Submitted:
26 February 2024
Posted:
27 February 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Patient Cohort
Definitions and Immunologic Profile Characterization
Multiplex Immunohistochemistry
Statistical Analysis
Results
Patient Cohort
Demographic and Clinicopathologic Characteristics of Upfront Surgery & NAC Cohorts
TIL and Molecular Profiles of Upfront Surgery & NAC Cohorts
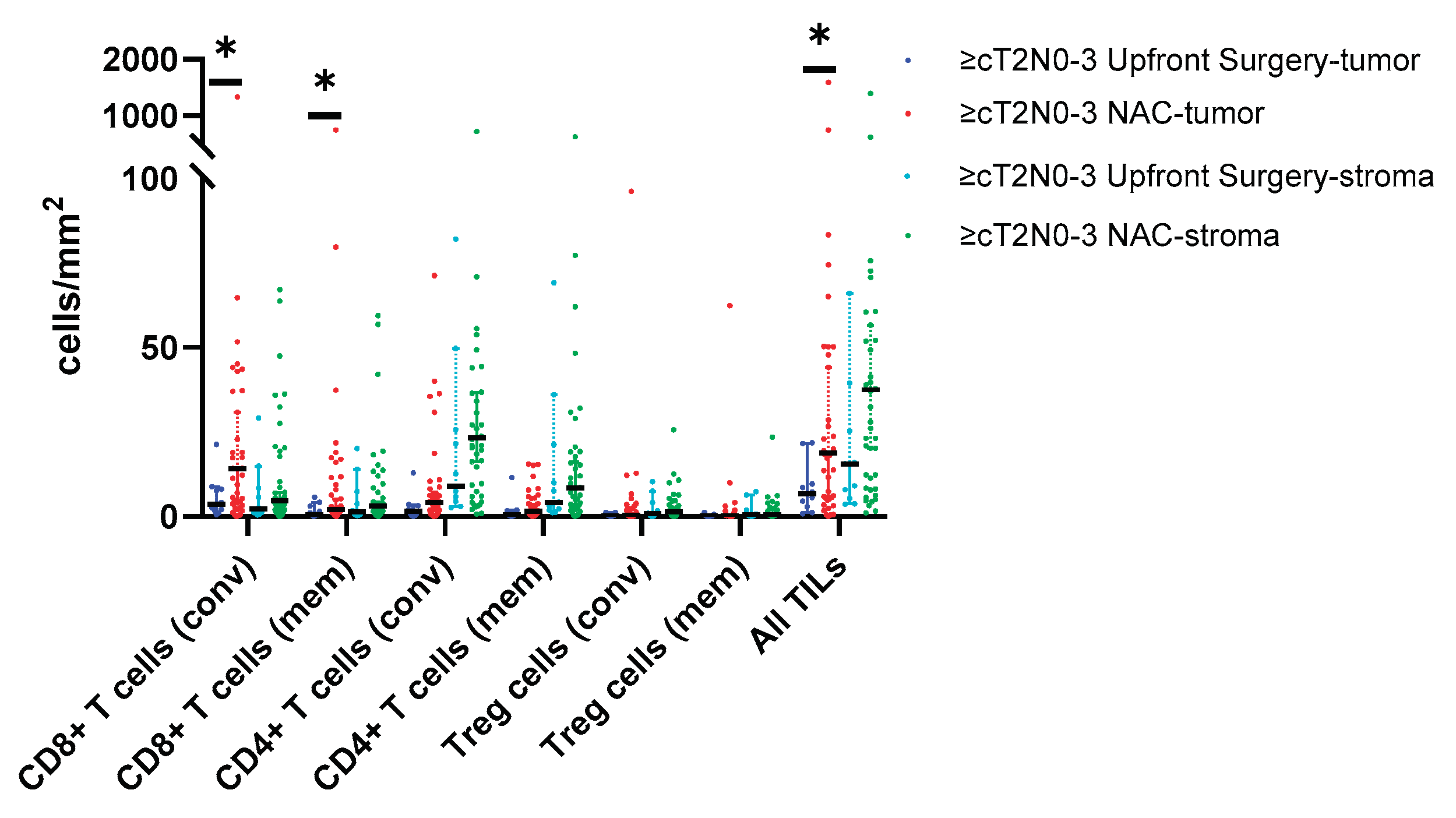
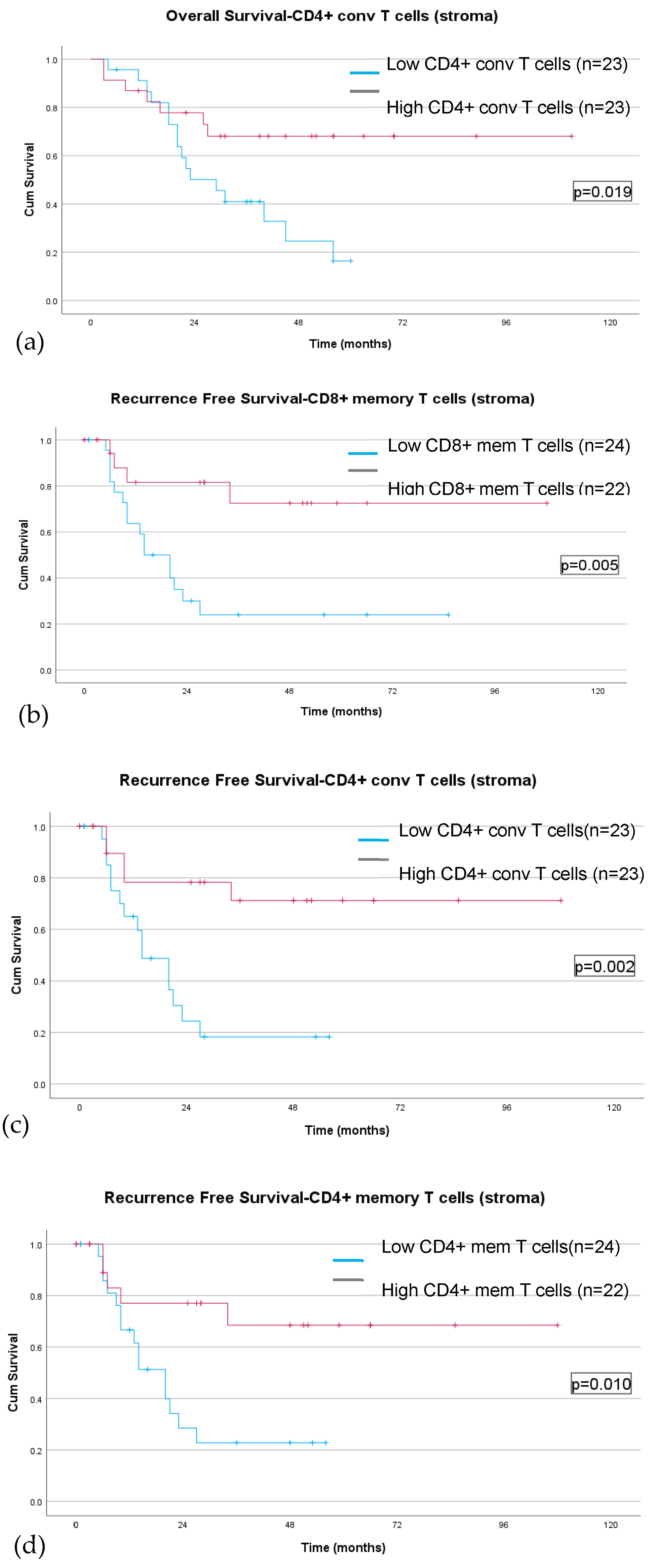
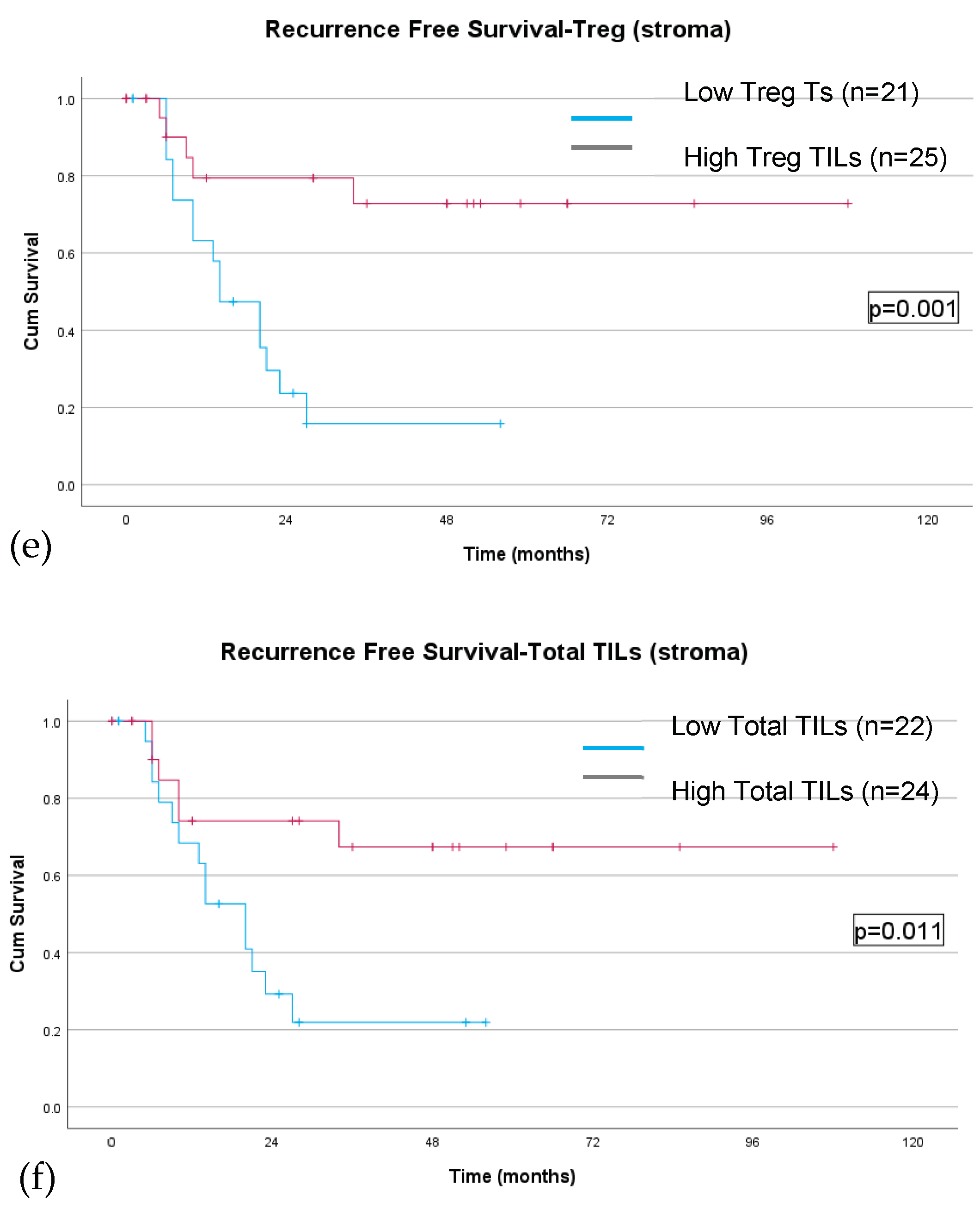
TIL density & Pathologic Response
Discussion
Conclusions
Supplementary Materials
Author Contributions
Funding
Ethical statement/consent
Acknowledgments
Disclosure/Conflicts of Interest
References
- de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol. Clin. N. Am. 2013, 42, 219–40. [CrossRef]
- Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [CrossRef]
- Morgan E, Arnold M, Camargo MC, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: A population-based modelling study. EClinicalMedicine 2022, 47, 101404. [CrossRef] [PubMed]
- Society AC. Cancer Facts & Figures 2024. Stomach Cancer Survival Rates. American Cancer Society. Available online: https://www.cancer.org/cancer/types/stomach-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 31 January 2024).
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [CrossRef] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016, 387, 1540–1550. [CrossRef] [PubMed]
- Bang YJ, Kang YK, Catenacci DV, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 2019, 22, 828–837. [CrossRef] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–39. [CrossRef]
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [CrossRef]
- NCCN Clinical Practice guidelines in Oncology: Gastric Cancer. NCCN Guidelines, (Version 1). 2023.
