1. Introduction
Between the major unmet medical needs that currently represent serious public health threats, Methicillin-resistant Staphylococcus aureus (MRSA) infections (that are associated with high mortality rates) and particularly medical device-associated infections (MDIs), are of major concern [
1,
2,
3,
4]. MDIs are caused by patient’s microbiota flora having biofilm-forming-ability.
Staphylococcus epidermidis and
S. aureus bacteria are the most common aetiologic pathogens for such infection [
5,
6].
Biofilm formation initiates by attachment of bacteria and proceeds by their proliferation and maturation in a matrix consisted of extracellular polymeric substances; this biofilm/matrix finally functions as a barrier to antibiotics and to host’s defence mechanisms [
6]. Biofilm bacteria develop resistance due to modification of their growth rate and other physiological functions and additionally because of the slow penetration of antimicrobials, Furthermore, biofilm matrices also block neutrophil attacks [
7]. In addition to the biofilm aggregate-caused tolerance to antibiotics most clinical strains of staphylococci are multi-resistant [
7,
8].
Daptomycin (Dapto) is an acidic cyclic lipopeptide antibiotic that, in the presence of calcium, forms oligomeric pores on membranes containing phosphatidylglycerol. It is clinically used against various Gram-positive bacteria such as
Staphylococcus aureus and
Enterococcus species, and in addition to its potent antimicrobial activity [
9], Dapto is known due to the unlikely development of daptomycin-resistant pathogens which is attributed to its unique mechanism of action [
9,
10]. The Infectious Disease Society of America proposes Dapto for therapy of enterococcal and staphylococcal infections of prosthetic joints [
11]. Another advantage of Dapto concerning the treatment of MDIs is its known high anti-biofilm activity [
12,
13,
14]. In one case it has been reported that Dapto rapidly penetrates a Staphylococcus epidermidis biofilm [
15]. It was also recently demonstrated in our laboratories that various Staphylococci strains showed lower MIC to Dapto, under biofilm-forming conditions, suggesting that Dapto is active in embedded cells [
16]. Nevertheless, development of some Dapto-insusceptible MRSA isolates have been reported [
17,
18], raising serious concerns and indicating the importance of developing novel strategies for antimicrobial therapeutics.
One such strategy currently being explored is the delivery of antibiotics or antimicrobials in general, via nanomedicines [
19,
20,
21]. For example, it has been reported that Dapto encapsulated in polymer-consisted nanoparticles demonstrated higher activity towards established Staphylococci biofilms, compared to free drug [
19].
In this context we investigate herein the development and optimization of liposomal Dapto and evaluated the antimicrobial activity of optimal Dapto-liposome types (compared to free drug) against four Staphylococci strains. The strains were selected between 20 clinical isolates that were previously characterized and also studied for their susceptibility towards Dapto; two strains that were found less susceptible towards Dapto and two that were more Dapto-susceptible, the three being additionally methicillin-resistant, were selected to be used in the current study [
16].
As nanomedicine type, liposomes were selected due to the numerous advantages that they possess as nanomedicines, giving their high biocompatibility, ability to be loaded with high amounts of any kind of drug (MW, solubility etc), versatility, etc., as described elsewhere [
22].
2. Materials and Methods
1,2-distearoyl-sn-glycerol-3-phosphatidylcholine (PC) and 1,2-distearoyl-sn-glycero -3-phospho-(19-rac-glycerol) (sodium salt) (PG), and 1,2-Distearoyl-sn-glycerol -3-phosphatidyl-ethanolamine-N-[methoxy (polyethylene-glycol)-2000] (PEG), were purchased from Lipoid, Germany. Cholesterol (Chol) was purchased from Sigma-Aldrich (Darmstadt, Germany). Dapto was purchased from Tocris Bioscience. All solvents and chromatography materilas used were of analytical or HPLC grade and purchased from Merck (Darmstadt, Germany). All other materials, such as salts used for buffer preparation, reagents for lipid concentration determination, etc., were of analytical grade and were purchased from Sigma–Aldrich (Darmstadt, Germany). Spectrapor® dialysis membrane with an MWCO of 12–14 kDa was from Serva, Germany. Materials used for antimicrobial activity evaluation are mentioned in the sections of the specific methods applied (below).
2.1. Preparation of Daptomycin-Loaded Liposomes
The lipid compositions used for liposome preparation where: PC/Chol (1:1 mole/mole) and PC/PG/Chol (8:2:10 mole/mole/mole). After preparation all liposome types were purified from non-encapsulated drug by size exclusion chromatography on Sepharose 4B-CL column (1 × 30), eluted with PBS, pH 7.40
2.1.1. Thin Film Method (TFM)
Appropriate amounts of lipid solutions (according to the lipid composition) in CH3Cl/MeOH (2/1 v/v) for a final lipid concentration of 10 mg/mL were placed in a 100 mL round-bottomed flask. Organic solvents were evaporated under vacuum at 41°C using a rotary evaporator until a thin lipid film was formed, and flasks were placed under N2 stream for 5 min to remove any residual traces of organic solvents. The dried lipid film was hydrated with a 250 ppm Dapto solution in PBS (1 mL) at 41°C and the dispersion vortexed and sonicated to form multilamellar vesicles (MLVs). Subsequent size reductions were carried out by sequential extrusion of the MLVs (10 times) through polycarbonate filters with pore diameter 0.4 µm and then 0.1 µm, fitted in a syringe-type extruder (Lipo-so-fast, Avestin, Ottawa, ON, Canada), to produce SUVs. After purification (as mentioned above), liposome dispersions were concentrated by ultrafiltration (MW cutoff 10,000 daltons) to bring to the required concentration. Samples were then stored at 4°C until further use.
2.1.2. DRV Method
For dehydration–rehydration vesicle (DRV) preparation, empty small unilamellar vesicles (SUV) were initially prepared as described in detail before [
23,
24]. Multilamellar vesicles (MLV) were prepared as described above (2.1.1), with the difference that the dried lipid film was hydrated using 1 mL solution of 10% PBS at 41°C. MLVs were then converted into SUVs using a microtip-probe sonicator (Vibra cell, Sonics and Materials, Suffolk, UK) at 26% amp for 10 min until we obtain a partial transparent solution. The next steps of the procedure are similar to the previous (thin film hydration), including sonication, annealing (1 h at 40 °C) and centrifugation at 15,000× g for 20 min in order to obtain liposome suspensions (SUV), free of titanium were left to stand for at least 1 h at 40°C, in order to anneal any structural defects. Any Ti-fragment and/lipid aggregate contaminants were removed from SUV suspensions by centrifugation at 15,000× g for 20 min. Then, 1 mL of the SUV suspension at a lipid concentration of 10 mg/ml was mixed with 1 mL of a 250 μg/ml Dapto solution (in distilled H
2O). The mixtures were then freezed at -80°C for 3 h and then dried under vacuum (below 5 Pa). The powder was then re-suspended initially in 100µL of dH
2O and incubated at RT for 30 min, which was repeated one more time and then finally 800µL of PBS were added and the vesicle dispersion was incubated at RT for 1 h. Subsequent size reductions were carried out as described above; extrusion was used as a size-reduction method in order to prevent disruption of the DRVs and leakage of the encapsulated drug. After extrusion, liposomes were purified (as above) from non-encapsulated Dapto.
2.1.3. MicroFluidics Mixing (MM)
Liposomes were prepared using the automated Nanoassemblr platform (Precision Nanosystems) with 2 input NxGen Cartridges. The lipid compositions tested was PC/Chol (1:1 mole/mole) starting with 20 mg/mL lipid in ethanol, 150ug/ml Dapto (in PBS). Two variables can be modulated in the MM apparatus: the total flow rate (TFR) and flow rate ratio (FRR) which is defined as the volumetric ratio of the aqueous phase stream (PBS-drug) to the organic phase stream (Ethanol-lipids). The flow rates of both solutions are controlled through the software. Initially the TFR was set at 12ml/min, and FRR was 5:1. For optimization studies, three Dapto concentrations were used ranging from 150, 250 and 500μg/ml. Also, FRR values tested were 1:1, 2.5:1 or 5:1, and TFR values of 4, 8 and 12mL/min were applied. In a second round of runs TFR values between 0.7–6 mL/min, were tested.
For removal of any solvent residuals, two rounds of ultrafiltration were performed using Molecular weight cut-off of 10,000 MWCO tubes. Liposomes were purified to remove non-encapsulated Dapto, as described above.
2.2. Physicochemical Characterization of Dapto-Liposomes
2.2.1. Dapto Encapsulation Efficiency
Dapto concentration in liposomes was quantified by isocratic high-performance liquid chromatography (HPLC) using a Shimadzu 20A5 Gradient HPLC system coupled to aSPD-20A Prominence UV/VIS detector operating at 223 nm. A RP-18e (100 A, 5μm, 125 x 4 mm; LiChrosphere®) was used; the mobile phase was a mixture of acidified water (0.1% trifluoroacetic acid) and acetonitrile at 60:40 v/v. The column was eluted at a flow rate of 1 mL/min at 30 ◦C, and Dapto was eluted at 5.01 min. Sample injection volume was 50 µL. Liposomes were analyzed after being totally lysed in methanol (one volume of sample was mixed with 10 volumes of methanol and the mixture was agitated by vortex). The final lipid concentration of analyzed samples was adjusted to be 0.3 mg/ml. A calibration curve in the range of 0.5–40 µg/mL was constructed by preparation of standard solutions of Dapto in media with similar composition as the samples (in MeOH and in the presence of 0.3 mg/ml lipid of composition PC/Chol 1:1 mole/mole or PC/PG/Chol 8:2:5 mole/mole/mole). Encapsulation efficiency (equivalent to drug loading capacity) was calculated using the following equation:
where D is drug concentration and L is lipid concentration; initial means before and final after, purification. Liposome lipid concentration was measured routinely by the Stewart assay [
25], a colorimetric method used for the quantification of phospholipids.
2.2.2. Liposome Size Distribution, Zeta-Potential and Morphology
The particle size distribution (mean hydrodynamic diameter and polydispersity index (PDI)) of Dapto loaded liposomes dispersed at 0.4 mg/mL lipid, in phosphate-buffered saline (10 mM) with pH 7.40, was measured by dynamic light scattering (DLS) (Malvern Nano-Zs, Malvern Instruments, Malvern, Worcestershire, UK) at 25 °C and a 173 ◦ angle [
24]. Each sample was measured 11 times in three independent measurements. The polydispersity index (PDI) was used as a measure of homogeneity of liposomal dispersions. Dispersions having a PDI of less than 0.200 or 0.250 are generally considered to have narrow size distribution. Zeta potential was measured in the same dispersions, at 25 ◦C, utilizing the Doppler electrophoresis technique, as recently reported [
24].
Liposome morphology was assessed by Transmission Electron Microscopy (TEM). For this liposomes (0.5–1 mg/ml) were re-suspended in 10 mM HEPES (to eliminate potential artifacts from phosphate salts). Then, samples were negatively stained with 1% phosphotungstic acid in dH2O (freshly prepared), washed 3 times with dH2O, drained with the tip of a tissue paper, and observed at 100,000 eV with JEM-2100 (Jeol, Tokyo, Japan) transmission electron microscope.
2.2.3. Drug Release and Stability Studies
A dialysis membrane method was used to follow the release kinetics of Dapto from the different liposome types. The experiment was performed at two different conditions; by keeping the lipid concentration constant or by keeping the drug concentration constant. Briefly, in the first case, 0.5 mL of Dapto-liposomes with 3.6 mg/mL lipid, were placed in a dialysis bag, while in the second case 0.5 ml of Dapto-liposomes with 12 μg/ml Dapto were used. Bags were immersed in 15 mL vials containing 15 mL PBS (pH 7.40) that were screw capped and placed in a shaking (50 rpm) incubator at 37°C for up to 240 h. At predetermined time intervals, the entire medium was withdrawn and replaced with fresh; samples were assayed by HPLC for Dapto concentration. A calibration curve in the range of 0.00625–0.2 µg/mL was constructed by preparation of standard solutions of Dapto in media with similar composition as the samples.
