Submitted:
27 February 2024
Posted:
27 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SPMA
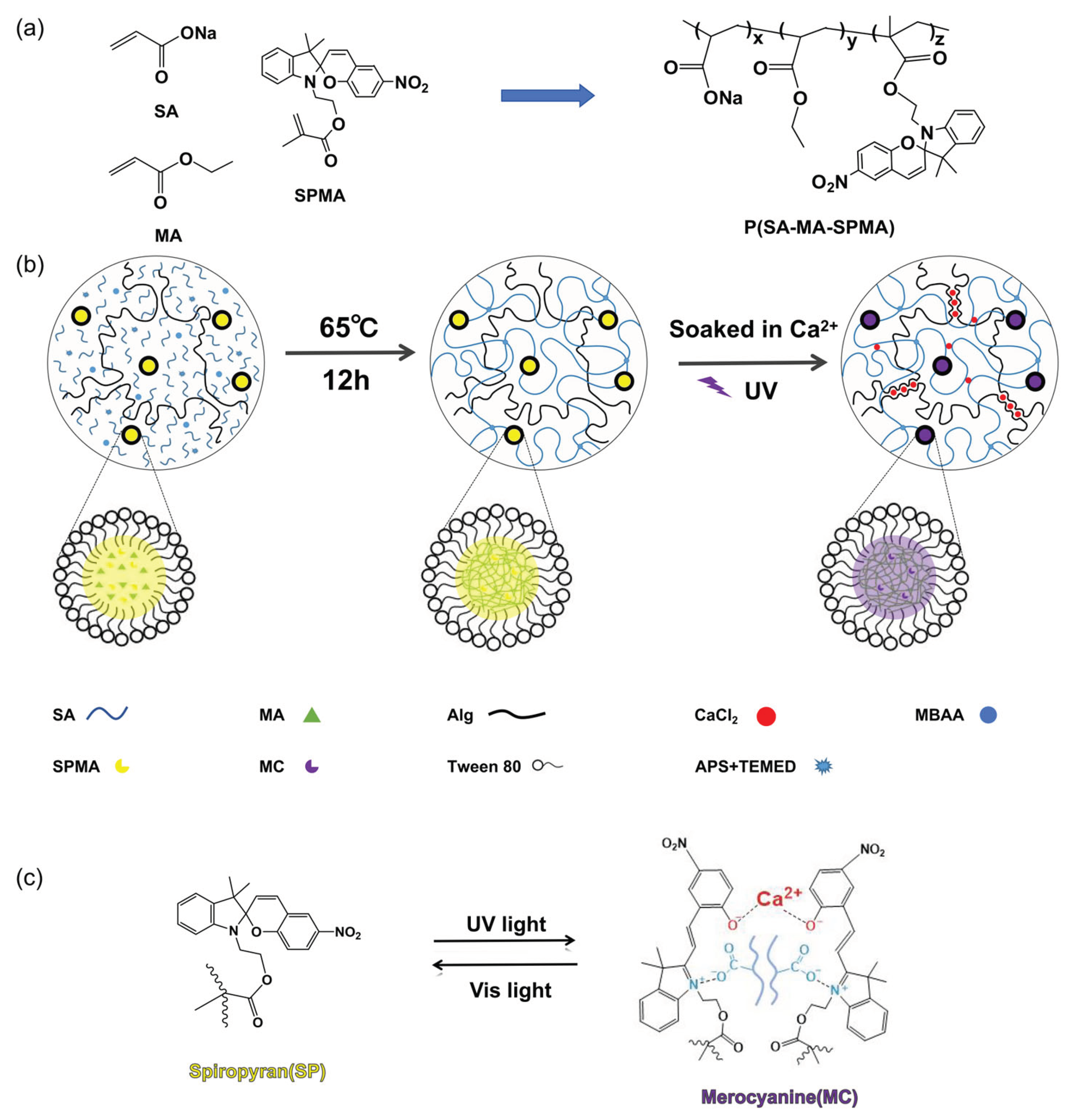
2.3. Preparation of Hybrid Cross-Linked Double-Network Photochromic Hydrogels
2.4. Measurements
2.4.1. Tensile Measurement
2.4.2. Fourier Transform Infrared Spectroscopy
2.4.3. UV–Vis Spectroscopy
2.4.4. Scanning Electron Microscopy Imaging
2.4.5. Water Content Measurement
2.4.6. The Shape Recovery Rate
3. Results and Discussion
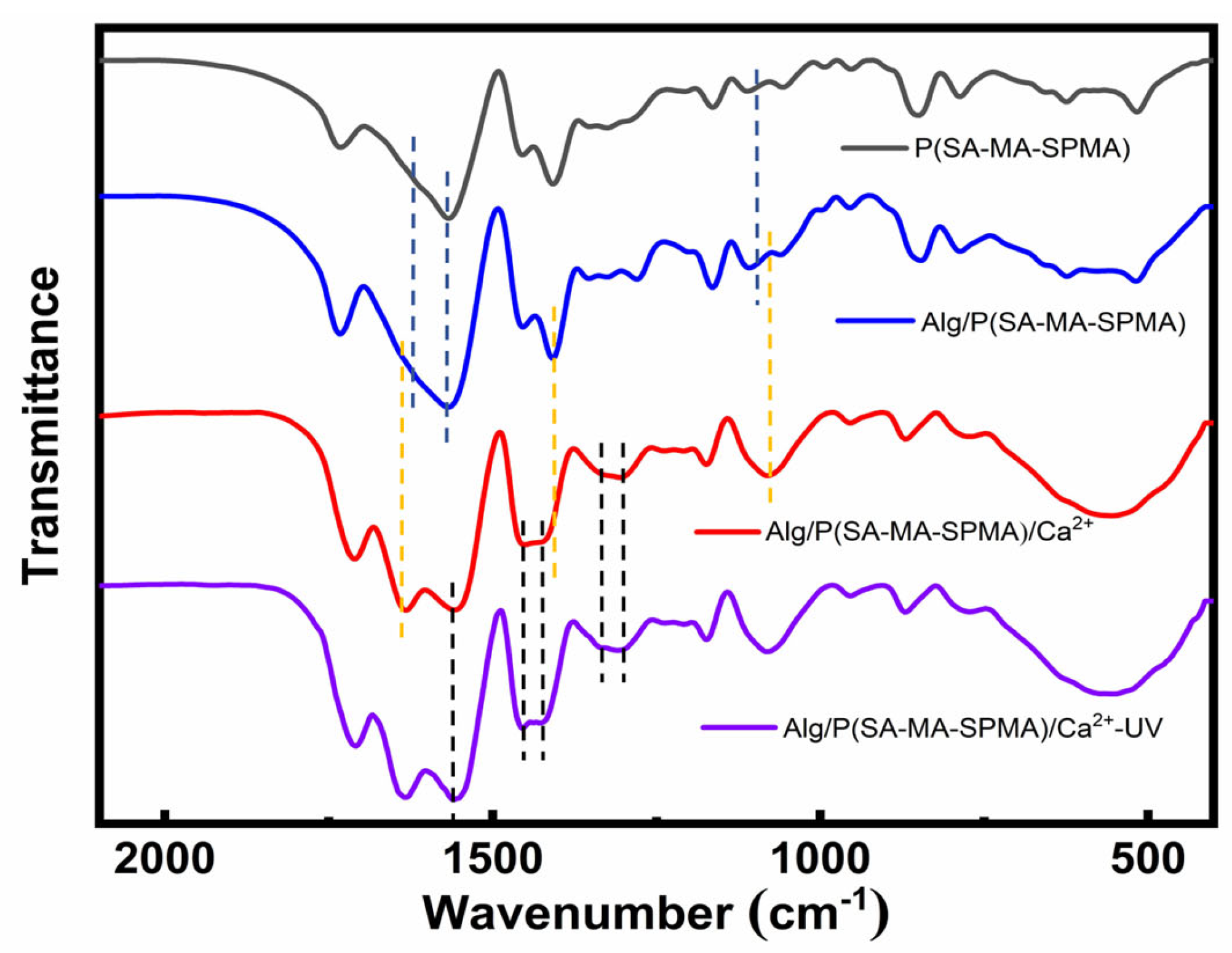
3.1. FTIR Spectroscopy
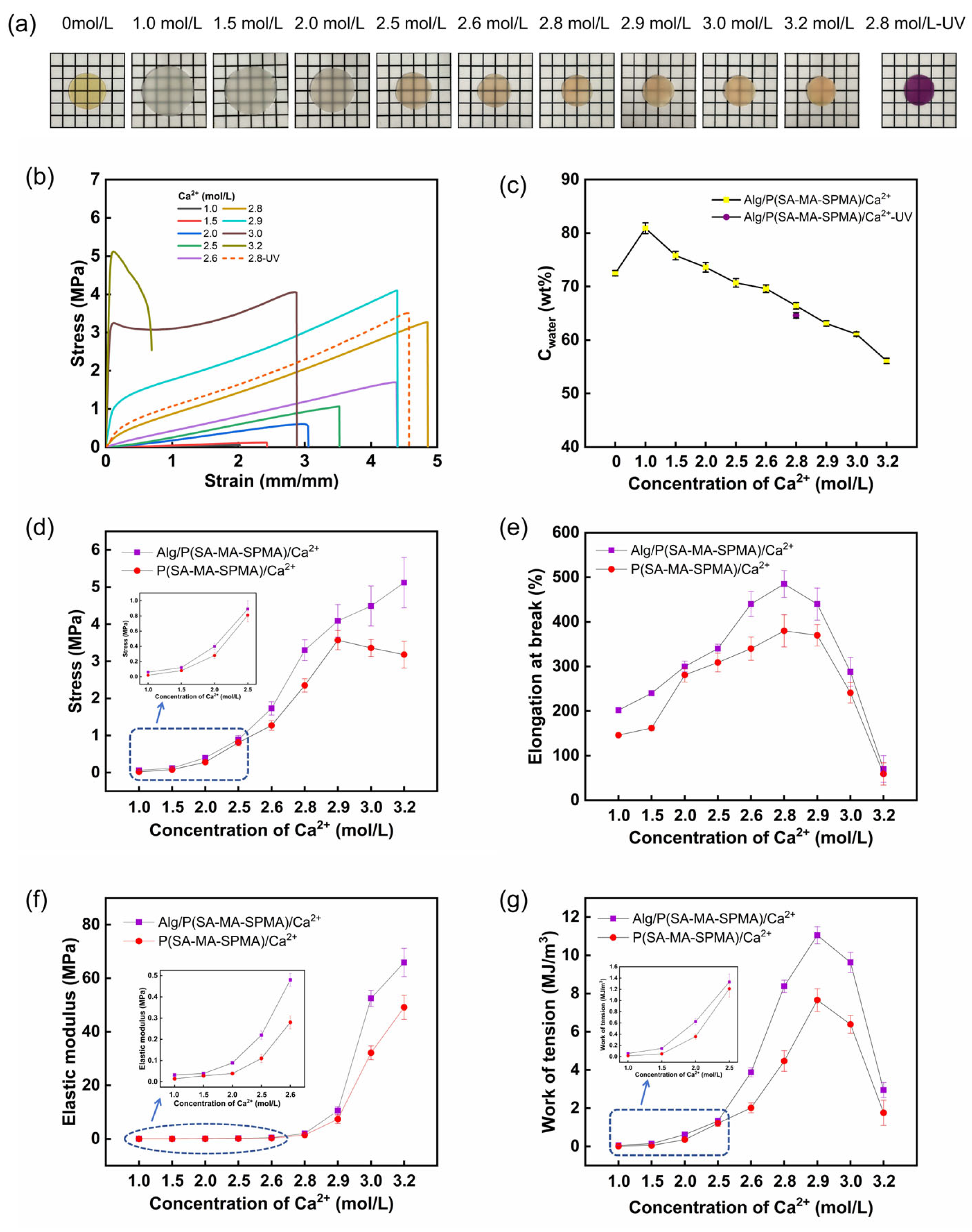
3.2. Mechanical Properties of the Hydrogels
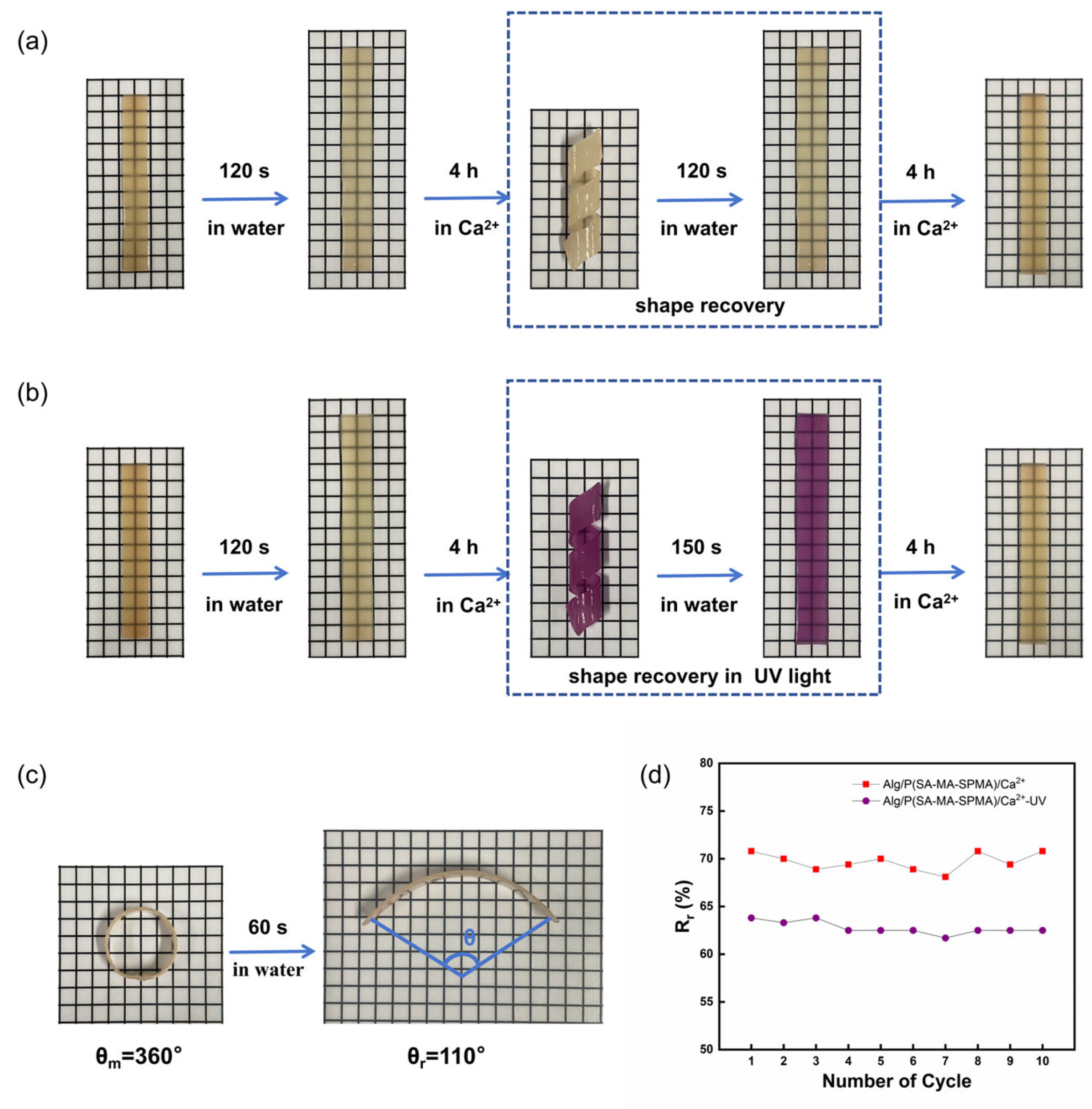
3.4. Shape Memory of the Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trung, T.; Lee, N. Recent progress on stretchable electronic devices with intrinsically stretchable components. Adv. Mater. 2017, 29, 1603167. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Jiao, D.; Zheng, Q.; Wu, Z. Recent progress in fabrications and applications of functional hydrogel films. J. Polym Sci. 2023, 61, 1026–1039. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Lee, H.; Kwon, S. Magnetochromatic microactuators for a micropixellated color-changing surface. Adv. Mater. 2013, 25, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, F.; Di, C.; Zhu, D. Advances of flexible pressure sensors toward artificial intelligence and health care applications. Mater. Horiz. 2015, 2, 140–156. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Chen, Y.; Zheng, X.; Liu, H.; Li, H. Multiple-stimuli-responsive and cellulose conductive ionic hydrogel for smart wearable devices and thermal actuators. ACS Appl. Mater. Interfaces 2021, 13, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.; Weaver, J.; Huebsch, N.; Lee, H.; Lippens, E.; Duda, G.; Mooney, D. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef]
