Submitted:
27 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Dietary Fibre and Phenolic Acids in Whole Grains and Their Health Potentials
3.1. Dietary Fibres Contents in Whole Grains
| Whole Grains | TDF | IDF | SDF | References |
|---|---|---|---|---|
| Rye (Secalecereale L.) | 15.2-20.9 | 11.1-15.9 | 3.7-4.5 | [37] |
| 14.7-20.9 | 10.8-15.9 | 3.4-4.6 | [38] | |
| Corn (Zea mays L.) | 3.7-8.6 | 3.1-6.1 | 0.5-2.5 | [39] |
| 13.1-19.6 | 11.6-14.0 | 1.5-3.6 | [31] | |
| Sorghum (Sorghum bicolor) | 7.55–12.3 | 6.52–7.90 | 1.05–1.23 | [40] |
| Millets (Eleusine coracana (L.) Gaertn.) | 13.0-13.8 | 12.5-13.5 | 0.52-0.59 | [41] |
| Triticale (Triticosecale Wittmack) | 14.5 | 0.7 | 6.7 | [42] |
| 14.6 | 12.0 | - | [43] | |
| Quinoa (Chenopodium quinoa Willd.) | 16.2-20.6 | - | - | [44] |
| 11.6-15.1 | 9.9-12.2 | 0.4-2.9 | [45] |
3.2. Health Potentials
| Grains | Dietary Fibres | Health Potentials | References |
|---|---|---|---|
| Whole grains | Arabinoxylans | Increase fecal biomass, enhance gut health, low LDL level, lipid metabolism | [55,56,57,58,59] |
| Whole grains | β-glucans | Anti-Inflammation, Decrease glycemic index; prebiotic effect, Blood cholesterol and glucose modulation, Immune function modulation | [60,61,62,63] |
| Whole grains | Total dietary fibres | Anti-Inflammation Lower risk of Anti-cardiovascular disease, Anti-diabetics, and certain Anti-cancers and Body weight regulation | [51,64,65,66,67] |
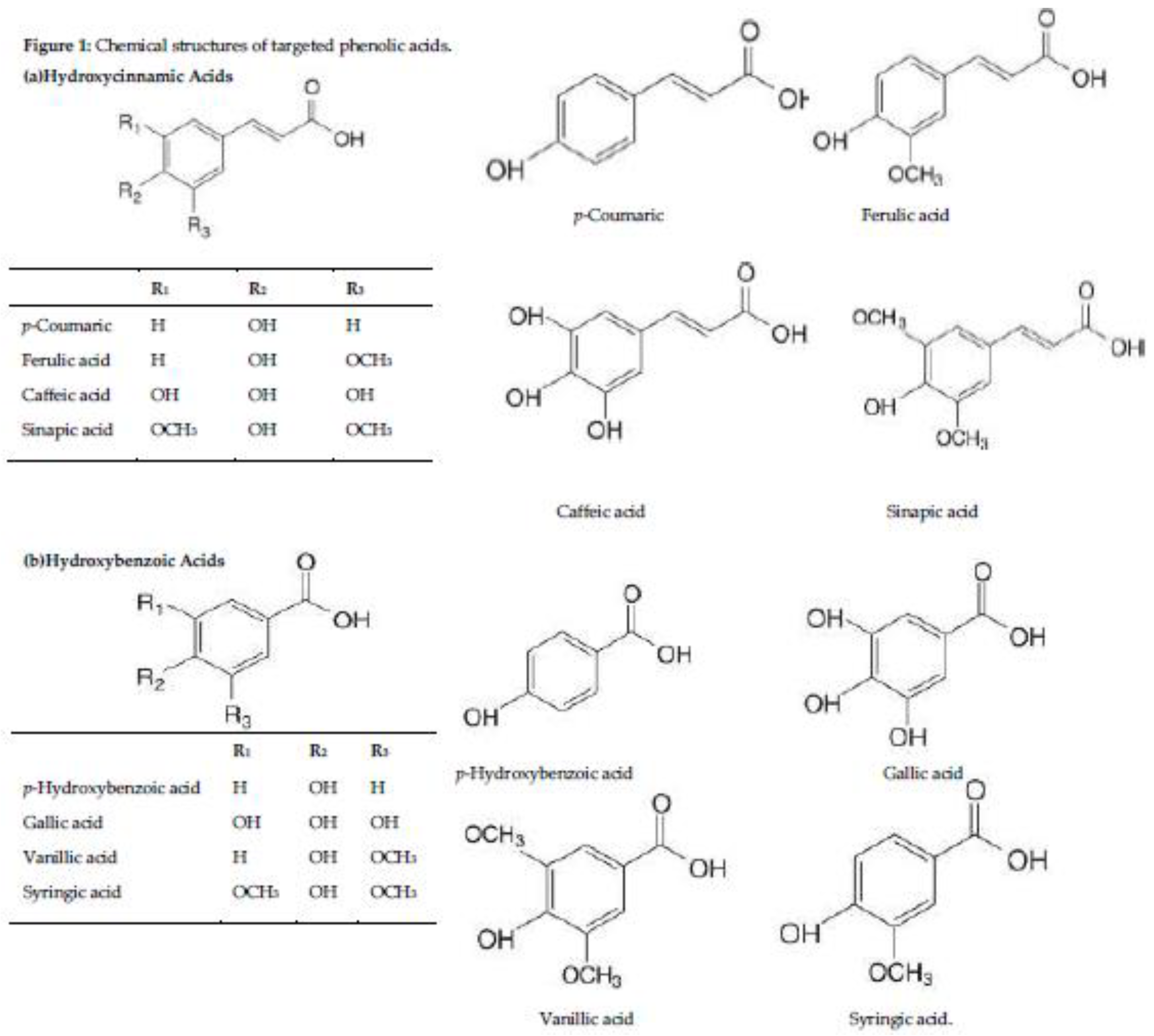
3.3. Phenolic Acids Contents in Whole Grains
| Hydroxycinnamic Acids in Targeted Cereal Grains µg/g of Dry Weight | |||||
|---|---|---|---|---|---|
| Whole grains | Ferulic acid | p-Coumaric | Caffeic acid | Sinapic acid | References |
| Rye (Secalecereale L.) | 827.2 (218.7–1170.0) | 49.0 (29.9–70.0) | 16.2 (12.3–20.0) | 94.2 (51.7–140.0) | [75,86] |
