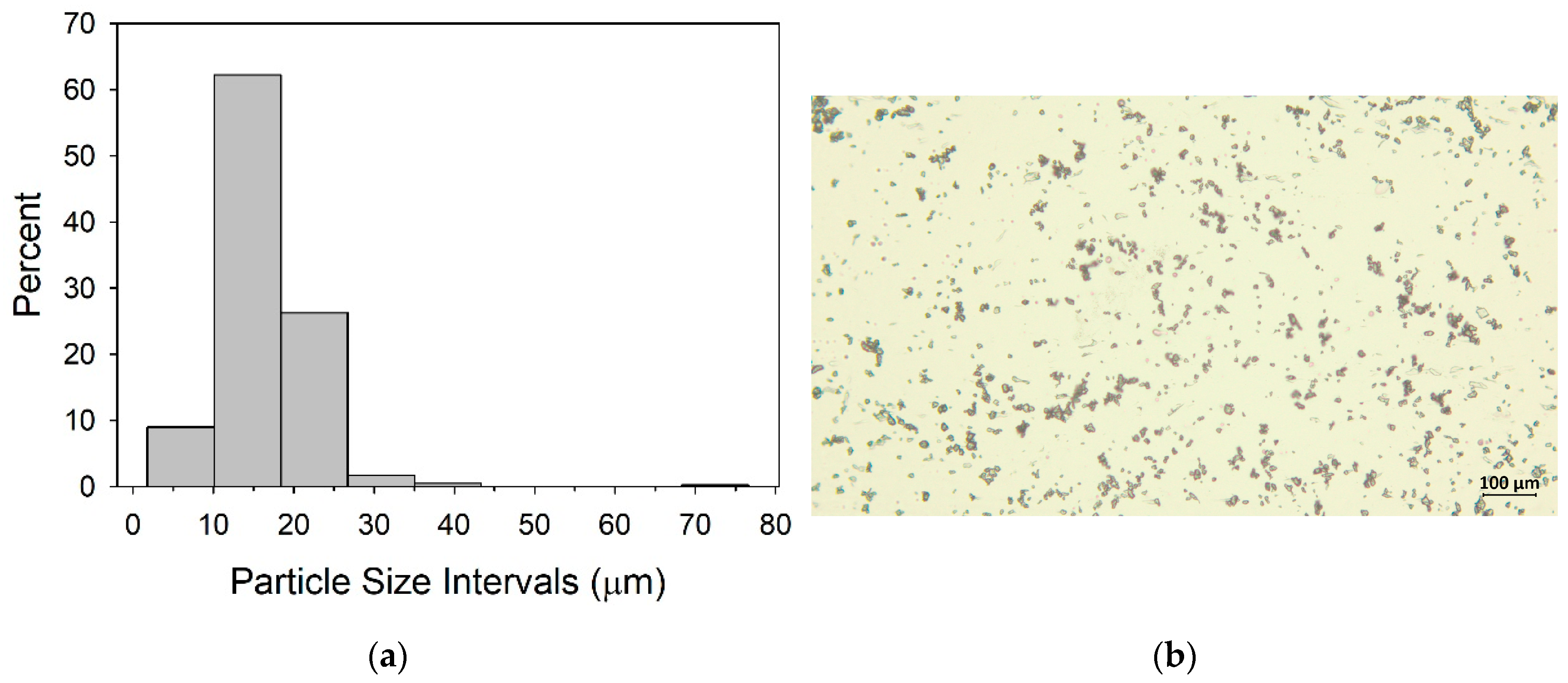
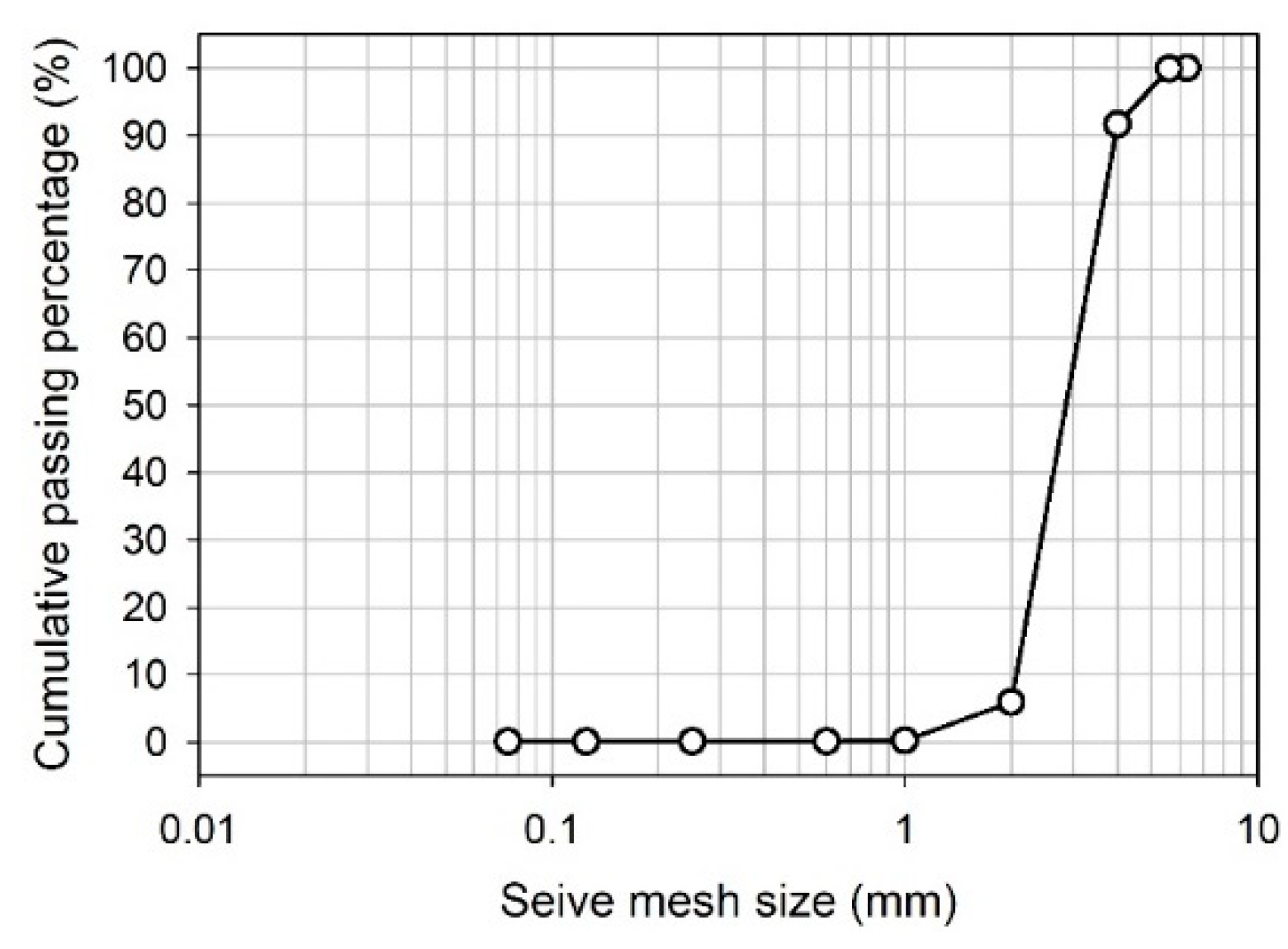
Transport experiments and constant head permeameter experiments were conducted in an acrylic column with 100 cm height and 7 cm inner diameter. The column has been packed with washed and dried sand at 105 ° C overnight. During the packaging process, water was pumped from bottom to top, and the column was gently tapped to prevent possible air bubbles.