The physical stability of Dapto-loaded liposomes during storage at 4 °C for up to 30 d was determined by measuring the vesicle mean diameter and PDI, of the two different Dapto-liposome types as described above, immediately after their preparation as well as after 15d and 30d.
It is known that Dapto is degraded in solution when stored at temperatures higher than 2-8°C [
26]. Dapto chemical stability studies were carried out in order to evaluate if liposome encapsulation preserves Dapto from degradation. Free Dapto (solution in PBS) and Dapto-liposomes containing 22 ± 2.1 μg/ml drug, were placed separately in hermetically sealed screw-tubes (to avoid evaporation) and then in an orbital incubator (Stuart S1500, UK) set at 50 rpm and 37°C for a period of up to 16d. A second batch of samples were stored at 4°C. At specific time intervals, samples were analyzed by HPLC, for quantification of intact Dapto, with the method mentioned above.
2.3. Antimicrobial Activity (In Vitro)
2.3.1. Bacterial Strains and Growth Conditions
Two well characterized MRSA strains of
S. aureus (71406 [MIC, 1 µg/ml] and 71221 [MIC, 0.38 µg/ml), and two strains of
S. epidermidis (9817 [MIC 0.75 µg/ml] which is also methicillin resistant, and 783 [MIC 0.19 µg/ml]) were used for antimicrobial studies, selected as mentioned above [
16]. All strains were grown aerobically in Tryptic soy broth (TSB, Oxoid CM0129, Oxoid Ltd., Basingstoke, UK) and on Tryptic soy agar plates (TSA, Oxoid) at 37°C overnight. CaCl
2-supplemented (1.25mM) Dapto solutions were used in all antimicrobial activity studies since Dapto acts by calcium-dependent potassium efflux from bacterial cell membranes [
12,
13].
The zeta-potential of the bacterial strains used in this study was measured by DLS, as previously described [
27,
28]. In brief, bacterial suspensions equivalent to the 0.5 MacFarland turbidity standard (~1.5 x 10
8 cfu/mL) were prepared in TSB broth. The bacterial suspensions were centrifuged at 10,000 rpm for 20 min and the supernatants were discarded. Then cell pellets were washed five times with 0.5mM potassium phosphate buffer solution (pH 7.4). Cell pellets were finally re-suspended in 1 ml of buffer and zeta potential measurements were performed.
2.3.2. Bacterial Growth Curve Assay
Bacterial Growth was spectrophotometrically monitored [
24] both in the presence and absence of Dapto liposomes, empty liposomes, mixtures of free Dapto + empty liposomes or free Dapto, at two concentrations 0.5 and 1 μg/ml. Briefly, overnight grown bacterial cells from a TSA agar plate were allowed to grow in fresh TSB broth (without glucose) to their early exponential phase. The broth containing bacteria was inoculated into 96-well flat-bottomed polystyrene plates with initial absorbance at λmax 570 nm ~0.01. The change in absorbance of each well was monitored each hour for a total of 24 h by a Fluostar (BMG LABTECH) microplate reader. A culture of the same strain without antibiotics was used as control. Experiments were performed in triplicate.
2.3.3. Biofilm Susceptibility Assays (Prevention and Treatment)
The antibiofilm activity was evaluated at 0.1 and 0.5 μg/ml drug concentrations for all the formulations, according to previously reported methods [
16,
24]. Crystal violet (CV) staining assay and a validated MTT [3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide, a yellow tetrazole] cell viability assay were used to assess biofilm susceptibility towards Dapto formulations. Briefly, one single bacteria colony isolated from fresh agar plates was inoculated into a tube filled with 5 mL sterile TSB and incubated at 37°C for 24 h. Fresh bacterial suspensions were prepared in TSB with 1% glucose from overnight cultures and adjusted to 0.5 MacFarland turbidity standard, followed by 1:10 dilution into fresh media. Then, 200µL of the suspension was added to 96 well sterile polystyrene plates and incubated at 37°C for 24 h.
For biofilm prevention studies, antimicrobial agents were added together with bacteria.
For biofilm reduction studies antimicrobial agents (Dapto formulations) were added in mature, preformed biofilms. For the later studies, following overnight incubation of bacteria in the plates, the plates were gently washed with 1x PBS (pH 7.4) to remove planktonic cells, and the well-formed biofilm was incubated with the Dapto formulations at 37°C for 24 h.
In both (prevention and treatment study) cases, following incubations the bacterial suspension of each well was gently spent, washed three times with PBS (pH 7.4) and stained with 195 µL of 0.1% Crystal Violet (Sigma-Aldrich, St. Louis, MO, USA) for 15 min at RT. Excess crystal violet was removed by washing with tap water, and biofilm was quantified by measuring the corresponding OD-570 nm of the supernatant following the solubilization of CV in 95% ethanol. For each sample (free or liposomal) tested, biofilm assays were performed in triplicate, and the mean biofilm absorbance value was determined.
In the MTT assay, biofilms were incubated with MTT (0.5 mg/mL) at 37°C for 1 h. After washing, the purple formazan crystals that formed inside the bacterial cells were dissolved by acidified isopropanol and then measured using a microplate reader by setting the detecting and reference wavelengths at 570 nm and 630 nm, respectively.
2.4. Statistical Analysis
IBM SPSS statistics pack was used for the statistical analysis of the results. All experiments were performed in triplicate. All data are presented as the mean ± standard deviation of the mean of independent experiments. Statistical significance was evaluated by one-way ANOVA or two-way ANOVA and LSD’s post hoc test with a significance level of p < 0.05.
3. Results
3.1. Dapto Liposome Physicochemical Properties
Dapto liposomes were prepared by three different methods. As seen in
Table 1 and
Table 2, the Thin Film hydration (TFH) and DRV methods resulted in similar encapsulation of Dapto (around 30%), for both lipid compositions tested. However, Dapto encapsulation was dramatically lower (around 4%) when liposomes were prepared by microfluidic mixing (MM) method, for both lipid compositions evaluated. Concerning Dapto liposome sizes, the vesicles prepared by TFH and DRV methods had mean diameters around 100nm (102 ± 12 nm) which is logical since they were extruded through 100nm pore membranes, while the liposomes prepared by MM had similar size in the case of PC/Chol composition, but smaller size when PG was included in the liposome membrane (
Table 2).
The zeta potential values of Dapto-liposomes composed of PC/Chol were slightly negative ranging between -3.7 to -8.8 (
Table 1). Liposome types that encapsulated higher amounts of Dapto (TFH and DRV) had higher negative zeta-potential values, compared to the MM liposomes, that encapsulated approx. 8 times lower amount of Dapto. The latter observation is logical since Dapto as an anionic lipopeptide antibiotic bears a negative charge at pH 7.4. When 20 mol% of PC is replaced with negative charged PG lipid (
Table 2), the charge of the lipid determines the liposome zeta potential which is around -20mV for all the liposome types (TFH, DRV, MM), irrespective of the amount of Dapto they incorporate.
As seen from the results of
Table 3, when PEG is included in the lipid composition of liposomes the encapsulation of Dapto is dramatically reduced. Such decreased drug loading into PEG liposomes has been reported before. In one case for vinorelbine loading, Chol-polyethylene glycol was used instead of PEG for preparation of polyethylene glycol coated liposomes with high drug loading [
29]. In our laboratory we have also observed similar reduction in drug loading into PEGylated liposomes especially when using the DRV method, for moxifloxacin and relaxin peptide [
24,
30], and found that post-PEGylation method could be applied, for preparation of PEGylated liposomes with high drug loading. In agreement with the previous cases, it was possible to apply post-pegylation procedure on pre-formed Dapto loaded liposomes, by incubating the liposomes with PEG micelles for 1h at 45°C. Indeed, the Dapto Liposomes retained most if not all of their Dapto content and no significant effect on their other physicochemical properties was conferred by post-PEGylation, with the exception of the high drop of the negative zeta potential value of the PG-containing Dapto-loaded liposomes that occurred after PEGylation (from -30.9 to -13.4), which in fact proves that the negative charged lipid containing membrane of the vesicles was successfully coated with the hydrophilic polymer chains, in agreement with other reports [
24,
30]. A similar drop of negative zeta potential was also observed when PEGylation was applied by adding the lipid-polymer conjugate in the lipid phase of the PG-containing liposomes (from -30.9 to -8.7) proving that PEGylation was achieved; however, in that case Dapto loading was highly reduced. Concluding, PEGylated Dapto-loaded liposomes with high Dapto loading could be achieved for non-charged and also for negative charge liposomes by post-PEGylation.
Due to the very low EE (%) of Dapto in liposomes prepared by MM, we decided to use the liposomes prepared by the TFH method for the remaining studies. MM method was further explored in order to investigate if by modulating the mixing parameters, or by using different organic solvents and/or initial drug concentration using higher Dapto amounts could be encapsulated in the liposomes, however increased Dapto loading in nanosized vesicles could not be achieved. Given the unique structure of this acidic lipopeptide drug, it will be interesting to further explore MM preparation of Dapto liposomes by applying design of experiment approaches.
The physical stability of liposomal Dapto formulations composed of PC/Chol (1:1) and PC/PG/Chol (8:2:10), and prepared by TFH, DRV method or MM method, was additionally studied, as mentioned in Methods section. Experimental results indicate that for both liposome membrane compositions tested, the liposomes prepared by TFH and DRV methods demonstrated high physical stability (mean diameter and PDI values did not change) for the one month period that was evaluated; oppositely, a gradual slight increase of the vesicle mean diameter was observed in the case of the liposomes prepared by MM method (
supplementary Figure S1).
