1. Introduction
Non-tuberculous mycobacteria (NTM) include many species of the genus Mycobacterium that have been known since the first years after the discovery of M. tuberculosis (1). Among these organisms, M. avium complex (MAC) includes several species of NTM (2), especially 3: M., M. intracellulare, and M. chimaera, that are considered the commonest NTM isolated throughout the world (3). In a multicenter and multinational study by Hoefsloot et al., MAC was the commonest NTM isolated in almost all countries (3), and in a more recent regional study in Madrid (Spain), MAC was also the most commonly isolated mycobacteria during all the years of the study (4).
MAC species are also important because they are a cause of human infection, especially respiratory infections among patients with underlying diseases at the present moment (2,5). It can also cause epidemic outbreaks, like those caused by M. chimaera related to heather-cooling machines used in cardiac surgery (6,7). These infections are currently considered biofilm-related infections, and this fact is of clinical importance because of the higher resistance against antibiotics of sessile bacteria compared with planktonic ones. However, despite these facts, there are only a few in vitro studies regarding biofilm formation by MAC (8–13), and most of them used only collection strains. Here we report a study about biofilm formation by the three most important species of MAC and the effect of clarithromycin on it, one antibiotic commonly used in these infections using not only collection strains but also clinical isolates of all of them.
2. Material and Methods
2.1. Strains
During the study the following strains were used: M. avium ATCC 25291 (type strain), M. intracellulare ATCC 13950 (type strain), and M. chimaera DSM 756 (type strain). Clinical strains of all these species were also included: M. avium (717 and 647), M. intracellulare (505 and 657), and M. chimaera (575 and 655). The clinical