Knee osteoarthritis (OA) is a common condition, with an estimated prevalence of 7.6% among the general population, i.e., around 600 million people worldwide (1). Although not directly life-threatening, knee OA can have a major impact on a patient’s quality of life, and indirectly on mortality, through disability, obesity and cardiovascular problems caused by a lack of physical activity and the use of non-steroidal anti-inflammatory drugs (NSAIDs) (2). Osteoarthritis is characterised by the degradation of hyaline articular cartilage, followed by damage to the various tissues that make up the joint (subchondral bone, synovium, capsule, etc.), leading to pain and functional impairment that can even lead to severe disability. To date, there is no treatment that can restore the cartilage or even halt its deterioration. The only definitive treatment is knee replacement, with the problems associated with surgery, its cost, the anaesthetic and the limited lifespan of the prosthesis, which often requires a repeat operation 1 or 2 decades later.
Conservative treatments for knee OA include a combination of pharmacological and non-pharmacological modalities (3, 4, 5), none of which is considered to be highly effective. Among the pharmacological methods, viscosupplementation by intra-articular (IA) injection(s) of hyaluronic acid (HA) has the highest effect size (0.63 - 95%, CrI :0.39 to 0.88) (6). The concept of viscosupplementation was introduced in the 1990s by EA. Balazs (7) who hypothesised that injecting high molecular weight HA intra-articularly would improve joint function by restoring the viscoelastic properties of the synovial fluid (SF). It has since been demonstrated that HA has not only a lubricating and shock-absorbing effect, but also other anti-inflammatory, analgesic, anti-apoptotic and anti-degenerative properties (8). Viscosupplementation is a symptomatic treatment for pain in knee OA, recommended by several learned societies (3-5) when first-line treatments (analgesics and NSAIDs) are not sufficiently effective. The safety of viscosupplementation is excellent; the relative risk of an adverse reaction versus saline is 1.01 (95%CI 0.96-1.07 P = 0.6) (9), making it the treatment of choice for a population that is often elderly and compromised by pre-existing conditions. However, although it is widely used in current practice and has produced good results in clinical practice (10), its actual level of efficacy remains controversial. This is why the Osteoarthritis Research Society International (OARSI) (11) and the American College of Rheumatology (12) do not recommend viscosupplementation in all situations and prefer, as first-line treatments, NSAIDs (topical and oral), corticosteroid IA injections, as well as non-pharmacological methods (weight loss, physical exercise, balance and proprioception training). HA injections are only recommended conditionally, in the event that first-line treatments fail or are unsuitable.
All the guidelines agree that knee OA treatment must be personalised and adapted to the needs and profile of the patient in order to achieve the most efficient response possible and provide the best possible advice to patients (3-5,11,12). We have previously shown that obesity and the radiographic severity of OA were independent factors in a poorer response to viscosupplementation (13, 14). The aim of this new study was to identify the predictive factors of the duration of viscosupplementation efficacy under “real-life” conditions, by not only studying the role of radiological and demographic characteristics, but also by including many other factors in the analysis, such as lifestyle habits, pre-existing conditions, treatments of comorbidities, and current and previous treatments for OA. We have chosen to describe the duration of response to treatment according to the patient’s clinical experience in order to reflect current clinical daily practice as closely as possible.
Patients and method.
PRESAGE (ClinicalTrials.gov Identifier: NCT04988698). was a single-centre, cross-sectional study, conducted in 2022 and 2023 at the Hôpital Nord Franche-Comté (HNFC- Belfort, France), aimed at studying factors predicting the duration of effectiveness (DE) of viscosupplementation in patients suffering from knee OA.
The study received approval from the Comité de Protection des Personnes Sud EST III (ID-CRB No. 2021-A00773-38). The trial was conducted in accordance with good clinical practice and the Declaration of Helsinki.
Patient inclusion criteria.
All adult outpatients in the rheumatology department of the Hôpital Nord Franche-Comté more than 2 months and less than 3 years after having been treated with viscosupplementation for symptomatic knee OA and who agreed to participate, were included in the study.
Patient exclusion criteria.
Patients who were unable to complete the questionnaire due to cognitive problems or language barriers were excluded from the study, as were patients who had not given their consent and those treated with viscosupplementation for a reason unrelated to knee OA.
Study progression
During a routine consultation, the investigator gave the patient an information document in order to obtain informed consent.