3.2. Liposomal Dapto Release and Chemical Stability Studies
The release of Dapto from the different types of liposome compositions (of
Table 1 and
Table 2) was studied under two conditions; either by keeping the drug concentration in the samples constant (
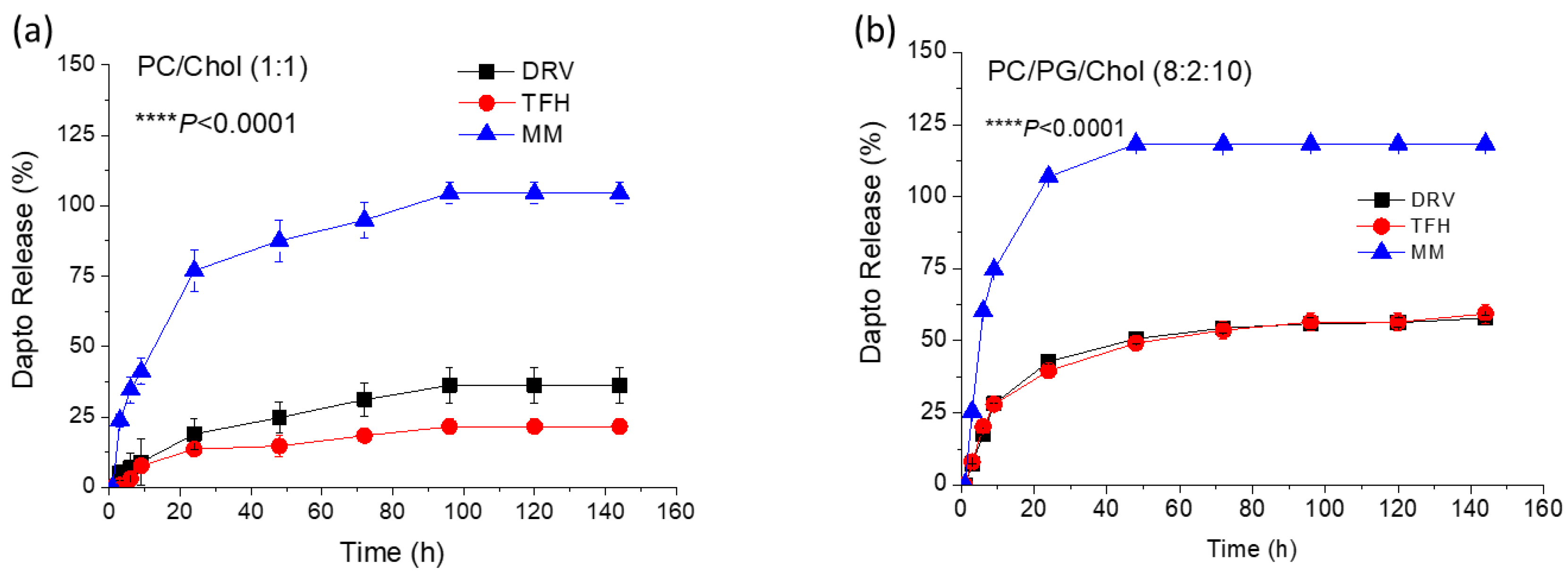
Figure 1), or by keeping the liposomal lipid concentration constant (
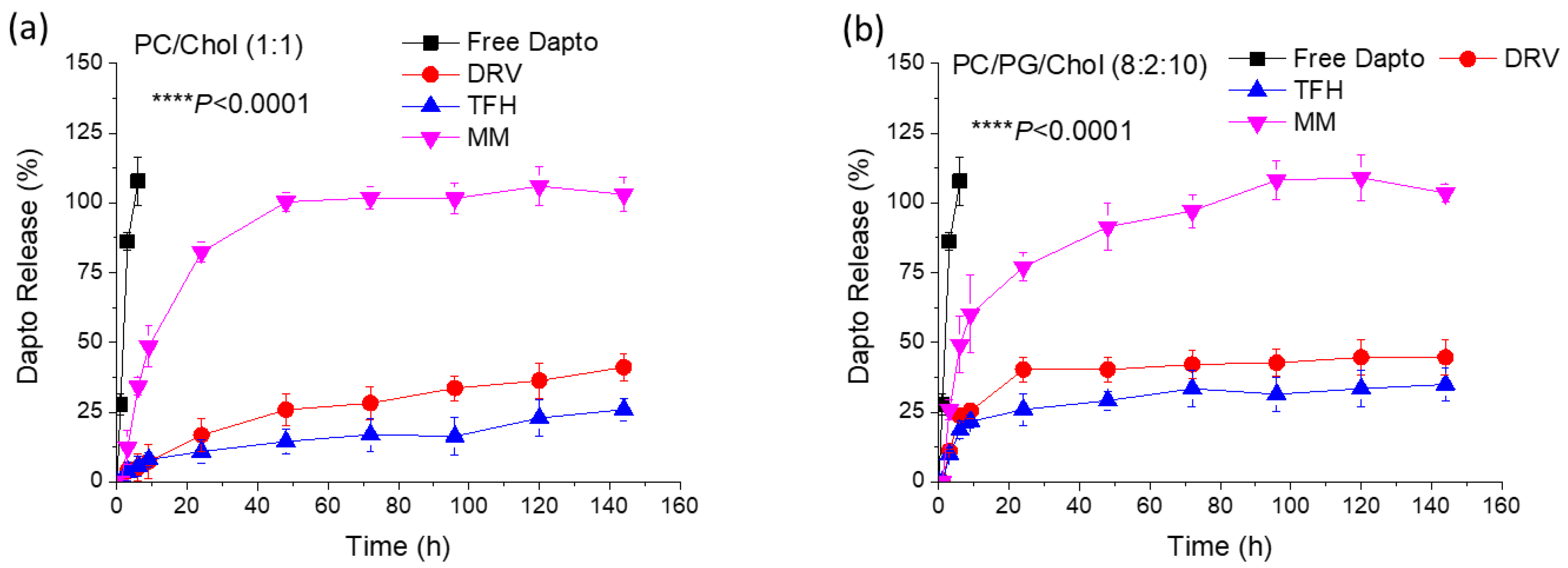
Figure 2).
In both cases sink conditions applied throughout the experiments. As seen, Dapto was released much faster from liposomes prepared by MM method, compared to the other two methods, regardless of the lipid composition. Dapto-liposomes prepared by TFH and DRV methods had similar Dapto releasing profiles, for both lipid compositions studied, but Dapto release from MM liposomes was significantly faster (for both liposome types). It is thus indicated that the very low amount of Dapto encapsulated in the ΜΜ liposomes is perhaps more loosely associated with the liposomes (or a different mode of association of the lipopeptide with the MM liposomes exists), an observation that requires further exploration. In any case the longer retention of Dapto in the DRV and THF liposomes is another reason that we selected TFH method for the following experiments, since we have recently observed a substantially higher antimicrobial activity of moxifloxacin liposomes from liposome types from which the release of antibiotic was slower (compared t other liposomes that released the drug faster) [
24].
Since it is known that Dapto is degraded in solution, we sought to investigate if perhaps liposomal encapsulation can provide protection towards Dapto degradation, as previously reported for other molecules, such as relaxin and curcumin [
30,
31]. Thereby, in another set of experiments the stability of liposomal Dapto was evaluated during incubation at 37° or 4°C for up to 16 days.
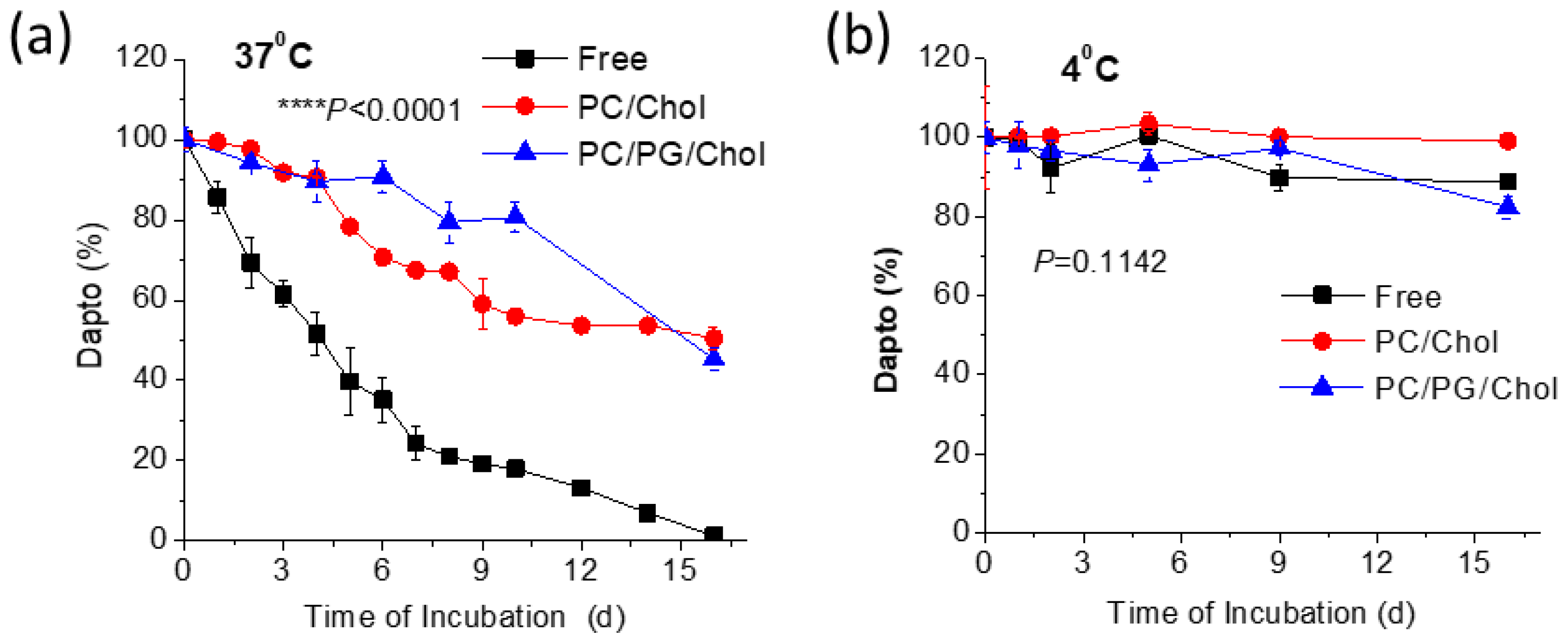
From the results reported in
Figure 3a, it becomes evident that liposomal encapsulation protects Dapto for degradation during incubation in aqueous media at 37°C. Indeed, free Dapto is degraded rapidly (
Figure 3a) resulting in more than 30% degradation after 48h, while after 16 days of incubation at 37°C no free Dapto is detected. Oppositely, under the same incubation conditions no degradation of liposomal Dapto is observed after 48h and more that 45% of (the initial amount) liposomal Dapto is detected (in both liposomes evaluated) after 16 days. On the contrary Dapto is stable, even as free drug solution when incubated at 4°C for the full period evaluated (
Figure 3b). These results prompted us to evaluate if the antimicrobial properties of liposomal Dapto were correspondingly retained for longer periods at 37°C, and a separate study was carried out, as mentioned below in
Section 3.5.
3.3. Transmission Electron Microscopy of Dapto Liposomes
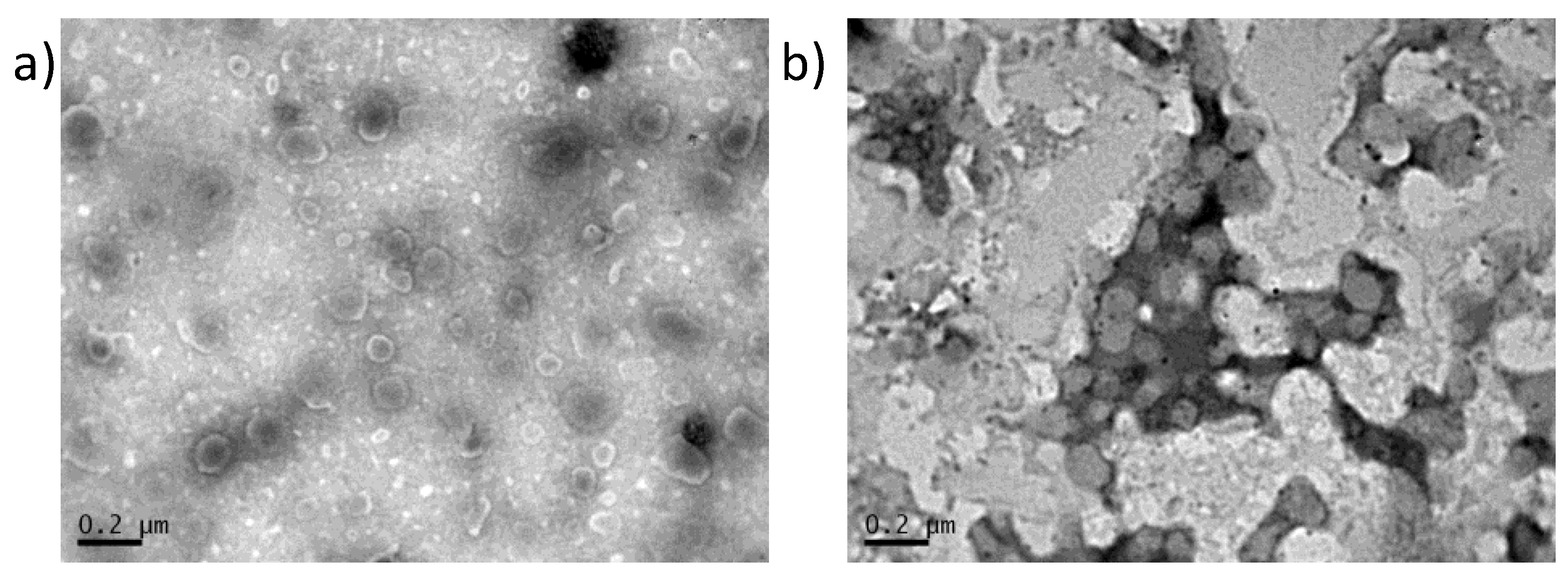
Transmission electron microscopy was performed to complete the characterization of Dapto liposomes. As seen in
Figure 4, both types of liposomes are round shaped and the vesicle diameters observed in TEM micrographs (around 100 nm) agree with the DLS measurements of
Table 1,
Table 2 and
Table 3.