significance of the clinical isolates was established according to the ATS-ERS-IDSA-ESCMID criteria (5). All the strains were maintained frozen at -80ºC. Before the experiments were performed, all the strains were defrosted, inoculated onto Middlebrook 7H10 agar plates, incubated at 37ºC for 15 days, and checked for purity.
2.2. Biofilm development
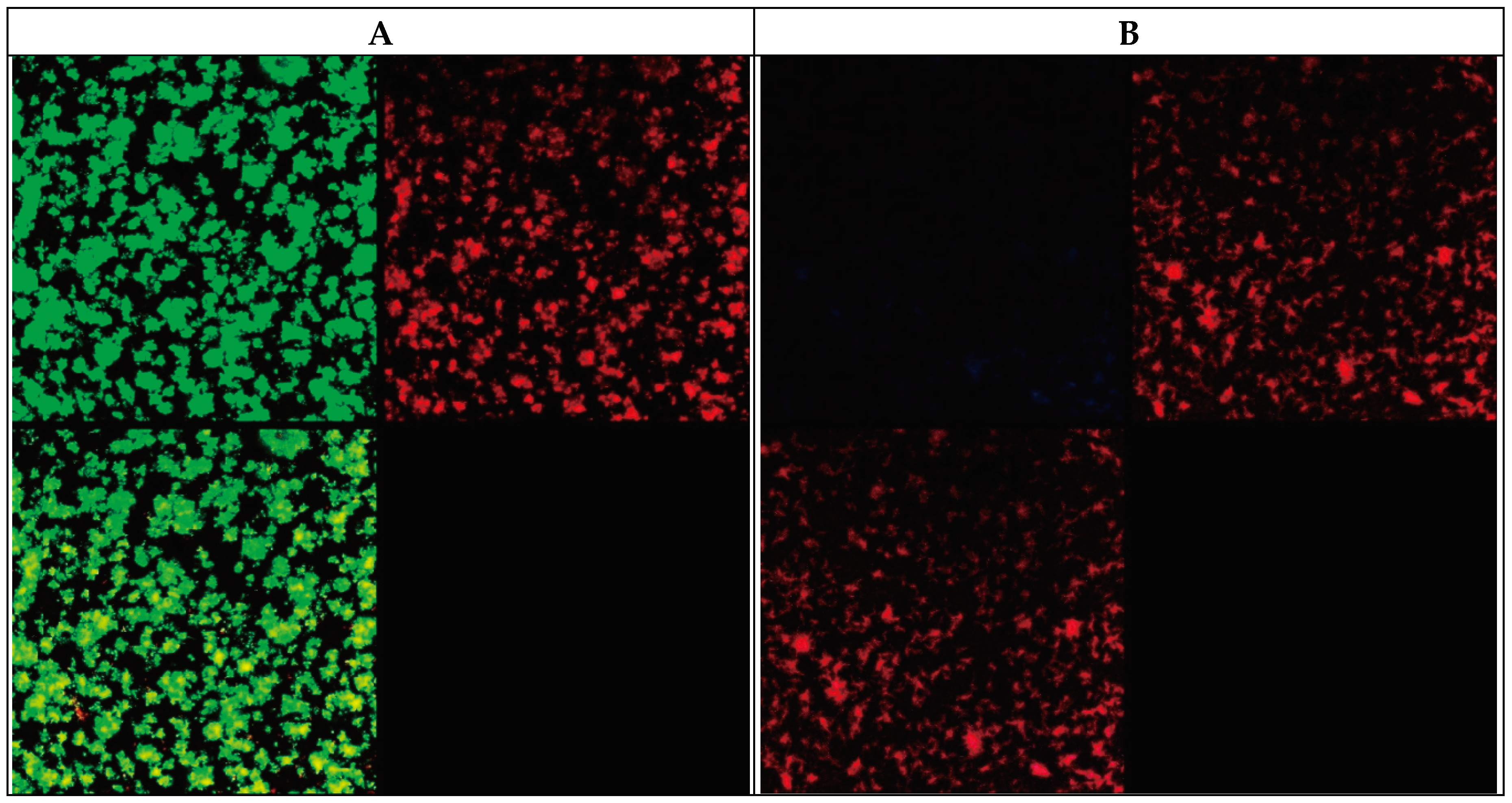
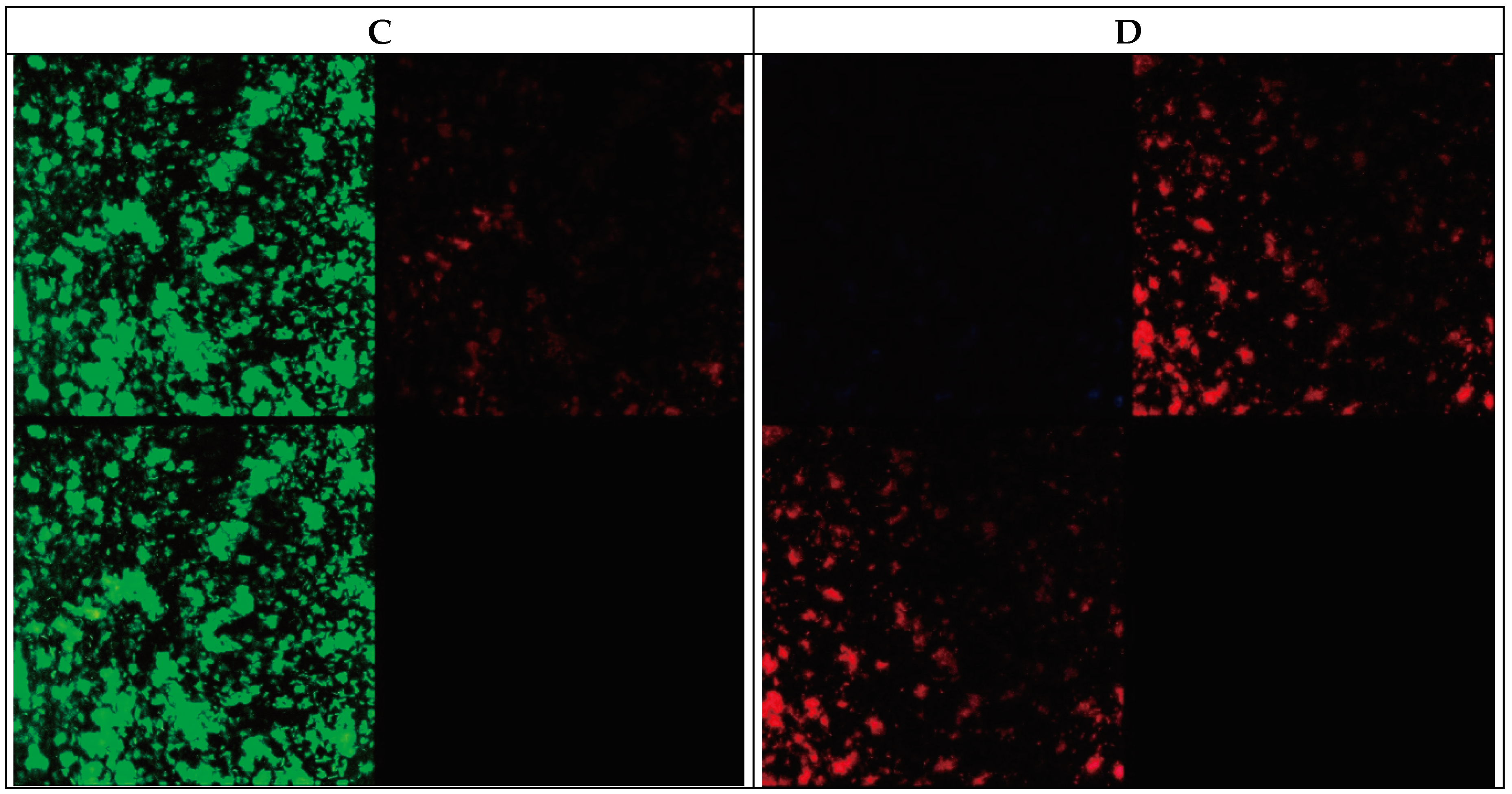
Biofilm development was performed using the technique described by Muñoz-Egea et al. (14,15) using 2 × 4-well plates with an uncoated hydrophobic surface (Ibidy GmbH, Martinsried, Germany) with a prolongation of the times for the study of biofilm development to 48, 120, 192 and 264 h. One group of 4 wells was stained with Nile Red© stain (Sigma-Aldrich Co., St. Louis, MO) and the other group was stained with Live/Dead BacLight© stain (Invitrogen, USA) according to the instructions provided by the manufacturers. Confocal Laser Scanning Microscopy (CLSM) was performed using a Leica DM IRB microscopy (Leica, Germany). Four parameters were evaluated each time: % of covered surface, thickness, viability, and presence of autofluorescence. The last parameter was analyzed as the percentage of fluorescence related to the number of bacterial cells detected with the Nile Red stain using the following formulation: (% autofluorescence of covered surface/% Nile Red covered surface) × 100. All the strains were tested in triplicates.
2.3. Effect of clarithromycin in biofilm ultrastructure
The same study described above was performed including clarithromycin in the culture medium at a concentration of 11.3 mg/L at different time points, using one row of wells as the control without antibiotic. This concentration is the concentration of clarithromycin reached in the lungs when a patient is receiving this treatment (16). After the treatment each well was stained with Live/Dead BacLight© stain. All the strains were tested in triplicate.
