1. Introduction
The term "heavy metals" encompasses a diverse group of elements with varied chemical compositions and significant biological roles. According to Ismanto, et al. [
1], heavy metals are defined as metals with a relative density higher than water, and often manifest as insoluble, toxic pollutants. These contaminants pose serious threats to human health, animal welfare, and ecosystem integrity, necessitating urgent remediation efforts. Moreover, the unchecked discharge of heavy metal ions into water bodies, stemming from industrial activities, mining operations, and agricultural practices, has led to widespread environmental degradation [
2,
3]. Soil and plants, in particular, accumulate heavy metals, compromising agricultural productivity and food safety [
4,
5]. Furthermore, heavy metal contamination in water sources, including sewage and stormwater, originates from diverse sources such as industrial runoff, vehicular emissions, and atmospheric deposition [
6]. To mitigate the adverse impacts of heavy metal pollution, effective remediation strategies are urgently needed. There are many techniques used today to treat wastewater. A few common techniques for cleaning wastewater include precipitation, neutralization, membrane filtration, ion exchange, flotation, and adsorption. Additionally, technologies for treatment have been developed that integrate physical and chemical processes, such as the electrochemical process [
7]. These are further elaborated below:
Chemical precipitation
It is an easy and popular technique to get removal of heavy metals because of how simple and inexpensive it is. Metal-containing wastewater is treated using the chemical precipitation process, which involves adding chemicals to produce an insoluble precipitate. Additionally, heavy metal chelating precipitation, hydroxide precipitation, and sulfide precipitation are used as chemical precipitation techniques. Because they are simple to use, convenient, and efficient at treating inorganic effluents at higher concentrations, lime and limestones are the most often employed precipitant agents in chemical precipitation. Despite the advantages, there are also disadvantages, such as the treatment requiring too many chemicals. The issue of sludge disposal into the environment and the overproduction of sludge are among the other issues [
8].
Ion exchange
The ability of wastewater to exchange cations for metals underlies the ion exchange technique. There are many different types of materials employed, including both man-made (zeolites and resins) and natural (alumina, carbon, and silicates). The one among them that is most frequently used in the ion exchange process is zeolites. In an aqueous medium, the ion exchange process involves the exchange of both cations and anions. The disadvantage of the method is that the ion exchange is not selective and that it is extremely pH-sensitive [
9].
Membrane process
The membrane filtration method used several types of membranes to filter out various heavy metals from aqueous solutions. With this technique, pollutants such oils, suspended particles, heavy metals, and both organic and inorganic contaminants are removed. Reverse osmosis, ultrafiltration, nanofiltration, and electrodialysis are some of the variations of this technology that are used depending on the kind of wastewater [
10].
1. Ultrafiltration
In this method, dissolved molecules, heavy metal ions, and other contaminants are filtered by a membrane according to their molecular size. Some membrane types prevent the passage of larger molecules and heavy metals; thus, they must be separated. These membranes are only able to pass low molecular weight solutes. It has also been divided into subcategories including complexation-ultrafiltration, micellar enhanced ultrafiltration, and chelating enhanced ultrafiltration [
11].
2. Nanofiltration
The membrane separation technique known as nanofiltration is used to remove heavy metals from aqueous solutions. It is utilized to get removal of a lot of heavy metals, like copper, nickel, arsenic, chromium, and nickel. According to Mohammad, et al. [
12], it is dependable, reasonably easy to use, and uses less energy than others.
3. Reverse osmosis
Reverse osmosis (RO) technology is generally used to separate and fractionate organic and inorganic chemicals, as well as heavy metals, in aqueous and nonaqueous solutions. The RO process can be used to treat a variety of industrial effluent types, including those from the chemical, textile, petrochemical, electrochemical, food, paper, and tannery sectors. When combined with the pilot membrane reactor, this technique is highly effective at removing metal. Furthermore, it has a number of disadvantages, including a high-power requirement during membrane repair and high pumping pressure [
13].
4. Electrodialysis
Dissolved ions are transported from one solution to another solution over a charged membrane while being exposed to an electric field in the electrodialysis (ED) separation process. It is used for salt production, wastewater treatment, the recovery of heavy metal ions, and the extraction of drinking water from seawater. According to Mei and Tang [
14], several researchers utilize it to remove off heavy metals including chromium, copper, and ferrous.
Flotation
Large-scale flotation is a method for removing toxic metal ions from wastewater. Additional flotation techniques include precipitate flotation, dissolved air flotation (DAF), and ion flotation. DAF is used more frequently for removing heavy metals from aqueous solutions as compared to other flotation techniques [
15].
Coagulation process
The coagulation process is used to produce colloids. Coagulate substances like aluminum, ferrous sulfate, and ferric chloride are used to neutralize contaminants in wastewater or water. Many researchers have shown that it is an important technique. Polyaluminum chloride (PAC) and ferric chloride solution are used as coagulants to remove heavy metals [
16].
Electrochemical process
In electrochemical processes, metal is removed from an electrolyte solution while being influenced by an external direct current. The coagulation process weakens colloidal particles and triggers the sedimentation process by adding a coagulant. An increase in the rate of coagulation requires the flocculation process, which quickens the conversion of weak particles into bulky floccules [
17].
Adsorption
Adsorption is a reasonably affordable, useful, and simple technology. It has excellent metal removal efficiency and is utilized as a quick method for treating different types of wastewaters. According to reports, adsorption is the most popular technique for removing various contaminants, particularly metal ions [
18,
19]. This is likely due to adsorption's high efficacy, ease of use, lack of waste production, and low cost. This technique is gaining acceptance because to the possibility of recovering the metal and reusing the adsorbent [
20]. Due to its low cost, high removal efficiency, adaptable working conditions, selectivity, and good stability to remove heavy metal ions, adsorption is a promising technology that has attracted interest [
21,
22].
1. Activated carbon
The most popular adsorbent in the world right now is activated carbon (AC). In addition to heavy metals, AC is excellent at removing a variety of other contaminants from water and wastewater. These can be used in batch mode operations and column mode operations because to their large surface area, microporous structure, and porosity features. Bagasse, coconut shell, leaf tea, peanut shells, apple waste, sawdust, rice husk, banana pith, tree bark, and activated cotton fibers are only a few of the agricultural waste biomasses that are used in the manufacturing of activated carbon. Adsorbents can be changed physically and chemically to increase the lignocellulosic material's capacity for adsorption. It is industrial in character and expensive. Because of this, scientists are focusing on using affordable adsorbents during the treatment procedure [
23].
2. Low-cost bio-absorbents
Due to the availability and affordability of various waste materials, industrial byproducts, agricultural wastes, and other natural waste materials, the low-cost technology has grown in favor in recent years. Due to the high cost of commercially generated activated carbon, consideration is given to selecting a less expensive absorbent. Researchers are developing cheap adsorbents from industrial by-products like pulp and paper waste, fertilizer waste, steel converter slag, steel production slag, commonly occurring sugarcane bagasse, and bagasse fly ash. Household wastes like fruit waste and adsorbents of marine origin are also used in the treatment in addition to peat and red mud. As less expensive adsorbents, non-agricultural materials such lignin, diatomic, clino-pyrrhotite, aragonite shells, natural zeolites, clay, kaolinite, and peat are also used [
24].
3. Bioadsorbents
For most of the time, agricultural and plant wastes were used as bioadsorbents for purifying wastewater since they are incredibly effective and have a lot of potential. The sources state that there are normally three types:(1) Non-living biomass, such as bark, lignin, squid, crab shells, shrimp, krill, etc. (2) Microbial biomass, which includes bacteria, fungi, yeast, and algae; and (3) Algal biomass. The development of bioadsorbents can also benefit from agricultural wastes like potato peel, sawdust, citrus peels, mango peel, corn cob, rice husk, tree fern, wheat bran, grape bagasse, coconut copra meal, orange waste, walnut, hazelnut, and almond shell, tea waste, dried parthenium powder, sugarcane bagasse, pine needles, peanut shell, tamarind seeds, sunflower stalk, and black gram husk are also promising for developing bioadsorbents [
25].
Among the above strategies, adsorption has gained considerable attention due to its simplicity, cost-effectiveness, and high efficiency in removing heavy metals from aqueous solutions. Particularly, cellulose and silica-based materials have emerged as promising adsorbents for heavy metal removal, owing to their abundant availability, low cost, and favorable adsorption properties [
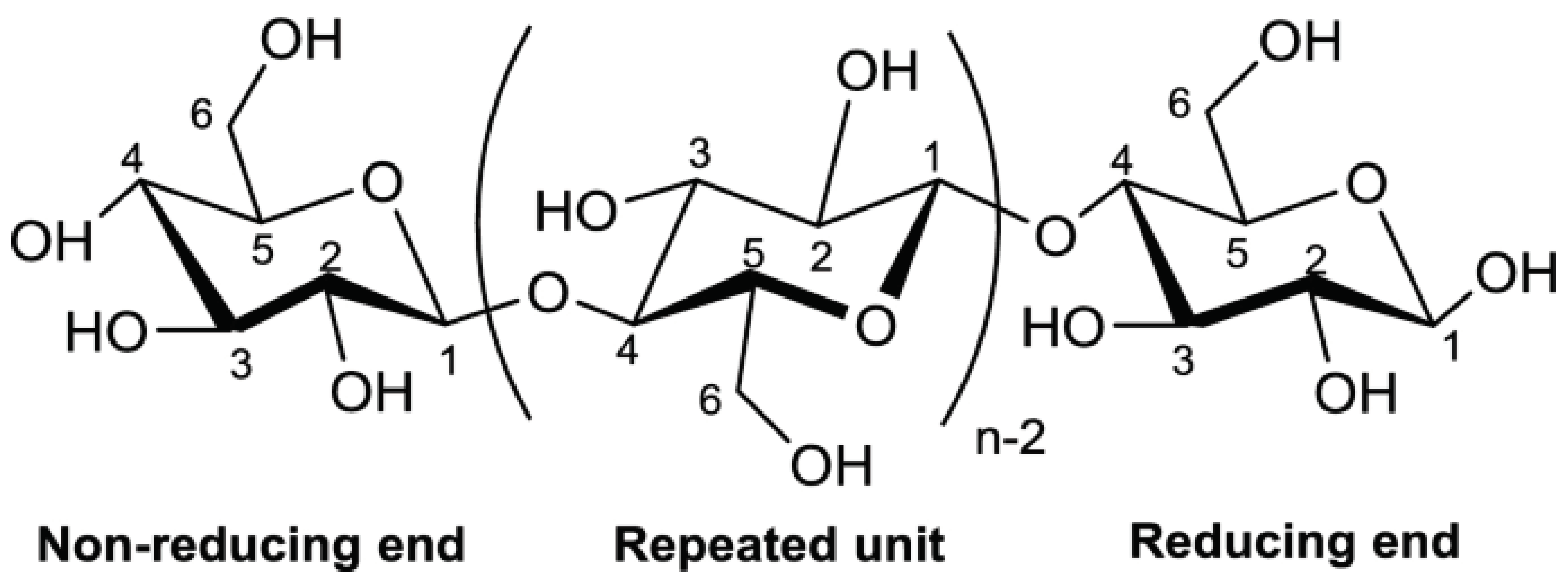
26]. Glucose units that are joined -(1,4) together make up the long linear polysaccharide polymer known as cellulose (C5H8O4)m (
Figure 1). It is an essential part of plants' cell walls. Along with lignin and hemicelluloses, it is present in plant cells. It is often insoluble in water. Materials having a high cellulose content include plant fibres, woods, stalks, stems, shells, straw, grasses, etc. [
27].
Cellulose is a substance of interest for a variety of applications because of its amazing physicochemical characteristics, which include colorlessness, a lack of odor or taste, and great mechanical strength, superior sorption capacity, hydrophilicity, and biocompatibility. Because of its low density and reactive surface with OH side groups, cellulose is able to graft chemical species onto its surface.
Figure 1.
The structure of cellulose (Source[
27]).
Figure 1.
The structure of cellulose (Source[
27]).
Moreover, the coupling of cellulose and silica with different functional groups further enhances their adsorption capacity and selectivity for heavy metals. While the understanding of heavy metal contaminants and their environmental implications has been extensively studied and reviewed in the scientific literature, previous reviews have often overlooked the potential synergistic effects of coupling cellulose and silica with different functional groups to enhance adsorption capacity and selectivity. While previous reviews have touched upon individual aspects of heavy metal remediation potential, there are notable gaps in the overall understanding of the field. For instance, while some reviews have examined the sources and environmental impacts of heavy metals [
2,
3], others have delved into the mechanisms of heavy metal adsorption by various materials [
28]. Additionally, many reviews have failed to systematically compare the adsorption capacities and efficiency of different adsorbents, hindering the identification of optimal materials for real-world applications. Despite these efforts, there remains a need for a comprehensive review that synthesizes the latest advancements in heavy metal adsorption using cellulose and silica coupling agents, addressing the limitations of previous reviews and offering new insights into the field.
Purpose of the review
This paper comprehensively reviews recent advancements in the field of heavy metal adsorption using cellulose and silica coupling agents. In this review, we aim to address these limitations by providing a comprehensive overview of recent advancements in heavy metal adsorption using cellulose and silica coupling agents. By synthesizing findings from a wide range of studies, we offer new insights into the potential applications of these materials for environmental remediation. Moreover, we systematically compare the adsorption capacities and efficiency of different cellulose and silica-based adsorbents, enabling researchers to identify the most promising materials for further investigation. Overall, our review aims to bridge the gap between theory and practice, offering practical guidance for researchers and policymakers working in the field of heavy metal remediation. The objectives of this review, therefore, include:
To review recent advancements in heavy metal adsorption using cellulose and silica coupling agents.
To compare the adsorption capacities and efficiency of different cellulose and silica-based adsorbents reported in recent literature.
To identify key research gaps and propose directions for future research in the field of heavy metal remediation.
3. Literature review
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.1. Natural fiber
The effects of toxic heavy metals on the environment and human health are among the biggest problems. The continued decrease of the water quality and the degree of contamination have been noted by scientists, who are concerned about them. Many methods for removing heavy metals from water and waste have recently undergone extensive research. Because non-renewable resources are used, and costs are high, heavy metal removal and remediation are provided at a high price in current technology. A variety of materials have frequently been employed as adsorbents, and many researchers are now utilizing adsorption techniques for this purpose. Due to its many advantages, such as stability, usability, cheap cost, simplicity of use, and performance, adsorption has proven to be a viable method for purification [
29].
In order to reduce the concentrations of heavy metal ions to extremely low levels, adsorption technology uses a variety of low-cost adsorbent materials such biosorbents, clays, activated carbons, zeolites, and metal oxides. Metal adsorption onto adsorbent material, particularly on agricultural wastes, is a more complicated process because it is influenced by a variety of different factors. This process includes the steps of complexation, chemisorption, adsorption-complexation on surface and pores, micro precipitation, and ion exchange. When biological materials are used in the adsorption process, specific functional groups, such as sulphydryl, amido, hydroxy, and carboxyl, attach metal ions from water [
29,
30].
Due to their low density, high strength to weight ratio, and reduction, natural fibers are vital light weight composite and reinforcement materials. The microstructure and chemical composition of fibers, with the fiber cross-sectional area having the largest degree of change, affect the mechanical properties of fibers. Natural fibers have hydrophilic properties because they include hemicellulose, which makes them easily absorb water. As a result, they are less suited to a matrix with hydrophobic characteristics [
30]. Higher cellulose concentration and crystallinity frequently result in better fiber strength, whereas lignin has the reverse effect. Additionally, variations in fiber anatomical qualities between and within species have an impact on the density and mechanical characteristics of fibers. The environment, the economy, and politics are some additional.
Recent years have seen an increase in interest in natural fiber reinforced composites because of their lightweight, nonabrasive, combustible, nontoxic, inexpensive, and biodegradable properties. Among the many natural fibers, flax, bamboo, sisal, hemp, ramie, jute, and wood fibers are particularly significant [
30]. The study of using natural fibers in place of synthetic fiber in fiber-reinforced composites has advanced and increased industrial alternatives. The advantages of natural fibers include their low cost, low density, and biodegradability. However, the main disadvantages of natural fibers in composites are their poor compatibility with the matrix and their relatively high moisture sorption. Chemical treatments are therefore considered for altering the characteristics of the fiber surface [
31].