3.4. Inhibition of Planktonic Bacterial Growth by Dapto Liposomes-Effect of Lipid Composition
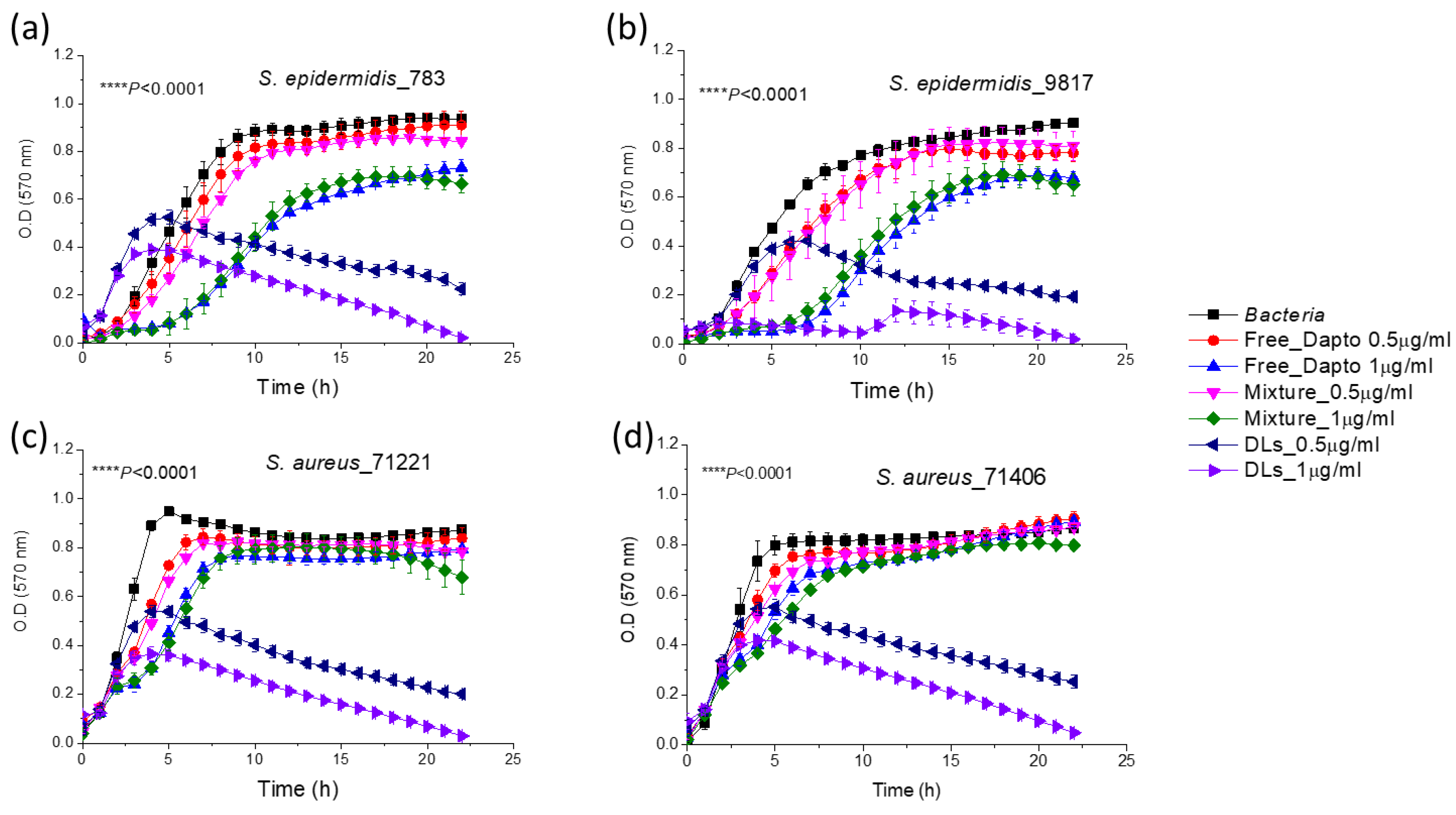
As shown in
Figure 5, the growth of the all four bacterial strains of
S. epidermidis and
S. aureus bacteria was substantially inhibited by PC/Chol Dapto-liposomes (DLs), at both drug doses (0.5 and 1 µg/ml) tested. Liposome formulations could inhibit bacterial growth significantly better compared to the same concentration of free drug in all cases. In fact, the bacteria count in the samples which were incubated for 24 h, in presence of the high dose of liposomal Dapto was below 2% (of the corresponding initial count) for all the bacterial strains studied, indicating a very high bacteriostatic activity of PC/Chol liposomal Dapto. On the other hand, empty liposomes (with the same lipid composition and at the lipid concentrations (94.7 and 189.6 µM lipid) corresponding to the two liposomal drug doses used, did not have any significant inhibitory effect on bacterial growth, for any of the bacteria (not shown in
Figure 4 for increased clarity). Finally, the mixtures of free drug (0.5 and 1 µg/ml) and empty liposomes (94.7 and 189.6 µM lipid, respectively), conferred (in all cases) similar effects on bacterial growth as that of the free drug. The latter observation indicates the importance of association of Dapto with the PC/Chol liposomes (and not just mixing of the two components together) for inhibition of bacterial growth.
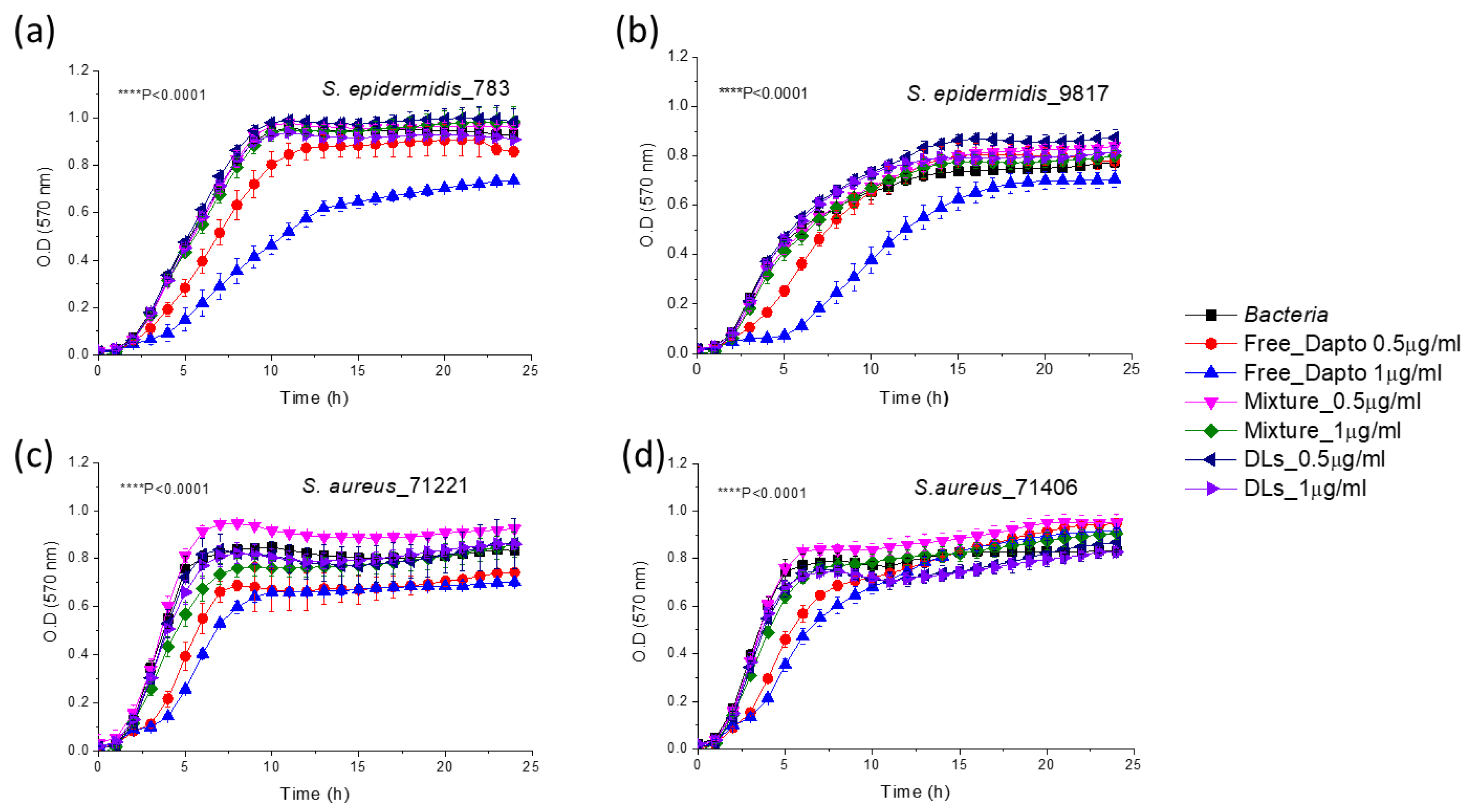
Surprisingly, when Dapto is encapsulated in PC/PG/Chol liposomes, the activity of liposomal Dapto against all the species of planktonic bacteria is diminished; no significant growth inhibition is demonstrated by liposomal Dapto for any of the bacterial strains tested (
Figure 6).
The main difference between the two liposomal Dapto formulations is the much higher negative charge of the PC/PG/Chol liposomes (-30.9 ± 1.6) (
Figure 6) compared to the PC/Chol liposomes (-8.8 ± 2.3) (
Figure 5), as reported in
Table 3. We thereby, hypothesize that perhaps the PC/PG/Chol liposomal Dapto does not demonstrate any inhibitory effect on the planktonic bacteria due to electrostatic repulsion between the suspended bacteria and liposome vesicles. This hypothesis is strengthened by the negative zeta potentials measured as described in the methods section, for all the bacterial strains used in growth inhibition studies (
Table 4).
Similar effects of liposome surface charge on liposomal antibiotic inhibitory action towards planktonic bacteria have been reported before. It has been recently reported that negatively charged gentamicin-loaded liposome exhibited the same bacteriostatic concentration as that of free gentamicin, while the minimum bactericidal concentration of neutral gentamicin-loaded liposomes towards planktonic
P. aeruginosa bacteria was twofold lower than that of free gentamicin [
32]. Similar enhanced antimicrobial activity of liposomal gentamycin was also reported elswhere [
33]. The different bacteriostatic activities of the two liposome types (neutral and negative) were attributed to “limitation of liposomal fusion with a negatively charged bacterial cell wall due to repulsive forces at close proximity”, in accordance with our suggestion. Similar results were also found by others and it was concluded that cationic and neutral liposomes could interact more with the negatively charged bacterial cell wall (of Gram-negative bacteria) or with the peptidoglycan (of Gram-positive bacteria) compared to negatively charged liposomes [
34].
