1. Introduction
Prostate cancer (PCa) is the second most common neoplasm diagnosed in men worldwide [
1].
Approximately half of the patients affected by PCa are found to have a low-risk progression PCa with a maximum Gleason score of 6 (Grade Group 1) with a mortality rate after 20 years of 0.2% [
2].
Furthermore, even in patients affected by Grade 2 prostate cancer (Gleason score 3+4), survival rates are not significantly different than in patients with Grade Group 1, despite the elevated risk of disease progression [
3].
Current standard therapy for PCa with radical intent usually consists of radical prostatectomy or radiotherapy, plus androgen deprivation therapy (ADT). Unfortunately, treatment-related morbidity is high. The ProtecT trial showed urinary incontinence rates of 3% and 20% and erectile dysfunction rates of 66% and 79% for radiotherapy and radical prostatectomy respectively [
4].
Consequently, over the past few years, there has been a notable escalation in scholarly attention towards the implementation of focal therapeutic interventions for patients diagnosed with low and intermediate-risk prostate cancer (PCa). This heightened focus has fostered the exploration and development of several focal treatment modalities, including high-intensity focal ultrasound (HIFU), irreversible electroporation, focal cryotherapy, and photodynamic therapy [
5].
According to the European Association of Urology (EAU), low-risk and intermediate-risk patients may undergo local procedures with the aim of achieving long-term cancer control and reducing morbidity associated with surgery and radiation therapy [
5].
Notwithstanding the considerable diversity in treatment techniques, it is noteworthy that each of these modalities is associated with a notable incidence of persistent cancer within the prostate upon rebiopsy, ranging from 24% to 49%. Importantly, these treatments are characterized by minimal occurrences of high-grade complications. Instances of incontinence are rare, and any reductions in erectile function are typically mild and transient. The most common adverse events are urinary tract infections, hematuria, and urinary retention [
6].
Focal laser ablation (FLA) is a safe and effective treatment of focal cancer lesions, not only prostatic; this is due to the very thin caliber of the applicators and the high precision of the delivered energy [
7,
8,
9]. This procedure involves a minimally invasive percutaneous thermoablation technique, which utilizes a 1064 nm laser light delivered into the target tissue through optical fibers for a brief duration, resulting in the heating of the affected tissue to the point of irreversible, in-situ damage, thus inducing coagulative necrosis.
Recently several pilot studies showed that transperineal laser ablation (TPLA) is feasible for the treatment of PCa under local anesthesia [
10,
11,
12].
In our study, we enrolled patients with prostate cancer (PCa) who exhibited intermediate-risk progression (defined as PSA levels below 20 ng/ml, Gleason Scores of 3+4 or 4+3, and clinical staging between cT2a and cT2b). Additionally, we included patients with low-risk progression (characterized by PSA levels below 10 ng/ml, Gleason score 3+3, and clinical staging ranging from cT1 to cT2a). These patients had expressed a preference to forego or declined participation in active surveillance protocols, as well as validated treatments for organ-confined cancer such as radical surgery and radiotherapy.
Our primary objectives encompassed a thorough assessment of the short-term (over a 3-year period) efficacy of magnetic resonance imaging (MRI)/ultrasound (US) fusion-guided focal laser ablation (FLA) for the treatment of low to intermediate-risk prostate cancer, as well as an evaluation of treatment-related complications. The effectiveness of this intervention was quantified by gauging cancer control, defined as the proportion of patients achieving disease-free survival as determined by target biopsy outcomes.
Concurrently, our secondary aim involved an appraisal of the utility of multiparametric magnetic resonance imaging (mpMRI) as a diagnostic tool during post-treatment follow-up to examine morphostructural alterations in the prostate gland resulting from FLA. Additionally, we assessed its reproducibility in determining the response to FLA therapy, correlating these findings with biopsy results, with the ultimate objective of establishing its predictive diagnostic value in identifying residual or recurring tumors.
2. Materials and Methods
Starting in 2018, we enrolled a cohort of forty male patients, aged between 46 and 86, all of whom were diagnosed with histologically confirmed, organ-confined prostate cancer displaying low to intermediate progression risk (as defined by Gleason scores ≤ 4+3, PSA levels below 20 ng/ml, and clinical staging below T2b).
Ethical approval for all research procedures was obtained in accordance to the ethical standards of the institutional and/or national research committee, and in accordance with the guidelines stipulated in the 1964 Helsinki Declaration and its later amendments (ClinicalTrials.gov ID NCT04045756).
The study unfolded across seven distinct phases. At Time 0, our focus was on patient selection, involving the recruitment process and a rigorous assessment of eligibility criteria. This step was undertaken by the Multidisciplinary Neoplasms Group specializing in uro-genital cases within our hospital. Patients were evaluated and recruited based on inclusion and exclusion criteria summarized in
Table 1.
The pre-procedural evaluation consisted in electrocardiogram, complete blood count, urine examination and urine culture, evaluation of post-voiding residue and uroflowmetry. Patients were also submitted to IPSS-QoL and IIEF-5 questionnaires at baseline and during follow-up to investigate any procedure-related erectile dysfunction or urinary symptoms. Given that the procedure was guided by MRI/US fusion images, a 3T multiparametric prostate resonance (mpMRI) scan was performed for each patient at Time 0, adhering to the protocol delineated in
Table 2 (refer also to
Figure 1).
Time 1: Interview and signing of informed consent.
Time 2: FLA treatment.
The procedure was performed by an expert radiologist on prostate MRI/US procedures, on a Day-Hospital setting, in a radiological interventional room. The patients were placed in the lithotomy position and Ciprofloxacin 500 mg was administered as antibiotic prophylaxis. The Echolaser system (Elesta s.r.l. - Calenzano (FI)) as laser light source operating at 1064 nm and MRI/US fusion imaging to identify the focal lesion to treat were used. Local superficial anesthesia of the perineal region, followed by a transrectal prostatic block with a lidocaine solution 2% (20 mL) were performed under US guidance. The laser light was conveyed from the source to the target through flexible, small caliber (300 micron) flat-tipped quartz optical fibers introduced transperineally by thin needles (21 Gauge) inserted previously under real-time US guidance. Up to three needles were used according to the lesion size because the multi fiber approach can extend the coagulation area. The laser fiber protrudes 10 mm from the needle tip. Each lighting lasted about 6 minutes and a maximum energy of 3600 j per fiber was delivered (1800 J per fiber with the pull-back technique which consists on pulling back the introducer by 1 cm and performing a second illumination to increase the ablation area with duration and power equal to the previous one) with a power of 3-5 Watts. The laser therapy was performed entirely under US guidance for real time monitoring of the correct positioning of applicators and the extension of the area of damage.
