1. Introduction
Photolithography utilizes light to project a designed pattern onto a photosensitive photoresist, and the light sensitivity of photoresists is of crucial importance which influences the efficiency of lithography and ultimately determines the yield of semiconductor manufacturing [
1]. Extreme ultraviolet (EUV) lithography [
2,
3] and electron beam lithography (EBL) [
4,
5,
6] possess state-of-the-art nanofabrication capabilities; however, stringent sensitivity requirements need to be met while maintaining the ability to print ever-shrinking feature sizes [
7,
8,
9]. For the time being, this remains a challenge.
Researchers have recently developed a variety of candidate photoresist materials, including chemically amplified photoresists (CARs) [
10,
11,
12], metal-based materials [
13,
14], and cleavage polymer-based non-CARs [
15,
16,
17]. Of these, polymer-based CARs are considered the latest qualifying EUV photoresist for realizing high-volume manufacturing [
18]. CARs involve resin with acid-sensitive leaving groups and PAGs which generate acids such as trifluoromethanesulfonic acid during exposure [
19,
20,
21]. The photoacid acts as a catalyst to catalyze the deprotection reaction, moreover, it plays the effect of chemical amplification and significantly improves the UV lithography sensitivity [
22,
23]. However, when the conventional CARs are applied to the EUV platform, the sensitivity is severely affected by the limited EUV absorption.
Various attempts at highly sensitive EUV photoresists using CARs have been conducted, yet most of these focused on the optimization of acid-sensitizing groups and acid-producing agents. However, with the addition of PAGs, a paradox arises where more PAG will significantly increase the sensitivity, yet the accompanying inevitable acid diffusion will bring deteriorations to the resolution. To address this issue, Ober et al. [
24] reported PAG-tethered self-immolated polymer resists and proposed the restricted diffusion of photogenerated acid to the reactive polymer backbone contributed to a 3-4-fold enhancement in sensitivity. Researchers have also developed a variety of alternative materials by introducing metals [
25] and halogens [
26,
27]. Yamamoto et al. [
28] improved the sensitivity by 43% by adding a metal sensitizer and suggested the improvement of sensitivity was mainly dependent on the increase in the yield of photoacid and photoelectron. Jiang et al. [
29] demonstrated that sensitizers containing F and I provide a significant advantage in absorption and electron generation, but the chemical environment where the halogens are bonded has a major impact on sensitivity. These studies have provided critical insights into the design of EUV photoresists to improve photosensitivity. However, radiation-induced chemistry is not well understood, and it is difficult to associate them directly with the solubility switches only through the absorption properties featured by metals and halogens.
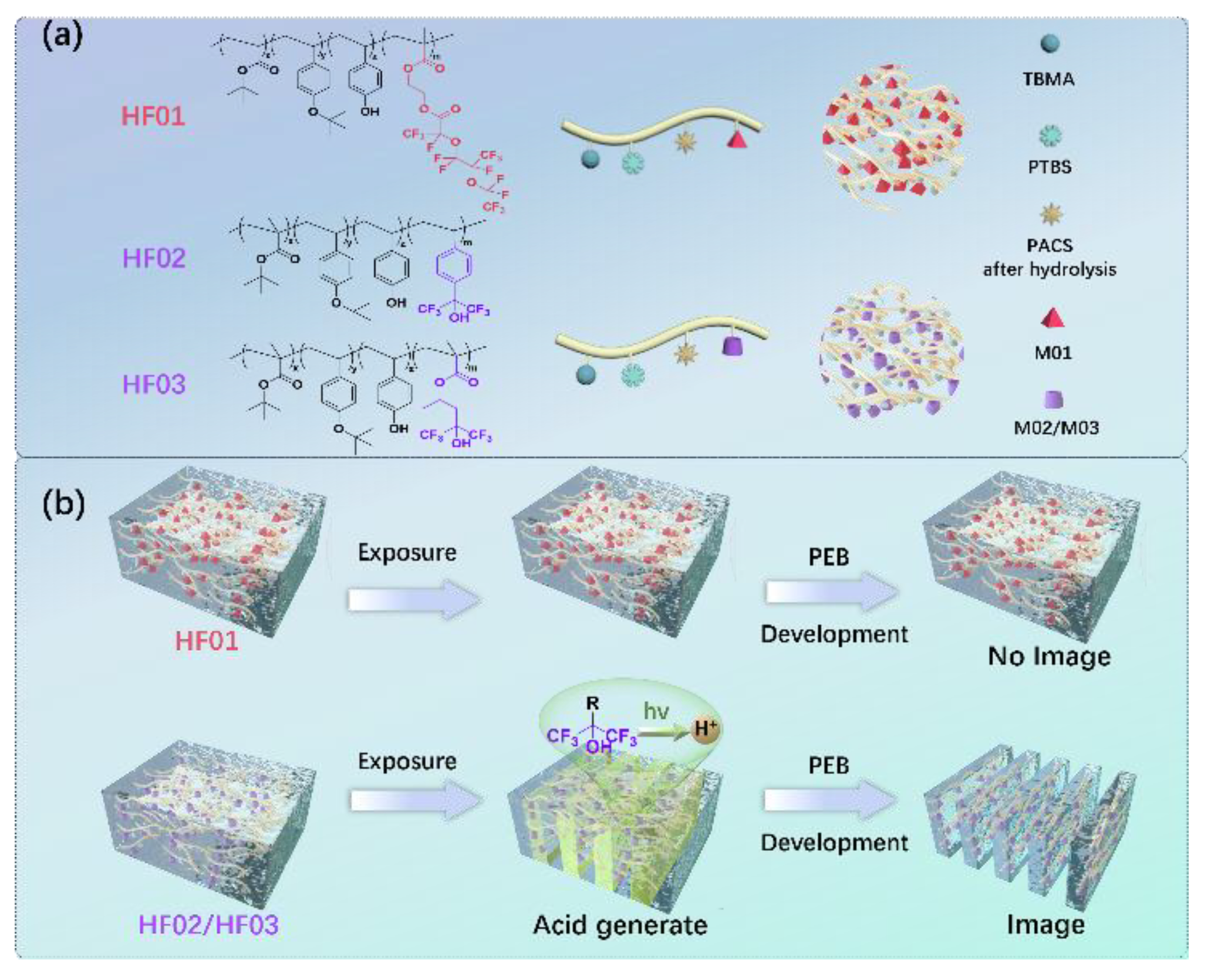
Here in our study, inspired by the high EUV absorption and strong electrophilic property provided by F, we have developed an innovative approach to the highly sensitive nanopatterning with a series of fluoropolymer photoresists (
Scheme 1). Surprised to find that the HFIP in photoresist can serve as an acid-generating agent and achieve patterning, which brings a broad research field for PAGs and enriches the CAR family. This acid-producing mechanism has been validated in both UV and EBL exposure approaches. The HFIP-containing photoresist shows superior patterning ability versus conventional PAGs-involved systems with exposure doses of down to 3 μC/cm
2 which is almost the minimum dose of the equipment. To the best of our knowledge, there is no research has been conducted to demonstrate HFIP serving as an acid-generating agent during exposure and significantly boosting the sensitivity. Our work will open a new way to higher-performance imaging beyond conventional formulation tuning.