- Zhang, Y.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Sun, Y.; Le, X.; Zhou, S.; Chen, T. Recent progress in smart polymeric gel-based information storage for anti-counterfeiting. Adv. Mater. 2022, 34, e2201262. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shi, H.; Lu, W.; Wei, S.; Shang, H.; Liu, H.; Si, M.; Le, X.; Yin, G.; Theato, P.; Chen, T. Aggregation-induced emissive carbon dots gels for octopus-inspired shape/color synergistically adjustable actuators. Angew. Chem. Int. Ed. 2021, 60, 21890–21898. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, F.; Chen, Q.; Zheng, J. A novel design of multi-mechanoresponsive and mechanically strong hydrogels. Adv. Mater. 2017, 29, 1606900. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yang, G.; Chen, A.; Zheng, Z.; Zhang, C.; Zhang, Y.; Liao, L. Multi-responsive chromatic hydrogel exhibiting reversible shape deformations. Dyes Pigm. 2022, 204, 110364. [Google Scholar] [CrossRef]
- Long, S.; Huang, J. Xiong, J.; Liu, C.; Chen, F.; Shen, J.; Huang, Y.; Li, X. Designing Multistimuli-Responsive Anisotropic Bilayer Hydrogel Actuators by Integrating LCST Phase Transition and Photochromic Isomerization. Polymers 2023, 15, 786. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, F.; Wang, F.; Wang, J.; Dong, R.; Zhuang, X.; Schmidt, O.; Feng, X. Stimulus-responsive micro-supercapacitors with ultrahigh energy density and reversible electrochromic window. Adv. Mater. 