A mpMRI was performed immediately after the treatment to evaluate the extent of the ablation zone according to the protocol in
Table 2. At the end of the procedure all patients were observed for a minimum period of 2 hours. The resumption of spontaneous diuresis was checked out. No cases of acute urinary retention or clinically significant hematuria that required a bladder catheter insertion occurred.
An oral cortisone drug as anti-edema therapy was prescribed.
Patients were discharged with antibiotic therapy, pain relief drugs if needed and gastroprotective for 7 days.
Time 3-4-5-6-7: follow-up at 1 (T3), 6 (T4), 12 (T5), 24 (T6) and 36 (T7) months with clinical evaluation performed by the UOSD Urology, PSA measurement and mpMRI according to the protocol in
Table 2. At 36 months, the IPSS and IIEF-5 questionnaires were repeated, and a systematic and target MRI/US fusion-guided prostate biopsy was performed.
Treatment success was operationally defined as the absence of any anomalies in MRI examinations conducted during the follow-up period, in conjunction with a negative target biopsy at the conclusion of the 36-month period. The presence of residual lesions within the treatment field was regarded as an indicator of treatment failure. Notably, a positive random biopsy indicating the existence of out-of-field lesions was not construed as a treatment failure.
2.1. Statistical Analysis
A statistical analysis has been realized on the cohort of 20 patients treated with TPLA, has been performed using Visual Basic (VB) as code and Excel has platform to create graphs and tables to investigate changes over time in PSA and necrotic cavity volume during 36 months follow-up (as seen respectively in
Table 3 and
Table 4) after the procedure. The authors have tested different fitting algorithms to obtain equations predicting the trend of the variables over the 36 months. The tests have been done for the first 3 patients with some trendline options: linear, logarithmic, polynomial, power, moving average, and exponential. The exponential trendline showed a better fit with data and it has been applied to all the patients both for PSA and cc cavity. In
Table 5 and
Table 6 you can find the trend equations and the related R2.
The authors have also evaluated clinical data and QoL variations of patients, through the IPSS score (
Table 7) and the IIEF-5 score (
Table 8), measured at time 0 and 36 months respectively. Functional outcome was evaluated by comparing IPSS and IIEF-5 mean values at baseline with 36 months after using the Student’s t-test.
Statistical tests were performed using IBM SPSS statistics, version 25 (IBM SPSS, IBM Corp., Armonk, NY, USA), and a p-value < 0.05 was considered as statistically significant difference and has been calculated accordingly to literature [28,29].
3. Results
All procedures were executed with technical proficiency, ensuring successful completion in all instances. Following their treatment, all patients were discharged on the same day.
Clinical follow-up entailed the monitoring of potential complications associated with focal laser ablation (FLA), including perineal pain, perineal hematoma, hematuria, acute urinary retention, hematospermia, erectile dysfunction, and urinary incontinence. It is noteworthy that none of these complications were observed during the follow-up and clinical observation, signifying a lack of any such occurrences, which aligns with Clavien-Dindo grade I classification.
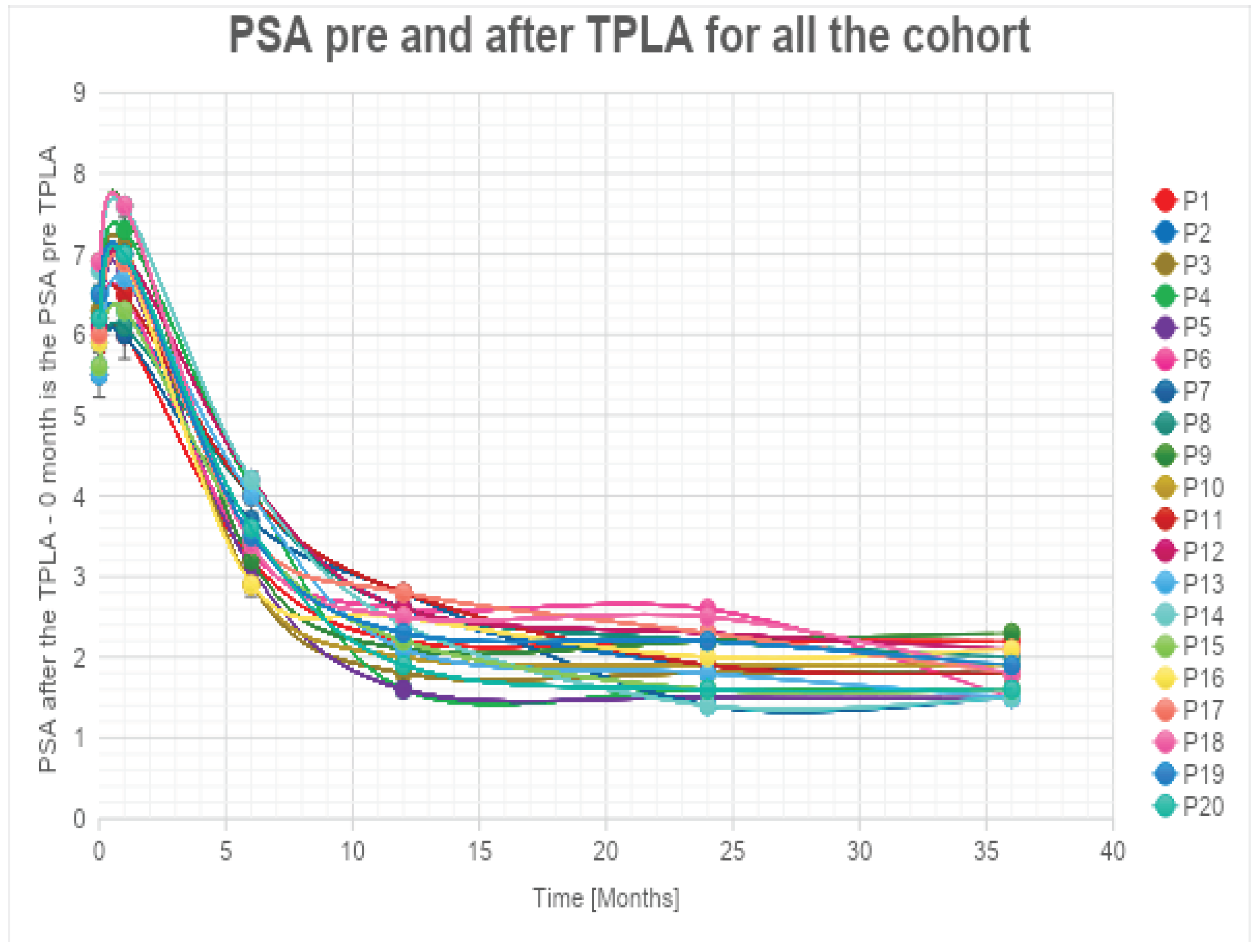
We assessed the temporal changes in PSA levels among our cohort.
