1. Introduction
More than three decades have passed since the pivotal International Cooperative Study on the Timing of Aneurysm Surgery [
1], which conclusively demonstrated that vasospasm stands as the predominant cause of death in cases of aneurysmal subarachnoid hemorrhage (aSAH). Despite the strides made in understanding and managing this condition, complications arising from aSAH, particularly delayed cerebral ischemia (DCI), persist as the primary sources of concern within the realm of neurocritical care for patients who successfully survive the initial hemorrhage and are promptly transferred to specialized centers [
2].
DCI remains the single most significant contributor to both mortality and morbidity among individuals recovering from aSAH, underscoring the imperative need for comprehensive research and therapeutic interventions in this domain [
3]. The multifaceted nature of DCI is underscored by a myriad of contributing factors, with vasospasm emerging as a central and pivotal element. In addition to vasospasm, other crucial determinants encompass microcirculatory dysfunction, glymphatic impairment, inflammatory responses, and neuroelectric disruptions [
4].
As we delve into the intricate landscape of DCI, it becomes evident that a thorough understanding of its pathophysiology is paramount for advancing effective treatment strategies. The interplay among various factors underscores the complexity of DCI, urging researchers and clinicians alike to explore novel avenues for intervention and management. This expanded perspective not only acknowledges the historical context established by earlier studies but also highlights the ongoing challenges and opportunities for furthering our comprehension and, ultimately, improving outcomes for patients grappling with the aftermath of aSAH.
The ability to predict which patients will develop clinically significant vasospasm and its consequential complications could have a huge impact on treatment and possible escalation of prevention strategy of vasospasm-related delayed cerebral ischaemia. This has been a point of research interest for some time. Various tests and predictors have been suggested in an attempt to predict which patients will develop clinically relevant vasospasm. For example, Fisher scale and later modified Fisher scale use computed-tomographic (CT) characteristics of the hemorrhage, like amount of blood-products, or the presence of intraventricular hemorrhage [
5]. The modified Fisher scale has remained a significant predictor of clinically relevant vasospasm even after the correction for other known risk factors like early angiographic vasospasm, history of hypertension, Hunt and Hess grade, and elevated mean arterial pressure during admission [
6].
The Monitoring and Imaging of SAH and Aneurysm (MISA) study is a prospective cohort trial in patients undergoing treatment for non - ruptured and ruptured aneurysms [
7]. Besides analysis of various biomarkers and CSF components, a phosphorous magnetic resonance spectroscopy (31P-MRS) was conducted after therapy in every patient.
Phosphorous magnetic resonance spectroscopy (31P-MRS) is a spectroscopy technique that enables in-vivo and non-invasive measurements of energy metabolites and membrane turnover metabolites dicertly, and Mg and pH indirectly in a voxel of tissue. [
7].
An especially important factor in post-hemorrhagic vasospasm could be intracerebral magnesium (Mg). Hypomagnesemia occurs in approximately 50 % of patients with aSAH. The addition of Mg to the therapy protocol showed reduction in stroke size and severity in aSAH stroke models [
8,
9,
10]. Recent studies showed potentially reduced morbidity and mortality of aSAH-patients after introducing magnesium sulfate therapy intravenously, however most of these studies were inconclusive with mixed results [
11,
12,
13,
14]. Further studies suggested intrathecal magnesium sulphate injections with somewhat better results [
15,
16]. Apart from nimodipine, no drugs showed benefit in phase III trials, despite promising results in phase II trials. One of the reasons for the mixed results could be the observed incongruence in design of phase II and phase III trials [
17]. One of the therapy regimens that showed enormous potential in phase II trials is the intravenous magnesium at a steady rate of 64 mmol/l/day [
18]. However, the phase III trials did not confirm these results [
19,
20].
Another factor in this examined cohort could be brain tissue pH. pH, together with CO2 is a significant contributing factor to the cerebral blood flow [
21]. Charbel et al. showed significantly lower brain tissue pH in patients experiencing vasospasm using invasive monitoring [
22]. On the other hand, some studies found higher CSF pH to be a contributing factor in the development of delayed cerebral ischaemia [
23].
In the pursuit of advancing our understanding of aSAH, the primary objective of this study was to undertake a comprehensive examination of cerebral magnesium (Mg) levels and cerebral pH in affected individuals. To achieve this, we employed advanced imaging techniques, specifically 31-phosphorus Magnetic Resonance Spectroscopy (31P-MRS), allowing for a nuanced exploration of these parameters across multiple dimensions.
In this study we quantify cerebral Mg levels and pH not only in the entire brain (cumulative results) but also with a focused analysis on the affected side of the brain. Additionally, we sought to delineate variations in these crucial metrics within the affected arterial territories, aiming to capture localized changes that may provide valuable insights into the pathophysiology of aSAH.
By employing 31P-MRS, we aimed to unravel the intricate interplay between cerebral Mg levels and pH, shedding light on potential correlations and patterns that could contribute to our understanding of the underlying mechanisms associated with aSAH. This comprehensive approach may contribute to possible targeted interventions and improved patient outcomes in this area.
2. Materials and Methods
Inclusion criteria for this study were aneurysmal subarachnoidal hemorrhage, MRI with 31P-MRS sequence and sufficient clinical and imaging follow-up data. The exclusion criteria were the age under 18 years, hemorrhage due to other causes, or repeated aneurysm rupture. 13 patients with aSAH who were admitted to our neurosurgery department between July 2016 and October 2017 were scanned using magnetic resonance imaging (MRI) in the first week after the hemorrhage (mean 4 days after the event, always within 72 hours from surgical or endovascular therapy). This corresponds with the time before the occurrence of clinically significant vasospasmus, which develops at around 4-15 days after the initial bleeding [
24]. Besides the standard sequences according to the institutional protocol, the 31P-MRS was conducted. The patients were hospitalised in the neurosurgical intensive care unit and closely monitored. They were then divided into a group with and a group without clinically relevant vasospasm (defined by well-established clinical criteria). The most important criterion defining the clinically relevant vasospasmus was the development of delayed cerebral ischemia confirmed by the MRI, or the need to undergo a repeated conventional angiography and intra-arterial therapy (nimodipine application or percutaneous transluminal balloon angioplasty). We analysed the cumulative results (all investigated voxels together), only voxels from the affected brain territory, and voxels from the affected side exclusively.
The study was approved by the local Ethics Committee (AN2016-0032 359/4.8), and completed in compliance with the Declaration of Helsinki. The informed consent was obtained from each patient or hers/his legal guardian.
2.1. Magnetic resonance imaging
MRI scans were performed on a 3T MRI scanner (Verio, Siemens Medical AG, Erlangen, Germany), and for the 31P-MRS sequence, a double-tuned 1H/31P volume head coil (Rapid Biomedical, Würzburg, Germany) was employed. The 3D 31P-MRS block was planned on a sagitally oriented T2-weighted sequence, acquired in advance and covering the entire cerebrum (3D T2 space with a voxel size of 1.2 × 1.2 × 1.2 mm³, repetition time (TR) = 3000 ms, echo time (TE) = 412.0 ms, acquisition time (TA) = 2:50). Air-filled cavities, bone, fat, and boundary regions were omitted, in order to avoid voxel contamination, performed as described in detail by Grams et al. [
25]. The volume of interest (VOI) was acquired with an 8 × 8 × 8 mm matrix, a field of view of 240 × 240 × 200 mm
3, resulting in a 30 × 30 mm
2 voxel size, and a slice thickness of 25 mm. The sequence was executed with a WALTZ 4 proton decoupling, a repetition time of 2000 ms (TR), an echo time of 2.3 ms (TE), a flip angle of 60
∘ (FA), and the number of averages was 10. Similar variations of this sequence have already been carried out in various studies [
26,
27,
28,
29,
30,
31,
32,
33].