2. Materials and Methods
The synthesis of copolymers was performed via the free radical polymerization of monomers in a solvent of 1,4-dioxane with azobisisobutyronitrile as a radical initiator at 80 °C for 6 hours, followed by an ammonolysis [
30] of acetoxy groups to phenolic hydroxyl groups (
Figure S1).
Scheme 1a shows the structures of fluoropolymers (abbreviated as HF01~03, Fourier transform infrared (FT-IR) spectra in
Figures S2–S5, Nuclear magnetic resonance (NMR) spectra in
Figures S6–S13). Fluorinated monomers mainly include those without (M01) and with (M02 and M03) HFIP structures. Gel permeation chromatography (GPC) analysis reveals that the Mw of the fluoropolymers ranges from 10000 to 13000 g/mol, meanwhile, HF02 and HF03 exhibit superior polymerization efficiency and dispersion (
Figure S14 and
Table S1). The fluoropolymers show good thermal stability (the temperature at 5 wt.% weight loss is 200 °C approximately,
Figures S16 and S17) and outstanding alkali resistance and film retention rate (
Figure S15).
3. Results
Preliminary EBL results show that the HFIP-containing photoresists (HF02 and HF03) can be patterned even without the addition of conventional PAGs, but photoresists using fluoropolymer HF01 cannot under identical conditions. As illustrated in
Scheme 1b, we propose the introduction of HFIP structure will not only enhance absorption and electron yield but also participate in chemical reactions which are critical to achieving imaging. To further investigate the imaging properties, we designed a variety of photoresists (abbreviated as S01~15) and evaluated them utilizing different exposure approaches, and the results are summarized in
Table 1.
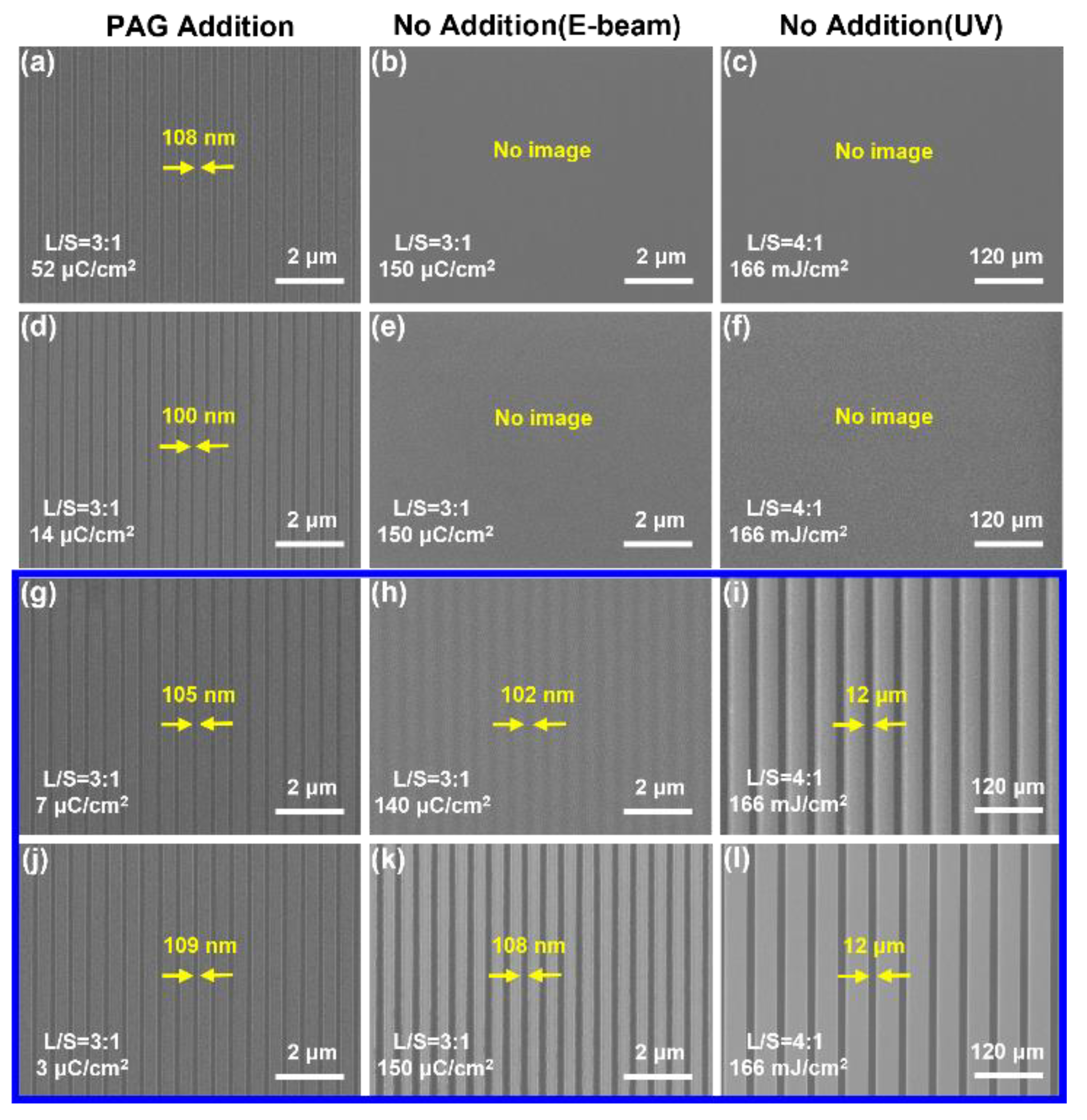
The scanning electron microscopy (SEM) images of photoresists S01~04 exposed by EBL showed clear line and space patterns with a trench of ~100 nm approximately (
Figure 1a,d,g,j). Compared to S01 using non-fluorinated polymer, photoresists S02~04 using fluorinated polymers exhibited higher sensitivity with the optimized dose reduced from 52 µC/cm