Notably, at the 1-month follow-up, every patient exhibited an increase in PSA levels compared to the baseline, attributed to the release of enzymes into the bloodstream following the necrotic process. However, subsequent to this initial increase, we observed a consistent and statistically relevant decline in PSA levels, with a mean reduction exceeding 60% at the 36-month follow-up for all patients (
Table 3, Graphic 1).
The authors have observed a significant decrease in PSA values that were always higher than 55% at 1 year after the treatment (with a peak of 75%) for PSA (
Table 3, Graphic 1). These percentages increased and are always higher than 60% (with a peak of 80%) at 2 years after the treatment. The percentage of decrease in PSA values is always lower than the 5% between the 2 and the 3 years. The authors can affirm that the decrease is significant in the first 24 months. The authors have estimated an error of 5% during the data acquisition phase.
Graphic 1.
PSA levels are reduced during follow-up.
Graphic 1.
PSA levels are reduced during follow-up.
The exponential trendlines show an estimated decrease higher than 87% for all the patients at 48 months from the treatment.
It is noteworthy that five out of 25 patients did not exhibit a substantial decrease in PSA levels during follow-up. Subsequent MRI and target biopsy identified the presence of a residual lesion in close proximity to the treatment area in these cases.
An imaging follow-up through 3T mpMRI was performed.
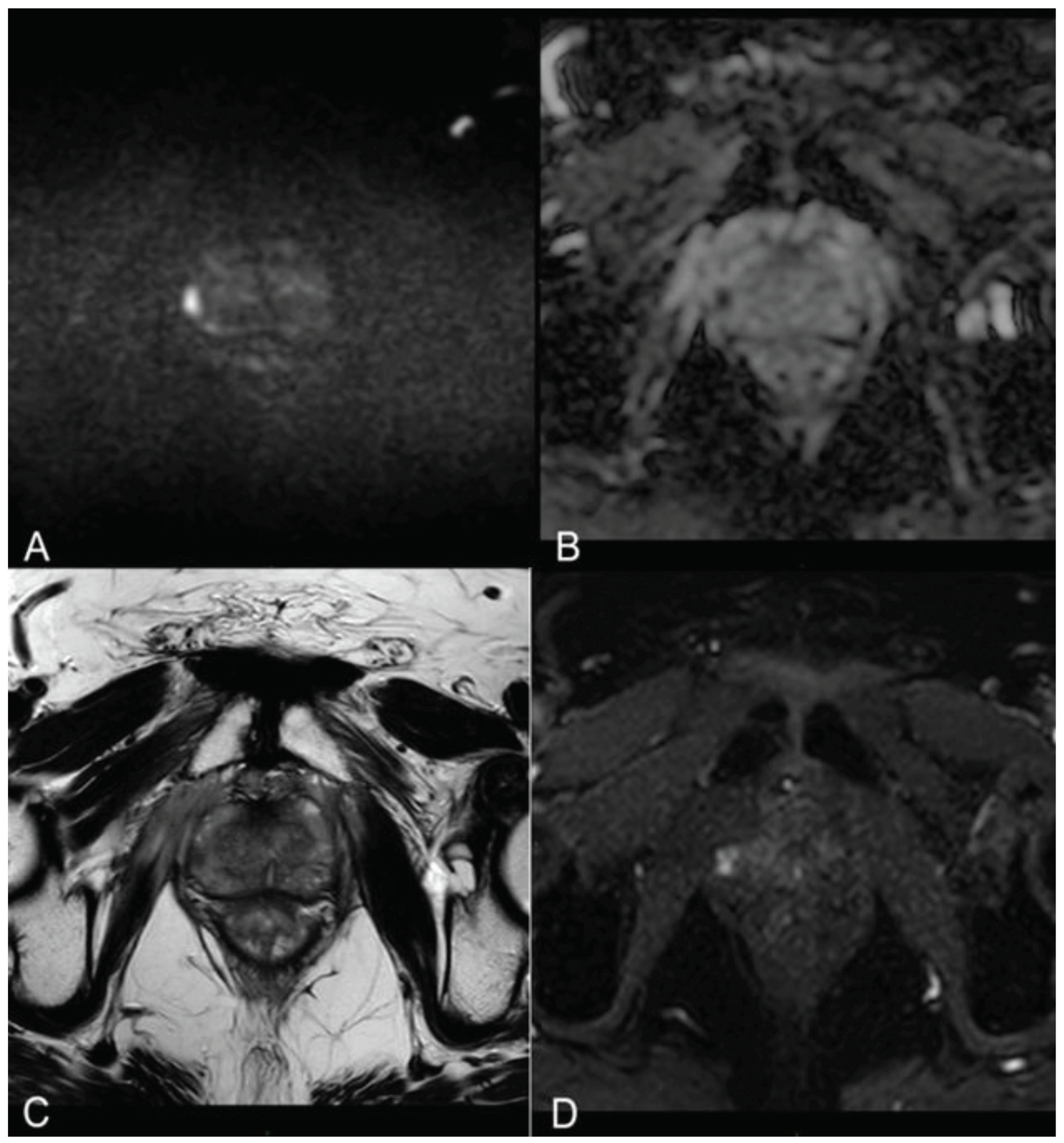
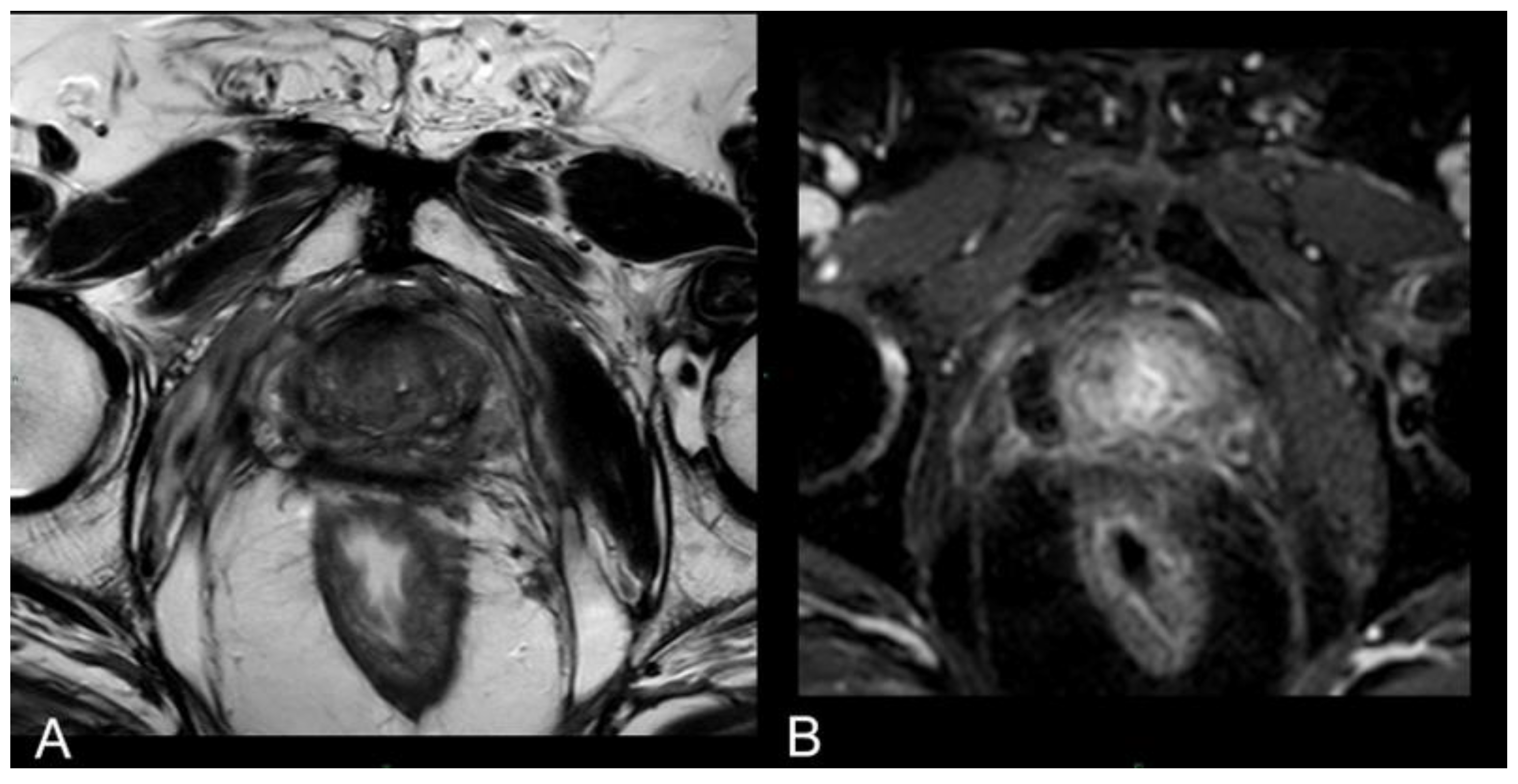
Immediate post-treatment mpMRI examinations consistently revealed the presence of a devascularized ablation cavity characterized by coagulative necrosis in all cases, and notably, this cavity exhibited a volume approximately three times larger than the original cancer lesion (as illustrated in
Figure 2).
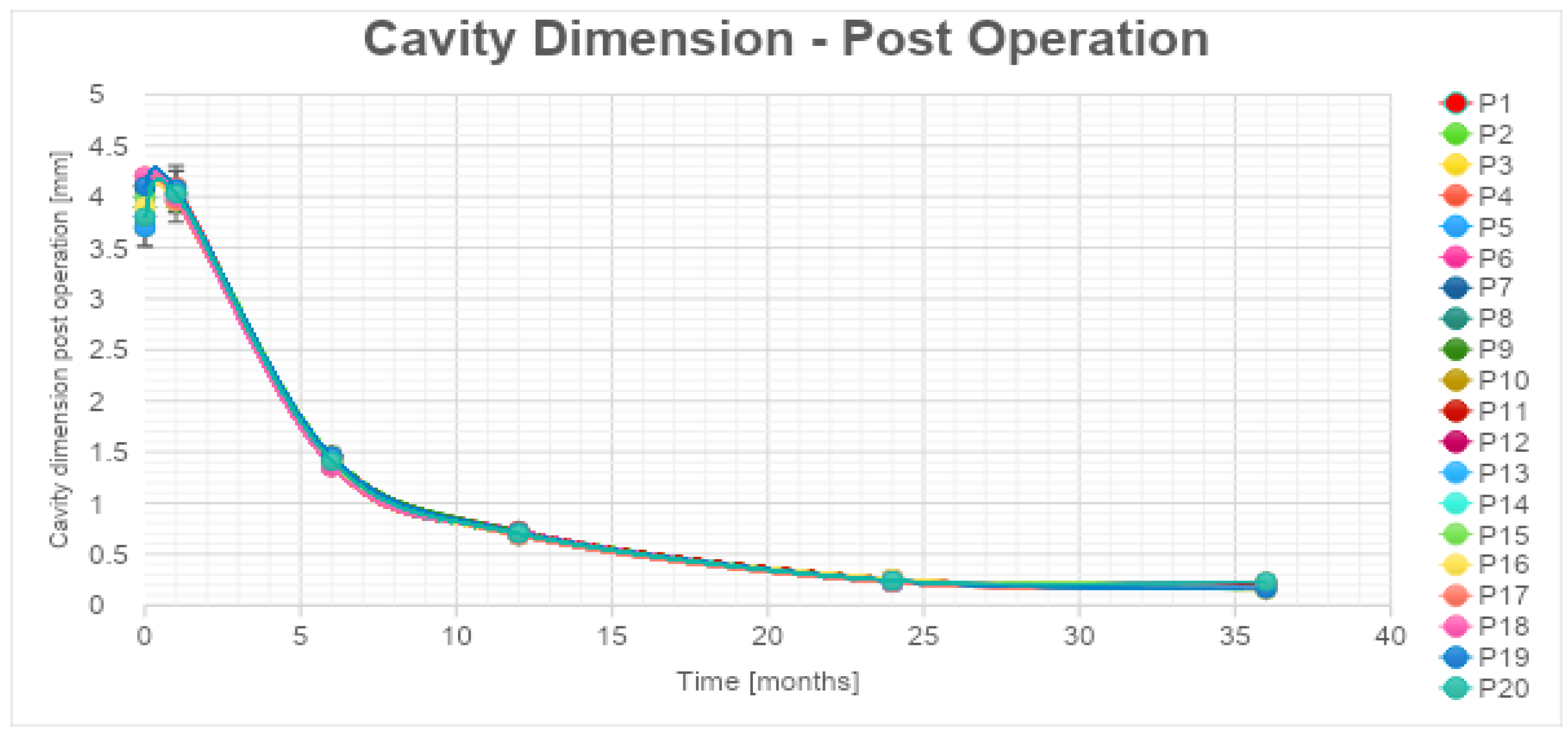
Subsequent mpMRI assessments conducted during the follow-up period portrayed the temporal evolution of this cavity, which began to exhibit a noticeable reduction in size after the 6-month follow-up, with an average volume decrease exceeding 80% by the 36-month mark (
Table 4, Graphic 2).