The investigator collected demographic data (age, sex, weight, height, body mass index - BMI) and radiographic data on the knees, including the Kellgren-Lawrence grades modified by Felson (15) and the compartments affected by OA (i.e. patellofemoral [PF], medial tibiofemoral [MTF] and lateral tibiofemoral [LTF]). The radiographs were centrally read by the same experienced investigator (TC).
The investigator also reported the treatment regimen performed, single or repeated injections: single-injection procedures were performed exclusively with cross-linked HA (HappyCross®, Synvisc-One®, Durolane®, Hymovis®). Patients who had been injected with linear HA (Happyvisc®, Arthrum®, Synolis-VA®) systematically underwent a procedure consisting of 3 injections separated by 7 days.
The presence and volume of any joint effusion on the day HA was administered was recorded, as was the number of previous viscosupplementations, previous IA corticosteroid injections, or IA steroid injections concomitant with viscosupplementation.
Finally, the patient completed a questionnaire including:
- Self-assessment of the duration of treatment efficacy (DE = number of weeks during which viscosupplementation was effective on symptoms)
- The degree of satisfaction with the treatment on a numerical scale from 0 to 10.
- Activity level: sedentary, active, athletic
- Physical activity practised and intensity: light, moderate and intense.
For the statistical analysis, the physical activity practised was divided into two categories; “low impact” on the knee joint and “high impact” comprising sports with a moderate and/or high impact on the knee according to Buckwalter and Jane’s classification (16). The “low impact” group includes walking, hiking, Pilates, swimming, downhill skiing and occasional cycling. The “high impact” group includes moderate to intense running, trail running, skating cross-country skiing, tennis and football, and intense cycling. Based on these considerations, the strain on the knees has been classified as low, light, normal or heavy.
Statistical analysis
The data were analysed using the R++ software (C. Genolini, A. Evain, J.Lopez, M.Heinrich, Released 2023-12-13, "R++, l’essentiel" for Windows, Version 1.6.15, Toulouse, France). A descriptive statistical analysis was carried out on the population, expressed as headcount, mean, standard deviation for quantitative variables and percentage, confidence interval for qualitative variables.
For the bivariate analysis, we used Welch’s t-test, Student’s t-test, Mann-Whitney U-test or one-way ANOVA, as appropriate. The primary endpoint was the DE self-reported by the patient. The significance level was set at 5%. A multivariate analysis was then performed using linear regression according to factors with a p-value < 0.1 found in the bivariate analysis.
Results.
After inclusion, 105 patients completed the questionnaire, the general characteristics of which are shown in
Table 1. The mean age was 66.1 ± 13.2 years, and the mean BMI was 27.5 ± 7.5 kg/m
2. Our population included a larger proportion of women (62%) and retired people (57%).
For the statistical analysis of the primary endpoint, we studied this population by knee treated, with a total of 149 knees studied, the details of which are given in
Table 2. The male/female distribution remained similar, as did the proportion of athletes.
The mean and median radiological grade was 3, representing 27.5% of the knees. The early radiological grades, 1-2, accounted for 43% of the knees, compared with 57% for grades 3 and 4. 60% of patients exhibited a unicompartmental TF or PF form, while 40% were affected in 2 or 3 compartments.
There was a high prevalence of oral treatment, mainly level I analgesics (45%), while 25% of our patients were taking symptomatic slow-acting drugs for osteoarthritis (SYSADOA). With regard to viscosupplementation, 89% of injections were performed with cross-linked HA using a single-injection procedure. Only 11% were performed with linear hyaluronic acid over 3 injections. On the day of viscosupplementation, the majority of knees studied (68%) had an effusion, most of which was small (mean 1.98ml ± 4.47mL [range 0.1-50]), requiring concomitant IA long-lasting corticosteroid injection in only 10 cases.
The mean duration of efficacy of viscosupplementation in the whole population was 48.2 + 24.8 weeks; the median was 48 weeks. The detailed characteristics of the population are shown in
Table 2.
