1. Introduction
Tendinopathy is a common, often disabling condition associated with tendon pain, functional decline and reduced exercise tolerance of the tendon and surrounding tissue structures [
1,
2,
3,
4,
5,
6]. Tendinopathy is also referred to as tendinitis, tendinosis, and tenosynovitis [
7].
Histopathological evaluation of tendinopathic biopsies using light microscopy demonstrates cell proliferation and morphological alteration of tenocytes, where they appear prominent and round, as identified by Zhang & Wang (2010) [
8]. Electron microscopy shows the presence of disorganisation and calcification of collagen fibres, elevated levels of ground substance, morphological alterations of mitochondria and nuclei, intracytoplasmic and mitochondrial calcification, mucoid patches, and the presence of vacuoles and lipid cells between fibres [
9,
10,
11,
12,
13,
14,
15,
16].
In chronic tendon disease, repeated trauma causes collagen fibres to become disorganised and fragmented, inducing a stem cell driven adaptive metaplastic response, away from a fibroblastic phenotype, toward a more chondroid phenotype, evidenced by collagen degeneration and increased proteoglycan production [
16].
The pathophysiology of tendinopathy remains largely undetermined; however recent studies have identified upregulation of pro-inflammatory and apoptotic cytokines in tendinopathic tendon tissue [
15,
17,
18,
19,
20]. These inflammatory cytokines (IL-1, IL-6, IL-8, IL-10, IL-17, IL-33, COX-1, COX-2, TGF, FGF) are produced by macrophages predominantly [
21]. However recent in vitro studies have demonstrated an active role of tenocytes under pro-inflammatory conditions, in propagating an inflammatory response. For, example, Stolk et al 2017 demonstrated that tenocytes in vitro responded to inflammatory environments with altered surface marker and cytokine profiles (IL-6, IL-8 and monocyte chemoattractant protein 1), inducing a pro-inflammatory macrophage polarization, characterised by CD 80 expression and a decreased HLA-DR-expression [
22]. Another study by Giancola et al 2022 demonstrated the role of tenocytes (tendon stem cell/progenitor cells) in releasing CD 200 ligand which binds to CD 200 receptors located on immune cells such as macrophages resulting in an upregulation of inflammatory processes during tendon injury/repair [
23].
One of the major limitations of human tendon studies is that tendon biopsies are usually obtained when patients have already torn their tendons or have had symptoms for at least 6 months. Consequently, biopsy material is likely to represent chronic, rather than early-stage processes. Previously, our research group (Miller et al) has shown that matched intact subscapularis tendons from patients with full-thickness supraspinatus tears display milder tendinopathic changes (Bonar Score grade 2-3), and increased levels of cytokines and apoptotic markers, thereby serving as an effective model for early stage human tendinopathy disease [
18,
24].
Molecular studies in tendinopathy by Dakin et al showed that acute and subacute stages of tendinopathy are associated with an upregulation of inflammatory cytokines (IL-1, IL-6, IL-17, IL-23, TGF) and cells (macrophages, mast cells, granulocytes and lymphocytes) [
25,
26]. Millar et. al. demonstrated an upregulation of inflammatory cells (macrophages, mast cells and T cells) in early human tendinopathy stages compared to both late-stage tendinopathy and normal healthy tendon samples [
24].
Millar et. al. (2017) hypothesised that in tendinopathy mast cells and macrophages, activated by damage and pathogen associated molecular patterns, produce cytokines which recruit lymphocytes and activate tenocyte surface receptors [
4]. These inflammatory cytokines such as IL-6, IL-15, and IL-18, stimulate reactive O
2 metabolites via the Fas-Ligand pathways, which in turn contributes to oxidative stress induced apoptosis of tenocytes [
26,
27].
Of note, our research group has demonstrated elevated IL-17A mRNA expression in early stages of tendinopathy compared to the late stage tendinopathy (torn supraspinatus) and healthy tendon samples [
18]. IL-17A did not just enhanced collagen synthesis but altered the synthesis from type I to type III collagen in human tenocyte cultures. Additionally, it regulated apoptosis through the overstimulation of caspase molecules and induced the synthesis of inflammatory cytokines such as TNF-α, MIP1α, IL-6, IL-8 and MCP-1 [
18].
IL-17, a potent inflammatory cytokine primarily produced by Th17 cells, has been implicated in autoimmune and inflammatory diseases such as inflammatory bowel disease, multiple sclerosis and rheumatoid arthritis wherein cells such as monocytes, dendritic cells, macrophages, and fibroblasts produce a milieu of chemicals (TNF-α, IL-1β, IL-6, IL-8, GM-CSF, MMP, CCL and PGE2) that generate a Th17 cell immune response [
28,
29,
30,
31].