2.4. Clarithromycin susceptibility of planktonic and sessile bacteria
Minimum inhibitory concentrations (MIC) were determined using the previously described broth microdilution method (17) with one modification. The MIC is the minimum concentration required to inhibit the bacterial visible growth. In brief, a series of concentrations of clarithromycin ranging from 512 to 0.5 µg/mL with a two-fold dilution were added to cation adjusted Müller-Hinton broth (Sigma Aldrich, USA) (CAMHB) supplemented with oleic, albumin, dextrose, catalase (OADC) to a final volume of 100 µL per well. One hundred microlitres of bacterial suspension in CAMHB from 1-MacFarland suspension were added to a Costar 96-well round-bottom polypropylene plate (Corning Inc., USA) followed by static incubation at 37 °C and 5% CO2 for at least 10 days. After incubation, MIC was determined by the naked eye as the well with the lowest concentration of clarithromycin where no bacterial growth was observed. Minimum bactericidal concentration (MBC) was determined using the flash microbiocide method (18). The MBC is defined as the minimum concentration required to kill a certain bacterial concentration. Briefly, 20 µL of each well from the MIC 96-wells plate were mixed after 24-h incubation with 180 µL of CAMHB supplemented with OADC in a new 96-well plate, which was incubated statically at 37 °C and 5% CO2 for 10 days. After incubation, MBC was determined by the naked eye as the well with the lowest concentration of clarithromycin where no bacterial growth was observed. The experiments were performed three times.
Minimal biofilm inhibitory concentrations (MBIC) and minimal biofilm eradication concentrations were determined. The MBIC is the minimum concentration required to inhibit the visible growth of a bacterial biofilm. For MBIC, biofilms were developed on untreated 96-well flat-bottom plates (Thermo Fisher Scientific, United States) by adhering previously 150 µL of a suspension of 1.00±0.02 MacFarland of each strain in sterile tap water for 3 h at 37ºC and CO2. After incubation, each well was washed with 150 µL of sterile tap water and 150 µL of Middlebrook 7H9 supplemented with OADC were deposited per well. The plate was statically incubated at 37 °C and 5% CO2 for 7 days. Afterwards biofilm growth, each well was washed with 150 µL of sterile phosphate buffer saline and filled with 200 µL of CAMHB supplemented with OADC containing different concentrations of clarithromycin, ranging from 512 to 0.5 µg/mL with a two-fold dilution. The plate was statically incubated at 37 °C and 5% CO2 for at least 10 days. After incubation, MBIC was determined by the naked eye as the well with the lowest concentration of clarithromycin where no planktonic growth was observed. The MBEC is the minimum concentration required to kill a bacterial biofilm. For MBEC, the bottom of each well was scrapped with a 100-µL tip to detach physically the biofilm from the bottom surface of each well and 20 µL of each well were transferred to a new well containing 180 µL of CAMHB supplemented with OADC. The plate was incubated statically at 37 °C and 5% CO2 for 10 days. After incubation, MBEC was determined by the naked eye as the well with the lowest concentration of antibiotic where no bacterial growth was observed. The experiments were performed three times.
2.5. Statistical analysis
Statistical studies were performed with Stata Statistical Software Release 11 (StataCorp 2009). The normality of data was evaluated using a Shapiro-Wilk test. Descriptive data are cited as median and interquartile range in case of non-normal distribution for each of the variables was calculated. A non-parametric Wilcoxon test was used to compare the differences between the different parameters evaluated in the biofilm structural study two groups, whiles a non-parametric Kruskal-Wallis test was used to compared more than two groups. The correlation between the bacterial viability in the biofilm and the treatment time was evaluated using a Spearman’s rank correlation coefficient (ρ). For absolute values of ρ, 0–0.19 is regarded as very weak, 0.2–0.39 as weak, 0.40–0.59 as moderate, 0.6–0.79 as strong, and 0.8–1 as very strong correlation.
3. Results
3.1. Strains
The clinical significance of the clinical isolates was established according to the ATS-ERS-IDSA-ESCMID criteria being the strains M. avium 647, M. intracellulare 657 and M. chimaera 655 considered as clinically significant. Clinical strains M. avium 717, M. intracellulare 505 and M. chimaera 575 were found to be non-clinically significant.
3.2. Biofilm forming studies.
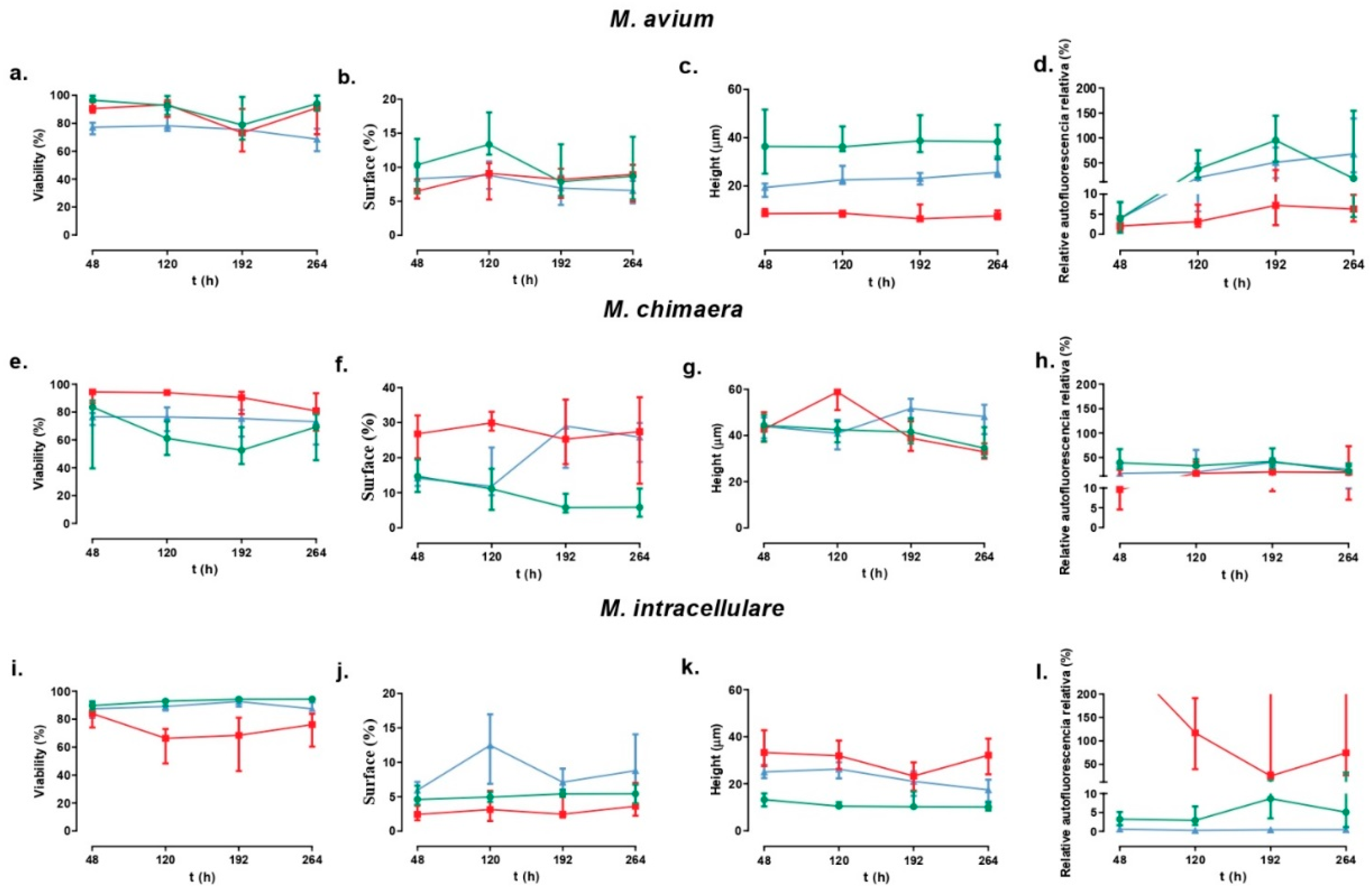
The results obtained are represented in
Figure 1. Examples of the images are shown in
Figure 2.