3.5. Antibiofilm Activity of Dapto Liposomes-Effect of Lipid Composition
The effect of liposome lipid composition on the anti-biofilm activity of Dapto was also studied by performing bacterial biofilm susceptibility assays with the same bacterial strains and the same liposomal preparations as those used for bacterial growth inhibition studies. Dapto concentrations of 0.1µg/ml (0.061μM) and 0.5 μg/ml (0.308μM), and corresponding lipid concentrations of 18.9μM and 94.7μM for PC/Chol liposomes, and slightly lower 16.3μM and 81.6μM for PC/PG/Chol liposomes (due to their slightly higher encapsulation efficiency (
Table 4)) were used. Two sets of experiments were carried out, biofilm prevention experiments (where therapeutics were incubated with the bacteria before biofilm formation) and biofilm treatment experiments (where therapeutics were incubated with pre-formed biofilms). The biofilm prevention study results are presented in
Figure 6, and the results of biofilm treatment studies are seen in
Figure 7. In both cases the results are expressed as percent Reduction of biofilm mass (CV) and percent Reduction of biofilm bacterial viability (MTT).
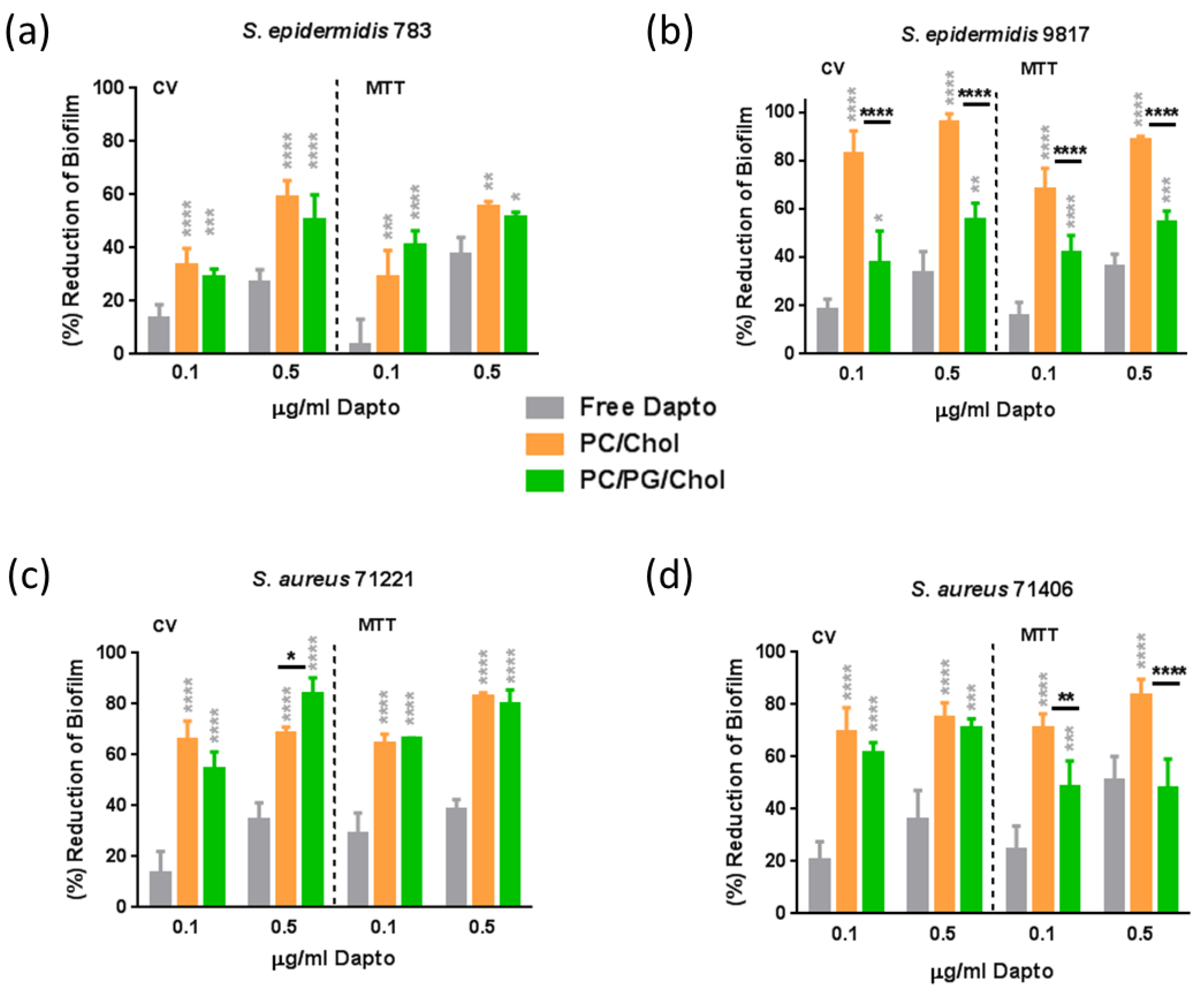
From
Figure 7, it is observed that free Daptomycin has significant activity against biofilm (compared to untreated samples) for all of the bacteria tested, especially at the highest dose used. Indeed, when the bacteria biofilm is formed in presence of 0.5 μg/ml free Dapto, both the mass of the biofilm and the biofilm (bacteria) viability is reduced by 27.5% to 36.2% (mass) and by 36.4% to 51.1% (viability) compared to biofilms that formed in absence of Dapto, in accordance to previous studies that report higher activity of Dapto towards biofilm bacteria compared to planktonic bacteria [
9,
16]. Moreover similar biofilm reductions were observed towards all the bacterial strains tested, the more susceptible bacteria Se 783 and Sa 71221 (
Figure 7a and
Figure 7c, respectively) and the more resistant strains Sa9817 and Sa71406 (
Figure 7b and
Figure 7d, respectively), proving the potential of liposomal Dapto also towards biofilms of resistant bacterial strains.
Nevertheless, biofilm mass (CV) as well as biofilm viability values were reduced several times more when Dapto-loaded liposomes were incubated with the bacteria, compared to the corresponding reductions conferred by free Dapto. Dapto liposomes (both lipid compositions tested) demonstrated dramatically higher biofilm prevention activity compared to free Dapto towards all the bacterial strains evaluated. Indeed, when the bacterial biofilms are formed in presence of 0.5 μg/ml liposomal Dapto both the mass of the biofilm and the biofilm (bacteria) viability is reduced by 59.5% to 96.2% (mass) and by 55.7% to 88.1% (viability) by PC/Chol liposomes and by 50.2% to 83.8% (mass) and by 48.1% to 80.3% (viability) by PC/PG/Chol liposomes (compared to biofilms that formed in absence of Dapto). Especially at the lower Dapto concentration studied (0.1 µg/ml), where the reductions conferred by the free drug (in biofilm mass and viability) were <30% for all bacteria, liposomal Dapto formulations conferred between 1.9 to 7.5 times higher reduction of biofilm mass and between 1.6 to 6.3 times higher reduction of biofilm viability, compared to free drug.
Even more interesting is the fact that very high reductions in biofilm masses and viabilities are conferred by liposomal Dapto towards the resistant strains Se 9817 and Sa 7 1406. In fact the Dapto liposomes demonstrated lowest biofilm prevention activity towards the less resistant Se 783 strain (when comparing the biofilm reductions conferred towards the different bacterial strains tested), which is also the only methicillin susceptible strain.
From
Figure 7, it is also evident that in some cases significant differences in the Dapto-liposome-conferred biofilm prevention activity are noticed between the two types (lipid compositions) of liposomes used in the study. In fact PC/Chol liposomes demonstrate highest activities, the only exception being the slightly higher (and marginally statistically significant) reduction of
Sa 71221 bacteria biofilm mass by PC/PG/Chol liposomes. It may be postulated that since in the biofilm prevention studies the liposomes are mixed with bacteria before the formation of the biofilm, any initial electrostatic repulsion between the negatively charged bacteria and the PC/PG/Chol liposomes may result in fewer liposomes being integrated into the biofilms, reducing thereby the action of these liposomes.
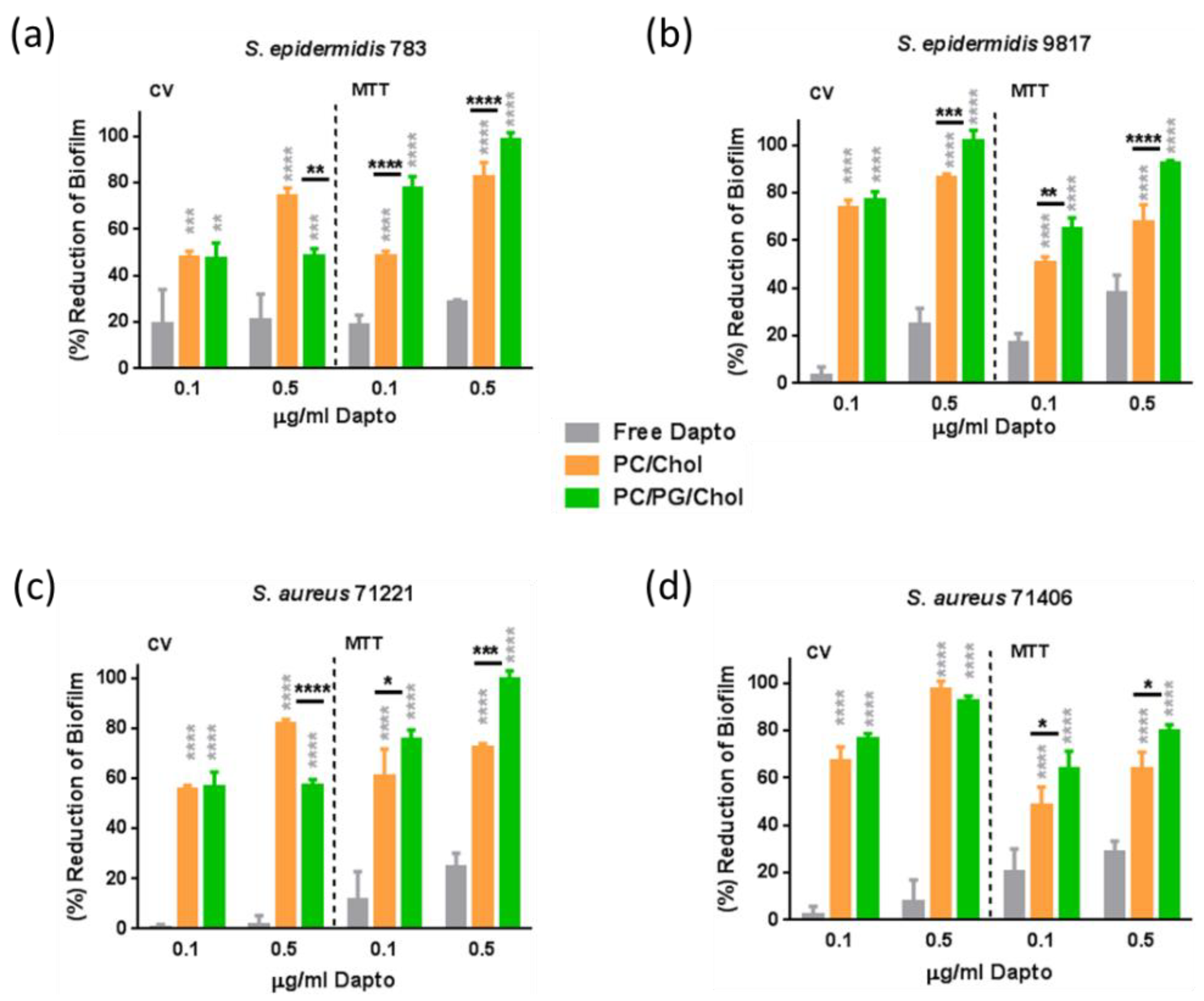
As seen in
Figure 8, in established biofilms, the anti-biofilm effect of free Dapto is lower compared to that observed in the biofilm prevention studies (
Figure 7). Indeed, when the bacteria biofilm is formed in presence of 0.5 μg/ml free Dapto, both the mass of the biofilm and the biofilm (bacteria) viability is reduced by 1.5% to 24.6.% (mass) and by 24.8% to 38.1% (viability) compared to biofilms that formed in absence of Dapto. This is logical since pre-established mature biofilms are much more difficultly treated by antibiotics, posing a well known unmet medical need.