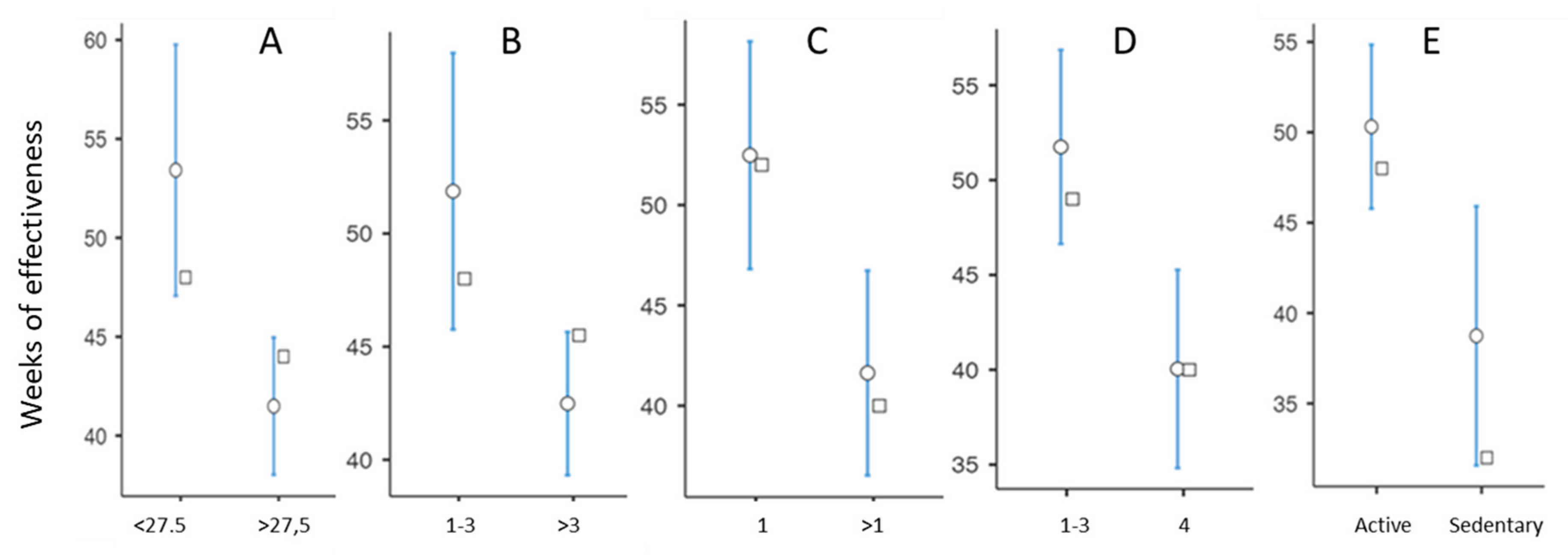
In a bivariate analysis of the factors associated with DE, we found a strong influence of BMI, with a mean DE of 53.4
+ 29.7 weeks in patients with a BMI <27.5kg/m2 compared with 41.16 ± 14.36 weeks in patients with a BMI >27.5kg/m2 (p=0.002) With regard to the number of viscosupplementations performed, we found a significant reduction in DE from the 4th cycle of injections onwards (
Figure 1B).
The location of the OA also had a statistically significant impact. OA having the best DE was the isolated MTF involvement, which had an average DE of 57.3 ± 31.8 weeks. More generally, the unicompartmental forms had a DE that was 11 weeks longer on average than the pluri-compartmental forms (p = 0.01) (
Figure 1C). In the unicompartmental forms, subjects with MTF OA had a longer mean DE than those with PF and LTF OA, at the limit of statistical significance (p = 0.10).
Radiographic grade 4 was associated with a decrease in DE of around 12 weeks, with a mean DE of 40.0 ± 17.9 weeks, compared with 51.8 ±26.6 for grades <4. (
Figure 1 D). There was no statistically significant difference in DE between grades 1, 2 and 3. Active patients had a mean DE of 50.3 ± 25.5 weeks, 12 weeks longer than sedentary patients, whose mean DE was 38.7 ± 19.0 weeks (
Figure 1E). It should be noted that there was no statistical difference in DE between patients practising sports and those not practising sports, or between patients practising high-impact and low-impact sports.
There was no statistically significant difference in DE according to the duration of symptoms, use of SYSADOAs or NSAIDs, presence and volume of effusion, dose regimen, or concomitant injection of corticosteroids. Women had a moderately shorter DE, but this was not statistically significant (p = 0.138). The presence of one or more pre-existing conditions did not affect DE. Surprisingly, however, we found a borderline statistically significant association between treated arterial hypertension and DE, with a longer DE in hypertensive patients compared with non-hypertensive patients (53,1 ± 31,3 versus 45,4 ± 19,8 weeks; p =0.068). Details of these data are summarised in
Table 3 and
Table 4.
