Submitted:
26 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Device
2.3. Intervention
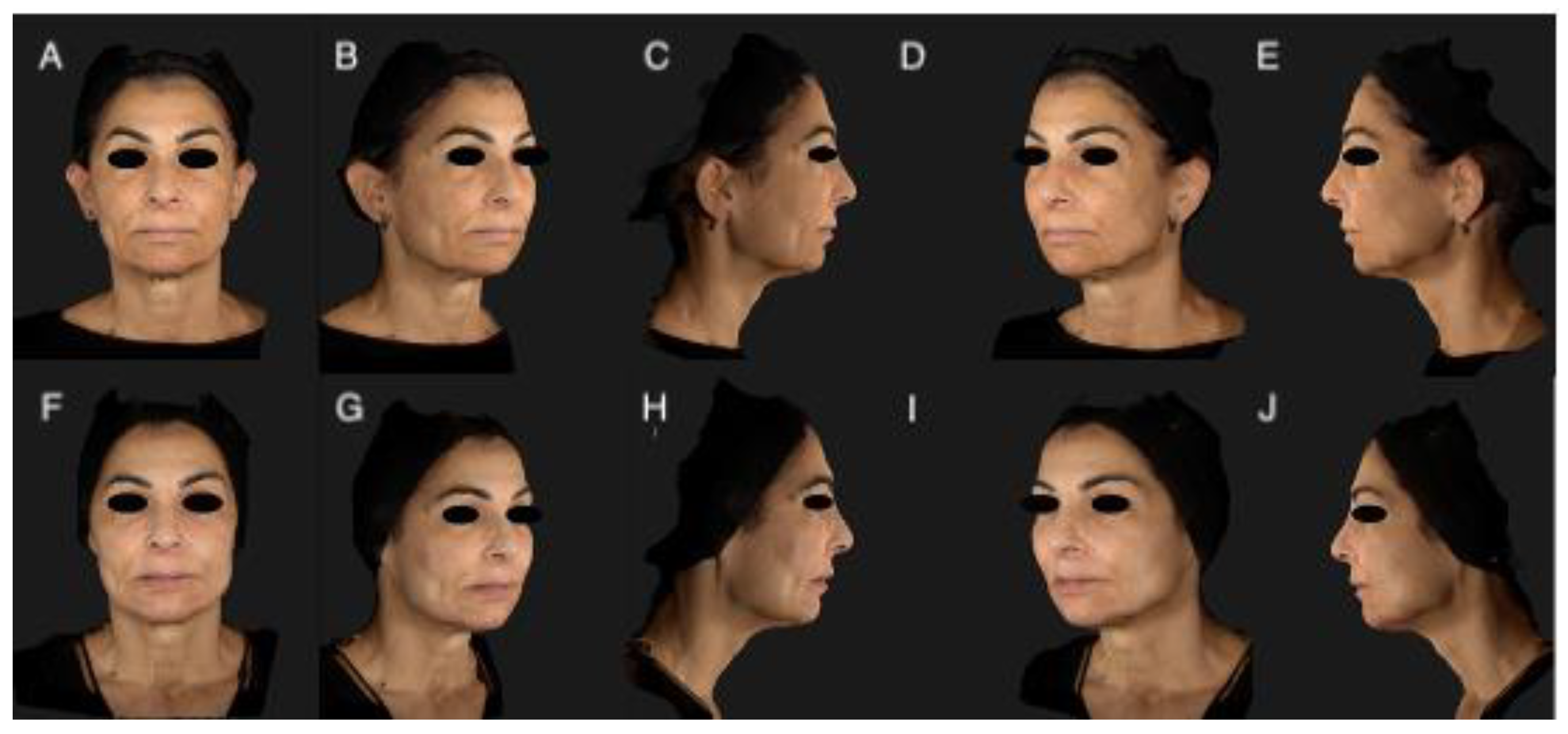
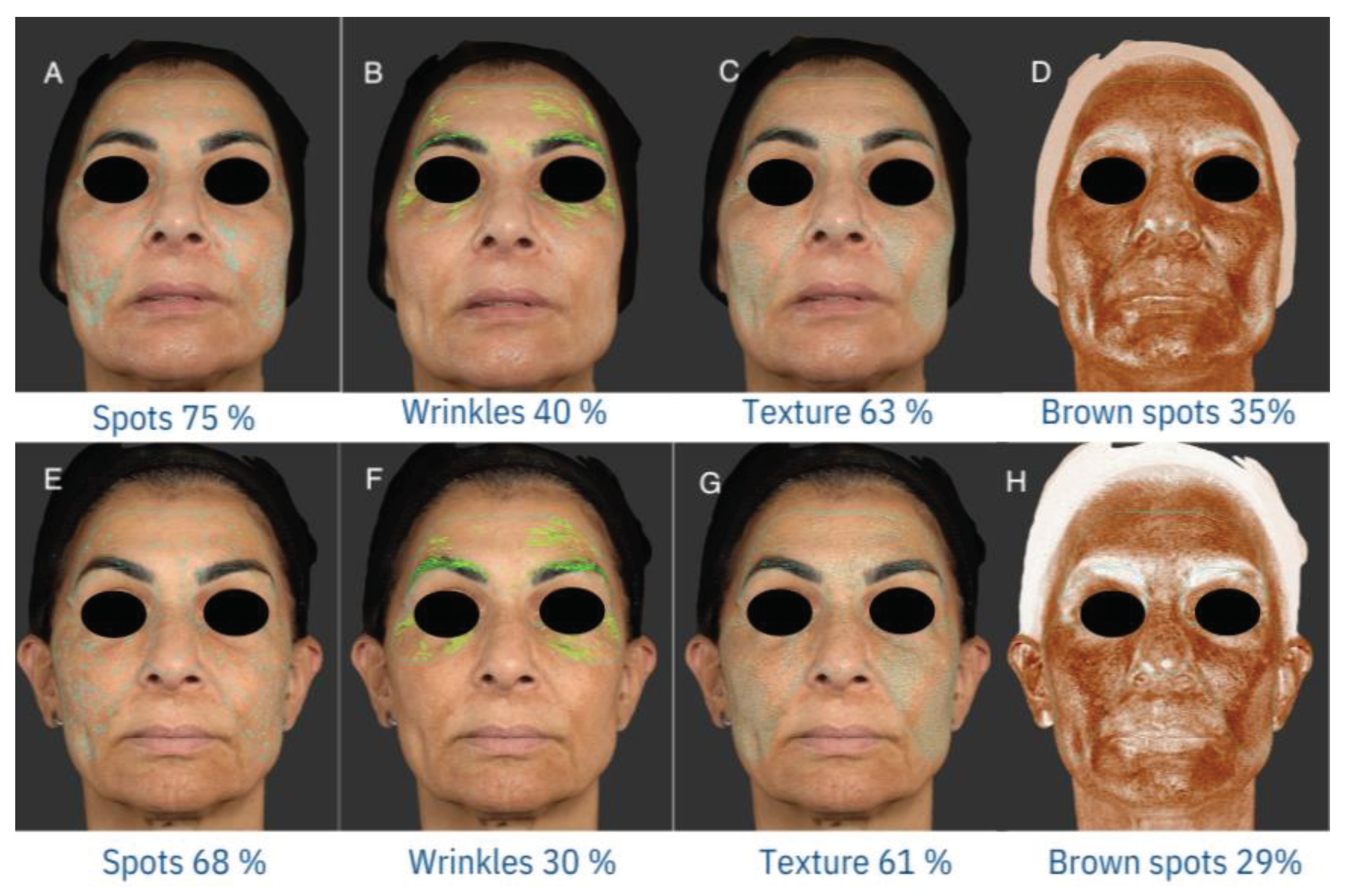
2.4. Photographic Documentation
2.5. mMASI
2.6. GAIS
2.7. Evaluation of Tolerability
2.8. Statistical Analysis
3. Results
3.1. Subjects
| Number of subjects (%) | ||
|---|---|---|
| Age | 18-40 | 2 (10) |
| 41-50 | 8 (40) | |
| 51-60 >60 |
6 (30) 4 (20) |
|
| Sex | Male | 4 (20) |
| Female | 16 (80) | |
| Fitzpatrick Phototype | I | 1 (5) |
| II | 12(60) | |
| III | 7(35) | |
| Number of sessions | 4 | 7 (35) |
| 5 | 13 (65) |
3.2. mMASI
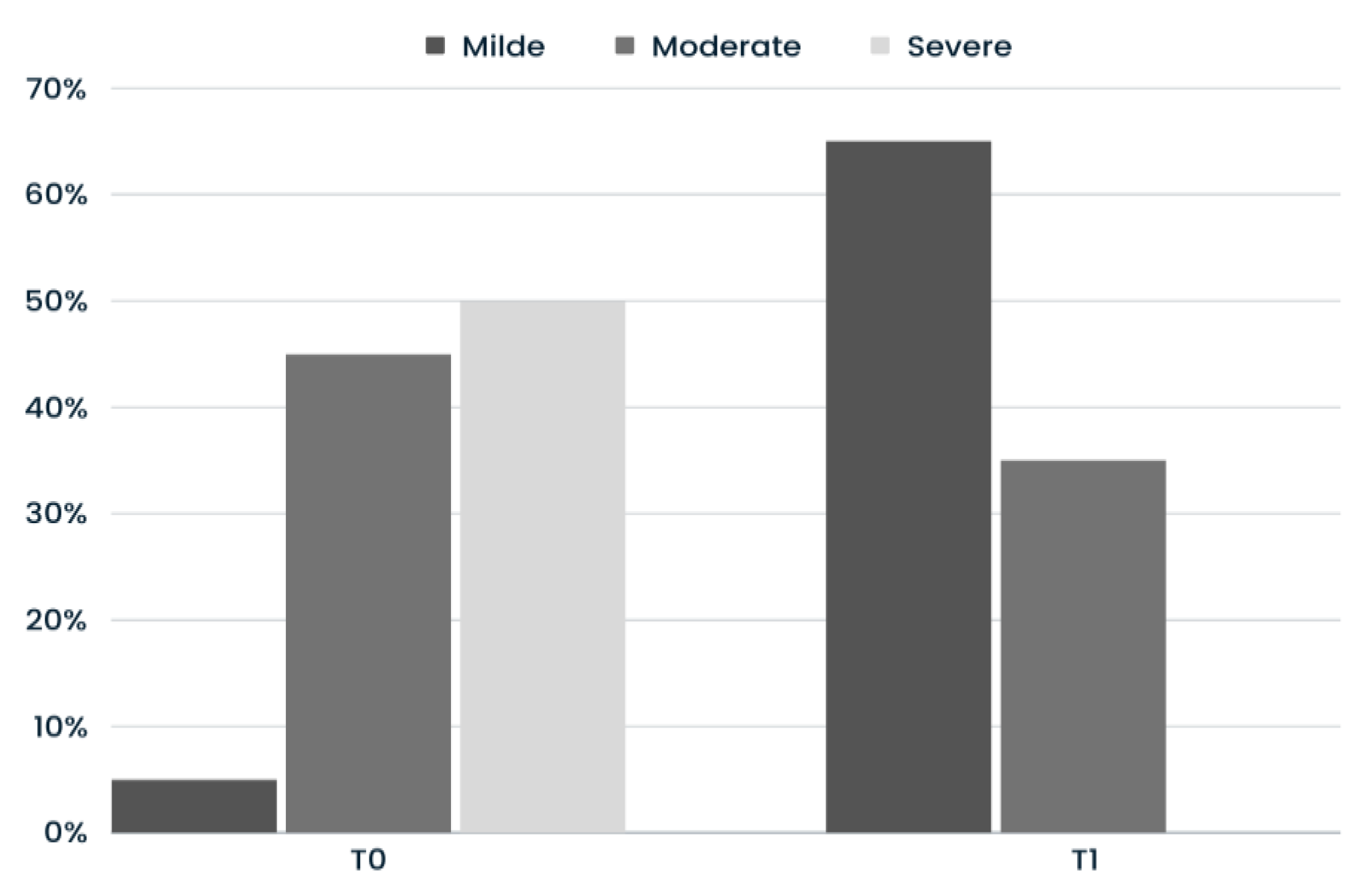
3.3. GAIS
|
Much Improved 4 (20%) |
GAIS Improved 14 (70%) |
No Change 2 (10%) |
||
|---|---|---|---|---|
| Age (yrs) | 18-40 | 0 (0.0%) | 1 (7.1%) | 1 (50.0%) |
| 41-50 | 3 (75.0%) | 5 (35.7%) | 0 (0.0%) | |
| 51-60 >60 |
1 (25.0%) 0 (0.0%) |
4 (28.6%) 4 (28.6%) |
1 (50.0%) 0 (0.0%) |
|
| Sex | Male | 1 (25.0%) | 3 (25.0%) | 0 (0.0%) |
| Female | 3 (75.0%) | 11 (78.6%) | 2 (100%) | |