| Corn (Zea mays L.) | 94.2 (51.7–140.0) | 340.5 (97.0–584.0) | 15.0 (5.7–24.4) | 66.1 (52.9–79.3) | [73,75,78,84,85] |
| Sorghum (Sorghum bicolor) | 66.1 (52.9–79.3) | 43.6 (3.8–83.4) | 32.1 (1.9–62.4) | 8.2 | [79] |
| Millets (Eleusine coracana (L.) Gaertn.) | 233.4 (20.0–571.3) | 46.0 (18.0–118.3) | 4.6 (1.1–8.2) | 46.7 (21.3–72.1) | [80,81,82] |
| Triticale (Triticosecale Wittmack) | 46.7 (21.3–72.1) | 139.8 (21.2–258.5) | 9.9 (6.0–13.9) | 83.0 (50.0–140.0) | [75,85] |
| Quinoa (Chenopodium quinoa Willd.) | 87.7 (23.7–150.0) | 48.6 (17.1–80.0) | 7.0 | - | [83,84] |
| Hydroxybenzoic acids in targeted cereal grains µg/g of Dry Weight | |||||
| p-Hydroxybenzoic acid | Gallic acid | Vanillic acid | Syringic acid | ||
| Rye (Secalecereale L.) | 14.1 (8.1–20.0) | 7.7 | 18.0 (15.9–20.0) | 6.3 | [75] |
| Corn (Zea mays L.) | 8.2 (4.9–11.6) | 55.4 (0.5–116.5) | 10.3 (5.4–15.4) | 45.3 (4.3–108.4) | [75,85] |
| Sorghum (Sorghum bicolor) | 36.2 (6.1–148.0) | 28.0 (13.2–46.0) | 23.2 (8.3–50.7) | 16.9 (15.6–19.7) | [78,88] |
| Millets (Eleusine coracana (L.) Gaertn.) | 3.0 | 68.6 (38.7–109.0) | 22.2 (11.0–33.3) | 13.1 (2.1–24.0) | [73,80,81] |
| Triticale (Triticosecale Wittmack) | 7.1 (6.9–7.4) | 333.7 (123.4–544.0) | 446.0 (154.0–738.0) | 173.2 (5.3–341.0) | [75,85] |
| Quinoa (Chenopodium quinoa Willd.) | 21.7 (13.8–29.0) | - | 30.4 (11.7–110.0) | - | [84] |
Health Potentials
4. Effect of Consuming Whole Grains on Inflammation Active Components
4.1. Evidences from Epidemiological Studies
4.2. Dietary Fibres
4.3. Phenolic Acids
4.4. Proposed Mechanism of Anti-Inflammatory Properties of Whole Grains Dietary Fibres and Phenolic Acids; and Involvement of Gut Microbiota
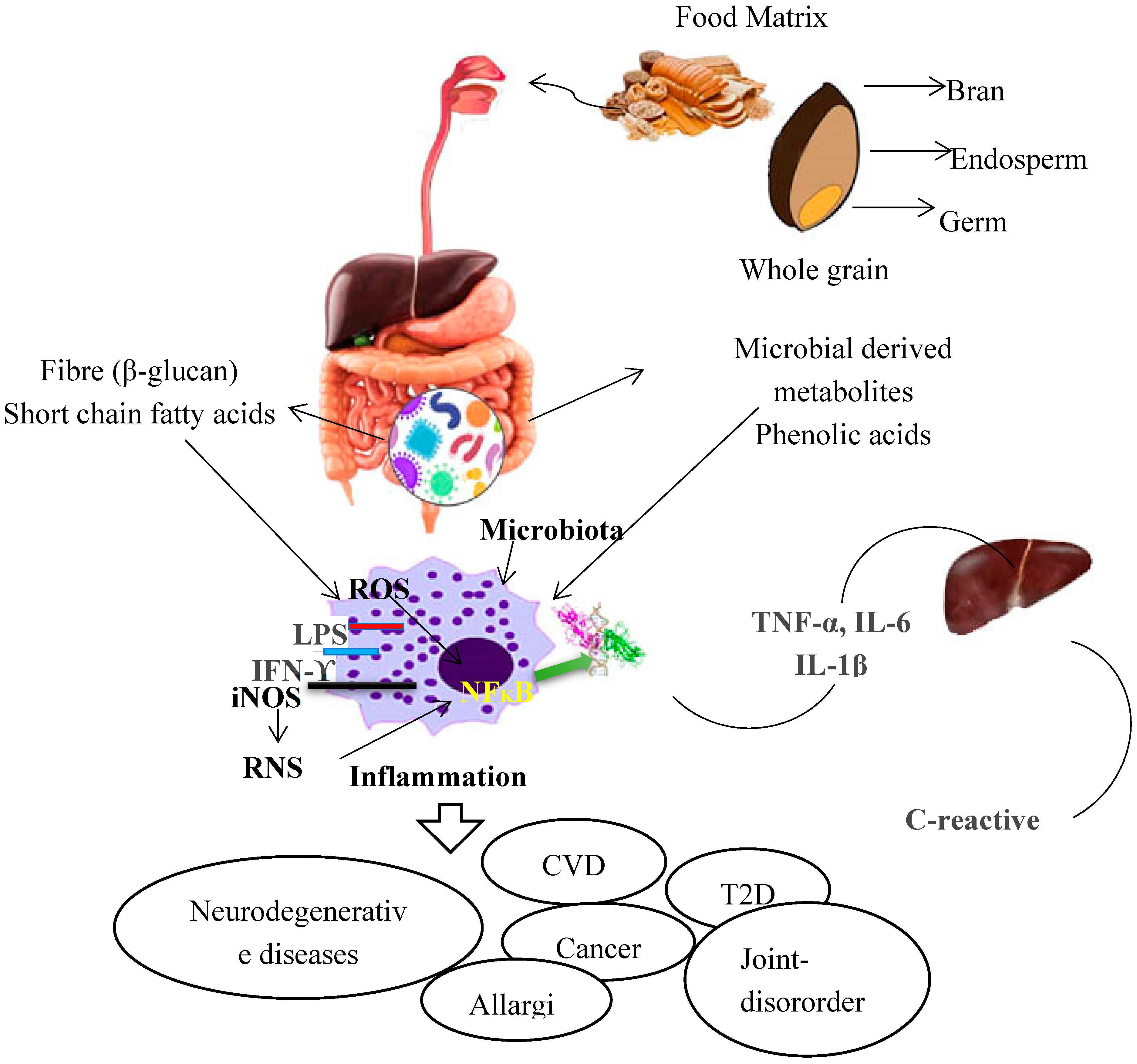
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AACCI. Definition of Whole Grain. 1999. Available online: http://www.aaccnet.org/definitions/wholegrain.asp (accessed on 15 February 2024).
