1. Introduction
In the European Union, the Waste Framework Directive 2008/98/EC (Article 3) defines waste as “any substance or object which the holder discards or intends or is required to discard” it stipulates that the priorities of waste management must be prevention and recycling as alternatives to landfill disposal (1). The EU directives also set targets for the types and tonnages of collected, sorted and recycled waste and a timetable for achieving these targets. They are transposed into the legislation of the various EU Member States that organise waste treatment and disposal on their own territory.
In France, municipal waste is waste collected by or for local authorities and includes (i) waste from households, including bulky waste, (ii) waste produced by small businesses (or administrations) and collected at the same time as household waste (so-called "assimilated waste") and (iii) waste from municipalities (maintenance of green spaces, road cleaning, market waste). Local authorities also collect 55 kg/inhabitant/year of rubble, bringing the total to 580 kg/inhabitant/year. Of the waste managed by local authorities, approximately 80% comes from households and 20% is produced by companies or public bodies (2). In 2016, about 399 waste sorting Plants (WSP) treated 11.6 million tons of non-hazardous household or industrial waste over the year (2). The population of workers employed in WSPs was estimated at about 7000 persons in 2013, 5,500 of whom work on the sorting chain (3), involved in different tasks such as sorting waste manually, cleaning and maintaining facilities, driving handling or loading machines.
Sorting activities and process are known for their potency to emit dust as well as biological and chemical agents in the air of WSP. Indeed, the exposure of workers to bioaerosols including dust, bacteria and fungi, as well as microbial compounds and metabolites has been document in WSPs from numerous countries. The occupational exposure reported in WSPs has been associated to respiratory, gastrointestinal or dermatologic symptoms among workers handling of household waste (Poole and Basu, 2017; Walser et al., 2015). However, carried out epidemiological studies did not allow clearly linking the measured exposures to the observed symptoms, and Occupational Exposure Limit values (OELs) are still not available for airborne biological agents. Thus, the interpretation of measurement results in term of risk is still delicate.
Previous published studies brought significant knowledge regarding the exposure of workers in WPs. Thus, ambient concentrations and individual exposures to airborne dust, microorganisms (bacteria, fungi), microbial compounds (endotoxins, (1,3)-β-D-Glucans) and metabolites (mycotoxins) found in WSPs to improve diagnosis were documented (4-8). Knowledge about taxa composition of microbial communities in bioaerosols from WSPs were improved through studies using methods based on the identification of cultivated isolates (9-11) and based on high throughput sequencing (HTS) (12, 13). In addition, studies allowed investigating the size distribution of airborne microorganisms (SDAM) found in the air of WSPs and their deposition in lungs (14-16). Several studies also encourage completing the measurement strategy by health effect indicators (17-19). Indeed, gathering information on exposure levels, SDAM and taxa composition of microbial communities in bioaerosols from WSPs during a measurement campaign appears to be a helpful approach for improving the interpretation of measurement in term of risk. However, the deployment of such strategies remains relatively undocumented in the literature.
The aim of the study was to assess the extent to which the measurement of concentration, species composition and particle size distribution can contribute to a better assessment of the biological risks associated with exposure to fungi.
2. Materials and Methods
2.1. General Information about the Investigated Waste Sorting Plant (WSP)
The investigated Waste Sorting Plant (WSP) was an industrial plant located in France. It treated dry recyclable household waste (DRHW) as well as dry recyclable commercial and industrial waste (DRCIW), both collected in the surrounding municipalities. It employed about 50 workers mainly dedicated to manual sorting of waste. Sorted DRHW included paper (newspapers, magazines, journals, leaflets, envelopes, catalogues, etc.), cartons and cardboard boxes for packaging, plastic bottles and flasks, food bricks, steel and aluminium metal packaging (metals beverage can and tins). It accounted for approximately 4000 tons per month when measurements were made and came from selective collection of source-separated waste. The DRCIW consisted of papers, cardboards, plastics and non-dangerous waste coming from industries, artisans and shops; it accounted for approximately 1200 tons per month.
The WSP also treated glass packaging (jars, bottles and pots) that were only collected as a voluntary contribution in the glass columns set up in each municipality. The corresponding activity was performed outdoor and was not in the scope of the present paper.
2.2. Description of the WSP and the Sorting Process
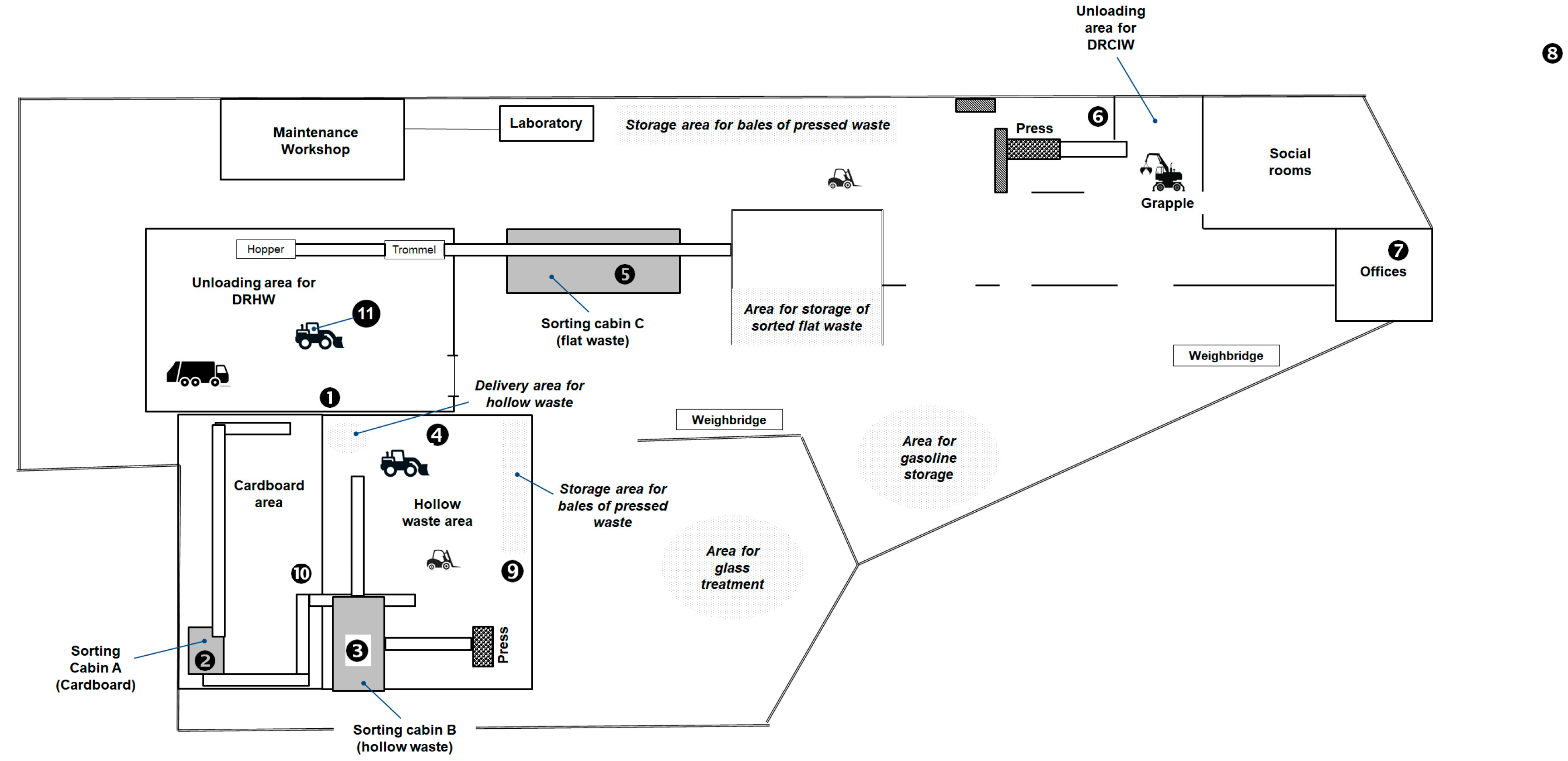
The investigated WSP was a totally indoor plant and consisted of a large hangar-like building in which the different sorting operations were performed. The organization of the different work areas is shown in
Figure 1 (a network of conveyor belts, that does not appear on the plan, connects the different production areas of the company) and the workflow for waste sorting is schematized in
Figure S1. DRHW and DRCIW were sorted in two different workflows.
DRCIW were unloaded from dump trucks in a dedicated area (
Figure 1). It comes from separate collections or from containers containing only one type of waste. It was checked, possibly manually sorted, loaded onto a conveyor belt using a grapple and then, baled using a bale press (
Figure S1). At the exit of the baler, a mechanical loader stored the bales in a dedicated storage area. The activity employed 2 sorting operators and 3 machine operators and drivers.
Mixed DRHW was brought to the sorting center by dump trucks. It was received either in plastic bags (door-to-door collection from residents) or in bulk (collection at voluntary drop-off points) and was deposited in a dedicated large area called the "unloading area for DRHW" (
Figure 1). A mechanical loader introduced the waste into a bag-opening hopper and the waste was then transported on a conveyor belt to a first trommel sieve (
Figure S1). The rotating sieve drum allows sorting the waste into three main fractions. The coarsest fraction corresponded to large pieces of cardboards and torn plastic bags. It was sent to sorting cabin A (two workstations), where the operators manually removed the pieces of cardboard as well as the torn plastic bags and then, the two types of waste were sent separately to a press to be baled. A first part of the intermediate fraction corresponded to the hollow waste which included cardboard boxes for packaging, steel and aluminium metal packaging (metals beverage can and tins), plastic bottles and flasks as well as food bricks (
Figure S1). It was discharged in a dedicated area receiving materials from cardboard sorting line and hollow wastes was from voluntary drop-off points (
Figure 1). A mechanical loader loaded the bulk hollow waste on a conveyor belt which conveys them to a packaging sorting process. The process comprised:
(i) a magnetic belt for the removal of metal;
(ii) an eddy current separator for the separation of non-ferrous metal packaging (aluminium, zinc, copper, etc.),
(iii) an optical sorting line which separates the plastic films and some plastic packaging from the rest of the waste stream and finally,
(iv) a manual sorting step of the different plastic containers which was carried out in the sorting cabin B (4 workstations). The sorted waste was baled using a press and stored in the same building. The other part of intermediate fraction was conveyed to the "flat waste" area. Flat waste corresponds to paper, magazines, newspapers and others (flattened paper and small cardboard packaging). The area housed the flat waste sorting line that handled waste from the cardboards line. The line included mechanical sorting as well as a large manual sorting cabin (Sorting cabin C, 12 workstations.). The sorted flat waste was stored in bulk in a dedicated area adjacent to the DRCIW area.
2.3. Measurement Strategy
Stationary and personal bioaerosol and airborne dust samples were collected in 2014, for two consecutive days in July (D1 and D2) and for two other ones in October (D3 and D4). The characterization of the aerosol was more complete in the sorting cabin A (cardboards), by adding several measurements of particle size distributions and real-time concentrations.
2.3.1. Stationary Measurements
Stationary measurements were carried out in different working areas of the company (
Figure 1) in order to assess the general contamination of the air, with both on-line and off-line methods. This was done in order to assess
(i) the ambient concentration levels of airborne culturable fungi and inhalable dust,
(ii) the real-time total number concentration of airborne particles,
(iii) the biodiversity of fungal communities in bioaerosols and,
(iv) the size distribution of airborne fungi. For that purpose, the sampling and measuring devices were placed at the height of the respiratory tract, at about 1.7 m from the floor. The stationary sampling plan was designed in order to make at least three measurements for each investigated area, spread over the 4 days of the campaign.
The description of the sampling points as well as the detail of the sampling plan are given in
Table S1. Thus, measurements were carried out in the unloading area for DRHW (sampling point ➊), in the main building of the cardboard area (sampling point ➓) and in the sorting cabin A (sampling point ➋), in the hollow waste area (main building: sampling point ➍; near the compactor: sampling point ➒) and in the sorting cabin B (sampling point ➌). Sampling devices were placed near the compactor (sampling point ➏) for investigating the DRCIW activities and in the sorting cabin C (sampling point ➎) for investigating the flat waste sorting ones. Measurements were also carried out in the cabins of the motorised vehicles operating in the different working areas (sampling point 11); the vehicles were the grapple of the DRCIW waste area and those circulating (loaders, pallet trucks, etc.) in the hollow waste area and in the unloading area for DRHW. For each day, stationary samples were also collected in two reference areas assumed to be not under influence of the waste sorting activities, that were located outside the company (sampling point ➑) and in an administrative office (sampling point ➐).