| Fitzpatrick Phototype | I | 0 (0.0%) | 1 (7.1%) | 0 (0.0%) |
| II | 4 (100%) | 6 (42.9%) | 2 (100%) | |
| III | 0 (0.0%) | 7 (50.0%) | 0 (0.0%) | |
| Number of sessions | 4 | 1 (25.0%) | 4 (28.6%) | 2 (100%) |
| 5 | 3 (75.0%) | 10 (71.4%) | 0 (0.0%) |
3.4. Evaluation of Tolerability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou LL, Baibergenova A. Melasma: systematic review of the systemic treatments. Int J Dermatol. 2017 Sep;56(9):902-908. Epub 2017 Feb 27. PMID: 28239840. [CrossRef]
- Kang WH, Yoon KH, Lee ES, Kim J, Lee KB, Yim H, Sohn S, Im S. Melasma: histopathological characteristics in 56 Korean patients. Br J Dermatol. 2002 Feb;146(2):228-37. PMID: 11903232. [CrossRef]
- Espósito ACC, Cassiano DP, da Silva CN, Lima PB, Dias JAF, Hassun K, Bagatin E, Miot LDB, Miot HA. Update on Melasma-Part I: Pathogenesis. Dermatol Ther (Heidelb). 2022 Sep;12(9):1967-1988. Epub 2022 Jul 29. PMID: 35904706; PMCID: PMC9464278. [CrossRef]
