical symptoms and quality of life of patients with metastatic prostate cancer26, and decreased pain in difficult-to-treat end-stage cancer patients27. Mathematical model simulations as well as experimental observations have demonstrated that bicarbonate functions as an effective agent to increase extra-tumoral pH, and that chronic oral bicarbonate is effective enough to be a potential anticancer treatment28. Furthermore, a phase 0/1 clinical trial using sodium bicarbonate for cancer treatment proposed a specific prescription regimens29.Definition of alkalization therapy.
We define alkalization therapy as a combination of an alkalizing diet and oral intake of baking soda and/or citric acid as an alkalizing agent7. An "alkalizing diet" is defined as a diet with a high amount of fruits and vegetables, with blue-back fish as the main source of protein, and as little meat and dairy as possible. Specifically, patients are instructed to consume 400 g of fruits and vegetables daily and to keep a dietary record for the first 4 weeks. In addition, when patients visit our clinic, the doctor and nurses review their dietary records to ensure that their diet meets our criteria of an alkalizing diet, and advise patients on dietary adjustments that need to be made. Ultimately, the choice of diet is left to the patient. We believe that any drug therapy or additional intake of natural products will have little effect if a proper alkaline diet is not followed, and this is the treatment we focus on the most.
2.2. Alkalization therapy; Our clinical experience to date
In our clinic, we have empirically combined alkalization therapy with chemotherapy, and found that some patients with various solid tumors and hematologic tumors show substantial therapeutic effects7. In a comparative study of advanced pancreatic cancer patients treated with conventional chemotherapy plus alkalization therapy versus chemotherapy alone group, the median overall survival (OS) was significantly longer (15.4 months vs. 10.8 months; p < 0.005) and the mean urine pH was significantly higher in the alkalization therapy group (6.38 ± 0.85 vs. 6.80 ± 0.71; p < 0.05). This suggested that alkalization therapy contributed to the increase in urine pH by acting as a systemic buffering therapy, and had a synergistic effect with chemotherapy, thus leading to the prolongation of patient OS6. In a study on patients with hepatocellular carcinoma, patients treated with alkalization therapy were divided into 2 groups, namely, a group with a mean post-treatment urine pH of ≥ 7.0, and the other with a pH of < 7.0. The results showed that the median OS from the start of alkalization therapy of patients with a urine pH of ≥ 7.0 was not reached (n = 12, 95% confidence interval(CI) = 3.0-not reached), which was significantly longer than that of patients with a pH of < 7.0 (15.4 months, n = 17, 95% CI = 5.8-not reached, p < 0.05)8. Furthermore, in an observational study of small cell lung cancer patients, alkalization therapy and intravenous vitamin C combined with chemotherapy (intervention group) was compared with chemotherapy alone (control group), and the mean urine pH of the intervention group was significantly higher than that of the control group (7.32 ± 0.45 vs. 6.44 ± 0.74; p < 0.05). The median OS of the intervention group was 44.2 months (95% CI = 22.0-not reached), compared with 17.7 months for the control group (95% CI = 13.5-not reached; p < 0.05)5. These results suggest that alkalization therapy may have an effect on anticancer drug resistance, and a synergistic effect with natural products may also be expected.
3. Natural products for the treatment of cancer
3.1. Triterpenoids
The triterpenoid compounds ursolic acid and oleanolic acid are isomers, and are widely found in foods, medicinal herbs, and other plants30. Ursolic acid and oleanolic acid tend to be discussed as a pair, but here we would like to focus on ursolic acid, which can be extracted from Japanese plums, which have long been used as a health food in Japan. Ursolic acid has long been used in folk medicine, and is also found in apples, basil, bilberries, cranberries, peppermint, rosemary, oregano, etc. Pharmacologically, ursolic acid is known for its hepatoprotective, anti-inflammatory, antihyperlipidemic, and anticancer effects 30. In terms of anticancer effects, its ability to affect the activity of several intracellular enzymes enables it to modulate processes that occur within tumor cells, and activate pathways leading to apoptosis(programmed cell death), including inhibition of the MAPK/ERK and PI3K/ACT/mTOR signaling cascades31. This activation of pathways that lead to apoptosis is the most important function of ursolic acid’s anticancer activity, which leads to the inhibition of pathways that lead to cancer proliferation, growth, and metastasis. Other anticancer activities of ursolic acid have also been reported, such as its effects in response to the exposure of cells to carcinogenic chemicals (e.g., benzo(a)pyrene32 and substances extracted from tobacco smoke33), reactive oxygen species (ROS)34, ionizing radiation35, and Epstein-Barr Virus36.