2017, 29, 1604491. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Lu, J.; Kong, F.; Liu, K.; Wei, H.; Si, C. Reversible photo-controlled release of bovine serum albumin by azobenzene-containing cellulose nanofibrils-based hydrogel. Adv. Compos. Hybrid Mater. 2019, 2, 462–470. [Google Scholar] [CrossRef]
- Boelke J, Hecht S. Designing molecular photoswitches for soft materials applications. Adv. Opt. Mater. 2019, 7, 1900404. [Google Scholar] [CrossRef]
- Weng, G.; Thanneeru, S.; He, J. Dynamic coordination of Eu-iminodiacetate to control fluorochromic response of polymer hydrogels to multistimuli. Adv. Mater. 2018, 30, 1706526. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Sun, S.; Hou, L.; Wu, P.; Wu, Z.; Zheng, Q. A tough and stiff hydrogel with tunable water content and mechanical properties based on the synergistic effect of hydrogen bonding and hydrophobic interaction. Macromolecules 2018, 51, 8136–8146. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Zeng, H.; Ji, X.; Xie, T.; Yan, X.; Wu, Z.; Huang, F. Reversible ion-conducting switch in a novel single-ion supramolecular hydrogel enabled by photoresponsive host-guest molecular recognition. Adv. Mater. 2019, 31, 1807328. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, F.; Chen, Q.; Zheng, J. A novel design of multi-mechanoresponsive and mechanically strong hydrogels. Adv. Mater. 2017, 29, 1606900. [Google Scholar] [CrossRef]
- Meng, X.; Qi, G.; Zhang, C.; Wang, K.; Zou, B.; Ma, Y. Visible mechanochromic responses of spiropyrans in crystals via pressure-induced isomerization. Chem. Commun. 2015, 51, 9320–9323. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 45, 148–184. [Google Scholar] [CrossRef]