The time evaluation of viability for M. avium strains showed that no differences can be detected for the reference strain (p-value=0.1212), but there are differences for the clinical strains (p-values=0.0052 and 0.0018 for strains 647 and 717 respectively). For M. intracellulare, the variation was observed for the reference strain (p-value<0.0001) and the strain 657 (p-value=0.0248), but not for the strain 505. For M. chimaera the variation showed similar characteristics that for M. intracellulare (p-values=0.0.0227 for the reference strain and 0.0003 for the strain 655, but no significative for strain 575).
For covered surface, we observed differences for M. avium reference strain (p-value=0.0202) and strain 717 (p-value=0.0.0001), for M. intracellulare strains 657 (p-value=0.0234) and 505 (p-value=0.0042), and for M. chimaera reference strain (p-value<0.0001) and strain 575 (p-value=0.0426).
Differences for height evolution during time were observed for M. avium clinical strain (p-value=0.0913 and <0.0001 for increase in height for strains 647 and 717 respectively), for height decrease in M. intracellulare reference strain and strain 505 (p<0.0001 for both of them), and an increase in M. chimaera strain 575 (p-value=0.0001) but a decrease for M. chimaera reference strain (p-value=0.0019) and strain 655 (p-value<0.0001). No differences in heigh were detected for the other strains.
Differences in autofluorescence evolution were also detected for all strains of M. avium (p-values=0.0004 for the reference strain and <0.0001 for both clinical strains), for M. intracellulare reference strain (increase, p-value=0.0101) and strain 655 (decrease, p-value=0.0002), and for M. chimaera reference strain (decrease, p-value=0.0426) and strain 655 (increase, p- value=0.0007).
We also analysed the intraspecies variations, with the following results:
3.2.1. For M. avium:
At 48 hours: The viability of the three strains was significantly different (p-value=0.001 for the Kruskal-Wallis test). In descending order: reference strains, strain 647 and strain 717. Each strain was statistically different from the others (p-value <0.05 for the Wilcoxon test). The surface area covered by the reference and 717 strains was similar (p-value=0.1345 for the Wilcoxon test). The surface area covered by the strain 647 was significantly less than the reference and 717 strains. (p-value <0.05 for the Wilcoxon test). The autofluorescence of the three strains was significantly different (p-value=0.0003 for the Kruskal-Wallis test). In descending order: strain 647, reference strain and strain 717. There were no significant differences between the reference and CON strains (p-value=0.7092 for the Wilcoxon test). The biofilm height of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: reference strain, strain 647 and strain 717.
At 120 hours: The viability of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). The viability of the strain 717 was significantly lower than the rest of the strains (p-value<0.001). There were no significant differences between the reference strain and the strain 647 (p-value=0.3472). The surface area covered by the reference and 707 strains was similar (p-value=0.0001 for the Wilcoxon test). The surface area covered by the reference strain was significantly greater than that of the clinical strains. (p-value<0.00001 for the Wilcoxon test). There were no significant differences between the strain 717 and the strain 647 (p-value=0.4688). The autofluorescence of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: reference strain, strain 717 and strain 647. The biofilm height of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: reference strain, strain 717 and strain 647.
At 192 hours: The viability of the three strains was similar (p-value=0.1995 for the Kruskal-Wallis test), as well as the covered surface area of the three strains (p-value=0.2198 for the Kruskal-Wallis test). The autofluorescence of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: reference strain, strain 717 and strain 647. Each strain was statistically different from the others (p-value <0.05 for the Wilcoxon test). The biofilm height of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: reference strain, strain 717 and strain 647. Each strain was statistically different from the others (p-value <0.05 for the Wilcoxon test).
At 264 hours: The viability of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: reference strain, strain 647 and strain 717. Each strain was statistically different from the others (p-value <0.05 for the Wilcoxon test). The surface area covered by the reference and 717 strains was similar (p-value=0.0152 for the Wilcoxon test). The surface area covered by the strain 717 was significantly greater than the 647 and reference strains. (p-value <0.05 for the Wilcoxon test). There were no significant differences between the reference strain and the strain 647 (p-value=0.5642). The biofilm height of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: reference strain, strain 717 and strain 647. The autofluorescence of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 717, reference strain and strain 647. Each strain was statistically different from the others (p-value <0.05 for the Wilcoxon test).
