Submitted:
22 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Muscle degradation, inflammation and increase of TNF-α level
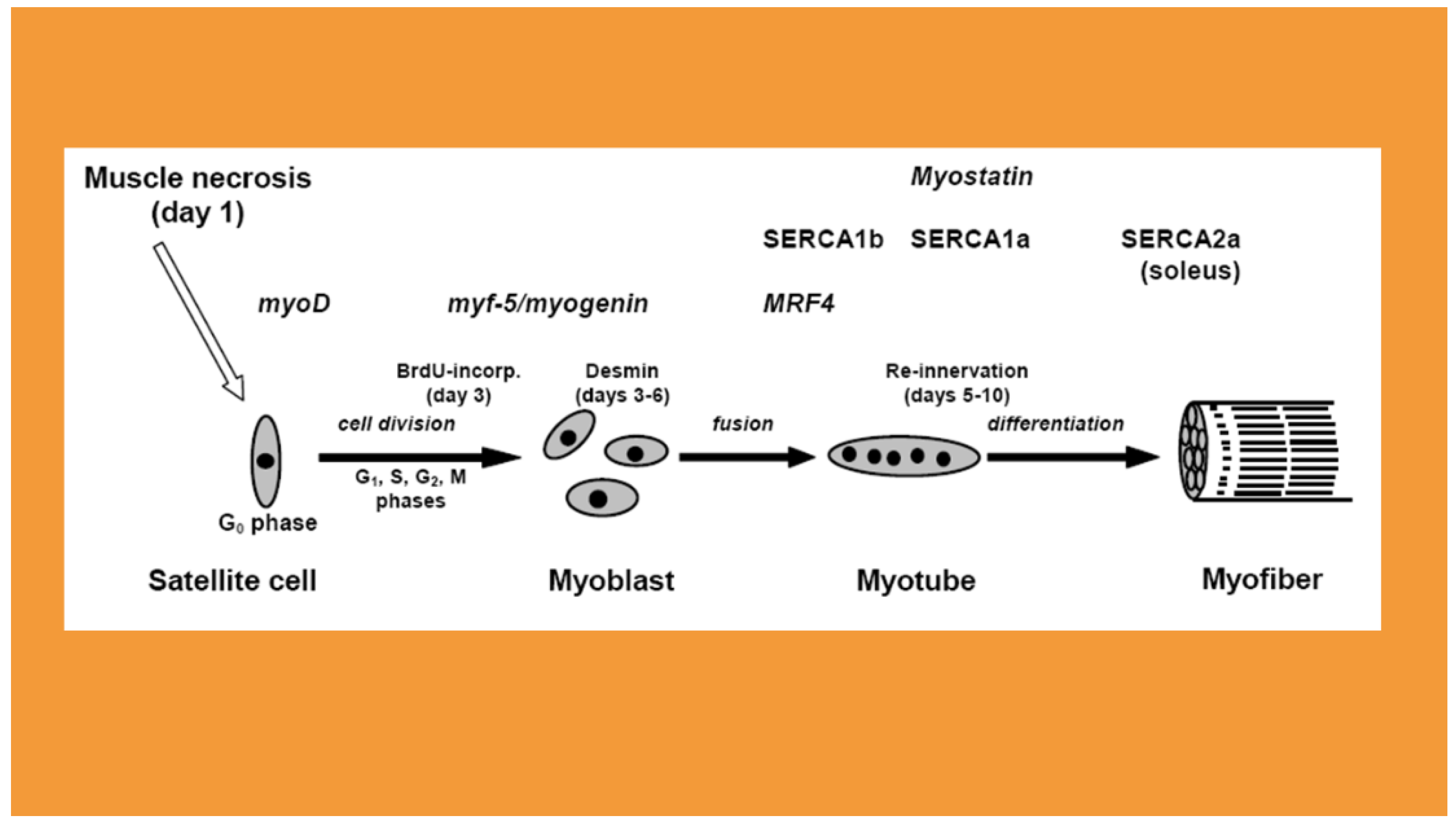
3. Myogenic regulatory factors
4. The expression of sarco/endoplasmic reticulum Ca2+ ATPases (SERCAs) in regeneration
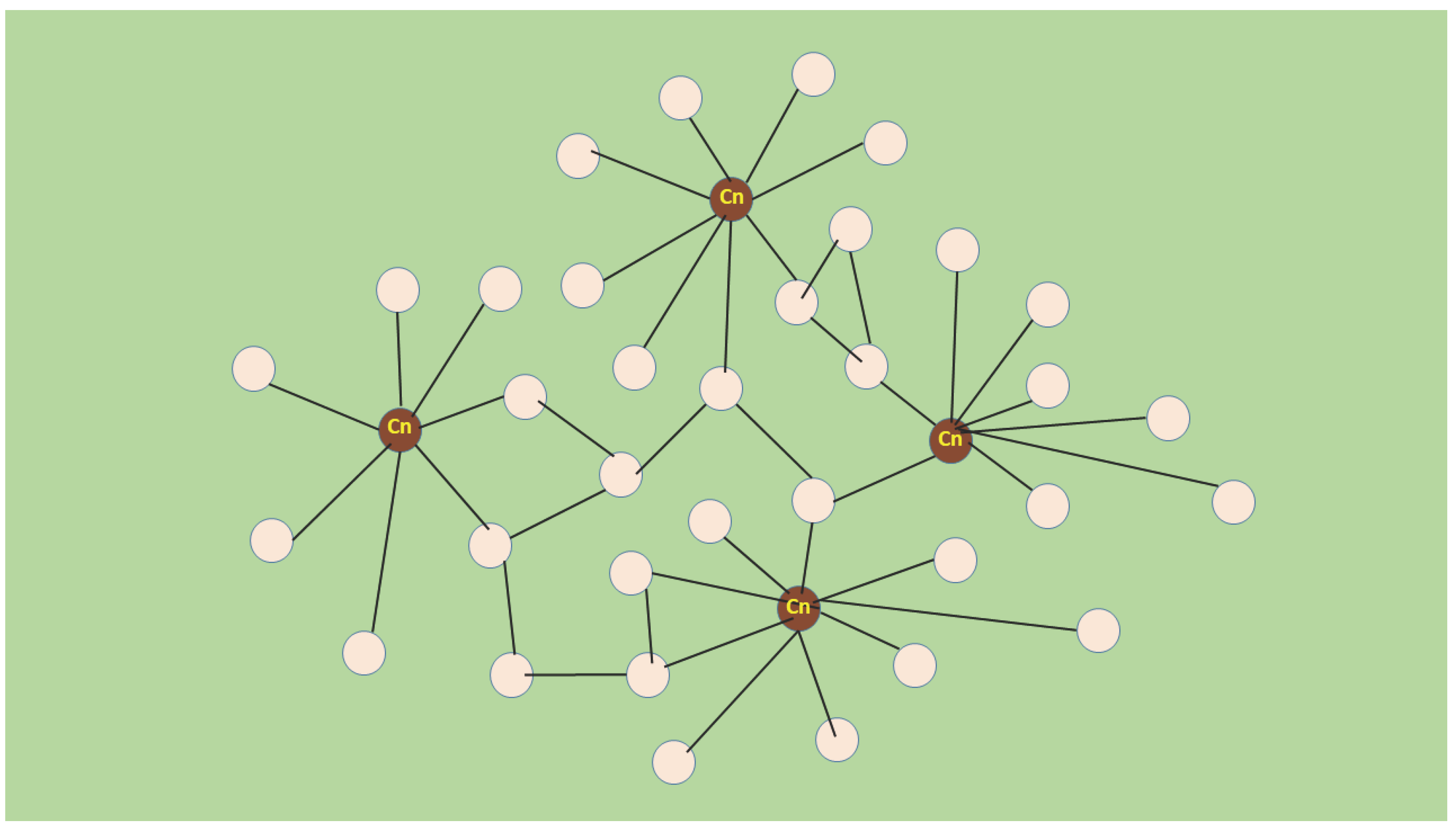
5. A novel muscle growth regulation revealed by molecular acupuncture in regeneration takes after scale-free network
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med 2016, 31, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Henrot, P.; Blervaque, L.; Dupin, I.; Zysman, M.; Esteves, P.; Gouzi, F.; Hayot, M.; Pomiès, P.; Berger, P. Cellular interplay in skeletal muscle regeneration and wasting: Insights from animal models. J Cachexia Sarcopenia Muscle 2023, 14, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.L.; Roberts, T.J.; Blemker, S.S.; Lee, S.S.M.; Herzog, W. Skeletal muscle mechanics, energetics and plasticity. J Neuroeng Rehabil 2017, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.M.; Haddad, F. The Evolution of Skeletal Muscle Plasticity. in Response to Physical Activity and Inactivity; Muscle and Exercise Physiology, Zoladz, J.A., Ed.; Academic Press, 2019, 347–377. [CrossRef]
- Laumonier, T.; Menetrey, J. Muscle injuries and strategies for improving their repair. J Exp Orthop 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A. Satellite cells of skeletal muscle fibers. J Biophys Biochem Cytol 1961, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Rudnicki, M.A. A new look at the origin, function, and ‘‘stem-cell’’ status of muscle satellite cells. Dev Biol 2000, 218, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.B.; Cheng, R.Y.; Davoudi, S.; Gilbert, P.M. Biomechanical Origins of Muscle Stem Cell Signal Transduction. J Mol Biol 2016, 428, 1441–1454. [Google Scholar] [CrossRef]
- Fukada, S.I.; Akimoto, T.; Sotiropoulos, A. Role of damage and management in muscle hypertrophy: Different behaviors of muscle stem cells in regeneration and hypertrophy. Biochim Biophys Acta Mol Cell Res 2020, 1867, 118742. [Google Scholar] [CrossRef]
- Ferrari, G.; Cusella-De Angelis, G.; Coletta, M.; Paolucci, E.; Stornaiuolo, A.; Cossu, G.; Mavilio, F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998, 279, 1528–1530. [Google Scholar] [CrossRef]
- Schulze, M.; Belema-Bedada, F.; Technau, A.; Braun, T. Mesenchymal stem cells are recruited to striated muscle by NFAT/IL-4-mediated cell fusion. Genes Dev 2005, 19, 1787–1798. [Google Scholar] [CrossRef]
- Andrade, B.M.; Baldanza, M.R.; Ribeiro, K.C.; Porto, A.; Peçanha, R.; Fortes, F.S.; Zapata-Sudo. G.; Campos-de-Carvalho, A.C.; Goldenberg, R.C.; Werneck-de-Castro, J.P. Bone marrow mesenchymal cells improve muscle function in a skeletal muscle re-injury model. PLoS ONE. 2015, 10, e0127561. [Google Scholar] [CrossRef]
- Gibson, A.J.; Karasinski, J.; Relvas, J.; Moss, J.; Sherratt, T.G.; Strong, P.N.; Watt, D.J. Dermal fibroblasts convert to a myogenic lineage in mdx mouse muscle. J Cell Sci 1995, 108, 207–214. [Google Scholar] [CrossRef]
- Chapman, M.A.; Meza, R.; Lieber, R. L Skeletal muscle fibroblasts in health and disease. Differentiation. 2016, 92, 108–115. [Google Scholar] [CrossRef]
- Giuliani, G.; Rosina, M.; Reggio, A. Signaling pathways regulating the fate of fibro/adipogenic progenitors (FAPs) in skeletal muscle regeneration and disease. FEBS J. 2022, 289, 6484–6517. [Google Scholar] [CrossRef]