- AACCI. 2006. Available online: http://www.aaccnet.org/definitions/pdfs/AACCIntlWholeGrainComments.pdf (accessed on 17 February 2024).
- Saleh, A. S.; Wang, P.; Wang, N.; Yang, S.; & Xiao, Z. Technologies for enhancement of bioactive components and potential health benefits of cereal and cereal-based foods: Research advances and application challenges. Crit. Rev. Food Sci. Nutr., 2019, 59(2), 207-227. [CrossRef]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Pasqualone, A.; Laddomada, B.; & Carluccio, M. A. Phenolic extracts from whole wheat biofortified bread dampen overwhelming inflammatory response in human endothelial cells and monocytes: Major role of VCAM-1 and CXCL-10.Eur. J. Nutr. 2020, 59, 2603-2615. [CrossRef]
- Milesi, G.; Rangan, A.; & Grafenauer, S. Whole Grain Consumption and Inflammatory Markers: A Systematic Literature Review of Randomized Control Trials. Nutr. 2022, 14, 374, 7. [CrossRef]
- Sang, S.; Idehen, E.; Zhao, Y.; & Chu, Y. Emerging science on whole grain intake and inflammation. Nutr. Rev., 2020, 78(1), 21-28. [CrossRef]
- Rahmani, S.; Sadeghi, O.; Sadeghian, M.; Sadeghi, N.; Larijani, B.; Esmaillzadeh, A. The effect of whole-grain intake on biomarkers of subclinical inflammation: A comprehensive meta-analysis of randomized controlled trials. Adv. Nutr. 2020, 11, 52–65. [CrossRef]
- Andersson, A.; Tengblad, S.; Karlstrom, B.; Kamal-Eldin, A.; Landberg, R., Basu, S. et al. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. The J. Nutr., 2007, 137(6), 1401-1407. [CrossRef]
- Roager, H. M.; Vogt, J. K.; Kristensen, M.; Hansen, L. B. S.; Ibrügger, S.; Mærkedahl, R. B et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut, 2019, 68(1), 83-93. [CrossRef]
- Vanegas, S. M.; Meydani, M.; Barnett, J. B.; Goldin, B.; Kane, A.; Rasmussen, H.; et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am. J. Clin. 2017, 105(3), 635-650. [CrossRef]
- Taskinen, R. E.; Hantunen, S.; Tuomainen, T. P.; & Virtanen, J. K. The associations between whole grain and refined grain intakes and serum C-reactive protein.Eur. J. Clin. Nutr, 2022, 76(4), 544-550. [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.; & Vatten, L. J. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis of cohort studies. Eur. J. Epid., 2013, 28, 845-858. [CrossRef]
- Jones, J. M.; García, C. G.; & Braun, H. J. Perspective: Whole and refined grains and health—Evidence supporting “make half your grains whole”. Adv Nutr. 2020, 11(3), 492-506.
- Sanders, L. M.; Zhu, Y.; Wilcox, M. L.; Koecher, K.; & Maki, K. C. Whole grain intake, compared to refined grain, improves postprandial glycemia and insulinemia: a systematic review and meta-analysis of randomized controlled trials. Crt. Rev.Food Sci. Nutr. 2023, 63(21), 5339-5357. [CrossRef]
- Arabzadegan, N.; Daneshzad, E.; Fatahi, S.; Moosavian, S. P.; Surkan, P. J.; & Azadbakht, L. Effects of dietary whole grain, fruit, and vegetables on weight and inflammatory biomarkers in overweight and obese women. Eat. Wei. Dis. Stud. Anor. Bul. Obes., 2020, 25, 1243-1251. [CrossRef]
- Browning, L. M.; Krebs, J. D.; & Jebb, S. A. Discrimination ratio analysis of inflammatory markers: implications for the study of inflammation in chronic disease. Meta. 2004, 53(7), 899-903. [CrossRef]
- Wang, W. Y.; Tan, M. S.; Yu, J. T.; & Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3(10).
- Agunloye, O. M.; Oboh, G.; Ademiluyi, A. O.; Ademosun, A. O.; Akindahunsi, A. A.; Oyagbemi, A. A. et al. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Bio. Phar., 2019, 109, 450-458. [CrossRef]
- Lee, Y. M.; Han, S. I.; Song, B. C.; & Yeum, K. J. Bioactives in commonly consumed cereal grains: implications for oxidative stress and inflammation. J. Med. Food, 2015, 18(11), 1179-1186. [CrossRef]
- Del Giudice, M.; & Gangestad, S. W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain, Beh. Imm, 2018, 70, 61-75. [CrossRef]
- Fink-Neuboeck, N.; Lindenmann, J.; Bajric, S.; Maier, A.; Riedl, R.; Weinberg, A. M.; & Smolle-Juettner, F. M. Clinical impact of interleukin 6 as a predictive biomarker in the early diagnosis of postoperative systemic inflammatory response syndrome after major thoracic surgery: a prospective clinical trial. Surg, 2016, 160(2), 443-453. [CrossRef]
- Chen, W. W.; Zhang, X. I. A.; & Huang, W. J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med Rep, 2016, 13(4), 3391-3396. [CrossRef]
- Hawkey, C. J.; & Langman, M. J. S. Non-steroidal anti-inflammatory drugs: overall risks and management. Complementary roles for COX-2 inhibitors and proton pump inhibitors. Gut, 2003, 52(4), 600. [CrossRef]
- Tomas-Barberan, F. A.; & Andres-Lacueva, C. Polyphenols and health: current state and progress. J. Agr. Food Chem, 2012, 60(36), 8773-8775. [CrossRef]
- Zeng, Z.; Liu, C.; Luo, S.; Chen, J., & Gong, E. The profile and bioaccessibility of phenolic compounds in cereals influenced by improved extrusion cooking treatment. PloS one, 2016, 11(8), e0161086. [CrossRef]
- Tian, M.; Pak, S.; Ma, C.; Ma, L.; Rengasamy, K. R.; Xiao, J. et al. Chemical features and biological functions of water-insoluble dietary fiber in plant-based foods. Crit. Rev. Food Sci. Nutr., 2022, 1-15. [CrossRef]
- Ciudad-Mulero, M.; Fernández-Ruiz, V.; Matallana-González, M. C.; & Morales, P. Dietary fiber sources and human benefits: The case study of cereal and pseudocereals. Adv. Food Nutr, Res., 2019, (90), 83-134. Academic Press. [CrossRef]
- Zhang, X.; Li, L.; & Xu, F. Chemical characteristics of wood cell wall with an emphasis on ultrastructure: A mini-review. Forests, 2022, 13(3), 439. [CrossRef]
- Van der Kamp, J. W.; Poutanen, K.; Seal, C. J.; & Richardson, D. P. The HEALTHGRAIN definition of ‘whole grain’. Food Nutr. Res., 2014, 58(1), 22100.