In composite materials, lignocellulosic natural fibers like sisal, coir, jute, ramie, pineapple leaf (PALF), and kenaf have the potential to replace glass or other common reinforcement materials. These fibers are an attractive alternative to traditional materials because of a number of characteristics. They have high particular properties like modulus and are rigid, impact-resistant, flexible, and flexible. They are also widely accessible, renewable, and biodegradable. Additional desirable characteristics include affordability, low density, decreased equipment abrasion, reduced skin and respiratory irritation, vibration damping, and enhanced energy recovery [
32]
3.1.1. Natural fiber and heavy metal adsorption capacity
The adsorption of heavy metals from wastewater has been done using a variety of natural plant fibres. Natural plant fibres are composed primarily of cellulose, a few extractives, and amorphous polymers (hemicelluloses and lignin). agricultural waste (cellulosic, non-wood) from crops that generate a lot of waste after processing. Waste frequently contains a lot of cellulose. As a result, heavy metal adsorbents made from agricultural waste are increasingly being used in research and development. Agricultural by-products are frequently utilized for adsorption, either modified or unmodified. The adsorption of heavy metal ions from contaminated water has been successfully applied using banana, orange, and lemon peels, rice husk, rice straw, peanut shell, bran, and sugar cane bagasse [
33,
34,
35].
Environmental pollution is a big problem in many highly populated regions of the world because of dangerous heavy metals. The industrial and domestic wastes that causes numerous environmental problems have a negative impact on human health. It is necessary to remove specific metals from industrial discharges before dumping aqueous waste into the environment. This monitor was used to compare the adsorption of lead (Pb2+) and zinc (Zn2+) ions on banana fiber in order to assess the efficacy of uptakes. The banana stem's fiber was mechanically removed before being softened with alkali (NaOH) treatment. The needle-punched fabrics were developed from the parallel-laid webs. When first basic research on heavy phase adsorption were carried out [
36].
To achieve the desired effluent discharge limitations, one of the key challenges in wastewater treatment plants has been the optimization of numerous variables. The optimization study conducted by Afolabi et al. in 2021 evaluated the removal of lead (II) from wastewater using banana peels. The central composite design was utilized to explore the interactive effects of the operating parameters (starting concentration, pH of the solution, adsorbent dosage, and particle size) using batch adsorption tests. With an initial concentration of 100 mg/L, a pH of 5, adsorbent dosage of 0.55 g, and particle size of 75 m, the response surface design produced the best results, with a removal percentage of 98.146%.
With the exception of acid pretreatment, heat and chemical preparation of banana peels were found to improve metal removal effectiveness in a related study by Cheah, et al. [
37]. The findings suggested that detergent pretreatment, similar to other chemicals, was a more useful and economical way than heat treatments to successfully improve metal biosorption by banana peels for wastewater treatment. According to the study, pretreatment can increase the effectiveness of removing metal from banana peels, with chemical pretreatment being preferable to heat treatment and detergent being identified as a workable and helpful agent for pretreatment. The efficiency of banana peel ftrip (BP) in removing copper and zinc ions from water is investigated by Bhagat, et al. [
38]. A cheap source of biomass generated from agricultural waste, BP demonstrated good adsorption capabilities when absorbing Cu2+ and Zn2+ from wastewater effluent.
The ability of banana fiber needle felted fabric to adsorb heavy metal ions (Pb2+ and Zn2+) under several conditions, including pH, contact time, and concentration, was examined. It was found that the amount of Pb2+ and Zn2+ adsorbed varied with the pH of the initial solution, the quantity of adsorbent utilized, the contact time, and the concentration. As the solution pH, concentration, and contact time increased, so did the amount of Pb2+ and Zn2+ ions taken up (mg/g). It was found that lead and zinc are highly adsorbed at pH 7, independent of time or concentration. The employment of chemicals (NaOH, HCl) to change the pH of the aqueous solution is the cause of the low adsorption at other pH levels. Banana fiber was therefore successful in eliminating 95.5% of lead and 98% of zinc, according to Batch tests.
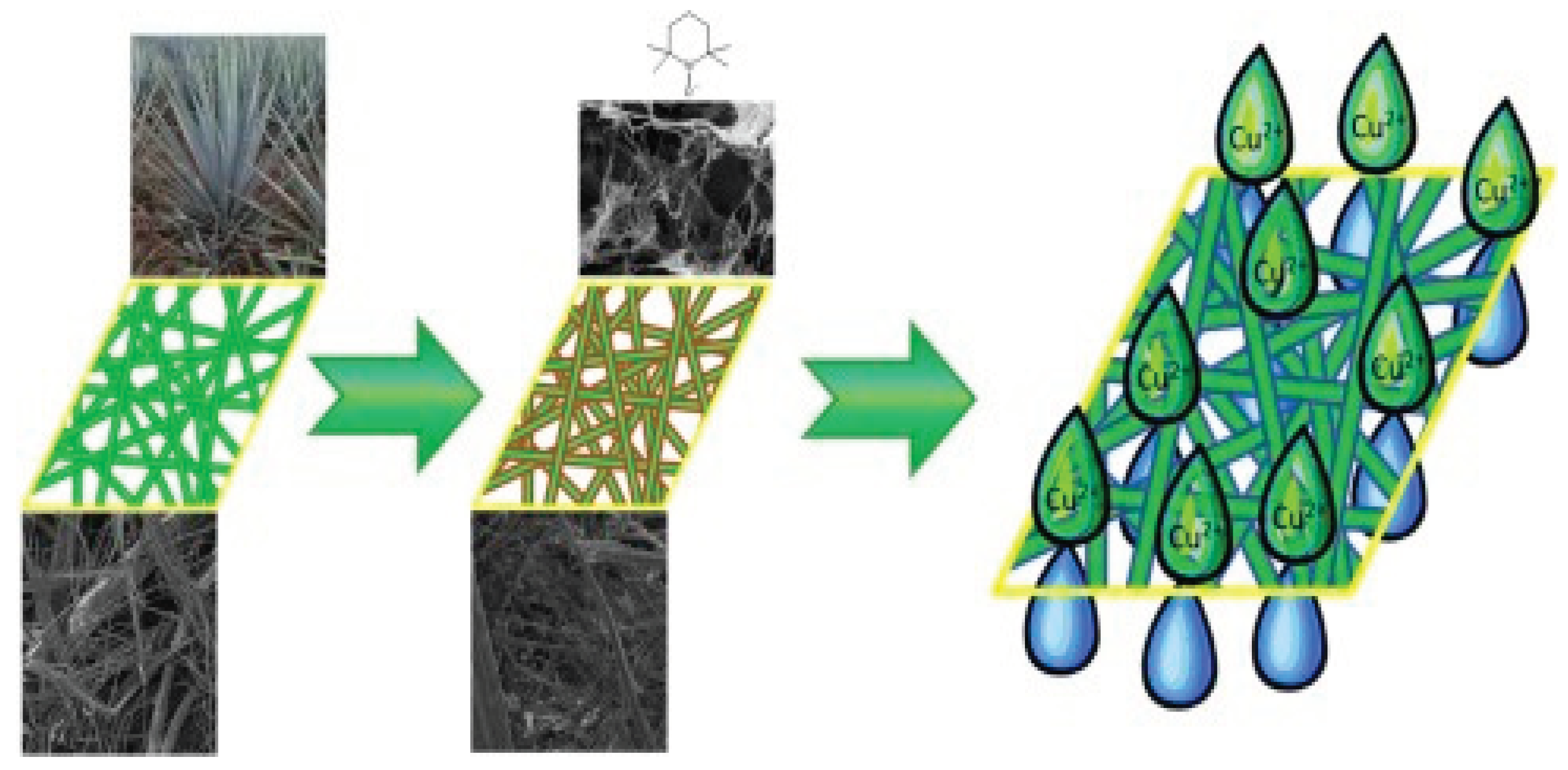
Heavy metal ion contamination of fresh water sources represents a serious risk to the safety of drinking water. Therefore, it is essential to treat water to remove specific contaminants, such as copper ions. Adsorbents or continuous membrane/filter procedures are frequently used to do this. For example, alternative combinations of these processes that could be applied as a treatment in this case include adsorption or ion-exchange membranes/filters. As a result, environmentally friendly alternatives to traditional adsorption membrane/filter materials include those made from renewable resources. For instance, filters made of natural fibers and nanocellulose were coated with (2,2,6,6-Tetramethylpiperidin-1-yl)oxy (TEMPO) and made from flax and agave fibers as well as other kinds of CNF. Although it has been demonstrated that anionic TEMPO-oxidized cellulose nanofibrils (TCNF) have a considerable affinity for heavy metal ions, their usage in nanopaper membranes is limited by their low permeance. Additionally, pure nanopapers have not yet been employed to effectively and efficiently adsorb metal ions [
39].
Figure 2.
Natural fibre-nanocellulose composite filters for the removal of heavy metal ions from water (Source [
39]).
Figure 2.
Natural fibre-nanocellulose composite filters for the removal of heavy metal ions from water (Source [
39]).
Pure nanopapers have yet to be used successfully to adsorb metal ions with great permeance and efficiency. The permeance and copper adsorption capacity of these filters were used to evaluate how well they performed. This novel kind of filter made from industrial crop residue was demonstrated to have extremely high permeances, which allowed it to adsorb large amounts of Cu2+ ions during a continuous filtration process. For the active adsorption agent TEMPO-CNF, this translates to an adsorption capacity of more than 60 mg/g.
Due to the presence of hydroxyl functional groups, cellulosic biomaterials have strong adsorption potential. Banana fibres and other lignocellulosic fibres are made of cellulose, hemicellulose, lignin, pectin, wax, and water-soluble components. This fiber is readily available and easy to produce, which is an appealing quality that makes it an appropriate substitute for synthetic fibres that might be dangerous [
40]. Sheng, et al. [
41] modified banana pseudostem fiber using cellulose xanthogenation and steam explosion techniques to improve the capacity of the fiber to absorb heavy metal ions Pb2+ and Cd2+. Steam explosion banana fibre cellulose xanthogenate's (SEBF-CX) adsorption kinetics fit in with the pseudo-second order model. Pb2+ and Cd2+ adsorption in the pseudo-second-order model, however, were 99.0099 mg/g and 67.3401 mg/g, respectively. Compared to raw banana fibre (RBF), the metal ion adsorption capacities of SEBF-CX and SEBF were higher. The three fibres tested, among them SEBF-CX, were the best and might be employed as an effective absorbent to remove heavy metal ions from contaminated water.
Additionally, Selambakkannu, et al. [
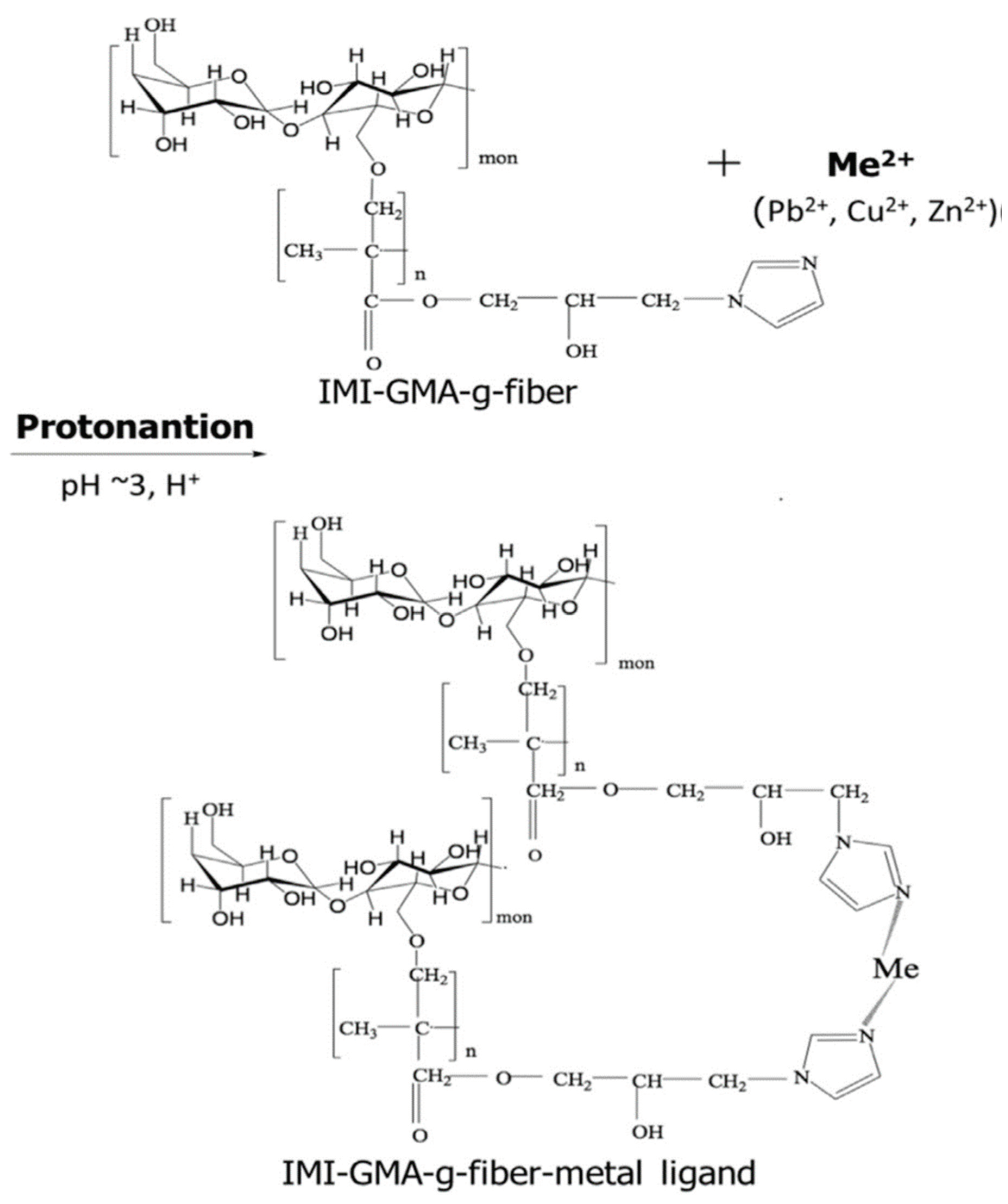
42], developed a synthetically modified fibrous bio-adsorbent obtained from banana trunk for potential usage in the adsorption of heavy metal from wastewater. The fiber was first given a graft of glycidyl methacrylate (GMA) polymer using an electron beam irradiation technique. The GMA-grafted fiber was then functionalized with an imidazole (IMI) group using an epoxide ring-opening procedure, resulting in an amine density of 2.00 mmol/g.
Figure 3.
Metal ion adsorption mechanism by IMI-functionalized GMA-grafted. banana fibre. (Source [
42])
Figure 3.
Metal ion adsorption mechanism by IMI-functionalized GMA-grafted. banana fibre. (Source [
42])
Figure 1. This is a figure. Schemes follow the same formatting.
The effective removal of metal ions from the aqueous solutions was carried out by the IMI-functional group. This comprehensive kinetic and mechanistic study employs IMI-functionalized GMA-grafted banana fibre to remove metal ions (Cu2+, Pb2+, and Zn2+) via adsorptive removal. The saturation of the adsorbent, however, caused the removal % to decline as the initial metal ion concentration rose. The adsorption capacities for the metal ions Cu2+, Pb2+, and Zn2+ were 71.6 mg/g, 84.2 mg/g, and 60.1 mg/g, respectively, using IMI-GMA-grafted fibre. On real sewage water that had been collected from a nearby household area, IMI-functionalized GMA-grafted fibres were tested as a metal ion adsorbent. Within one hour of contact time, a 100% removal of the target metal ions was accomplished. This test demonstrated that biosorbent made from fodder crops, in this case the banana trunk, may be adapted and used as a cost-efficient treatment for threatening environmental issues.