- Lee DJ, Park KC, Ortonne JP, Kang HY. Pendulous melanocytes: a characteristic feature of melasma and how it may occur. Br J Dermatol. 2012 Mar;166(3):684-6. Epub 2012 Feb 6. PMID: 21936855. [CrossRef]
- Kwon SH, Hwang YJ, Lee SK, Park KC. Heterogeneous Pathology of Melasma and Its Clinical Implications. Int J Mol Sci. 2016 May 26;17(6):824. PMID: 27240341; PMCID: PMC4926358. [CrossRef]
- Kang HY, Hwang JS, Lee JY, Ahn JH, Kim JY, Lee ES, Kang WH. The dermal stem cell factor and c-kit are overexpressed in melasma. Br J Dermatol. 2006 Jun;154(6):1094-9. PMID: 16704639. [CrossRef]
- Kapoor R, Dhatwalia SK, Kumar R, Rani S, Parsad D. Emerging role of dermal compartment in skin pigmentation: comprehensive review. J Eur Acad Dermatol Venereol. 2020 Dec;34(12):2757-2765. Epub 2020 May 13. PMID: 32243635. [CrossRef]
- Rendon M, Berneburg M, Arellano I, Picardo M. Treatment of melasma. J Am Acad Dermatol. 2006 May;54(5 Suppl 2):S272-81. PMID: 16631968. [CrossRef]