In the multivariate analysis, we identified 4 independent factors associated with a shorter DE: BMI >27.5 kg/m2, knee multi-compartment damage, number of viscosupplements >3, sedentary lifestyle. We also found a statistically highly significant association between a longer DE and treated arterial hypertension (p<0.001). It is important to emphasize that in multivariate analysis, the radiological grade no longer appeared associated with DE (p = 0.22). (Table 5)
Discussion.
This study confirms once again that overweight is associated with a shorter duration of efficacy of viscosupplementation, as it has already been described in obesity (13,14). In our study, we showed that the DE decreased even in cases where the patient was moderately overweight, from a BMI of 27.5 kg/m2. It is well demonstrated that an increase in body fat is associated with higher levels of pro-inflammatory cytokines, increased production of adipokines with deleterious effects on articular cartilage, upregulation of proteolytic enzymes such as matrix metalloproteinases and agrecanases, and increased production of reactive oxygen species, all of which are involved in the pathophysiology of OA (16). This reinforces the importance of weight loss in patients suffering from OA and could be one explanation for the potentially reduced efficacy of this treatment in North America, where the prevalence of obesity is around 32%, compared with 17% in France (17). Wang et al reported poorer efficacy in patients aged over 65 years (18), which we did not find in terms of duration of efficacy. However, although 41% of our patient population was under 65 years of age, there were very few patients under 50 (8%). A case-control study could reveal a hidden difference in our study. Another explanation could be that older patients are more satisfied with a more modest effect, as has already been pointed out (19).
Although we found in a bivariate analysis that advanced radiological grade was a factor in the reduced response to viscosupplementation we did not find any statistical difference in a multivariate analysis between DE and radiological grade 4, unlike Eymard and al (13), Altman et al (20) and Perruchet et al (21). This may be explained by the fact that, in the present study, which was carried out under everyday conditions, patients with radiological grade 4 were likely mildly or moderately symptomatic, whereas patients with more severe symptoms were referred to a surgeon for arthroplasty. Although we found a better DE for viscosupplementation in unicompartmental OA, particularly MTF, the DE in multi-compartmental damage remained satisfactory (average 41.6 weeks).
We found no difference between a triple-injection dosing regimen with linear HA and a single-injection approach with cross-linked HA, demonstrating that cross-linking is a valid method that allows for a single injection, which is beneficial for the patient, the doctor’s schedule, indirect costs and carbon footprint.
It is interesting to note that we observed a constant DE over time until the fourth treatment cycle, beyond which DE decreased slightly but remained still satisfactory. The presence of an effusion at the time of viscosupplementation did not affect the DE. However, only 3 knees from our population had an effusion of more than 10mL. It is interesting to underline that the only patient with a large effusion of 50mL reported an efficacy of only 34 weeks, despite being active and having a BMI<27.5, two factors contributing to long DE. We did not observe that an injection of corticosteroids concomitant with the injection of HA significantly increased the DE of the latter. However, the small number of patients who received a corticosteroid injection was too small to draw definitive conclusions. In terms of physical activity, we found no reduction in the DE in athletic patients, including in activities that put a lot of strain on the knees. However, these patients are probably more demanding in terms of joint function, with a potentially lower DE than non-athletic patients. Although the DE was not significantly different in athletic and active patients, we found a significantly shorter DE in sedentary patients than in active or athletic patients, including in the multivariate analysis. This justifies the value of physical activity in the management of knee OA as already established, and not to restrict the exercise of patients suffering from OA. Concerning the practice of “extreme” sports such as very long distance running (only 1 patient practised endurance trail running in our population), which is very popular at the moment and which is likely to be common practice among patients with OA in a few years’ time, it is advisable to be cautious and a specific case-control study could be useful, given the limited number of people with OA currently practising these sports.