- Molina, T.; Fabre, P.; Dumont, N.A. Fibro-adipogenic progenitors in skeletal muscle homeostasis, regeneration and diseases. Open Biol 2021, 11, 210110. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.M. Muscle regeneration in animal models. in: Skeletal Muscle Repair and Regeneration. Springer; Schiaffino, S.; Partridge, T.; 2008, pp. 163–180. [CrossRef]
- Mendler, L.; Zádor, E.; Dux, L.; Wuytack, F. mRNA levels of myogenic regulatory factors in rat slow and fast muscles regenerating from notexin-induced necrosis. Neuromusc Disorders 1998, 8, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Jomard, C.; Chazaud, B.; Gondin, J. Kinetics of skeletal muscle regeneration after mild and severe muscle damage induced by electrically-evoked lengthening contractions. FASEB J. 2023, 37, e23107. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Dorshkind, K.; Wehling-Henricks, M. Shared signaling systems in myeloid cell-mediated muscle regeneration. Development 2014, 141, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Khuu, S.; Fernandez, J.W.; Handsfield, G.G. Delayed skeletal muscle repair following inflammatory damage in simulated agent-based models of muscle regeneration. PLoS Comput Biol 2023, 19, e1011042. [Google Scholar] [CrossRef] [PubMed]
- Bordon, K.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A. , Cardoso, I.A., Ferreira, I.G.; de Oliveira, I.S.; Boldrini-França, J.; Pucca, M.B., Baldo, M.A.; Arantes, E.C. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front Pharmacol 2020, 11, 1132. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon. 2013, 62, 27–39. [Google Scholar] [CrossRef]
- Benoit, P.W.; Belt, W.D. Destruction and regeneration of skeletal muscle after treatment with a local anaesthetic, bupivacaine (Marcaine). J Anat 1970, 107, 547–556. [Google Scholar] [PubMed]
- Chen, Y,; Li, X. ; Huo, Z.; Chen, H.; Zhang, L. An overview of bupivacaine-induced morphological changes: A novel animal model of skeletal muscle injury. Int J Clin Exp Med 2020, 13, 7–15. [Google Scholar]
- Harris, J.B.-; Johnson, M.A.; Karlsson, E. Pathological responses of rat skeletal muscle to a single subcutaneous injection of a toxin isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin Exp Pharmacol Physiol 1975, 2, 383–404. [Google Scholar] [CrossRef]
- Plant, D.R.; Colarossi, F.E.; Lynch, G.S. Notexin causes greater myotoxic damage and slower functional repair in mouse skeletal muscles than bupivacaine. Muscle Nerve 2006, 34, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Zádor, E.; Mendler, L.; Ver Heyen, M.; Dux, L.; Wuytack, F. Changes in mRNA levels of the sarcoplasmic/endoplasmic-reticulum Ca(2+)-ATPase isoforms in the rat soleus muscle regenerating from notexin-induced necrosis. Biochem, J. 1996, 320, 107–113. [Google Scholar] [CrossRef]
- Murgia, M.; Serrano, A.L.; Calabria, E.; Pallafacchina, G.; Lomo, T.; Schiaffino, S. Ras is involved in nerve-activity-dependent regulation of muscle genes. Nat Cell Biol 2000, 2, 142–147. [Google Scholar] [CrossRef]
- Zhou, K.; Luo, W.; Liu, T.; Ni, Y.; Qin, Z. Neurotoxins Acting at Synaptic Sites: A Brief Review on Mechanisms and Clinical Applications. Toxins 2022, 15, 18. [Google Scholar] [CrossRef]