- Gu, H.; Wang, G.; Cao, X. Thermoresponsive nanocomposite hydrogels with high mechanical strength and toughness based on a dual crosslinking strategy. J. Appl. Polym. Sci. 2021, 138, 51509. [Google Scholar] [CrossRef]
- Mu, Q.; Cui, K.; Wang, Z.; Matsuda, T.; Cui, W.; Kato, H.; Namiki, S.; Yamazaki, T.; Frauenlob, M.; Nonoyama, T.; Tsuda, T.; Tanaka, S.; Nakajima, T.; Gong, J. Force-triggered rapid microstructure growth on hydrogel surface for on-demand functions. Nat. Commun. 2022, 13, 6213–6223. [Google Scholar] [CrossRef]
- Meng, X.; Qiao, Y.; Do, C.; Bras, W.; He, C.; Ke, Y.; Russell, T.; Qiu, D. Hysteresis-free nanoparticle-reinforced hydrogels. Adv. Mater. 2022, 34, 2108243. [Google Scholar] [CrossRef]
- Li, X.; Xu, D.; Wang, H.; Gong, C.; Li, H.; Huang, Y.; Long, S.; Li, D. Programmed transformations of strong polyvinyl alcohol/sodium alginate hydrogels via ionic crosslink lithography. Macromol. Rapid Commun. 2020, 41, 2000127. [Google Scholar] [CrossRef]
- Huang, G.; Tang, Z.; Peng, S.; Zhang, P.; Sun, T.; Wei, W.; Zeng, L.; Guo, H.; Guo, H.; Meng, G. Modification of hydrophobic hydrogels into a strongly adhesive and tough hydrogel by electrostatic interaction. Macromolecules 2022, 55, 156–165. [Google Scholar] [CrossRef]
- Jiang, Z.; Song, P. Strong and fast hydrogel actuators. Science 2022, 376, 245. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, S.; Fan, D. A physicochemical double cross-linked multifunctional hydrogel for dynamic burn wound healing: shape adaptability, injectable self-healing property and enhanced adhesion. Biomaterials 2021, 276, 120838. [Google Scholar]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of double network hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Zhang, D.; Xu, Z.; Liu, W. A highly tough and stiff supramolecular polymer double network hydrogel. Polymer 2018, 153, 193–200. [Google Scholar] [CrossRef]
- Yu, H.; Zheng, S.; Fang, L.; Ying, Z.; Du, M.; Wang, J.; Ren, K.; Wu, Z.; Zheng, Q. Reversibly transforming a highly swollen polyelectrolyte hydrogel to an extremely tough one and its application as a tubular grasper. Adv. Mater. 2020, 32, 2005171. [Google Scholar] [CrossRef]
- Zheng, S.; Ding, H.; Qian, J.; Yin, J.; Wu, Z.; Song, Y.; Zheng, Q. Metal-coordination complexes mediated physical hydrogels with high toughness, stick-slip tearing behavior, and good processability. Macromolecules 2016, 49, 9637–9646. [Google Scholar] [CrossRef]
- Yang, C.; Wang, M.; Haider, H.; Yang, J.; Sun, J.; Chen, Y.; Zhou, J.; Suo, Z. Strengthening alginate/polyacrylamide hydrogels using various multivalent cations. ACS Appl. Mater. Interfaces 2013, 5, 10418–10422. [Google Scholar] [CrossRef]
- Yasin, A.; Li, H.; Zhao, L.; Rehman, S.; Siddiq, M.; Yang, H. A shape memory hydrogel induced by the interactions between metal ions and phosphate. Soft Matter 2014, 10, 972–977. [Google Scholar] [CrossRef]