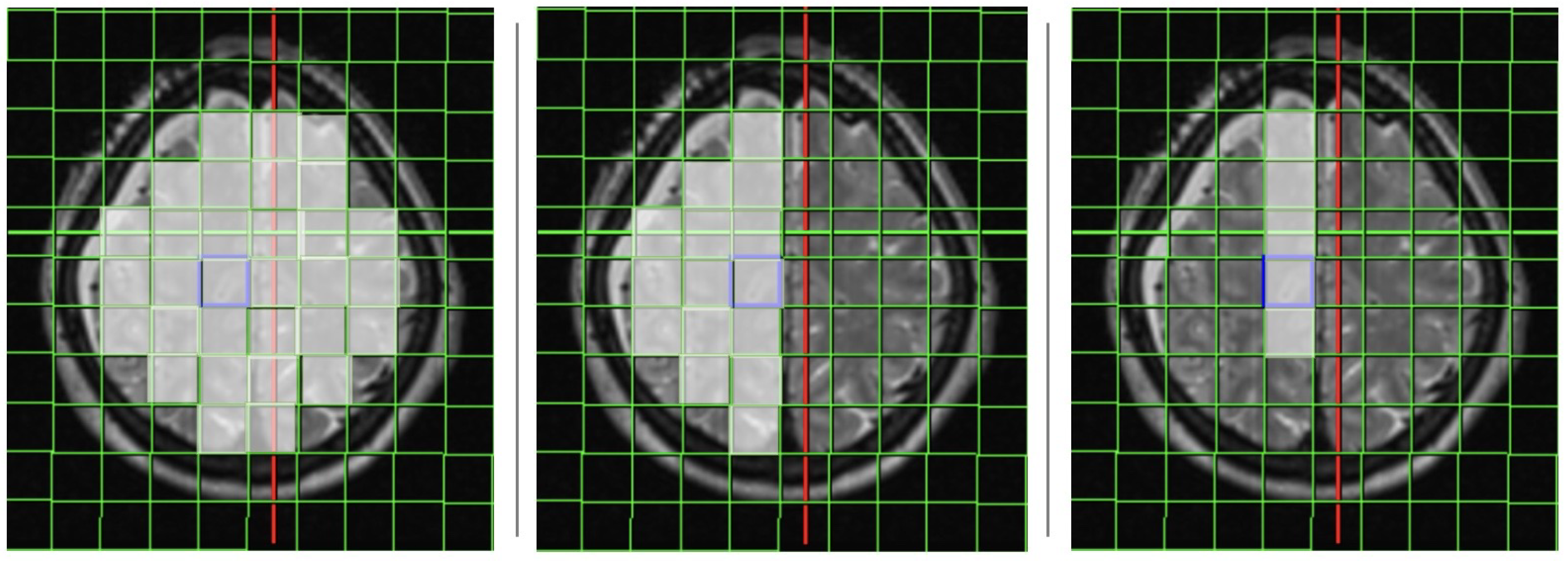
Voxels, which were included for the 31P MRS analyses were chosen from the following vascular territories: basal ganglia (BA), anterior cerebral arteries (ACA), middle cerebral arteries (MCA) and the posterior cerebral arteries (PCA). Predetermined by the size of the measured voxels we chose at least 4 voxels on each side of the brain within the regions of interest and averaged the calculated values for both hemispheres, respectively. Furthermore, every spectrum in each single voxel was visually assessed and checked regarding the quality of the spectra according to the criteria of existing literature [
34]. Voxels that did not fulfill these quality criteria were excluded from further analysis.
Data post-processing of the 31P-MRS .rda data files (Siemens Medical AG, Erlangen, Germany) was performed offline with jMRUI (version 5.0, MRUI Consortium, available at
http://www.mrui.uab.es, accessed 1 October 2020). The fitting process employed the non linear square fitting algorithm AMARES and involved 12 Lorentzian-shaped exponentially decaying sinusoids, representing metabolites, including phosphocholine and phospho-ethanolamine (summarized as phsophomonoesters—PME), Pi, glycerophosphocholine and glycerophosphoethanolamine (summarized as phosphodiesters—PDE), PCr, and ATP.
We calculated the pH and Mg2+ values as described in detail by Hattingen et al. [
30]. The changes in pH were estimated from the signal position of inorganic phosphate and the PCr peak (
Pi), and the changes in Mg
2+ concentrations were assessed from the chemical shift difference between the
-ATP peak and the PCr signal (
). Both parameters were calculated according the formulae given by Iotti et al. [
35], as described in detail in [
30] and [
36].
The analysis of the 31P-MRS data took place in all investigated voxels (cumulative results), and separately only in the affected side, and in the arterial territory of the affected artery. The positioning of the voxels is shown in
Figure 1.
2.2. Statistical analysis
Statistical analysis was performed using R (R Core Team v. 3.6.1). The Shapiro-Wilk test and one-sample Kolmogorov-Smirnov test were used to assess the normality of data, which was presented with quantile-comparison plots and histograms. The data was not normally distributed. Wilcoxon signed-rank test was used for comparison between groups. For the analysis of more than two groups ANOVA and Tukey’s post-hoc test were used. p-values < 0.05 were considered statistically significant. The results are presented using boxplots. Additionally, sensitivity and specificity analysis was calculated and the results were presented using receiver operating characteristics curves. The cut-off values were determined using the greatest Youden’s J index. The statistics was done using the cumulative results of all measurements, and using measurements from the affected side and arterial territory exclusively.
3. Results
3.1. Patient characteristics
This study incorporated a total of 13 patients, with a mean age of 55 years (± standard deviation 9.66 years), who had experienced aSAH. The characteristics of the participants are outlined in
Table 1. More details concerning the patient group can be found in [
7].