Surprisingly, we also found a longer DE in patients treated for arterial hypertension. As it is unlikely that hypertension has a positive effect on OA symptoms, the most logical hypothesis is that certain anti-hypertensive medications could have a beneficial effect. We did not record the medication taken by the patients, but in terms of frequency, the most frequently prescribed medications in France are angiotensin II receptor blockers and diuretics followed by beta-blockers, calcium channel blockers and Angiotensin-converting enzyme (ACE) inhibitors (22). Several experimental studies (23-32) have shown that certain anti-hypertensives have potentially beneficial effects in OA, whether through an anti-inflammatory, antioxidant, analgesic or even anti-degenerative effect. Our work is therefore in line with the literature on the potential chondroprotective effect of certain anti-hypertensive medications, but as we did not record the medications, we cannot draw any conclusions in this respect. Other studies specifically designed for this purpose will have to be carried out to confirm or refute the beneficial role of anti-hypertensive treatment on symptoms of knee OA.
Our study has obviously several strengths and limitations. The main strength is that it evaluates the DE of viscosupplementation in real-life conditions, without selecting age, BMI or radiographic grade. In addition, all the patients evaluated were treated according to the recommended procedures, i.e., a single injection of cross-linked product or a triple injection of linear product with a 7-day interval between each injection, in a centre specialising in the treatment of OA patients and injected by highly experienced senior rheumatologists, with a centralised reading of the radiographs. Finally, to our knowledge, this is the first study to look at such a large number of parameters, in particular patients’ lifestyle habits. The decision to choose “patient self-assessment of DE” as the primary endpoint was a pragmatic one. Although the notion of effectiveness is subjective and varies from subject to subject, depending on their expectations, it corresponds to clinical practice where composite scores (e.g., Western Ontario and McMaster Universities Arthritis score -WOMAC, Knee Injury and Osteoarthritis Outcome -KOOS) (33) are rarely used in routine consultations. However, efficacy is well correlated with patient satisfaction and a reduction in the patient’s global assessment and WOMAC score (34).
One of the weaknesses of our study is that it is mono-centric. The results therefore reflect the habits of a single centre. However, as pointed out above, the investigators had considerable experience of viscosupplementation. In addition, the cross-sectional nature of the study means that we have no quantified assessment of changes in disability and pain over time. However, as mentioned above, the patient’s feelings have been shown to be well correlated with these values (34). We also have certain information biases. The data on physical activity and intensity were reported by the patient and we had no objective data to corroborate them. We also had a patient recruitment bias, with some patients being systematically called back at 1 year, resulting in the DE being capped at 52 weeks, whereas some patients had opted not to return until the pain had returned. Finally, we were unable to assess the number of patients who met the inclusion criteria but were not seen again, either because of treatment failure or because efficacy was still in progress and did not justify a return visit.
In conclusion, despite its limitations, our work provides new useful information concerning the predictors of success of viscosupplementation, as one of the doctor’s duties is to accurately inform patients about the treatment offered to them. While it confirms the harmful influence of an increased BMI, the novelty of this study lies in the fact that the negative influence of BMI appears as early as 27.5 kg/m2, which corresponds to moderately overweight patients. It also shows that viscosupplementation is more effective in unicompartmental OA, while radiological grade 4 appears to have a lesser influence than previously published. Other points worth highlighting are the reduction in the duration of efficacy with repeated cycles of injections and the absence of any difference between patients practising sport and those who regularly use their lower limbs in their everyday activities. Once again, this confirms the value of physical activity in the overall management of OA of the knee. Finally, contrary to what we might have thought, our results do not show a shorter DE in patients practising high-impact sports than in athletic people engaged in activities that place less strain on the knees.
Author Contributions
TC conceptualized and designed the study; AL, FB and TC recruited the patients and collected their data. CB and CR performed data entry and statistical analysis. CR wrote the manuscript. TC reviewed and revised the draft. All authors have read and accepted the published version of the manuscript.
Funding
The “Hôpital Nord Franche-Comté” (HNFC) was the sponsor of the study and covered the expenses related to the latter. No investigators were paid for this work.
Institutional Review Board Statement
The study received approval from the Comité de Protection des Personnes Sud EST III , France (ID-CRB No. 2021-A00773-38). The trial was conducted in accordance with good clinical practice and the Declaration of Helsinki.
Informed Consent Statement
The patient’s informed consent was obtained before patients were included in the trial.
Data Availability Statement
Data are available at the Clinical Research Unit of Hôpital Nord Franche-Comté (URC HNFC), Trevenans, France.
Acknowledgments
The authors thank Elodie Bouvier (URC HNFC) for coordinating the regulatory aspect of research.