- Montecucco, C.; Gutiérrez, J.M.; Lomonte, B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: Common aspects of their mechanisms of action. Cell Mol Life Sci 2008, 65, 2897–2912. [Google Scholar] [CrossRef]
- Cintra-Francischinelli, M.; Pizzo, P.; Rodrigues-Simioni, L.; Ponce-Soto, L.A.; Rossetto, O.; Lomonte, B.; Gutiérrez, J.M.; Pozzan, T.; Montecucco, C. Calcium imaging of muscle cells treated with snake myotoxins reveals toxin synergism and presence of acceptors. Cell Mol Life Sci 2009, 66, 1718–1728. [Google Scholar] [CrossRef]
- Whalen, R.G.; Harris, J.B.; Butler-Browne, G.S.; Sesodia, S. Expression of myosin isoforms during notexin-induced regeneration of rat soleus muscles. Dev Biol 1990, 141, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Herrera, C.; Fernández, J.; Lomonte, B.; Fox, J.W. Unresolved issues in the understanding of the pathogenesis of local tissue damage induced by snake venoms. Toxicon. 2018, 148, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B.; Scott-Davey, T. Secreted phospholipases A2 of snake venoms: Effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins 2013, 5, 2533–2571. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B. Myotoxic phospholipases A2 and the regeneration of skeletal muscles. Toxicon 2003, 42, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Gasanov, S.E.; Dagda, R.K.; Rael, E.D. Snake Venom Cytotoxins, Phospholipase A2s, and Zn2+-dependent Metalloproteinases: Mechanisms of Action and Pharmacological Relevance. J Clin Toxicol 2014, 4, 1000181. [Google Scholar] [CrossRef] [PubMed]
- Bickler, P.E. Amplification of Snake Venom Toxicity by Endogenous Signaling Pathways. Toxins. 2020, 12, 68. [Google Scholar] [CrossRef]
- Delp, M.D.; Duan, C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 1996, 80, 261–270. [Google Scholar] [CrossRef]
- Zádor, E.; Wuytack, F. Expression of SERCA2a is independent of innervation in regenerating soleus muscle. Am J Physiol -Cell Physiol 2003, 285, C853–C861. [Google Scholar] [CrossRef]
- Zádor, E.; Szakonyi, G.; Rácz, G.; Mendler, L.; Ver Heyen, M.; Lebacq, Dux, L. ; Wuytack, F. Expression of the sarco/endoplasmic reticulum Ca2+-transport ATPase protein isoforms during regeneration from notexin induced necrosis of rat muscle. Acta Histochem 1998, 100, 355–369. [Google Scholar] [CrossRef]
- Zádor E, Mendler L, Takács V, De Bleecker J and Wuytack, F. Regenerating soleus and EDL muscles of the rat show elevated levels of TNF-α and its receptors, TNFR-60 and TNFR-80. Muscle and Nerve, 2001, 24, 1058–1067. [Google Scholar] [CrossRef]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 2010, 298, R1173–R1187. [Google Scholar] [CrossRef]
- Mourkioti, F.; Rosenthal, N. NF-kappaB signaling in skeletal muscle: Prospects for intervention in muscle diseases. J Mol Med 2008, 86, 747–759. [Google Scholar] [CrossRef]
- Webster, J.D.; Vucic, D. The Balance of TNF Mediated Pathways Regulates Inflammatory Cell Death Signaling in Healthy and Diseased Tissues. Front Cell Dev Biol 2020, 8, 365. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Panguluri, S.K.; Gupta, S.K.; Dahiya, S.; Lundy, R.F.; Kumar, A. Tumor necrosis factor-α regulates distinct molecular pathways and gene networks in cultured skeletal muscle cells. PLoS ONE. 2010, 5, e13262. [Google Scholar] [CrossRef]
- Langen, R.C.; Van der Velden, J.L.; Schols, A.M.; Kelder, M.C.; Wouters, E.F. Janssen-Heininger, Y.M. Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J 2004, 18, 227–237. [Google Scholar] [CrossRef]