3.2. Cumulative results
3.2.1. Magnesium levels
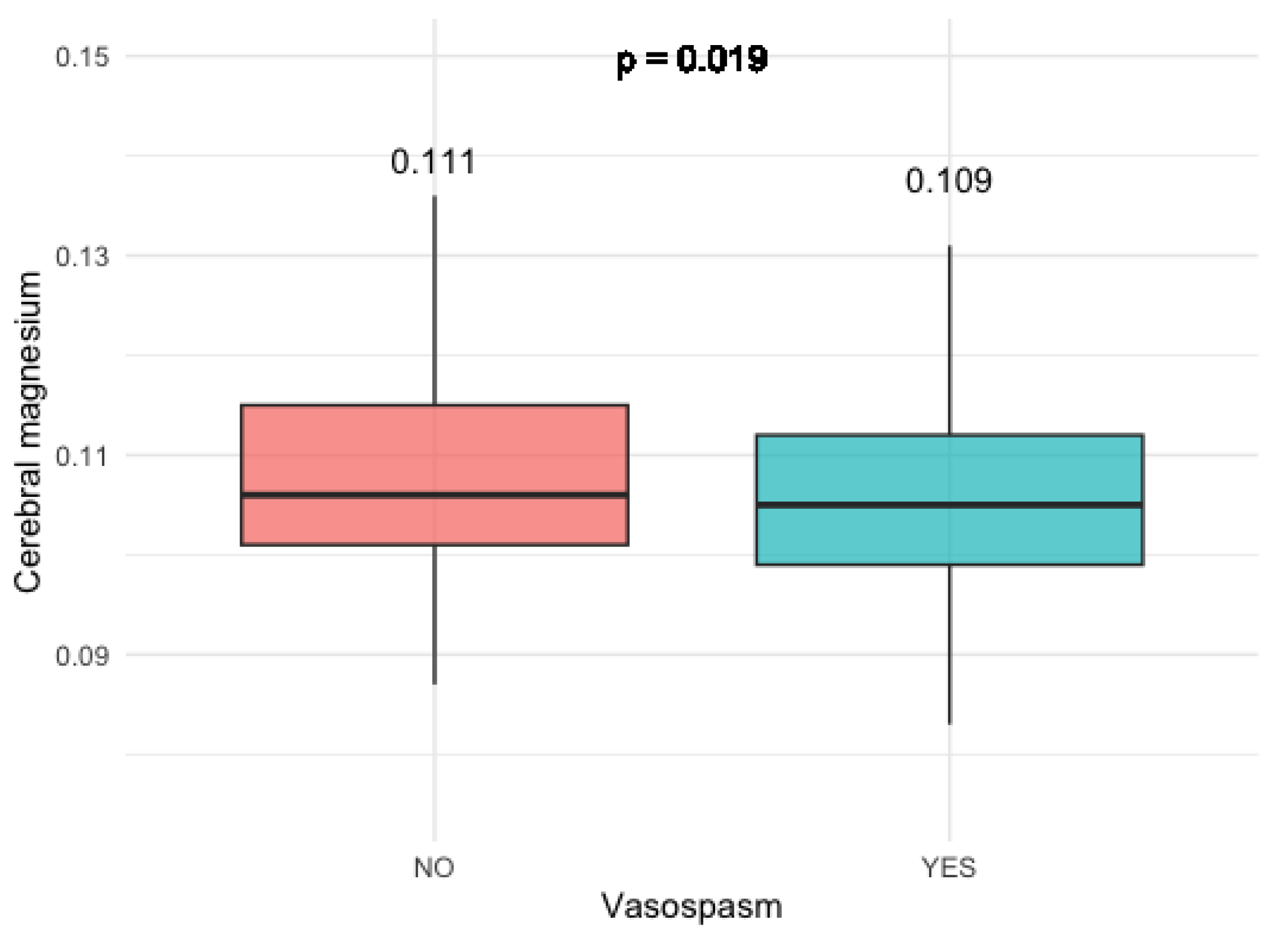
In individuals who experienced clinically relevant vasospasm, there was a notable and statistically significant reduction in cumulative cerebral magnesium levels across all investigated brain voxels (p = 0.019). This observation is visually represented in
Figure 2.
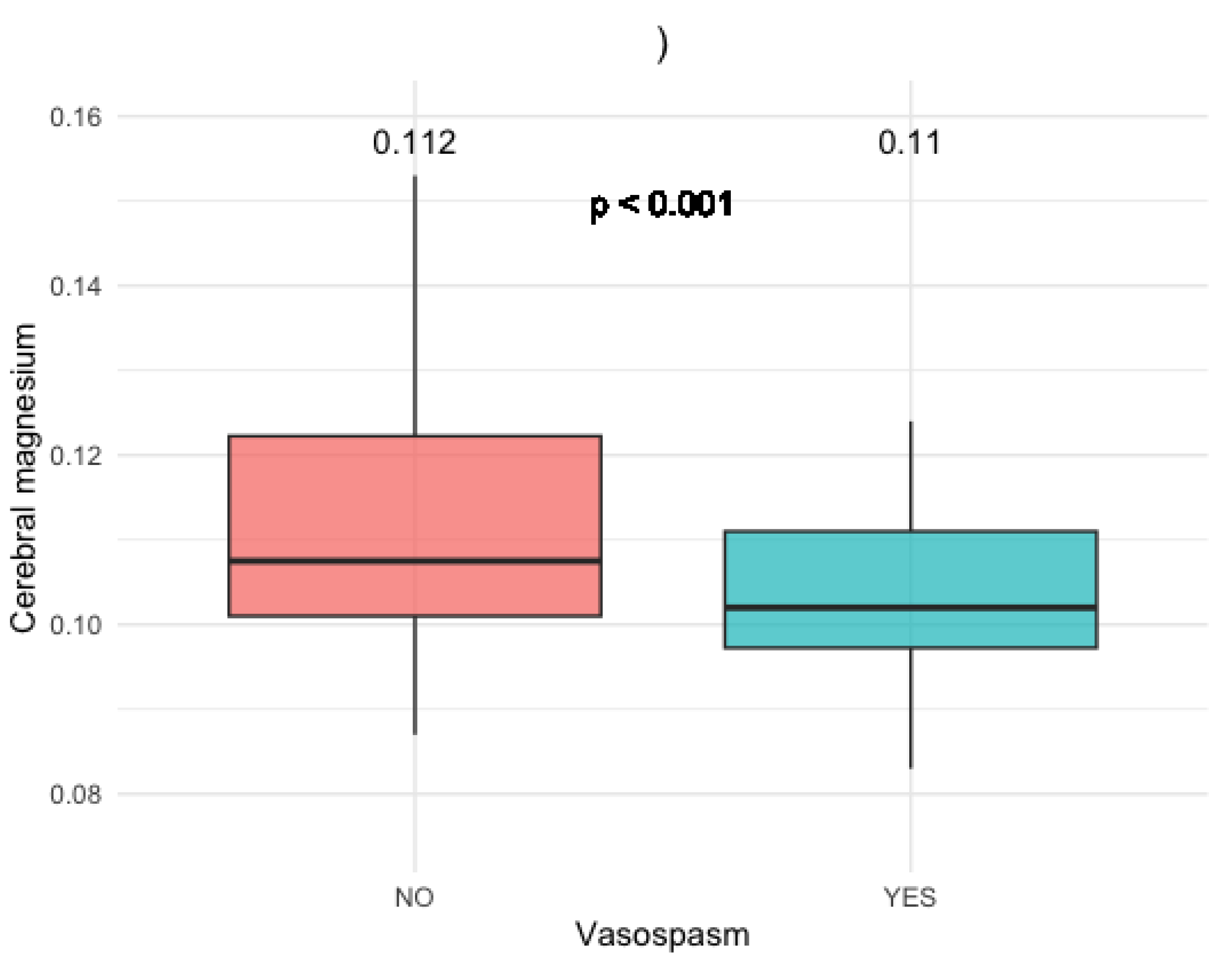
This was especially pronounced in patients with anterior communicating artery (AComm) and anterior cerebral artery (ACA) aneurysms (p < 0.05) (
Figure 3).