3.2. Parthenolide
Parthenolide is a type of sesquiterpenoid derived from the leaves and flowers of feverfew, and is a natural compound that has been used as a herbal remedy since ancient times37. Parthenolide causes anti-inflammatory and anticancer effects by strongly inhibiting the activity of nuclear factor kappa-B (NF-κB)38. NF-κB is an important transcription factor involved in cancer cell proliferation, survival, invasion, metastasis, and evasion of anti-tumor immunity. To date, parthenolide has been shown to have high cytotoxicity and apoptosis-inducing effects against malignant tumors, such as breast cancer and chronic myeloid leukemia, and to inhibit the proliferation and self-renewal ability of cancer stem cells, suggesting its potential usefulness for the curative treatment of cancer39, 40. In addition, there is also a study that parthenolide has anticancer effects by targeting epidermal growth factor receptor (EGFR) in non-small cell lung cancer41. Furthermore, parthenolide inhibits the mitochondrial respiratory chain and increases the production of ROS42. ROS cause DNA damage and apoptosis in cancer cells, and hence parthenolide can also be expected to have anticancer effects by reducing the expression of antioxidant enzymes and increasing the sensitivity of cancer cells to oxidative stress.
3.3. Fulvic acid
Fulvic acid is a high-molecular weight organic acid resulting from the decomposition of ancient plants and animals by microorganisms43. Fulvic acid is also the main component of Shilajit, which has been applied in the field of Ayurvedic traditional medicine for a long time44. The actions of fulvic acid in the body have been summarized in previous reports as immunomodulatory, oxidation-regulating, and gastrointestinal-activating, and it has been found to promote the activation of various physiological functions45. Regarding anticancer effects, fulvic acid is considered to prevent the progression of cancer by inhibiting the proliferation of cancer cells and inducing apoptosis 46, 47. In addition, a study has suggested that the binding of fulvic acid to transferrin in human serum enables more efficient delivery of anti-tumor drugs to the target tumor48.
3.4. Taxus yunnanensis (Taxus plant)
Paclitaxel, extracted from plant called Taxus brevofolia, was originally known as the anticancer drug taxol49, and is widely used in large quantities in both clinical and basic research, and is well-known as a clinically effective anticancer molefule50, 51. One of its sources, T. yunnanensis, is endemic to China, but is an endangered species and is being depleted as a biological resource52. Unlike other species of the Taxus genus, T. yunnanensis has a high taxol concentration53, and it has unique polysaccharides that have been shown to substantially inhibit the proliferation of HeLa and HT1080 cells in a concentration-dependent manner54. T. yunnanensis has a special position within the genus, as it is a plant that contains a large amount of α-conidendrin, which induces apoptosis in breast cancer cell lines55. There are also reports that T. yunnanensis has significantly higher oral absorption and bioavailability than pure extracted paclitaxel in rat studies56, and it has been associated with the induction of tumor cell apoptosis via multiple pathways57.
3.5. Apple pectin
Pectin is a substance obtained from the remains of the extraction of sugar and juice from fruits, and it plays an important role as a soluble plant fiber. However, many preclinical studies have shown that pectin and its derivatives have anticancer effects against breast, stomach, colon, pancreatic, hepatocellular, bladder, prostate, ovarian, leukemia, myeloma, skin, brain, and lung cancer58, 59. For example, pectin and pectin oligosaccharides increased the number of apoptotic cells in human colon cancer cell lines60. In a study using human breast cancer cell lines and mice, it was also reported that pectin induces apoptosis in vitro, suppresses cell proliferation and cell adhesion, and suppresses p53 expression in vivo, thereby reducing tumor growth and increasing the number of apoptotic cells61. Clinical studies to date include a phase II study reporting that the oral administration of modified citrus pectin reduced prostate specific antigen (PSA) doubling time in prostate cancer patients62.
4. Hypothesis: Can the combination of “alkalization therapy” and natural products have a positive effect on cancer patients?