The experiments adhered to a sequence commencing with constant-head permeameter tests to ascertain hydraulic conductivity. Subsequently, dye tracer experiments were conducted to evaluate the hydrodynamic properties of the porous media, and the sequence concluded with MPls tracer experiments aimed at determining sorption parameters and retention efficiencies of the porous media. This order was consistently followed for each column packing, and after completing the series of experiments, the porous media were changed for a new set of tests. In total, four sets of experiments were carried out.
2.2.2. Transport Experiments
Subsequently, the column underwent a thorough flushing with distilled water, reaching a state of saturation characterized by effluent exhibiting negligible turbidity measurements. The operational pH and electrical conductivity values were measured following each MPls transport experiment, with mean values determined as 7 ± 0.2 for pH and 57 ± 5 µS cm-1 for electrical conductivity.
The volume of one pore volume in each sand-packed column varies slightly depending on the sand height and porosity. Specifically, the average one pore volume of the sand-packed column is calculated to be 1664 ± 31 ml. The amount equivalent to 1.8 pore volume of fluorescent dye tracer was continuously injected, followed using at least 2 pore volumes for the 65 ± 0.3 ml min⁻¹ flow rate, and 5 pore volumes for the 31 ± 0.4 ml min⁻¹ flow rate of distilled water to flush the column. This injection procedure was followed for continuous injection of MPls. The flushing process was conducted at a constant flow rate generated by a peristaltic pump 65 ± 0.5 ml min⁻¹ and 31 ± 0.2 ml min⁻¹ flow rates. Relative to the flow rate employed, samples of 4 ml each were collected every two minutes, commencing from the 8th minute post-injection for a flow rate of 65 ml min⁻¹, and every three minutes, beginning from the 27th minute post-injection for a flow rate of 31 ml min⁻¹. Each flow rate condition was replicated.
The flow rates of 31 ml min⁻¹ and 65 ml min⁻¹ correspond to linear velocities (v) of 0.4 cm min⁻¹ and 0.8 cm min⁻¹, respectively. Groundwater moves from higher elevations to lower elevations and from locations of higher pressure to locations of lower pressure. Typically, this movement is quite slow, ranging from less than 0.3 meters per day to a few tens of meters per day. For karst aquifers, which are not considered porous medium, it could be faster [
21].Typical groundwater velocity in a sandy or gravelly aquifer may range from approximately 0.01 to 1 cm per minute [
22].
There is nearly a twofold difference between the two flow rates, which allows for the distinction of their effects on the experiment. When the flow rate is too fast, advective transport becomes dominant, overshadowing the dispersion and sorption effects. Therefore, a maximum flow rate of 65 ml min⁻¹ was chosen. For the selection of the lowest flow rate, the reliability of operational conditions was considered, particularly focusing on the duration of the experiment, which was set to six hours for a flow rate of 31 ml min⁻¹. These selected flow rates align with the general conditions of groundwater flow.
The Aquaflour
® Handheld dual-channel fluorometer and turbidity device were utilized for concentration determination. For fluorescent dye tracer experiments, the concentration measurements were calibrated using known concentrations of dye solutions in the green channel. Following the calibration of the fluorometer at a concentration of 400 µg/L rhodamine WT for effluent measurements, dilutions of rhodamine WT were prepared for each experiment by deriving calibration curves from the injection solution. (
Figure S1) For MPl tracer experiments, the turbidity channel was employed. The device was initially calibrated using a 1000 NTU calibration standard, followed by the creation of a calibration curve using MPls and surfactant-containing suspension of different concentrations to convert NTU to concentration values (
Figure S2). The injection concentration is selected 2 g/L which is approximately equal to the 1000 NTU to reduce the measurement errors due to impurities caused by sand or water at the low concentrations in the experiment.