- Li, Y.P. TNF-α is mitogen in skeletal muscle. Am J Physiol-Cell Physiol 2003, 285, C370–C376. [Google Scholar] [CrossRef]
- O'Brien, M.E.; Londino, J.; McGinnis, M.; Weathington, N.; Adair, J.; Suber, T.; Kagan, V.; Chen, K.; Zou, C.; Chen, B.; Bon, J.; Mallampalli, R.K. Tumor Necrosis Factor Alpha Regulates Skeletal Myogenesis by Inhibiting SP1 Interaction with cis-Acting Regulatory Elements within the Fbxl2 Gene Promoter. Mol Cell Biol 2020, 40, e00040–20. [Google Scholar] [CrossRef]
- Chen, S.E.; Jin, B.; Li, Y.P. TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am J Physiol Cell Physiol 2007, 292, C1660–C1671. [Google Scholar] [CrossRef]
- Careccia, G.; Mangiavini, L.; Cirillo, F. Regulation of Satellite Cells Functions during Skeletal Muscle Regeneration: A Critical Step in Physiological and Pathological Conditions. Int J Mol Sci. 2023, 25, 512. [Google Scholar] [CrossRef]
- Ceafalan, L.C.; Popescu, B.O.; Hinescu, M.E. Cellular players in skeletal muscle regeneration. Biomed Res Int 2014, 2014, 957014. [Google Scholar] [CrossRef]
- Tu, H.; Li, Y.L. Inflammation balance in skeletal muscle damage and repair. Front Immunol 2023, 14, 1133355. [Google Scholar] [CrossRef]
- Kovács, E.; Zádor, E. The effect of a TNF-α inhibiting drug on skeletal muscle regeneration. XXXIV. European Muscle Conference, Hungary, Hortobágy J Muscle Res Cell Motil 2005, 26, p89 (Abstract). [Google Scholar]
- Ermolova, N.V.; Martinez, L.; Vetrone, S.A.; Jordan, M.C.; Roos, K.P.; Sweeney, H.L.; Spencer, M.J. Long-term administration of the TNF blocking drug Remicade (cV1q) to mdx mice reduces skeletal and cardiac muscle fibrosis, but negatively impacts cardiac function. Neuromuscul Disord. 2014, 24, 583–595. [Google Scholar] [CrossRef]
- McCroskery, S.; Thomas, M.; Maxwell, L.; Sharma, M.; Kambadur, R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 2003, 162, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Mendler, L.; Zador, E.; Ver Heyen, M.; Dux, L.; Wuytack, F. Myostatin in regenerating rat muscles and in myogenic cell cultures. J Muscle Res and Cell Mot 2000, 21, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Outeiriño, L.; Hernandez-Torres, F.; Ramírez-de Acuña, F.; Matías-Valiente, L.; Sanchez-Fernandez, C.; Franco, D.; Aranega, A.E. Muscle Satellite Cell Heterogeneity: Does Embryonic Origin Matter? Front Cell Dev Biol 2021, 9, 750534. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, T.B.; Rhodes, S.J.; Moore, J.L.; Sharman, D.A.; Konieczny, S.F.; Taparowsky, E.J. Isolation and structural analysis of the rat myoD gene. Gene 1992, 116, 223–230. [Google Scholar] [CrossRef]
- Miller, J.B.; Everitt, E.A.; Smith, T.H.; Block, N.E.; Dominov, J.A. Cellular and Molecular Diversity in Skeletal Muscle Development: News from in vitro and in vivo. BioEssays 1993, 15, 191–195. [Google Scholar] [CrossRef]
- Olson, E.N.; Klein, W.H. bHLH factors in muscle development: Dead lines and commitments, what to leave in and what to leave out. Genes and Devel 1994, 8, 1–8. [Google Scholar] [CrossRef]
- Motohashi, N.; Asakura, A. Muscle satellite cell heterogeneity and self-renewal. Front Cell Dev Bio. 2014, 2, 1. [Google Scholar] [CrossRef]
- Ludolph, D.C.; Konieczny, S.F. Transcription factor families: Muscling in on the myogenic program. FASEB J 1995, 9, 1595–1604. [Google Scholar] [CrossRef]