However the biofilm reduction activities demonstrated by liposomal Dapto were surprisingly very high, suggesting that Dapto liposomes may be considered for treatment of resistant biofilms. Indeed, as depicted from
Figure 8 results, Dapto liposomes (both lipid compositions tested) demonstrated dramatically higher biofilm reduction activity compared to free Dapto, towards all the bacterial strains evaluated. Indeed, when the pre-formed bacterial biofilms are treated with 0.5 μg/ml liposomal Dapto, both the mass of the biofilm and the biofilm (bacteria) vitality is dramatically reduced (eradicated in some cases) by 74.6% to 97.6% (mass) and by 63.7.7% to 82.2% (viability) by PC/Chol liposomes and by 48.4% to 100% (mass) and by 80.2% to 100% (viability) by PC/PG/Chol liposomes (compared to biofilms that formed in absence of Dapto). At the lower Dapto concentration studied (0.1 µg/ml), where the reductions conferred by the free drug (in biofilm mass and viability) were <20% for all bacteria, liposomal Dapto formulations conferred between 2.6 to 73 times higher reduction of biofilm mass and between 2.6 to 6.6 times higher reduction of biofilm viability, compared to free drug.
Furthermore, very high antibiofilm activity is conferred by liposomal Dapto towards all bacterial strains evaluated and particularly towards the more resistant strains
Se 9817 (
Figure 8b) and
Sa 71406 (
Figure 8d), for which the high liposomal-Dapto dose resulted in complete eradication of the pre-formed biofilm masses. In fact the Dapto liposomes demonstrated lowest biofilm prevention activity towards the less resistant
Se 783 strain (when comparing the biofilm reduction conferred towards all the strains tested).
It should be clarified at this point that empty liposomes (PC/Chol and PC/PG/Chol) at similar concentrations with those used for Dapto liposome samples were also evaluated for potential biofilm prevention and treatment , and did not demonstrate any significant difference regarding biofilm mass and viability, compared to untreated biofilms (
see Supplementary Figure S2).
Another very interesting observation from the results of the biofilm treatment study (
Figure 8) it that oppositely to what was seen in the biofilm prevention studies, here in most cases the PC/PG/Chol negative charge liposomes demonstrated highest activities, with the exceptions of biofilm mass reduction of (less resistant)
Se 783 (
Figure 8a) and
Sa 71221 (
Figure 8c) bacteria (where PC/Chol liposomes acted better).
Perhaps PC/PG/Chol liposomes allow better penetration into some biofilms since in the biofilm treatment studies the drug formulations are added on already formed biofilms and thereby the charge of the biofilm components and not the charge of the bacteria should be more important. In fact, similar results were previously reported about the effect of liposome charge for treatment of bacterial biofilm. In one study negatively charged clarithromycin-loaded liposomes were reported to have increase activity of against
P. aeruginosa biofilm [
35]. In another study negatively charged tobramycin-loaded liposomes were observed to be immobilized close to a biofilm cluster due to electrostatic attraction between the cluster and the liposomes. This leaded to penetration of the liposomes into the biofilm and to subsequent bacteria killing [
36]. Additionally, negatively charged gentamycin-loaded liposomes exhibited higher antibiofilm activity against
P. aeruginosa and
K. oxytoca compared to the free drug but also to neutral liposomes [
34].
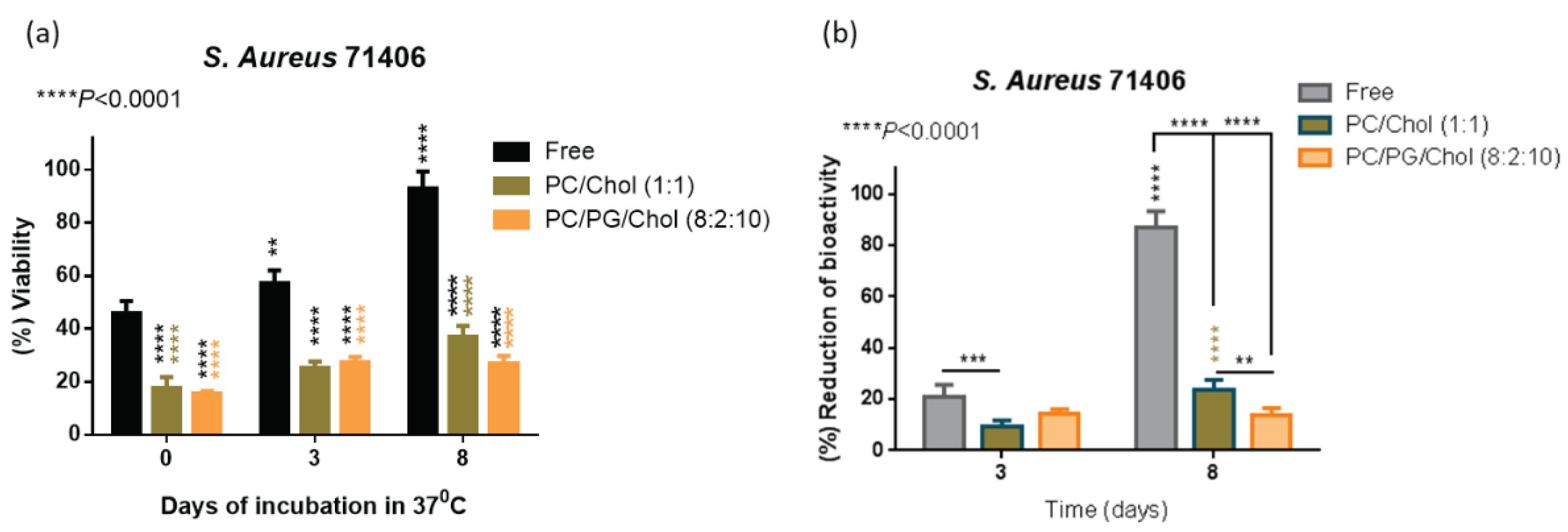
3.6. Preservation of Antibiofilm Activity of Dapto by Liposome Encapsulation
As mentioned above, we proved that liposomal encapsulation of Dapto protects the drug from chemical degradation (
Figure 3), and a question was posed regarding the antimicrobial activity of free and liposomal Dapto following subjection of Dapto formulations at 37°C that will take place after
in vivo administration. For this, an additional experiment was carried out to measure the bioactivity of free and liposomal Dapto formulations after incubation at 37°C for 3 d and 8 d. More specifically, the reduction of the viability of
S. aureus 71406 pre-formed biofilm was assessed, with the same methodologies used for biofilm treatment studies (
Figure 9).
As seen in
Figure 9b the reduction of the bioactivity of Dapto after 3 and 8 days is highest for free Dapto (21%) and similar for the two liposome types at day 3 (approx. 10%). At the day 8 time point, most of the bioactivity of free Dapto (~80%) is lost, while PC/Chol liposomes lose significantly more bioactivity compared to PC/PG/Chol liposomes. The later results correlate well with the Dapto degradation study results (
Figure 3a), suggesting that bioactivity of Dapto is reduced linearly with its integrity. From the current results it is proven that liposomal encapsulation preserves not only the chemical integrity but also the biofilm reduction activity of Dapto liposomes, suggesting that the higher activity of liposomal Dapto (compared to free drug) is at least partly attributed to the protection provided by liposomal encapsulation; of course this is not the only mechanism involved, as already discussed before.
4. Discussion
Summarizing the findings of the current report, three methods were used for the formation of nanosized Dapto-encapsulating liposomes. THF and DRV methods showed similar Dapto loading ability and sustained release of the drug from the lipsoomes, while MM methods could not provide liposomes with similar EE% and release profile of Dapto, although their size and other properties were similar; further investigations are needed for elucidation of the involved mechanisms.
Concerning the antibacterial activity of Dapto liposomes, neutral charge liposomes conferred significantly increased bacteriostatic activity (compared to free drug) towards planktonic bacteria of two
S. epidermidis and two
S. aureus clinical strains (most being methicillin resistant), while negative charge liposomes had no activity, in agreement with previous reports in the relevant literature for other liposomal drugs [
32,
33,
34]. Furthermore, biofilm prevention and biofilm treatment studies revealed the high potential of Dapto liposomes to reduce biofilm mass and viability towards all the bacterial strains studied, compared to free Dapto. Interestingly the charge of liposomes seemed to determine their antibiofilm activity in an opposite way to that demonstrated for their bacteriostatic activity against planktonic bacteria, a phenomenon also reported before for other drugs [
34,
35,
36]. Thus, the previous theories to explain the effect of liposomal antibiotic surface charge on their inhibitory activity towards planktonic bacteria growth, as well as on their biofilm treatment efficacy are further strengthened.
Dapto integrity and bioactivity preservation studies during incubation of free and liposomal drug at 37°C showed that liposome encapsulation protects the lipopeptide drug from chemical degradation and reserves its bioactivity in a similar manor, providing one potential mechanism for the higher potency of liposomal Dapto, compared to free drug. We cannot be sure which other mechanisms previously proposed to explain the high potency of other liposomal antibiotics, such as fusion between liposome and bacterial cells that results in antibiotic direct delivery in bacterial cytoplasm [
37] and/or enhanced penetration of liposomes into bacterial biofilms [
36] are implicated in the current findings, however the current results provide another example about the high potential of liposomal antimicrobials.
Especially for Daptomycin that is a particular drug being a large cyclic lipopeptide and having good activity to reduce persistent and methicillin resistant biofilms related to medical devise infections, simple liposome formulations such as the ones studies herein could find applications as potent therapeutic solutions for treating persistent biofilms; additionally, liposomal Dapto integration into medical devices could be considered [
38].
In fact, in the two studies concerning Daptomycin liposomes reported before, targeted liposomes were considered in which Dapto was conjugated with PEG and attached on liposome surface to assist their targeting to bacteria and also loaded alone or with other drug in the liposomes [
39,
40]. In the earlier study such Dapto liposomes demonstrated specific binding to MRSA by flow cytometry and good targeting capabilities
in vivo to MRSA-infected lungs in a pneumonia model [
38]. In the second more recent report the targeted liposomes were also loaded with Vancomycin and were additionally coated with erythrocyte ghosts, being thus a particularly complicated formulation. In comparison to free drugs, the formulations sustained the release of drugs for 3 days and evaded detection by macrophages. Additionally, the targeted liposomes reduced the MIC and significantly increased bacterial permeability (compared to free drug), resulting in more than 80% bacterial death within 4 h.
In light of the current findings that planktonic bacteria as well as matured biofilms of MRSA and MRSE bacterial strains (that also have low susceptibility towards Dapto) were almost completely eradicated by the liposomal Dapto formulations developed herein, perhaps the use of more simple Dapto liposomes alone or in combination with other antimicrobials should be considered in designing novel antimicrobial therapeutic systems against medical device-associated infections. Such simple liposomal Dapto formulations have the additional benefit of being more easily translatable into drug products.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: Physical stability of Dapto liposomes; Figure S2: Control study to evaluate Empty Liposome effect on Prevention and Treatment of Bacterial Biofilms.