In simulation computations, MT3DMS where MT3D stands for the Modular 3-Dimensional Transport model, and MS denotes the multi-species structure for accommodating add-on reaction packages is commonly employed alongside MODFLOW which is a block-centered finite-difference flow model. Following the development and calibration of a flow model, the required data for the transport model was retrieved accordingly. [
23,
24]. The transport of fluorescent dye was simulated using advection-dispersion equations corporation first-order reversible kinetic (non-equilibrium) sorption, while the transport of MPls was modeled with advection-dispersion equations incorporating first-order reversible kinetic (non-equilibrium) sorption and first-order irreversible sorption terms. The numerical models from the observed values were created with Groundwater Vistas Version 7 (student license) which provides user interfaces for MODFLOW and MT3D modules of USGS [
24]. The MT3D module is applied for modeling advection, dispersion, and chemical reactions within groundwater systems.
The partial differential equation governing the fate and transport of contaminants in one-dimensional, transient groundwater flow systems can be expressed as follows [
24] :
where θ is porosity (-), C is concentration (ML
-3), t is time (T), x is distance (L), D is hydrodynamic dispersion coefficient (L
2T
-1), v is linear pore water velocity (LT
-1), q is volumetric flow rate per unit volume of aquifer representing fluid sources (positive) and sinks (negative) (T
-1), C
s is concentration of the source or sink flux (ML
-3), and ΣRn is chemical reaction term (ML
-3T
-1).
In instances where the local equilibrium cannot be attained, it is posited that the sorption process may be effectively delineated through a reversible kinetic reaction of the first order as follows [
24]:
Where ρb is bulk density (M/L3), β is the first-order mass transfer rate between the dissolved (C) and sorbed (C’) phases (T-1), and Kd is the distribution coefficient (L3M-1).