Conflicts of Interest
TC reports grants from LABRHA SAS, MEDAC, SYMATESE. The remaining authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
References
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [CrossRef]
- Cleveland, R.J.; Nelson, A.E.; Callahan, L.F. Knee and hip osteoarthritis as predictors of premature death: a review of the evidence. Clin Exp Rheumatol. 2019, 37 Suppl 120, 24-30. PMID: 31621563; PMCID: PMC6934074.
- Sellam, J.; Courties, A.; Eymard, F.; Ferrero, S.; Latourte, A.; Ornetti, P.; et al. French Society of Rheumatology Recommendations of the French Society of Rheumatology on pharmacological treatment of knee osteoarthritis. .Joint Bone Spine 2020, 87, 548–55. [Google Scholar] [CrossRef]
- Bruyère Cooper, C.; Pelletier, J.P.; Branco, J.; Luisa Brandi, M.; Guillemin, F.; et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: A report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2014 S0049-0172(14)00108-5. [CrossRef]
- Jordan, K.M.; Arden, N.K.; Doherty, M.; Bannwarth, B.; Bijlsma, J.W.; Dieppe, P.; et al. Standing committee for international clinical studies including therapeutic trials ESCISIT. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003, 62, 1145–55. [Google Scholar]
- Bannuru, R.R.; Schmid, C.H.; Kent, D.M.; Vaysbrot, E.E.; Wong, J.B.; McAlindon, T.E. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015, 162, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A.; Denlinger, J.L. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993, 39, 3–9 PMID: 8410881. [Google Scholar] [PubMed]
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Bhattacharyya, S.; Parrish, W.R.; Fredericson, M.; Bisson, B.; Altman, R.D. Safety of Intra-Articular Hyaluronic Acid for Knee Osteoarthritis: Systematic Review and Meta-Analysis of Randomized Trials Involving More than 8,000 Patients. Cartilage. 2019:1947603519888783. [CrossRef]
- Migliore, A.; Bizzi, E.; Herrero-Beaumont, J.; Petrella, R.J.; Raman, R.; Chevalier, X. The discrepancy between recommendations and clinical practice for viscosupplementation in osteoarthritis: mind the gap! Eur Rev Med Pharmacol Sci. 2015, 19, 1124–9 PMID: 25912569. [Google Scholar] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019 11, 1578–1589. [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis & Rheumatology. 2020, 72, 220–33. [Google Scholar] [CrossRef]
- Eymard, F.; Chevalier, X.; Conrozier, T. Obesity and radiological severity are associated with viscosupplementation failure in patients with knee osteoarthritis. J Orthop Res. 2017, 35, 2269–2274. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T.; Eymard, F.; Chouk, M.; Chevalier, X. Impact of obesity, structural severity and their combination on the efficacy of viscosupplementation in patients with knee osteoarthritis. BMC Musculoskelet Disord. 2019, 20, 376. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Niu, J.; Guermazi, A.; Sack, B.; Aliabadi, P. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis. 2011, 70, 1884–6. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Lane, N.E. Athletics and osteoarthritis. Am J Sports Med. 1997, 25, 873–81. [Google Scholar] [CrossRef] [PubMed]
- Shumnalieva, R.; Kotov, G.; Monov, S. Obesity-Related Knee Osteoarthritis-Current Concepts. Life (Basel). 2023, 13, 1650. [Google Scholar] [CrossRef]
- Matta, J.; Carette, C.; Rives Lange, C.; Czernichow, S. Épidémiologie de l’obésité en France et dans le monde [French and worldwide epidemiology of obesity]. Presse Med. 2018, 47, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; Lin, J.; Chang, C.J.; Lin, Y.T.; Hou, S.M. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee: a meta-analysis of randomized controlled trials. J Bone Joint Surg Am 2004, 86-A, 538-45. [CrossRef] [PubMed]
- Conrozier, T.; Mathieu, P.; Schott, A.M.; Laurent, I.; Hajri, T.; Crozes, P.; et al. Factors predicting long-term efficacy of Hylan GF-20 viscosupplementation in knee osteoarthritis. Joint Bone Spine. 2003, 70, 128–33. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Farrokhyar, F.; Fierlinger, A.; Niazi, F.; Rosen, J. Analysis for prognostic factors from a database for the intra-Articular hyaluronic acid (Euflexxa) treatment for osteoarthritis of the knee. Cartilage 2016, 7, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Perruchet, S.; Balblanc, J.C.; Rapp, C.; Bourgoin, C.; Guillochon, C.; Lohse, A.; et al. The Association between Radiographic Features and the Duration of Effectiveness of a Single Injection of Extended-Release Hyaluronic Acid (HANOX-M-XL) in Patients with Knee Osteoarthritis: Preliminary Results of a Prospective Trial. Cartilage. 2023, 14, 136–143. [Google Scholar] [CrossRef]
- HAS, Haute Autorité de Santé. Évaluation par classe des médicaments antihypertenseurs. https://www.has-sante.fr/upload/docs/application/pdf/2013-05/rapport_evaluation_medicaments_antihypertenseurs.