3.2.2. For M. intracellulare:
At 48 hours: The viability of the three strains was significantly different (p-value=0.0004 for the Kruskal-Wallis test). In descending order: reference strain, strain 505 and strain 657. The viability of strain 657 was significantly lower than the rest of the strains (p-value<0.05). There were no significant differences between the reference strain and strain 505 (p-value=0.1242). The covered surface of the strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strains 505, reference strain and strain 657. Each strain was statistically different from the others (p-value <0.05 for the Wilcoxon test). The autofluorescence of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 657, reference strain and strain 505. Each strain was statistically different from the others (p-value <0.0001 for the Wilcoxon test). The biofilm height of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 657, reference strain and strain 505. Each strain was statistically different from the others (p-value <0.001 for the Wilcoxon test).
At 120 hours: The viability of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: reference strain, strain 505 and strain 657. The viability of the 657 strain was significantly higher than the reference strains and 505. (p-value <0.0001 for the Wilcoxon test). There were no significant differences between strain 505 and the reference strain (p-value=0.0863). The surface area covered by the reference and 505 strains was similar (p-value=0.0001 for the Wilcoxon test). In descending order: strain 505, reference strain and strain 657. Each strain was statistically different from the others (p-value <0.01 for the Wilcoxon test). The autofluorescence of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 657, strain 505 and reference strain. Each strain was statistically different from the others (p-value <0.01 for the Wilcoxon test). The biofilm height of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 657, reference strain and strain 505. Each strain was statistically different from the others (p-value <0.01 for the Wilcoxon test).
At 192 hours: The viability of the three strains was statistically different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: reference strain, strain 505 and strain 657. The viability of strain 657 was significantly lower than the other two strains (p-value <0.0001 for the Wilcoxon test). There were no significant differences between strain 505 and the reference strain (p-value=0.1354). The surface area covered by the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 505, reference strain and strain 657. Each strain was statistically different from the others (p-value <0.001 for the Wilcoxon test). The autofluorescence of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 657, reference strain and strain 505. Each strain was statistically different from the others (p-value <0.001 for the Wilcoxon test). The biofilm height of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 657, strain 505 and reference strain. The biofilm height of the reference strain was significantly lower than the other two strains (p-value <0.0001 for the Wilcoxon test). There were no significant differences between strain 657 and strain 505 (p-value=0.11896).
At 264 hours: The viability of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: reference strain, strain 505 and strain 657. Each strain was statistically different from the others (p-value <0.001 for the Wilcoxon test). The covered surface was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 505, reference strain, and strain 657. Each strain was statistically different from the others (p-value <0.001 for the Wilcoxon test). The autofluorescence of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 657, reference strain, and strain 505. Each strain was statistically different from the others (p-value <0.001 for the Wilcoxon test). The biofilm height of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 657, strain 505 and reference strain. Each strain was statistically different from the others (p-value <0.001 for the Wilcoxon test).
3.2.3. For M. chimaera:
At 48 hours: The viability of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 655, reference strain and strain 575. The viability of strain 655 was significantly higher than the rest of the strains (p-value<0.0001). There were no significant differences between the reference strain and strain 575 (p-value=0.2254). The covered surface of the strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). The surface area covered by strain 655 was significantly greater than the reference strains and 575 (p-value <0.05 for the Wilcoxon test). There were no significant differences between the reference strain and strain 575 (p-value=0.6256). The autofluorescence of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: reference strains, strain 575 and strain 655. Each strain was statistically different from the others (p-value <0.01 for the Wilcoxon test). The biofilm height of the three strains was not significantly different (p-value=0.963 for the Kruskal-Wallis test).
At 120 hours: The viability of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strains 575, reference strain and strain 655. Each strain was statistically different from the others (p-value <0.001 for the Wilcoxon test). The surface area covered by the reference and 575 strains was similar (p-value=0.0001 for the Wilcoxon test). The surface area covered by strain 655 was significantly greater than the reference strains and 575. (p-value <0.0001 for the Wilcoxon test). There were no significant differences between strain 575 and the reference strain (p-value=0.1184). The autofluorescence of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: reference strains, strain 575 and strain 655. The autofluorescence of the reference strain was significantly higher than that of strain 655 (p-value=0.0232 for the Wilcoxon test). There were no significant differences between strain 575 and strain 655 or between strain 575 and the reference strain (p-value>0.15). The biofilm height of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). The biofilm height of strain 655 was significantly higher than the other two strains (p-value <0.0001 for the Wilcoxon test). There were no significant differences between strain 575 and the reference strain (p-value=0.6492).
At 192 hours: The viability of the three strains was statistically different (p-value=0.0001 for the Kruskal-Wallis test). In descending order: strain 655, strain 575 and reference strain. Each strain was statistically different from the others (p-value <0.0001 for the Wilcoxon test). The surface area covered by the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). The surface covered by biofilm of the reference strain was significantly lower than the other two strains (p-value <0.0001 for the Wilcoxon test). There were no significant differences between strain 575 and strain 655 (p-value=0.9411). The autofluorescence of the three strains was significantly different (p-value=0.0475 for the Kruskal-Wallis test). The autofluorescence of the reference strain was significantly higher than that of strain 655 (p-value=0.0232 for the Wilcoxon test). There were no significant differences between strain 575 and strain 655 or between strain 575 and the reference strain (p-value>0.15). The biofilm height of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). The biofilm height of strain 655 was significantly lower than the other two strains (p-value <0.001 for the Wilcoxon test). There were no significant differences between strain 655 and the reference strain (p-value=0.1958).