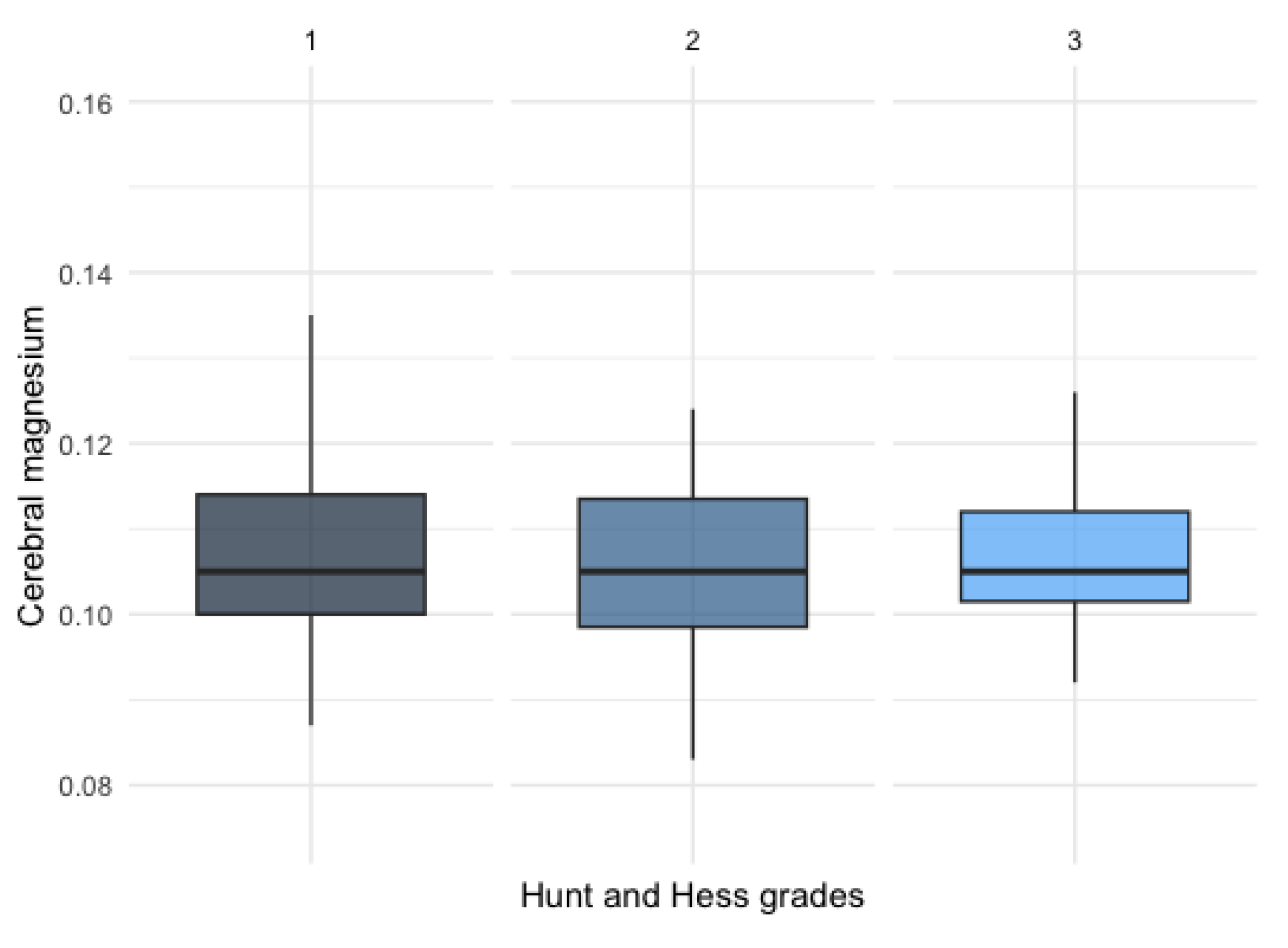
No significant difference was observed between patients with different Hunt and Hess clinical grades (
Figure 4).
There was no statistically significant difference detected among patients with varying Hunt and Hess clinical grades, as illustrated in
Figure 4.
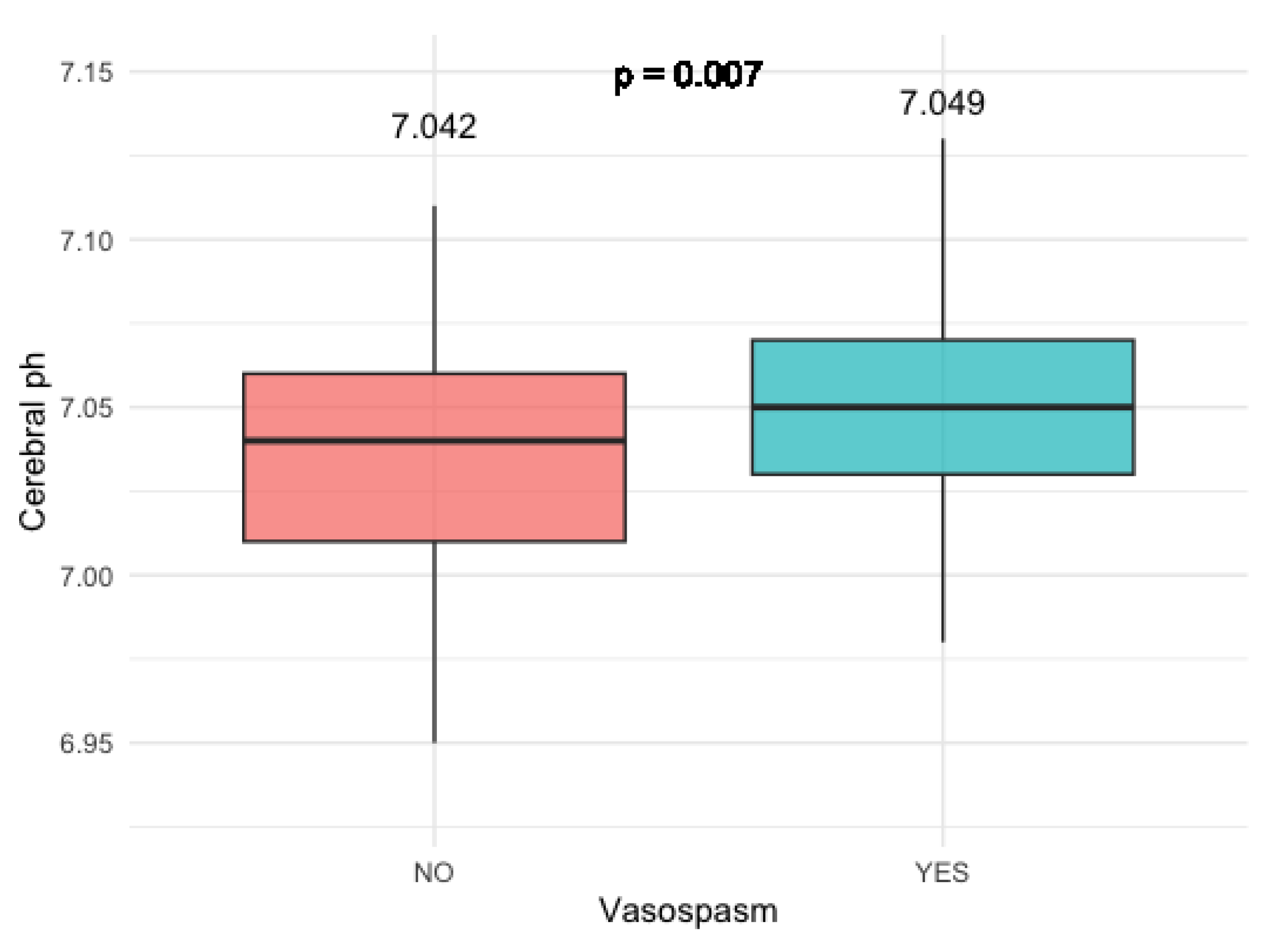
3.2.2. Cerebral pH levels
Patients who developed clinically relevant vasosopasm had significantly higher cerebral pH levels compared to the patients who did not develop vasosopasm (p = 0.007) (
Figure 5).