- Fang, Y.; Al-Assaf, S.; Phillips, G.; Nishinari, K.; Funami, T.; Williams, P.; Li, L. Multiple steps and critical behaviors of the binding of calcium to alginate. J. Phys. Chem. B 2007, 111, 2456–2462. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Y.; Li, D.; Zhang, G.; Long, S.; Wang, H. Hybrid dual crosslinked polyacrylic acid hydrogels with ultrahigh mechanical strength, toughness and self-healing properties via soaking salt solution. Polymer 2017, 121, 55–63. [Google Scholar] [CrossRef]
- Wu, X.; Deng, X.; Song, Y.; Zhang, Z.; Su, H.; Han, Y.; Shen, Y.; Liu, S.; Sun, K.; Yao, H.; Guan, S. Polyacrylamide/sodium alginate photochromic hydrogels with enhanced toughness and fast response for optical display and rewritable information record. Dyes Pigm. 2023, 210, 111009. [Google Scholar] [CrossRef]
- Long, S.; Ye, Z.; Jin, Y.; Huang, J.; Huang, Y.; Liao, Y.; Li, X. High-performance photochromic hydrogels for rewritable information record. Macromol. Rapid Commun 2021, 42, 2000701. [Google Scholar] [CrossRef]
- Fries, K.; Driskell, J.; Samanta, S.; Locklin, J. Spectroscopic analysis of metal ion binding in spiropyran containing copolymer thin films. Anal. Chem. 2010, 82, 3306–3314. [Google Scholar] [CrossRef] [PubMed]
- Fries, K.; Sheppard, G.; Bilbrey, J.; Locklin, J. Tuning chelating groups and comonomers in spiropyran-containing copolymer thin films for color-specific metal ion binding. Polym. Chem 2014, 5, 2094–2102. [Google Scholar] [CrossRef]
- Li, C.; Iscen, A.; Palmer, L.; Schatz, G.; Stupp, S. Light-driven expansion of spiropyran hydrogels. J. Am. Chem. Soc. 2020, 142, 8447–8453. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Le, X.; Zhang, J.; Chen, T.; Theato, P. Trends in polymeric shape memory hydrogels and hydrogel actuators. Polym. Chem. 2019, 10, 1036–1055. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