- Singh, A.; & Eligar, S. M. Feruloylated oligosaccharides-emerging natural oligosaccharides for human health: Production, structural characterization, bioactive potential, and functional food applications. Res. Tech. Adv. Food Sci, 2022, 141-173.
- Whole Grain Poster. Available online: https://nutritioneducationstore.com/products/go-for-thewhole-grain-poster (accessed on 18 February 2024).
- Laddomada, B.; Durante, M.; Minervini, F.; Garbetta, A.; Cardinali, A.; D’Antuono, I. et al. Phytochemical composition and anti-inflammatory activity of extracts from the whole-meal flour of Italian durum wheat cultivars. Int. J. Mol. Sci, 2015, 16(2), 3512-3527. [CrossRef]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A. A.; Giacco, R.; Ercolini, D. et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Amer. J. Clin. Nutr., 2015, 101(2), 251-261. [CrossRef]
- Mateo Anson, N.; Aura, A. M.; Selinheimo, E.; Mattila, I.; Poutanen, K.; Van Den Berg, R. et al. Bioprocessing of wheat bran in whole wheat bread increases the bioavailability of phenolic acids in men and exerts antiinflammatory effects ex vivo. J. Nutr., 2011, 141(1), 137-143. [CrossRef]
- Sánchez-Maldonado, A. F.; Schieber, A.; & Gänzle, M. G. Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. App. Mic., 2011, 111(5), 1176-1184. [CrossRef]
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; & Delikanli, B. Phenolics in human health. Int. J. Chem. Emg. Appl., 2014, 5(5), 393-396. [CrossRef]
- Anson, N. M.; Hemery, Y. M.; Bast, A.; & Haenen, G. R. Optimizing the bioactive potential of wheat bran by processing. Food Func, 2012, 3(4), 362-375. [CrossRef]
- Li, L.; Shewry, P. R.; & Ward, J. L. Phenolic acids in wheat varieties in the HEALTHGRAIN diversity screen. J. Agri. Food Chem., 2008, 56(21), 9732-9739. [CrossRef]
- Rawat, N.; Laddomada, B.; & Gill, B. S. Genomics of cereal-based functional foods. Cereal Gen. II, 2013, 247-274. [CrossRef]
- Verma, B.; Hucl, P.; & Chibbar, R. N. Phenolic content and antioxidant properties of bran in 51 wheat cultivars. Cereal Chem, 2008, 85(4), 544-549. [CrossRef]
- Juurlink, B. H.; Azouz, H. J.; Aldalati, A. M.; AlTinawi, B. M.; & Ganguly, P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr. J., 2014, 13, 1-10. [CrossRef]
- Rakha, A.; Åman, P.; & Andersson, R. Dietary fiber in triticale grain: Variation in content, composition, and molecular weight distribution of extractable components. J.Cereal Sci., 2011, 54(3), 324-331. [CrossRef]
- Biel, W.; Kazimierska, K.; & Bashutska, U. Nutritional value of wheat, triticale, barley and oat grains. Acta. Sci. Pol. Zootech., 2020, 19(2), 19-28.
- Fernandez-Orozco, R.; Li, L.; Harflett, C.; Shewry, P. R.; & Ward, J. L. Effects of environment and genotype on phenolic acids in wheat in the HEALTHGRAIN diversity screen. J. Agr. Food Chem., 2010, 58(17), 9341-9352. [CrossRef]
- Laddomada, B.; Durante, M.; Mangini, G.; D’Amico, L.; Lenucci, M. S.; Simeone, R. et al. Genetic variation for phenolic acids concentration and composition in a tetraploid wheat (Triticum turgidum L.) collection. Genet Resour Crop Evol Title, 2017, 64, 587-597. [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; & Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem., 2018, 240, 212-221. [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L. T.; Boffetta, P.; Greenwood, D. C. et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. bmj, 2016, 353. [CrossRef]
- Saini, P.; Islam, M.; Das, R.; Shekhar, S.; Sinha, A. S. K.; & Prasad, K. Wheat bran as potential source of dietary fiber: Prospects and challenges. J. Food Comp. Anal., 2023, 116, 105030. [CrossRef]
- Chauhan, M.; Mahanty, J.; Kumar, S.; Singh, H.,; & Sharma, A. An Insight into the Functional Benefit of Phenolic Acids from Whole Grains: An Update. Cur. Nutr. Food Sci., 2023, 19(9), 906-921. [CrossRef]
- Khan, J.; Khan, M. Z.; Ma, Y.; Meng, Y.; Mushtaq, A.; Shen, Q.; & Xue, Y. Overview of the composition of whole grains’ phenolic acids and dietary fibre and their effect on chronic non-communicable diseases. Int. J. Env. Res. and Pub. Heal., 2022, 19(5), 3042. [CrossRef]
- Waddell, I. S.; & Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr., 2023, 63(27), 8752-8767. [CrossRef]
- Snauwaert, E.; Paglialonga, F.; Vande Walle, J.; Wan, M.; Desloovere, A.; Polderman, N. et al. The benefits of dietary fiber: the gastrointestinal tract and beyond. Pediatric Nephrology, 2023, 38(9), 2929-2938. [CrossRef]
- Wood, P. J. Cereal β-glucans in diet and health. J. Cereal Sci., 2007, 46(3), 230-238.
- Mathews, R.; Shete, V.; & Chu Y. The effect of cereal Β-glucan on body weight and adiposity: a review of efficacy and mechanism of action. Crit. Rev. Food Sci. Nut., 2023, 63(19), 3838-3850. [CrossRef]
- Neyrinck, A. M.; Possemiers, S.; Druart, C.; Van de Wiele, T.; De Backer, F.; Cani, P. D. et al. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PloS one, 2011, 6(6), e20944. [CrossRef]
- Rosicka-Kaczmarek, J.; Komisarczyk, A.; Nebesny, E.; & Makowski, B. The influence of arabinoxylans on the quality of grain industry products. Eur. J. Food Res. Nutr., 2016, 242, 295-303. [CrossRef]
- Kellow, N. J.; & Walker, K. Z. Authorised EU health claim for arabinoxylan. In Foods, nutrients and food ingredients with authorised eu health claims, 2018, 201-218.