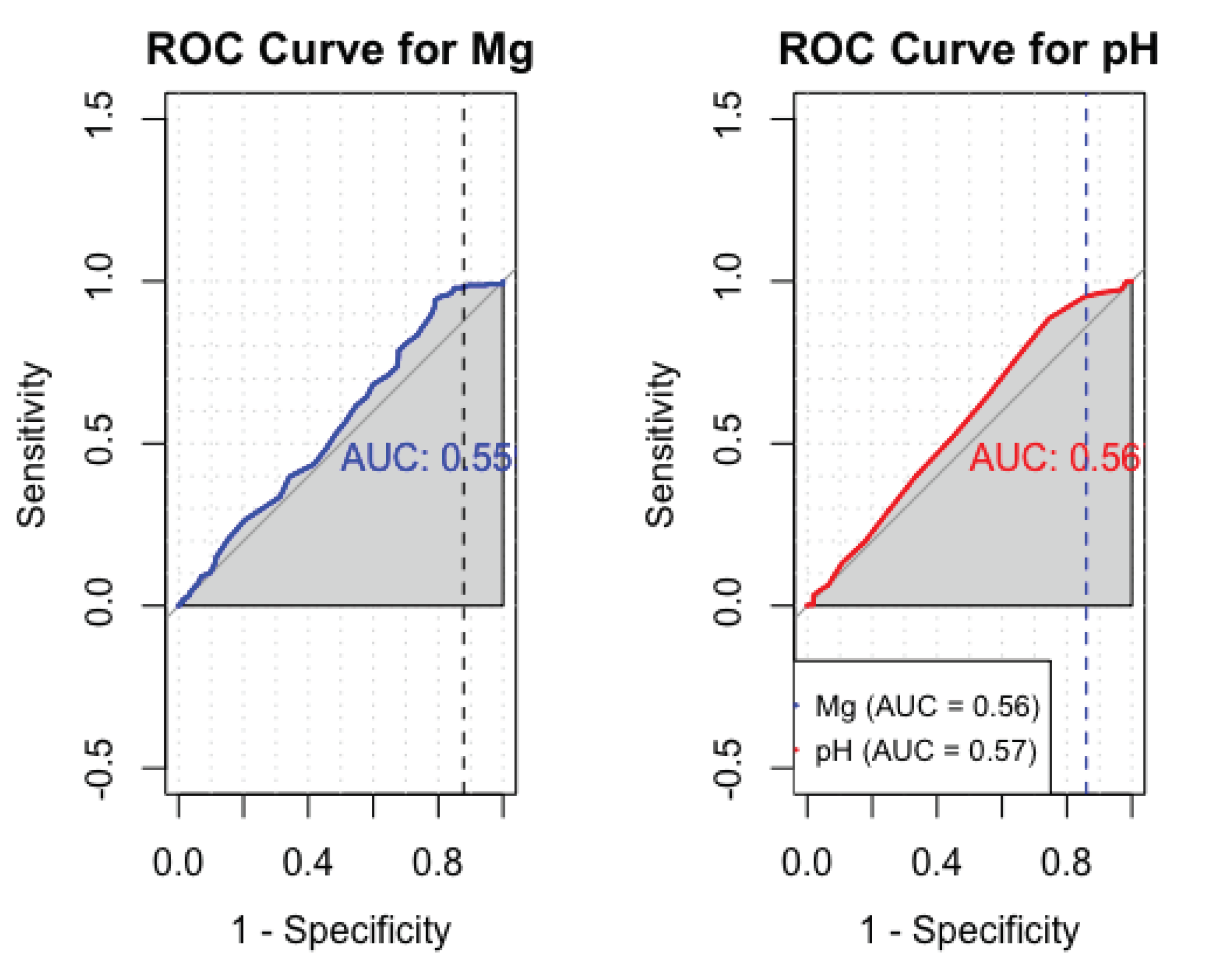
3.2.3. Sensitivity and specificity analysis of cerebral Mg and pH
The analysis for sensitivity and specificity derived an AUC value of 0.55 for Mg and 0.56 for pH (
Figure 6).
3.3. Results from the affected arterial territory and affected side
No significant difference could be derived in Mg or pH levels when comparing only voxels in the affected side or affected arterial territory. There was no significant difference in the results when comparing the two different treatments arms (coiling and clipping).
4. Discussion
Aneurysmal subarachnoidal hemorrhage stays one of the most deadly neurological diseases with high morbidity and mortality rates. For those who survive the initial subarachnoidal bleeding and seek medical attention, vasospasm emerges as a predominant concern, with its complications, particularly the onset of DCI, being a leading cause of adverse outcomes. In the quest to enhance neurocritical care, predicting which patients are at risk of developing clinically significant vasospasm and implementing timely preventive measures has become an imaportant goal.
Numerous studies have diligently explored various clinical tools to achieve this objective. Notably, Toi et al. employed transcranial Doppler ultrasound at different time points throughout the neurocritical care continuum following aSAH. Their findings revealed an elevation in mean flow velocity (MFV) within the horizontal portion of the middle cerebral artery (MCA) in patients who later manifested clinically relevant vasospasm, showcasing the potential of this modality for early detection [
37]. Building on this approach, Matamoros et al. expanded the scope to include bilateral anterior cerebral arteries (ACA) in their assessment. Their results echoed those of Toi et al., demonstrating increased blood flow in patients predisposed to significant vasospasm [
38].
The cumulative findings from these studies underscore the pivotal role of advanced imaging techniques and diagnostic tools in early identification of vasospasm risk following aneurysmal subarachnoid hemorrhage (aSAH). The pursuit of predictive strategies not only promises potential improvements in patient outcomes but also signifies a critical advancement in the ongoing evolution of neurocritical care. By proactively identifying and addressing the risk of vasospasm, these studies contribute to a more comprehensive and personalized approach to managing individuals grappling with the consequences of aSAH. This forward-looking perspective holds the potential to enhance both the understanding and the treatment of this complex neurological condition, ultimately paving the way for more effective and tailored interventions in the field of neurocritical care.