- Maione, R.; Amati, P. Interdependence between muscle differentiation and cell cycle control. Biochim Biophys Acta 1997, 1322, M19–M30. [Google Scholar] [CrossRef]
- Tapscott, S.J. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 2005, 132, 2685–2695. [Google Scholar] [CrossRef]
- Lowe, D.A.; Lund, T.; Alway, S.E. Hypertrophy-stimulated myogenic regulatory factor mRNA increases are attenuated in fast muscle of aged quails. Am J Physiol-Cell Physiol 1998, 275, C155–C166. [Google Scholar] [CrossRef]
- Singh, K.; Dilworth, F.J. Differential modulation of cell cycle progression distinguishes members of the myogenic regulatory factor family of transcription factors. FEBS J 2013, 280, 3991–4003. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Rudnicki, M.A. ,.; Schnegelsberg, P.N.J.; Stead, R.H.; Braun, T.; Arnold, H.-H.,; Jaenisch, R. MyoD and myf-5 is required for the formation of skeletal muscle. Cell 1993, 75, 1351–1359. [Google Scholar] [CrossRef]
- Gerber, A.N.; Klesert, T.R.; Bergstrom, D.A.; Tapscott, S.J. Two domains of MyoD mediate transcriptional activation of genes in repressive chromatin: A mechanism for lineage determination in myogenesis. Genes Dev 1997, 11, 436–450. [Google Scholar] [CrossRef]
- Londhe, P.; Davie, J.K. Sequential association of myogenic regulatory factors and E proteins at muscle-specific genes. Skelet Muscle 2011, 1, 14. [Google Scholar] [CrossRef]
- de Martin, X.; Sodaei, R.; Santpere, G. Mechanisms of Binding Specificity among bHLH Transcription Factors. Int. J. Mol. Sci. 2021, 22, 9150. [Google Scholar] [CrossRef]
- Sabourin, L.A.; Rudnicki, M.A. The molecular regulation of myogenesis. Clin Genet 2000, 57, 16–25. [Google Scholar] [CrossRef]
- Zammit, P.S. All muscle satellite cells are equal, but are some more equal than others? J Cell Sci 2008, 121, 2975–2982. [Google Scholar] [CrossRef]
- Günther, S.; Kim, J.; Kostin, S.; Lepper, C.; Fan, C.M.; Braun, T. Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell 2013, 13, 590–601, Erratum in: Cell Stem Cell 2013, 13, 769. [Google Scholar] [CrossRef]
- Zádor, E.; Bottka, S.; Wuytack, F. Antisense inhibition of myoD expression in regenerating rat soleus muscle is followed by an increase in the mRNA levels of myoD, myf-5 and myogenin and by a retarded regeneration. Biochim Biophys Acta Mol Cell Res 2002, 1590, 52–63. [Google Scholar] [CrossRef]
- Grubb, B.; Harris, J.; Schofield, I. Neuromuscular transmission of newly formed neuromuscular junctions in the regenerating soleus muscle. J Physiol 1991, 441, 405–421. [Google Scholar] [CrossRef]
- Abu Hatoum, O.; Gross-Mesilaty, S.; Breitschop, K.; Hoffman, A.; Gonen, H.; Ciechanover, A.; Bengal, E. Degradation of myogenic transcription factor myoD by the ubiquitin pathway in vivo and in vitro: Regulation by specific DNA binding. Mol Cell Biol 1998, 18, 5670–5677. [Google Scholar] [CrossRef]
- Bisbal, C.; Silhol, M.; Laubenthal, H.; Kaluza, T.; Carnac, G.; Milligan, L.; Le Roy, F.; Salehzada, T. The 2V–5Voligoadenylate/RNase L/RNase L inhibitor pathway regulates both myoD mRNA stability and muscle cell differentiation. Mol Cell Biol 2000, 20, 4959–4969. [Google Scholar] [CrossRef]
- Phillis, M.I.; Gyurko, R. Antisense oligonucleotides: New tools for physiology. News Physiol Sci 1997, 12, 99–105. [Google Scholar] [CrossRef]