- Chen, Z.; Li, S.; Fu, Y. Li, C.; Chen, D.; & Chen, H. Arabinoxylan structural characteristics, interaction with gut microbiota and potential health functions. J. Func. Food, 2019, 54, 536-551. [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to arabinoxylan produced from wheat endosperm and reduction of post-prandial glycaemic responses (ID 830) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA Journal, 2011, 9(6), 2205.
- Zhong, Y.; Marungruang, N.; Fåk, F.; & Nyman, M. Effects of two whole-grain barley varieties on caecal SCFA, gut microbiota and plasma inflammatory markers in rats consuming low-and high-fat diets. Brit. J. Nutr, 2015, 113(10), 1558-1570.
- Liu Bo, L. B.; Lin QinLu, L. Q.; Yang Tao, Y. T.; Zeng LinNa, Z. L.; Shi LiMin, S. L.; Chen Yaya, C. Y.; & Luo FeiJun, L. F. Oat β-glucan ameliorates dextran sulfate sodium (DSS)-induced ulcerative colitis in mice. Food Func. 2015 3454-3463.
- Keegstra, K.; & Walton, J. β-Glucans--brewer’s bane, dietician’s delight. Science, 2006, 311(5769), 1872-1873.
- Slavin, J. L.; Tucker, M.; Harriman, C.; & Jonnalagadda, S. S. Whole grains: Definition, dietary recommendations, and health benefits. Cereal Foods Wor. 2013, 58(4), 191-198. [CrossRef]
- Hu, J.; Wang, J.; Li, Y.; Xue, K.; & Kan, J. Use of Dietary Fibers in Reducing the Risk of Several Cancer Types: An Umbrella Review. Nutr., 2023, 15(11), 2545. [CrossRef]
- Mao, L.; Kitani, A.; Strober, W.; & Fuss, I. J. The role of NLRP3 and IL-1β in the pathogenesis of inflammatory bowel disease. Front. Immunol., 2018, 9, 2566. [CrossRef]
- Zhang, H.; Gong, C.; Qu, L.; Ding, X.; Cao, W.; Chen, H.; ... & Zhou, G. Therapeutic effects of triptolide via the inhibition of IL-1β expression in a mouse model of ulcerative colitis. Exp Ther Med , 2016, 12(3), 1279-1286. [CrossRef]
- Shmuel-Galia, L.; Aychek, T.; Fink, A.; Porat, Z.; Zarmi, B.; Bernshtein, B.; ... & Shai, Y. Neutralization of pro-inflammatory monocytes by targeting TLR2 dimerization ameliorates colitis. The EMBO J. 2016, 35(6), 685-698. [CrossRef]
- Zagoskina, N. V.; Zubova, M. Y.; Nechaeva, T. L.; Kazantseva, V. V.; Goncharuk, E. A.; Katanskaya, V. M.; ... & Aksenova, M. A. Polyphenols in plants: structure, biosynthesis, abiotic stress regulation, and practical applications. Int. J. Mol. Sci. 2023, 24(18), 13874. [CrossRef]
- Sahu, R.; Mandal, S.; Das, P.; Ashraf, G. J.; Dua, T. K.; Paul, P.; ... & Khanra, R. The bioavailability, health advantages, extraction method, and distribution of free and bound phenolics of rice, wheat, and maize: A review. Food Chem. Adv., 2023, 100484. [CrossRef]
- Perez-Jimenez, J.; Neveu, V.; Vos, F.; & Scalbert, A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the phenol-explorer database. J. Agri. Food Chem., 2010, 58(8), 4959-4969. [CrossRef]
- Ragaee, S.; Seetharaman, K.; & Abdel-Aal, E. S. M. The impact of milling and thermal processing on phenolic compounds in cereal grains. Crit. Rev. Food. Sci. Nutr, 2014, 54(7), 837-849. [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; & Jiménez, L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79(5), 727-747. [CrossRef]
- Obayiuwana, O. A. Phenolic acid content and fibre in Nigerian wholegrains; their metabolism, and potential cardiovascular benefits (Doctoral dissertation, University of Roehampton London). 2023.
- Liu, R. H. Health Benefits of Dietary Phytochemicals in Whole Foods. In Nutritional Health: Strategies for Disease Prevention, 2023, 177-190.
- Irakli, M. N.; Samanidou, V. F.; Biliaderis, C. G.; & Papadoyannis, I. N. Development and validation of an HPLC-method for determination of free and bound phenolic acids in cereals after solid-phase extraction. Food Chem, 2012, 134(3), 1624-1632. [CrossRef]
- Hassan, S. M. Nutritional, Functional and Bioactive Properties of Sorghum (Sorghum Bicolor I. Moench) with its Future Outlooks: A Review. 2023.
- Chandrasekara, A.; & Shahidi, F. Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. J. Func. Food, 2011b, 3(3), 144-158. [CrossRef]
- Žilić, S.; Serpen, A.; Akıllıoğlu, G.; Gökmen, V.; & Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agr. Food Chem., 2012, 60(5), 1224-1231.
- Hithamani, G.; & Srinivasan, K. Bioaccessibility of polyphenols from wheat (Triticum aestivum), sorghum (Sorghum bicolor), green gram (Vigna radiata), and chickpea (Cicer arietinum) as influenced by domestic food processing. J. Agr. Food Chem. 2014, 62(46), 11170-11179.
- N’Dri, D.; Mazzeo, T.; Zaupa, M.; Ferracane, R.; Fogliano, V.; & Pellegrini, N. Effect of cooking on the total antioxidant capacity and phenolic profile of some whole-meal African cereals. J. Sci. Food Agri., 2013, 93(1), 29-36. [CrossRef]
- Viswanath, V.; Urooj, A.; & Malleshi, N. G. Evaluation of antioxidant and antimicrobial properties of finger millet polyphenols (Eleusine coracana). Food Chem., 2009, 114(1), 340-346. [CrossRef]
- Chandrasekara, A.; & Shahidi, F. Bioactivities and antiradical properties of millet grains and hulls. Journal of Agricultural and Food Chem. 2011a, 59(17), 9563-9571. [CrossRef]
- Gómez-Caravaca, A. M.; Iafelice, G.; Verardo, V.; Marconi, E.; & Caboni, M. F. Influence of pearling process on phenolic and saponin content in quinoa (Chenopodium quinoa Willd). Food Chem. 2014, 157, 174-178. [CrossRef]
- Repo-Carrasco-Valencia, R.; Hellström, J. K.; Pihlava, J. M.; & Mattila, P. H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120(1), 128-133. [CrossRef]
- Kandil, A.; Li, J.; Vasanthan, T.; & Bressler, D. C. Phenolic acids in some cereal grains and their inhibitory effect on starch liquefaction and saccharification. J. Agri. Food Chem. 2012, 60(34), 8444-8449. [CrossRef]
- Van Hung, P. Phenolic compounds of cereals and their antioxidant capacity. Crit. Rev. Food Sci. Nutr, 2016, 56(1), 25-35.