The bulk density ρ
b is calculated from the density ρ of sand grains (M/L
3) which is calculated with a pycnometer. Then the bulk density is calculated as follows [
20]:
In some situations, once the solute is sorbed on the solid phase, it cannot be desorbed. The reaction is irreversible and leads to a mass loss of the dissolved phase. This process can be described through the first-order irreversible kinetic sorption model, with the following equation [
25]:
where K
1 (T
-1) is the first order irreversible kinetic sorption rate constant.
The first-order irreversible kinetic sorption model was used by Lee et al., (2021) to analyze the migration of toluene contaminant plume in 3D flow conditions. This last kinetic model was also applied to simulate radioactive decay or biodegradation [
27]. In such cases, the constant rate (K
1) is related to the half-life (t
1/2) of the materials as follows (Equation 5):
where t
1/2 is conventionally denoting half-life (T
-1), was employed to signify the retained mass rather than its conventional application to represent the temporal duration associated with the decay of a substance by half.
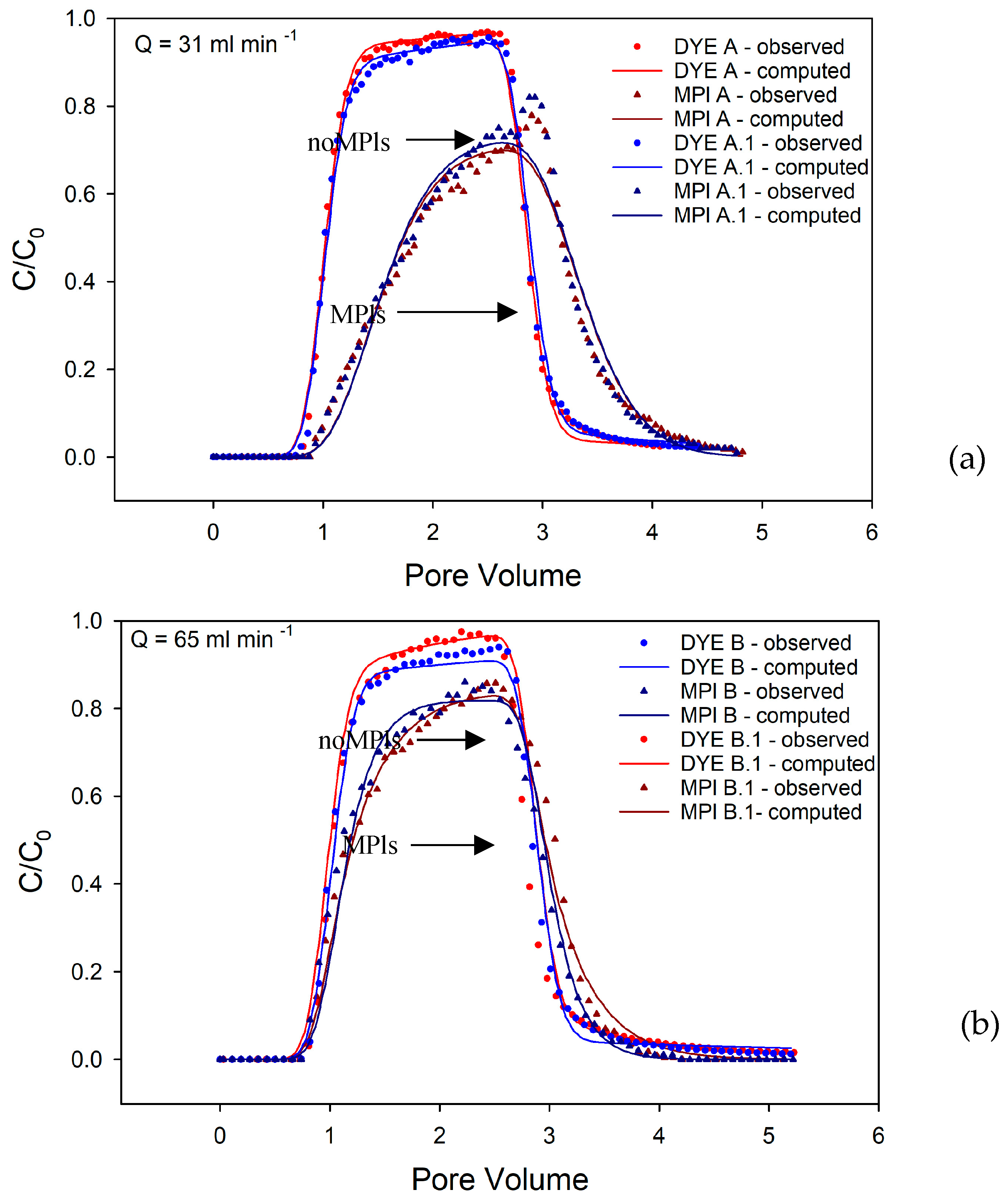
Rhodamine WT might not behave entirely as a conservative tracer, especially when applied to the used quartz sand [
28,
29]. Consequently, a numerical solution incorporating sorption was chosen as a better fit, enhancing the alignment of calculated BTCs with observed BTCs. Through fluorescent dye experiments, kinematic porosity (θ), dispersivity (α), distribution coefficient for the sorbed phase (K
d), and the first-order mass transfer rate (β) values that characterize properties of porous media were estimated. The obtained θ and α values were subsequently utilized as inputs for analyzing MPl experiments. In the MPl experiments, the parameters under investigation include the distribution K