- Tu, G.-C.; Cao, Q.-N.; Zhou, F.; Yedy, I. Tetranucleotide GGGA motif in primary RNA transcripts. Novel target site for antisense design. J Biol Chem 1998, 273, 25125–31. [Google Scholar] [CrossRef]
- Huang, Y.C.; Dennis, R.G.; Baar, K. Cultured slow vs. fast skeletal muscle cells differ in physiology and responsiveness to stimulation. Am J Physiol Cell Physiol 2006, 291, C11–C17. [Google Scholar] [CrossRef]
- Gorbe, A.; Becker, D.L.; Dux, L.; Stelkovics, E.; Krenacs, L.; Bagdi, E.; Krenacs, T. Transient upregulation of connexin43 gap junctions and synchronized cell cycle control precede myoblast fusion in regenerating skeletal muscle in vivo. Histochem Cell Biol 2005, 123, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Gorbe, A.; Becker, D.L.; Dux, L.; Krenacs, L.; Krenacs, T. In differentiating prefusion myoblasts connexin43 gap junction coupling is upregulated before myoblast alignment then reduced in post-mitotic cells. Histochem Cell Biol 2006, 125, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Gorbe, A.; Krenacs, T.; Cook, J.E.; Becker, D.L. Myoblast proliferation and syncytial fusion both depend on connexin43 function in transfected skeletal muscle primary cultures. Exp Cell Res. 2007, 313, 1135–1148. [Google Scholar] [CrossRef]
- Ishido, M.; Kasuga, N. Characteristics of the Localization of Connexin 43 in Satellite Cells during Skeletal Muscle Regeneration In Vivo. Acta Histochem Cytochem 2015, 48, 53–60. [Google Scholar] [CrossRef]
- Vitale, G.; Ferrantini, C.; Piroddi, N.; Scellini, B.; Pioner, J.M.; Colombini, B.; Tesi, C.; Poggesi, C. The relation between sarcomere energetics and the rate of isometric tension relaxation in healthy and diseased cardiac muscle. J Muscle Res Cell Motil 2021, 42, 47–57. [Google Scholar] [CrossRef]
- Xu, H.; Van Remmen, H. The SarcoEndoplasmic Reticulum Calcium ATPase (SERCA) pump: A potential target for intervention in aging and skeletal muscle pathologies. Skelet Muscle 2021, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Brandl, C.J.; Green, N.M.; Korczak, B.; MacLennan, D.H. Two Ca2+ ATPase genes: Homologies and mechanistic implications of deduced amino acid sequences. Cell 1986, 44, 597–607. [Google Scholar] [CrossRef]
- Brandl, C.J.; DeLeon, S. , Martin, D.R.; MacLennan, D.H. Adult forms of the Ca2+ ATPase of sarcoplasmic reticulum. Expression in developing skeletal muscle. J Biol Chem 1987, 262, 3768–3774. [Google Scholar] [CrossRef]
- Korczak, B.; Zarain-Herzberg, A.; Brandl, C.J.; Ingles, C.J.; Green, M.N.; MacLennan, D.H. Structure of the rabbit fast-twitch skeletal muscle Ca2+ ATPase gene. J Biol Chem 1988, 263, 4813–4819. [Google Scholar] [CrossRef]
- Zádor, E.; Vangheluwe, P.; Wuytack, F. The expression of the neonatal sarcoplasmic reticulum Ca2+ pump (SERCA1b) hints to a role in muscle growth and development. Cell Calcium. 2007, 41, 379–388. [Google Scholar] [CrossRef]
- Zador, E.; Dux, L.; Wuytack, F. Prolonged passive stretch of rat soleus muscle provokes an increase in the mRNA levels of the muscle regulatory factors distributed along the entire length of the fibers. J. Muscle Res. and Cell Mot 1999, 20, 395–402. [Google Scholar] [CrossRef]
- Szabó, A.; Wuytack, F.; Zádor, E. The effect of passive movement on denervated soleus highlights a differential nerve control on SERCA and MyHC isoforms. J Histochem Cytochem. 2008, 56, 1013–1022. [Google Scholar] [CrossRef]