2 to less than 14 µC/cm
2, among which the doses for S03 and S04 were even lower at 7 µC/cm
2, and 3 µC/cm
2, respectively. We also evaluated the imaging properties of photoresists S05~08 without the addition of PAGs. The results showed that the photoresists using fluorine-free polymer (S05) and HF01 (S06) were unable to obtain line patterns (
Figure 1b,e), while photoresists (S07~08) with HFIP structures can achieve imaging (
Figure 1h,k) under identical exposure conditions, especially S08 using HF03, demonstrates a better resolution (
Figure 1k). However, the optimized dose was shifted to 150 µC/cm
2, approximately. As well, this phenomenon was further verified during the UV exposure. After being exposed to a UV lamp (254 nm) for 166 mJ/cm
2, HFIP-containing photoresists (S07~08) yielded clear line patterns (
Figure 1i,l), while photoresists (S05~06) gave no image (
Figure 1c,f). Utilizing HF00 and BJ3015 and HFIP-containing materials such as fluorinated polymers (HF02 and HF03) and monomers (M02 and M03), we also prepared several two-component photoresists (S09~15), which all gave discernible patterns (
Figure S18). Since this type of photoresist follows the imaging mechanism of acid-induced deprotection, we propose that upon irradiation the introduced HFIP structure can sever as an acid generator, significantly increase the acidity of photoresist and subsequently catalyze the acidogenic deprotection to generate positive tone image.
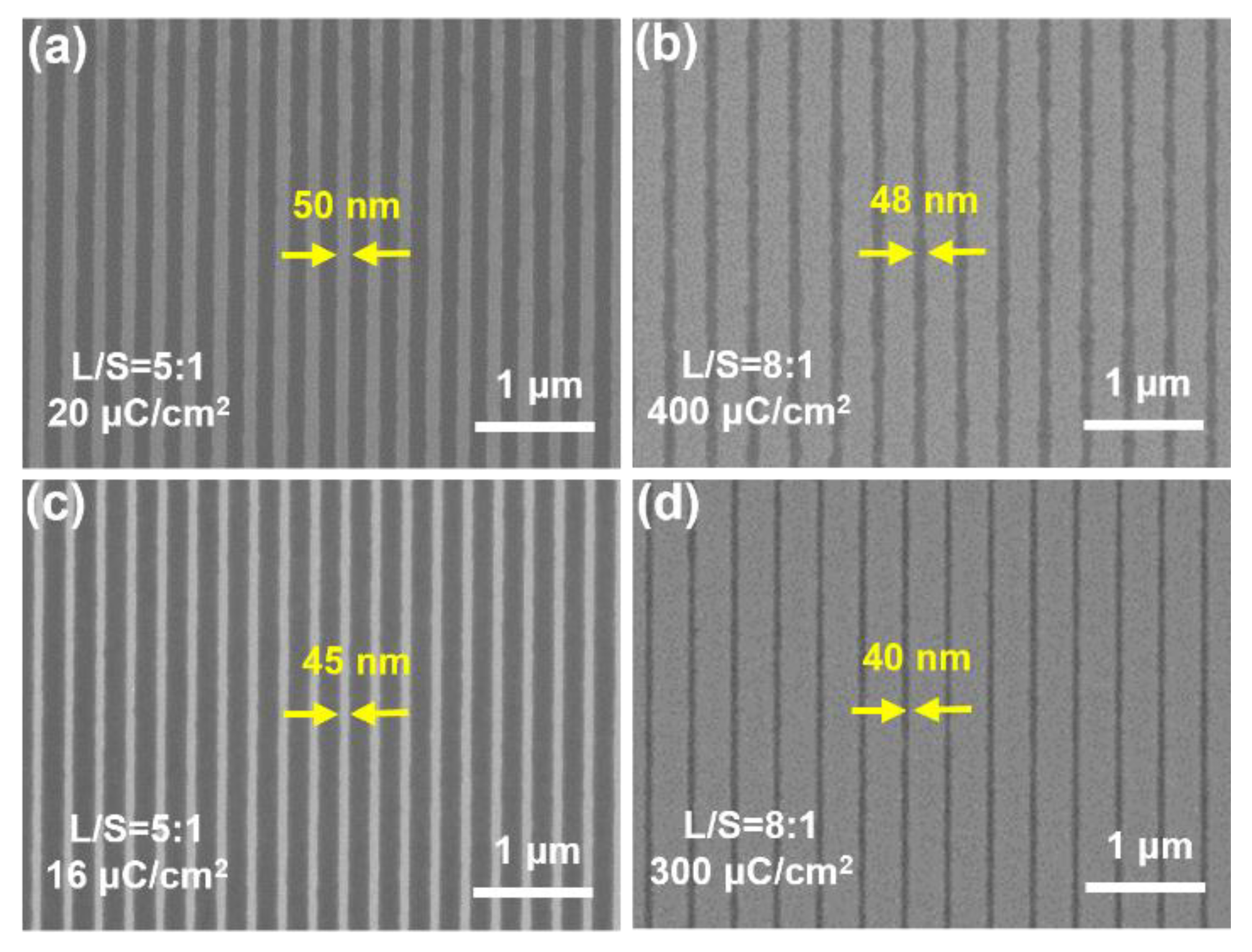
We further evaluated the ability of such fluoropolymer photoresists to achieve smaller feature sizes. The results showed that line and space patterns with feature sizes down to 40 nm can be obtained by optimization (
Figure 2d). Compared with the single-component fluoropolymer photoresist (
Figure 2b,d), the two-component photoresists composed of fluoropolymer and PAGs (
Figure 2a,c) showed significant advantages in terms of sensitivity and roughness, which may result from the higher contrast in development.