- Li F, Li C, Cai X, et al. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. EClinicalMedicine 2021, 41, 101134. [CrossRef]
- Loupakis F, Depetris I, Biason P, et al. Prediction of Benefit from Checkpoint Inhibitors in Mismatch Repair Deficient Metastatic Colorectal Cancer: Role of Tumor Infiltrating Lymphocytes. Oncologist 2020, 25, 481–487. [CrossRef]
- Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol. Immunol. 2021, 18, 842–859. [CrossRef]
- Uryvaev A, Passhak M, Hershkovits D, Sabo E, Bar-Sela G. The role of tumor-infiltrating lymphocytes (TILs) as a predictive biomarker of response to anti-PD1 therapy in patients with metastatic non-small cell lung cancer or metastatic melanoma. Med. Oncol. 2018, 35, 25. [CrossRef]
- Emens LA, Cruz C, Eder JP, et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019, 5, 74–82. [CrossRef]
- Hurkmans DP, Kuipers ME, Smit J, et al. Tumor mutational load, CD8(+) T cells, expression of PD-L1 and HLA class I to guide immunotherapy decisions in NSCLC patients. Cancer Immunol. Immunother. 2020, 69, 771–777. [CrossRef]
- Wang Y, Gao P, Hao Z, et al. The effect of neoadjuvant chemotherapy on the tumor immune microenvironment in gastrointestinal tumors. Front. Oncol. 2022, 12, 1054598. [CrossRef]
- Merlano MC, Denaro N, Galizia D, et al. How Chemotherapy Affects the Tumor Immune Microenvironment: A Narrative Review. Biomedicines 2022, 10. [CrossRef]
- Yu X, Zhang Z, Wang Z, Wu P, Qiu F, Huang J. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin. Transl. Oncol. 2016, 18, 497–506. [CrossRef]
- Xing X, Shi J, Jia Y, et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in gastric cancer as determined by multiplex immunofluorescence and T cell receptor repertoire analysis. J. Immunother Cancer 2022, 10. [CrossRef]
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [CrossRef]
- Fuchs CS, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric. Cancer. 2022, 25, 197–206. [CrossRef]
- Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [CrossRef]
- Shitara K, Rha SY, Wyrwicz LS, et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 2024, 25, 212–224. [CrossRef]
- Presti D, Dall’Olio FG, Besse B, Ribeiro JM, Di Meglio A, Soldato D. Tumor infiltrating lymphocytes (TILs) as a predictive biomarker of response to checkpoint blockers in solid tumors: A systematic review. Crit. Rev. Oncol. Hematol. 2022, 177, 103773. [CrossRef]
- Iwahori K, Uenami T, Yano Y, et al. Peripheral T cell cytotoxicity predicts the efficacy of anti-PD-1 therapy for advanced non-small cell lung cancer patients. Sci. Rep. 2022, 12, 17461. [CrossRef]
- Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [CrossRef]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–21. [CrossRef] [PubMed]
- Wei Q, Xu Q, Yuan X, et al. Immunological impact of chemotherapy on the tumor microenvironment in gastric cancer. J. Surg. Oncol. 2021, 123, 1708–1715. [CrossRef]
- Garcia-Martinez E, Gil GL, Benito AC, et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014, 16, 488. [CrossRef] [PubMed]
- James FR, Jiminez-Linan M, Alsop J, et al. Association between tumour infiltrating lymphocytes, histotype and clinical outcome in epithelial ovarian cancer. BMC Cancer. 2017, 17, 657. [CrossRef]
- Jary M, Liu WW, Yan D, et al. Immune microenvironment in patients with mismatch-repair-proficient oligometastatic colorectal cancer exposed to chemotherapy: the randomized MIROX GERCOR cohort study. Mol. Oncol. 2022, 16, 2260–2273. [CrossRef]
- Qi J, Liu X, Yan P, et al. Analysis of Immune Landscape Reveals Prognostic Significance of Cytotoxic CD4(+) T Cells in the Central Region of pMMR CRC. Front. Oncol. 2021, 11, 724232. [CrossRef] [PubMed]
- Gaudreau PO, Negrao MV, Mitchell KG, et al. Neoadjuvant Chemotherapy Increases Cytotoxic T Cell, Tissue Resident Memory T Cell, and B Cell Infiltration in Resectable NSCLC. J. Thorac. Oncol. 2021, 16, 127–139. [CrossRef] [PubMed]
- Hu M, Li K, Maskey N, et al. Decreased intratumoral Foxp3 Tregs and increased dendritic cell density by neoadjuvant chemotherapy associated with favorable prognosis in advanced gastric cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 4685–94.
- Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 2005, 353, 2654–66. [CrossRef] [PubMed]
- Brightman SE, Becker A, Thota RR, et al. Neoantigen-specific stem cell memory-like CD4(+) T cells mediate CD8(+) T cell-dependent immunotherapy of MHC class II-negative solid tumors. Nat. Immunol. 2023, 24, 1345–1357. [CrossRef]
- Huang Q, Wu X, Wang Z, et al. The primordial differentiation of tumor-specific memory CD8(+) T cells as bona fide responders to PD-1/PD-L1 blockade in draining lymph nodes. Cell 2022, 185, 4049–4066. [CrossRef]
- Zuazo M, Arasanz H, Fernandez-Hinojal G, et al. Functional systemic CD4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO Mol. Med. 2019, 11, e10293. [CrossRef]
- Perez EA, Ballman KV, Tenner KS, et al. Association of Stromal Tumor-Infiltrating Lymphocytes With Recurrence-Free Survival in the N9831 Adjuvant Trial in Patients With Early-Stage HER2-Positive Breast Cancer. JAMA Oncol. 2016, 2, 56–64. [CrossRef]
- Chen L, Gibbons DL, Goswami S, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014, 5, 5241. [CrossRef]
- Sun XF, Zhang H. Clinicopathological significance of stromal variables: angiogenesis, lymphangiogenesis, inflammatory infiltration, MMP and PINCH in colorectal carcinomas. Mol. Cancer 2006, 5, 43. [CrossRef]
- Guo S, Deng CX. Effect of Stromal Cells in Tumor Microenvironment on Metastasis Initiation. Int. J. Biol. Sci. 2018, 14, 2083–2093. [CrossRef] [PubMed]
- Zhu Y, Tzoras E, Matikas A, et al. Expression patterns and prognostic implications of tumor-infiltrating lymphocytes dynamics in early breast cancer patients receiving neoadjuvant therapy: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 999843. [CrossRef]
- Liu H, Zhang T, Ye J, et al. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol. Immunother. 2012, 61, 1849–56. [CrossRef]
- Kong JCH, Guerra GR, Millen RM, et al. Tumor-Infiltrating Lymphocyte Function Predicts Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. JCO Precis Oncol. 2018, 2, 1–15. [CrossRef]
- Sirody J, Kaji AH, Hari DM, Chen KT. Patterns of gastric cancer metastasis in the United States. Am. J. Surg. 2022, 224, 445–448. [CrossRef] [PubMed]
| Characteristic |
Overall cohort (n=68) |
Upfront Surgery (n=18) |
NAC (n=50) |
p value |
≥cT2N0-3 US (n=11) |
≥cT2N0-3 NAC (n=46) |
p value |
| Demographic characteristics | |||||||
| Sex, n (%) Male Female |
40 (58.8) 28 (41.2) |
8 (44.4) 10 (55.6) |
32 (64.0) 18 (36.0) |
0.148 |
7 (63.6) 4 (36.4) |
30 (65.2) 16 (34.8) |
0.921 |