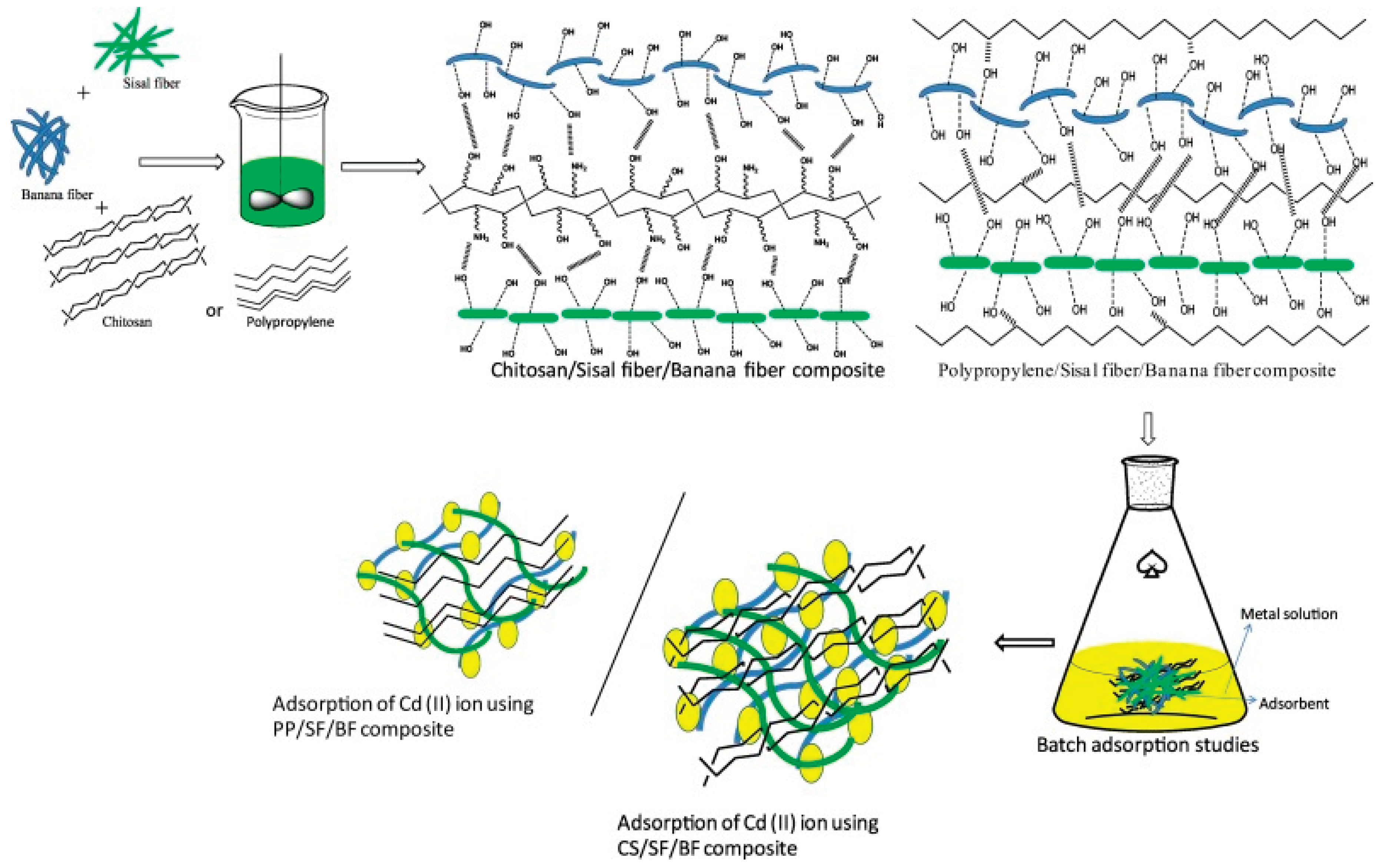
Polypropylene is also used because of its importance in producing strong composite materials for a variety of applications. The chitosan biopolymer is composed of a number of amino and hydroxyl groups and efficiently removes Cd ions from wastewater. For the adsorptive removal of cadmium (Cd) ions from water waste, Alaswad, et al. [
43] prepared the performance of polypropylene (PP)/sisal fiber (SF)/banana fiber (BF) and chitosan-based hybrid (chitosan (CS)/SF)/BF) composite materials.
Figure 4.
Schematic representation for the preparation of ternary chitosan/sisal fibre(SF)/banana fibre (BF) composite, polypropylene (PP)/SF/BF fibre composite (Source [
43]).
Figure 4.
Schematic representation for the preparation of ternary chitosan/sisal fibre(SF)/banana fibre (BF) composite, polypropylene (PP)/SF/BF fibre composite (Source [
43]).
The developed ternary hybrid composites were characterized, and the sample with the best amorphous/less crystalline nature was chosen for the adsorption tests. For PP/SF/BF composite and bio (CS/SF/BF) composite, the Cmax value for Cd (II) ion was determined to be 304 mg/g and 419 mg/g, respectively. Pseudo-1st-order kinetics, pseudo-second-order kinetics, and intraparticle diffusion studies were used to analyze the kinetic adsorption data. For pseudo-2nd-order kinetics, PP/SF/BF and CS/SF/BF fibre composites both exhibit very high R2 values. As a result, the biocomposite developed from chitosan, sisal, and banana fibres showed the greatest ability to adsorb and remove Cd (II) ions.
In the current work, the removal of lead ions from solid forms (as strips) by adsorption on green cellulosic fiber/polyacrylamide (GCFP) was investigated. Solid strips were developed in place of hydrogel to carry out the adsorption process without losing any water. The investigations included a number of operating parameters, including contact time, initial Pb concentration, adsorbent dosage, and pH value. The findings of the kinetic investigation demonstrated that for the adsorption of Pb ions, the pseudo-second-order kinetic model offered improved correlation. The results showed that GCFP films are highly effective at removing lead ions from water. At pH=7, an adsorbent dosage of 0.4 g, an initial Pb concentration of 50 ppm, and a contact time of 60 min, GCFP removed 98% (about 128 mg/g) of the Pb. These findings supported the GCFP film's strong attraction to Pb, which caused the metal to adsorb from the solution and fill the pores and active surface areas of the film [
44].
3.1.2. Cellulose and heavy metal adsorption capacity
Cellulose and heavy metal adsorption capacity
Numerous sources, including plants, animals, aquatic life, and microorganisms, can yield cellulose. Wood pulp is one of the most widely used sources of cellulose [
45]. The ecosphere's most readily available and renewable source of organic polymer is cellulose [
46]. An organic polymer called cellulose holds the anhydroglucose units of a long, straight chain molecule together. The -(1,4)-glycosidic connections bind these anhydroglucose units together [
45,
47]. The abundance of OH groups in the cellulose structure allows for a number of processes to take place that can change the surface charge. This suggests that cellulose performance in various applications will be enhanced.
Cellulose has been derived from a variety of naturally occurring substances, including bacteria and plants, that are typically receptive and biodegradable. The fact that cellulose can be extracted from every portion of a fruit indicates that the fruit truly contains some cellulose and is therefore safe to eat. A new bacterial cellulose composite has also been shown to be effective at adsorbing heavy metals like Pb2+, Cu2+, Ni2+, and Cr(VI). Su, et al. [
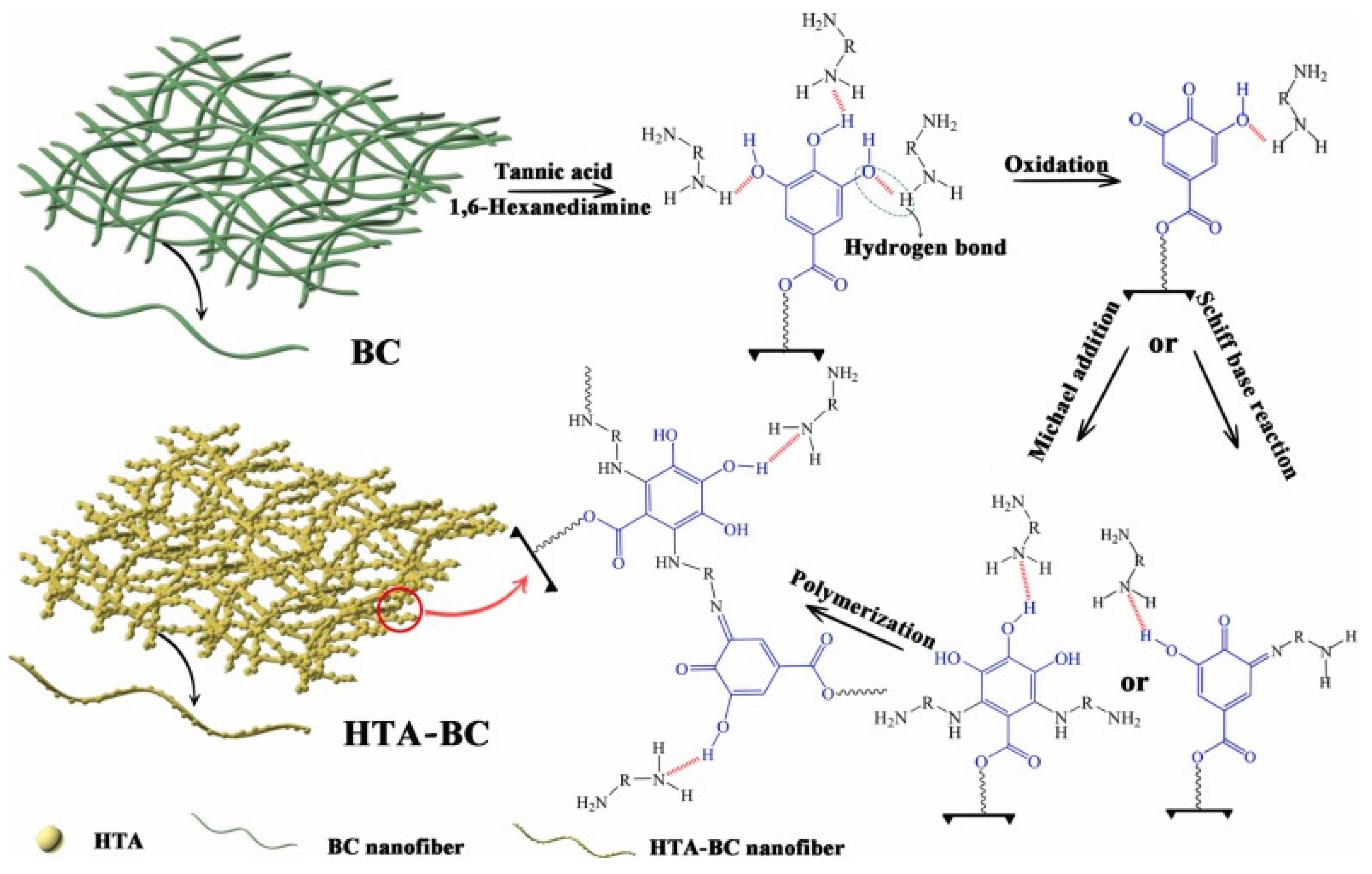
48] developed a poly(hexamethylenediamine-tannic acid)-bacterial cellulose (HTA-BC) composite using abundant, inexpensive, and nontoxic natural material in a simple one-step technique for the decontamination of highly poisonous Cr(VI). The as-synthesized HTA-BC had exhibited great capacity (534.8 mg/g) and good selectivity for Cr(VI) scavenging. It was functionalized with rich amino and phenolic hydroxyl groups.
Figure 5.
Schematic illustration of the possible polymerization mechanism of the HTA and the formation of HTA-BC (Source [
48]).
Figure 5.
Schematic illustration of the possible polymerization mechanism of the HTA and the formation of HTA-BC (Source [
48]).
The HTA-BC composite, as it developed, had a strong capacity for adsorption and great selectivity for the removal of Cr(VI). The second-pseudo-order kinetics model had higher regression coefficients (R2 > 0.987) for the adsorption kinetic parameters of Cr(VI) by HTA-BC. The findings also showed that HTA-BC's adsorption behaviour toward Cr(VI) fitted in with the Langmuir model. The HTA-BC composite could effectively lower the content of the spiked Cr(VI) below the allowable levels for drinking water by treating real water samples.
The literature indicates that cellulose is a novel class of adsorbent substance that has demonstrated the ability to successfully extract heavy metals from aqueous solution. cellulose, the most well-known of which are adsorption membranes [
49,
50]. These nanopapers, however, were too dense to permit high water permeability, making the materials ineffective for removing heavy metals [
39]. Therefore, modifying cellulose may greatly improve its capacity for adsorption and encourage adsorption processes such ion exchange, complexation, and electrostatic interaction. Very high adsorption capabilities were obtained by functionalizing cellulose with amine groups, thiol groups, and other substances like nanobentonite and chitosan [
51].
The heavy metals in wastewater and natural water bodies have significantly harmed ecosystems and reduced the quality of the water environment. A unique efficient Activated Carbon/Carborundum@Microcrystalline Cellulose core shell nano-composite (AC/CB/MCC) was developed by Mubarak, et al. [
52] to be used for the detoxification of As3+ and Cu2+ ions from aqueous solutions. The kinetic analyses showed that, among the models considered, the pseudo-second-order model is the most advantageous for ion adsorption. The maximal adsorption capabilities for the adsorption of Cu2+ and As2+ ions were 423.55 and 422.9 mg/g, respectively, at pH 7.0, 298 K, and 500 mg of the AC/CB/MCC adsorbent dose. It was indicated that the AC/CB/MCC nano-composite can be employed as a substitute and effective nano-adsorbent for the removal of hazardous heavy metal ions from polluted effluents, such as Cu2+ and As3+ ions.
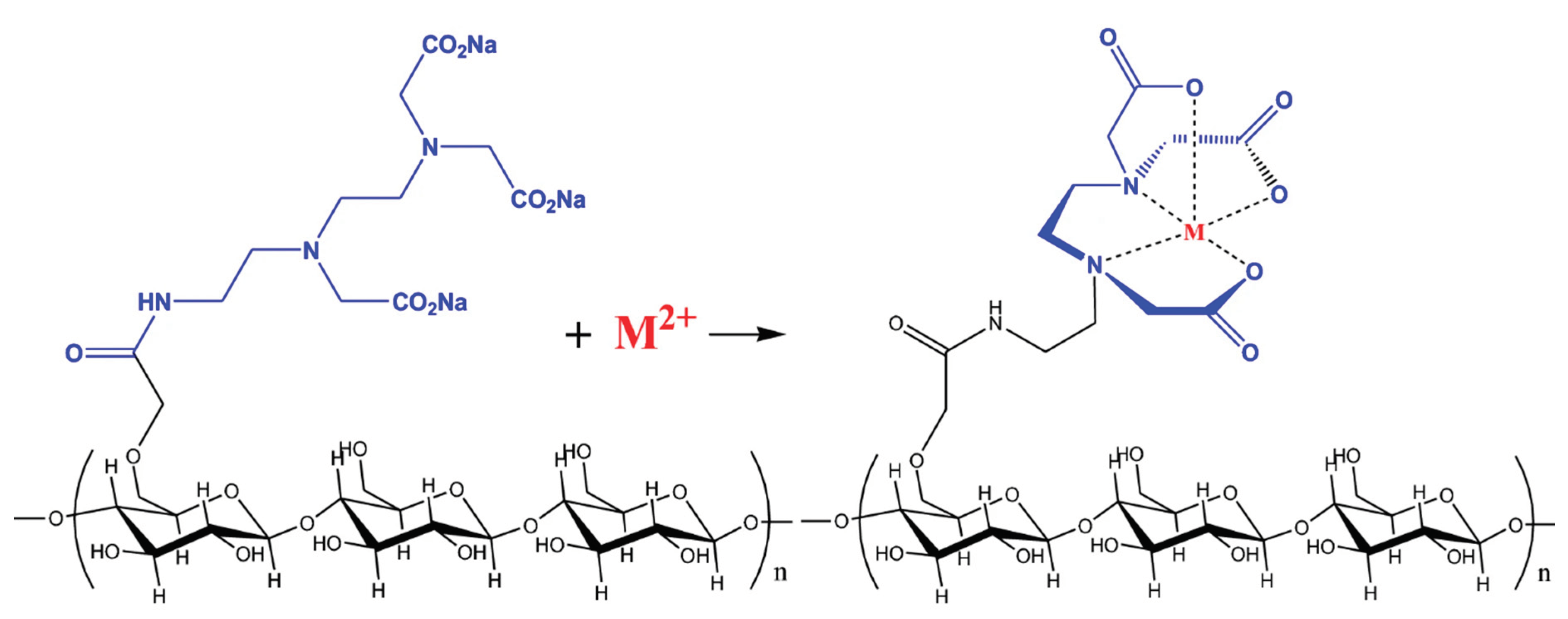
Numerous researches have discussed the chemical modification of cellulose by esterification with EDTA for the recovery of metal ions, as well as the removal of metal ions from aqueous solutions by materials that link EDTA to cellulose derivatives [
53,
54]. In-situ synthesised chelating (amino acetic acid groups) adsorbent grafted on cellulose and its heavy metal ion adsorption capabilities from aqueous solution were developed by Hu, et al. [
55].
Figure 6.
Proposed structure of the Modified cellulose with Amino acetic acid group and its chelating center with Metal ions being adsorbed (Source [
55]).
Figure 6.
Proposed structure of the Modified cellulose with Amino acetic acid group and its chelating center with Metal ions being adsorbed (Source [
55]).
The findings showed that this modified cellulose had good adsorption capability for a variety of metal ions and displayed adsorption characteristics comparable to those of EDTA. The maximum adsorption capacity for Cu2+ on modified cellulose with an amino acetic acid group is 80.3 mg/g, while the maximum adsorption capacity for Pb2+ is 266.7 mg/g. By using the Freundlich and Langmuir models, the adsorption of Cu2+ and Pb2+ from single metal ion aqueous solutions onto this adsorbent was assessed and examined. The adsorption of both Cu2+ and Pb2+ ions was found to best fit the Langmuir isotherm model. The adsorbent saw a maximum quantity of Cu2+ and Pb2+ adsorption at pH 5.