As an adjuvant therapy for cancer, would alkalization therapy and natural products have an anticancer effect, either individually or in combination? We believe that they have the potential to alleviate the side effects of current chemotherapies, and that their anti-inflammatory, antioxidant, and immunostimulatory effects may complement the effects of chemotherapy and contribute to improving the overall health of patients. Indeed, some researchers have expressed skepticism about the anticancer effects of alkalization therapy63, 64. However, if an alkalizing diet and the oral administration of alkalizing agents, which have very limited side effects, can produce even a small benefit, then alkalization therapy may become a mainstream option in cases in which the effects of conventional therapy are not sufficient, therapeutic effects have not been achieved, or when therapeutic effects are desired at a low cost.
Natural products, on the other hand, are less likely to be used as conventional drugs owing to their complex nature and the high costs associated with their development as pharmaceuticals. However, they have the potential to support the improvement, maintenance, and recovery of health, to enhance the effectiveness of standard treatments, and to reduce their side effects. Natural products are usually taken in the form of supplements or dietary supplements and are expected to play an adjunctive role in supporting treatment. It is noteworthy that many of the natural products used as anticancer treatments are used in folk medicine, and their safety is empirically assured because they are very familiar in the human diet. Consuming whole plants rather than extracted, refined, and medicated versions of their constituents is a good risk management measure in terms of side effects. Whereas we expect extracted components to have anticancer effects, the bioavailability of the raw material may be higher than that of the extracted component, as in the case of T. yunnanensis mentioned above. As some of the natural products mentioned here have strong anticancer effects, they may be effective adjuvant therapies that can reduce the serious side effects of chemotherapy, although they cannot be used in combination with chemotherapy as safely as alkalization therapy. However, natural products can be used safely and effectively in clinical applications in combination with alkalization therapy.
5. Long-term survival of patients with advanced cancer treated with alkalization therapy and natural products
5.1. Study outline