- Shao, Y.; & Bao, J. Polyphenols in whole rice grain: Genetic diversity and health benefits. Food Chem, 2015, 180, 86-97. [CrossRef]
- Sène, M.; Gallet, C.; & Doré, T. Phenolic compounds in a Sahelian sorghum (Sorghum bicolor) genotype (CE145–66) and associated soils. J. Chem. Ecol, 2001, 27, 81-92. [CrossRef]
- Gade, A.; & Kumar, M. S. Gut microbial metabolites of dietary polyphenols and their potential role in human health and diseases. J. Physiol. Biochem., 2023, 79(4), 695-718. [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P. C.; & Ribeiro, M. H. Polyphenols in health and disease: Gut microbiota, bioaccessibility, and bioavailability. Compounds, 2023, 3(1), 40-72. [CrossRef]
- Anantharaju, P. G.; Gowda, P. C.; Vimalambike, M. G.; & Madhunapantula, S. V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15(1), 1-16. [CrossRef]
- Grande, T.; Souid, A.; Ciardi, M.; Della Croce, C. M.; Frassinetti, S;, Bramanti, E.; ... & Pozzo, L. Evaluation of antioxidant and antimicrobial activities of whole flours obtained from different species of Triticum genus. Eur. Food Res. Technol., 2023, 249(6), 1575-1587. [CrossRef]
- Abdel-Wahab, M. H.; El-Mahdy, M. A.; Abd-Ellah, M. F.; Helal, G. K.; Khalifa, F.; & Hamada, F. M. A. Influence of p-coumaric acid on doxorubicin-induced oxidative stress in rat’s heart. Pharmacol. Res., 2003, 48(5), 461-465. [CrossRef]
- Janicke, B.; Hegardt, C.; Krogh, M.; Önning, G.; Åkesson, B.; Cirenajwis, H. M.; & Oredsson, S. M. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr. Canc., 2011, 63(4), 611-622.
- Chao, C. Y.; Mong, M. C.; Chan, K. C.; & Yin, M. C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nut. Food Res., 2010, 54(3), 388-395. [CrossRef]
- Fahrioğlu, U.; Dodurga, Y.; Elmas, L.; & Seçme, M. Ferulic acid decreases cell viability and colony formation while inhibiting migration of MIA PaCa-2 human pancreatic cancer cells in vitro. Gene, 2016, 576(1), 476-482. [CrossRef]
- Hou, Y. Z.; Zhao, G. R.; Yang, J.; Yuan, Y. J.; Zhu, G. G.; & Hiltunen, R. Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sci. 2004, 75(14), 1775-1786. [CrossRef]
- Narasimhan, A.; Chinnaiyan, M.; & Karundevi, B. Ferulic acid regulates hepatic GLUT2 gene expression in high fat and fructose-induced type-2 diabetic adult male rat. Eur. J. Pharmacol., 2015, 761, 391-397. [CrossRef]
- Eitsuka, T.; Tatewaki, N.; Nishida, H.; Kurata, T.; Nakagawa, K.; & Miyazawa, T. Synergistic inhibition of cancer cell proliferation with a combination of δ-tocotrienol and ferulic acid. Biochem. Biophys. Res. Commun, 2014, 453(3), 606-611. [CrossRef]
- Ji, L.; Deng, H.; Xue, H.; Wang, J.; Hong, K.; Gao, Y.; ... & You, Y. Research progress regarding the effect and mechanism of dietary phenolic acids for improving nonalcoholic fatty liver disease via gut microbiota. Compr. Rev. Food Sci. Food Saf.., 2023, 22(2), 1128-1147. [CrossRef]
- Roney, M.; Dubey, A.; Zamri, N. B.; & Aluwi, M. F. F. M. Inhibitory effect of Sinapic acid derivatives targeting structural and non-structural proteins of dengue virus serotype 2: An in-silico assessment. Mol Aspects Med, 2023, 2, 100028. [CrossRef]
- DMoreno, D. A.; Pérez-Balibrea, S.; Ferreres, F.; Gil-Izquierdo, Á.; & García-Viguera, C. Acylated anthocyanins in broccoli sprouts. Food Chem, 2010, 123(2), 358-363. [CrossRef]
- Maddox, C. E.; Laur, L. M.; & Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol, 2010, 60, 53-58. [CrossRef]
- Tuncer, S. Ç.; Akarsu, S. A.; Küçükler, S.; Gür, C.; & Kandemir, F. M. Effects of sinapic acid on lead acetate-induced oxidative stress, apoptosis and inflammation in testicular tissue. Environ. Toxicol., 2023, 38(11), 2656-2667.
- Yun, K. J.; Koh, D. J.; Kim, S. H.; Park, S. J.; Ryu, J. H.; Kim, D. G.; ... & Lee, K. T. Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-κB inactivation. J. Agric. Food Chem, 2008, 56(21), 10265-10272. [CrossRef]
- Taştemur, Ş.; Hacısüleyman, L.; Karataş, Ö.; Yulak, F.; & Ataseven, H. Anticancer activity of sinapic acid by inducing apoptosis in HT-29 human colon cancer cell line. Canadian Journal of Physiology and Pharmacology. 2023.
- Kanchana, G.; Shyni, W. J.; Rajadurai, M.; & Periasamy, R. Evaluation of antihyperglycemic effect of sinapic acid in normal and streptozotocin-induced diabetes in albino rats. World J. Pharmacol., 2011, 5(1), 33-39.
- Sun XiuLan, S. X.; Ito, H.; Masuoka, T.; Kamei, C.; & Hatano, T. Effect of Polygala tenuifolia root extract on scopolamine-induced impairment of rat spatial cognition in an eight-arm radial maze task. Biol. Pharm. Bull. Biological 2008 1727-1731. [CrossRef]
- Yoon, B. H.; Jung, J. W.; Lee, J. J.; Cho, Y. W.; Jang, C. G.; Jin, C. B.; ... & Ryu, J. H. Anxiolytic-Like effects of sinapic acid in mice. Pharm. Sci, 2008, 35(1), 67-67. [CrossRef]
- He, L.; Li, H. T.; Guo, S. W.; Liu, L. F.; Qiu, J. B.; Li, F.; & Cai, B. C. Inhibitory effects of sinapine on activity of acetylcholinesterase in cerebral homogenate and blood serum of rats. China Journal of Chinese Materia Medica, 2008, 813-815.