2.3.2. Personal Measurements
Personal measurements were carried out by placing the sampling heads of the devices directly on the workers, as close as possible to the breathing zone for the assessment of exposure to culturable bacteria and fungi, dust and endotoxins. The personal sampling plan was designed in order to make several measurements for the main working tasks, spread over the 4 days of the campaign. This was done in order to assess the exposure of worker to airborne endotoxins and culturable microorganisms as well as to inhalable dust during their work shift. The tasks considered were sorting operations, drivers, operation of compactors, and maintenance. The detail of the sampling plan for personal exposure is given in
Table S2.
2.4. Air Sampling Methods
An overview of the measurement process used during the campaign is provided in
Figure S2.
Samples for the measurement of culturable fungi and Eucaryota biodiversity in bioaerosols were taken with 37-mm CFCs mounted with a sterile polycarbonate filter (Whatman®, Nuclepore® polycarbonate membrane, 0.8 µm pore size) and a backing cellulose pad (Millipore®, thick cellulose absorbent pad) as previously described (12, 20). The duration of the culturable fungi sampling (minimum = 187 min; median = 364 min; maximum = 494 min) often corresponded to a large proportion of the work shift, but was sometimes adapted to assess specific shorter activities. For samples dedicated to the study of biodiversity, the durations of sampling were: minimum = 205 min; median = 350 min; maximum = 500 min.
Inhalable dust were sampled using the sampling device CIP 10-I (Tecora, Fontenay sous Bois, France) operating at 10 L/min. The durations of sampling for inhalable dust have also been adapted to follow specific tasks or the entire duration of the workstation: minimum = 192 min; median = 358 min; maximum = 495 min. The CIP 10-I was equipped with an omni-directional particle selector targeting the conventional inhalable fraction (21) and a plastic rotating cup containing a polyurethane foam filter [grade 60 pores per linear inch (22)] for aerosol collection. After sampling, the rotating cup was removed from the CIP 10-I and closed with its lid in order to be transported to the laboratory at room temperature
The size distribution of the workplace aerosol carrying airborne fungi was assessed using a Marple Cascade Impactor (MCI, model 298) as previously described (20). In order to maximise the recovery efficiency of microorganisms during the extraction step, no grease was applied to the Mylar collection media in the case of culturable analysis. The Marple impactor was connected to a sampling pump (Gilian®, GilAir-3 R, Sensidyne, USA) to achieve an overall flow rate of 2 L/min and the durations of sampling were between 259 and 409 min. At the end of each sampling day, the collection supports of the MCI dedicated to the enumeration of culturable microorganisms were carefully removed from the device, in aseptic conditions, and transferred to empty Greiner tubes (50 mL).
For sampling with the MCI and the CFC, the flow rate of the device (sampling head connected to a pump) was calibrated and measured before and after sampling using a soap film bubble flowmeter (Gilian, Gilibrator, USA – 2 L/min) or a mass flow meter (Mass Flow Meter 4140, TSI Inc., USA). For sampling with the CIP-I, the required rotation speed of the cup to achieve a 10 L/min flow rate was 6750 +/- 50 rotations per minute (rpm); it was controlled with an optical tachymeter (ST-6236B, Tecora, France) before and after each sampling day as described previously (23).
2.5. Real-Time Measurement of Airborne Particles Number Concentration and Size Distribution
The monitoring over time of the number concentration of airborne particles was carried out using the GRIMM® 1.109 optical particle counter (OPC GRIMM® 1.109, GRIMM Aerosol Technik GmbH, Germany). The ambient aerosol is captured through a dedicated omnidirectional annular slot, with a 1.2 L/min air flow rate, and each individual particle is exposed to an incident laser beam in an optical measuring cell ; the detection and the measurement of particles number concentration and particles sizes are based on the principle of light scattering providing the optical diameter (dopt) of particles (24, 25). The GRIMM® 1.109 allows the classification of the sampled particles into 31 measuring channels between 0.25 and 32 µm and particle counts were integrated over a period of 6 seconds during the measurements and results were expressed in number of particles per cubic meter of air (#/m3).
2.6. Sampling of Settled Dust
Settled dust was also collected in the cardboard sorting area for biodiversity analysis, using a sterile 50 mL tube.
2.7. Transport and Preservation of Samples
Samples dedicated to microbial analysis were transported to the laboratory, after each day of sampling, using a cold box and were then stored at 4°C until analysis. Samples dedicated to gravimetric analysis were transported to the laboratory after the two sampling days of July and October and stored at room temperature.
2.8. Measurement of Temperature and Relative Humidity
Temperature and relative humidity of the air were monitored using a portable device (Thermohygrometer B6285C Pocket, Fischer, France) at each sampling point.
2.9. Sample Analysis
Microbiological samples (culturable fungi and Eucaryota biodiversity) were analysed within 24 hours after being collected. All equipment and dilution water used in our experiments were sterile and DNA free when required. The analyses were performed in aseptic conditions, in a biological safety cabinet. Gravimetric samples were analysed a few days later, after staying in the weighing room. An overview of the samples process used for assessing the concentration levels and size distribution of airborne particles is provided in
Figure S2.
2.9.1. Cullturable Microorganisms
Culturable mesophilic fungi were enumerated in the CFCs and the MCI samples as described previously (20). For CFCs, a 10 mL volume of a sterile extraction solution was introduced into the cassette by the inlet. Tubes containing the Mylar collection media and the terminal filter from the MCI received a 10 mL volume of the same sterile extraction solution. The CFCs and the tubes were recapped and then shaken for 20 min at 2000 rpm (Multi-Reax®, Heildolph). The extracts were serially diluted 1/10th in a sterile tryptone salt solution and a 100 µL aliquot of each dilution was spread on two Petri dishes containing the Malt Extract Agar for mesophilic moulds. This was done for three successive dilutions and the inoculated Petri dishes were incubated at 25°C for 5 days. The number of grown colonies on the surface of the culture media was counted every day for five days to determine the microbial concentration in the extract and then in the sample. Results were expressed in Colony Forming Unit (CFU) per cubic meter of air (CFU/m3).
2.9.2. Analysis of Fungal Biodiversity in Samples
The genomic DNA from bioaerosol and dust samples was extracted using the with FastDNA® SPIN kit for soil kit (MP Biomedicals, Illkirch, France) according to the manufacturer’s instructions. After DNA concentration measurement by spectrophotometry (Nanodrop 2000c, Thermo Fischer Scientific, Illkirch, France), the samples were stored at -20°C until their sequencing.
The DNA sequencing was performed by INRA Transfert Environnement (Narbonne, France) as described previously (12). Briefly, the V1 variable region of Eukaryota 18S rDNA were sequencing using MiSeq technology (Illumina, San Diego, CA USA) and GS-FLX pyrosequencer (454 Life Sciences, Branford, CT, USA) respectively. The preprocessing of sequence analysis (trimming, denoising and removing of barcodes, primers, and homopolymers longer than 8 pb) was performed using a Mothur pipeline version 1.33.2 (26) developed by INRA Transfert Environnement. Reads with 100% of identity were clustered into a unique sequence. Then, sequences were clustered into operational taxonomic units (OTU) at a threshold of 97% sequence similarity. The dominant eukaryotic OTUs were identified at the genus rank at 95% sequence similarity using BLASTn algorithm in Genbank (NCBI database;
https://blast.ncbi.nlm.nih.gov).
2.9.3. Gravimetric Dust Analysis
The mass of the collected particles was determined in all the CIP 10-I samples. The substrates weighing was achieved using a 10.0 µg precision balance (AE163, Mettler-Toledo, Switzerland) for the CIP 10-I rotating cup containing the polyurethane foam. Prior to weighing, the substrates were dried in an oven at 50°C for at least 4 hours and were then left for at least one night in the weighing room. Electrostatic charges were neutralized just before the weighing (anti-static ionizing bars, Elcowa or Haug). Weight differences between final and initial weighing operations were corrected for weight variations observed in the field blanks (caused by different environmental conditions or handling of the substrates for example).
2.10. Data Analysis
The statistical analysis of data, including linear regression and ANOVA at the 95% confidence level, were done using the StatGraphics 5.1 software (Statistical Graphics Corp.,USA) and OriginPro® (OriginPro 2019b - 9.6.5.169, OriginLab® Corporation, USA).
3. Results
3.1. Sampling Conditions
The measurement campaign allow collecting 41 stationary (including 8 references) and 16 personal samples for culturable fungi and, 16 stationary and 8 personal samples for inhalable dust. For investigations of the biodiversity of microbial communities in bioaerosols, 21 stationary air samples were collected inside the WSP; 1 at the indoor reference and 2 at the outdoor reference, in summer and in fall. One settled dust sample has also been collected in the plant for biodiversity analysis. Four measurements were also carried out with the Marple cascade impactor.
The measurement campaign took place under normal operating conditions for the company. A few production stoppages were observed during the measurement days, but these did not exceed half an hour.
The monitoring of climatic conditions in July (D1 and D2) revealed temperature values of the air at the different working area were between 24.0 and 31.3°C inside the WSP (
Table S3). In October (D3 and D4), the temperature and relative humidity inside the WSP were between around 21 and 25°C and the relative humidity of air were between around 55 and 65%. The outdoor air temperature and relative humidity values are in good agreement with the measurements recorded by the nearest weather station (
Table S3).
3.2. Real Time Number Concentration of Airborne Particles
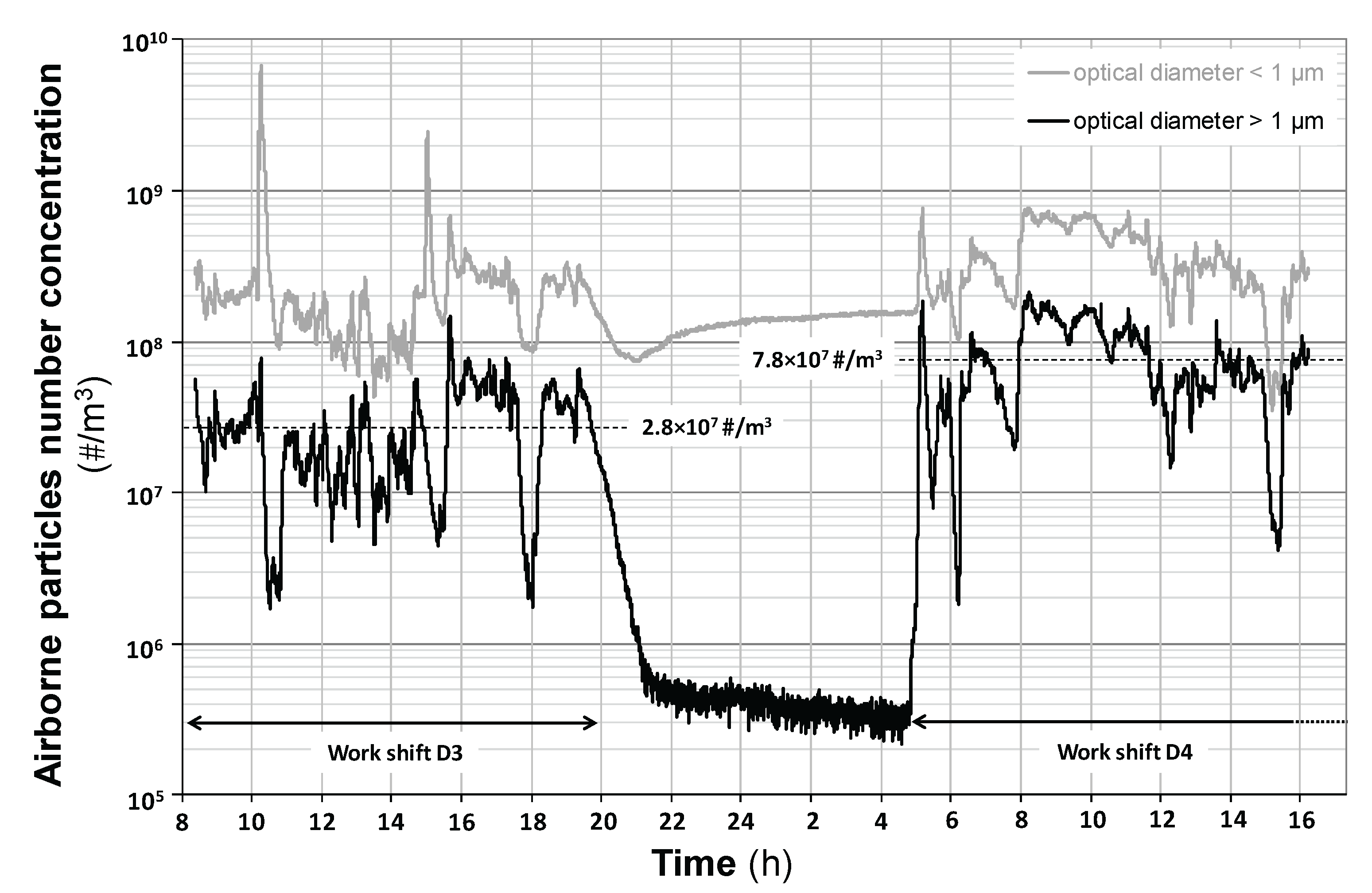
The real-time monitoring of airborne particles with the OPC has been done in the sorting cabin A for the four sampling days, close to the manual cardboard sorting activity. As an example,
Figure 2 shows the evolution of the particle number concentration over the entire duration of days D3 and D4 (including during the intermediate night).