Here, we report cases of patients with advanced cancer, who were treated with triterpenoids, parthenolides, fulvic acid, T. yunnanensis, and apple pectin. Data were extracted retrospectively from the medical records of patients with advanced cancer (postoperative recurrent or metastatic cancer) who visited Karasuma Wada Clinic between January 1, 2011 and September 30, 2018, who were taking natural products continuously while receiving alkalization therapy in addition to standard treatment, and who survived for at least 5 years after starting treatment. The data includes each patient’s age, sex, type of cancer diagnosed, cancer stage, type of natural products taken, and survival time (as of September 30, 2023) since the start of treatment.
These cases are comprehensively included in the research project "Investigation of survival factors for cancer patients using data science methods" approved by the Institutional Review Board of the Japan-Multinational Trial Organization (UMIN000047446).
5.2. Patient characteristics
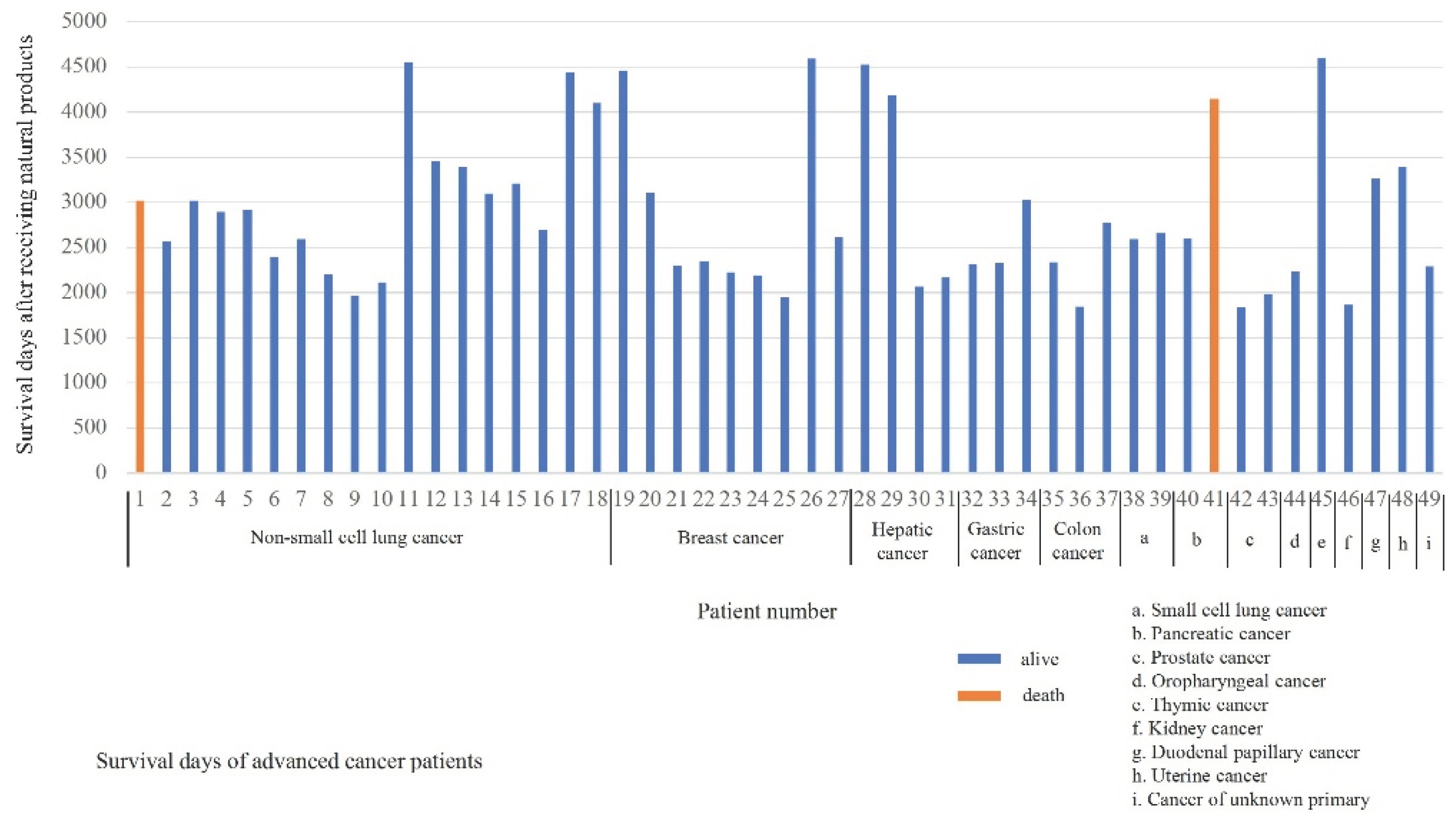
Table 1 shows the characteristics of a cohort of 49 advanced cancer patients treated with alkalization therapy and natural products. The group includes 22 men and 27 women, with a mean age at the first clinic visit of 62.2 years (range: 39–86 years). Metastasis was observed in 31 patients, and 18 experienced recurrence after surgery. The cancers diagnosed included non-small cell lung cancer in 18 patients, breast cancer in 9, hepatic cancer in 4, gastric cancer and colon cancer in 3 each, small cell lung cancer, pancreatic cancer, and prostate cancer in 2 each, and oropharyngeal, thymic, kidney, duodenal papillary, uterine, and unknown primary cancers in 1 patient each. Triterpenoid was the most utilized natural product, used by 48 patients, followed by parthenolide in 19, fulvic acid in 11,
T. yunnanensis in 5, and apple pectin in 2.
5.3. Outcomes of patients treated with alkalization therapy and natural products in combination with standard treatments
Figure 1 illustrates the survival duration for each patient following treatment with a combination of natural compounds and alkalization therapy, together with standard treatments. As of September 30, 2023, the average survival time across the cohort was 2,886 days, ranging from 1,840 to 4,592 days. At the specified date, only 2 patients—one with non-small cell lung cancer and the other with pancreatic cancer—had died from their illnesses.
This figure displays the individual survival durations (days) of each patient who underwent treatment combining natural compounds and alkalization therapy, in addition to standard cancer treatments.
6. Summary and limitations
Here we have summarized the anticancer effects of alkalization therapy and natural products, and reported cases of advanced cancer patients who were treated with alkalization therapy and natural products in addition to standard therapies and achieved long-term survival. On the basis of our clinical data, it is possible that the combination of alkalization therapy and natural products with standard therapies may have a higher anticancer effect than existing therapies on their own.