3.2. Silica and heavy metal adsorption capacity
Due to their distinctive characteristics, such as a larger surface area, tunable pore diameters, excellent chemical and thermal stability, selectivity towards heavy metal ions, and ease of surface modification, silica nanoparticles from renewable sources have emerged as a leader in the various groups of nanoparticles. Studies [
56,
57] reported that silica nanoparticles can be synthesized from a variety of agricultural by-products and plants, including rice husk, maize cob, sugarcane bagasse, and bamboo residues.
Traditional mesoporous silica adsorbents had limited capacities or slow kinetics [
58]. Mesoporous silica is thought to be effective substrates for capturing pollutants as well as excellent catalyst carriers because of its large surface areas, rapid mass transfer inside nanostructures, excellent hydrothermal stabilities, tunable pore sizes, and ease of organic modification. Especially, the organic modification and inorganic hybridization could be approached under relatively mild preparation conditions [
59,
60]. However, due to its capacity to remove heavy metal ions, silica has been used in adsorption techniques as a heavy metals’ remover in aqueous solution. Due to its high efficiency, simplicity, and ease of use, adsorption has been widely used as a successful approach for removing a lot of heavy metal ions [
61,
62,
63].
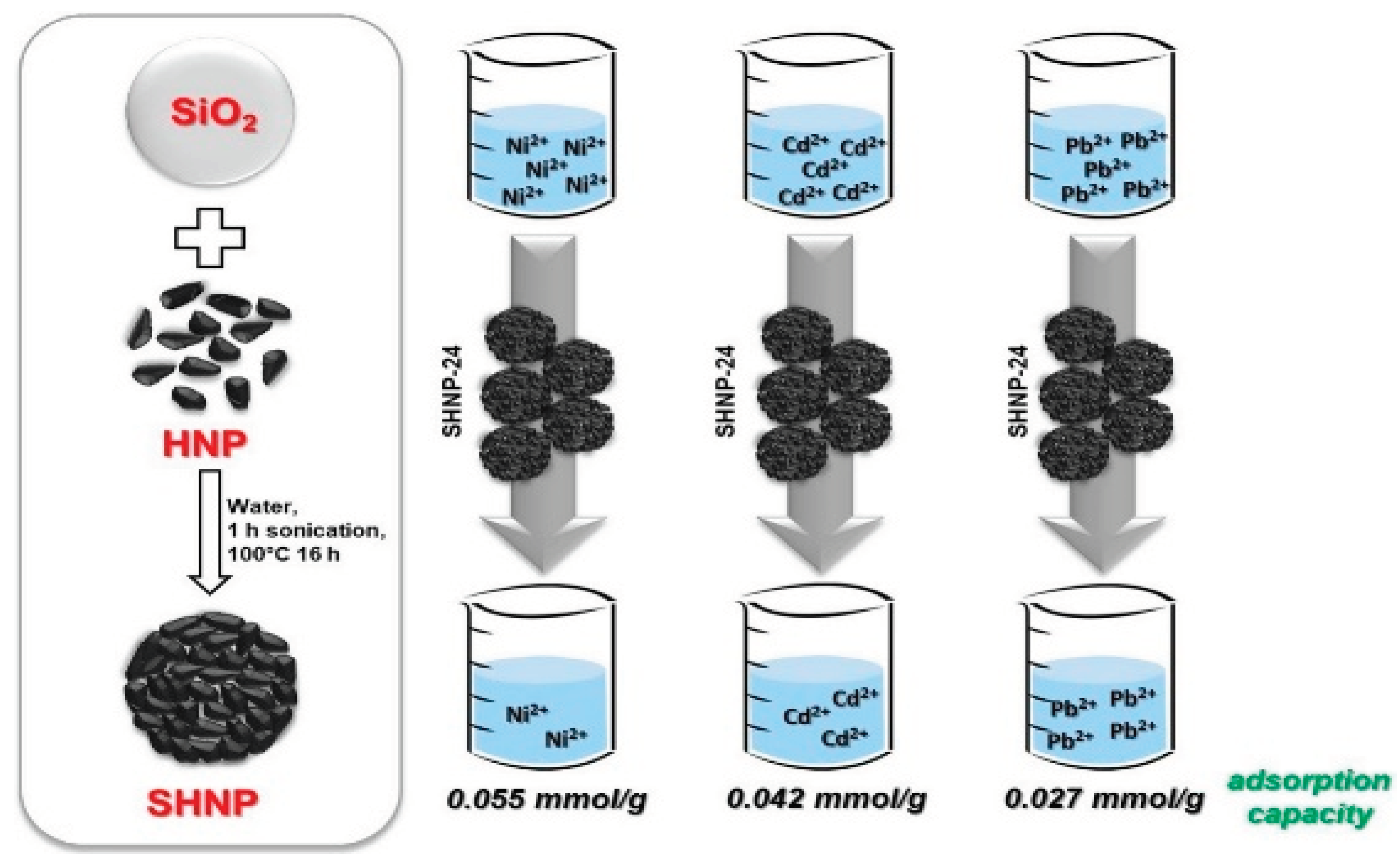
The testing of synthetic sorbents (SHNPs), developed by mixing colloidal hydrophilic nanoparticles (HNPs) with silica for useful water treatment applications, is described in Di Natale, Gargiulo and Alfè [
58]. Silica improves both the functionality and ease of removal of the used sorbent from the HNPs. The supported HNPs (SHNPs) show remarkable removal efficiency, particularly at neutral pH and low temperature (10 °C), which are typical conditions for natural water remediation and some types of industrial wastewater. At a reference value of 0.2 mM, the SHNPs' highest adsorption capacity for Cd2+, Pb2+, and Ni2+ ions are 0.042 mmol g1, 0.027 mmol g1, and 0.055 mmol g1, respectively.
Figure 7.
Adsorption of heavy metals on silica-supported hydrophilic carbonaceous nanoparticles (SHNPs) (Source [
58]).
Figure 7.
Adsorption of heavy metals on silica-supported hydrophilic carbonaceous nanoparticles (SHNPs) (Source [
58]).
The sorbents demonstrate significant adsorption capabilities per gram of active phase (0.54 mg/g for Cd2+ ions, 13.48 mg/g for Ni2+ ions, and 8.87 mg/g for Pb2+ ions) at the corresponding quality level accepted by Italian legislation on wastewater, indicating a potential application for them in water treatment operations.
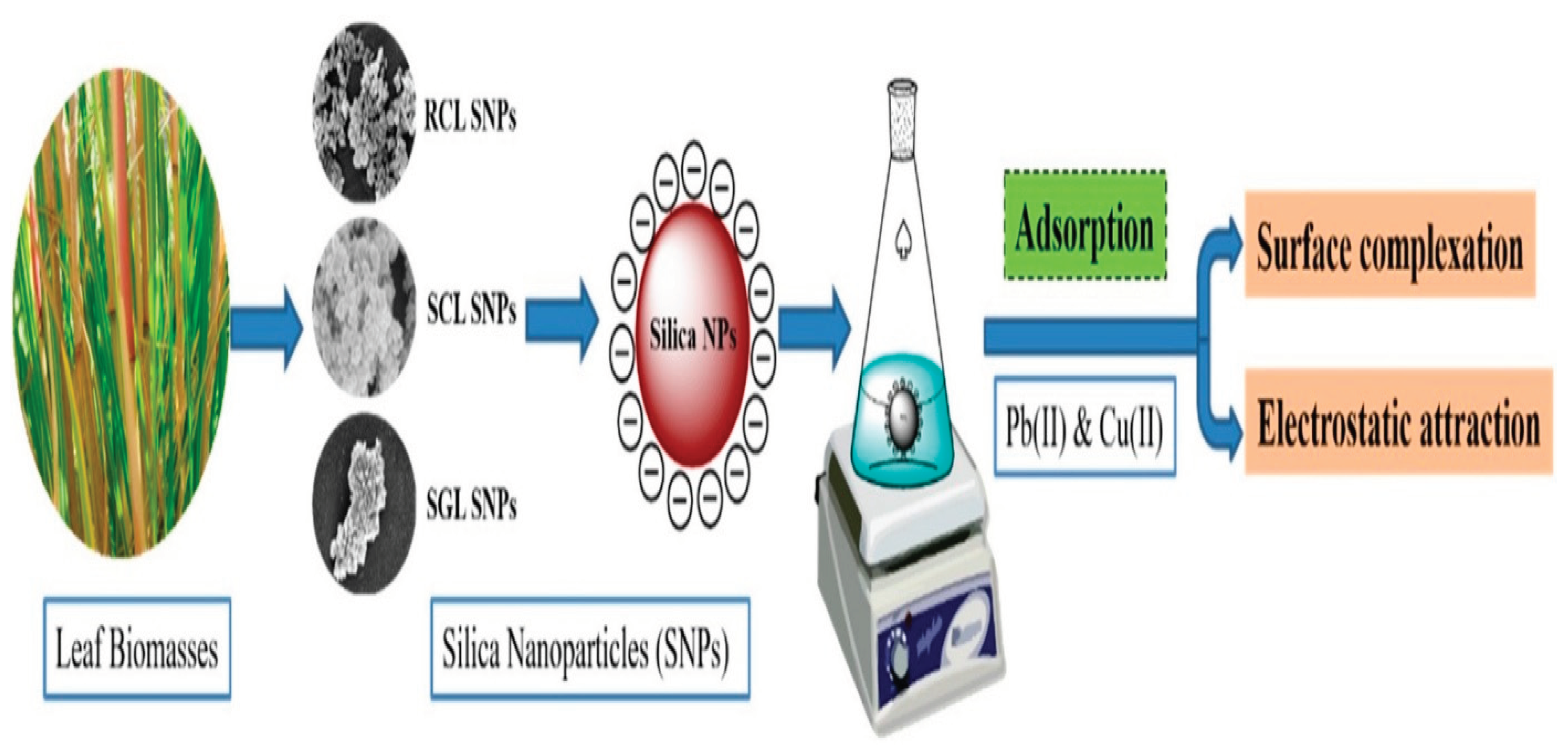
As a possible absorbent for heavy metal ions, Sachan, Ramesh and Das [
28] produced silicon nanoparticles (SNPs) from the dried leaf biomass of Saccharum ravannae (SRL), Saccharum officinarum (SOL), and Oryza sativa (OSL). SNPs were also used to remove copper (Cu2+) and lead (Pb2+) from wastewater as suitable adsorbent materials.
Figure 8.
Green synthesis of silica nanoparticles from leaf biomass and its application to remove heavy metals from synthetic wastewater: A comparative analysis. (Source [
28]).
Figure 8.
Green synthesis of silica nanoparticles from leaf biomass and its application to remove heavy metals from synthetic wastewater: A comparative analysis. (Source [
28]).
The adsorption results reported high adsorption capacity for heavy metal ions (Pb2+ and Cu2+), (140.06 mg/ g and 149.25 mg/g SRL SNPs); (338.55 mg/g and 179.45 mg/g for SOL SNPs), (334.7 mg/g and 274.02 mg/g for OSL SNPs). The synthesized SNPs have shown high adsorption capacity with high reusability for the removal of heavy metal ions from synthetic wastewater. The SNPs were able to remove more than 95 % of heavy metal ions from synthetic wastewater. The adsorption isotherm was well explained by Freundlich isotherm model and kinetics via pseudo-second-order kinetics for Pb2+ and Cu2+ using SRL, SOL and OSL SNPs.
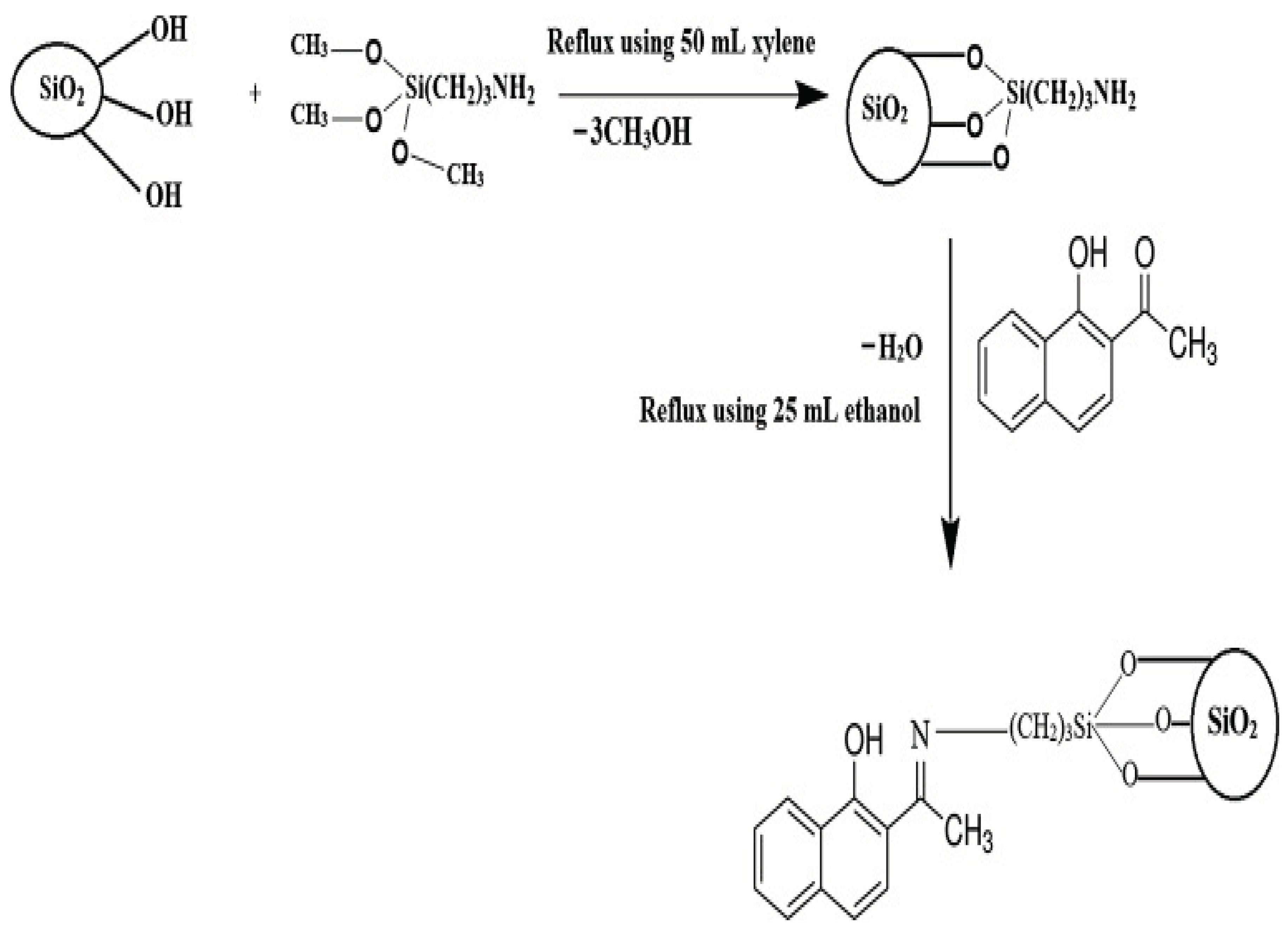
Al-Wasidi, et al. [
64] synthesized unique composite based on Schiff base synthesis on silica nanoparticles. First, 3-aminopropyltrimethoxysilane was used to modify silica nanoparticles, which contain silanol groups (Si-OH). A new Schiff base/silica composite developed after the modified silica reacted with 1-hydroxy-2-acetonaphthone.
Figure 9.
The synthetic steps of the composite (Source [
64]).
Figure 9.
The synthetic steps of the composite (Source [
64]).
For the effective removal of Ni2+, Cu2+, Zn2+, and Hg2+ions from aqueous solutions, the synthesized composite was used. The composite has a maximal absorption capacity of 68.630, 50.942, 45.126, and 40.420 mg/g for Cu2+, Hg2+, Zn2+, and Ni2+ ions, respectively. The findings demonstrated that at pH = 6.5, contact time = 90 min, and adsorption temperature = 298 K, the greatest percentage removal of the examined metal ions was accomplished.