| Age at diagnosis, mean (SD) | 62.8 (53.3, 73.3) | 64.6 (+/-18.0) | 65.5 (+/-13.5) | 0.531 | 69.8 (+/-16.6) | 63.0 (+/-13.4) | 0.156 |
| Race, n (%) White Black/African American Asian American Indian/Alaskan native Other |
46 (67.6) 6 (8.8) 8 (11.8) 1 (1.5) 7 (10.3) |
13 (72.2) 1 (5.6) 2 (11.1) - 2 (11.1) |
33 (66.0) 5 (10.0) 6 (12.0) 1 (2.0) 5 (10.0) |
0.944 |
7 (63.6) 1 (9.1) 1 (9.1) - 2 (18.2) |
31 (67.4) 4 (8.7) 6 (13.0) - 5 (10.9) |
0.944 |
| Clinicopathologic characteristics | |||||||
| Clinical T stage, n (%) T1a T1b T2 T3 T4 T4a T4b Missing |
3 (4.4) 7(10.3) 12 (17.6) 35 (51.5) 1 (1.5) 7 (10.3) 2 (2.9) 1 (1.5) |
2 (11.1) 5 (27.8) 7 (38.9) 3 (16.7) - - 1 (5.6) - |
1 (2.0) 2 (4.0) 5 (10.0) 32 (64.0) 1 (2.0) 7 (14.0) 1 (2.0) 1 (2.0) |
<0.001 |
- - 7 (63.6) 3 (75.0) - - 1 (9.1) - |
- - 5 (10.9) 32 (69.6) 1 (2.2) 7 (15.2) 1 (2.2) - |
<0.001 |
| Clinical N stage, n (%) N0 N1-2 Missing |
39 (57.4) 28 (41.2) 1 (1.5) |
16 (88.9) 2 (11.1) - |
23 (46.9) 26 (52.0) 1 (2.1) |
0.002 |
9 (81.8) 2 (18.2) - |
20 (43.5) 25 (54.3) 1 (2.2) |
0.026 |
| Overall clinical stage, n (%) Stage I Stage II Stage III Stage Iva Missing |
19 (27.9) 21 (30.9) 25 (36.8) 67 (98.5) 1 (1.5) |
14 (77.8) 2 (11.1) 1 (5.6) 1 (5.6) |
5 (10.2) 19 (38.0) 24 (48.0) 1 (2.0) 1 (2.0) |
<0.001 |
7 (63.6) 2 (18.2) 1 (9.1) 1 (9.1) - |
3 (6.5) 18 (39.1) 24 (52.2) 1 (2.2) - |
<0.001 |
| Tumor location, n (%) Distal Proximal Linitis plastica Undefined |
45 (66.2) 18 (26.5) 4 (5.9) 1 (1.5) |
15 (75.0) 2 (10.0) - 1 (5.0) |
30 (60.0) 16 (32.0) 4 (8.0) - |
0.091 |
10 (90.9) - - 1 (9.1) |
26 (56.5) 16 (34.8) 4 (8.7) - |
0.034 |
| Histologic subtype, n (%) Intestinal Diffuse/Signet-ring Mixed Neuroendocrine |
24 (35.3) 39 (57.4) 3 (4.4) 2 (2.9) |
9 (50.0) 8 (44.4) - 1 (5.6) |
15 (30.0) 31 (62.0) 3 (6.0) 1 (2.0) |
0.277 |
7 (63.6) 4 (36.4) - - |
15 (32.6) 28 (60.9) 2 (4.3) 1 (2.2) |
0.275 |
| Preoperative and intraoperative characteristics | |||||||
| Neoadjuvant regimen, n (%) Other FOLFOX FLOT |
17 (25.0) 25 (36.8) 6 (8.8) |
- - - |
- - - |
- |
- - - |
17 (37.0) 23 (50.0) 6 (13.0) |
- |
| Rounds of chemotherapy | 3.89 (+/-1.7) | - | - | - | 4.0 (3.0-4.0) | - | |
| Neoadjuvant radiation, n (%) No Yes |
65 (95.6) 3 (4.4) |
- - |
45 (93.8) 3 (6.3) |
0.288 |
11 (100) |
43 (93.5) 3 (6.5) |
0.288 |
| Type of resection, n (%) Partial gastrectomy Total gastrectomy EMR/ESD* |
40 (58.8) 23 (33.8) 5 (7.3) |
9 (50.0) 5 (27.8) 4 (20.0) |
31 (62.0) 18 (36.0) 1 (2.0) |
0.019 |
7 (63.6) 3 (27.3) 1 (9.1) |
28 (60.9) 17 (37.0) 1 (2.2) |
0.482 |
| Pathologic tumor characteristics | |||||||