At 264 hours: The viability of the three strains was significantly different (p-value=0.0031 for the Kruskal-Wallis test). In descending order: strain 655, strain 575 and reference strain. The viability of strain 655 was significantly higher than the other two strains studied (p-value <0.05 for the Wilcoxon test). There were no significant differences between strain 575 and the reference strain (p-value=0.2544). The surface area covered by the reference and 575 strains was similar (p-value=0.0001 for the Wilcoxon test). The surface area covered by the reference strain was significantly smaller than the other two strains studied (p-value <0.0001 for the Wilcoxon test). There were no significant differences between strain 575 and strain 655 (p-value=0.9411). The autofluorescence of the three strains was not significantly different (p-value=0.5166 for the Kruskal-Wallis test). The biofilm height of the three strains was significantly different (p-value=0.0001 for the Kruskal-Wallis test). The height of strain 575 was significantly greater than the other two strains studied (p-value <0.0001 for the Wilcoxon test). There were no significant differences between strain 575 and strain 655 (p-value=0.1858).
3.3. Antimicrobial susceptibility of planktonic and sessile bacteria
The results of susceptibility testing of all strains appear in the
Table 1.
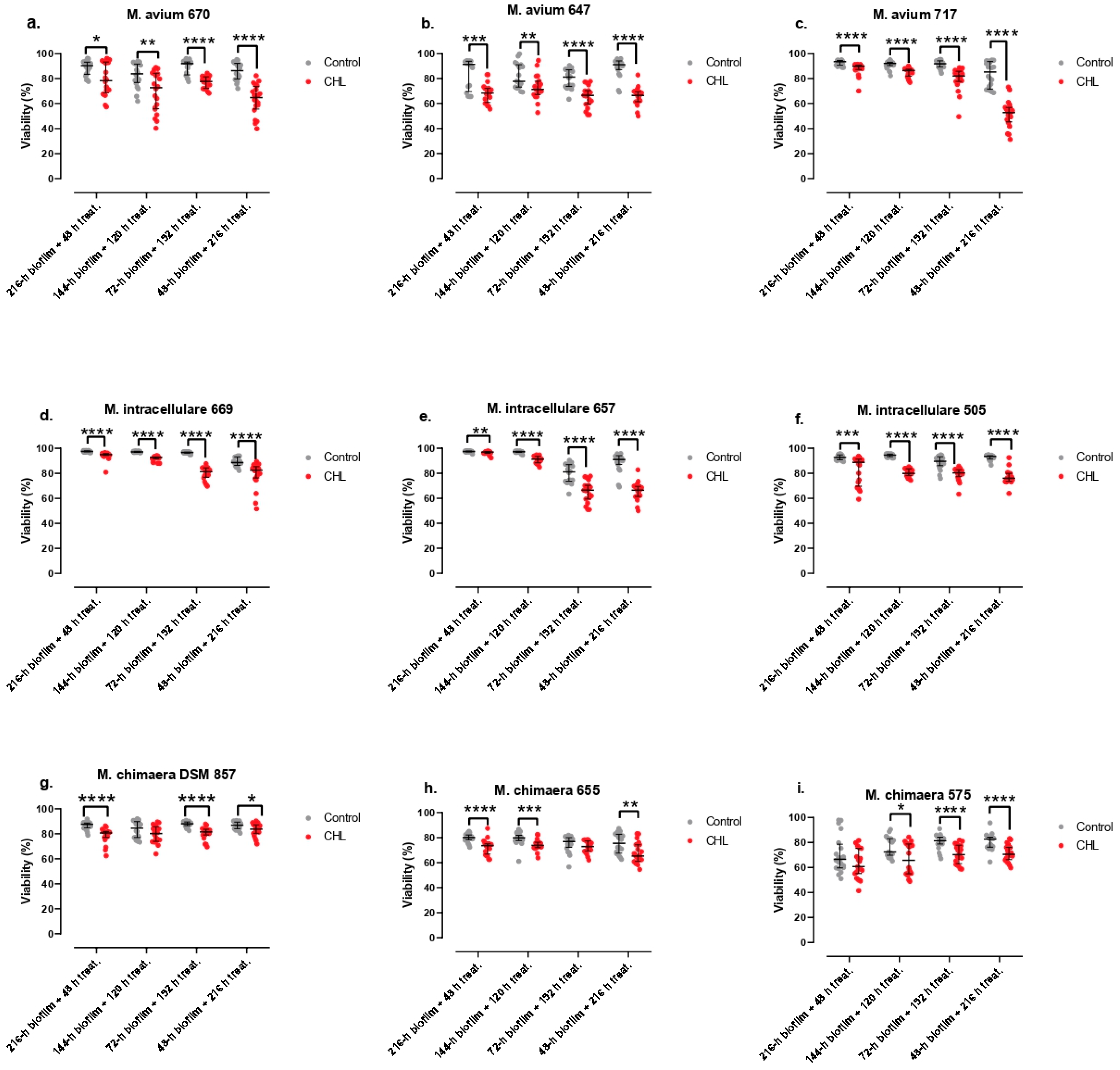
3.4. Effect of clarithromycin in biofilm ultrastructure
The results of this study are shown in
Figure 3.
The viability of bacteria contained in the biofilm of M. avium strain 717 showed a strong negative correlation with treatment time (ρ=-0.8211; p-value<0.0001). The viability of bacteria contained in the biofilm of M. avium reference strain showed a weak negative correlation with treatment time (ρ=-0.3239; p-value=0.0033). The viability of bacteria contained in the biofilm of M. avium strain 647 did not show a clear correlation with treatment time (p-value=0.0714). The viability of bacteria contained in the biofilm of M. intracellulare reference strain showed a very strong positive correlation with treatment time (ρ=-0.8090; p-value<0.0001). The viability of bacteria contained in the biofilm of M. intracellulare strain 505 showed a weak negative correlation with treatment time (ρ=-0.3045; p-value=0.0060). The viability of bacteria contained in the biofilm of M. intracellulare strain 657 showed a very strong negative correlation with treatment time (ρ=-0.9281; p-value<0.0001). The viability of bacteria contained in the biofilm of M. chimaera reference strain showed a weak positive correlation with treatment time (ρ=0.2783; p-value=0.0124). The viability of bacteria contained in the biofilm of M. chimaera strain 575 showed a weak positive correlation with treatment time (ρ=0.2871; p-value=0.0103). The viability of bacteria contained in the biofilm of M. chimaera strain 655 showed a weak negative correlation with treatment time (ρ=-0.2271; p-value=0.0428).