Author Contributions
Conceptualization, Iris Spiliopoulou and Sophia Antimisiaris; Data curation, Foteini Gkartziou, Chariklia Kipraiou, Iti Gauttam, Pavlos Klepetsanis and Sophia Antimisiaris; Formal analysis, Foteini Gkartziou, Pavlos Klepetsanis and Sophia Antimisiaris; Funding acquisition, Iris Spiliopoulou and Sophia Antimisiaris; Investigation, Foteini Gkartziou, Maria Plota, Chariklia Kipraiou, Iti Gauttam and Sophia Antimisiaris; Methodology, Foteini Gkartziou, Maria Plota, Kolonitsiou Fevronia, Pavlos Klepetsanis and Sophia Antimisiaris; Project administration, Foteini Gkartziou, Iris Spiliopoulou and Sophia Antimisiaris; Resources, Maria Plota, Kolonitsiou Fevronia, Iris Spiliopoulou and Sophia Antimisiaris; Supervision, Foteini Gkartziou, Kolonitsiou Fevronia, Pavlos Klepetsanis, Iris Spiliopoulou and Sophia Antimisiaris; Validation, Maria Plota and Sophia Antimisiaris; Visualization, Iris Spiliopoulou and Sophia Antimisiaris; Writing – original draft, Foteini Gkartziou; Writing – review & editing, Kolonitsiou Fevronia, Pavlos Klepetsanis, Iris Spiliopoulou and Sophia Antimisiaris. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Committee of the University of Patras, Greece, grant number 39540000 awarded to I.S. and co-funded (including APC) by the following grants received by Institute of Chemical Engineering Sciences, FORTH/ICE-HT: (1) the Operational Program “Human Resources Development, Education and Lifelong Learning 2014–2020”, co-financed by Greece and the European Union (European Social Fund-ESF) through in the context of the subproject “Innovative lipid nanoparticles for treatment of staphylococcal biomembranes” (MIS 5049223), grant number 39540000 awarded to S.G.A and I.S, and (2) the Operational Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020), co-financed by Greece and the European Union (European Regional Development Fund) subproject “Preclinical development of INNOvative FORmulations of antibiotics for intraocular administration for the treatment/prevention of postoperative endophthalmtis-Innofor I” (MIS 5031792) which is implemented under the Special Service of the Operational Program Competitiveness Entrepreneurship and Innovation.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
Authors and especially SGA dedicate this work to the loving memory of a valuable colleague and a good friend Pavlos Klepetsanis who very sadly passed during the preparation of this manuscript. Iti Gauttam is a funded student by the Education, Audiovisual and Culture Executive Agency (EACEA) of the European Commission in the field of the Erasmus Mundus Joint Master Degree (EMJMD) of Nanomedicine for Drug Delivery (NANOMED). The help provided in the TEM studies by Dr. Mary Kollia, Laboratory of Electron Microscopy and Microanalysis (L.E.M.M.), Faculty of Natural Sciences, University of Patras, is highly acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Klein, E.Y.; Jiang, W.; Mojica, N.; Tseng, K.K.; McNeill, R.; Cosgrove, S.E.; Perl, T.M. National Costs Associated With Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus Hospitalizations in the United States, 2010-2014. Clin Infect Dis. 2019 Jan 1;68(1):22-28. [CrossRef]
- Abebe, A.A.; Birhanu, A.G. Methicillin Resistant Staphylococcus aureus: Molecular Mechanisms Underlying Drug Resistance Development and Novel Strategies to Combat. Infect Drug Resist. 2023 Dec 14;16:7641-7662. [CrossRef]
- Wi, Y.M.; Patel, R. Understanding Biofilms and Novel Approaches to the Diagnosis, Prevention, and Treatment of Medical Device-Associated Infections. Infect Dis Clin North Am. 2018 Dec;32(4):915-929. [CrossRef]
- Dubey, A.K.; Sharma, M.; Parul Raut, S.; Gupta, P.; Khatri, N. Healing wounds, defeating biofilms: Lactiplantibacillus plantarum in tackling MRSA infections. Front Microbiol. 2023 Dec 5;14:1284195. [CrossRef]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of Medical Devices by Staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal Biofilms. Microbiol. Spectr. 2018, 6, 6–4. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-Resistant Staphylococcus aureus. Nat. Rev. Dis. Primer 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Fischer A, Yang SJ, Bayer AS, et al. (2011). Daptomycin resistance mechanisms in clinically derived Staphylococcus aureus strains assessed by a combined transcriptomics and proteomics approach. J Antimicrob Chemother 66:1696–711. [CrossRef]
- Lambert, M. IDSA Guidelines on the Treatment of MRSA Infections in Adults and Children. Am. Fam. Physician 2011, 84, 455–463. [Google Scholar]
- Heidary M, Khosravi AD, Khoshnood S, Nasiri MJ, Soleimani S, Goudarzi M. Daptomycin. J Antimicrob Chemother. 2018 Jan 1;73(1):1-11. [CrossRef]
- Tzalis S, Ioannou P, Billiari E, Kofteridis DP, Karakonstantis S. Daptomycin as an option for lock therapy: a systematic literature review. Future Microbiol. 2023 Sep;18:917-928. [CrossRef] [PubMed]
- Pai L, Patil S, Liu S, Wen F. A growing battlefield in the war against biofilm-induced antimicrobial resistance: insights from reviews on antibiotic resistance. Front Cell Infect Microbiol. 2023 Dec 19;13:1327069. [CrossRef]
- Stewart PS, Davison WM, Steenbergen JN. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm. Antimicrob Agents Chemother. 2009 Aug;53(8):3505-7. [CrossRef]
- Plota M, Sazakli E, Giormezis N, Gkartziou F, Kolonitsiou F, Leotsinidis M, Antimisiaris SG, Spiliopoulou I. In Vitro Anti-Biofilm Activity of Bacteriophage K (ATCC 19685-B1) and Daptomycin against Staphylococci. Microorganisms. 2021 Aug 31;9(9):1853. [CrossRef]
- Cunha BA, Pherez FM. (2009). Daptomycin resistance and treatment failure following vancomycin for methicillin-resistantStaphylococcusaureus(MRSA) mitral valve acute bacterial endocarditis (ABE). Eur JClin Microbiol Infect Dis 28:831–3. [CrossRef]
- Mangili A, Bica I, Snydman DR, Hamer DH. (2005). Daptomycin-resistant, methicillin-resistantStaphylococcus aureusbacteremia. ClinInfect Dis 40:1058–60. [CrossRef]
- Santos Ferreira I, Kikhney J, Kursawe L, Kasper S, Gonçalves LMD, Trampuz A, Moter A, Bettencourt AF, Almeida AJ. Encapsulation in Polymeric Microparticles Improves Daptomycin Activity Against Mature Staphylococci Biofilms-a Thermal and Imaging Study. AAPS PharmSciTech. 2018 May;19(4):1625-1636. [CrossRef]
- Andrade S, Ramalho MJ, Santos SB, Melo LDR, Santos RS, Guimarães N, Azevedo NF, Loureiro JA, Pereira MC. Fighting Methicillin-Resistant Staphylococcus aureus with Targeted Nanoparticles. Int J Mol Sci. 2023 ;24(10):9030. 20 May. [CrossRef]
- Arroyo-Urea EM, Lázaro-Díez M, Garmendia J, Herranz F, González-Paredes A. Lipid-based nanomedicines for the treatment of bacterial respiratory infections: current state and new perspectives. Nanomedicine (Lond). 2024 Jan 25. [CrossRef]
- Antimisiaris SG, Marazioti A, Kannavou M, Natsaridis E, Gkartziou F, Mourtas S. Overcoming barriers by local drug delivery with liposomes. Advanced drug delivery reviews 174, 53-86. [CrossRef]
- Antimisiaris, SG. Preparation of DRV Liposomes. Methods Mol Biol. 2023;2622:21-47. [CrossRef] [PubMed]
- Natsaridis E, Gkartziou F, Mourtas S, Stuart MCA, Kolonitsiou F, Klepetsanis P, Spiliopoulou I, Antimisiaris SG. Moxifloxacin Liposomes: Effect of Liposome Preparation Method on Physicochemical Properties and Antimicrobial Activity against Staphylococcus epidermidis. Pharmaceutics. 2022 Feb 7;14(2):370. [CrossRef]
- Stewart, J. C. M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980, 104, 10–4. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rubio Ferrández J, Vázquez Sánchez R, Córdoba Díaz D, Córdoba Díaz M, Lozano Estevan MC, Molina García T. Stability of daptomycin reconstituted vials and infusion solutions. Eur J Hosp Pharm. 2018 Mar;25(2):107-110. [CrossRef]
- Halder S, Yadav KK, Sarkar R, Mukherjee S, Saha P, Haldar S, Karmakar S, Sen T. Alteration of Zeta potential and membrane permeability in bacteria: a study with cationic agents. Springerplus. 2015 Nov 4;4:672. [CrossRef]
- Wilson WW, Wade MM, Holman SC, Champlin FR. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J Microbiol Methods. 2001 Jan;43(3):153-64. [CrossRef]
- Li C, Cui J, Wang C, Zhang L, Xiu X, Li Y, Wei N, Li Y, Zhang L. Encapsulation of vinorelbine into cholesterol-polyethylene glycol coated vesicles: drug loading and pharmacokinetic studies. J Pharm Pharmacol. 2011 Mar;63(3):376-84. [CrossRef]
- Kogkos G, Gkartziou F, Mourtas S, Barlos KK, Klepetsanis P, Barlos K, Antimisiaris SG. Liposomal Entrapment or Chemical Modification of Relaxin2 for Prolongation of Its Stability and Biological Activity. Biomolecules. 2022 Sep 24;12(10):1362. [CrossRef]
- Matloob AH, Mourtas S, Klepetsanis P, Antimisiaris SG. Increasing the stability of curcumin in serum with liposomes or hybrid drug-in-cyclodextrin-in-liposome systems: a comparative study. Int J Pharm. 2014 Dec 10;476(1-2):108-15. [CrossRef]
- Alhariri M, Majrashi MA, Bahkali AH, Almajed FS, Azghani AO, Khiyami MA, Alyamani EJ, Aljohani SM, Halwani MA. Efficacy of neutral and negatively charged liposome-loaded gentamicin on planktonic bacteria and biofilm communities. Int J Nanomedicine. 2017 Sep 18;12:6949-6961. [CrossRef]
- Rukholm G, Mugabe C, Azghani AO, Omri A. Antibacterial activity of liposomal gentamicin against Pseudomonas aeruginosa: a time-kill study. Int J Antimicrob Agents. 2006;27(3):247–252. [CrossRef]
- Drulis-Kawa Z, Gubernator J, Dorotkiewicz-Jach A, Doroszkiewicz W, Kozubek A. A comparison of the in vitro antimicrobial activity of liposomes containing meropenem and gentamicin. Cell Mol Biol Lett. 2006;11(3):360-75. [CrossRef] [PubMed] [PubMed Central]
- Alhajlan M, Alhariri M, Omri A. Efficacy and safety of liposomal clarithromycin and its effect on Pseudomonas aeruginosa virulence factors. Antimicrob Agents Chemother. 2013;57(6):2694–2704. [CrossRef]
- Messiaen AS, Forier K, Nelis H, Braeckmans K, Coenye T. Transport of nanoparticles and tobramycin-loaded liposomes in Burkholderia cepacia complex biofilms. PLoS One. 2013;8(11):e79220. [CrossRef]
- Mugabe C, Halwani M, Azghani AO, Lafrenie RM, Omri A. Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50(6):2016–2022. [CrossRef]
- Natsaridis E, Mouzoura P, Gkartziou F, Marazioti A, Antimisiaris SG. Development of growth factor-incorporating liposomes for integration into scaffolds as a method to improve tissue regeneration. Int J Dev Biol. 2022;66(1-2-3):137-154. [CrossRef]
- Jiang H, Xiong M, Bi Q, Wang Y, Li C. Self-enhanced targeted delivery of a cell wall- and membrane-active antibiotics, daptomycin, against staphylococcal pneumonia. Acta Pharm Sin B. 2016 Jul;6(4):319-28. [CrossRef]
- Rani NNIM, Chen XY, Al-Zubaidi ZM, Azhari H, Khaitir TMN, Ng PY, Buang F, Tan GC, Wong YP, Said MM, Butt AM, Hamid AA, Amin MCIM. Surface-engineered liposomes for dual-drug delivery targeting strategy against methicillin-resistant Staphylococcus aureus (MRSA). Asian J Pharm Sci. 2022 Jan;17(1):102-119. [CrossRef]
Figure 1.