- Zádor, E.; Kósa, M. The neonatal sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA1b): A neglected pump in scope. Pflugers Arch 2015, 467, 1395–1401. [Google Scholar] [CrossRef]
- Kósa, M.; Brinyiczki, K.; van Damme, P.; Goemans, N.; Hancsák, K.-; Mendler, L.; Zádor, E. The neonatal sarcoplasmic reticulum Ca(2+)-ATPase gives a clue to development and pathology in human muscles. J Muscle Res Cell Motil 2015, 36, 195–203. [Google Scholar] [CrossRef]
- Fodor, J.; Gomba-Tóth, A.; Oláh, T.; Zádor, E.; Tóth, Z.C.; Ioannis, I.; Molnár, B.; Kovács, I.; Csernoch, L. Alteration of sarcoplasmic reticulum Ca(2+) ATPase expression in lower limb ischemia caused by atherosclerosis obliterans. Physiol Int 2017, 104, 183–192. [Google Scholar] [CrossRef]
- Zhao, Y.; Ogawa, H.; Yonekura, S.; Mitsuhashi, H.; Mitsuhashi, S.; Nishino, I.; Toyoshima, C.; Ishiura, S. Functional analysis of SERCA1b, a highly expressed SERCA1 variant in myotonic dystrophy type 1 muscle. Biochim Biophys Acta 2015, 1852, 2042–2047. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, V.; Oosterhof, A.; Voermans, N.C.; Cardani, R.; Molenaar, J.P.; van Kuppevelt, T.H.; Meola, G.; van Engelen, B.G.; Tomelleri, G.; Vattemi, G. Characterization of sarcoplasmic reticulum Ca(2+) ATPase pumps in muscle of patients with myotonic dystrophy and with hypothyroid myopathy. Neuromuscul Disord 2016, 26, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Mendler, L.; Szakonyi, G.; Zádor, E.; Görbe, A.; Dux, L.; Wuytack, F. Expression of sarcoplasmic/endoplasmic reticulum Ca2+ ATPases in the rat extensor digitorum longus (EDL) muscle regenerating from notexin-induced necrosis. J Muscle Res Cell Mot 1998b, 19, 777–785, *Authors contributing equally. [Google Scholar] [CrossRef]
- Kiss, G.; Zádor, E.; Szalay, J.; Somogyi, F.; Vér, A. Molecular forms of acetylcholinesterase in rat extensor digitorum longus and soleus muscles regenerating from notexin induced necrosis. J Muscle Res Cell Motil 2004, 25, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, R.; Rácz, G.; Wuytack, F.; Zádor, E. The calcineurin activity and MCIP1.4 mRNA levels are increased by innervation in regenerating soleus muscle. Biochem Biophys Res Com 2004, 320, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Zádor, E.; Fenyvesi, R.; Wuytack, F. Expression of SERCA2a is not regulated by calcineurin or upon mechanical unloading in skeletal muscle regeneration. FEBS Letters 2005, 579, 749–752. [Google Scholar] [CrossRef]
- Serrano, A.L.; Murgia, M.; Pallafacchina, G.; Calabria, E.; Coniglio, P.; Lømo, T.; Schiaffino, S. Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc Natl Acad Sci U S A 2001, 98, 13108–13113. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, K.J.; Calabria, E.; Pallafacchina, G.; Ciciliot, S.; Serrano, A.L.; Argentini, C.; Kalhovde, J.M.; Lømo, T.; Schiaffino, S. NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching. Proc Natl Acad Sci USA 2004, 101, 10590–10595. [Google Scholar] [CrossRef]
- Launay, T.; Noirez, P.; Butler-Browne, G.; Agbulut, O. Expression of slow myosin heavy chain during muscle regeneration is not always dependent on muscle innervation and calcineurin phosphatase activity. Am J Physiol-Regul Integr Comp Physiol. 2006, 290, R1508–R1514. [Google Scholar] [CrossRef]