d, β, and K
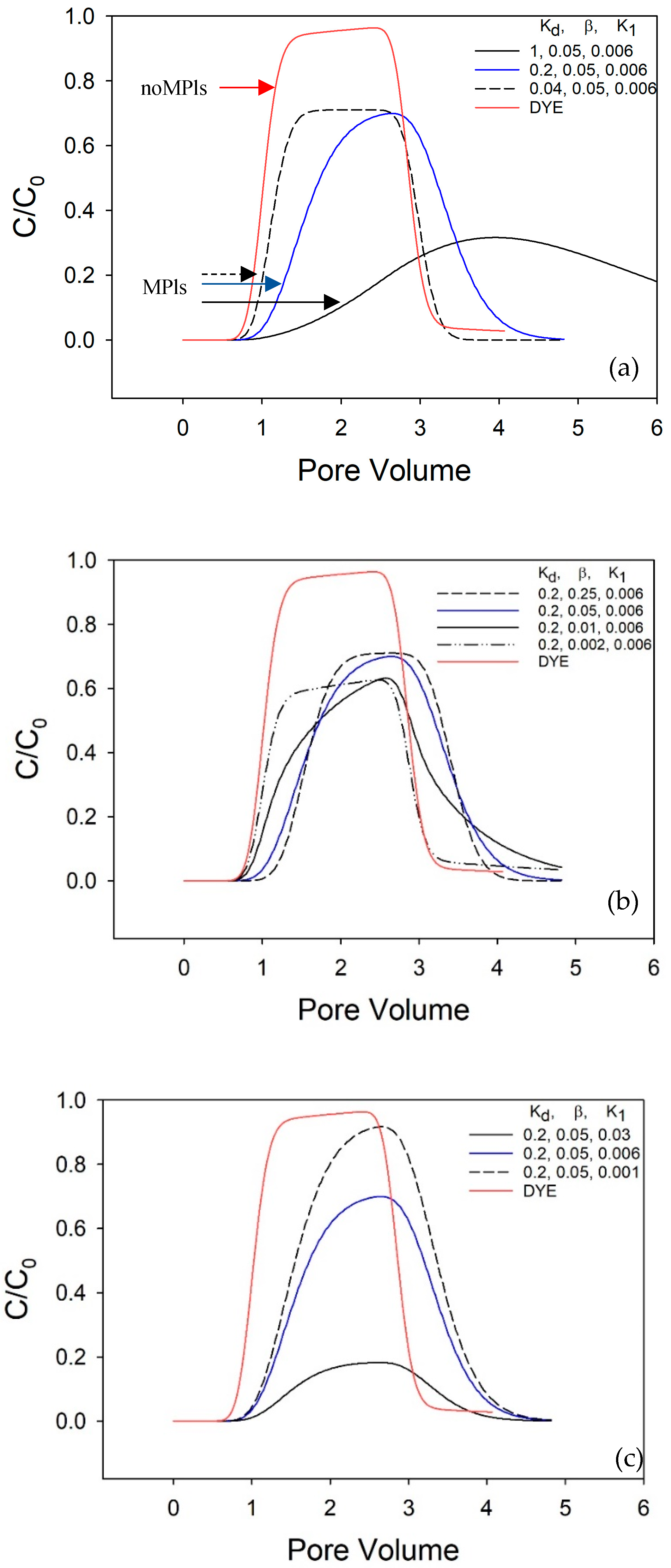
1. The identification of optimal values necessitates the utilization of a trial-and-error approach. The parameters Kd, β, and K
1 were adjusted iteratively to align the curve with the calculated values to the curve with observed values. Following each parameter adjustment, the residual sum of squares (RSS) error was scrutinized.
2.2.3. Retention Efficiencies
The quantity of MPls transported through the porous media was assessed through numerical model mass calculations and by measuring the mass of particles at the conclusion of experiments. The numerical model evaluation involves accounting for mass released from storage as a result of a decrease in sorbed concentration and mass accumulation in storage due to an increase in sorbed concentration, incorporating considerations for first-order irreversible reactions.
Experimental measurements were conducted by collecting the transported effluent with a known volume at the conclusion of experiments. The concentration of accumulated particles was determined using a turbidimeter and then converted to concentration units. By utilizing the known volume, the transported mass was calculated. Subsequently, the initially transported quantity was subtracted from the total injected amount, thereby yielding insights into the retention rates of MPls in porous media.
The Mean Absolute Error (MAE) was calculated by comparing the retention values obtained numerically with those measured experimentally, aiming to evaluate the precision of the numerical model. MAE provides a direct measure of the average absolute deviation between predicted and actual values. It is frequently employed in scenarios where all errors carry equal significance, rendering it more resilient to the influence of outliers compared to other metrics such as RMSE. Additionally, MAE shares the same unit of measurement as the original data, further enhancing its interpretability.