For particles with optical diameters greater than 1.0 µm, the working periods were characterized by airborne particles concentrations between around 2×10
6 and 2×10
8 #/m
3. On D3, the average concentration between 8:15 a.m. and 7:30 p.m. was equal to 2.8×10
7 #/m
3 with a series of concentration peaks up to 5×10
7 #/m
3. The occurrence of concentrations peaks was related with the types and the amount of waste entering the cabin as well as with the work rate. The end of the work-shift on day D3 led to a drop of particles concentration. Between 10:00 p.m. on day D3 and 4:00 a.m. on day D4, working operations ceased and the average measured concentration dropped to approximately 4×10
5 #/m
3 (
i.e. 69 and 195 times lower than during the periods of activity on the days D3 and D4, respectively). D4 started with a high-level concentration peak monitored at the beginning of the work shift (about 5 a.m.). The average concentration between 5:00 a.m. and 4:15 p.m. was equal to 7.8×10
7 #/m
3 on day D4 with peaks of concentration which often reach higher levels than those monitored on day D3 (
Figure 2). For submicronic particles with optical diameters below 1.0 µm, the time profile is similar to that of micronic particles, but with number concentrations that are higher and between around 5×10
7 and more than 1×10
9 #/m
3 during the working periods of D3 and D4.
The measurements carried out on days D1 and D2 were not organized in such a way as to cover the night and the entire working days. They covered only limited periods of the two working days. However, the measurements indicate results similar to those observed for days D3 and D4 (data not shown).
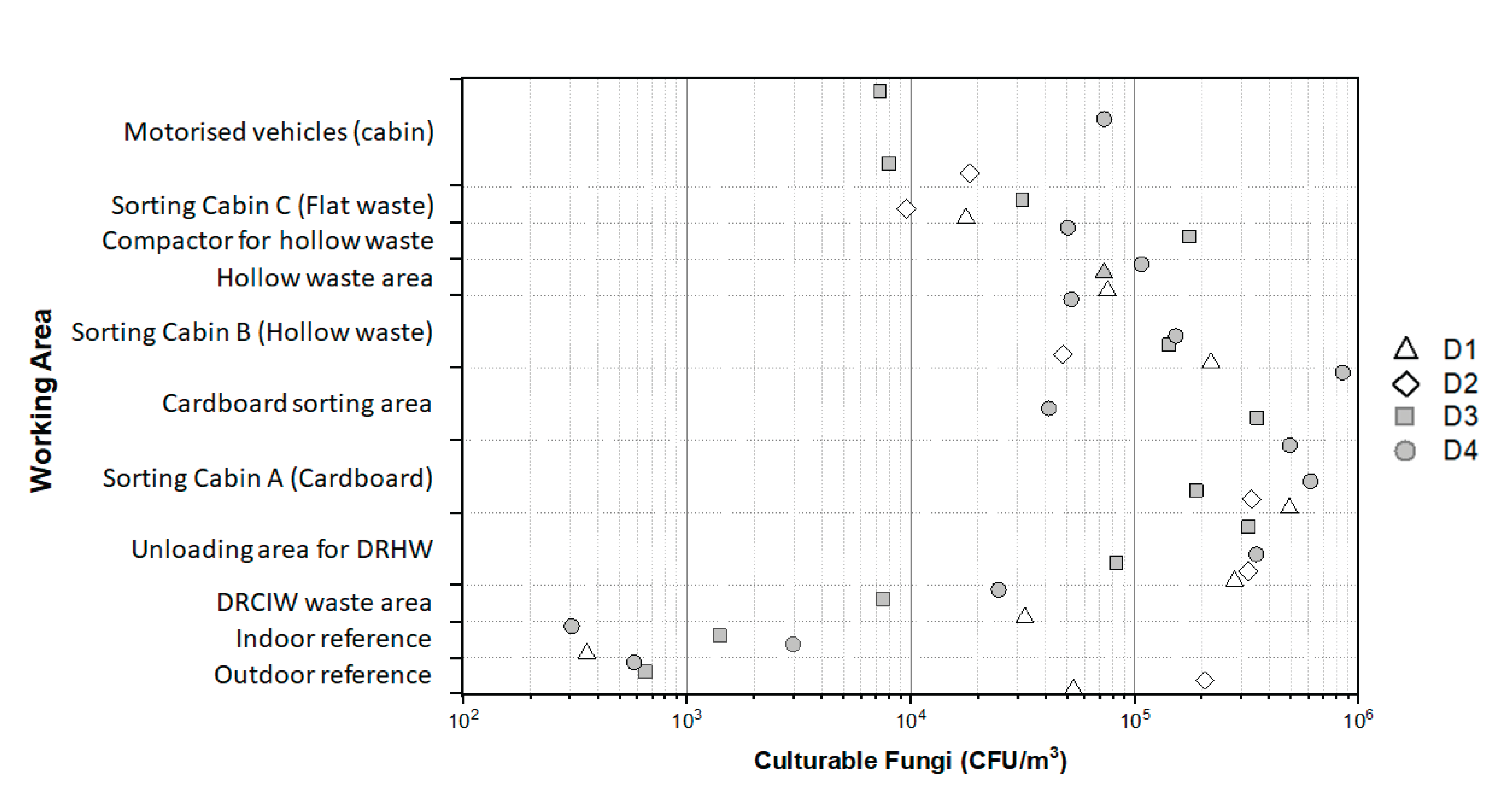
3.3. Exposure Levels to Airborne Fungi
In October, the ambient concentrations of airborne culturable moulds were measured under 1.0×10
3 CFU/m
3 in the outdoor air (
Figure 3). In contrast, those measured in July were 5.3×10
4 and 2.1×10
5 CFU/m
3 for the first and second day, respectively. In the meeting room, the measured concentrations ranged from 3.1×10
2 to 3.0×10
3 CFU/m
3. Measurements in the different working area of the WSP showed ambient concentrations of culturable moulds ranging from 7.3×10
3 to 8.5×10
5 CFU/m
3 with a median concentration of 8.3×10
4 CFU/m
3 (
Figure 3). The highest ambient concentrations were measured in the cardboard area (8.5×10
5 CFU/m
3) and all values obtained in DRHW unloading area and in the sorting cabin A (cardboards) were higher than 8.5×10
4 CFU/m
3. The ambient concentrations measured in the working area for a given day are from ~3 to ~1470 times higher than those measured at the outdoor reference point (or in the meeting room for July measurements) on the same day.
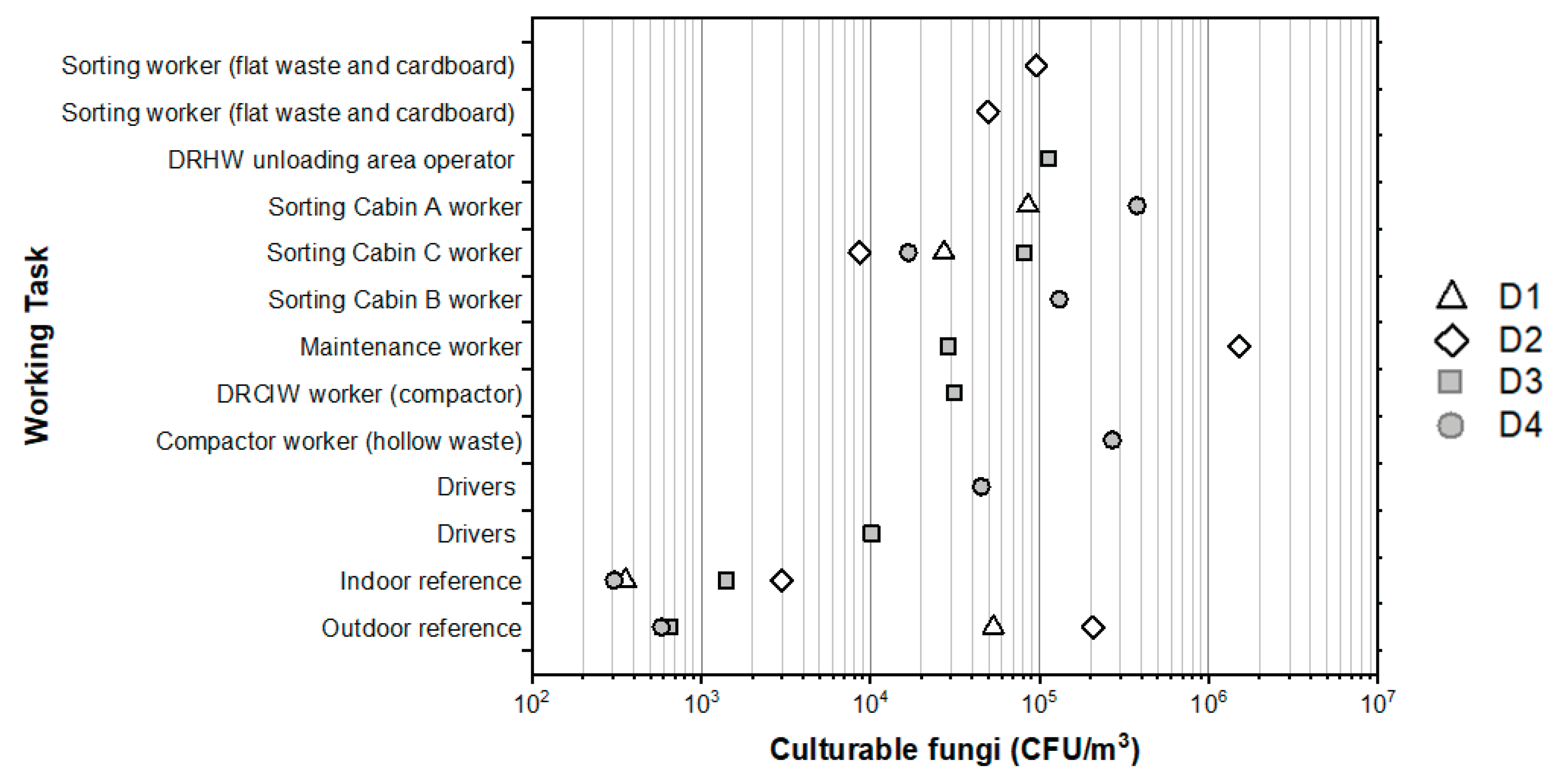
Personal exposures of workers to airborne culturable fungi ranged from 8.6×10
3 to 1.5×10
6 CFU/m
3 (
Figure 4). The highest personal exposures were measured for a maintenance operator cleaning in the cardboard sorting area (1.5×10
6 CFU/m
3). Exposures above 1.0×10
5 CFU/m
3 were also found for operators working at the hollow waste press, for sorting workers in the sorting Cabin B (hollow waste) and A (cardboards) as well as for operator in the DRHW unloading area. For a given day, the measured personal exposures were from ~3 to ~650 times times higher than those measured at the outdoor reference.