- Tang, Y.; Hu, X.; Lu, X. Captopril, an angiotensin-converting enzyme inhibitor, possesses chondroprotective efficacy in a rat model of osteoarthritis through suppression local renin-angiotensin system. Int J Clin Exp Med. 2015, 8, 12584–92 PMID: 26550169; PMCID: PMC4612854. [Google Scholar]
- Wang, Y.; Kou, J.; Zhang, H.; Wang, C.; Li, H.; Ren, Y.; Zhang, Y. The renin-angiotensin system in the synovium promotes periarticular osteopenia in a rat model of collagen-induced arthritis. Int Immunopharmacol. 2018, 65, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Silveira, K.D.; Coelho, F.M.; Vieira, A.T.; Barroso, L.C.; Queiroz-Junior, C.M.; Costa, V.V.; et al. Mechanisms of the anti-inflammatory actions of the angiotensin type 1 receptor antagonist losartan in experimental models of arthritis. Peptides. 2013, 46, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.M.; Mansour, M. Effects of captopril on interleukin-6, leukotriene B(4), and oxidative stress markers in serum and inflammatory exudate of arthritic rats: evidence of antiinflammatory activity. Toxicol Appl Pharmacol. 2000, 168, 123–30. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Abhishek, A.; Muir, K.; Zhang, W.; Maciewicz, R.A.; Doherty, M. Association of Beta-Blocker Use With Less Prevalent Joint Pain and Lower Opioid Requirement in People With Osteoarthritis. Arthritis Care Res (Hoboken). 2017, 69, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Kwoh, C.K.; Ran, D.; Ashbeck, E.L.; Lo-Ciganic, W.H. Lack of evidence that beta blocker use reduces knee pain, areas of joint pain, or analgesic use among individuals with symptomatic knee osteoarthritis. Osteoarthritis Cartilage. 2020, 28, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Uzieliene, I.; Bernotiene, E.; Rakauskiene, G.; Denkovskij, J.; Bagdonas, E.; Mackiewicz, Z.; et al. The Antihypertensive Drug Nifedipine Modulates the Metabolism of Chondrocytes and Human Bone Marrow-Derived Mesenchymal Stem Cells. Front Endocrinol (Lausanne). 2019, 10, 756. [Google Scholar] [CrossRef]
- Yao, J.; Long, H.; Zhao, J.; Zhong, G.; Li, J. Nifedipine inhibits oxidative stress and ameliorates osteoarthritis by activating the nuclear factor erythroid-2-related factor 2 pathway. Life Sci. 2020, 253, 117292. [Google Scholar] [CrossRef] [PubMed]
- Aleksiuk, V.; Baleisis, J.; Kirdaite, G.; Uzieliene, I.; Denkovskij, J.; Bernotas, P.; et al. Evaluation of Cartilage Integrity Following Administration of Oral and Intraarticular Nifedipine in a Murine Model of Osteoarthritis. Biomedicines. 2023, 11, 2443. [Google Scholar] [CrossRef] [PubMed]
- Daniilidis, K.; Georges, P.; Tibesku, C.O.; Prehm, P. Positive side effects of Ca antagonists for osteoarthritic joints-results of an in vivo pilot study. J Orthop Surg Res. 2015, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jones, M.H.; Khair, M.M.; Miniaci, A. Patient-reported outcome measures for the knee. J Knee Surg. 2010, 23, 137–51. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T.; Monet, M.; Lohse, A.; Raman, R. Getting Better or Getting Well? The Patient Acceptable Symptom State (PASS) Better Predicts Patient’s Satisfaction than the Decrease of Pain, in Knee Osteoarthritis Subjects Treated with Viscosupplementation. Cartilage 2018, 9, 370–377. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).