Graphic 2.
Necrotic cavity volume reduction during follow-up.
Graphic 2.
Necrotic cavity volume reduction during follow-up.
According to the statistical analysis, the authors have observed a significant decrease also in cavity volume values that were, respectively, always between 80%-83% for all the patients at 1 year after the treatment (
Table 4, Graphic 2). The percentages of cc cavity decreasing are always between 93%-95% at 2 years from the treatment. The percentage of decrease in cc cavity values is always lower than the 3% between the 2 and the 3 years. The decrease is considered significant in the first 24 months. The authors have estimated an error of 5% during the data acquisition phase.
The exponential trendlines show for cavity volume an estimated decrease higher than 98% for all the patients at 48 months from the treatment.
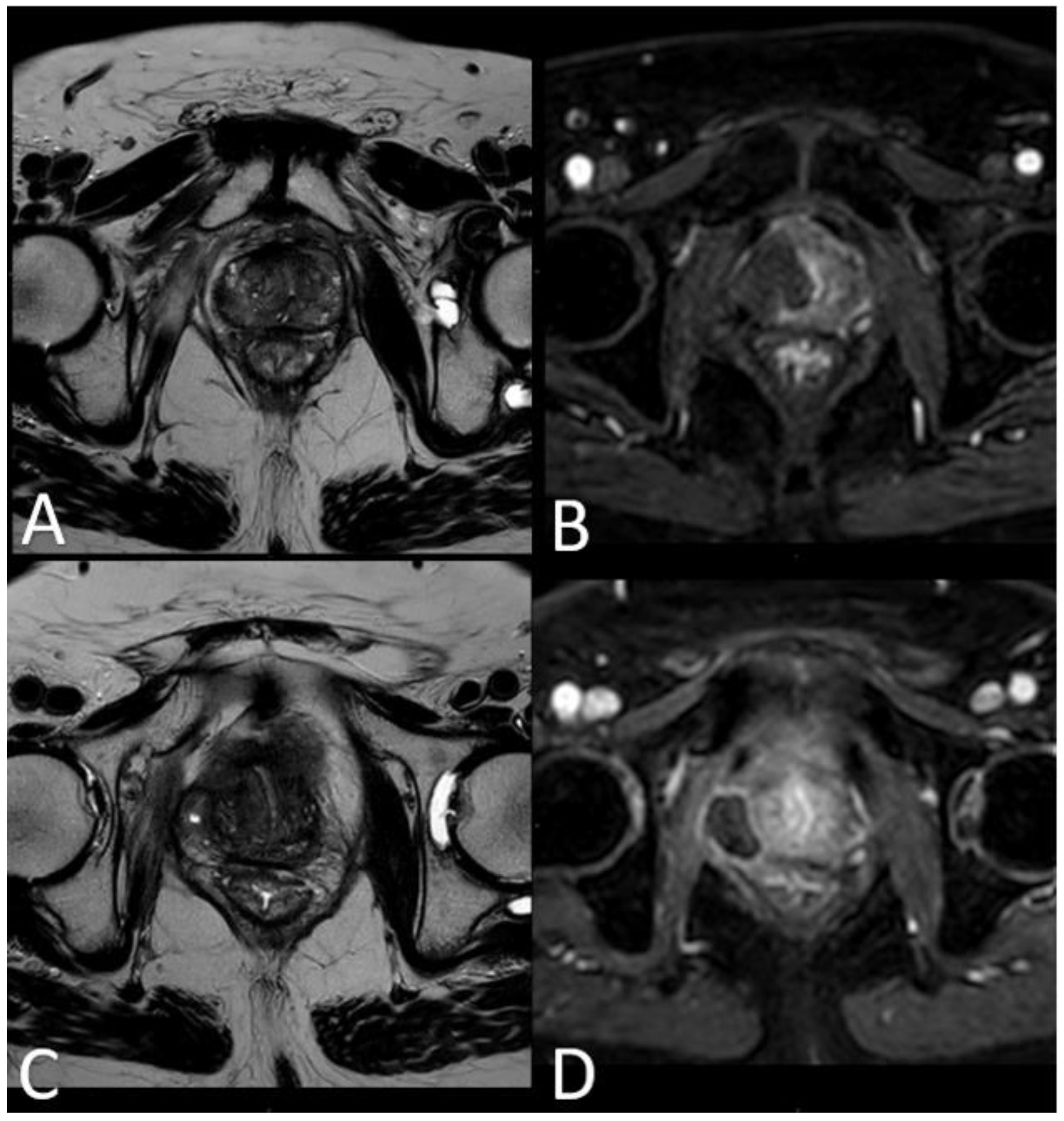
In 25% of the cases, the necrotic coagulation cavity was entirely reabsorbed and replaced by a fibrotic scar, exemplified in Figure 4. Among the 25 patients, 20 did not exhibit any reliable focal contrast enhancement indicative of residual or recurrent neoplastic lesions within the treated area. This absence of neoplasia was subsequently confirmed by MRI/US fusion-guided target biopsies conducted at the 36-month follow-up.
Importantly, although already known in literature [
8], the IIEF-5 and IPSS scores demonstrated not statistically significant variations (p-value >0,05) between the pre-procedural values and those recorded at the 36-month mark (as described below in Statistical analysis results paragraph,
Table 7 and
Table 8).
Mean IPSS scores PRE and 36 months after the procedure decreased from 7.35 to 6.70. The difference between mean IPSS scores PRE and POST (after 36 months) analysed with a t-test for paired samples resulted in a t-statistic of approximately 1.82 with a p-value of 0.085 (
Table 9). This p-value is above the conventional significance level of 0.05, suggesting that there is no statistically significant difference between IPSS scores before and 36 months after. Erectile function measured by the mean PRE IIEF-5 scores and 36 months after the procedure increased from 14.20 to 15.50. The difference between mean IIEF-5 scores PRE and POST (after 36 months) analysed with a t-test for paired samples yielded a t-value of approximately -1.70 with a p-value of 0.105 (
Table 9). This p-value is above the conventional significance level of 0.05, suggesting that there is no statistically significant difference between IIEF-5 scores before and 36 months after.
However, for the remaining 5 out of 25 patients, mpMRI at the 12-month follow-up identified a focal lesion adjacent to the ablation cavity in all cases (as depicted in Figure 5), promptly confirmed by target biopsy. In all these instances, the biopsy results indicated Gleason Score 3+4 or 4+3 neoplasm (
Table 10). Given the proximity of these lesions to the necrotic cavity, they were all interpreted as indicative of residual cancer.