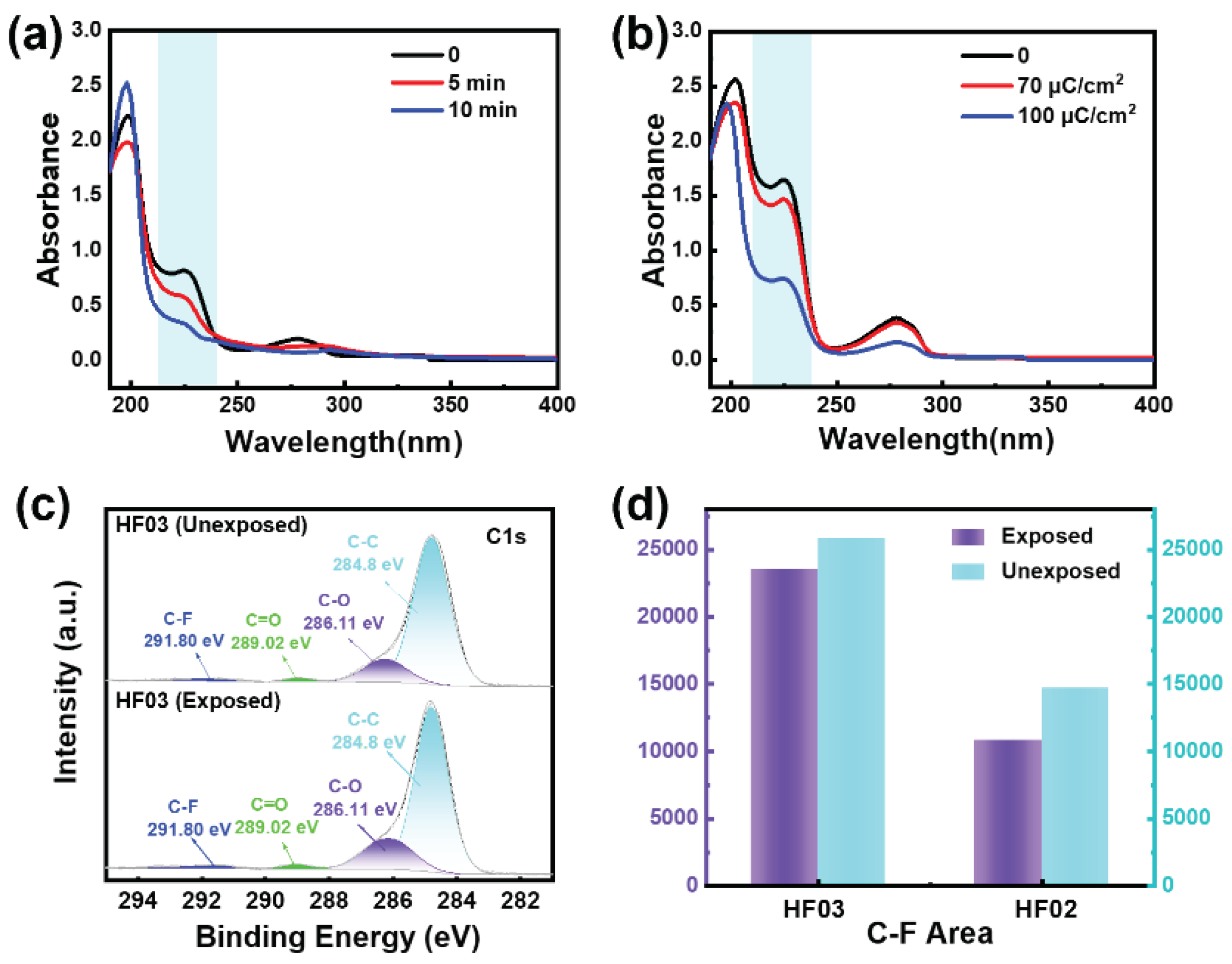
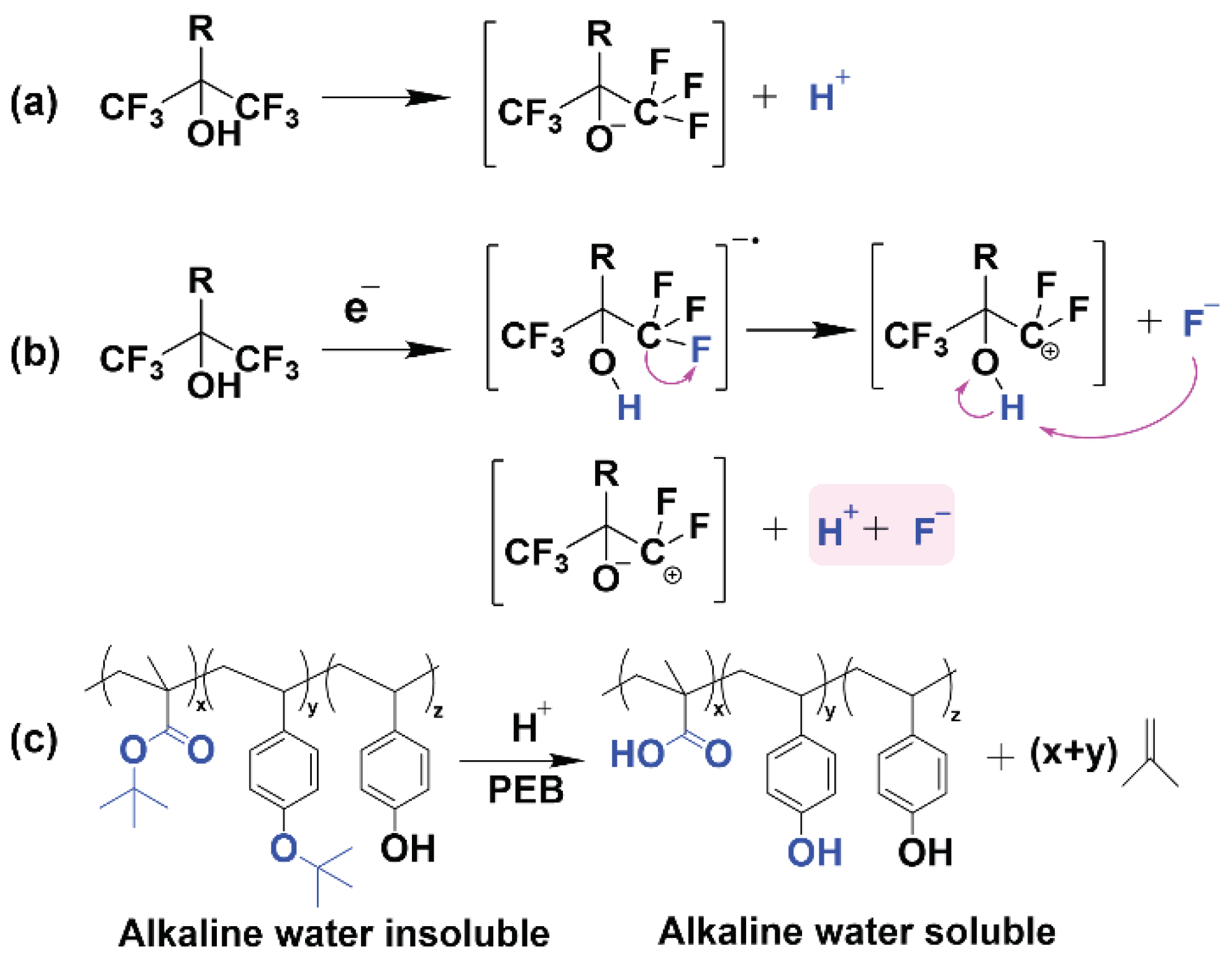
To further investigate the acid-producing properties, we added a pH indicator bromocresol green into the fluoropolymer film and measured the change in UV absorption. An increase in acidity will cause the indicator’s absorption at 220 nm to be reduced due to conjugate addition
1,2 (
Figures S19 and S20). As decided in
Figure 3a,b, after UV or electron beam irradiation, there is a significant decrease in the absorbance at around 220 nm, resulting in a change greater than 50%, which is attributed to the proton gain. It has been observed that the exposed polymer can release strong acids, which are critical for the deprotection of photoresist resins. Combined with the above lithography results, only the HFIP-containing fluoropolymer photoresists can be imaged, suggesting that HFIP is the source of the photogenerated acid. X-ray photoelectron spectroscopy (XPS) results indicated a 35 % decrease in C-F (291.8 ± 0.25 eV)
3 and a 14 % decrease in C-O (286.1 ± 0.21 eV) for the exposed film compared to the unexposed film (
Figures S21–S26), suggesting the cleavage of the C-F bonds and the deprotection of tert-butoxy groups. Therefore, we hypothesize that the C-F bonds in polymers are involved in chemical reactions in the presence of secondary electrons, and the chemical environment generated by C-F cleavage and the dissociation of hydrogen ions in the HFIP groups contribute together to the solubility transition caused by the acid-induced deprotection in the exposed region (
Scheme 2c).
The well-known dissociative electron attachment (DEA) reaction is often reported for halogen-containing molecules
4,5. The dissociative electron attachment reaction involves not only simple bond cleavage, but also multiple bond cleavages, rearrangement of precursor ions, and formation of new molecules, in which the electron attachment proceeds through the formation of transient anions
6. As illustrated in
Scheme 2a,b, attributed to the electron-withdrawing -CF3 groups located close to the hydroxyl group, the HFIP group could effectively capture thermal electrons and is easy to be excited by electrons
7-10, and dissociates to generate H+ and anion fragments, leading to acid formation and enhancement in acidity. Meanwhile, electron ionization events lead to the breaking of the C-F bond and the production of F-, whose nucleophilic attack will also accelerate the dissociation of H+. The presence of F- and H+ produces hydrofluoric acid (HF), which is much stronger than trifluoromethanesulfonic acid (a common photogenerated acid), leading to highly efficient acidogenic deprotection of tert-butoxy (
Scheme 2c), ultimately enabling higher sensitivity.