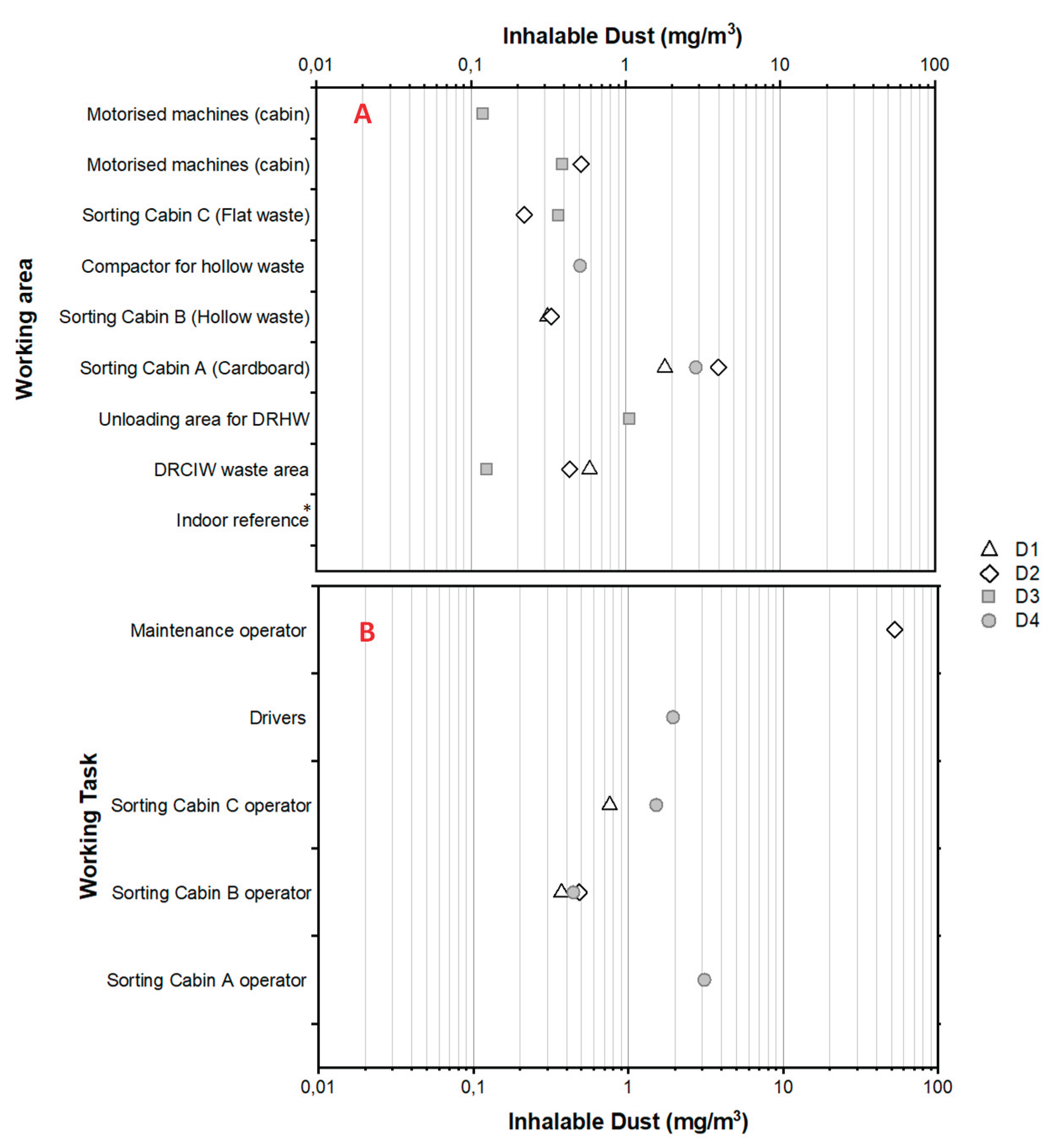
3.4. Exposure Levels to Inhalable Dust
The ambient concentrations of inhalable dust were found at a level less than or equal to 1 mg/m
3 in most the investigated working areas of the WSP, with the exception of the sorting cabin A in which they were found between 1.8 and 4.0 mg/m
3 (
Figure 5A). A measurement carried out at the indoor reference indicates below the Limit of Quantification of 0.1 mg/m
3 (data not shown). The personal exposure to airborne dust were between ~0.1 and 2.0 mg/m
3 for drivers as well as for workers involved in sorting hollow wastes (sorting cabin B) and flat wastes (sorting cabin C).
One personal exposure measurement carried out for a worker involved in cardboard sorting (sorting cabin A), revealed a concentration of 3.0 mg/m
3. Another one revealed an exposure level of to 52.5 mg/m
3 for a worker in charge of the maintenance/cleaning activity in the cardboard sorting area (
Figure 5B). The ambient concentration levels measured with a CIP 10-I inhalable sampler in sorting cabins A and C were in good agreement with those measured with MCI when taking into account the sum of the 9 collection stages (
Figure 5).
3.5. Biodiversity among Fungal Communities in the Emitted Bioaerosols
3.5.1. General Data and Alpha Biodiversity
All the 21 samples were successfully sequenced for the genetic target 18S rDNA . Reads number was from 858 to 23,952 (hollow waste sorting area) for eukaryota. Fungal OTUs number was lower in samples with a minimum at the outdoor reference (15 OTUs) and a maximum in the sorting cabin A (436 OTUs) for a median value of 59 (
Table 1). The Simpson index for fungal alpha biodiversity was between 0.0105 and 0.4 (median = 0.1) and the Shannon index was between 1.70 and 4.40 (median = 2.34). These values indicate that all fungal OTUs were not present in equal abundance, which means that some fungal genera were overrepresented as compared to other ones (
Table 1).
3.5.2. Biodiversity among Eukaryota Communities
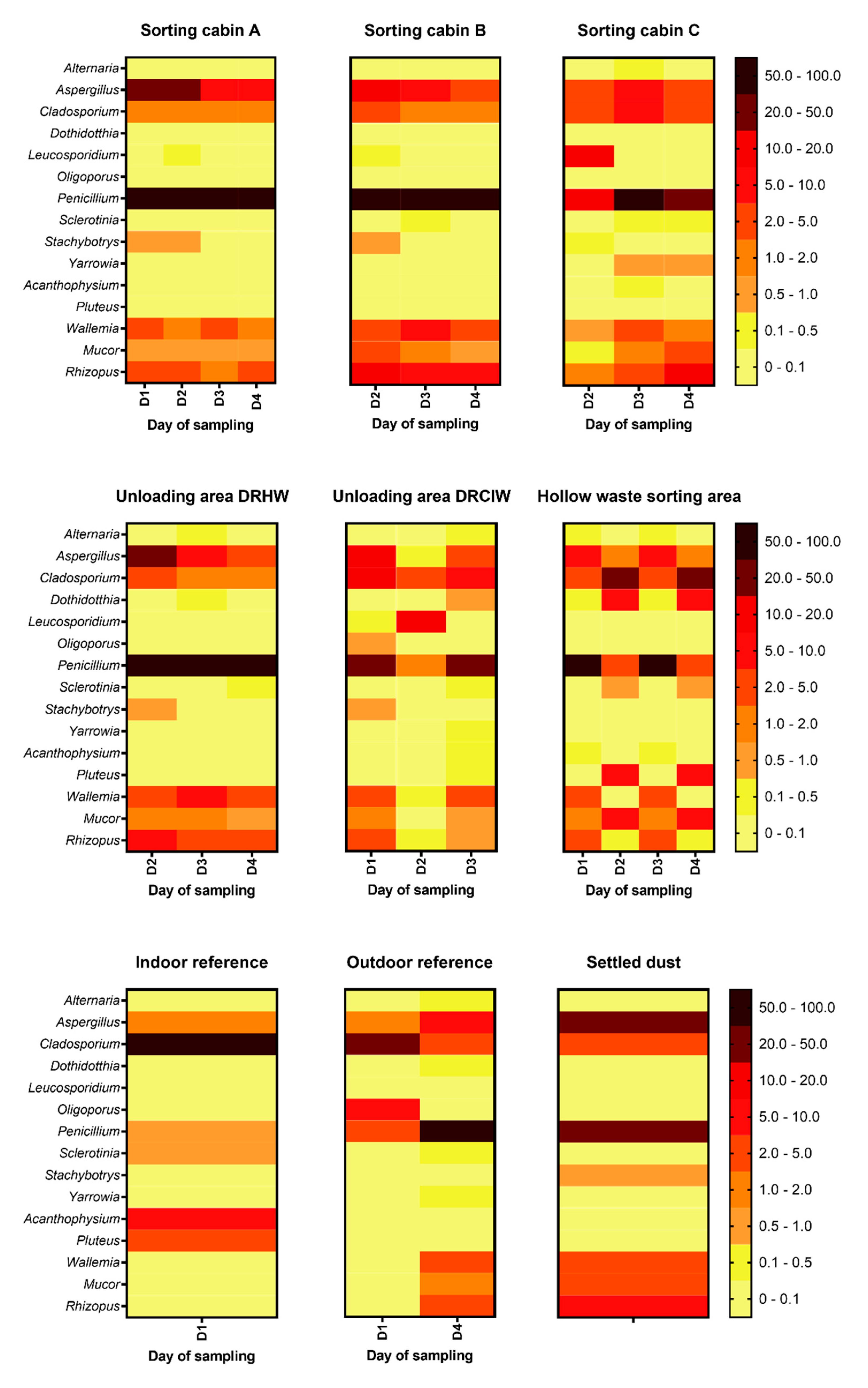
Most of the Eukaryota sequences were assigned to fungi. Ascomycota was the main fungal phylum with a median value of 85% of relative abundance (from 21% to 93%) followed by Basidiomycota with 5% (from 2% to 27%). The same trend was observed for both months. As for bacterial biodiversity, few fungal genera represented most of fungal sequences. Fungal core microbiome consisted of 15 genera representing 92% (18% to 96%) of the Eukaryota sequences in the WSP, 70% (58% to 91%) in the references and 92% in the settled dust sample (
Figure 6).
Penicillium was the predominant fungal genera in the WSP with a relative abundance higher than 20% in most of collected samples with a median value of 62% (10 to 80%). The
Aspergillus genus was also present in high relative abundance followed by
Cladosporium,
Wallemia,
Mucor, and
Rhizopus. Fungal biodiversity in settled dust mainly consisted of
Penicillium and
Aspergillus. At the indoor reference,
Cladosporium and
Acanthophysium were in majority whereas
Cladosporium and
Penicillium were the most present fungi at the outdoor reference.
Furthermore, the composition of the airborne fungal communities showed significant similarities from one day of sampling to another one in the sorting cabin A (cardboards), the sorting Cabin B (hollow waste), the sorting cabin C (Flat waste) and the unloading area for DRHW. On the contrary, it was more variable for the unloading area for DRCIW and the hollow waste sorting area (
Figure 6).
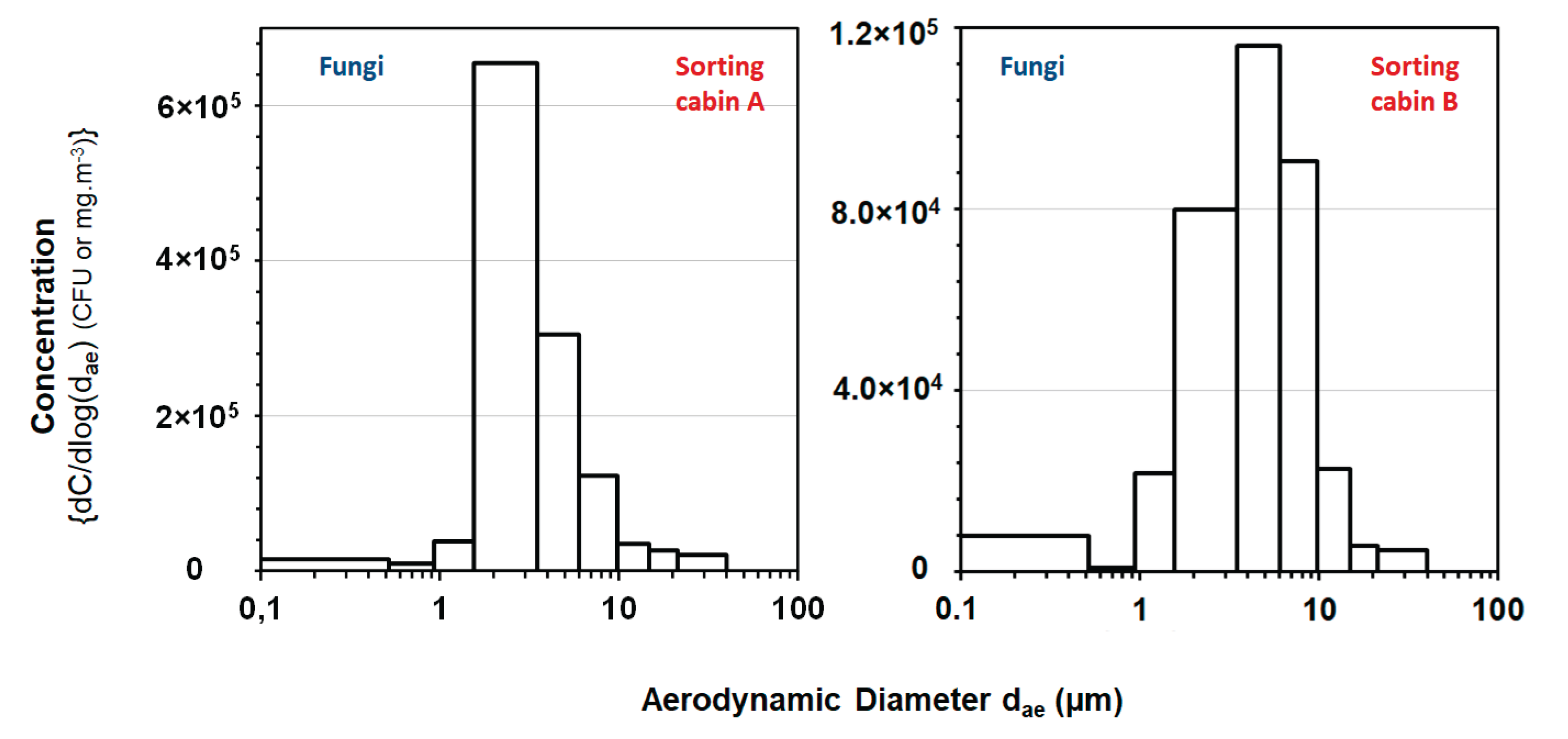
3.6. Size Distribution of Airborne Culturable Fungi
The size distribution of airborne culturable fungi and gravimetric dust were investigated with the MCI in sorting cabins in which cardboard (sorting cabin A) and hollow waste (sorting cabin B) were manually sorted. In the sorting cabin A, the distribution of culturable fungi according to the aerodynamic diameter indicates a monomodal population with a median aerodynamic diameter close to 3.0 μm and a geometric standard deviation of about 1.7 (
Figure 7). In the sorting cabin B, the size distribution of culturable fungi is also a monomodal population with larger particles. Indeed, the airborne fungal entities emitted in the sorting cabins A and B have aerodynamic diameters mainly between around 2.0 and 10.0 µm. Other measurements done in the sorting cabin A (cardboard) on days D1 and D2 and in the sorting cabin B (hollow waste) on day D4 revealed similar patterns regarding the size distribution of airborne culturable fungi and gravimetric dust (data not shown).