| Pathologic overall stage, n (%) Stage I Stage II Stage III Stage IV |
21 (30.9) 20 (29.4) 20 (29.4) 7 (10.3) |
11 (61.1) 1 (5.6) 5 (27.8) 1 (5.6) |
10 (20.0) 19 (38.0) 15 (30.0) 6 (12.0) |
0.006 |
4 (36.4) 1 (9.1) 5 (45.5) 1 (9.1) |
9 (19.6) 18 (39.1) 15 (32.6) 4 (8.7) |
0.270 |
| Clinical to pathologic stage change, n (%) No change Downstage Upstage Missing |
35 (51.5) 17 (25.0) 15 (22.1) 1 (1.5) |
13 (72.2) 1 (5.6) 4 (22.2) - |
22 (44.0) 16 (32.0) 11 (22.0) 1 (2.0) |
0.058 |
6 (54.5) 1 (9.1) 4 (36.4) - |
21 (45.7) 16 (34.8) 9 (19.6) - |
0.201 |
| Histologic subtype, n (%) Intestinal Diffuse/signet-ring Mixed Neuroendocrine |
24 (35.3) 39 (57.4) 3 (4.4) 2 (2.9) |
9 (45.0) 10 (50.0) 1 (5.0) |
15 (31.3) 29 (60.4) 3 (6.3) 1 (2.1) |
0.443 |
7 (63.6) 4 (36.4) - - |
15 (32.6) 28 (60.9) 2 (4.3) 1 (2.2) |
0.275 |
| Histologic differentiation, n (%) Poor Poor-moderate Moderate Mod to well Well |
42 (61.8) 5 (7.4) 17 (25.0) 1 (1.5) 3 (4.4) |
7 (38.9) 2 (11.1) 6 (33.3) 1 (5.6) 2 (11.1) |
35 (70.0) 3 (6.0) 11 (22.0) - 1 (2.0) |
0.075 |
3 (27.3) 2 (18.2) 5 (45.5) 1 (9.1) - |
31 (67.4) 3 (6.5) 11 (23.9) - 1 (2.2) |
0.047 |
| Margin status, n (%) R0 R1 R2 |
58 (85.3) 10 (14.7) - |
18 (100) - - |
40 (80.0) 10 (20.0) - |
0.040 |
11 (100) - |
37 (80.4) 9 (19.6) - |
0.040 |
| Treatment effect, n (%) Minimal residual disease (CRS 3) Moderate response (CRS 2) Poor response (CRS 1) Unknown |
4 (8.0) 21 (42.0) 22 (44.0) 3 (6.0) |
- |
4 (8.0) 21 (42.0) 22 (44.0) 3 (6.0) |
- |
- |
4 (8.7) 20 (43.5) 19 (41.3) 3 (6.5) |
- |
| Molecular phenotype & tumor infiltrating lymphocyte profiles | |||||||
|
Overall cohort (n=68) |
Upfront Surgery (n=18) |
NAC (n=50) |
p value |
≥cT2N0-3 US (n=11) |
≥cT2N0-3 NAC (n=46) |
p value | |
| EBV status, n (%) Negative Positive |
65 (95.6) 3 (4.4) |
18 (100) - |
47 (94.0) 3 (6.0) |
0.288 |
11 (100) - |
43 (93.5) 3 (6.5) |
0.384 |
| MMR, n (%) Proficient Deficient |
60 (88.2) 8 (11.8) |
17 (94.4) 1 (5.6) |
43 (86.0) 7 (14.0) |
0.340 |
10 (90.9) 1 (9.1) |
40 (87.0) 6 (13.0) |
0.720 |
| PD-L1 status, n (%) Negative Positive |
41 (60.3) 27 (39.7) |
11 (61.1) 7 (38.9) |
30 (60.0) 20 (40.0) |
0.934 |
7 (63.6) 4 (36.4) |
27 (58.7) 19 (41.3) |
0.764 |
| Tumor infiltrating lymphocytes densities – intratumoral | |||||||
| CD8+ T cells, cells/mm2 Conventional (CD8+) Memory (CD8+/CD45RO+) |
8.6 (3.4, 37.1) 1.8 (0.8, 9.0) |
5.1 (2.1, 8.5) 1.0 (0.6, 4.8) |
14.25 (4.3, 43.7) 2.3 (1.1, 10.8) |
0.024 0.119 |
3.6 (2.0, 8.1) 0.7 (0.4, 3.1) |
14.2 (4.0, 43.7) 2.0 (1.0, 11.6) |
0.019 0.050 |