There are several limitations to this review. First, this review was based on a single-center, retrospective, long-term survival analysis, and does not compare patients with and without alkalization therapy or natural products. Therefore, the possibility that only cases of long-term survival owing to factors not attributable to alkalization therapy or natural products were selected cannot be denied. Second, the natural products used and the combination therapy differ depending on the type of cancer and the case, making generalization difficult, and therefore, the results cannot be evaluated straightforwardly. Finally, it is difficult to provide any evidence regarding the degree of compliance of patients undergoing alkalization therapy, and furthermore, the degree to which the body and TME actually become alkalized remains unclear, and no investigation has been conducted to date on this point.
7. Conclusion
Alkalization therapy and natural products have been suggested to have anti-cancer effects, but combining them may have even higher anti-cancer effects. The combination of these 2 therapies with existing standard therapies may be a new treatment strategy for advanced cancer, but further studies are needed to confirm the efficacy of this strategy.
Author Contributions
Conceptualization and methodology, M.I., R.H., and H.W.; Formal analysis, data curation, and investigation, R.N., R.H., and H.M.; writing—original draft preparation, M.I. and R.H.; writing—review and editing, R.H.; supervision, T.O. and H.W. Project administration, H.W.; All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no external funding for this research.
Institutional Review Board statement
The case series presented in this study are comprehensively included in "Investigation of survival factors for cancer patients using data science methods" approved by the Institutional Review Board of the Japan-Multinational Trial Organization (UMIN000047446).
Informed Consent Statement
This research was conducted using retrospective data. Patients were provided the option to opt out from the study. Therefore, written informed consent was not obtained.
Data Availability Statement
All data are contained within the article.
Acknowledgments
The authors thank Helena Akiko Popiel of Tokyo Medical University for her editing of this article.
Conflicts of Interest
The authors declare no conflicts of interest associated with this study.
References
- Siegel, R. L.; Miller, K. D.; Wagle, N. S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D. B.; Johnston, P. G. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef]
- Koppenol, W. H.; Bounds, P. L.; Dang, C. V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Hamaguchi, R.; Narui, R.; Morikawa, H.; Wada, H. Improved chemotherapy outcomes of patients with small-cell lung cancer treated with combined alkalization therapy and intravenous vitamin C. Cancer Diagn Progn 2021, 1, 157–163. [Google Scholar] [CrossRef]
- Hamaguchi, R.; Ito, T.; Narui, R.; Morikawa, H.; Uemoto, S.; Wada, H. Effects of alkalization therapy on chemotherapy outcomes in advanced pancreatic cancer: A retrospective case-control study. In Vivo 2020, 34, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Hamaguchi, R.; Narui, R.; Morikawa, H. Meaning and significance of "alkalization therapy for cancer". Front Oncol 2022, 12, 920843. [Google Scholar] [CrossRef] [PubMed]
- Isowa, M.; Hamaguchi, R.; Narui, R.; Morikawa, H.; Wada, H. Effects of alkalization therapy on hepatocellular carcinoma: a retrospective study. Front Oncol 2023, 13, 1179049. [Google Scholar] [CrossRef] [PubMed]