4. Discussion
4.1. Ambient Concentration Levels in the WSP
4.1.1. Level of Total Airborne Particles Measured with the OPC
The results from the OPC clearly show that working periods were responsible for the release of large amounts of particles in the air of the sorting cabin A (
Figure 2). The airborne particles number concentration was highly variable (between 5×10
7 and >10
9 #/m
3 for particles with d
opt > 0.25 µm, between 2×10
6 and 2×10
8 #/m
3 for d
opt > 1.0 µm). Such a variability and the additional occurrence of several peaks of concentration depend on the work rate, on the occasional stoppages in the processing chain or on the type and amount of waste handled in the cabin.
The above-mentioned levels are comparable with the number concentrations measured with similar or different OPCs in other companies belonging to the waste treatment sector. OPC GRIMM® 1.108 (dopt > 0.3 µm) number concentrations were measured between 2×107 and >108 #/m3 in composting enclosed facilities, with also the existence of concentration peaks related to activities such as wastes delivery and shredding (27, 28). OPC GRIMM® 1.108 (dopt > 0.3 µm) number concentrations remained greater than 107 #/m3 at 100 m upwind and 50 m downwind distances from a green waste composting open plant (29). A 4-channel handheld OPC (Met One Instruments, model 804) measured particle number concentration values (dopt > 0.3 µm) between ~3×107 and >8×107 #/m3 in the waste processing shed of a materials recycling facility (30). Number concentrations (particle size mainly between 0.3 and 5 µm) were measured near 1.3×106 #/m3 in a dry waste treatment plant in Finland using an Airborne Particle Counter APC-plus 1000 (31). In the same article, Tolvanen specified that he previously measured much higher number concentrations (107 to 108 #/m3) in other Finnish waste treatment plants.
The OPCs’ measurements are non-specific and do not provide any information on the nature, the chemical or biological composition, the shape or the density of the numerous detected particles. Therefore, the particles counted are not all microorganisms or biological components but are mixed in a complex way with other organic (or inorganic) dust present in the workplace. Moreover, because the different real-time instruments do not necessarily have the same diameter measurement ranges or specifications, it remains all the more difficult to compare studies with each other. However, the COP real-time measurements used in the present study demonstrated the existence of a significant (concentration ~70 to ~200 times higher than during the night; sometimes >109 #/m3 which corresponds to the upper range of number concentrations found in the literature) and variable particle emissions in the air of cabin A during the work shift and the progress of waste sorting activities. The release of such a large amounts of various particles was unsurprisingly associated with high inhalable gravimetric dust mass concentrations that ranged from 1.8 to 4.0 mg/m3 (section 3.4) In addition, the COP confirmed that the work periods were indeed at the origin of the emission of particles into the air in cabin A (day/night difference). The real time measurement also allows to identify the most emissive tasks or work events (concentration peaks observed). The information collected is essential for establishing appropriate prevention strategies.
4.1.2. Airborne Inhalable Gravimetric Dust
Our results confirm that WSPs can be dusty work environments. The sorting cabin A (cardboards) was the area where concentrations of inhalable gravimetric dust mass concentrations were the highest (up to 4 mg/m
3 Figure 5A). In the other areas, mass concentration were between >0.1 and 1 mg/m
3. These stationary sampling concentrations are close to some examples reported in previously published studies which reported ambient concentrations between LOD to 13.33 mg/m
3 in the waste treatment sector of different countries (4, 9, 18, 31-33).
As previously mentioned with the measurement results of the OPC, huge amounts of particles are emitted into the air during the working day from the treated waste and greatly contribute to the ambient dust pollution at the different workstations. Dust deposits, sometimes significant, were also observed during the sampling campaign on handrails, vehicles and on floors, especially in waste storage areas and under conveyor belts. Such settled dusts were also reported in previous studies (8, 33) and may be secondary sources of aerosol emission in the work area and may promote exposure through contact with hands or clothing.
4.1.3. Airborne Culturable Fungi
Measurements carried out in the different working area of the WSP revealed ambient concentrations ranging from 7.3×103 to 8.5×105 CFU/m3 for culturable moulds. The emission of culturable fungi into the ambient air of sorting centres has been reported in several previous studies. Indeed, the ambient concentration of culturable mesophilic fungi was measured between 4.5×102 and 2.2×106 CFU/m3 in the air of household waste treatment plants located in Germany, Finland, the Netherlands, Denmark, Portugal and Canada (4, 9, 31, 32, 34-39). In the waste processing shed of a materials recycling Brazilian facility, using a combined settle plate passive method and a 100 L/min MAS-100 active sampling, the mean fungal concentration values ranged from 1.6×103 to 4.7×103 CFU/m3, depending on the season (30). Thus, our findings corroborate results from previously published studies indicating the occurrence of fungi in the ambient air of WSPs, at concentration levels that vary over a wide range.
These results confirm that waste and waste sorting activities are sources of airborne fungi. Firstly, waste is a favourable environment for the growth and survival of micro-organisms. Indeed, the sorted dry recyclable household waste delivered at the WSP consists of many different elements and matters such as plastic, paper, cardboard, metal, glass, organic waste and residual, which depend on the national or local consumer habits as well as sorting regulation for household waste collection. It usually contain significant amounts of microorganisms ; for example, microbial concentration in waste was found over 107 CFU/g of matter in freshly collected waste in India (40). The survival and growth of microbial communities in waste is favoured by the materials that constitutes the waste but also by the residual organic matters that remains on the packaging when it is thrown in the bin. Previous studies carried out during waste collection (11, 41) and sorting (42) also suggested that they were influenced by season and prolonged duration of waste storage. Secondly, sorting activities (moving conveyor belts, workers' gestures, vehicle traffic etc.) are conducive to the emission of particles from contaminated waste or deposited dust.
Thus, dry household waste generally contains a significant amount of microorganisms that become airborne when the waste is handled. In the present study, significant concentrations of airborne dust and microorganisms were found in all the working areas, especially in the unloading area for DRHW, in the main building of the cardboard area and in the associated sorting cabin A.
4.2. Biodiversity of Airborne Fungal Communities
4.2.1. Overview of Fungal Biodiversity in the Air of the WSP
Biodiversity data form the present study revealed high number of OTUs in bioaerosol samples collected in the WSP, which suggest a high diversity even if only 15 taxa accounted for the majority. This is far higher than the richness observed in studies carried out in the same occupational environment using culture-based methods (9, 10, 14, 31, 43-45), for which usually less than 10 genera were detected, and in the range of the published ones carried out using molecular biology-based methods (12, 13), for which up to 430 genera were reported. Such differences in results from the different methods has already been reported and discussed and it is acknowledged that culture-based methods and molecular biology-based ones are complementary for the assessment of biodiversity in bioaerosols (46, 47).
4.2.2. Dominant Fungal Taxa and Possible Origins
The present study also revealed an overwhelming prevalence of the Penicillium fungal genera in both bioaerosols and settled dust samples taken in the WSP, followed by the Aspergillus, Cladosporium, Wallemia, Mucor, and Rhizopus genera. The genera Stachybotrys, Oligoprus and Leucosporidium were also detected in relevant proportions but for some sampling days only. These findings corroborate the biodiversity among microbial communities found in bioaerosols emitting in WSPs that were reported over the world. Indeed, Published studies carried out with the culture-based method in Finland (9, 31), Portugal (43, 44) , Denmark (10), Czech Republic (45), Poland (14), revealed that bioaerosols emitted in municipal solid waste treatment plants were dominated by cultivated fungal species belonging to the genus Penicillium, with other common genera including Aspergillus, Rhizopus, Cladosporium, Geotrichum, and Chrysonilia. In studies that have been able to identify isolates to species level, cultivated fungal species mentioned were Aspergillus niger (9, 43), A. fumigatus (10, 31, 43), A. flavus (43), Chrysonilia sitophila (31), Cladosporium cladosporioides (45) and a series of more or less well identified species belonging to the genus Penicillium including Penicillium nalgiovense (31, 45). In a study on household waste collection, Madsen et al., have found that the most common culturable fungi in the air of the delivery waste area of the WSP were Penicillium species belonging to P. brevicompactum, P. commune, P. expansum and P. italicum (11). Biodiversity of culturable fungi were also investigated on surfaces in a WSP facilities (43).
Fewer biodiversity studies were carried out in WSPs with the molecular-based methods. A study reported the occurrence of Stachybotrys chartarum and Aspergillus fumigatus using direct qPCR in bioaerosol samples taken from a WSP in Portugal (44). This was not the case in another WPS investigated with the same method in the same country (43). Two studies carried out in France reported the biodiversity of airborne fungal communities assessed by high-throughput sequencing. A first one based on 18S sequencing revealed 22 identified fungal genera belonging to Ascomycota, an early diverging fungal lineage and Basidiomycota in bioaerosol samples (12). A second one, based on ITS1 sequencing, reported 592 identified fungal genera in bioaerosol samples, with a fungal core microbiome composed of 5 genera, mainly belonging to Ascomycota, Basidiomycota, and Mucoromycota (13). In both studies, the dominant fungal genera were Cladosporium, Alternaria, Debaryomyces, Penicillum, Candida, Wallemia, Cryptococcus, Rhizopus and Mucor, which is in the line of the findings in the present study. The same taxa were also found by NGS among microbial communities sampled in the filtration systems of forklifts used in a WSP (48).
The composition of microbial communities in bioaerosols from WSPs may be determined by numerous factors that are not fully elucidated. These factors include the waste and its history, the WSP (geographical location, organisation, etc.), season and associated meteorological conditions, sorting activity (amount of treated waste, intensity of production, etc.), settled dust, possible external sources as well as methodological aspects. They can explain the differences observed between studies.
The taxa airborne microbiome in WPS is originated from waste and waste handling during the sorting process, the surrounding air, microorganisms generated from workers and other sources and activities. Interestingly, the dominant fungal genera in bioaerosols from the WSP reported in the present study are the ones previously reported in the air of WSPs in studies carried out for waste collection (49). This suggest that the main source of airborne fungal communities is waste and waste handling during the sorting process. Dominance of some fungal taxa can be explained by both nutrient and environmental conditions occurring in the waste and WSPs, which would be favourable for the growth and survival of fungal species belonging to the taxa concerned. The materials that constitutes the waste as well as the presence of food packaging among waste (trays, yoghurt pots and sauce jars, beverage bottles, etc.) on which food residue remains, would create favourable conditions for growth and survival of microorganisms. Residues coming from food stuffs with initially low water activity (aw) or with aw reduced by dehydration before sorting may be in favour of microorganisms able to grow at low aw. This is the case for species belonging to Penicillium and Cladosporium, which were shown to be able to grow at low aw and a large range of temperatures (50). Their growth and survival on paper and cardboard have also been shown (51). The case of Wallemia is interesting as the genera was found in studies carried out with molecular biology-based methods (12, 13) and not in those conducted with culture-based ones. Wallemia was also found by NGS among microbial communities sampled in the filtration systems of forklifts used in a WSP (48). Reasons for discrepancies between biology-based studies and culture-based ones were previously detailed (12, 13). They were mainly attributed to the slow growth rate of Wallemia species as well as their nutrient requirements their growth on culture media. The several species belonging to Wallemia are xerophilic (52) and are reported as spoiler microorganisms of food stuffs with low aw (53). Some were reported in bioaerosols and in settled dust from both occupational environments and dwellings (54-56).