4. Conclusions
In summary, we have designed and synthesized a novel type of HFIP-containing fluoropolymer photoresists and revealed the combination of the high absorption property provided by F, the strong electron-withdrawing effect of -CF3, and the cleavage of C-F bond in electron ionization events will both contribute to the significant enhancement in sensitivity. Upon irradiation, the HFIP structure can dissociate to generate strong acid and amplify the acidolytic deprotection reaction. Thanks to this, monomers or copolymers containing the HFIP structure can also be used as PAGs for CARs. This work may not only structurally enrich the family of PAGs but also provide insights into the rational design of the next-generation photoresists.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, J.L. and W.K.; methodology, J.L., Q.W., and W.K.; software, J.L.; validation, J.L. and W.K.; formal analysis, J.L., Q.W., and W.K.; investigation, J.L., D.W., T.L., H.W., H.C., and W.K.; resources, W.K.; data curation, J.L. and W.K.; writing—original draft preparation, J.L.; writing—review and editing, J.L., Q.W., and W.K.; visualization, J.L. D.W., T.L., H.W., H.C., and W.K.; supervision, W.K.; project administration, W.K.; funding acquisition, W.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors acknowledge the National Engineering Research Center for Colloidal Materials for support, the School of Chemistry and Chemical Engineering and Shandong University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Y. Mei, W. Huang, W. Di, X. Wang, Z. Zhu, Y. Zhou, F. Huo, W. Wang, Y. Cao, Mechanochemical Lithography, J. Am. Chem. Soc., 2022, 144, 9949-9958.
- G. Tallents, E. Wagenaars, G. Pert, Lithography at EUV wavelengths, Nat. Photonics, 2010, 4, 809-811.
- C. Wagner, N. Harned, Lithography gets extreme, Nat. Photonics, 2010, 4, 24-26.
- J. Fischer, M. Wegener, Three-dimensional optical laser lithography beyond the diffraction limit, Laser Photonics Rev., 2013, 7, 22-44.
- Z. Gan, Y. Cao, R.A. Evans, M. Gu, Three-dimensional deep sub-diffraction optical beam lithography with 9 nm feature size, Nat. Commun., 2013, 4, 1-7.
- J. Shi, A. Ravi, N.E. Richey, H. Gong, S.F. Bent, Molecular Layer Deposition of a Hafnium-Based Hybrid Thin Film as an Electron Beam Resist, ACS Appl. Mater. Interfaces, 2022, 14, 27140-27148.
- X. Wang, P. Tao, Q. Wang, R. Zhao, T. Liu, Y. Hu, Z. Hu, Y. Wang, J. Wang, Y. Tang, H. Xu, X. He, Trends in photoresist materials for extreme ultraviolet lithography: A review, Mater. Today, 2023, 67, 299-319.
- J. Gao, L. Chen, J. Yu, X. Guo, R. Hu, S. Wang, J. Chen, Y. Li, G. Yang, Research Progress on High Resolution Extreme Ultraviolet Photoresist, Chin. J. Appl. Chem., 2021, 38, 1138-1153.
- P. Tao, Q. Wang, M. Vockenhuber, D. Zhu, T. Liu, X. Wang, Z. Hu, Y. Wang, J. Wang, Y. Tang, Y. Ekinci, H. Xu, X. He, Charge Shielding-Oriented Design of Zinc-Based Nanoparticle Liquids for Controlled Nanofabrication, J. Am. Chem. Soc., 2023, 145, 23609-23619.
- S. Hu, J. Chen, T. Yu, Y. Zeng, G. Yang, Y. Li, Chemically Amplified Resist Based on Dendritic Molecular Glass for Electron Beam Lithography, Chem. Res. Chin. Univ., 2023, 39, 139-143.
- M. Guo, X.Y. Liu, T. Li, Q. Duan, X.Z. Dong, J. Liu, F. Jin, M.L. Zheng, Cross-Scale Topography Achieved by MOPL with Positive Photoresist to Regulate the Cell Behavior, Small, 2023, 19, 2303572-2303581.
- J. Sha, J.-K. Lee, S. Kang, V.M. Prabhu, C.L. Soles, P.V. Bonnesen, C.K. Ober, Architectural Effects on Acid Reaction-Diffusion Kinetics in Molecular Glass Photoresists, Chem. Mater., 2010, 22, 3093-3098.
- Q. Wang, H. Cui, X. Wang, Z. Hu, P. Tao, M. Li, J. Wang, Y. Tang, H. Xu, X. He, Exceptional Light Sensitivity by Thiol–Ene Click Lithography, J. Am. Chem. Soc., 2023, 145, 3064-3074.
- Y. Wang, J. Chen, Y. Zeng, T. Yu, S. Wang, X. Guo, R. Hu, P. Tian, M. Vockenhuber, D. Kazazis, Nonchemically Amplified Molecular Resists Based on Sulfonium-Functionalized Sulfone Derivatives for Sub-13 nm Nanolithography, ACS Appl. Nano Mater., 2023, 6, 18480-18490.
- J. Gao, S. Zhang, X. Cui, X. Cong, X. Guo, R. Hu, S. Wang, J. Chen, Y. Li, G. Yang, Effective Optimization Strategy for Electron Beam Lithography of Molecular Glass Negative Photoresist, Adv. Mater. Interfaces, 2023, 2300194.
- B. Cardineau, P. Garczynski, W. Earley, R.L. Brainard, Chain-Scission Polyethers for EUV Lithography, J. Photopolym. Sci. Technol., 2013, 26, 665-671.
- Z. Wang, J. Chen, T. Yu, Y. Zeng, X. Guo, S. Wang, T. Allenet, M. Vockenhuber, Y. Ekinci, G. Yang, Y. Li, Sulfonium-Functionalized Polystyrene-Based Nonchemically Amplified Resists Enabling Sub-13 nm Nanolithography, ACS Appl. Mater. Interfaces, 2022, 15, 2289-2300.
- D.L. Goldfarb, R. Wang, C. Thomas, H. Polgrean, M. Lawson, A. Hess, A. De Silva, R. Gronheid, D.P. Sanders, EUV chemically amplified resist component distribution and efficiency for stochastic defect control, in: Advances in Patterning Materials and Processes XXXVII, 2020.
- K. Arimitsu, M. Yonekura, M. Furutani, Acid-amplifying polymers: synthesis, characterization, and application to environmentally stable chemical amplification positive (ESCAP) resists, RSC Adv., 2015, 5, 80311-80317.
- J. Liu, W. Kang, New Chemically Amplified Positive Photoresist with Phenolic Resin Modified by GMA and BOC Protection, Polymers, 2023, 15, 1598.