Souissi et al. used jugular bulb oximetry monitoring and found increased cerebral oxygen extractions (AVDO2) 12 hours before the significant vasospasm occured clinically [
39]. Similar results were achieved in a study by Heran et al. [
40]. Various clinical predictors can also be used in order to predict clinically relevant vasospasm. Csok et al. showed very good results using Hijdra sum score, early sonographic vasospasm, and a simplified binary level of consciousness score between admission and day 5 after the event[
41]. Various studies demonstrated hypomagnesemia at some timepoint following aSAH [
42,
43]. It is hypothesised that the low Mg levels are caused to the intracellular shift of Mg to the cells with a poor energy state and less ATP, like brain cells in aSAH [
44]. To our knowledge, this is the first study using intracellular Mg levels measured by 31P-MRS in assessment of clinically relevant vasospasm in patients with SAH. The results of this study showed the potential of 31P-MRS for indicating the presence of clinically relevant vasospasm in the first few days after aSAH, using Mg and pH. This was especially true for the aneurysms of the AComm and ACA. This correlation was neither associated with the various Hunt and Hess scores in clinical presentation, nor with various Fisher grades of the amount of extravasated blood on imaging. Even though the results of this study show some significant difference in cerebral Mg and pH in patients who developed clinically relevant vasospasm, the sensitivity and specificity analysis shows no statistical significance. We then undertook the analysis of the results using only voxels from the affected arterial territory and only voxels from the side of the bleeding. These results showed no significant difference between the two patient groups. This could be due to a number of reasons. We hypothesise that the main reason for this is the change of the microenviroment in these regions due to the oedema and blood products. Secondly, our study included only 13 patients who showed great heterogeneity in imaging features, ranging from different affected arteries, the amount of extravasated blood expressed by a Fisher scale, to the various clinical settings expressed by a different Hunt and Hess grades, and type of undertaken treatment. Also, it would be interesting to see the differences between various grades of vasospasm. As our patient cohort was not big enough for this analysis we omitted it from this publication. However, this issue will be addressed in one of our follow up studies. In our patient group there weren’t any differences in two treatment arms (clipping vs coiling). In the future studies we plan to test a vasospasmus grading system, like CTA vasospasm score developed by van der Harst et al. [
45], or grading on digital subtraction angiography developed by Merkel et al. [
46]. Also, the longitudinal analysis of the data would also of interest. As the vasospasmus peaks at around 4-15 day after bleed [
24], and our imaging occurred at the mean of 4 day after the bleeding event, this could also have influenced the results. The possibility of 31P-MRS to indicate the presence of clinically significant vasospasm could be hugely beneficial, as it provides a non-invasive approach. Further, the exact pathophysiological mechanism in the changes of pH and Mg after aSAH is not yet understood. To our knowledge, this is the first study using 31P-MRS nad measurements of intracellular Mg and pH in assessment of clinically relevant vasospasm in patients with aSAH. The results of this study showed the potential of 31P-MRS for indicating the presence of clinically relevant vasospasm in the first few days after aSAH, using Mg and pH. This was especially true for the aneurysms of the AComm and ACA. This correlation was neither associated with the various Hunt and Hess scores in clinical presentation, nor with various Fisher grades of the amount of extravasated blood on imaging. Even though the results of this study show some significant difference in cerebral Mg and pH in patients who developed clinically relevant vasospasm, the sensitivity and specificity analysis shows no statistical significance. We then undertook the analysis of the results using only voxels from the affected arterial territory and only voxels from the side of the bleeding. These results showed no significant difference between the two patient groups. This could be due to a number of reasons. First, our study included only 13 patients. Furthermore, these patients showed great heterogeneity in imaging features, ranging from different affected arteries, the amount of extravasated blood expressed by a Fisher scale, to the type of undertaken treatment. Also, it would be interesting to see the differences between various grades of vasospasm. As our patient cohort was not big enough for this analysis we omitted it from this publication. However, this issue will be addressed in one of our follow up studies. In our patient group there weren’t any differences in two treatment arms (clipping vs coiling). In the future studies we plan to test a vasospasmus grading system, like CTA vasospasm score developed by van der Harst et al. [
45], or grading on digital subtraction angiography developed by Merkel et al. [
46]. Also, the longitudinal analysis of the data would also of interest. As the intensity of vasospasmus peaks at around 4-15 day after bleed [
24], and our imaging occurred at the mean of 4 day after the bleeding event, this could also have influenced the results. The potential utility of 31P-MRS in indicating the presence of clinically significant vasospasm is highly promising, especially considering its non-invasive nature. This method could offer valuable insights into the physiological changes associated with vasospasm after subarachnoid hemorrhage. However, it’s crucial to acknowledge that the precise pathophysiological mechanisms underlying the alterations in pH and magnesium levels following subarachnoidal hemorrhage are not yet fully understood. The complex interplay of various factors in this context necessitates further exploration to unravel the intricate details of these changes. Moreover, it’s essential to recognize certain limitations in the present study. The size and positioning of the 31P-MRS voxels pose challenges, as it may not always be feasible to position a voxel exclusively within the affected arterial territory. The inherent size constraints could lead to partial volume effects, potentially contaminating some of the voxels and influencing the accuracy of the measurements. These considerations emphasize the need for continued refinement and optimization of imaging techniques to overcome such limitations and enhance the reliability of 31P-MRS as a diagnostic tool in the assessment of vasospasm after aSAH.
5. Conclusions
This pilot study has illuminated the potential of employing magnesium (Mg) and pH measurements through 31P-MRS in the context of aSAH. The findings reveal a discernible trend, indicating lower magnesium levels and higher pH in patients who subsequently developed clinically relevant vasospasm. However, it’s crucial to acknowledge that the study’s conclusions are constrained by a limited number of subjects, impacting statistical power.
Despite these limitations, the study suggests a promising option for further exploration. The measured Mg and pH levels could potentially serve as valuable additions to other diagnostic tests, contributing to a more personalized approach in anti-vasospasm therapy for patients after aSAH. To solidify these preliminary findings and establish their clinical relevance, it is imperative to conduct larger-scale prospective clinical studies. These studies should adhere to strictly defined imaging and clinical criteria to identify the most promising and feasible tests for predicting clinically relevant vasospasm and informing the treatment strategies for vasospasm and its associated complications. This iterative process will ultimately enhance our understanding and management of aSAH, potentially leading to improved outcomes and individualized care for affected patients.
Author Contributions
Conceptualization, M.G. and R.S.; methodology, M.G., S.A.T., W.M.H.; software, M.R.; validation, B.P., A.E.G., O.P., C.T., E.R.G.; formal analysis, M.G.; investigation, R.S., S.A.T.; resources, E.R.G.; data curation, M.G.; writing—original draft preparation, M.G.; writing—review and editing, M.G., R.S., S.M., V.L., J.D., L.G., M.O., L.L.; visualization, M.G.; supervision, E.R.G.; project administration, C.T., E.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Medical University of Innsbruck (AN2016-0032 359/4.8).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study or their legal guardian.
Data Availability Statement
The research data will be made available upon request at the corresponding author.