The composition of microbial communities in settled dust samples reflect the one in bioaerosol samples which is not suppressing since dust deposited on the floor and on the machinery of the WSP from waste and waste processing. On the other hand, it can also be assumed that the resuspension of settled dust into the air would contribute partly to the airborne microbiome. However, the collection of additional dust samples is necessary to comfort the tendencies. We were not able to find published data regarding biodiversity among microbial communities in settled dust from WSPs. However, a study revealed that the composition of culturable fungal communities was very close for both bioaerosol and surface samples collected in a WSP in Portugal (43).
4.2.3. Spatio-Temporal Variation in Biodiversity
We’ve observed spatio-temporal variations of fungal biodiversity in bioaerosls from the WSP. Several previously published studies reported seasonal variations of microbial biodiversity in bioaerosols from outdoor air (56, 57), in swine houses (58, 59) and during sorting of households waste (13). However, no general tendencies can be deduced in regards with the role of environmental parameters in observed variations. Degois et al., (13) have investigated the airborne fungal communities during one year in a WSP located in France and showed that biodiversity was significantly affected by season of sampling irrespective to the working areas where samples were taken.
Indeed, the composition of microbial communities in bioaerosols from WSPs may be determined by numerous factors that are not fully elucidated. These factors include waste and its history (which may vary from a geographical area to another due to consumers habits), the WSP (location, organisation, etc.), season and associated meteorological conditions, geographical area, sorting activity (amount of treated waste, intensity of production, etc.), settled dust, possible external sources as well as methodological aspects. They can explain the discrepancies between the studies.
4.3. Size Distribution of Bioaerosols
The results from our study revealed two size distribution patterns for airborne fungi (median aerodynamic diameter = 3.0 µm).
The size distribution of particles carrying microbial entities in bioaerosols emitted in WSPs has received little attention over the past. Airborne fungal entities were associated to particles <5 µm in aerodynamic diameters in municipal solid waste treatment plants in Finland (9) and in the range of 3.3-7.0 µm in Poland (15, 60). Thus, results from published studies are contradictory regarding the size of particles carrying microorganisms and are not totally in the line of our findings. On the other hand, our results are consistent with those published in the waste sector, which indicate that the size distribution of airborne particles carrying bacteria and those carrying fungi is strongly different (61). The sources of the inconsistencies between the studies regarding the size distribution of particles carrying fungi are probably to be found in the multiple and varied factors that affect size distribution. These factors include the studied environment, the season and more generally climatic conditions, time of the day, type of activities, sampling methods etc. (61). It should also be noted that all the cited published studies carried out in WSP used the six-stage Andersen sampler for the investigation of SDAM.
Our findings confirm that the emitted bioaerosols are complex with different patterns for bacteria and fungi. The measured SDAM suggest that airborne microorganisms from bioaerosols sampled in the WSP, once inhaled, can be deposited in different region of the human respiratory tract (62, 63).
4.4. Personal Exposure Levels
4.4.1. Personal Exposure for Inhalable Dust
We were able to collect only few personal samples for the analysis of gravimetric dust but they indicate that workers of the WSP were exposed to inhalable dust mainly between 0.3 and 3 mg/m3. A specific case of excessive exposure level to dust was also highlighted by our personal measurements with a mass concentration of 52.5 mg/m3 during a maintenance and cleaning activity in the cardboard area, close to the sorting cabin A.
Such levels of personal exposure to inhalable dust for workers in WSPs corroborate previous findings. Indeed, the personal exposure to inhalable dust among WPS workers has been investigated for WSPs located in Denmark, Poland, Germany, England and Wales, Korea, the Netherlands, France and Canada (4, 6, 8, 10, 18, 35-37, 42, 64-66). They revealed personal exposure levels from 0.1 to 62.6 mg/m3 with a wide range of variation between studies.
The heterogeneity of mass concentrations measured between the previous published articles is not surprising and can be explained by the diversity of the situations encountered, in particular the nature of the handled waste, the aerosol samplers used or the quality of the ventilation system. For example, Schlosser et al. (42) demonstrated that a reduction in dust exposure by a factor of ~3 occurred when the investigated sorting room was fitted with a ventilation system that enabled operators to work directly under a unidirectional clean air flow as opposed to a less-effective ventilation system. Likewise, their results also showed that the age of the sorted waste (since collection) and the order in which waste is treated could lead to variability in the exposure to dust in sorting rooms.
Our results also confirmed the conclusions of some other previous articles on the fact that workers performing mobile, and possibly dusty, tasks away from the rooms (i.e. cleaning or maintenance) may be exposed to personal mass concentrations as abnormally high as 10 mg/m3, or even 50 mg/m3.
Some areas or personal exposures may present low dust levels but, at the same time, very high concentrations of cuturable microorganisms. This result highlights the fact that only using the regulatory limit for dust with no specific effects to assess the risk associated with bioaerosol emitted in WSP is not a suitable approach.
4.4.2. Personal Exposure for Culturable Fungi
The results indicate that personal exposures to airborne cultuable fungi for workers of the WSP varied in a very wide range. The highest exposure levels were found for workers involved maintenance and cleaning, but workers in the sorting cabins, at the hollow waste press, in the DRHW unloading area, as well as drivers, were also found to be exposed to airborne fungi. Indeed, personal exposure to airborne cultuable fungi is well documented and our results corroborate those from previously published studies. Especially in France, a recent study measured personal exposures between 910 and 2.7×106 CFU/m3 for moulds (42). In the same country, Duquenne and Facon reported personal exposures between 240 and 9.1 x 106 CFU/m3 for culturable fungi (8). Similar exposure levels were reported for fungi in Germany (4), in Denmark (10) and in (66). These published studies indicate that the most exposing tasks in WPSs are unloading, shredding and sorting of waste as well as maintenance operations.
4.5. Risks Associated with the Measured Exposures
4.5.1. Risks Associated to the Exposure to Airborne Dust
The decree 2021-1763 of 23 December 2021 amends article R 4222-10 of the French Labour Code, setting the concentrations of total and respirable dust not to be exceeded in specific pollution areas at 4 and 0.9 mg/m3 respectively (67). Indeed, most the personal inhalable dust mass concentrations measured in the investigated WSP were over one tenth of this proposed value. The maintenance/cleaning activity in the cardboard area was associated with a very high exposure level to dust, equal to 52.5 mg/m3, more than 10 times over the French value. Moreover, the dust produced during such a cleaning activity of the zone has undeniably degraded the air quality in the sorting cabin A (cardboards) at the same time.
4.5.2. Risks Associated to the Exposure to Airborne Fungi
Levels of exposure to airborne fungi reached high levels as compared to other working situations. However, the lack of any there is not OEL occupational exposure limit value (OELV) makes it difficult to interpret the results in terms of risk. Several guide values were proposed to assist the experts in judging exposure levels for moulds ; the corresponding values generally vary from country to country, are not available in all countries and not health bases values (68). For example; in Switzerland, the guide values that can be described as acceptable at workplaces are 103 CFU/m3 moulds (69). In Germany, a guide value of 5×104 CFU/m3 is proposed for moulds in the air of household waste sorting plants (70). In Canada, the proposed guide value for industrial environments is 104 moulds spores/m3 (71). There is no guide value for moulds yet proposed in France. Furthermore, a scientific synthesis of numerous works (including a large number of different sectors) concludes that the majority of effects related to mould exposure are observed from levels of approximately 105 spores/m3 (72).In the present study, about 62 % of measured personal exposures were over 104 CFU/m3 and about 31 % were over 105 CFU/m3. Thus, most of the personal exposures for bacteria during exceeded the proposed guide values, which indicate significant exposures for the concerned workers.
Data from the present study regarding the size distribution fugal airborne particles in the air of sorting cabins indicate that the majority of inhaled particles carrying moulds deposit in the respiratory tract and especially in the lower airways (
Figure 7).
The information provided by the analysis of biodiversity in fungal aerosol contribute to a better appreciation of risk. However, it should be remembered that the biodiversity measurements were made in an ambient environment and do not correspond to measurements of personal worker exposure. Moreover, the identification was only made at the genera level. Since the main pathogenic effects of fungi on health are often species-related, the scope of the interpretation is limited to qualitative speculation. Anyway, the composition of airborne microbiome shows a mixture of microbial genera that may be involved in symptoms among workers. Indeed, several dominant fungal genera identified in the air of the investigated WSP, as well as in settled dust, include species that are opportunistic pathogens of allergenic ones for humans or known as MVOC or mycotoxin producers. Thus, the exposure to several Penicillium species such as P. nalgiovense in the food industry (73) and P. glabrum in the cork one (74), was associated with respiratory symptoms. Species belonging to the Aspergillus genera such as A. fumigatus and several other several Aspergillus species are opportunistic fungal pathogens that cause allergic and invasive diseases (aspergillosis) especially among immunocompromised hosts (75). The exposure to airborne A. fumigatus has been associated to respiratory disease among compost workers (76). Species belonging to the Cladosporium genera such as Cladosporium herbarum are major source of inhaled allergens and often associated to allergic symptoms of the respiratory tract (77). W. sebi, W. mellicola and W. muriae are species belonging to the genus Wallemia that were reported in lungs diseases such as farmer’s lung disease, and also rare subcutaneous and cutaneous infections (78). Weber (79) reviewed the health effects induce by the occupational exposure to Mucor species and especially asthma among workers handling contaminated Esparto fibres, hypersensitivity pneumonitis in a cork worker and allergic alveolitis among teachers due to the inhalation of contaminated sugarcane dust. The exposure of Norwegian sawmills workers to high concentrations of airborne spores of Rhizopus microsporus has been associated to R. microsporus-specific antibody production against a widespread range of antigens (80). In addition, many fungal species that are found in the air of WSPs carry allergens or/and are mycotoxins producers and are associated with allergenic and toxinic diseases (77, 81, 82).
The lack of VELP makes it difficult to interpret the measured concentration levels of airborne microorganisms in terms of risk. Indeed, the present study indicates that the combined measurement of exposure levels, particle size distribution (and their further deposition in lungs) and species composition helps to understand the risks faced by exposed workers. Several published studies have also proposed the measurement of indicators of health effects such as total inflammatory potential and cytotoxicity in parallel to the measurement of exposure levels (17). In fact, such indicators could be integrated into measurement strategies of epidemiological studies with exposure levels as well as size distribution and biodiversity of bioaerosols in order to advance the assessment of risks associated with exposure to biological agents.
4.6. Prevention Means
The results from the present study provide the evidence that overall emission and dispersion of aerosols should be minimized during the sorting process of waste. When source control is not sufficient or possible for design reasons and exposure issues cannot be resolved by general ventilation, preventive measures must be deployed at least on certain worker populations or workstations.
Motorised vehicles are commonly used in the waste management industry for the transport, transfer and handling of waste. They include grapples, forklifts, wheel loaders etc., which may or may not be equipped with a ventilated cabin. In any case, the waste workers in charge of the corresponding tasks may spend most of their working time driving these vehicles. Results from the present study indicate significant ambient concentration levels in cabins of motorised vehicles and associated personal exposures for drivers regarding airborne culturable fungi and inhalable dust. This corroborates the previously published results in the same occupational environment (8, 33) and was also observed during waste collection (83) and waste composting (84). In the absence of ventilated cabins, drivers are exposed to the ambient aerosol and their level of exposure depends on the work areas into which they escape. When they are in a ventilated cabin, WSP drivers can also be exposed if the ventilation or air conditioning is defective or poorly maintained (85) and if it is used improperly (opening of doors etc.). These results indicate that it is necessary to equip motorised vehicles of WSP with ventilated and air-conditioned cabs and to recall good practices for the use of these cabs and in particular for the maintenance of ventilation and air-conditioning systems (8, 42).
In some work situations a respiratory protective device, such as filtering mask, may be recommended, e.g. during particularly exposing maintenance operations such as maintenance ones (86). Indeed, recent studies indicate that filter masks can be contaminated with fungi and are favourable to the development of toxigenic species (87, 88); they encourage further reflection as well staff training regarding the frequency of changing the respiratory protective masks in WSPs. The recently published findings that mechanical gloves and work clothes can be contaminated with fungi and mycotoxins when handling household waste (89) are a reminder of the need to apply strict hygiene guidelines in WSPs. Finally, particular attention should be paid to the maintenance of odour treatment systems, such as activated carbon filters, in order to prevent the growth of micro-organisms in the facilities and their release into the air in the WSPs and in the neighbourhood (90).