- M.S. Ober, D.R. Romer, J. Etienne, P. Thomas, V. Jain, J.F. Cameron, J.W. Thackeray, Backbone degradable poly (aryl acetal) photoresist polymers: synthesis, acid sensitivity, and extreme ultraviolet lithography performance, Macromolecules, 2019, 52, 886-895.
- J.H. Jung, M.J. Kim, K.H. Sohn, H.N. Kang, M.K. Kang, H. Lee, Enhanced Acid Diffusion Control by Using Photoacid Generator Bound Polymer Resist, J. Nanosci. Nanotechnol., 2015, 15, 1764-1766.
- J.Y. Deng, S. Bailey, S.Y. Jiang, C.K. Ober, High-Performance Chain Scissionable Resists for Extreme Ultraviolet Lithography: Discovery of the Photoacid Generator Structure and Mechanism, Chem. Mater., 2022, 34, 6170-6181.
- J. Deng, S. Bailey, S. Jiang, C.K. Ober, Modular Synthesis of Phthalaldehyde Derivatives Enabling Access to Photoacid Generator-Bound Self-Immolative Polymer Resists with Next-Generation Photolithographic Properties, J. Am. Chem. Soc., 2022, 144, 19508-19520.
- H. Xu, K. Sakai, K. Kasahara, V. Kosma, K. Yang, H.C. Herbol, J. Odent, P. Clancy, E.P. Giannelis, C.K. Ober, Metal–Organic Framework-Inspired Metal-Containing Clusters for High-Resolution Patterning, Chem. Mater., 2018, 30, 4124-4133.
- P.J. Evans, C.M. Brick, A. Bell, P. Kandanarachchi, J. Thoresen, L.F. Rhodes, O. Onishi, H. Ikeda, G.M. Benedikt, E. Koronich, Polymers of norbornenyl-4-phenol: Dissolution rate characteristics, positive tone photo-patterning, and polymer properties, J. Appl. Polym. Sci., 2017, 134, 44952-44960.
- Kostko, B. Xu, M. Ahmed, D.S. Slaughter, D.F. Ogletree, K.D. Closser, D.G. Prendergast, P. Naulleau, D.L. Olynick, P.D. Ashby, Y. Liu, W.D. Hinsberg, G.M. Wallraff, Fundamental understanding of chemical processes in extreme ultraviolet resist materials, J. Chem. Phys., 2018, 149, 154305-154314.
- H. Yamamoto, Y. Vesters, J. Jiang, D. De Simone, G. Vandenberghe, T. Kozawa, Role of Metal Sensitizers for Sensitivity Improvement in EUV Chemically Amplified Resist, J. Photopolym. Sci. Technol., 2018, 31, 747-751.
- J. Jing, G. Giordano, R. Fallica, D. DeSimone, G. Vandenberghe, Sensitizer for EUV Chemically Amplified Resist: Metal versus Halogen, J. Photopolym. Sci. Technol., 2019, 32, 15-19.
- H. Li, J. Liu, X. Zheng, C. Ji, Q. Mu, R. Liu, X. Liu, Synthesis of chemically amplified photoresist polymer containing four (Meth) acrylate monomers via RAFT polymerization and its application for KrF lithography, J. Polym. Res., 2016, 23, 1-7.
- L. Wu, M. Baljozovic, G. Portale, D. Kazazis, M. Vockenhuber, T. Jung, Y. Ekinci, S. Castellanos, Mechanistic insights in Zr-and Hf-based molecular hybrid EUV photoresists, J. Micro/Nanolithogr., MEMS, MOEMS, 2019, 18, 013504-013504.
- E.C. Mattson, Y. Cabrera, S.M. Rupich, Y. Wang, K.A. Oyekan, T.J. Mustard, M.D. Halls, H.A. Bechtel, M.C. Martin, Y.J. Chabal, Chemical modification mechanisms in hybrid hafnium oxo-methacrylate nanocluster photoresists for extreme ultraviolet patterning, Chem. Mater., 2018, 30, 6192-6206.
- N. Thakur, R. Bliem, I. Mochi, M. Vockenhuber, Y. Ekinci, S. Castellanos, Mixed-ligand zinc-oxoclusters: efficient chemistry for high resolution nanolithography, J. Mater. Chem. C, 2020, 8, 14499-14506.
- C. Jiang, P. Chen, G. Liu, Cu/photoredox-catalyzed decarboxylative radical C(sp3)-C(sp3) cross-coupling reactions, Sci. China: Chem, 2023, 66, 2858-2862.
- M. Zawadzki, A. Chachereau, J. Kocisek, C.M. Franck, J. Fedor, Electron attachment to hexafluoropropylene oxide (HFPO), J. Chem. Phys., 2018, 149, 204305-204312.
- E. Böhler, J. Warneke, P. Swiderek, Control of chemical reactions and synthesis by low-energy electrons, Chem. Soc. Rev., 2013, 42, 9219-9231.
- Y. Ikari, K. Okamoto, A. Konda, T. Kozawa, T. Tamura, Heating effect of the radiation chemistry of polyhydroxystyrene-type chemically amplified resists, Jpn. J. Appl. Phys., 2020, 59, 086506-086514.
- L. Wang, J. Han, Q. Yuan, W. Cao, X. Zhou, S. Liu, X.-B. Wang, Electron Affinity and Electronic Structure of Hexafluoroacetone (HFA) Revealed by Photodetaching the [HFA]•–Radical Anion, J. Phys. Chem. A, 2020, 125, 746-753.
- Martin, J. Langer, M. Stano, E. Illenberger, Reactions in clusters of acetone and fluorinated acetones triggered by low energy electrons, Int. J. Mass Spectrom., 2009, 280, 107-112.
- C. Szmytkowski, P. Mozejko, E. Ptasinska-Denga, Electron scattering from hexafluoroacetone molecules: cross section measurements and calculations, J. Phys. B: At., Mol. Opt. Phys., 2011, 44, 205202-205.