Acknowledgments
The authors would like to thank patients and their families for agreeing to participate in this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| aSAH |
aneurysmal subarachoidal hemorrhage |
| DCI |
delayed cerebral ischemia |
| CT |
computerised tomography |
| MRI |
magnetic resonance imaging |
| MISA |
Monitoring and Imaging of SAH and Aneurysms |
| 31P-MRS |
phosphorous magnetic resonance spectrocopy |
| CSF |
cerebrospinal fluid |
References
- Kassell, N.; Torner, J. The International Cooperative study on timing of aneurysm surgery. Acta neurochirurgica 1982, 63, 119–23. [Google Scholar] [CrossRef]
- Lantigua, H.; Ortega-Gutierrez, S.; Schmidt, J.M.; Lee, K.; Badjatia, N.; Agarwal, S.; Claassen, J.; Connolly, E.; Mayer, S. Subarachnoid hemorrhage: Who dies, and why? Critical care (London, England) 2015, 19, 309. [Google Scholar] [CrossRef]
- Rowland, M.; Hadjipavlou, G.; Kelly, M.; Westbrook, J.; Pattinson, K. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. British journal of anaesthesia 2012, 109, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Dodd, W.S.; Laurent, D.; Dumont, A.S.; Hasan, D.M.; Jabbour, P.M.; Starke, R.M.; Hosaka, K.; Polifka, A.J.; Hoh, B.L.; Chalouhi, N. Pathophysiology of delayed cerebral ischemia after subarachnoid hemorrhage: a review. Journal of the American Heart Association 2021, 10, e021845. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.; Claassen, J.; Schmidt, J.M.; Wartenberg, K.; Temes, R.; Connolly, E.; Mayer, S. Prediction of Symptomatic Vasospasm after Subarachnoid Hemorrhage: The Modified Fisher Scale. Neurosurgery 2006, 59, 21–7, discussion 21. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Claassen, J.; Schmidt, J.M.; Wartenberg, K.E.; Temes, R.; Connolly, E.S.; Macdonald, R.L.; Mayer, S.A. Prediction of symptomatic vasospasmafter subarachnoid hemorrhage: the modified fisher scale. Neurosurgery 2006, 59, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Treichl, S.A.; Ho, W.M.; Steiger, R.; Grams, A.E.; Rietzler, A.; Luger, M.; Gizewski, E.R.; Thomé, C.; Petr, O. Cerebral energy status and altered metabolism in early brain injury after aneurysmal Subarachnoid hemorrhage: A prospective 31P-MRS pilot study. Frontiers in Neurology 2022, 319. [Google Scholar] [CrossRef] [PubMed]
- Marinov, M.B.; Harbaugh, K.S.; Hoopes, P.J.; Pikus, H.J.; Harbaugh, R.E. Neuroprotective effects of preischemia intraarterial magnesium sulfate in reversible focal cerebral ischemia. Journal of neurosurgery 1996, 85, 117–124. [Google Scholar] [CrossRef]
- van den Bergh, W.M.; Zuur, J.K.; Kamerling, N.A.; van Asseldonk, J.T.H.; Rinkel, G.J.; Tulleken, C.A.; Nicolay, K. Role of magnesium in the reduction of ischemic depolarization and lesion volume after experimental subarachnoid hemorrhage. Journal of neurosurgery 2002, 97, 416–422. [Google Scholar] [CrossRef]
- Ram, Z.; Sadeh, M.; Shacked, I.; Sahar, A.; Hadani, M. Magnesium sulfate reverses experimental delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Stroke 1991, 22, 922–927. [Google Scholar] [CrossRef]
- Bergh, W.; Algra, A.; Kooten, F.; Dirven, C.; van Gijn, J.; Vermeulen, M.; Rinkel, G. Magnesium sulfate in aneurysmal subarachnoid hemorrhage. Stroke; a journal of cerebral circulation 2005, 36, 1011–5. [Google Scholar] [CrossRef]
- Soliman, R.; Zohry, G. Effect of magnesium sulphate and milrinone on cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a randomized study. Brazilian Journal of Anesthesiology (English Edition) 2018, 69. [Google Scholar] [CrossRef]
- Chen, T.; Carter, B. Role of magnesium sulfate in aneurysmal subarachnoid hemorrhage management: A meta-analysis of controlled clinical trials. Asian journal of neurosurgery 2011, 6, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Sheen, S.; Hwang, G.; Kang, S.H.; Heo, D.; Cho, Y.J. Intravenous Magnesium Infusion for the Prevention of Symptomatic Cerebral Vasospasm after Aneurysmal Subarachnoid Hemorrhage. Journal of Korean Neurosurgical Society 2012, 52, 75–9. [Google Scholar] [CrossRef]
- Mori, K.; Yamamoto, T.; Miyazaki, M.; Hara, Y.; Aiko, Y.; Koike, N.; Sakamoto, S.; Nakao, Y.; Esaki, T. Effect of intrathecal magnesium sulfate solution injection via a microcatheter in the cisterna magna on cerebral vasospasm in the canine subarachnoid haemorrhage model. British journal of neurosurgery 2011, 26, 64–8. [Google Scholar] [CrossRef]
- Grossen, A.; Ernst, G.; Bauer, A. Update on intrathecal management of cerebral vasospasm: a systematic review and meta-analysis. Neurosurgical Focus 2022, 52, E10. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Lobanova, I.; Huang, W.; Ishfaq, M.F.; Broderick, J.P.; Cassarly, C.N.; Martin, R.H.; Macdonald, R.L.; Suarez, J.I. Lessons learned from phase II and phase III trials investigating therapeutic agents for cerebral ischemia associated with aneurysmal subarachnoid hemorrhage. Neurocritical care 2022, 36, 662–681. [Google Scholar] [CrossRef]
- Van den Bergh, W.; Albrecht, K.; Berkelbach Van Der Sprenkel, J.; Rinkel, G. Magnesium therapy after aneurysmal subarachnoid haemorrhage a dose-finding study for long term treatment. Acta neurochirurgica 2003, 145, 195–199. [Google Scholar] [CrossRef]
- Mees, S.M.D.; Algra, A.; Vandertop, W.P.; van Kooten, F.; Kuijsten, H.A.; Boiten, J.; van Oostenbrugge, R.J.; Salman, R.A.S.; Lavados, P.M.; Rinkel, G.J.; others. Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): a randomised placebo-controlled trial. The Lancet 2012, 380, 44–49. [Google Scholar] [CrossRef]
- Leijenaar, J.F.; Mees, S.M.D.; Algra, A.; Van Den Bergh, W.M.; Rinkel, G.J. Effect of magnesium treatment and glucose levels on delayed cerebral ischemia in patients with subarachnoid hemorrhage: a substudy of the Magnesium in Aneurysmal Subarachnoid Haemorrhage trial (MASH-II). International Journal of Stroke 2015, 10, 108–112. [Google Scholar] [CrossRef]
- Yoon, S.; Zuccarello, M.; Rapoport, R.M. pCO2 and pH regulation of cerebral blood flow. Frontiers in physiology 2012, 3, 365. [Google Scholar] [CrossRef]