- Block, K. I.; Koch, A. C.; Mead, M. N.; Tothy, P. K.; Newman, R. A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic toxicity: a systematic review of the evidence from randomized controlled trials. Int J Cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M. H.; Bahramsoltani, R.; Rahimi, R. Phytochemicals as adjunctive with conventional anticancer therapies. Curr Pharm Des 2016, 22, 4201–4218. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Thews, O.; Riemann, A. Tumor pH and metastasis: a malignant process beyond hypoxia. Cancer Metastasis Rev 2019, 38, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Asgharzadeh, M. R.; Barar, J.; Pourseif, M. M.; Eskandani, M.; Jafari Niya, M.; Mashayekhi, M. R.; Omidi, Y. Molecular machineries of pH dysregulation in tumor microenvironment: potential targets for cancer therapy. Bioimpacts 2017, 7, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Parks, S. K.; Chiche, J.; Pouyssegur, J. pH control mechanisms of tumor survival and growth. J Cell Physiol 2011, 226, 299–308. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Sattler, U. G.; Mueller-Klieser, W. Lactate: a metabolic key player in cancer. Cancer Res 2011, 71, 6921–6925. [Google Scholar] [CrossRef] [PubMed]
- Pötzl, J.; Roser, D.; Bankel, L.; Hömberg, N.; Geishauser, A.; Brenner, C. D.; Weigand, M.; Röcken, M.; Mocikat, R. Reversal of tumor acidosis by systemic buffering reactivates NK cells to express IFN-γ and induces NK cell-dependent lymphoma control without other immunotherapies. Int J Cancer 2017, 140, 2125–2133. [Google Scholar] [CrossRef]
- Robey, I. F.; Nesbit, L. A. Investigating mechanisms of alkalinization for reducing primary breast tumor invasion. Biomed Res Int 2013, 2013, 485196. [Google Scholar] [CrossRef]
- Robey, I. F.; Baggett, B. K.; Kirkpatrick, N. D.; Roe, D. J.; Dosescu, J.; Sloane, B. F.; Hashim, A. I.; Morse, D. L.; Raghunand, N.; Gatenby, R. A.; et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res 2009, 69, 2260–2268. [Google Scholar] [CrossRef]
- Azzarito, T.; Lugini, L.; Spugnini, E. P.; Canese, R.; Gugliotta, A.; Fidanza, S.; Fais, S. Effect of Modified alkaline supplementation on syngenic melanoma growth in CB57/BL Mice. PLoS One 2016, 11, e0159763. [Google Scholar] [CrossRef]
- Spugnini, E. P.; Buglioni, S.; Carocci, F.; Francesco, M.; Vincenzi, B.; Fanciulli, M.; Fais, S. High dose lansoprazole combined with metronomic chemotherapy: a phase I/II study in companion animals with spontaneously occurring tumors. J Transl Med 2014, 12, 225. [Google Scholar] [CrossRef]
- Gillies, R. J.; Ibrahim-Hashim, A.; Ordway, B.; Gatenby, R. A. Back to basic: Trials and tribulations of alkalizing agents in cancer. Front Oncol 2022, 12, 981718. [Google Scholar] [CrossRef]
- Ando, H.; Eshima, K.; Ishida, T. Neutralization of acidic tumor microenvironment (TME) with daily oral dosing of sodium potassium citrate (K/Na citrate) increases therapeutic effect of anti-cancer agent in pancreatic cancer xenograft mice model. Biol Pharm Bull 2021, 44, 266–270. [Google Scholar] [CrossRef]
- Ribeiro, M. D.; Silva, A. S.; Bailey, K. M.; Kumar, N. B.; Sellers, T. A.; Gatenby, R. A.; Ibrahim-Hashim, A.; Gillies, R. J. Buffer therapy for cancer. J Nutr Food Sci 2012, 2, 6. [Google Scholar] [CrossRef]
- Robey, I. F. Examining the relationship between diet-induced acidosis and cancer. Nutr Metab (Lond) 2012, 9, 72. [Google Scholar] [CrossRef]
- Park, Y. M.; Steck, S. E.; Fung, T. T.; Merchant, A. T.; Elizabeth Hodgson, M.; Keller, J. A.; Sandler, D. P. Higher diet-dependent acid load is associated with risk of breast cancer: Findings from the sister study. Int J Cancer 2019, 144, 1834–1843. [Google Scholar] [CrossRef]
- Hoang, B. X.; Le, B. T.; Tran, H. D.; Hoang, C.; Tran, H. Q.; Tran, D. M.; Pham, C. Q.; Pham, T. D.; Ha, T. V.; Bui, N. T.; et al. Dimethyl sulfoxide-sodium bicarbonate infusion for palliative care and pain relief in patients with metastatic prostate cancer. J Pain Palliat Care Pharmacother 2011, 25, 350–355. [Google Scholar] [CrossRef]