4. Discussion
M. avium complex is a group of nontuberculous mycobacteria that includes mainly 3 species (M. avium, M. intracellulare and M. chimaera) that have been described as human pathogens, especially in the respiratory tract in patients with underlying diseases, as well as disseminated disease in immunosuppressed patients, especially aids patients (19).
The infections caused by these organisms are difficult to diagnose, because in some cases they can appear as colonizations or contaminations from environmental sources. For this purpose, several guidelines have been published that included specific criteria for the diagnosis of M. avium complex infections. Many of these infections (especially lung ones) have been considered as biofilm-related ones. The ability of develop biofilms by these mycobacteria have been proved several years ago, and this property is now considered an essential pathogenic factor for these infections. Moreover, because sessile bacteria appear to be more resistant to antibiotics than planktonic ones, therapy of these patients must be influenced by this finding, and prolonged treatment with a combination of different antimicrobials is mandatory. However, despite these recommendations, in some cases treatment failures appear, and these failures could be related with the existence of biofilms in the patient (20,21). Previous studies have shown that M. avium complex can for biofilm in vitro (10,11), and some techniques have been tested aimed to minimize the colonization and subsequent development of biofilms in biomedical surfaces (22). However, most of these studies have been performed with collection strains. In this study we have tested clinical strains not only of different species, but of different clinical relevance, so our results are of special interest because the potential differences that can appear.
The main aim of this study was to characterize the biofilm formation using Nile Red© stain and Live/Dead BacLight© stain with a previously described protocol in order to analyze several parameters (% of covered surface, thickness, viability, and presence of autofluorescence) with and without antibiotic to determine their relative importance in the treatment resistance and looking for know if there are species specific patterns. In our study, we found many differences in the different parameters between the different species. However, the obtained values do have neither a species specific nor a clinical significance specific pattern (22,23). Other studies relating biofilm formation for M. avium and M. intracellulare clinical strains, found M. avium to produce stronger biofilm than M. intracellulare (8), but we have not detected this difference when all the strains of the same species were analyzed together. Although more studies are needed in order to understand MAC biofilm formation to determine more specific treatments for patients with MAC related infections, we can conclude that biofilm formation is strain-dependent, as no clear differences between species can be detected.
Experiments were also carried out on the nine clinical isolates treated with clarithromycin using Live/Dead BacLight© stain to differentiate the live and dead cells from biofilm and also determine the MIC, MBC and MBIC of these strains. In these experiments, again we do not find any common pattern among the different isolates, while most isolates showed higher activity against younger biofilms+prolonged treatment that against older ones+short treatment, with only 2 strains (both of them M. chimaera, DSM756 and strain 575) showing only a weak positive correlation with time. In this study, it is difficult to know with this methodology if the effect is due because the treatment time or the age of the biofilm, but the percentage of biofilm viability of the strains evaluated was not less than 40% at best, even with the highest time of clarithromycin treatment, which suggest that biofilm is an extremely important parameter in the resistance of these organisms and the need of prolonged treatments, as recommended in the guidelines (5). The high degree of resistance against clarithromycin in the static studies suggest that the age of the biofilm could be the parameter of highest importance in the results. However, the finding of almost lack of effect in 2 of the M. chimaera strains in different times suggests that other parameters probably influence the effect of the antibiotic in the biofilms, and the differences between this species and the other ones form the complex need further studies with more clinical strains. Mycobacterium avium complex strains, when appear as biofilms attached to other surfaces, show more resistance against clarithromycin than in conventional studies using planktonic cells against clarithromycin and also rifampin (24). This increased resistance have been demonstrated also in other species of mycobacteria using a similar methodology (15,25) and could be one explanation for the problems in the treatment of the infections caused by these organisms.
In conclusion, biofilm forming ability in M. avium complex appears to be as a strain-specific property that does not depend on the species or any other characteristic. The effect of antibiotic therapy with clarithromycin is also strain-specific, while treatment of older biofilms seems to have less effect that that of treatment of younger biofilms.
Funding
The present study was funded by a grant from the Libyan Government. AA was funded also by a grant from the Libyan Government.
Acknowledgments
We also wish to acknowledge María del Mar González García-Parreño and Dr Juana Serrano-López from IIS- Fundación Jimenez Diaz for their help with the use of the confocal laser-scanning microscope.
Conflicts of Interest
No conflicts of interest for all authors regarding the present study.