4. Discussion
Currently, a myriad of focal treatment modalities is available for the ablation of prostate cancer. These encompass cryotherapy, high-intensity focused ultrasound (HIFU), irreversible electroporation, photodynamic therapy, and focal brachytherapy [
13].
Compared to Focal Laser Ablation (FLA), cryotherapy necessitates the use of larger needle calibers and deploys multiple probes situated within the gland, strategically positioned between the prostate and the rectum for temperature monitoring. Additionally, a transurethral warming device is employed to safeguard against urethral damage. However, a drawback of cryotherapy lies in the challenge of precisely controlling the size of the ice ball, often leading to a wider treatment area than required, which can inadvertently result in the ablation of the ipsilateral neurovascular bundle [
14,
15].
HIFU is a relatively new treatment option for prostate cancer, and limited data are available regarding its safety. It requires general anesthesia and a transrectal approach being then able to treat only posterior lesions [
6,
16,
17].
Photodynamic therapy (PDT) shares some similarities with FLA, as it also employs transperineally inserted optical fibers directed at the prostate target. However, it's noteworthy that Azzouzi et al. reported a notable rate of 38% for erectile dysfunction and urinary complications associated with PDT [
13].
A study on the irreversible electroporation investigated 63 patients and reported a 16% of in-field recurrence with a mild decline in the sexual quality of life score [
13]. Within focal brachytherapy, seeds are transperineally inserted into the prostate. King et al. conducted an evaluation of 354 patients who underwent brachytherapy focused on the peripheral zone, revealing concerning long-term oncologic results. Specifically, they reported a 10-year biochemical progression-free survival rate of only 28% for patients with intermediate-risk prostate cancer [
13].
Focal Laser Ablation (FLA) represents a minimally invasive percutaneous procedure that leverages laser light transmitted through optical fibers to selectively elevate the temperature within tissue. This thermal intervention results in protein denaturation and the induction of irreversible coagulative necrosis. Notably, FLA is versatile in its application, capable of ablating cancer lesions in any region of the prostate. Furthermore, it offers the advantage of MRI compatibility, enabling real-time, in-bore MRI guidance during the procedure [
16,
18].
Moreover, this approach can be performed on an outpatient basis and under local anesthesia. The limited side-effect profile due to the excellent precision of the ablation obtainable with laser energy delivery are great advantages of FLA [
19].
The effectiveness of focal laser ablation (FLA) has been previously documented in studies focusing on the treatment of benign prostatic hyperplasia (BPH) and benign thyroid and hepatic nodules [
7,
9,
20,
21].
There are few studies about FLA and most of them have small sample size and short follow-up.
In a 2016 published phase II trial, Eggener et al. treated 27 men with a Gleason 7 or less prostate cancer. At 3 and 12 months they found respectively a 4 % and 11% percentage of disease recurrence within the ablation zone (in-field recurrence). However, 37% of patients were found to have some residual cancer within the prostate gland on a systematic 12-cores re-biopsy (out-of-field recurrence) done at 12 months [
6,
10,
18].
Lepor et al. published their results of 25 consecutive patients with low-intermediate risk PCa treated with MRI-guided FLA [
22,
23]. Post ablation biopsy at 3 months showed no evidence of cancer in 96% of the patients without a compromise in the functional outcomes.
In 2018, interim findings from the most extensive study evaluating Focal Laser Ablation (FLA) were disclosed (Feller et al.) [
19]. This study encompassed the treatment of 98 patients and addressed 138 tumor foci, all performed under real-time MRI guidance. Their report highlighted a 23% rate of in-field cancer recurrence, a notable outcome, while reassuringly, there were no reports of serious adverse events.
Forty cases of low to intermediate progression risk prostate cancer were selected and treated with focal laser ablation in our hospital. Multiparametric magnetic resonance imaging (mpMRI) was used for initial assessment of the lesions, pre-procedural planning and post-treatment imaging follow-up. Ultrasound/magnetic resonance (US/MRI) fusion guided biopsies were performed at 36 months. In 5 cases the biopsy was performed between 12 months and 24 months because mpMRI showed an in-field recurrence.
Throughout the follow-up period, no patients exhibited statistically significant alterations in urinary symptoms or erectile dysfunction.
In almost all patients, we witnessed stability or even slight improvement in urinary symptoms and sexual function over time; in cases of worsening, however, patients were seen to fall into the same severity class. This finding, although already described in the short term in the literature [
8], is particularly important because it is the first time it has been evaluated in the medium to long term (36 months after the procedure).
This favorable outcome can be attributed to several reasons, first of all the specific location of the lesions, which were consistently situated within the peripheral zone of the prostate and the operator’s expertise; a psychological factor cannot be excluded. Consequently, the procedure effectively preserved the integrity of the urethra and the bladder neck in all cases.
One month after the procedure, there was a transient increase in PSA levels. This is a common occurrence after localized prostatic procedures attributed to the sudden release of PSA into the bloodstream by necrotic cells, and it is considered a favorable prognostic factor (24). Beginning in the third month of the follow-up period, a notable decline in PSA levels became evident. By the time we reached the 36-month mark, we had observed a consistent and statistically significant reduction of over 60% in PSA levels.
We attained an impressive 80% success rate with the procedure, encountering only five cases with residual lesions. Remarkably, these patients opted for radical prostatectomy as a salvage treatment option, even though a second FLA could have been a viable alternative. Another notable advantage of FLA is its flexibility, allowing patients to explore more invasive and well-established treatments in the event of treatment failure. Importantly, throughout the entire 36-month follow-up period, we did not record any instances of complications or side effects linked to the procedure. The transperineal approach, which safeguards the urethra and rectum, played a pivotal role in minimizing potential complications.
While other studies on the subject of FLA have been conducted, to the best of our knowledge, our study represents a pioneering effort in investigating focal laser ablation (FLA) for the treatment of low to intermediate-risk prostate cancer. A distinctive feature of our study is the consistent utilization of multiparametric magnetic resonance imaging (mpMRI) as an imaging tool throughout the follow-up period, which distinguishes it from previous research in this field. The use of mpMRI as the primary follow-up imaging method resulted in high accuracy for the validation of the procedure [
25,
26,
27].
We conducted a qualitative and quantitative evaluation of the main imaging findings at each follow-up step.
Immediately following the procedure we observed elliptical hypointense ablation cavities, roughly three times the size of the original lesions. Some of these cavities contained fluid and/or complex fluid (comprising blood and proteinaceous material), with hyperintense fiber tracks discernible within the treated area (as depicted in
Figure 2a, b).