- Hoang, B. X.; Tran, D. M.; Tran, H. Q.; Nguyen, P. T.; Pham, T. D.; Dang, H. V.; Ha, T. V.; Tran, H. D.; Hoang, C.; Luong, K. N.; et al. Dimethyl sulfoxide and sodium bicarbonate in the treatment of refractory cancer pain. J Pain Palliat Care Pharmacother 2011, 25, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Martin, N. K.; Robey, I. F.; Gaffney, E. A.; Gillies, R. J.; Gatenby, R. A.; Maini, P. K. Predicting the safety and efficacy of buffer therapy to raise tumour pHe: an integrative modelling study. Br J Cancer 2012, 106, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Robey, I. F.; Lopez, A. M.; Roe, D. J. Safety and tolerability of long-term sodium bicarbonate consumption in cancer care. Journal of Integrative Oncology 2015, 4, 128. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 1995, 49, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic acid--A pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef]
- Huang, M. T.; Ho, C. T.; Wang, Z. Y.; Ferraro, T.; Lou, Y. R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J. D.; Conney, A. H. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res 1994, 54, 701–708. [Google Scholar]
- Liu, W.; Tan, X.; Shu, L.; Sun, H.; Song, J.; Jin, P.; Yu, S.; Sun, M.; Jia, X. Ursolic acid inhibits cigarette smoke extract-induced human bronchial epithelial cell injury and prevents development of lung cancer. Molecules 2012, 17, 9104–9115. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, R.; Priya, D. K.; Gunassekaran, G. R.; Sakthisekaran, D. Ursolic acid attenuates oxidative stress-mediated hepatocellular carcinoma induction by diethylnitrosamine in male Wistar rats. Asian Pac J Cancer Prev 2009, 10, 933–938. [Google Scholar] [PubMed]
- Hsu, H. Y.; Yang, J. J.; Lin, C. C. Effects of oleanolic acid and ursolic acid on inhibiting tumor growth and enhancing the recovery of hematopoietic system postirradiation in mice. Cancer Lett 1997, 111, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ohigashi, H.; Takamura, H.; Koshimizu, K.; Tokuda, H.; Ito, Y. Search for possible antitumor promoters by inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation; ursolic acid and oleanolic acid from an anti-inflammatory Chinese medicinal plant, Glechoma hederaceae L. Cancer Lett 1986, 30, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, A.; Sinjab, A.; Herceg, Z.; Darwiche, N. Parthenolide: from plant shoots to cancer roots. Drug Discov Today 2013, 18, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Hehner, S. P.; Hofmann, T. G.; Dröge, W.; Schmitz, M. L. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J Immunol 1999, 163, 5617–5623. [Google Scholar] [CrossRef] [PubMed]
- D’Anneo, A.; Carlisi, D.; Lauricella, M.; Puleio, R.; Martinez, R.; Di Bella, S.; Di Marco, P.; Emanuele, S.; Di Fiore, R.; Guercio, A.; et al. Parthenolide generates reactive oxygen species and autophagy in MDA-MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis 2013, 4, e891. [Google Scholar] [CrossRef]
- Flores-Lopez, G.; Moreno-Lorenzana, D.; Ayala-Sanchez, M.; Aviles-Vazquez, S.; Torres-Martinez, H.; Crooks, P. A.; Guzman, M. L.; Mayani, H.; Chávez-González, A. Parthenolide and DMAPT induce cell death in primitive CML cells through reactive oxygen species. J Cell Mol Med 2018, 22, 4899–4912. [Google Scholar] [CrossRef]
- Li, X.; Huang, R.; Li, M.; Zhu, Z.; Chen, Z.; Cui, L.; Luo, H.; Luo, L. Parthenolide inhibits the growth of non-small cell lung cancer by targeting epidermal growth factor receptor. Cancer Cell Int 2020, 20, 561. [Google Scholar] [CrossRef]
- Mathema, V. B.; Koh, Y. S.; Thakuri, B. C.; Sillanpää, M. Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation 2012, 35, 560–565. [Google Scholar] [CrossRef]
- Pettit, R. E. Organic matter, humus, humate, humic acid, fulvic acid and humin: their importance in soil fertility and plant health. CTI Research 2004, 10, 1–7. [Google Scholar]
- Ghosal, S. Chemistry of shilajit, an immunomodulatory Ayurvedic rasayan. Pure and Applied Chemistry 1990, 62, 1285–1288. [Google Scholar] [CrossRef]