- Charbel, F.T.; Du, X.; Hoffman, W.E.; Ausman, J.I. Brain tissue PO2, PCO2, and pH during cerebral vasospasm. Surgical neurology 2000, 54, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Shiba, M.; Nakatsuka, Y.; Nakano, F.; Nishikawa, H. Higher Cerebrospinal Fluid pH may Contribute to the Development of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. Translational Stroke Research 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mascia, L.; Del Sorbo, L. Diagnosis and management of vasospasm. F1000 medicine reports 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Grams, A.E.; Mangesius, S.; Steiger, R.; Radovic, I.; Rietzler, A.; Walchhofer, L.M.; Galijašević, M.; Mangesius, J.; Nowosielski, M.; Freyschlag, C.F.; others. Changes in brain energy and membrane metabolism in glioblastoma following chemoradiation. Current Oncology 2021, 28, 5041–5053. [Google Scholar] [CrossRef] [PubMed]
- Hattingen, E.; Magerkurth, J.; Pilatus, U.; Mozer, A.; Seifried, C.; Steinmetz, H.; Zanella, F.; Hilker, R. Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain 2009, 132, 3285–3297. [Google Scholar] [CrossRef]
- Wenger, K.J.; Hattingen, E.; Franz, K.; Steinbach, J.P.; Bähr, O.; Pilatus, U. Intracellular pH measured by 31P-MR-spectroscopy might predict site of progression in recurrent glioblastoma under antiangiogenic therapy. Journal of Magnetic Resonance Imaging 2017, 46, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Hattingen, E.; Jurcoane, A.; Bähr, O.; Rieger, J.; Magerkurth, J.; Anti, S.; Steinbach, J.P.; Pilatus, U. Bevacizumab impairs oxidative energy metabolism and shows antitumoral effects in recurrent glioblastomas: a 31P/1H MRSI and quantitative magnetic resonance imaging study. Neuro-oncology 2011, 13, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Walchhofer, L.M.; Steiger, R.; Rietzler, A.; Kerschbaumer, J.; Freyschlag, C.F.; Stockhammer, G.; Gizewski, E.R.; Grams, A.E. Phosphorous magnetic resonance spectroscopy to detect regional differences of energy and membrane metabolism in naïve glioblastoma multiforme. Cancers 2021, 13, 2598. [Google Scholar] [CrossRef]
- Hattingen, E.; Lanfermann, H.; Menon, S.; Neumann-Haefelin, T.; DuMesnil de Rochement, R.; Stamelou, M.; Höglinger, G.; Magerkurth, J.; Pilatus, U. Combined 1 H and 31 P MR spectroscopic imaging: impaired energy metabolism in severe carotid stenosis and changes upon treatment. Magnetic Resonance Materials in Physics, Biology and Medicine 2009, 22, 43–52. [Google Scholar] [CrossRef]
- Hattingen, E.; Magerkurth, J.; Pilatus, U.; Hübers, A.; Wahl, M.; Ziemann, U. Combined 1H and 31P spectroscopy provides new insights into the pathobiochemistry of brain damage in multiple sclerosis. NMR in biomedicine 2011, 24, 536–546. [Google Scholar] [CrossRef]
- Wenger, K.J.; Hattingen, E.; Franz, K.; Steinbach, J.; Bähr, O.; Pilatus, U. In vivo metabolic profiles as determined by 31 P and short TE 1 H MR-spectroscopy: no difference between patients with IDH wildtype and IDH mutant gliomas. Clinical Neuroradiology 2019, 29, 27–36. [Google Scholar] [CrossRef]
- Galijašević, M.; Steiger, R.; Radović, I.; Birkl-Toeglhofer, A.M.; Birkl, C.; Deeg, L.; Mangesius, S.; Rietzler, A.; Regodić, M.; Stockhammer, G.; et al. Phosphorous magnetic resonance spectroscopy and molecular markers in IDH1 wild type glioblastoma. Cancers 2021, 13, 3569. [Google Scholar] [CrossRef] [PubMed]
- Kreis, R. Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR in Biomedicine 2004, 17, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Iotti, S.; Frassineti, C.; Alderighi, L.; Sabatini, A.; Vacca, A.; Barbiroli, B. In vivo assessment of free magnesium concentration in human brain by 31P MRS. A new calibration curve based on a mathematical algorithm. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In Vivo 1996, 9, 24–32. [Google Scholar] [CrossRef]
- Galijašević, M.; Steiger, R.; Regodić, M.; Waibel, M.; Sommer, P.J.D.; Grams, A.E.; Singewald, N.; Gizewski, E.R. Brain Energy Metabolism in Two States of Mind Measured by Phosphorous Magnetic Resonance Spectroscopy. Frontiers in Human Neuroscience 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Toi, H.; Matsumoto, N.; Yokosuka, K.; Matsubara, S.; Hirano, K.; Uno, M. Prediction of cerebral vasospasm using early stage transcranial Doppler. Neurol Med Chir (Tokyo) 2013, 53, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, C.E.; Samaniego, E.; Sam, K.; Roa, J.; Perez-Nellar, J.; Rodríguez, D. Prediction of Symptomatic Vasospasm in Patients with Aneurysmal Subarachnoid Hemorrhage Using Early Transcranial Doppler. Journal of vascular and interventional neurology 2020, 11, 19–26. [Google Scholar]
- Souissi, R.; Boubakker, A.; Souissi, H.; Abdelrazek, A.; Badri, M.; Bziouech, S.; Jemel, H. Prediction of cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage using jugular bulb oximetry monitoring: preliminary results. Critical Care 2010, 14, 1–1. [Google Scholar] [CrossRef]
- Heran, N.S.; Hentschel, S.J.; Toyota, B.D. Jugular bulb oximetry for prediction of vasospasm following subarachnoid hemorrhage. Canadian journal of neurological sciences 2004, 31, 80–86. [Google Scholar] [CrossRef]
- Csók, I.; Grauvogel, J.; Scheiwe, C.; Bardutzky, J.; Wehrum, T.; Beck, J.; Reinacher, P.C.; Roelz, R. Basic Surveillance Parameters Improve the Prediction of Delayed Cerebral Infarction After Aneurysmal Subarachnoid Hemorrhage. Frontiers in Neurology 2022, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.; Muizelaar, J.; Choi, S. Magnesium’s role in cerebral vasospasm and outcome in patients with aneurysmal subarachnoid haemorrhage. Cerebral Vasospasm 1993, 401–404. [Google Scholar]
- van den Bergh, W.M.; Algra, A.; van der Sprenkel, J.W.; Tulleken, C.A.; Rinkey, G.J. Hypomagnesemia after aneurysmal subarachnoid hemorrhage. Journal of Neurosurgical Anesthesiology 2003, 15, 291–292. [Google Scholar] [CrossRef]
- van den Bergh, W.M. Magnesium in subarachnoid haemorrhage. Magnesium in the Central Nervous System [Internet] 2011. [Google Scholar]
- Harst, J.; Luijckx, G.J.; Elting, J.; Lammers, T.; Bokkers, R.; Bergh, W.; Eshghi, O.; Metzemaekers, J.; Groen, R.; Mazuri, A.; Veeger, N.; van Dijk, J.M.C.; Uyttenboogaart, M. The Predictive Value of the CTA Vasospasm Score on Delayed Cerebral Ischemia and Functional Outcome after aneurysmal Subarachnoid Hemorrhage. European journal of neurology 2021, 29. [Google Scholar] [CrossRef]
- Merkel, H.; Lindner, D.; Gaber, K.; Ziganshyna, S.; Jentzsch, J.; Engelmann, S.; Gerhards, T.; Sari, S.; Stock, A.; Vothel, F.; Falter, L.; Quäschling, U.; Hoffmann, K.T.; Meixensberger, J.; Halama, D.; Richter, C. Standardized Classification of Cerebral Vasospasm after Subarachnoid Hemorrhage by Digital Subtraction Angiography. Journal of Clinical Medicine 2022, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).