5. Conclusions
The investigated WSP is characterised by relatively high ambient concentrations of fungi and dust and the study shows that workers in charge of sorting activities can be exposed to high levels of fungi and inhalable dust. The tasks most prone to moulds are waste sorting in the first cabin, driving vehicles and maintenance operations. The workers exposure levels found in the investigated WSP were in the range of the previously published ones in WSP. The new findings dealing with size distribution and biodiversity of bioaerosols suggest that employees are exposed to complex bioaerosols during their work and helps to make a finer diagnosis of the risks involved, which is often difficult in the absence of any OEL for bioaerosols in general.
The results of the study encourage supplementing the assessment of individual exposure levels to biological agents with data concerning the species composition of bioaerosols and the size of particles carrying microorganisms that are inhaled. Measurement and markers of health effects would further refine the diagnosis of biological risk for WSP workers. This would provide helpful data for defining accurate strategies for the assessment bioaerosol exposure and for a better understanding of biological risks at the workplaces.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Schematic representation of the workflow for waste sorting in the WSP; Figure S2: Schematic representation of the off-line measurement process used for assessing the concentration levels of airborne culturable bacteria and fungi, endotoxins, inhalable dust as well as the biodiversity of microbial communities in bioaerosols and the size distribution of airborne microorganisms and dust in the WSP; Table S1: Details of the sampling plan designed for the stationary assessment of bioaerosols and airborne dust in the investigated WSP; Table S2: Details of the sampling plan designed for the assessment of personal exposure to bioaerosols and airborne dust in the investigated WSP; Table S3: Data of temperature and relative humidity of air at the sampling points in the investigated WSP.
Author Contributions
Conceptualization, P. Duquenne, X. Simon and B. Facon; Samples collection and analysis as well as real-time measurements, all the authors; Data analysis and representation, P. Duquenne, X. Simon and J. Degois; writing—original draft preparation, P. Duquenne, X. Simon and J. Degois, writing—review and editing, P. Duquenne, X. Simon and J. Degois. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Dominique Rousselle and Olivier Deloras, from the INRS, for their valuable technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- EU, EU. 2008. Directive 2008/98/EC of the European Parliament and of the Council of on waste and repealing certain Directives (http://data.europa.eu/eli/dir/2008/98/oj). 19 November.
- ADEME (Agence De l'Environnement et de la Maîtrise de l'Energie). 2020. Déchets : chiffres-clés. ADEME (Agence De l'Environnement et de la Maîtrise de l'Energie), Anger, France.
- Cabaret, M.; Folley, S. État des lieux du parc des centres de tri de recyclables secs ménagers en France. TERRA, ADEME, 2013.
- Hebisch, R.; Linsel, G. Workers' exposure to hazardous substances and biological agents in recycling enterprises. Gefahrstoffe - Reinhaltung der Luft 2012, 72, 163–169. [Google Scholar]
- Schlosser, O.; Robert, S.; Noyon, N. Airborne mycotoxins in waste recycling and recovery facilities: Occupational exposure and health risk assessment. Waste Management 2020, 105, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Kozajda, A.; Jeżak, K.; Cyprowski, M.; Szadkowska-Stańczyk, I. Inhalable dust, endotoxins and (1-3)-β-d-glucans as indicators of exposure in waste sorting plant environment. Aerobiologia 2017, 33, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Cyprowski, M.; Ławniczek-Wałczyk, A.; Górny, R.L. Occupational exposure to anaerobic bacteria in a waste sorting plant. Journal of the Air & Waste Management Association 2021.
- Duquenne, P.; Facon, B. 2018. Exposition aux bioaérosols dans les centres de tri des déchets ménagers recyclables. Hygiène et Sécurité du Travail HST n°252 - Juillet / Août / Septembre 2018 44-50.
- Lehtinen, J.; Tolvanen, O.; Nivukoski, U.; Veijanen, A.; Hänninen, K. Occupational hygiene in terms of volatile organic compounds (VOCs) and bioaerosols at two solid waste management plants in Finland. Waste Management 2013, 33, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Breum, N.O.; Würtz, H.; Midtgaard, U.; Ebbehøj, N. Dustiness and bio-aerosol exposure in sorting recyclable paper. Waste management and research 1999, 17, 100–108. [Google Scholar] [CrossRef]
- Madsen, A.M.; Frederiksen, M.W.; Mahmoud Kurdi, I.; Sommer, S.; Flensmark, E.; Tendal, K. Expanded cardboard waste sorting and occupational exposure to microbial species. Waste Management 2019, 87, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Degois, J.; Clerc, F.; Simon, X.; Bontemps, C.; Leblond, P.; Duquenne, P. First metagenomic survey of the microbial diversity in bioaerosols emitted in waste sorting plants. Annals of Work Exposures and Health 2017, 61, 1076–1086. [Google Scholar] [CrossRef]
- Degois, J.; Simon, X.; Clerc, F.; Bontemps, C.; Leblond, P.; Duquenne, P. One-year follow-up of microbial diversity in bioaerosols emitted in a waste sorting plant in France. Waste Management 2021, 120, 257–268. [Google Scholar] [CrossRef]
- Bragoszewska, E. The dose of fungal aerosol inhaled by workers in a waste-sorting plant in Poland: A case study. International Journal of Environmental Research and Public Health 2020, 17, 10. [Google Scholar] [CrossRef]
- Bulski, K.; Frączek, K.; Chmiel, M. Microbiological air quality at municipal waste sorting plant. Ochrona Srodowiska i Zasobów Naturalnych 2016, 27.
- Madsen, A.M.; Beswick, A.; Oppliger, A.; Kolk, A.; Crook, B.; Tendal, K.; Hinker, M.; Cyprowsky, M.; Raulf, M.; Duquenne, P.; Graff, P.; Laitinen, S. Occupational exposure to microorganisms as related to new waste sorting instructions - a PEROSH project, p. In.
- Madsen, A.M.; Frederiksen, M.W.; Jacobsen, M.H.; Tendal, K. Towards a risk evaluation of workers’ exposure to handborne and airborne microbial species as exemplified with waste collection workers. Environmental Research 2020, 183, 109177. [Google Scholar] [CrossRef]
- Cyprowski, M.; Stobnicka-Kupiec, A.; Górny, R.L.; Gołofit-Szymczak, M.; Ptak-Chmielewska, A.; Ławniczek-Wałczyk, A. Across-shift changes in upper airways after exposure to bacterial cell wall components. Annals of agricultural and environmental medicine 2019, 26, 236–241. [Google Scholar] [CrossRef]
- Viegas, S.; Osteresch, B.; Almeida, A.; Cramer, B.; Humpf, H.-U.; Viegas, C. Enniatin B and ochratoxin A in the blood serum of workers from the waste management setting. Mycotoxin Research 2018, 34, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Simon, X.; Duquenne, P. Assessment of workers’ exposure to bioaerosols in a french cheese factory. annals of occupational hygiene 2014, 58, 677–692. [Google Scholar] [PubMed]
- Görner, P.; Wrobel, R.; Simon, X. High efficiency CIP 10-I personal inhalable aerosol sampler. Journal of Physics: Conference Series 2009, 151, 012061. [Google Scholar] [CrossRef]
- AFNOR. 2012. Air quality — Workplace air — Solid aerosol sampling with a rotating dish (respirable, thoracic and inhalable fractions). Norme NF X 43-262.
- Simon, X.; Bau, S.; Boivin, A.; Duquenne, P.; Witschger, O.; Görner, P. Physical performances and kinetics of evaporation of the CIP 10-M personal sampler's rotating cup containing aqueous or viscous collection fluid. Aerosol Science and Technology 2016, 50, 507–520. [Google Scholar] [CrossRef]
- Burkart, J.; Steiner, G.; Reischl, G.; Moshammer, H.; Neuberger, M.; Hitzenberger, R. Characterizing the performance of two optical particle counters (Grimm OPC1. 108 and OPC1.109) under urban aerosol conditions. Journal of Aerosol Science 2010, 41, 953–962. [Google Scholar] [PubMed]
- Görner, P.; Simon, X.; Bémer, D.; Lidén, G. Workplace aerosol mass concentration measurement using optical particle counters. Journal of Environmental Monitoring 2012, 14, 420–428. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; Sahl, J.W.; Stres, B.; Thallinger, G.G.; Van Horn, D.J.; Weber, C.F. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Duquenne, P.; Simon, X.; Koehler, V.; Goncalves-Machado, S.; Greff, G.; Nicot, T.; Poirot, P. Documentation of bioaerosol concentrations in an indoor composting facility in France. Journal of Environmental Monitoring 2012, 14, 409–419. [Google Scholar] [CrossRef]
- Byeon, J.H.; Park, C.W.; Yoon, K.Y.; Park, J.H.; Hwang, J. Size distributions of total airborne particles and bioaerosols in a municipal composting facility. Bioresource Technology 2008, 99, 5150–5154. [Google Scholar] [CrossRef]
- Galès, A.; Bru-Adan, V.; Godon, J.-J.; Delabre, K.; Catala, P.; Ponthieux, A.; Chevallier, M.; Birot, E.; Steyer, J.-P.; Wéry, N. Predominance of single bacterial cells in composting bioaerosols. Atmospheric Environment 2015, 107, 225–232. [Google Scholar] [CrossRef]
- Wikuats, C.F.H.; Duarte, E.H.; Prates, K.V.M.C.; Janiaski, L.L.L.; de Oliveira Gabriel, B.; da Cunha Molina, A.; Martins, L.D. Assessment of airborne particles and bioaerosols concentrations in a waste recycling environment in Brazil. Scientific reports 2020, 10, 14812–14812. [Google Scholar] [CrossRef]
- Tolvanen, O.K. Airborne bio-aerosols and noise in a dry waste treatment plant in Pietarsaari, Finland. Waste management and research 2001, 19, 108–114. [Google Scholar] [CrossRef]
- Tolvanen, O.K. Exposure to bioaerosols and noise at a Finnish dry waste treatment plant. Waste Management & Research 2004, 22, 346–357. [Google Scholar]
- Karamkhani, M.; Asilian-Mahabadi, H.; Daraei, B.; Seidkhani-Nahal, A.; Noori-Zadeh, A. Liver and kidney serum profile abnormalities in workers exposed to aflatoxin B1 in urban solid waste management centers. Environmental Monitoring and Assessment 2020, 192, 472. [Google Scholar] [CrossRef]
- Marchand, G.; Lavoie, J. ; LazureL. Evaluation of bioaerosols in a municipal solid waste recycling and composting plant. Journal of the Air & Waste Management Association 1995, 45, 778–781. [Google Scholar]
- Van Tongeren, M.; Van Amelsvoort, L.; Heederik, D. Exposure to organic dusts, endotoxins, and microorganisms in the municipal waste industry. International Journal of Occupational and Environmental Health 1997,3:30-36.