- Winkler, J.; Ghosh, S. Therapeutic Potential of fulvic acid in chronic inflammatory diseases and diabetes. J Diabetes Res 2018, 2018, 5391014. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, R. G. P. T.; Dilshara, M. G.; Kang, C. H.; Lee, S.; Choi, Y. H.; Jeong, Y. K.; Kim, G. Y. Fulvic acid promotes extracellular anti-cancer mediators from RAW 264.7 cells, causing to cancer cell death in vitro. Int Immunopharmacol 2016, 36, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Piri, H.; Farasat, A.; Pakbin, B.; Gheibi, N. Activation of apoptosis and G0/G1 cell cycle arrest along with inhibition of melanogenesis by humic acid and fulvic acid. Iran J Basic Med Sci 2022, 25, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. F.; Yang, G.; Dong, Y.; Zhao, Y. Q.; Sun, X. R.; Chen, L.; Chen, H. B. Studies on the binding of fulvic acid with transferrin by spectroscopic analysis. Spectrochim Acta A Mol Biomol Spectrosc 2015, 137, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K. C.; Yang, Z.; Liu, J. J.; Ueno, H.; Nantermet, P. G.; Guy, R. K.; Claiborne, C. F.; Renaud, J.; Couladouros, E. A.; Paulvannan, K. Total synthesis of taxol. Nature 1994, 367, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett 2019, 24, 40. [Google Scholar] [CrossRef]
- Cragg, G. M.; Newman, D. J. Plants as a source of anti-cancer agents. J Ethnopharmacol 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Su, J.; Liu, W.; Li, S. Dormancy release and germination of Taxus yunnanensis seeds during wet sand storage. Sci Rep 2018, 8, 3205. [Google Scholar] [CrossRef]
- Yu, C.; Luo, X.; Zhan, X.; Hao, J.; Zhang, L.; L Song, Y. B.; Shen, C.; Dong, M. Comparative metabolomics reveals the metabolic variations between two endangered Taxus species (T. fuana and T. yunnanensis) in the Himalayas. BMC Plant Biol 2018, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yin, Y.; Zhang, D.; Yang, W.; Yu, R. Structural characterization and in vitro antitumor activity of a novel polysaccharide from Taxus yunnanensis. Carbohydr Polym 2013, 96, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, K.; Hemmati, A. A.; Abbaszadeh, H.; Valizadeh, A.; Makvandi, M. Anticancer activity and molecular mechanisms of α-conidendrin, a polyphenolic compound present in Taxus yunnanensis, on human breast cancer cell lines. Phytother Res 2020, 34, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Cai, D.; Bi, H.; Zhong, G.; Zeng, H.; Gu, L.; Huang, Z.; Huang, M. Comparative pharmacokinetics of paclitaxel after oral administration of Taxus yunnanensis extract and pure paclitaxel to rats. Fitoterapia 2013, 90, 1–9. [Google Scholar] [CrossRef]
- Akao, Y.; Terazawa, R.; Sugito, N.; Heishima, K.; Morikawa, K.; Ito, Y.; Narui, R.; Hamaguchi, R.; Nobukawa, T. Understanding of cell death induced by the constituents of Taxus yunnanensis wood. Sci Rep 2022, 12, 6282. [Google Scholar] [CrossRef]
- Emran, T. B.; Islam, F.; Mitra, S.; Paul, S.; Nath, N.; Khan, Z.; Das, R.; Chandran, D.; Sharma, R.; Lima, C. M. G.; et al. Pectin: A bioactive food polysaccharide with cancer preventive potential. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Leclere, L.; Cutsem, P. V.; Michiels, C. Anti-cancer activities of pH- or heat-modified pectin. Front Pharmacol 2013, 4, 128. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Rimbach, G. H.; Gibson, G. R.; Rastall, R. A. Pectin and pectic-oligosaccharides induce apoptosis in in vitro human colonic adenocarcinoma cells. Anticancer Res 2003, 23, 341–346. [Google Scholar]
- Delphi, L.; Sepehri, H. Apple pectin: A natural source for cancer suppression in 4T1 breast cancer cells in vitro and express p53 in mouse bearing 4T1 cancer tumors, in vivo. Biomed Pharmacother 2016, 84, 637–644. [Google Scholar] [CrossRef]
- Keizman, D.; Frenkel, M.; Peer, A.; Kushnir, I.; Rosenbaum, E.; Sarid, D.; Leibovitch, I.; Mano, R.; Yossepowitch, O.; Margel, D.; et al. Modified citrus pectin treatment in non-metastatic biochemically relapsed prostate cancer: Results of a prospective phase II study. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D. R.; O’Riordan, E. Starving cancer and other dangerous dietary misconceptions. Lancet Oncol 2023, 24, 1177–1178. [Google Scholar] [CrossRef] [PubMed]
- Zick, S. M.; Snyder, D.; Abrams, D. I. Pros and cons of dietary strategies popular among cancer patients. Oncology (Williston Park) 2018, 32, 542–547. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).