- Ferreres, F.; Fernandes, F.; Sousa, C.; Valentao, P.; Pereira, J. A.; & Andrade, P. B. Metabolic and bioactivity insights into Brassica oleracea var. acephala. J. Agric. Food Chem., 2009, 57(19), 8884-8892. [CrossRef]
- Wakamatsu, D.; Morimura, S.; Sawa, T.; Kida, K.; Nakai, C.; & Maeda, H. Isolation, identification, and structure of a potent alkyl-peroxyl radical scavenger in crude canola oil, canolol. Biosci. Biotechnol. Biochem., 2005, 69(8), 1568-1574. [CrossRef]
- Kuwahara, H.; Kanazawa, A.; Wakamatu, D.; Morimura, S.; Kida, K.; Akaike, T.; & Maeda, H. Antioxidative and antimutagenic activities of 4-vinyl-2, 6-dimethoxyphenol (canolol) isolated from canola oil. J. Agric. Food Chem., 2004, 52(14), 4380-4387. [CrossRef]
- Park, J.; Lee, B.; Choi, H.; Kim, W.; Kim, H. J.; & Cheong, H. Antithrombosis activity of protocatechuic and shikimic acids from functional plant Pinus densiflora Sieb. et Zucc needles. J. Nat. Med.2016, 70, 492-501. [CrossRef]
- Choi, J. H.; & Kim, S. In vitro antithrombotic, hematological toxicity, and inhibitor studies of protocatechuic, isovanillic, and p-hydroxybenzoic acids from Maclura tricuspidata (Carr.) Bur. Molecules, 2022, 27(11), 3496.
- Yeh, R. D.; Chen, J. C.; Lai, T. Y.; Yang, J. S.; Yu, C. S.; Chiang, J. H.; ... & Chung, J. G. Gallic acid induces G0/G1 phase arrest and apoptosis in human leukemia HL-60 cells through inhibiting cyclin D and E, and activating mitochondria-dependent pathway. Anticancer Res., 2011, 31(9), 2821-2832.
- Veluri, R.; Singh, R. P.; Liu, Z.; Thompson, J. A.; Agarwal, R.; & Agarwal, C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinog. 2006, 27(7), 1445-1453. [CrossRef]
- Kamatham, S.; Kumar, N.; & Gudipalli, P. Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol. Rep.2015, 2, 520-529. [CrossRef]
- Kumar, R.; Khurana, N.; & Kaur, B. Effect of INM 176 on ischemia reperfusion injury using rat heart model. 2017. [CrossRef]
- Kumar, R.; Kumar, R.; Anand, A.; Mahajan, R.; Khatik, GL.; Duggal, N.; Mehta, M.; Satija, S.; Sharma, N.; Khurana, N. Potential of Prediction of Activity Spectra of Substances Software to Justify 3Rs Ethics for In Vivo Anti-Alzheimer’s Studies of Phytochemicals. Int. J. Green Pharm. 2018, 66-72.
- Sharma, N.; Tiwari, N.; Vyas, M.; Khurana, N.; Muthuraman, A.; & Utreja, P. An overview of therapeutic effects of vanillic acid. Plant Arch, 2020, 20(2), 3053-3059.
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C. M.; & Kumar, C. S. Syringic acid (SA)‒a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomedicine & Pharmacotherapy, 2018, 108, 547-557.
- Della Pepa, G.; Vetrani, C.; Vitale, M.; & Riccardi, G. Wholegrain intake and risk of type 2 diabetes: evidence from epidemiological and intervention studies. Nutr. 2018, 10(9), 1288. [CrossRef]
- Sang, S.; & Chu, Y. Whole grain oats, more than just a fiber: Role of unique phytochemicals. Mol. Nutr. Food Res., 2017, 61(7), 1600715. [CrossRef]
- Sang, S.; & Landberg, R. The chemistry behind health effects of whole grains. Mol. Nutr. Food Res., 2017, 61(7), 1770074. [CrossRef]
- Xu, Y.; Wan, Q.; Feng, J.; Du, L.; Li, K.; & Zhou, Y. Whole grain diet reduces systemic inflammation: A meta-analysis of 9 randomized trials. Med. 2018, 97(43).
- Hajihashemi, P.; & Haghighatdoost, F. Effects of whole-grain consumption on selected biomarkers of systematic inflammation: a systematic review and meta-analysis of randomized controlled trials. J. Am. Coll. Nutr. 2019, 38(3), 275-285. [CrossRef]
- Zamaratskaia, G.; Omar, N. A. M.; Brunius, C.; Hallmans, G.; Johansson, J. E.; Andersson, S. O.; ... & Landberg, R. Consumption of whole grain/bran rye instead of refined wheat decrease concentrations of TNF-R2, e-selectin, and endostatin in an exploratory study in men with prostate cancer. Clin. Nutr. 2020, 39(1), 159-165. [CrossRef]
- Brownlee, I. A.; Moore, C.; Chatfield, M.; Richardson, D. P.; Ashby, P.; Kuznesof, S. A.; ... & Seal, C. J. Markers of cardiovascular risk are not changed by increased whole-grain intake: the WHOLEheart study, a randomised, controlled dietary intervention. Br. J. Nutr. 2010, 104(1), 125-134. [CrossRef]
- Kwok, C. S.; Gulati, M.; Michos, E. D.; Potts, J.; Wu, P.; Watson, L.; ... & Mamas, M. A. Dietary components and risk of cardiovascular disease and all-cause mortality: a review of evidence from meta-analyses. Eur. J. Prev. Cardiol., 2019, 26(13), 1415-1429. [CrossRef]
- Neuenschwander, M.; Ballon, A.; Weber, K. S.; Norat, T.; Aune, D.; Schwingshackl, L.; & Schlesinger, S. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. bmj, 2019, 366. [CrossRef]
- Tieri, M.; Ghelfi, F.; Vitale, M.; Vetrani, C.; Marventano, S.; Lafranconi, A.; ... & Grosso, G. Whole grain consumption and human health: an umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020, 71(6), 668-677. [CrossRef]
- Ampatzoglou, A.; Atwal, K. K.; Maidens, C. M.; Williams, C. L.; Ross, A. B.; Thielecke, F.; ... & Yaqoob, P. Increased whole grain consumption does not affect blood biochemistry, body composition, or gut microbiology in healthy, low-habitual whole grain consumers. J. Nutr, 2015, 145(2), 215-221. [CrossRef]