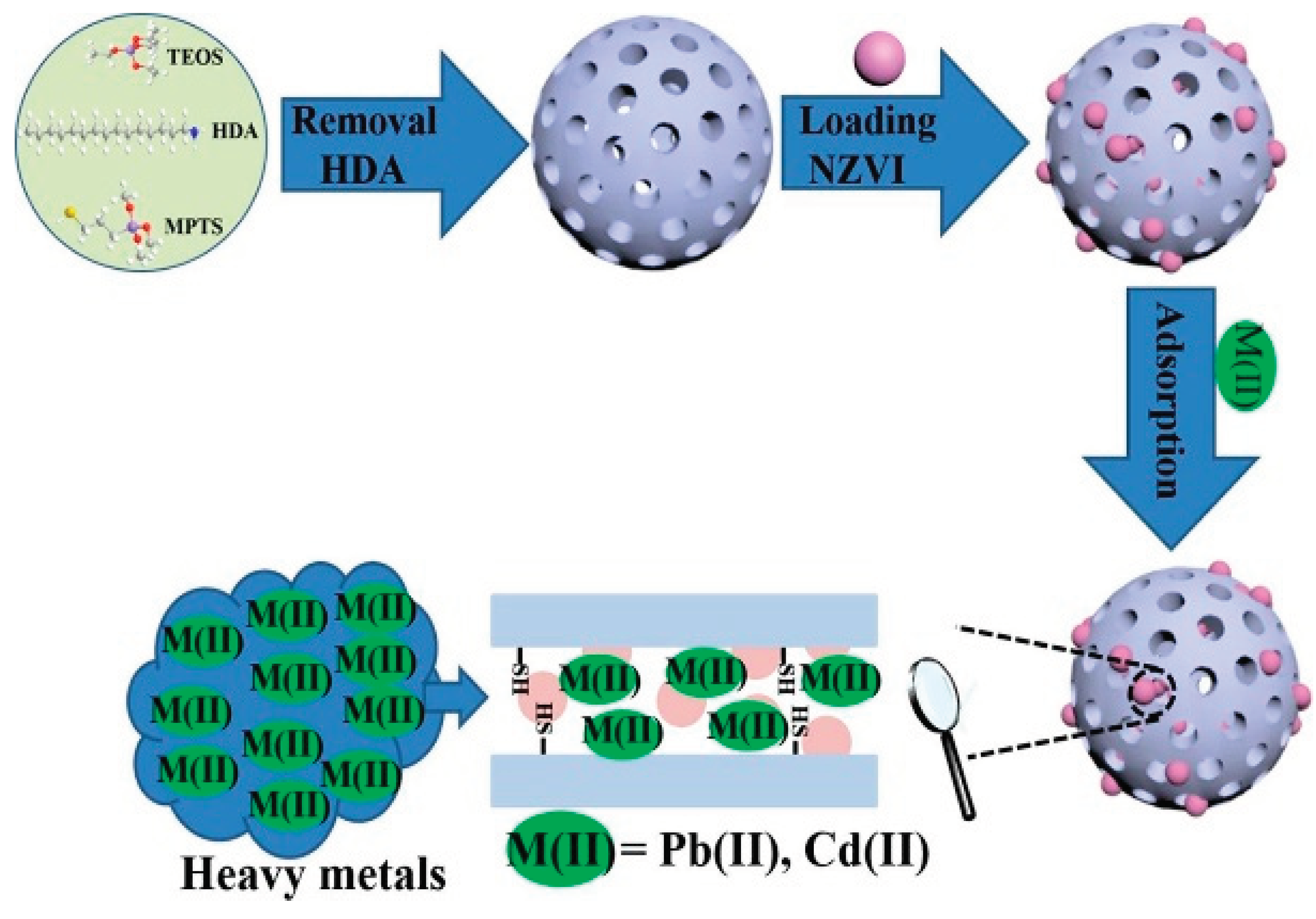
Bifunctional magnetic mesoporous silica (NZVI-SH-HMS) was successfully synthesized by Li, et al. [
65] for the efficient removal of Pb2+ and Cd2+ ions from aqueous solution. First, a bifunctionalized magnetic mesoporous silica (NZVI-SH-HMS) material was manufactured at room temperature using the sol-gel and wet impregnation processes, with thiol and nanoscale zero-valent iron bound on the surface.
Figure 10.
Highly effective removal of lead and cadmium ions from wastewater by bifunctional magnetic mesoporous silica (Source [
65]).
Figure 10.
Highly effective removal of lead and cadmium ions from wastewater by bifunctional magnetic mesoporous silica (Source [
65]).
NZVI-SH-HMS had maximal adsorption capabilities of 487.8 mg/g for Pb2+ and 330.0 mg/g for Cd2+, respectively. Data on the pseudo-second-order kinetics and isotherm of adsorption were well fitted to the Langmuir isotherm model. The reusability and regeneration studies showed NZVI-SH-HMS had a large capability for removing heavy metals from the actual water environment and retained outstanding adsorption performance.
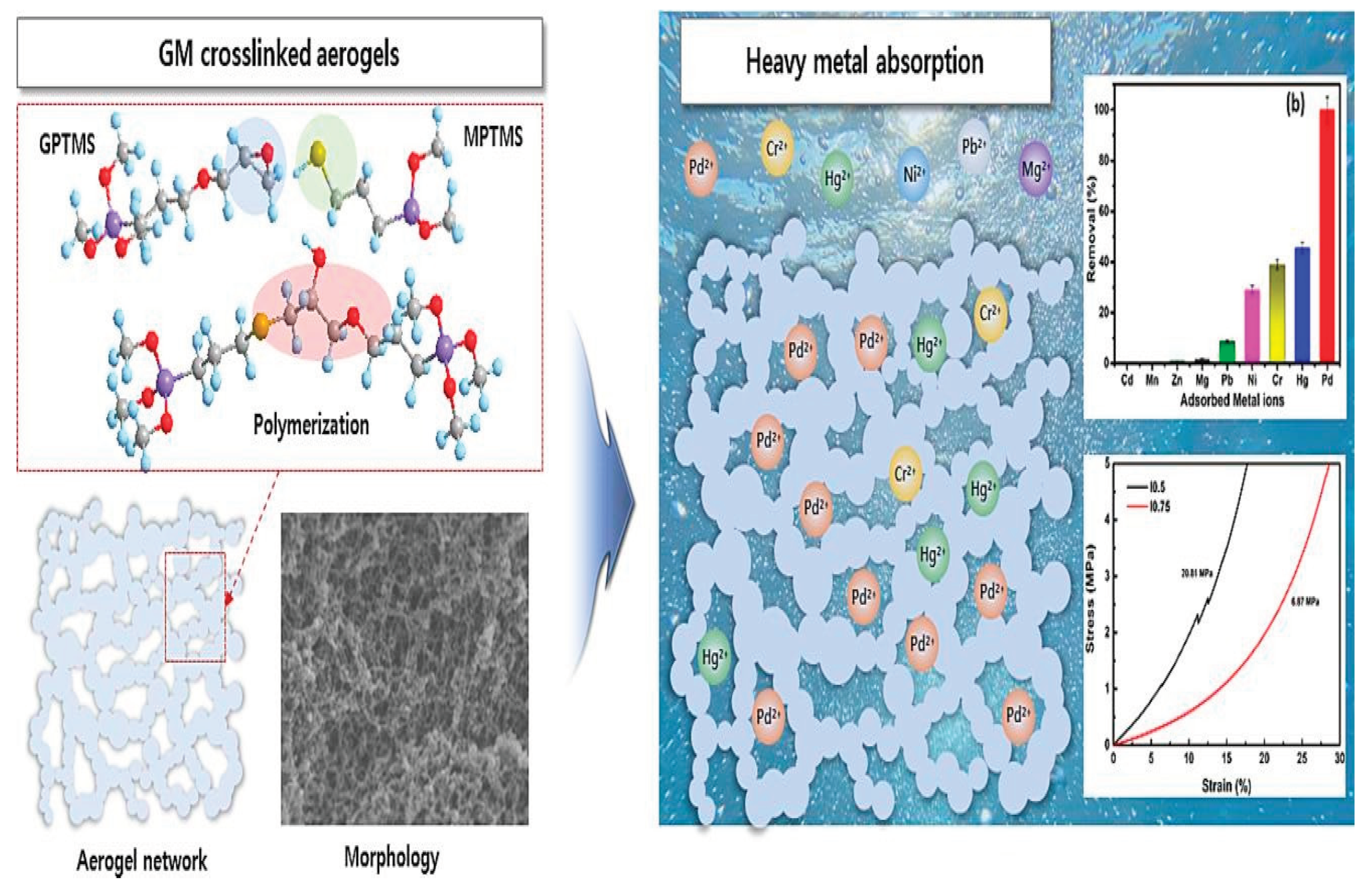
To increase the mechanical strength and heavy metal ion adsorption of silica aerogel, Parale, et al. [
66] developed a novel in situ sulfur-doping manufacturing process. Three-dimensional monolithic silica aerogels were initially developed by polymerizing (3-glycidoxypropyl) trimethoxysilane and (3-mercaptopropyl)trimethoxysilane using an adjustable one-pot epoxy-thiol reaction, then by using the sol-gel method and supercritical drying.
Figure 11.
Cross-linked silica aerogels were synthesized using one-pot epoxy-thiol polymerization and a sol-gel process and heavy metal adsorption ([
66]).
Figure 11.
Cross-linked silica aerogels were synthesized using one-pot epoxy-thiol polymerization and a sol-gel process and heavy metal adsorption ([
66]).
The heavy metals were eventually removed using the produced aerogels, with Pd2+ being adsorbed nearly 100% of the time. The Langmuir adsorption model was able to fit an adsorption capacity of up to 689.65 mg/g. The suggested technique is a novel approach for the in-situ synthesis of sulfur-doped silica aerogels with improved mechanical properties for heavy metal removal.
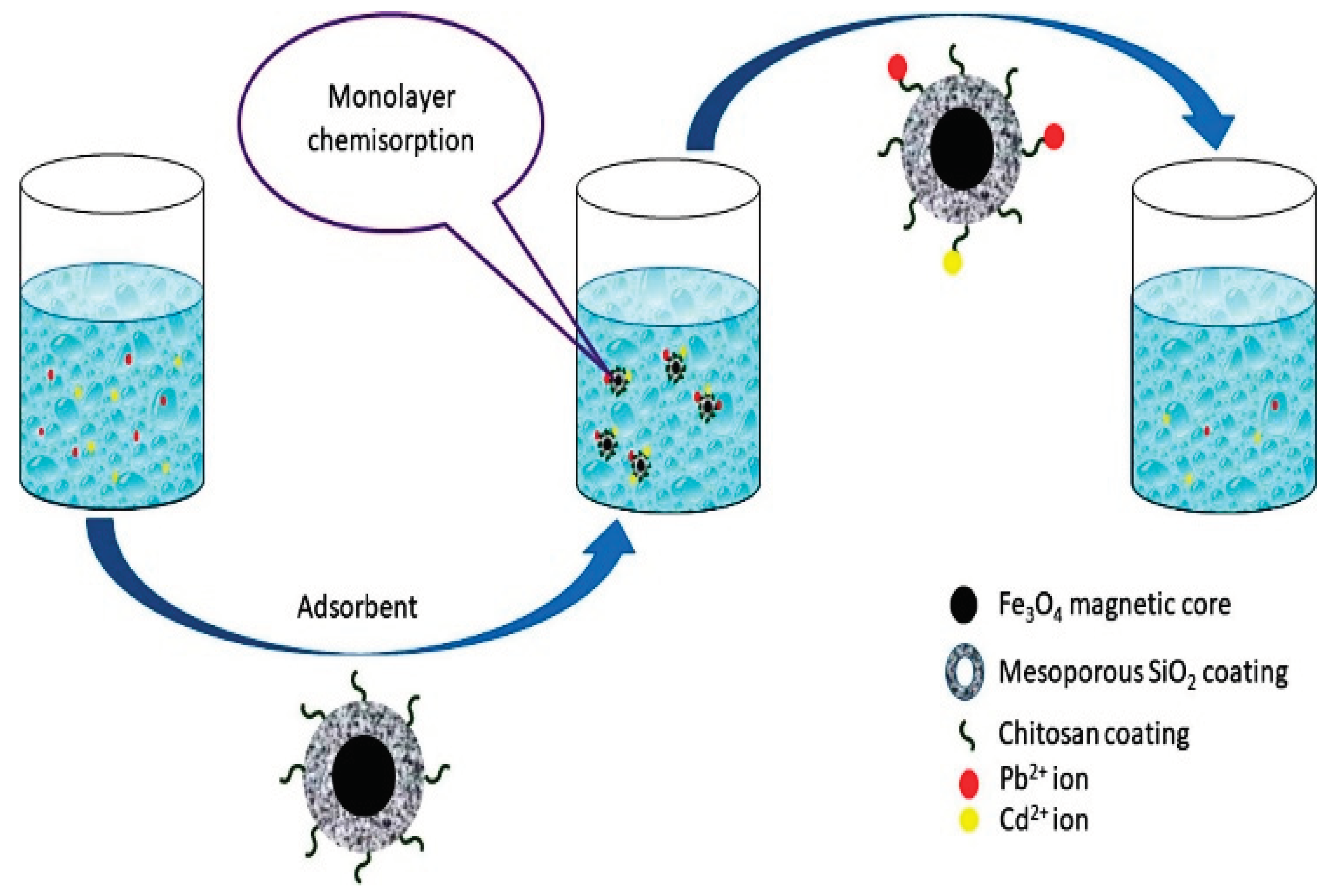
Groundwater contamination by heavy metals in the environment has grown to be a major threat to the health of living things. In order to reduce the hazards involved, heavy metals in contaminated water must be removed. Amin, et al. [
67] Mesoporous silica and chitosan nanoparticles that have been synthesized are coated with magnetite to absorb heavy metals from wastewater. First, due of the monodispersity offered by this approach, magnetite nanoparticles (MNPs) developed through the thermal degradation of the iron oleate precursor. The particles were given a mesoporous silica layer coating using the surfactant cetyltrimethylammonium bromide (CTAB). By stirring in chitosan solution, the coated particles were subjected to chitosan coating.
Figure 12.
Heavy metal adsorption from wastewater by mesoporous silica and chitosan-coated magnetite nanoparticles (Source [
67]).
Figure 12.
Heavy metal adsorption from wastewater by mesoporous silica and chitosan-coated magnetite nanoparticles (Source [
67]).
In known concentrations of heavy metal salt solutions, the particles were utilized as an adsorbent. It is a favourable sign for future investigation and analysis because the produced adsorbent shown a reasonable adsorption capacity of 150.33 mg/g and 126.26 mg/g for Pb2+ and Cd2+, respectively. The pseudo second order kinetic model and the Langmuir isotherm model are superior fits for the adsorption data of Pb2+ and Cd2+ based on the value of R2 (R2 ≥ 0.99).
A new composite was developed in this study by Al-Wasidi, Naglah, Saad and Abdelrahman [
64], based on the production of Schiff base on silica nanoparticles. A silanol group (Si-OH) was added to silica nanoparticles using (3-aminopropyl)trimethoxysilane. A novel Schiff base/silica composite was made by mixing the modified silica with 4,6-diacetylresorcinol.
Figure 13.
The synthetic steps of the composite (Source [
64]).
Figure 13.
The synthetic steps of the composite (Source [
64]).
Pb2+, Cu2+, Co2+, and Ni2+ ions were efficiently removed from aqueous solutions using the produced composite. The composite has a maximal absorption capacity of 107.066, 89.767, 80.580, or 70.972 mg/g for Pb2+, Cu2+, Co2+, and Ni2+ ions, respectively. The examined metal ions' adsorption processes were chemical, spontaneous, and well matched with the pseudo-second-order kinetic model and Langmuir equilibrium isotherm.
3.3. Cellulose, silica nanoparticles and heavy metal adsorption (Cd, Pb, Cr)
Recent research has shown that nanotechnology has enormous potential for improving the efficiency of water prevention and purification while reducing costs. In this field, nanocellulose has attracted attention for two applications where it has effectively eliminated pollutants as a membrane and an adsorbent. It has this potential as a result of its high aspect ratio, huge specific surface area, excellent water retention, and environmental inertness. In addition to the advantages already mentioned, the presence of active sites enables the incorporation of chemical moieties that might increase the effectiveness of pollutants binding to the surface [
68].
Heavy metals such Cd2+, Pb2+, Mn2+, and others pose a serious threat to the environment since they are nonbiodegradable. Using environmentally acceptable nanomaterials, heavy metal pollution can be minimized thanks to the development of nanobiotechnology. Specially designed bionanomaterials usually exhibit properties like a longer shelf life, self-healing nature, adaptability in varied environments, and cost effectiveness in comparison to nanoparticles generated using physicochemical procedures. Due to their excellent selectivity and adsorption capacity, bionanomaterials can effectively remove heavy metals from wastewater, even when they are present in incredibly low quantities. In order to reduce expenses and create a way for environmental sustainability, bionanotechnology is applied in their cleanup [
69].