Release of Dapto from PC/Chol (1:1) (A) or PC/PG/Chol (8:2:10) (B) formulated by TFH, DRV or MM method. Sample drug concentration was adjusted at 12 μg/ml. Results are expressed as percentage of initial drug for each sample. Experiments were carried out in triplicate; mean values ± SD are reported. ANOVA p values of liposome-type effect are reported.
Figure 1.
Release of Dapto from PC/Chol (1:1) (A) or PC/PG/Chol (8:2:10) (B) formulated by TFH, DRV or MM method. Sample drug concentration was adjusted at 12 μg/ml. Results are expressed as percentage of initial drug for each sample. Experiments were carried out in triplicate; mean values ± SD are reported. ANOVA p values of liposome-type effect are reported.
Figure 2.
Release of Dapto from liposomes consisted of: (A) PC/Chol (1:1) and (B) PC/PG/Chol (8:2:10) formulated by TFH, DRV or MM method. Sample lipid concentration was 3.6 mg/ml. Results are expressed as percentage of initial drug for each sample. Experiments were carried out in triplicate, mean values ± SD are reported. ANOVA p values of liposome-type effect are reported.
Figure 2.
Release of Dapto from liposomes consisted of: (A) PC/Chol (1:1) and (B) PC/PG/Chol (8:2:10) formulated by TFH, DRV or MM method. Sample lipid concentration was 3.6 mg/ml. Results are expressed as percentage of initial drug for each sample. Experiments were carried out in triplicate, mean values ± SD are reported. ANOVA p values of liposome-type effect are reported.
Figure 3.
Kinetics of liposomal and free Dapto (solution) degradation during incubation at: (A) 37°C, and (B) 4 °C for up to 16 d. Results are expressed as percentage of initial drug for each sample. Experiments were carried out in triplicate; mean values ± SD are reported. ANOVA p values of formulation-type effect are reported.
Figure 3.
Kinetics of liposomal and free Dapto (solution) degradation during incubation at: (A) 37°C, and (B) 4 °C for up to 16 d. Results are expressed as percentage of initial drug for each sample. Experiments were carried out in triplicate; mean values ± SD are reported. ANOVA p values of formulation-type effect are reported.
Figure 4.
TEM micrographs of Dapto liposomes a) PC/Chol (1:1); b) PC/PG/Chol (8:2:10).
Figure 4.
TEM micrographs of Dapto liposomes a) PC/Chol (1:1); b) PC/PG/Chol (8:2:10).
Figure 5.
Growth curves of S. epidermidis 783 (a) and 9817 (b), as well as 71221 (c) and 71404 (d) in presence and absence of 0.5µg/ml (0.308 μM) and 1 μg/ml (0.616 μM) Dapto as free or liposomal drug. Mixtures of empty liposomes (94.7 and 189.6 uM lipid) with free Dapto were also used as controls; Lipid composition of liposomal formulations applied was PC/Chol (1:1).
Figure 5.
Growth curves of S. epidermidis 783 (a) and 9817 (b), as well as 71221 (c) and 71404 (d) in presence and absence of 0.5µg/ml (0.308 μM) and 1 μg/ml (0.616 μM) Dapto as free or liposomal drug. Mixtures of empty liposomes (94.7 and 189.6 uM lipid) with free Dapto were also used as controls; Lipid composition of liposomal formulations applied was PC/Chol (1:1).
Figure 6.
Growth curves of S. epidermidis 783 (a) and 9817 (b), as well as 71221 (c) and 71404 (d) in presence and absence of 0.5µg/ml (0.308 μM) and 1 μg/ml (0.616 μM) Dapto as free or liposomal drug. Mixtures of empty liposomes (94.7 and 189.6 uM lipid) with free Dapto were also used as controls; Lipid composition of liposomal formulations applied was PC/PG/Chol (8:2:10).
Figure 6.
Growth curves of S. epidermidis 783 (a) and 9817 (b), as well as 71221 (c) and 71404 (d) in presence and absence of 0.5µg/ml (0.308 μM) and 1 μg/ml (0.616 μM) Dapto as free or liposomal drug. Mixtures of empty liposomes (94.7 and 189.6 uM lipid) with free Dapto were also used as controls; Lipid composition of liposomal formulations applied was PC/PG/Chol (8:2:10).
Figure 7.
Biofilm Prevention studies: Reduction (% compared to untreated control) of biofilm mass (CV) and biofilm bacteria viability (MTT) of (a) S.epidermidis 783, (b) S.epidermidis 9817, (c) S. aureus 71221 and (d) S. aureus 71406, by Dapto solution (Free) and two types of Liposomal Dapto (PC/Chol and PC/PG/Chol), at doses of 0.1 and 0.5 µg/ml. Significance of difference from Free is presented as grey asterisks (*) on top of corresponding bars, and separately between liposome types.
Figure 7.
Biofilm Prevention studies: Reduction (% compared to untreated control) of biofilm mass (CV) and biofilm bacteria viability (MTT) of (a) S.epidermidis 783, (b) S.epidermidis 9817, (c) S. aureus 71221 and (d) S. aureus 71406, by Dapto solution (Free) and two types of Liposomal Dapto (PC/Chol and PC/PG/Chol), at doses of 0.1 and 0.5 µg/ml. Significance of difference from Free is presented as grey asterisks (*) on top of corresponding bars, and separately between liposome types.
Figure 8.
Biofilm Treatment studies: Reduction (% compared to untreated control) of biofilm mass (CV) and biofilm bacteria viability (MTT) of (a) S.epidermidis 783, (b) S.epidermidis 9817, (c) S. aureus 71221 and (d) S. aureus 71406, by Dapto solution (Free) and two types of Liposomal Dapto (PC/Chol and PC/PG/Chol), at doses of 0.1 and 0.5 µg/ml. Significance of difference from Free is presented as grey asterisks (*) on top of corresponding bars, and separately between liposome types.
Figure 8.
Biofilm Treatment studies: Reduction (% compared to untreated control) of biofilm mass (CV) and biofilm bacteria viability (MTT) of (a) S.epidermidis 783, (b) S.epidermidis 9817, (c) S. aureus 71221 and (d) S. aureus 71406, by Dapto solution (Free) and two types of Liposomal Dapto (PC/Chol and PC/PG/Chol), at doses of 0.1 and 0.5 µg/ml. Significance of difference from Free is presented as grey asterisks (*) on top of corresponding bars, and separately between liposome types.
Figure 9.
a) Bioactivity (expressed as cell viability of S. aureus 71406 biofilm after treatment of with 0.5 μg/ml of free or liposomal Dapto formulations, following their incubation for various durations at 37°C) and b) Bioactivity reduction (expressed as reduction of bioactivity from initial value at time 0).
Figure 9.
a) Bioactivity (expressed as cell viability of S. aureus 71406 biofilm after treatment of with 0.5 μg/ml of free or liposomal Dapto formulations, following their incubation for various durations at 37°C) and b) Bioactivity reduction (expressed as reduction of bioactivity from initial value at time 0).
Table 1.
Dapto-liposome (PC/Chol (1:1 mol/mol)) EE (%), mean diameter, PDI, and zeta potential. TFH, DRV and MM methods were used. Each value is the mean of three different samples ± the corresponding SD of each mean.
Table 1.
Dapto-liposome (PC/Chol (1:1 mol/mol)) EE (%), mean diameter, PDI, and zeta potential. TFH, DRV and MM methods were used. Each value is the mean of three different samples ± the corresponding SD of each mean.
| Method |
EE (%) |
Mean Hydr. Diameter (nm) |
pdi |
ζ-pot (mV) |
| TFH |
27.9 ± 1.8 |
119.2 ± 6.5 |
0.198 |
-8.7 ± 2.3 |
| DRV |
31.7 ± 4.0 |
102.6 ± 5.7 |
0.068 |
-8.80 ± 0.17 |
| MM |
8.1 ± 1.0 |
113.8 ± 7.6 |
0.282 |
-3.7 ± 1.4 |
Table 2.
Dapto-liposome (PC/PG/Chol 8:2:5 mol/mol/mol) EE (%), mean diameter, PDI, and zeta potential. TFH, DRV and MM methods were used. Each value is the mean of three different samples ± the corresponding SD of each mean.
Table 2.
Dapto-liposome (PC/PG/Chol 8:2:5 mol/mol/mol) EE (%), mean diameter, PDI, and zeta potential. TFH, DRV and MM methods were used. Each value is the mean of three different samples ± the corresponding SD of each mean.
| Method |
EE (%) |
Mean Hydr. Diameter (nm) |
pdi |
ζ-pot (mV) |
| TFH |
30.1±6.6 |
98.3 ± 6.3 |
0.065 |
-23.2 ± 1.1 |
| DRV |
37.6 ± 7.3 |
103.5 ± 1.77 |
0.046 |
-21.9 ± 1.8 |
| MM |
4.2±0.5 |
83.8 ± 4.4 |
0.192 |
-20.5 ± 2.3 |
Table 3.
Properties of Dapto liposomes (TFH) with different lipid compositions. Each value reported is the mean of three different samples and the corresponding SD of each mean is reported. Post-PEG stands for post-PEGylation of pre-formed liposomes without PEG.
Table 3.
Properties of Dapto liposomes (TFH) with different lipid compositions. Each value reported is the mean of three different samples and the corresponding SD of each mean is reported. Post-PEG stands for post-PEGylation of pre-formed liposomes without PEG.
| Lipid Composition |
EE
(% D/L) |
Mean Hydr. Diameter (nm) |
PDI |
ζ-Potential (mV) |
| PC/Chol (2:1) |
25.8 ± 5.8 |
122.2 ± 9.5 |
0.93 |
-6.2 ± 2.9 |
| PC/Chol (1:1) |
30.06 ± 5.2 |
119.23 ± 6.48 |
0.198
|
-8.8 ± 2.3 |
| PC/Chol/PEG (1:1:0.17) |
3.56 ± 0.009 |
117.1 ± 8.4 |
0.125 |
-7.7 ± 0.6 |
| PC/PG/Chol (8:2:5) |
37.2 ± 9 |
127 ± 12 |
0.182 |
-21.60 ± 0.75 |
| PC/PG/Chol (8:2:10) |
31.2 ± 5 |
120.3 ± 9.3 |
0.182 |
-30.9 ± 1.6 |
| PC/PG/Chol/PEG (8:2:10:1.7) |
3.56 ± 0.21 |
98.6 ± 5.3 |
0.187 |
-8.7 ± 1.0 |
|
PC/Chol/PEG (1:1: 0.17) Post-PEG* |
25.6 ± 2.0 |
106.7 ± 6.3 |
0.159 |
-8.93 ± 0.47 |
| PC/PG/Chol/PEG (8:2:10:1.7) Post-PEG* |
32.2 ± 1.5 |
138.7 ± 8.8 |
0.175 |
-13.4 ± 3.0 |
Table 4.
Zeta potential values of planktonic bacteria. Each value reported is the mean of three different samples and the corresponding SD of each mean is reported. Post-PEG stand for post-PEGylation of pre-formed liposomes without PEG.
Table 4.
Zeta potential values of planktonic bacteria. Each value reported is the mean of three different samples and the corresponding SD of each mean is reported. Post-PEG stand for post-PEGylation of pre-formed liposomes without PEG.
| Bacterial Strain |
ζ-potential (mV) |
|
S. epidermidis _783 |
-28.7 ± 1.30 |
|
S. epidermidis _9817 |
-20.8 ± 1.48 |
|
S. aureus _71221 |
-11.7 ± 3.44 |
|
S. aureus _71406 |
-23.8 ± 1.88 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).