References
- Wolinsky, E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. enero de 1979;119(1):107-59. [CrossRef]
- Daley, C.L. Mycobacterium avium Complex Disease. Microbiol Spectr. abril de 2017;5(2). [CrossRef]
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Angeby, K.; Bauriaud, R.; Bemer, P.; et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. diciembre de 2013;42(6):1604-13. [CrossRef]
- Lopez-Roa, P.; Aznar, E.; Cacho, J.; Cogollos-Agruna, R.; Domingo, D.; Garcia-Arata, M.I.; et al. Epidemiology of Non-Tuberculous Mycobacteria isolated from clinical specimens in Madrid, Spain, from 2013 to 2017. Eur J Clin Microbiol Infect Dis. junio de 2020;39(6):1089-94. [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin Infect Dis. agosto de 2020;71(4):e1-36. [CrossRef]
- Kasperbauer, S.H.; Daley, C.L. Mycobacterium chimaera Infections Related to the Heater-Cooler Unit Outbreak: A Guide to Diagnosis and Management. Clin Infect Dis. 19 de marzo de 2019;68(7):1244-50. [CrossRef]
- Marra, A.R.; Diekema, D.J.; Edmond, M.B. Mycobacterium chimaera Infections Associated With Contaminated Heater-Cooler Devices for Cardiac Surgery: Outbreak Management. Clin Infect Dis. 15 de agosto de 2017;65(4):669-74. [CrossRef]
- Portell-Buj, E.; López-Gavín, A.; González-Martín, J.; Tudó, G. In Vitro Biofilm Formation in Mycobacterium avium-intracellulare Complex. Arch Bronconeumol (Engl Ed). febrero de 2021;57(2):140-1. [CrossRef]
- Portell-Buj E, González-Criollo C, López-Gavín A, Fernández-Pittol M, Busquets MA, Estelrich J, et al. Activity of Antibiotics and Potential Antibiofilm Agents against Biofilm-Producing Mycobacterium avium-intracellulare Complex Causing Chronic Pulmonary Infections. Antibiotics (Basel). 27 de abril de 2022;11(5):589. [CrossRef]
- Carter, G.; Wu, M.; Drummond, D.C.; Bermudez, L.E. Characterization of biofilm formation by clinical isolates of Mycobacterium avium. J Med Microbiol. septiembre de 2003;52(Pt 9):747-52. [CrossRef]
- Carter, G.; Young, L.S.; Bermudez, L.E. A subinhibitory concentration of clarithromycin inhibits Mycobacterium avium biofilm formation. Antimicrob Agents Chemother. diciembre de 2004;48(12):4907-10. [CrossRef]
- Keefe, B.F.; Leestemaker-Palmer, A.; Bermudez, L.E. Mycobacterium avium subsp. hominissuis (MAH) Microaggregate induction of host innate immunity is linked to biofilm formation. Microb Pathog. agosto de 2021;157:104977. [CrossRef]
- Yamazaki, Y.; Danelishvili, L.; Wu, M.; Hidaka, E.; Katsuyama, T.; Stang, B.; et al. The ability to form biofilm influences Mycobacterium avium invasion and translocation of bronchial epithelial cells. Cell Microbiol. mayo de 2006;8(5):806-14. [CrossRef]
- Muñoz-Egea, M.C.; García-Pedrazuela, M.; Mahillo, I.; García, M.J.; Esteban, J. Autofluorescence as a tool for structural analysis of biofilms formed by nonpigmented rapidly growing mycobacteria. Appl Environ Microbiol. febrero de 2013;79(3):1065-7. [CrossRef]
- Muñoz-Egea, M.C.; García-Pedrazuela, M.; Mahillo, I.; Esteban, J. Effect of ciprofloxacin in the ultrastructure and development of biofilms formed by rapidly growing mycobacteria. BMC Microbiol. 4 de febrero de 2015;15:18. [CrossRef]
- Fish, D.N.; Gotfried, M.H.; Danziger, L.H.; Rodvold, K.A. Penetration of clarithromycin into lung tissues from patients undergoing lung resection. Antimicrob Agents Chemother. abril de 1994;38(4):876-8. [CrossRef]
- Woods, G.L.; Brown-Elliott, B.A.; Conville, P.S.; Desmond, E.P.; Hall, G.S.; Lin, G.; et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes [Internet]. 2nd ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2011 [citado 4 de agosto de 2020]. (CLSI Standards: Guidelines for Health Care Excellence). Disponible en: http://www.ncbi.nlm.nih.gov/books/NBK544374/. [PubMed]
- Hernandes C, Coppede J da S, Bertoni BW, Fran?a S de C, Pereira AMS. Flash microbiocide: A Rapid and Economic Method for Determination of MBC and MFC. American Journal of Plant Sciences. 11 de abril de 2013;04(04):850. [CrossRef]
- Meier, E.; Pennington, K.; Gallo de Moraes, A.; Escalante, P. Characteristics of Mycobacterium avium complex (MAC) pulmonary disease in previously treated lung cancer patients. Respir Med Case Rep [Internet]. 23 de junio de 2017 [citado 11 de enero de 2024];22:70-3. Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5491758/. [CrossRef]
- Falkinham, J.O. Mycobacterium avium complex: Adherence as a way of life. AIMS Microbiol. 2018;4(3):428-38. [CrossRef]
- Esteban, J.; García-Coca, M. Mycobacterium Biofilms. Front Microbiol. 2017;8:2651. [CrossRef]
- Pradal, I.; Esteban, J.; Mediero, A.; García-Coca, M.; Aguilera-Correa, J.J. Contact Effect of a Methylobacterium sp. Extract on Biofilm of a Mycobacterium chimaera Strain Isolated from a 3T Heater-Cooler System. Antibiotics (Basel) [Internet]. 3 de agosto de 2020 [citado 11 de enero de 2024];9(8):474. Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7460266/. [CrossRef] [PubMed]
- Johansen, T.B.; Agdestein, A.; Olsen, I.; Nilsen, S.F.; Holstad, G.; Djønne, B. Biofilm formation by Mycobacterium avium isolates originating from humans, swine and birds. BMC Microbiology [Internet]. 6 de agosto de 2009 [citado 11 de enero de 2024];9(1):159. Disponible en: https://doi.org/10.1186/1471-2180-9-159. [CrossRef]
- Mycobacterium biofilms: factors involved in development, dispersal, and therapeutic strategies against biofilm-relevant pathogens - PubMed [Internet]. [citado 25 de enero de 2024]. Disponible en: https://pubmed.ncbi.nlm.nih.gov/25072151/. [CrossRef] [PubMed]
- Muñoz-Egea, M.C.; Akir, A.; Esteban, J. Mycobacterium biofilms. Biofilm. diciembre de 2023;5:100107. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).