The characteristics of nanoparticles have been extensively investigated by their restricted capacity to adsorb some heavy metal ions. According to Rajendran, et al. [
70] there has been research on the effects of experimental conditions on the uptake of metal ions, including the dose of the adsorbent, pH, substrate concentration, response time, temperature, and electrostatic force. Furthermore, the use of polymeric membranes for the adsorption of dangerous particles may lead to the development of next-generation reusable and portable water filtration systems. Because they conduct both membrane filtration and adsorption, membranes for membrane adsorption (MA) are very effective at eliminating trace quantities of contaminants such cationic heavy metals, anionic phosphates, and nitrates [
71].
Utilizing carbohydrate biopolymers, several nanocomposite adsorbents for wastewater treatment have been developed. The effectiveness of extracting inorganic pollutants from aqueous solutions was investigated into using these adsorbents. Toxic metals may be efficiently absorbed by cross-linkers, which dissolve in aqueous solutions of divalent heavy metal ions to assess their polymer absorption capacity. These nanocomposites were used to remove Cd2+, Pb2+, and Zn2+, three very dangerous elements from water. To improve the effectiveness of heavy metal ion absorption, various functionalization techniques have been applied, such as grafting, blending, or mixing with other nanomaterials that have an extra functional group. The ability of an adsorbent to be recycled depends in part on its mechanical efficiency, which is raised by integrating the second component into the primary polymer chain. The method for removing metal ions from wastewater is less expensive as long as the adsorbent is recycled [
72].
To recover heavy metal ions and benzene fumes, Yarkulov et al., 2022 developed diacetate cellulose-silicon bionanocomposite adsorbent (DACSBNC). The observed findings validated the following: (i) the DACSBNC's adsorption capacitance for Cd2+, Hg2+, and Pb2+ ions were proven to be 12.23, 13.87, and 31.40 mg/g, respectively; and (ii) the DACSBNC's sorption capacitance for benzene vapors was 0.5618 mmol/g. For the extraction of heavy hazardous metal ions as Cd2+, Hg2+, and Pb2+ from galvanic aqueous solutions, the researched DACSBNC materials have been suggested as potential sorbents.
To develop a porous matrix with balanced surface characteristics, silica from rice husk was treated using the sol-gel method, and cellulose from Nata de Coco was added in the gelling process. Hazardous waste is laboratory waste that pollutes the environment and contains heavy metals as Cadmium, Chromium, Nickel, and Zinc. Adsorption is an efficient and inexpensive method to handle heavy metal waste. The material silica-cellulose was developed and used in this study by Royanudin, et al. [
73], as an adsorbent for heavy metals, including cadmium, chromium, nickel, and zinc, in laboratory waste. The percentages of remediation for the metal’s cadmium, chromium, nickel, and zinc were 95.09%, 58.77%, 94.56%, 94.56%, and 97.50%, respectively.
In the study by Yang, et al. [
74], the researchers created and made ferrocene-incorporated mesoporous organosilica nanoparticles, which is a novel inorganic-organic hybrid nanomaterial (MONs). Organoalkoxysilanes containing ferrocene have been synthesized as shown in
Figure 14a, and the raw material was used directly to synthesize MONs without any purification because 1,10-ferrocenedicarboxylic acid was not involved in the process. The results of the reaction are shown below.
The mesoporous structure, substantial surface area, and presence of the ferrocene group in the framework allowed MONs to more effectively adsorb the phosphate anion than surface-modified MSNs (SiO2-Fe) (488 mg/g). The results showed that, when compared to MSNs with ferrocene surface modifications, MONs is a better absorbent. Congo red (CR) and Pb2+ were also utilized as model pollutants.
Titanium dioxide (TiO2) and other inorganic nanoparticles were used to remove heavy metals. In comparison to bulk particles, TiO2 nanoparticles (nanoTiO2) also show a higher rate of adsorption [
75]. For the purpose of multiple heavy metal adsorption, Wang, Zhan, Wu, Shi, Du, Luo and Deng [
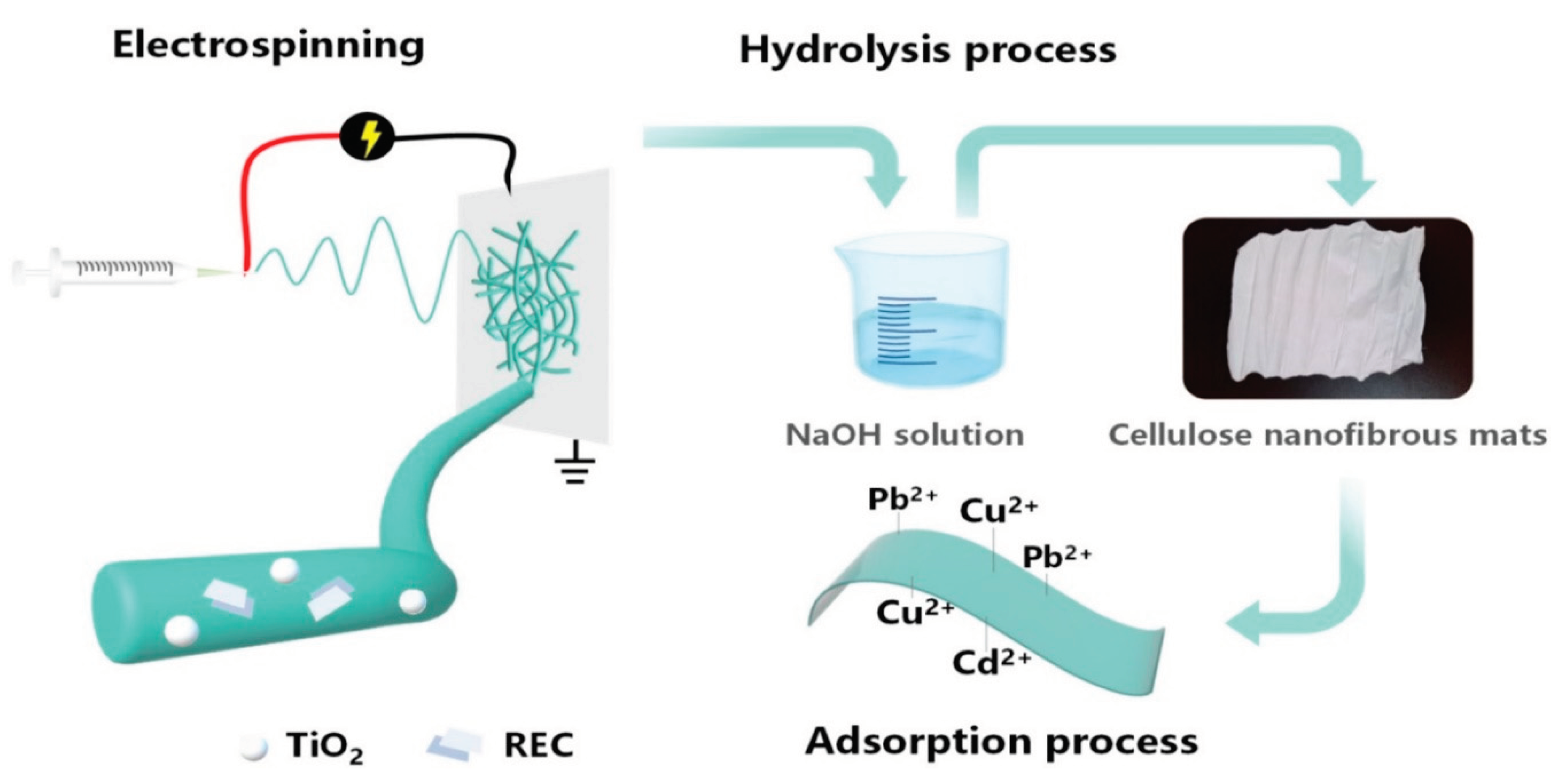
46] synthesized titanium dioxide (TiO2)/rectorite (REC)-trapped cellulose composite nanofibrous mats using electrospinning. The thermal stability, surface area, tensile strength, and adsorption capacity of the mats were all enhanced by the interactions between inorganic adsorbents and cellulose molecules.
Figure 15.
Schematic diagram of preparation of cellulose membrane via electrospinning of cellulose acetate with TiO2 nanoparticles and rectorite, followed by deacetylation to pure cellulose with increased absorption properties towards heavy metal ions Pb2+, Cu2+ and Cd2+ (Source [
46]).
Figure 15.
Schematic diagram of preparation of cellulose membrane via electrospinning of cellulose acetate with TiO2 nanoparticles and rectorite, followed by deacetylation to pure cellulose with increased absorption properties towards heavy metal ions Pb2+, Cu2+ and Cd2+ (Source [
46]).
The pseudosecond-order model and Langmuir isotherm model were mostly used in the adsorption of Cd2+ onto cellulose-TiO2/REC nanofibrous mats. The pH of the solution influenced the multiple adsorptions of Pb2+, Cu2+, and Cd2+ onto composite nanofibrous mats. Cellulose-TiO2/REC2:1 nanofibrous mats at pH 6 had the highest total adsorption capacity of 69.81 mg/g. The produced cellulose-TiO2/REC nanofibrous mats might be employed to extract heavy metals from acidic wastewater. The composite nanofibrous mats successfully captured TiO2 nanoparticles.
3.4. Cellulose and silica coupling agents in absorption of heavy metals
The functionalization of cellulose through a reaction with organosilanes has frequently been utilized to enhance its characteristics. For the improvement of the dispersions of the functional groups that serve as the adsorption sites for aqueous arsenate, the influence of substitutions prior to coupling with silanes has hardly been discussed [
76,
77]. The introduction of coordination groups containing coordination atoms, such as -OH, -COOH, -C-O, -C=N, -NH2, -NH, -C-S, -C=S, -S-S, and -S=O, into the cellulose-based adsorbent can result in an adsorbent with high adsorption performance toward heavy metal ions and other pollutants.
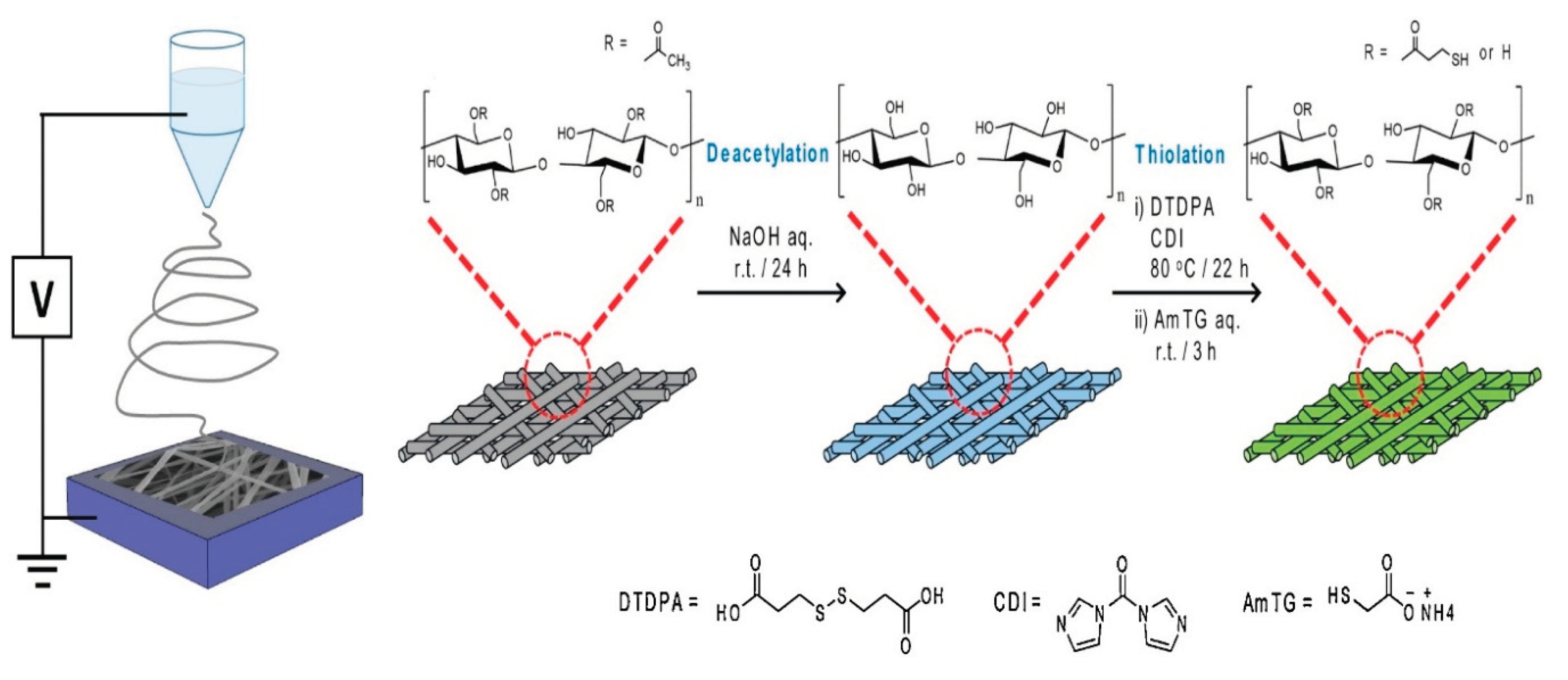
A cellulose nanofiber membrane that has been thiol-functionalized and is capable of successfully adsorbing heavy metal ions is described by Choi, et al. [
78]. As a result of the deacetylation of electrospun cellulose acetate nanofibers and subsequent esterification of a thiol precursor molecule, thiol was added to the surface of cellulose nanofibers.
Figure 16.
Schematic illustration depicting fabrication process for thiol-functionalized cellulose nanofiber membrane (Source [
78]).
Figure 16.
Schematic illustration depicting fabrication process for thiol-functionalized cellulose nanofiber membrane (Source [
78]).
The Langmuir isotherm provided a good description of adsorption capacity as a function of adsorbate concentration, indicating that metal ions form a surface monolayer with a homogeneous distribution of adsorption energy. Cu2+, Cd2+, and Pb2+ ions have maximum adsorption capabilities of 49.0, 45.9, and 22.0 mg/g in the Langmuir isotherm, respectively. According to the time-dependent adsorption capacities, which were consistent with a pseudo-second-order kinetic model, chemisorption of each doubly charged metal ion takes place when there are two thiol groups present on the surface.