Figure 1.
Comparison of UV and EBL Performance of the Fluoropolymer-based Photoresists with and without the Addition of Conventional PAGs. (a, d, g, j) Photoresists S01~04 composed of polymers HF00, HF01, HF02, HF03 and PAGs, respectively. (b, c) S05, (e, f) S06, (h, i) S07, and (k, l) S08 are photoresists composed of polymers HF00, HF01, HF02, HF03, respectively. The pitch and L/S of the designed layout are 400 nm and 3/1 (for EBL), 60 μm and 4/1 (for UV), respectively.
Figure 1.
Comparison of UV and EBL Performance of the Fluoropolymer-based Photoresists with and without the Addition of Conventional PAGs. (a, d, g, j) Photoresists S01~04 composed of polymers HF00, HF01, HF02, HF03 and PAGs, respectively. (b, c) S05, (e, f) S06, (h, i) S07, and (k, l) S08 are photoresists composed of polymers HF00, HF01, HF02, HF03, respectively. The pitch and L/S of the designed layout are 400 nm and 3/1 (for EBL), 60 μm and 4/1 (for UV), respectively.
Figure 2.
SEM images of photoresist patterns with a small feature size of sub-50 nm. (a) S03 and (c) S04 are photoresists using HF02 and PAGs, HF03 and PAGs, respectively. (b) S07 and (d) S08 are photoresists using HF02 and HF03, respectively.
Figure 2.
SEM images of photoresist patterns with a small feature size of sub-50 nm. (a) S03 and (c) S04 are photoresists using HF02 and PAGs, HF03 and PAGs, respectively. (b) S07 and (d) S08 are photoresists using HF02 and HF03, respectively.
Figure 3.
UV and XPS Investigations. UV absorbance spectra of films composed of HF03 and a pH indicator bromocresol green before and after exposure to (a) UV light and (b) Electron beam (30 kV). (c) C 1s spectra of HF03 film before and after exposure to electron beam (30 kV, 100 µC/cm2). (d) Calculated integral area of C-F from (c).
Figure 3.
UV and XPS Investigations. UV absorbance spectra of films composed of HF03 and a pH indicator bromocresol green before and after exposure to (a) UV light and (b) Electron beam (30 kV). (c) C 1s spectra of HF03 film before and after exposure to electron beam (30 kV, 100 µC/cm2). (d) Calculated integral area of C-F from (c).
Scheme 1.
Strategy for Fluoropolymer Photoresist Patterning. (a) Approach to synthesize
fluoropolymers (HF01~03): the copolymerization of tert-butyl methacrylate (TBMA), pacetoxystyrene
(PACS), p-tert-butoxystyrene (PTBS), and fluorinated monomers 2-((2,3,3,3-
tetrafluoro-2-(1,1,2,3,3,3-hexafluoro-2-(perfluoroethoxy)propoxy)propanoyl)oxy)ethyl methacrylate
(M01), alpha, alpha-bis(trifluoromethyl)-4-vinylbenzyl alcohol (M02), and 1,1,1-trifluoro-2-
trifluoromethyl-2-hydroxy-4-pentyl methacrylate (M03). (b) Comparison of lithographic imaging
performance of photoresists using different fluoropolymers.
Scheme 1.
Strategy for Fluoropolymer Photoresist Patterning. (a) Approach to synthesize
fluoropolymers (HF01~03): the copolymerization of tert-butyl methacrylate (TBMA), pacetoxystyrene
(PACS), p-tert-butoxystyrene (PTBS), and fluorinated monomers 2-((2,3,3,3-
tetrafluoro-2-(1,1,2,3,3,3-hexafluoro-2-(perfluoroethoxy)propoxy)propanoyl)oxy)ethyl methacrylate
(M01), alpha, alpha-bis(trifluoromethyl)-4-vinylbenzyl alcohol (M02), and 1,1,1-trifluoro-2-
trifluoromethyl-2-hydroxy-4-pentyl methacrylate (M03). (b) Comparison of lithographic imaging
performance of photoresists using different fluoropolymers.
Scheme 2.
Proposed Imaging Mechanism of HFIP-containing Fluoropolymer Photoresists. (a) Dissociation of hydrogen ions. (b) Electrolytic dissociation and C−F bond cleavage, showing the formation of HF. (c) Acid-induced deprotection reaction.
Scheme 2.
Proposed Imaging Mechanism of HFIP-containing Fluoropolymer Photoresists. (a) Dissociation of hydrogen ions. (b) Electrolytic dissociation and C−F bond cleavage, showing the formation of HF. (c) Acid-induced deprotection reaction.
Table 1.
Composition of Photoresist Samples and Corresponding Imaging Results.
Table 1.
Composition of Photoresist Samples and Corresponding Imaging Results.
| Sample No. |
Polymer |
PAGb
|
Additive |
Exposure approacha
|
| UV |
Electron beam |
| S01 |
HF00 |
3 wt.% |
- |
√ |
√ |
| S02 |
HF01 |
3 wt.% |
- |
√ |
√ |
| S03 |
HF02 |
0.5 wt.% |
- |
√ |
√ |
| S04 |
HF03 |
0.5 wt.% |
- |
√ |
√ |
| S05 |
HF00 |
- |
- |
× |
× |
| S06 |
HF01 |
- |
- |
× |
× |
| S07 |
HF02 |
- |
- |
√ |
√ |
| S08 |
HF03 |
- |
- |
√ |
√ |
| S09 |
BJ3015 |
- |
- |
× |
× |
| S10 |
BJ3015 |
- |
HF02c
|
√ |
√ |
| S11 |
BJ3015 |
- |
HF03c
|
√ |
√ |
| S12 |
BJ3015 |
- |
M02d
|
√ |
√ |
| S13 |
BJ3015 |
- |
M03d
|
√ |
√ |
| S14 |
HF00 |
- |
M02e
|
△ |
- |
| S15 |
HF00 |
- |
M03e
|
△ |
- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).