- Bertold C, Fontas E, Singh T, Gastaut N, Ruitort S, Wehrlen Pugliese S, Passeron T. Efficacy and safety of a novel triple combination cream compared to Kligman's trio for melasma: A 24-week double-blind prospective randomized controlled trial. J Eur Acad Dermatol Venereol. 2023 Dec;37(12):2601-2607. Epub 2023 Sep 4. PMID: 37620285. [CrossRef]
- Neagu, N., Conforti, C., Agozzino, M., Marangi, G. F., Morariu, S. H., Pellacani, G., Persichetti, P., Piccolo, D., Segreto, F., Zalaudek, I., & Dianzani, C. (2022). Melasma treatment: a systematic review. In Journal of Dermatological Treatment. [CrossRef]
- Abdel-Raouf Mohamed H, Ali Nasif G, Saad Abdel-Azim E, Abd El-Fatah Ahmed M. Comparative study of fractional Erbium: YAG laser vs combined therapy with topical steroid as an adjuvant treatment in melasma. J Cosmet Dermatol. 2019 Apr;18(2):517–23.
- Lee M-C, Chang C-S, Huang Y-L, Chang S-L, Chang C-H, Lin Y-F, et al. Treatment of melasma with mixed parameters of 1,064-nm Q-switched Nd:YAG laser toning and an enhanced effect of ultrasonic application of vitamin C: a split-face study. Lasers Med Sci. 2015 Jan;30(1):159–63.