- Misquitta, C.M.; Chen, T.; Grover, A.K. Control of protein expression through mRNA stability in calcium signalling. Cell Calcium 2006, 40, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Zádor, E. dnRas stimulates autocrine-paracrine growth of regenerating muscle via calcineurin-NFAT-IL-4 pathway. Biochem Biophys Res Com 2008, 375, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Kósa, M.; Zádor, E. Transfection efficiency along the regenerating soleus muscle of the rat. Mol Biotechnol 2013, 54, 220–227. [Google Scholar] [CrossRef]
- Horsley, V.; Jansen, K.M.; Mills, S.T.; Pavlath, G.K. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 2003, 113, 483–494. [Google Scholar] [CrossRef]
- Shaikh, S. , Lee, E.; Ahmad, K.; Ahmad, S.S.; Chun, H.; Lim, J.; Lee, Y.; Choi, I. Cell Types Used for Cultured Meat Production and the Importance of Myokines. Foods 2021, 10, 2318. [Google Scholar] [CrossRef]
- Horsley, V.; Friday, B.B.; Matteson, S.; Kegley, K.M.; Gephart, J.; Pavlath, G.K. Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J Cell Biol 2001, 153, 329–338. [Google Scholar] [CrossRef]
- Ichida, M.; Finkel, T. Ras regulates NFAT3 activity in cardiac myocytes. J Biol Chem 2001, 276, 3524–3530. [Google Scholar] [CrossRef]
- Sanna, B.; Bueno, O.F.; Dai, Y.S.; Wilkins, B.J.; Molkentin, J.D. Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2 signaling pathways regulate cardiac gene expression and cellular growth. Mol Cell Biol 2005, 25m, 865–78. [Google Scholar] [CrossRef]
- Zádor, E.; Owsianik, G.; Wuytack, F. Silencing SERCA1b in a few fibers stimulates growth in the entire regenerating soleus muscle. Histochem Cell Biol 2011, 135, 11–20. [Google Scholar] [CrossRef]
- Pan, Y.; Zvaritch, E.; Tupling, A.R.; Rice, W.J.; de Leon, S.; Rudnicki, M.; McKerlie, C.; Banwell, B.L.; MacLennan, D.H. Targeted disruption of the ATP2A1 gene encoding the sarco(endo)plasmic reticulum Ca2+ ATPase isoform 1 (SERCA1) impairs diaphragm function and is lethal in neonatal mice. The Journal of biological chemistry, 2003, 278, 13367–13375. [Google Scholar] [CrossRef]
- Anderson, D.M.; Anderson, K.M.; Chang, C.L.; Makarewich, C.A.; Nelson, B.R.; McAnally, J.R.; Kasaragod, P.; Shelton, J.M.; Liou, J.; Bassel-Duby, R.; Olson, E.N. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 2015, 160, 595–606. [Google Scholar] [CrossRef]
- Hansson, K.A.; Eftestøl, E.; Bruusgaard, J.C.; Juvkam, I.; Cramer, A.W.; Malthe-Sørenssen, A.; Millay, D.P.; Gundersen, K. Myonuclear content regulates cell size with similar scaling properties in mice and humans. Nature communications 2020, 11, 6288. [Google Scholar] [CrossRef]
- Bagley, J.R.; Denes, L.T.; McCarthy, J.J.; Wang, E.T.; Murach, K.A. The myonuclear domain in adult skeletal muscle fibres: Past, present and future. The Journal of physiology 2023, 601, 723–741. [Google Scholar] [CrossRef]
- Lin, J.G.; Kotha, P.; Chen, Y.H. ; Understandings of acupuncture application and mechanisms. American journal of translational research 2022, 14, 1469–1481. [Google Scholar]
- Rajula, H.S.R. , Mauri, M., & Fanos, V. Scale-free networks in metabolomics. Bioinformation, 2018, 14, 140–144. [Google Scholar] [CrossRef]
- Barabasi, A.L.; Albert, R. Emergence of scaling in random networks. Science, 1999, 286, 509–512. [Google Scholar] [CrossRef]
- Newlands, S. , Levitt, L.K., Robinson, C.S., Karpf, A.B., Hodgson, V.R., Wade, R.P., & Hardeman, E.C. Transcription occurs in pulses in muscle fibers. Genes & development, 1998, 12, 2748–2758. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