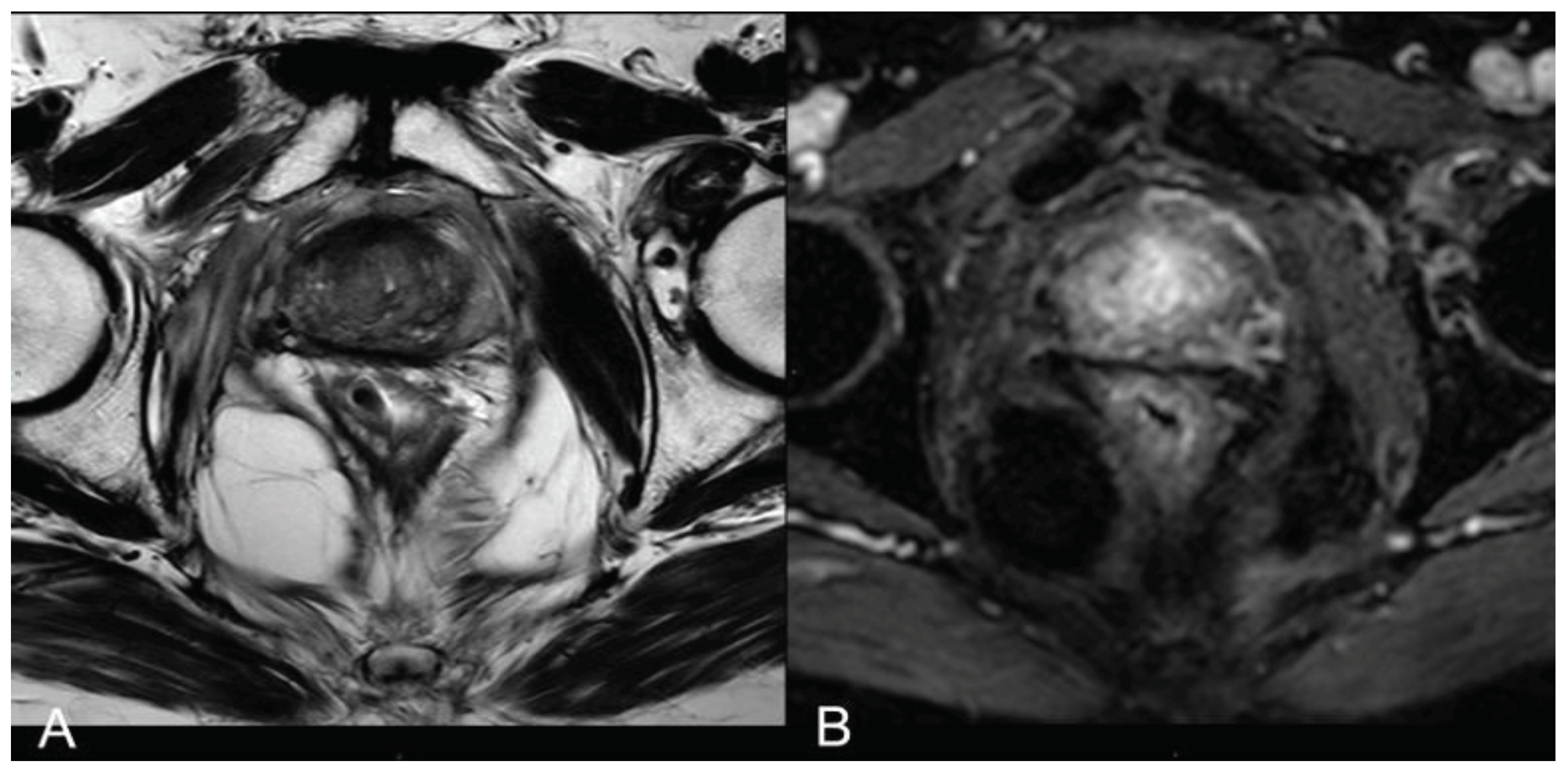
At 1 month - 6 months - 12 months: the main findings included the laser fiber tracks surrounded by a large elliptical-shaped necrotic tissue cavitation, which appeared hypointense on T2-weighted images (
Figure 2 c, d;
Figure 3).
At 24 months - 36 months: presence of a T2W hypointense scar tissue that almost completely replaced the original cavity (
Figure 4).
These imaging characteristics are similar to those documented in the TPLA of BPH [
7].
We observed a progressive decrease in ablation cavity volume and by 36 months it was less than 70% in all patients. It would be helpful to continue the follow-up of patients to determine if the decreasing trend in PSA levels and ablation cavity volume remain stable.
In our experience we reported 10 cases, accounting for 40% of patients, where new prostate cancer foci emerged outside the ablation zone. Importantly, these newly identified lesions consistently exhibited a Gleason Score of 3+3. Their presence was ascertained through the performance of random biopsies at the 36-month follow-up. Notably, the multiparametric magnetic resonance imaging (mpMRI) yielded negative results for all these cases, indicating the absence of clinically significant lesions.
To establish more robust statistical analyses, it would be advantageous to augment the patient cohort and potentially broaden the selection criteria. Similar to other innovative interventional techniques, focal laser ablation (FLA) mandates the expertise of trained operators. In our study, the operators possessed approximately 10 years of experience, which significantly contributed to reduced procedural durations and shorter hospital stays for the patients.
A further limitation of our study lies in the absence of an initial sample size calculation. Instead, we exclusively included patients who met our specified criteria, resulting in a relatively limited sample size. Consequently, our study can be regarded as an initial experience. Therefore, it is imperative to initiate multicenter investigations involving a more extensive patient pool and standardized selection criteria to substantiate FLA's efficacy as a standard treatment approach for low to intermediate-risk prostate cancer.
5. Conclusions
With a remarkable disease-free survival rate achieved in 80% of cases over a 5-year period and the absence of any complications among our patients, our study unequivocally demonstrates the effectiveness and safety of Focal Laser Ablation (FLA) for the treatment of low to intermediate-risk prostate cancer in the short term. Furthermore, our findings also attest to the favorable short- and mid-term quality of life and functional outcomes associated with FLA.
The complete alignment between MRI and histological findings, along with the high accuracy of MRI in assessing morphological changes in the prostate resulting from the procedure, underscores the optimal positive/negative predictive diagnostic value of this imaging modality in the context of residual/relapsing tumors and loco-regional complications.
However, it is imperative to acknowledge that further research and more extensive studies are requisite to validate these outcomes and establish the long-term efficacy and safety of Transperineal Prostate Laser Ablation (TPLA) as a viable treatment option for low to intermediate-risk prostate cancer.