- Jung, J. W., Kim, W. O., Jung, H. R., Kim, S. A., & Ryoo, Y. W. (2019). A face-split study to evaluate the effects of microneedle radiofrequency with Q-switched Nd:YAG laser for the treatment of melasma. Annals of Dermatology. [CrossRef]
- Cassiano DP, Esposito AC, Hassun KM, Lima EV, Bagatin E, Miot HA (2019). Early clinical and histological changes induced by microneedling in facial melasma: A pilot study. Indian J Dermatol Venereol Leprol 2019;85:638-641.
- Singh, A., & Yadav, S. (2016). Microneedling: Advances and widening horizons. Indian Dermatology Online Journal. [CrossRef]
- Saleh FY, Abdel-Azim ES, Ragaie MH, Guendy MG. Topicaltranexamic acid with microneedling versus microneedlingalone in treatment of melasma: clinical, histopathologic, and immunohistochemical study. J Egypt Women’s Dermatol Soc.2019;16(2). [CrossRef]
- Farshi S, Mansouri P. Study of efficacy of microneedling and mesoneedling in the treatment of epidermal melasma: A pilot trial. J Cosmet Dermatol. 2020; 19: 1093–1098. [CrossRef]
- Cohen, B. E., & Elbuluk, N. (2016). Microneedling in skin of color: A review of uses and efficacy. Journal of the American Academy of Dermatology. [CrossRef]
- Ha DH, Kim HK, Lee J, Kwon HH, Park GH, Yang SH, Jung JY, Choi H, Lee JH, Sung S, Yi YW, Cho BS. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells. 2020 May 7;9(5):1157. PMID: 32392899; PMCID: PMC7290908. [CrossRef]
- From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO); Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA, Shazam Hussain M, Jansen O, Jayaraman MV, Khalessi AA, Kluck BW, Lavine S, Meyers PM, Ramee S, Rüfenacht DA, Schirmer CM, Vorwerk D. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int J Stroke. 2018 Aug;13(6):612-632. Epub 2018 May 22. PMID: 29786478. [CrossRef]
- Bano R, Ahmad F, Mohsin M. A perspective on the isolation and characterization of extracellular vesicles from different biofluids. RSC Adv. 2021 Jun 1;11(32):19598-19615. PMID: 35479207; PMCID: PMC9033677. [CrossRef]
- Thakur, A.; Shah, D.; Rai, D.; Parra, D.C.; Pathikonda, S.; Kurilova, S.; Cili, A. Therapeutic Values of Exosomes in Cosmetics, Skin Care, Tissue Regeneration, and Dermatological Diseases. Cosmetics 2023, 10, 65. [CrossRef]
- Olumesi KR, Goldberg DJ. A review of exosomes and their application in cutaneous medical aesthetics. J Cosmet Dermatol. 2023 Oct;22(10):2628-2634. Epub 2023 Jul 27. PMID: 37498301. [CrossRef]
- Yin, L.; Liu, X.; Shi, Y.; Ocansey, D.K.W.; Hu, Y.; Li, X.; Zhang, C.; Xu, W.; Qian, H. Therapeutic Advances of Stem Cell-Derived Extracellular Vesicles in Regenerative Medicine. Cells 2020, 9, 707. [CrossRef]
- Xiong M, Zhang Q, Hu W, Zhao C, Lv W, Yi Y, Wang Y, Tang H, Wu M, Wu Y. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol Res. 2021 Apr;166:105490. Epub 2021 Feb 12. PMID: 33582246. [CrossRef]
- Gross, J., Chaudhary, V., Bartscherer, K. et al. Active Wnt proteins are secreted on exosomes. Nat Cell Biol 14, 1036–1045 (2012). [CrossRef]
- Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, Zhu Y, Wu L, Pan Z, Zhu W, Qian H, Xu W. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015 May;4(5):513-22. Epub 2015 Mar 30. PMID: 25824139; PMCID: PMC4414225. [CrossRef]
- Ku YC, Omer Sulaiman H, Anderson SR, Abtahi AR. The Potential Role of Exosomes in Aesthetic Plastic Surgery: A Review of Current Literature. Plast Reconstr Surg Glob Open. 2023 Jun 12;11(6):e5051. PMID: 37313480; PMCID: PMC10259637. [CrossRef]