- Andersen, J. L. M.; Halkjær, J.; Rostgaard-Hansen, A. L.; Martinussen, N.; Lund, A. S. Q.; Kyrø, C.; ... & Olsen, A. Intake of whole grain and associations with lifestyle and demographics: a cross-sectional study based on the Danish Diet, Cancer and Health—Next Generations cohort. Eur. J. Nutr. 2021, 60, 883-895. [CrossRef]
- Egeberg, R.; Frederiksen, K.; Olsen, A.; Johnsen, N. F.; Loft, S.; Overvad, K.; & Tjønneland, A. Intake of wholegrain products is associated with dietary, lifestyle, anthropometric and socio-economic factors in Denmark. Public Health Nutr. 2009, 12(9), 1519-1530. [CrossRef]
- Mazidi, M.; Kengne, A. P.; Mikhailidis, D. P.; Cicero, A. F.; & Banach, M. Effects of selected dietary constituents on high-sensitivity C-reactive protein levels in US adults. Ann. Med., 2018, 50(1), 1-6. [CrossRef]
- Qi, L.; Van Dam, R. M.; Liu, S.; Franz, M.; Mantzoros, C.; & Hu, F. B. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Car., 2006, 29(2), 207-211. [CrossRef]
- Wilczak, J.; Błaszczyk, K.; Kamola, D.; Gajewska, M.; Harasym, J. P.; Jałosińska, M.; ... & Gromadzka-Ostrowska, J. The effect of low or high molecular weight oat beta-glucans on the inflammatory and oxidative stress status in the colon of rats with LPS-induced enteritis. Food Fun. 2015, 6(2), 590-603. [CrossRef]
- Shahidi, F.; & Peng, H. Bioaccessibility and bioavailability of phenolic compounds. Journal of Food Bioactives, 2018, 4, 11-68. [CrossRef]
- Esmaillzadeh, A., Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F. B.; & Willett, W. C. Dietary patterns and markers of systemic inflammation among Iranian women. J. Nutr, 2007, 137(4), 992-998. [CrossRef]
- McRorie Jr, J. W. Evidence-based approach to fiber supplements and clinically meaningful health benefits, part 1: what to look for and how to recommend an effective fiber therapy. Nutr. Today, 2015, 50(2), 82.
- Jacobs Jr, D. R. The whole cereal grain is more informative than cereal fibre. Nat. Rev. Endocrinol., 2015, 11(7), 389-390.
- Pragasam, S. J.; Venkatesan, V.; & Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inf. 2013, 36, 169-176. [CrossRef]
- Ibitoye, O. B.; & Ajiboye, T. O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch. Physiol. Biochem. 2018, 124(5), 410-417. [CrossRef]
- Koenig, R.; Dickman, J. R.; Kang, C.; Zhang, T.; Chu, Y. F.; & Ji, L. L. Avenanthramide supplementation attenuates exercise-induced inflammation in postmenopausal women. Nutr. J. 2014, 13(1), 1-11. [CrossRef]
- Liu, L.; Zubik, L.; Collins, F. W.; Marko, M.; & Meydani, M. The antiatherogenic potential of oat phenolic compounds. Atherosclerosis, 2004, 175(1), 39-49. [CrossRef]
- Sur, R.; Nigam Sur, A.; Grote, D.; Liebel, F.; & Southall, M. D. Avenanthramides, polyphenols from oats, exhibit anti-inflammatory and anti-itch activity. Arch. Dermatol. 2008, 300(10), 569-574. [CrossRef]
- Tetens, I. Substituting whole grain for refined grain: what is needed to strengthen the scientific evidence for health outcomes? Am. J. Clin. 2017, 105(3), 545-546. [CrossRef]
- Vinolo, M. A.; Rodrigues, H. G.; Nachbar, R. T.; & Curi, R. Regulation of inflammation by short chain fatty acids. Nutr. 2011, 3(10), 858-876. [CrossRef]
- Fischer, N.; Sechet, E.; Friedman, R.; Amiot, A.; Sobhani, I.; Nigro, G. et al. Deacetylase inhibition enhances antimicrobial peptide but not inflammatory cytokine expression upon bacterial challenge. Proc. Natl. Acad. Sci. 2016, 113(21), 2993-3001. [CrossRef]
- Felice, C.; Lewis, A.; Armuzzi, A.; Lindsay, J. O.; & Silver, A. Selective histone deacetylase isoforms as potential therapeutic targets in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2015, 41(1), 26-38. [CrossRef]
- Lee, J. C. Y.; AlGhawas, D. S.; Poutanen, K.; Leung, K. S.; Oger, C.; Galano, J. M. et al. Dietary oat bran increases some proinflammatory polyunsaturated fatty-acid oxidation products and reduces anti-inflammatory products in apolipoprotein E−/− mice. Lipids, 2018, 53(8), 785-796. [CrossRef]
- Martínez, I.; Lattimer, J. M.; Hubach, K. L.; Case, J. A.; Yang, J.; Weber, C. G et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013, 7(2), 269-280. [CrossRef]
| Cereal | Botanical Name |
|---|---|
| Rye | Secalecereale L. |
| Corn | Zea mays L. |
| Sorghum | Sorghum bicolor. |
| Millets | Eleusine coracana (L.) Gaertn. |
| Triticale | Triticosecale Wittmack. |
| Pseudo cereal | Botanical name |
| Quinoa | Chenopodium quinoa Willd. |
| Phenolic Acids | Health Potentials | References |
|---|---|---|
| p-Coumaric | Anti-microbial, Anti-inflammatory, Anti-cancer, | [92,2] |
| Ferulic acid | Anti-cancer, Anti-hypertension, Anti-diabetes, Anti-inflammation | [95,96,97,98] |
| Caffeic acid | Anti-mutagenic, Andti-arcinogenic activity | [100] |
| Sinapic acid | Anti-oxidative , Anti-inflammation, Anti-cancer, Anti-diabetes, Anti-neurodegeneration, Anti-anxiety | [104,105,106,107,108,109] |
| p-Hydroxybenzoic acid | Anti-thrombotic and Anti-coagulant | [114,115] |
| Gallic acid | Anti-cancer HCV inhibition Anti-bacterial | [116,117,118] |
| Vanillic acid | Alzheimer’s disease and Parkinson’s Disease. Neurological disorders, Vascular dementia, Anti-inflammatory and Cerebrovascular insufficiency states. | [119,120,121] |
| Syringic acid | Antidiabetic, Anticancer, Cardioprotective, Anti-inflimation | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