Author Contributions
Conceptualization, G.M. and M.N.; methodology, A.Ma., T.P.; validation, G.M., F.G.; formal analysis, A.Ma, D.D.G.; investigation, F.R.F., B.C., P.E.G., A.Mi.; performing procedures T.P.; data curation, A.Ma., D.D.G.; writing—original draft preparation, P.E.G., B.C., F.R.F., A.Mi.; writing—review and editing, P.E.G., A.Mi., B.C., C.P.R.; supervision, G.M., M.N., F.G.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of University of Rome Tor Vergata (protocol code NCT04045756, date of approval: 12/17/2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020 GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [CrossRef]
- Ross HM, Kryvenko ON, Cowan JE et al (2012) Do adenocarcinomas of the prostate with gleason score (GS)_6 have the potential to metastasize to lymph nodes? Am J Surg Pathol 36:1346–1352. [CrossRef]
- Cooperberg MR, Cowan JE, Hilton JF et al (2011) Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol 29:228–234. [CrossRef]
- Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–37. [CrossRef]
- EAU Guidelines. Edn. presented at the EAU Annual Congress Milan 2021. ISBN 978-94-92671-13-4.
- Ahdoot M, Lebastchi AH, Turkbey B, Wood B, Pinto PA. Contemporary treatments in prostate cancer focal therapy. Curr Opin Oncol. 2019 May;31(3):200-206. [CrossRef]
- Manenti G, Perretta T, Calcagni A, et al. 3-T MRI and clinical validation of ultrasound-guided transperineal laser ablation of benign prostatic hyperplasia. Eur Radiol Exp. 2021;5(1):41. Published 2021 Sep 17. [CrossRef]
- Van Riel LAMJG, van Kollenburg RAA, Vis AN, et al. Safety and Feasibility of Soractelite Transperineal Focal Laser Ablation for Prostate Cancer and Short-term Quality of Life Analysis from a Multicenter Pilot Study. Eur Urol Open Sci. 2022;39:48-54. Published 2022 Apr 2. [CrossRef]
- Chai W, Zhao Q, Song H, Cheng C, Tian G, Jiang T. Treatment response and preliminary efficacy of hepatic tumour laser ablation under the guidance of percutaneous and endoscopic ultrasonography. World J Surg Oncol. 2019;17(1):133. Published 2019 Aug 5. [CrossRef]
- Eggener SE, Yousuf A, Watson S, Wang S, Oto A. Phase II evaluation of magnetic resonance imaging guided focal laser ablation of prostate cancer. J Urol 2016;196:1670–5. [CrossRef]
- Lindner U, Weersink RA, Haider MA, et al. Image guidedphotothermal focal therapy for localized prostate cancer: Phase I trial. J Urol 2009;182:1371–7. [CrossRef]
- Natarajan S, Raman S, Priester AM, et al. Focal laser ablation of prostate cancer: Phase I clinical trial. J Urol 2016;196:68–75. [CrossRef]
- Atluri S, Mouzannar A, Venkatramani V, Parekh DJ, Nahar B. Focal therapy for localized prostate cancer - Current status. Indian J Urol. 2022 Jan-Mar;38(1):7-14. [CrossRef]
- Lodeizen O, de Bruin M, Eggener S, et al. Ablation energies for focal treatment of prostate cancer. World J Urol. 2019;37(3):409-418. [CrossRef]
- da Silva RD, Kim FJ. Prostate Cancer - Local Treatment after Radiorecurrence: Salvage Cryoablation. Int Braz J Urol. 2018;44(3):435-439. [CrossRef]
- Cordeiro ER, Cathelineau X, Thuroff S, et al. High-intensity focused ultrasound (HIFU) for definitive treatment of prostate cancer. BJU Int 2012; 110:1228–1242. [CrossRef]
- Bakavicius A, Marra G, Macek P, et al. Available evidence on HIFU for focal treatment of prostate cancer: A systematic review. Int Braz J Urol. 2022;48(2):263-274. [CrossRef]
- Natarajan S, Jones TA, Priester AM, et al. Focal laser ablation of prostate cancer: Feasibility of magnetic resonance imaging-ultrasound fusion for guidance. J Urol 2017; 198:839–847. [CrossRef]
- Feller J, Greenwood B, Jones W, Toth R. Mp30-02 transrectally delivered, outpatient MRI-guided laser focal therapy of prostate cancer: Seven year interim results of NCT #02243033. The Journal of Urology 2018; 199:e374–e375. [CrossRef]
- C.M. Pacella; G. Patelli; G. Iapicca; G. Manenti; T. Perretta; C.P. Ryan; R. Esposito; G. Mauri. Transperineal laser ablation for percutaneous treatment of benign prostatic hyperplasia: A feasibility study. Results at 6 and 12 months from a retrospective multi-centric study. Prostate Cancer and Prostatic Diseases, 2020, 23, 356–363. [CrossRef]
- Patelli G, Ranieri A, Paganelli A, Mauri G, Pacella CM. Transperineal Laser Ablation for Percutaneous Treatment of Benign Prostatic Hyperplasia: A Feasibility Study. Cardiovasc Intervent Radiol. 2017 Sep;40(9):1440-1446. [CrossRef]
- Lepor H, Llukani E, Sperling D, Fütterer JJ. Complications, recovery, and early functional outcomes and oncologic control following in-bore focal laser ablation of prostate cancer. Eur Urol. 2015;68:924–6. [CrossRef]
- Hamdy FC, Donovan JL, Lane JA, et al.; ProtecT Study Group. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424. [CrossRef]
- Engeler DS, Schwab C, Thöni AF, et al. PSA bounce after ¹²⁵I-brachytherapy for prostate cancer as a favorable prognosticator. Strahlenther Onkol. 2015;191(10):787-791. [CrossRef]
- Di Trani MG, Nezzo M, Caporale AS, et al. Performance of Diffusion Kurtosis Imaging Versus Diffusion Tensor Imaging in Discriminating Between Benign Tissue, Low and High Gleason Grade Prostate Cancer. Acad Radiol. 2019;26(10):1328-1337. [CrossRef]
- Manenti G, Carlani M, Mancino S, et al. Diffusion tensor magnetic resonance imaging of prostate cancer. Invest Radiol. 2007;42(6):412-419. [CrossRef]
- Manenti G, Nezzo M, Chegai F, Vasili E, Bonanno E, Simonetti G. DWI of Prostate Cancer: Optimal b-Value in Clinical Practice. Prostate Cancer. 2014;2014:868269. [CrossRef]
- Ronald L. Wasserstein & Nicole A. Lazar (2016) The ASA Statement on p-Values: Context, Process, and Purpose, The American Statistician, 70:2, 129-133. [CrossRef]
- Karl Pearson F.R.S. (1900): X. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling, Philosophical Magazine Series 5, 50:302, 157-175. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).