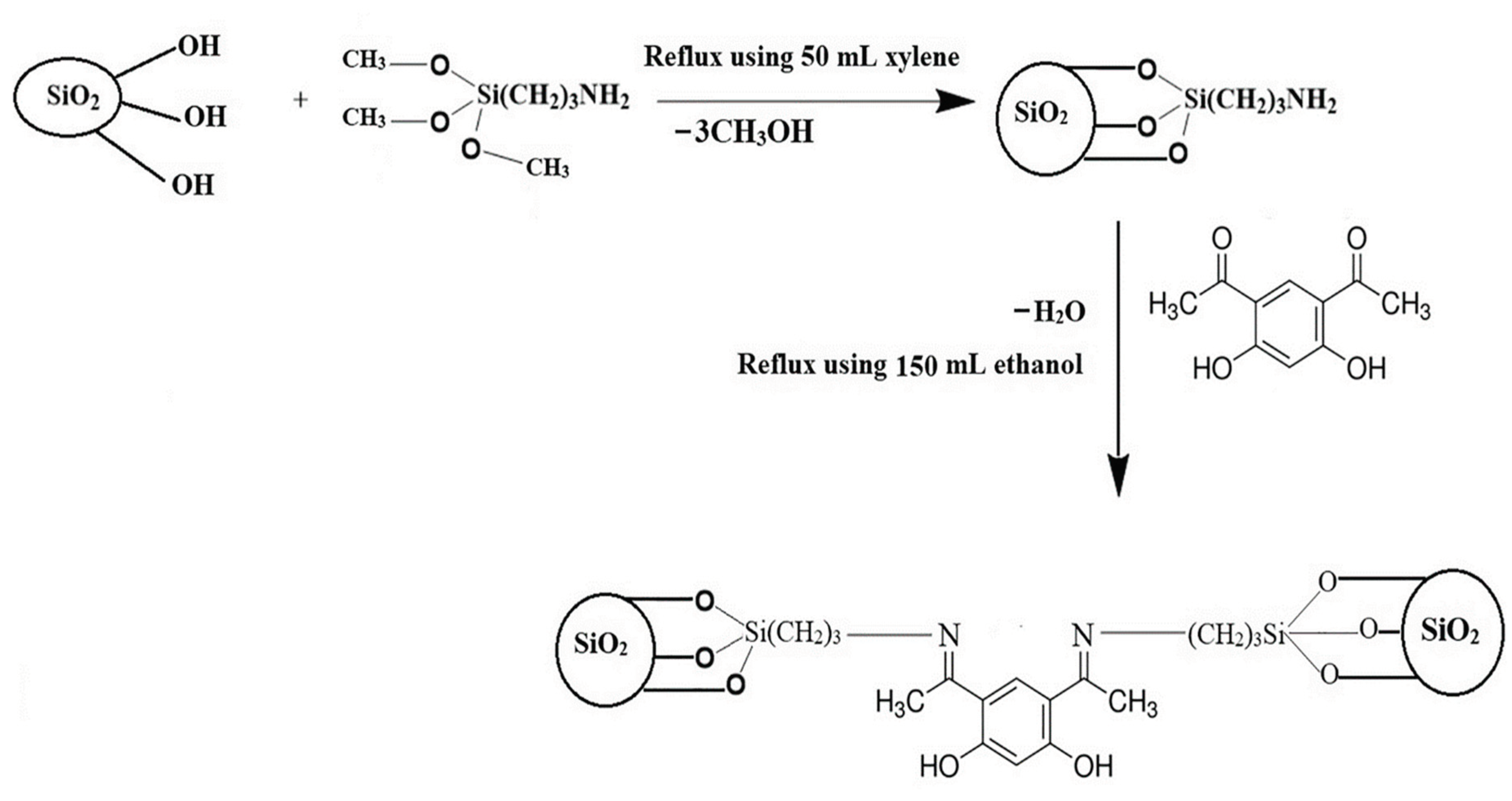
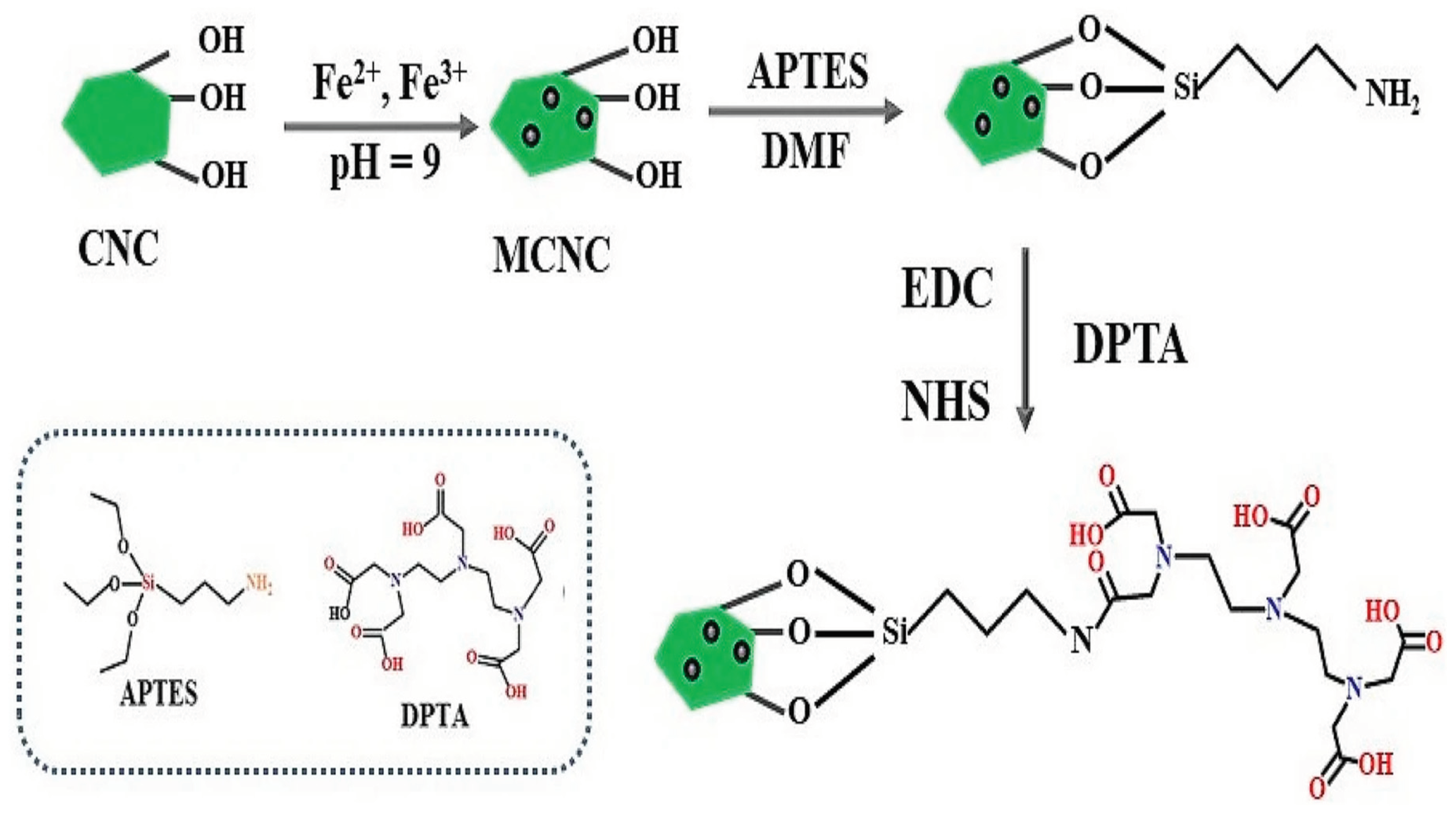
The study on the reaction of hydroxy groups with diethylenetriaminepentaacetic acid (DPTA)-modified aminosilane shows the usage of a silane containing both carboxy and amine for the chemical modification of cellulose [
79].
Figure 17.
Schematic representation of the preparation of MCNC-DPTA (Source [
79]).
Figure 17.
Schematic representation of the preparation of MCNC-DPTA (Source [
79]).
Higher temperatures were discovered to facilitate adsorption, with maximum Pb2+ adsorption capacities of 424.78, 424.67, and 440.0 mg/g being obtained at 293.3, 298.3, and 303.3 K, respectively. This material's adsorption behaviour was investigated, and the results showed that the natural initial pH of the aqueous Pb2+ solution was optimal for the adsorption process. Investigations were made on the isotherm and kinetic parameters for Pb2+ adsorption by MCNC-DPTA. The Langmuir isotherm model and pseudo-second order were thought to better represent the adsorption process of Pb2+ by MCNC-DPTA due to the greater fitting coefficients.
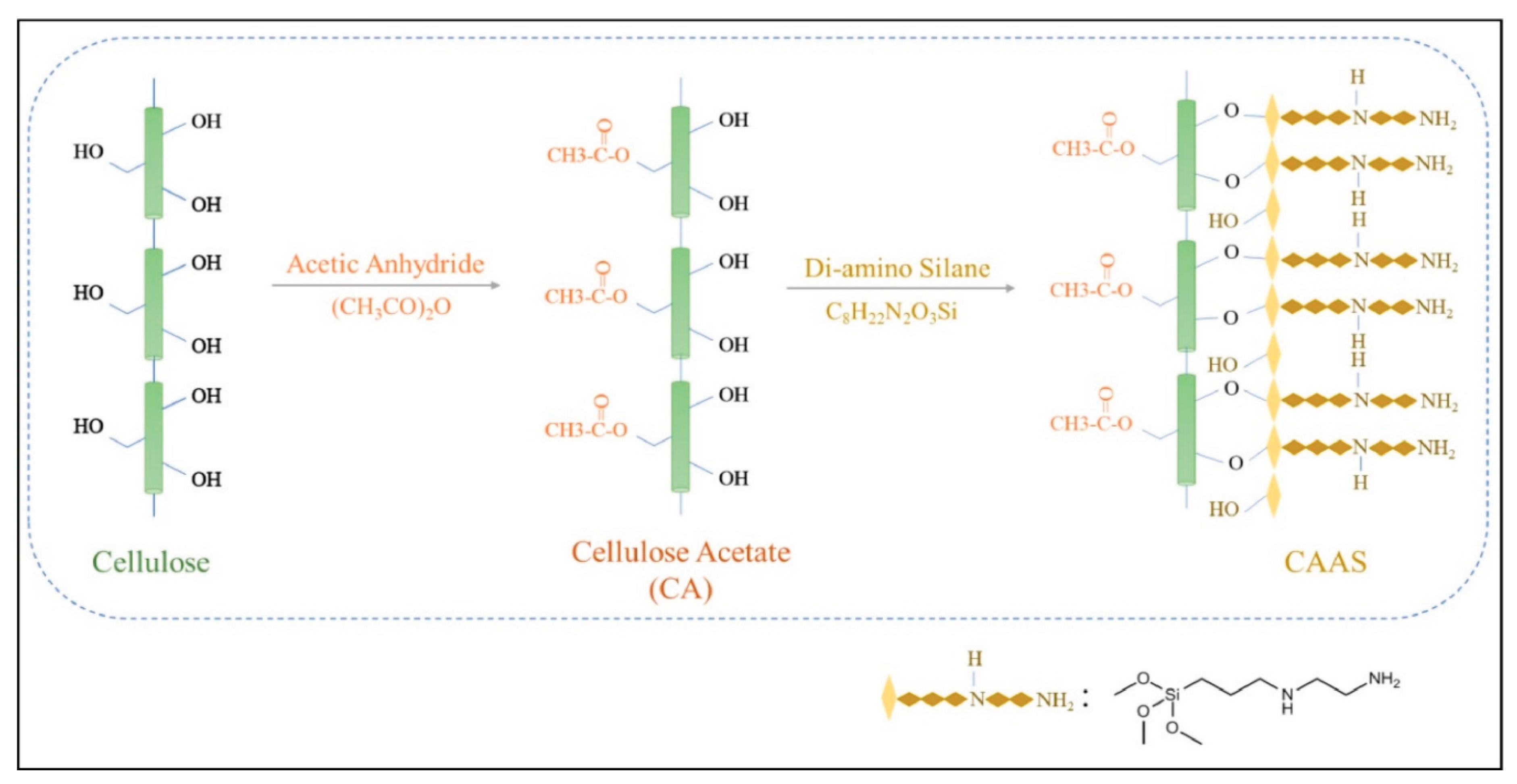
Using acetylated microcrystalline cellulose and 3-(2-aminoethylamino)propyltrimethoxysilane (AEAPTMS), Bisla, et al. [
80] prepared a new cross-linked polymer called cellulose acetate aminosilane (CAAS), where the acetyl group likely promotes the formation of polymer structure that makes aqueous ion diffusion easier during their adsorptions.
Figure 18.
Schematic illustration for preparation of CAAS (Source [
80]).
Figure 18.
Schematic illustration for preparation of CAAS (Source [
80]).
A significant quantity of arsenate was adsorbed by CAAS in a pH range of 2 to 10, indicating that the inclusion of both amine and acetyl groups broadens the pH range of the adsorption. The pseudo-second order kinetics and Langmuir isotherm models suit the adsorption data nicely. A remarkable capacity for the aminosilane-modified cellulose polymers, the adsorption reached 455 mg/g.
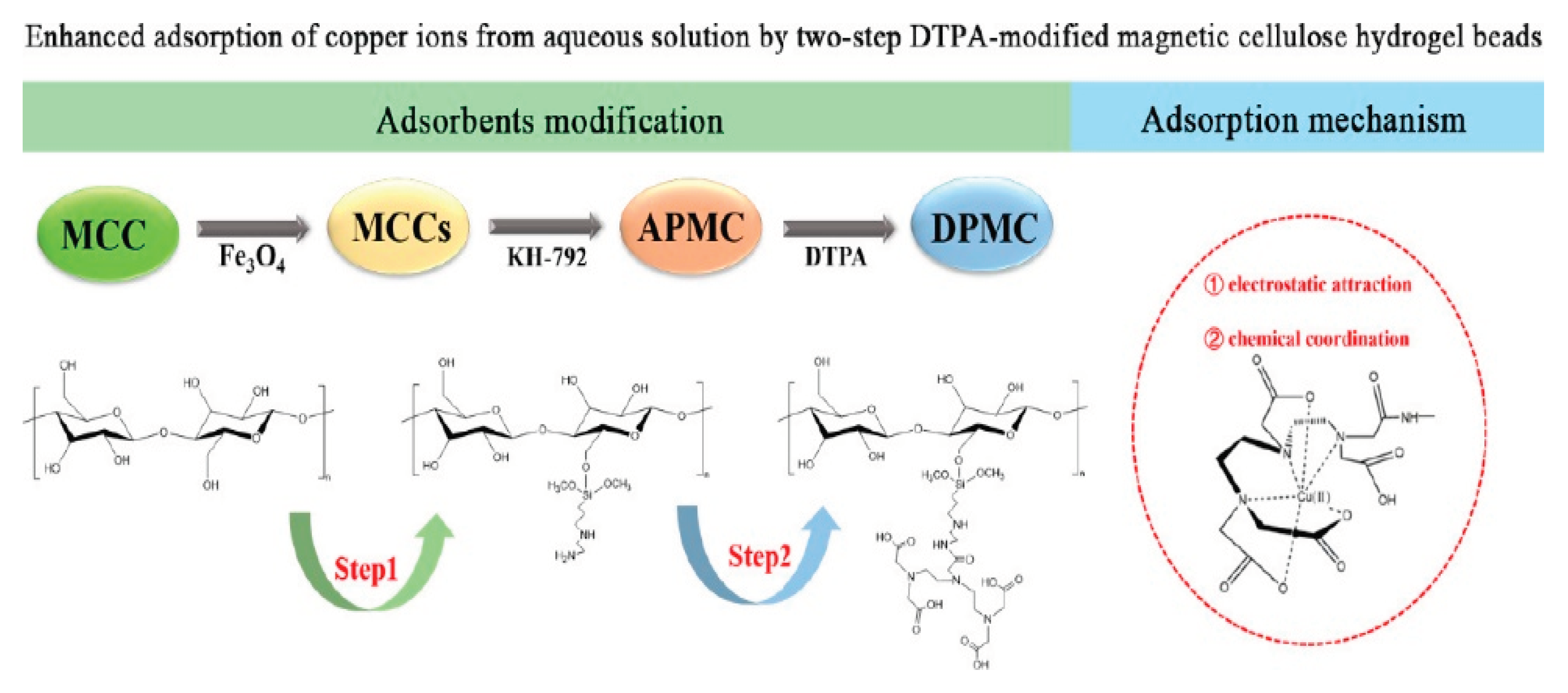
One of the most serious environmental issues that has generated a great deal of worry in recent decades is copper contamination of water. For the purpose of removing Cu2+ from aqueous solutions, Gao, et al. [
81] treated cellulose in two steps with N-[3-(trimethoxysilyl)propyl]ethylenediamine (KH-792) and diethylenetriaminepentaacetic acid (DTPA).
Figure 19.
Cu(II) adsorption process and related mechanism ([
81]).
Figure 19.
Cu(II) adsorption process and related mechanism ([
81]).
DPMC had more Cu(II) adsorption capacity than MCCs and APMC, with a maximum adsorption capacity of 298.62 mg/g at pH 6. The equilibrium adsorption data were well represented by the Langmuir model, and the adsorption process was found to fit better with the pseudo-second-order kinetics model, indicating that monolayer chemical adsorption dominated the process. In order to remove Cu(II) from aqueous media, DPMC composite exhibits great potential as an adsorbent.