- Sigsgaard, T.; Malmros, P.; Nersting, L.; Petersen, C. Respiratory disorders and atopy in Danish refuse workers. American Journal of Respiratory and Critical Care Medicine 1994, 149, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.; Guertin, S. Evaluation of health and safety risks in municipal solid waste recycling plants. Journal of the Air & Waste Management Association 2001, 51, 352–360. [Google Scholar]
- Rahkonen, P.; Ettala, M.; Laukkanen, M.; Salkinoja-Salonen, M. Airborne microbes and endotoxins in the work Environment of two sanitary landfills in Finland. Aerosol Science and Technology 1990, 13, 505–513. [Google Scholar] [CrossRef]
- Nadal, M.; Inza, I.; Schuhmacher, M.; Figueras, M.J.; Domingo, J.L. Health risks of the occupational exposure to microbiological and chemical pollutants in a municipal waste organic fraction treatment plant. International Journal of Hygiene and Environmental Health 2009, 212, 661–669. [Google Scholar] [CrossRef]
- Atalia, K.R.; Buha, D.M.; Joshi, J.J.; Shah, N.K. Microbial biodiversity of municipal solid waste of Ahmedabad. Journal of Materials and Environmental Science 2015, 6, 1914–1923. [Google Scholar]
- Gladding, T.L.; Gwyther, C.L. A study of the potential release of bioaerosols from containers as a result of reduced frequency residual waste collections. Science of The Total Environment 2017, 576, 481–489. [Google Scholar] [CrossRef]
- Schlosser, O.; Déportes, I.Z.; Facon, B.; Fromont, E. Extension of the sorting instructions for household plastic packaging and changes in exposure to bioaerosols at materials recovery facilities. Waste Management 2015, 46, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Gomes, A.Q.; Abegão, J.; Sabino, R.; Graça, T.; Viegas, S. Assessment of fungal contamination in waste sorting and incineration - Case study in portugal. Journal of Toxicology and Environmental Health, Part A 2014, 77, 57–68. [Google Scholar] [CrossRef]
- Malta-Vacas, J.; Viegas, S.; Sabino, R.; Viegas, C. Fungal and microbial volatile organic compounds exposure assessment in a waste sorting plant. Journal of Toxicology and Environmental Health, Part A 2012, 75, 1410–1417. [Google Scholar] [CrossRef]
- Černá, K.; Wittlingerová, Z.; Zimová, M.; Janovský, Z. Exposure to airborne fungi during sorting of recyclable plastics in waste treatment facilities. Med Pr 2017, 68, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Duquenne, P. On the identification of culturable microorganisms for the assessment of biodiversity in bioaerosols. Annals of Work Exposures and Health 2018, 62, 139–146. [Google Scholar] [CrossRef]
- Mbareche, H.; Brisebois, E.; Veillette, M.; Duchaine, C. Bioaerosol sampling and detection methods based on molecular approaches: No pain no gain. Science of the Total Environment 2017, 599, 2095–2104. [Google Scholar] [CrossRef]
- Viegas, C.; Caetano, L.A.; Cox, J.; Korkalainen, M.; Haines, S.R.; Dannemiller, K.C.; Viegas, S.; Reponen, T. The effects of waste sorting in environmental microbiome, THP-1 cell viability and inflammatory responses. Environmental Research 2020, 185, 109450. [Google Scholar] [CrossRef]
- Madsen, A.M.; Raulf, M.; Duquenne, P.; Graff, P.; Cyprowski, M.; Beswick, A.; Laitinen, S.; Rasmussen, P.U.; Hinker, M.; Kolk, A.; Górny, R.L.; Oppliger, A.; Crook, B. Review of biological risks associated with the collection of municipal wastes. Science of The Total Environment 2021. [CrossRef]
- Gunde-Cimerman, N.; Sonjak, S.; Zalar, P.; Frisvad, J.C.; Diderichsen, B.; Plemenitaš, A. Extremophilic fungi in arctic ice: a relationship between adaptation to low temperature and water activity. Physics and Chemistry of the Earth, Parts A/B/C 2003, 28, 1273–1278. [Google Scholar] [CrossRef]
- Das, M.K.L.; Prasad, J.S.; Ahmad, S.K. Endoglucanase production by paper-degrading mycoflora. Letters in Applied Microbiology 1997, 25, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Zalar, P.; Sybren de Hoog, G.; Schroers, H.-J.; Frank, J.M.; Gunde-Cimerman, N. Taxonomy and phylogeny of the xerophilic genus Wallemia (Wallemiomycetes and Wallemiales, cl. et ord. nov.). Antonie van Leeuwenhoek 2005, 87, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Zajc, J.; Gunde-Cimerman, N. The genus Wallemia - From contamination of food to health threat. Microorganisms 2018, 6:46.
- Li, L.; Qiu, Y.; Gustafsson, Å.; Krais, A.M.; Weiss, J.M.; Lundh, T.; Bergman, Å. Characterization of residential household dust from Shanghai by particle size and analysis of organophosphorus flame retardants and metals. Environmental Sciences Europe 2019, 31, 94. [Google Scholar] [CrossRef]
- Zeng, Q.; Westermark, S.; Rasmuson-Lestander, A.; Wang, X. Detection and quantification of Wallemia sebi in aerosols by real-time PCR, conventional PCR, and cultivation. Applied and environmental microbiology 2004, 70, 7295–7302. [Google Scholar] [CrossRef]
- Fröhlich-Nowoisky, J.; Pickersgill, D.A.; Després, V.R.; Pöschl, U. High diversity of fungi in air particulate matter. Proceedings of the National Academy of Sciences 2009, 106, 12814–12819. [Google Scholar] [CrossRef] [PubMed]
- Bowers, R.M.; McCubbin, I.B.; Hallar, A.G.; Fierer, N. Seasonal variability in airborne bacterial communities at a high-elevation site. Atmospheric Environment 2012, 50, 41–49. [Google Scholar] [CrossRef]
- Kumari, P.; Woo, C.; Yamamoto, N.; Choi, H.-L. Variations in abundance, diversity and community composition of airborne fungi in swine houses across seasons. Scientific Reports 2016, 6, 37929. [Google Scholar] [CrossRef] [PubMed]
- Nehme, B.; Gilbert, Y.; Letourneau, V.; Forster, R.J.; Veillette, M.; Villemur, R.; Duchaine, C. Culture-independent characterization of Archaeal biodiversity in swine confinement building bioaerosols. Applied and Environmental Microbiology 2009, 75, 5445–5450. [Google Scholar] [CrossRef]
- Brągoszewska, E. Exposure to Bacterial and Fungal Aerosols: Microorganism Indices in A Waste-Sorting Plant in Poland. International Journal of Environmental Research and Public Health 2019, 16, 3308. [Google Scholar] [CrossRef]
- Clauß, M. Particle size distribution of airborne microorganisms in the environment – a review. Landbauforschung Applied Agricultural and Forestry Research 2015, 65, 77–100. [Google Scholar]
- Sturm, R. Modeling the deposition of bioaerosols with variable size and shape in the human respiratory tract - A review. Journal of Advanced Research. 2012. [CrossRef]
- Hofmann, W. Modelling inhaled particle deposition in the human lung—A review. Journal of Aerosol Science 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Krajewski, J.A.; Tarkowski, S.; Cyprowski, M.; Szarapińska-Kwaszewska, J.; Dudkiewicz, B. Occupational exposure to organic dust associated with municipal waste collection and management. Int J Occup Med Environ Health 2002, 15, 289–301. [Google Scholar] [PubMed]
- Gladding, T.; Thorn, J.; Stott, D. Organic dust exposure and work-related effects among recycling workers. American Journal of Industrial Medicine 2003, 43, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-U.; Ryu, S.-H.; Kim, S.-B.; Yoon, C.-S. An assessment of dust, endotoxin, and microorganism exposure during waste collection and sorting. Journal of the Air & Waste Management Association 2011, 61, 461–468. [Google Scholar]
- Guillou, P.; Brunet, D.; Rousselle, C.; Binet, S. Recommandation de l’Anses en vue de la révision des valeurs limites d’exposition professionnelle (VLEP) pour les poussières dites sans effet spécifique (PSES). Archives des Maladies Professionnelles et de l'Environnement 2020, 81, 676–677. [Google Scholar] [CrossRef]
- Mandal, J.; Brandl, H. Bioaerosols in indoor environment - A review with special reference to residential and occupational locations. The Open Environmental & Biological Monitoring Journal 2011, 4, 83–96. [Google Scholar]
- SUVA (Schweizerische Unfallversicherungsanstalt). 2015. Valeurs limites d’exposition aux postes de travail 2015 - Edition 2015 - ( Référence 1903.f ; http://www.suva.ch.). SUVA, Luzern.
- Arbeit und Soziales im Gemeinsamen Ministerialblatt. TRBA (Technische Regel für Biologische Arbeitsstoffe) 214 - Abfallbehandlungsanlagen. Joint Ministerial Gazette 2013, 49, S. 978–989.
- Lavoie, J.; Cloutier, Y.; Lara, J.; Marchand, G. Guide sur la protection respiratoire contre les bioaérosols - Recommandations sur le choix et l'utilisation (IRSST). Rapport Etudes et Recherches RG- 2007, 497, 1–30. [Google Scholar]
- Eduard, W. A health-based criteria document on fungal spore exposure in the working population. Is it relevant for the general population? Indoor Air 2008, 18, 257–258. [Google Scholar]
- Rouzaud, P.; Soulat, J.M.; Trela, C.; Fraysse, P.; Recco, P.; Carles, P.; Lauque, D. Symptoms and serum precipitins in workers exposed to dry sausage mould: consequences of exposure to sausage mould. International Archives of Occupational and Environmental Health 2001, 74, 371–374. [Google Scholar] [CrossRef]
- Winck, J.C.; Delgado, L.; Murta, R.; Lopez, M.; Marques, J.A. Antigen characterization of major cork moulds in Suberosis (cork worker's pneumonitis) by immunoblotting. Allergy 2004, 59, 739–745. [Google Scholar] [CrossRef]
- Latge, J.-P. Aspergillus fumigatus and Aspergillosis. Clinical Microbiological Reviews 1999, 12, 310–350. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Broadbridge, C.; Murray, S.; Waghorn, D.; Mahoney, A. Gardening can seriously damage your health. The Lancet 2008, 371, 2056. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, A.P.; Bush, R.K.; Demain, J.G.; Denning, D.W.; Dixit, A.; Fairs, A.; Greenberger, P.A.; Kariuki, B.; Kita, H.; Kurup, V.P.; Moss, R.B.; Niven, R.M.; Pashley, C.H.; Slavin, R.G.; Vijay, H.M.; Wardlaw, A.J. Fungi and allergic lower respiratory tract diseases. Journal of Allergy and Clinical Immunology 2012, 129, 280–291. [Google Scholar] [CrossRef]
- Jančič, S.; Zalar, P.; Kocev, D.; Schroers, H.-J.; Džeroski, S.; Gunde-Cimerman, N. Halophily reloaded: new insights into the extremophilic life-style of Wallemia with the description of Wallemia hederae sp. nov. Fungal Diversity 2016, 76, 97–118. [Google Scholar] [CrossRef]
- Weber, R.W. Allergen of the Month—Mucor. Annals of Allergy, Asthma & Immunology 2015, 115, A15. [Google Scholar]
- Rydjord, B.; Eduard, W.; Stensby, B.; Sandven, P.; Michaelsen, T.E.; Wiker, H.G. Antibody response to long-term and high-dose mould-exposed sawmill workers. Scandinavian Journal of Immunology 2007, 66, 711–718. [Google Scholar] [CrossRef]
- Quirce, S.; Vandenplas, O.; Campo, P.; Cruz, M.J.; de Blay, F.; Koschel, D.; Moscato, G.; Pala, G.; Raulf, M.; Sastre, J.; Siracusa, A.; Tarlo, S.M.; Walusiak-Skorupa, J.; Cormier, Y. Occupational hypersensitivity pneumonitis: an EAACI position paper. Allergy 2016, 71, 765–779. [Google Scholar] [CrossRef]
- Egbuta, M.A.; Mwanza, M.; Babalola, O.O. Health Risks Associated with Exposure to Filamentous Fungi. International journal of environmental research and public health 2017, 14, 719. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Alwan, T.; Ørberg, A.; Uhrbrand, K.; Jørgensen, M.B. Waste workers’ exposure to airborne fungal and bacterial species in the truck cab and during waste collection. Annals of Occupational Hygiene 2016, 60, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, O.; Huyard, A.; Rybacki, D.; Do Quang, Z. Protection of the vehicle cab environment against bacteria, fungi and endotoxins in composting facilities. Waste Management 2012, 32, 1106–1115. [Google Scholar] [CrossRef]
- Viegas, C.; Faria, T.; de Oliveira, A.C.; Caetano, L.A.; Carolino, E.; Quintal-Gomes, A.; Twaruzek, M.; Kosicki, R.; Soszczynska, E.; Viegas, S. A new approach to assess occupational exposure to airborne fungal contamination and mycotoxins of forklift drivers in waste sorting facilities. Mycotoxin Research 2017, 33, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Wassiem, A.; Zaki, G.R.; Fahmy, F.C.; El-Gazzar, R. Biochemical changes among municipal solid waste sorting workers: implications for personal protective equipment availability and use. International Journal of Occupational Safety and Ergonomics 2019. [CrossRef]
- Viegas, C.; Dias, M.; Almeida, B.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Twaruzek, M.; Kosicki, R.; Grajewski, J.; Marchand, G.; Viegas, S. Are workers from waste sorting industry really protected by wearing Filtering Respiratory Protective Devices? The gap between the myth and reality. Waste Management 2020, 102, 856–867. [Google Scholar]
- Viegas, C.; Dias, M.; Almeida, B.; Carolino, E.; Gomes, A.Q.; Viegas, S. Aspergillus spp. burden on filtering respiratory protective devices. Is there an occupational health concern? Air Quality Atmosphere and Health 2020, 13, 187–196. [Google Scholar]
- Viegas, C.; Twarużek, M.; Dias, M.; Almeida, B.; Carolino, E.; Kosicki, R.; Soszczyńska, E.; Grajewski, J.; Caetano, L.A.; Viegas, S. Assessment of the microbial contamination of mechanical protection gloves used on waste sorting industry: A contribution for the risk characterization. Environmental Research 2020, 189, 109881. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Michalska, M.; Tankiewicz, M.; Staniszewski, A.; Ratajczyk, J.; Wolska, L. Effect of carbon filter usage period on the secondary emission of bioaerosols. Polish Journal of Environmental Studies 2020, 29, 3057–3069. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).