- Cho, B. S., Lee, J., Won, Y., Duncan, D. I., Jin, R. C., Lee, J., Kwon, H. H., Park, G. H., Yang, S. H., Park, B. C., Park, K. Y., Youn, J., Chae, J., Jung, M., & Yi, Y. W. (2020). Skin brightening efficacy of exosomes derived from human adipose tissue-derived stem/stromal cells: A prospective, split-face, randomized placebo-controlled study. Cosmetics. [CrossRef]
- Park GH, Kwon HH, Seok J, Yang SH, Lee J, Park BC, Shin E, Park KY. Efficacy of combined treatment with human adipose tissue stem cell-derived exosome-containing solution and microneedling for facial skin aging: A 12-week prospective, randomized, split-face study. J Cosmet Dermatol. 2023 Dec;22(12):3418-3426. Epub 2023 Jun 28. PMID: 37377400. [CrossRef]
- Wang T, Gao H, Wang D, Zhang C, Hu K, Zhang H, Lin J, Chen X. Stem cell-derived exosomes in the treatment of melasma and its percutaneous penetration. Lasers Surg Med. 2023 Feb;55(2):178-189. Epub 2022 Dec 27. PMID: 36573453. [CrossRef]
- Mu N, Li J, Zeng L, You J, Li R, Qin A, Liu X, Yan F, Zhou Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int J Nanomedicine. 2023 Sep 5;18:4987-5009. PMID: 37693885; PMCID: PMC10492547. [CrossRef]
- Won Yu Jin, Lee Esther, Min Seon Young, Cho Byong Seung (2023). Biological Function of Exosome-like Particles Isolated from Rose (Rosa Damascena) Stem Cell Culture Supernatant. InbioRxiv - Cell Biology 2023-10-19 . [CrossRef]
- Pandya, A. G., Hynan, L. S., Bhore, R., Riley, F. C., Guevara, I. L., Grimes, P., Nordlund, J. J., Rendon, M., Taylor, S., Gottschalk, R. W., Agim, N. G., & Ortonne, J. P. (2011). Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. Journal of the American Academy of Dermatology. [CrossRef]
- Ma W, Gao Q, Liu J, Zhong X, Xu T, Wu Q, Cheng Z, Luo N, Hao P. Efficacy and safety of laser-related therapy for melasma: A systematic review and network meta-analysis. J Cosmet Dermatol. 2023 Nov;22(11):2910-2924. Epub 2023 Sep 22. PMID: 37737021. [CrossRef]
- Zhu JW, Ni YJ, Tong XY, Guo X, Wu XP. Activation of VEGF receptors in response to UVB promotes cell proliferation and melanogenesis of normal human melanocytes. Exp Cell Res. 2020 Feb 15;387(2):111798. Epub 2019 Dec 23. PMID: 31874175. [CrossRef]
- ORENTREICH, D. S., & ORENTREICH, N. (1995). Subcutaneous Incisionless (Subcision) Surgery for the Correction of Depressed Scars and Wrinkles. Dermatologic Surgery. [CrossRef]
- Cassiano, D. P., Espósito, A. C. C., Hassun, K. M., de Andrade Lima, M. M. D., de Andrade Lima, E. V., Miot, L. D. B., Miot, H. A., & Bagatin, E. (2022). Histological changes in facial melasma after treatment with triple combination cream with or without oral tranexamic acid and/or microneedling: A randomised clinical trial. Indian Journal of Dermatology, Venereology and Leprology. [CrossRef]
- Hou, A., Cohen, B., Haimovic, A., & Elbuluk, N. (2017). Microneedling: A Comprehensive Review. In Dermatologic Surgery. [CrossRef]
- Brasil dos Santos, J., Nagem Lopes, L. P., de Lima, G. G., Teixeira da Silva, R., da Silva e Souza Lorca, B., Miranda Pinheiro, G., & Faria de Freitas, Z. M. (2022). Microneedling with cutaneous delivery of topical agents for the treatment of melasma: A systematic review. Journal of Cosmetic Dermatology. [CrossRef]
- Rivas, S., & Pandya, A. G. (2013). Treatment of melasma with topical agents, peels and lasers: An evidence-based review. In American Journal of Clinical Dermatology. [CrossRef]
- Alster, T. S., & Graham, P. M. (2018). Microneedling: A review and practical guide. In Dermatologic Surgery. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