| CD4+ T cells, cells/mm2 Conventional (CD4+) Memory (CD4+/CD45RO+) |
3.4 (0.8, 8.0) 1.6 (0.4, 5.0) |
1.7 (0.6, 5.7) 0.8 (0.3, 3.3) |
4.5 (0.8, 9.2) 2.1 (0.6, 5.6) |
0.182 0.254 |
1.5 (0.3, 3.2) 0.6 (0.2, 1.7) |
4.2 (0.9, 8.3) 1.6 (0.5, 5.4) |
0.089 0.119 |
| Treg cells, cells/mm2 Conventional (CD4+/FOXP3+) Memory (CD4+/CD45RO+) |
0.4 (0.1, 1.7) 0.2 (0.04, 1.7) |
0.5 (0.04, 1.5) 0.2 (0.02, 1.2) |
0.4 (0.1, 1.9) 0.2 (0.04, 0.9) |
0.671 0.950 |
0.3 (0.1, 1.1) 0.1 (0.04, 0.4) |
0.4 (0.1, 1.9) 0.2 (0.03, 0.8) |
0.442 0.754 |
| B cells, cells/mm2 Conventional (CD220+) Memory (CD220+/CD45RO+) |
0.02 (0.003, 0.15) 0 |
0.01 (0.01, 0.09) 0 |
0.04 (0.0, 0.16) 0 |
0.550 |
0.01 (0.0, 0.01) 0.0 |
0.03 (0.0, 0.16) 0.0 |
0.088 |
| All TIL (CD8+, CD4+, B cell) | 13.6 (5.5, 49.6) | 7.9 (4.1, 15.4) | 19.3 (5.6, 53.9) | 0.047 | 6.7 (2.8, 9.6) | 18.8 (5.4, 53.9) | 0.041 |
| ALL memory TILs | 0.3 (0.03, 2.3) | 0.11 (0.01, 0.49) | 0.28 (0.05, 3.48) | 0.098 | 0.05 (0.01, 0.3) | 0.2 (0.04, 3.1) | 0.048 |
| CD8:Treg ratio | 23.2 (6.6, 3.4) | 7.8 (3.7, 53.3) | 25.5 (12.4, 54.6) | 0.123 | 7.5 (3.1, 29.5) | 25.5 (7.0, 55.2) | 0.079 |
| Tumor infiltrating lymphocytes densities – stromal | |||||||
| CD8+ T cells, cells/mm2 Conventional (CD8+) Memory (CD8+/CD45RO+) |
4.9 (1.6, 19.0) 3.3 (1.0, 11.6) |
6.4 (1.2, 21.4) 4.4 (0.9, 13.8) |
4.6 (1.8, 18.1) 3.1 (1.0, 8.8) |
0.597 0.396 |
2.3 (1.1, 8.4) 1.45 (0.6, 7.3) |
4.6 (1.9, 19.6) 3.1 (1.0, 10.2) |
0.203 0.385 |
| CD4+ T cells, cells/mm2 Conventional (CD4+) Memory (CD4+/CD45RO+) |
23.2 (6.3, 53.1) 9.7 (2.4, 29.0) |
23.6 (7.1, 73.7) 11.1 (3.0, 40.1) |
23.2 (6.0, 50.4) 8.4 (2.3, 21.0) |
0.906 0.359 |
8.9 (4.5, 21.2) 4.2 (1.8, 21.2) |
23.2 (7.0, 54.2) 8.4 (2.6, 22.7) |
0.143 0.454 |
| Treg cells, cells/mm2 Conventional (CD4+/FOXP3+) Memory (CD4+/CD45RO+) |
1.3 (0.2, 3.4) 0.5 (0.1, 1.8) |
1.0 (0.5, 4.9) 0.6 (0.2, 1.7) |
1.4 (0.2, 2.8) 0.5 (0.1, 1.3) |
0.592 0.254 |
0.8 (0.2, 4.1) 0.5 (0.1, 1.8) |
1.4 (0.2, 3.3) 0.5 (0.1, 1.7) |
0.716 0.952 |
| B cells, cells/mm2 Conventional (CD220+) Memory (CD220+/CD45RO+) |
1.2 (04, 6.7) 0.1 (0.02, 1.2) |
1.3 (0.6, 10.0) 0.2 (0.02, 1.7) |
0.9 (0.3, 5.6) 0.1 (0.2, 0.6) |
0.294 0.555 |
0.9 (0.2, 1.3) 0.3 (0.02, 0.3) |
1.1 (0.3, 6.6) 0.1 (0.02, 0.9) |
0.379 0.201 |
| All TIL (CD8+, CD4+, B cell) | 36.1 (8.6, 74.9) | 31.0 (8.7, 97.8) | 37.4 (8.3, 71.1) | 0.889 | 15.6 (5.3, 39.4) | 37.4 (10.6, 73.3) | 0.110 |
| CD8:Treg ratio | 3.6 (2.3, 10.3) | 3.4 (1.4, 16.2) | 3.6 (2.4, 9.6) | 0.479 | 2.3 (1.2, 4.8) | 3.6 (2.3, 9.6) | 0.152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