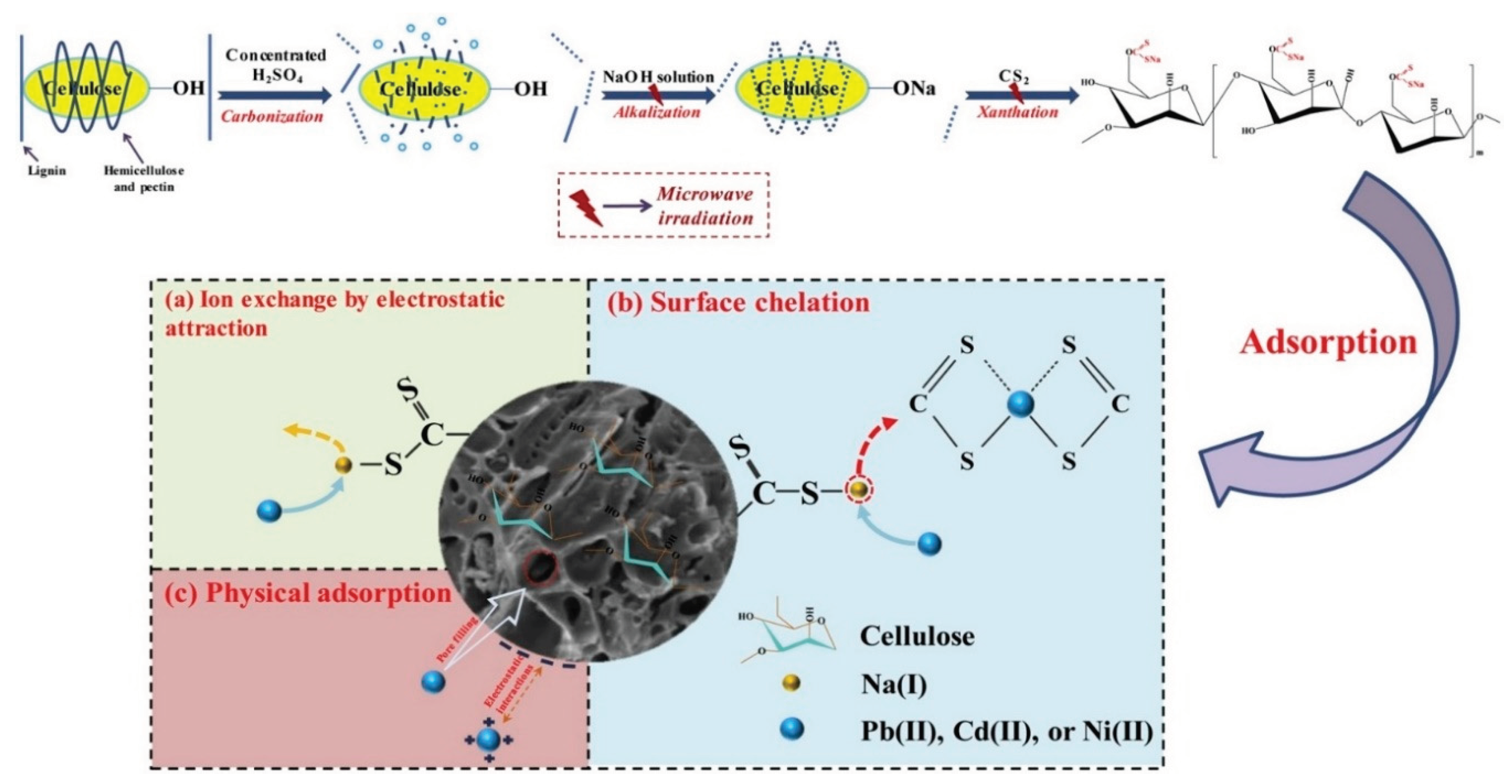
In mono- and multi-component systems, Pb2+, Cd2+, and Ni2+ removal were accomplished with the help of rice husk-derived microwave-functionalized cellulose developed by Qu, et al. [
82]. In order to prepare xanthate-modified cellulose, microwave (MW) irradiation methods were used, followed by the sequential functional stages of carbonization, alkalization, and xanthation via water-bath heating treatment. The functionalized process was completed in 6.5 min thanks to microwave irradiation, and the resulting RHMW-X had surprisingly high adsorption capacities of 295.20 mg/g for Pb2+, 151.51 mg/g for Cd2+, and 72.80 mg/g for Ni2+ during the equilibrium duration of 30 min.
Figure 20.
Schematic illustration of microwave-assisted modification of cellulose to produce RHMW-X; and Proposed adsorption mechanisms of Pb2+, Cd2+ and Ni2+ by RHMW-X: (a) ion exchange, (b) surface chelation and (c) physical adsorption (Source [
82]).
Figure 20.
Schematic illustration of microwave-assisted modification of cellulose to produce RHMW-X; and Proposed adsorption mechanisms of Pb2+, Cd2+ and Ni2+ by RHMW-X: (a) ion exchange, (b) surface chelation and (c) physical adsorption (Source [
82]).
Shaheen, et al. [
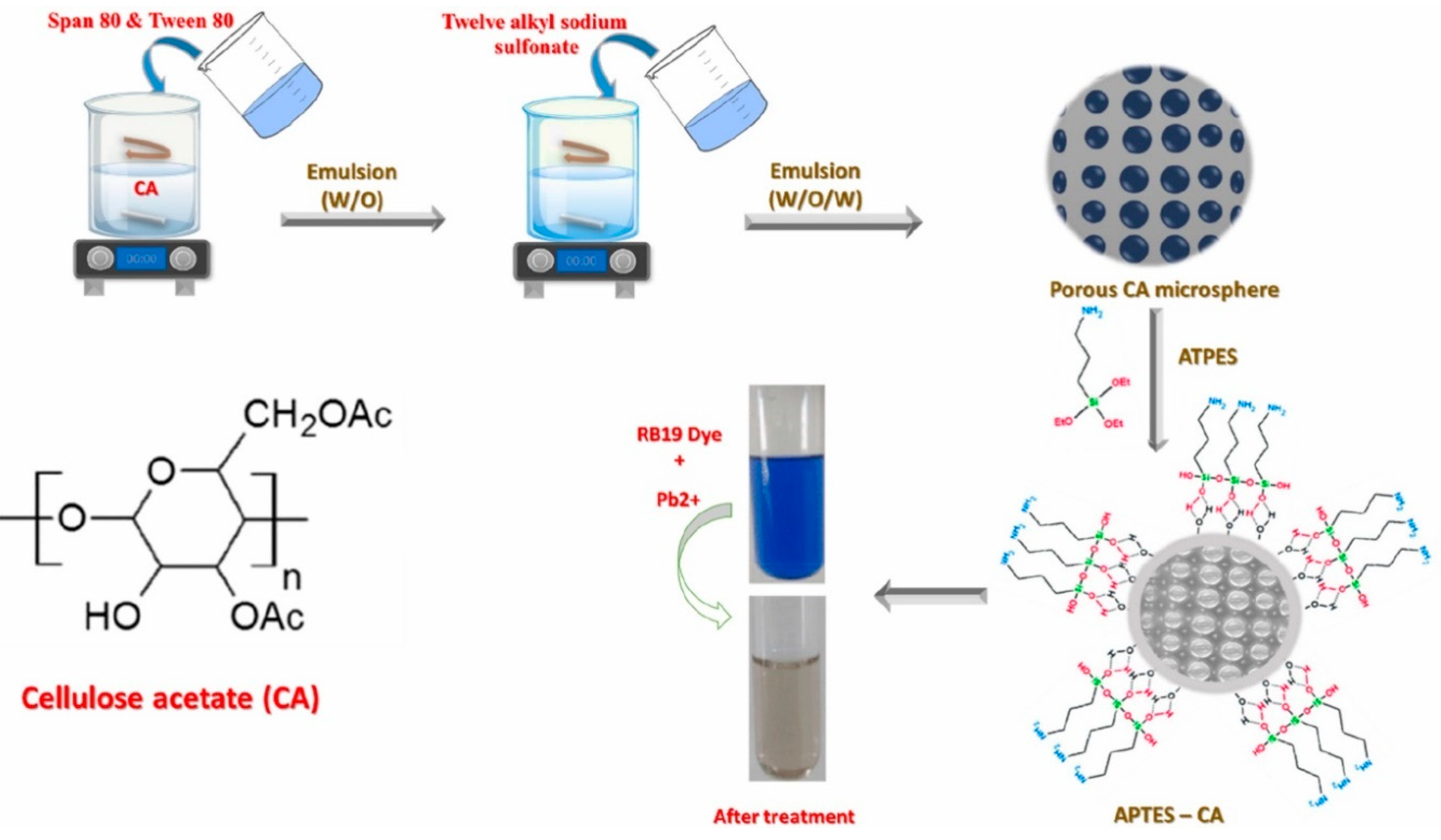
83] develop a new, inexpensive bio-based adsorbent for the successful removal of an anionic dye and a hazardous metal simultaneously. 3-aminopropyltriethoxysilane (APTES-CA) was used to modify porous cellulose acetate (CA) microspheres that had been synthesized utilizing a double emulsion (W/O/W)-solvents evaporation process.
Figure 21.
Schematic diagram for APTES-CA synthesis pathway and Reactive blue 19 (RB19) and Pb2+ adsorption (Source [
83]).
Figure 21.
Schematic diagram for APTES-CA synthesis pathway and Reactive blue 19 (RB19) and Pb2+ adsorption (Source [
83]).
According to the adsorption investigation, APTES modification increased the effectiveness of adsorption for RB19 and Pb2+. According to the kinetic investigation, APTES-CA adsorbs RB19 and Pb2+ by several processes, specifically chemisorption for RB19 and physisorption for Pb2+. A good efficiency of APTES-CA towards Pb2+ was demonstrated by the theoretical Langmuir monolayer saturation capacity (180 mg/g).
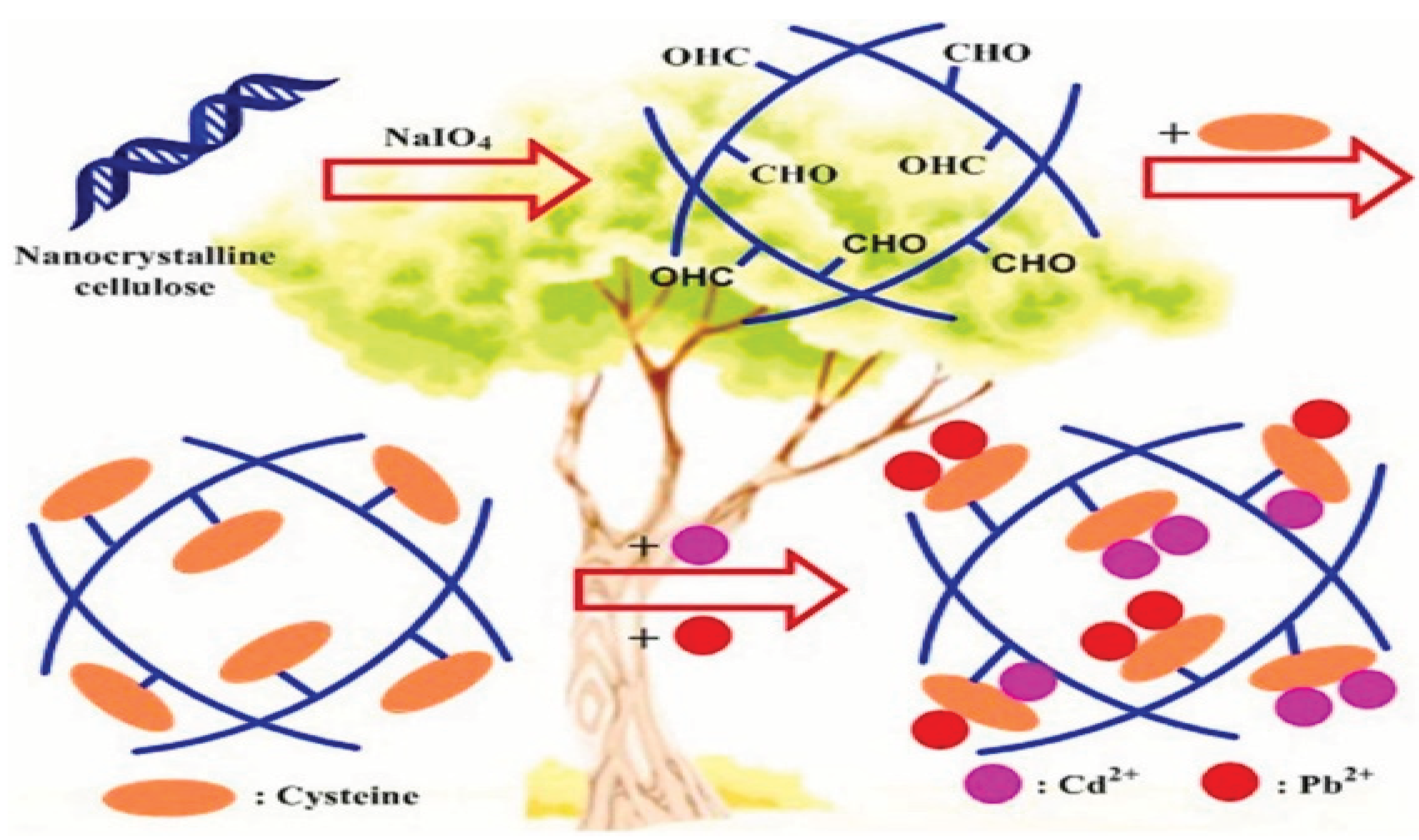
In order to improve the adsorptive removal of Cd2+ and Pb2+ from aqueous solution, chelating celluloses functionalized with the four amino acids cysteine, histidine, aspartic acid, and methionine (as named Cys-CL, His-CL, Asp-CL, and Met-CL) were prepared by Chen, et al. [
84] through a Schiff base reaction. These materials were then investigated by various means of characterization. Because it has a large number of active binding sites and uses complexation of its thiol, imine, and carboxyl groups with both metal ions as the adsorption mechanism, the Cys-CL stood out among the others for its excellent adsorptive capabilities for the improved adsorption of the Cd2+ and Pb2+ ions.
Figure 22.
synthesis of chelating celluloses functionalized with amino acid (cysteine) and heavy metals adsorption (Source[
84]).
Figure 22.
synthesis of chelating celluloses functionalized with amino acid (cysteine) and heavy metals adsorption (Source[
84]).
Table 1 summarizes the type of heavy metal ions and the capacity removal in mg/g and or percentage.
4. Discussion
The effectiveness of adsorbents in heavy metal removal is a crucial aspect of environmental remediation, with natural fiber-based, silica-based, and cellulose-silica coupling agents demonstrating notable performance in various studies (
Table 1). The Cd2+ and Pb2+ ions were shown to be adsorbed at 130.7 and 180.9 mg/g by the Cys-CL, respectively. All of these demonstrated the Cys-CL's potential to remove Cd2+ and Pb2+ ions from water as an effective adsorbent. The Langmuir and pseudo second-order models accurately predicted the spontaneous and endothermic character of the adsorption of both metal ions by the Cys-CL. The Cys-CL has a high potential for the adsorptive removal of Cd2+ and Pb2+ from aqueous solution due to its good adsorptive properties and recyclability.
Banana fiber needle felted fabric and banana peel have shown high removal percentages for heavy metal ions such as Pb2+, Zn2+, and Cd2+ (Ariharasudhan et al., 2022; Afolabi et al., 2021). These natural fiber-based materials offer several advantages, including their biodegradability, abundance, and low cost. The fibrous structure of these materials provides ample surface area for adsorption, while their inherent properties make them environmentally friendly options for heavy metal remediation in soil and water bodies (Sharma and Agrawal, 2005).
Silica nanoparticles and silica aerogels have exhibited remarkable adsorption capacities for a wide range of heavy metal ions, including Pb2+, Cu2+, and Pd2+ (Al-Wasidi et al., 2022a; Parale et al., 2023). The high surface area and porous structure of silica-based materials contribute to their excellent adsorption properties, making them suitable for applications in industrial wastewater treatment and groundwater purification. Additionally, the stability of silica-based adsorbents under varying environmental conditions enhances their potential for long-term use in heavy metal removal processes.
Furthermore, coupling cellulose and silica with different functional groups, such as thiol-functionalized cellulose nanofiber and MCNC-DPTA, has demonstrated promising adsorption capacities for various heavy metal ions (Choi et al., 2020; Shen et al., 2022). These hybrid materials combine the advantages of both cellulose and silica, including abundant availability, low cost, and favourable adsorption properties. The synergistic effects resulting from the combination of cellulose and silica further enhance the adsorption capacity and selectivity of these materials, making them suitable for tailored solutions in diverse environmental remediation scenarios.
The diverse range of adsorbents offers opportunities for their application in real-world scenarios, including industrial wastewater treatment, agricultural runoff remediation, and groundwater purification. Natural fiber-based adsorbents can be utilized in agricultural settings to mitigate heavy metal contamination in soil and water bodies, thereby promoting sustainable agriculture practices (Sharma and Agrawal, 2005). Silica-based materials hold promise for industrial applications due to their high adsorption capacities and stability under varying environmental conditions, making them suitable for large-scale heavy metal removal processes (Parale et al., 2023). Cellulose and silica coupling agents offer customizable adsorption properties, allowing for tailored solutions to specific environmental remediation challenges, including point-source and non-point-source pollution (Choi et al., 2020).
Further research is needed to optimize the synthesis methods and functionalization techniques of adsorbents to enhance their adsorption capacities and selectivity for specific heavy metal ions. Exploring novel hybrid materials and surface modification strategies could lead to the development of more efficient adsorbents with improved performance and stability. Long-term studies evaluating the ecological impacts and sustainability of adsorbent-based remediation strategies are essential for assessing their viability in large-scale applications. Collaborative efforts between researchers, industry stakeholders, and policymakers are necessary to translate laboratory findings into practical solutions for addressing heavy metal pollution on a global scale.