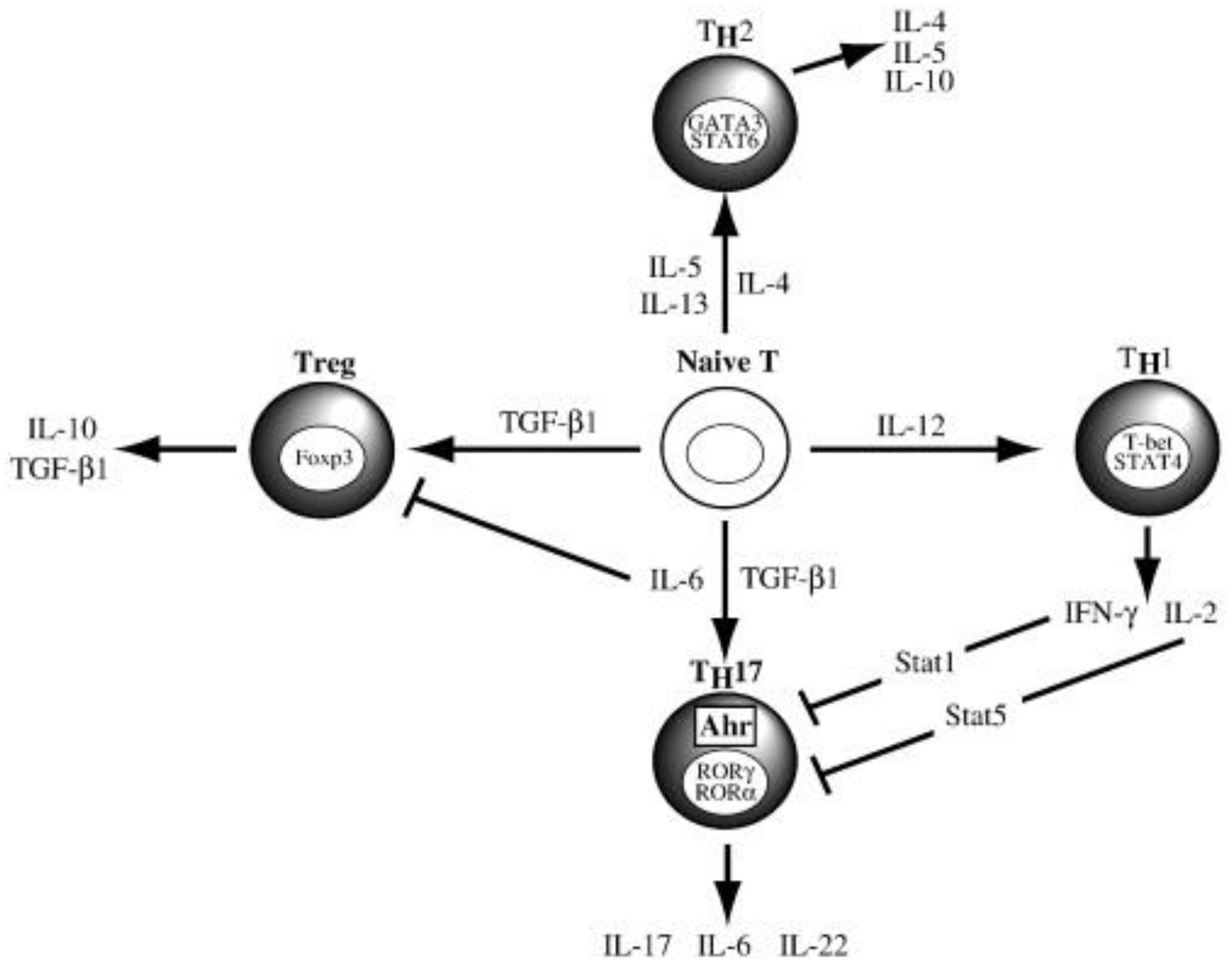
Th17 cells, a subset of CD4+ T cells, are pivotal in shaping an adaptive immune response [
32]. Naïve CD4+ T cells develop into different subsets (Th1, Th2, Th9, Th17, Tfh and Treg), based on the quantity and type of cytokines that interact with them (
Figure 1) [
28]. Kimura et. al. reported that TGF-β and IL-6 cytokines promote Th17 cell differentiation (
Figure 1) [
32].
Dendritic Cells (DCs) are known to initiate and regulate a T-cell adaptive immune response during pathogenic insults, tumour development and autoimmune responses [
33,
34]. Of note, monocyte-derived DCs (mo-DCs) have been found to activate Th17 cells and initiate IL-17 cytokine release (from Th 17 cells) in multiple myeloma [
33], ascites, breast cancer, arthritic joints [
35], systemic lupus erythematosus [
29], multiple sclerosis, autoimmune encephalitis [
36,
37], psoriasis [
38], and colitis [
39].
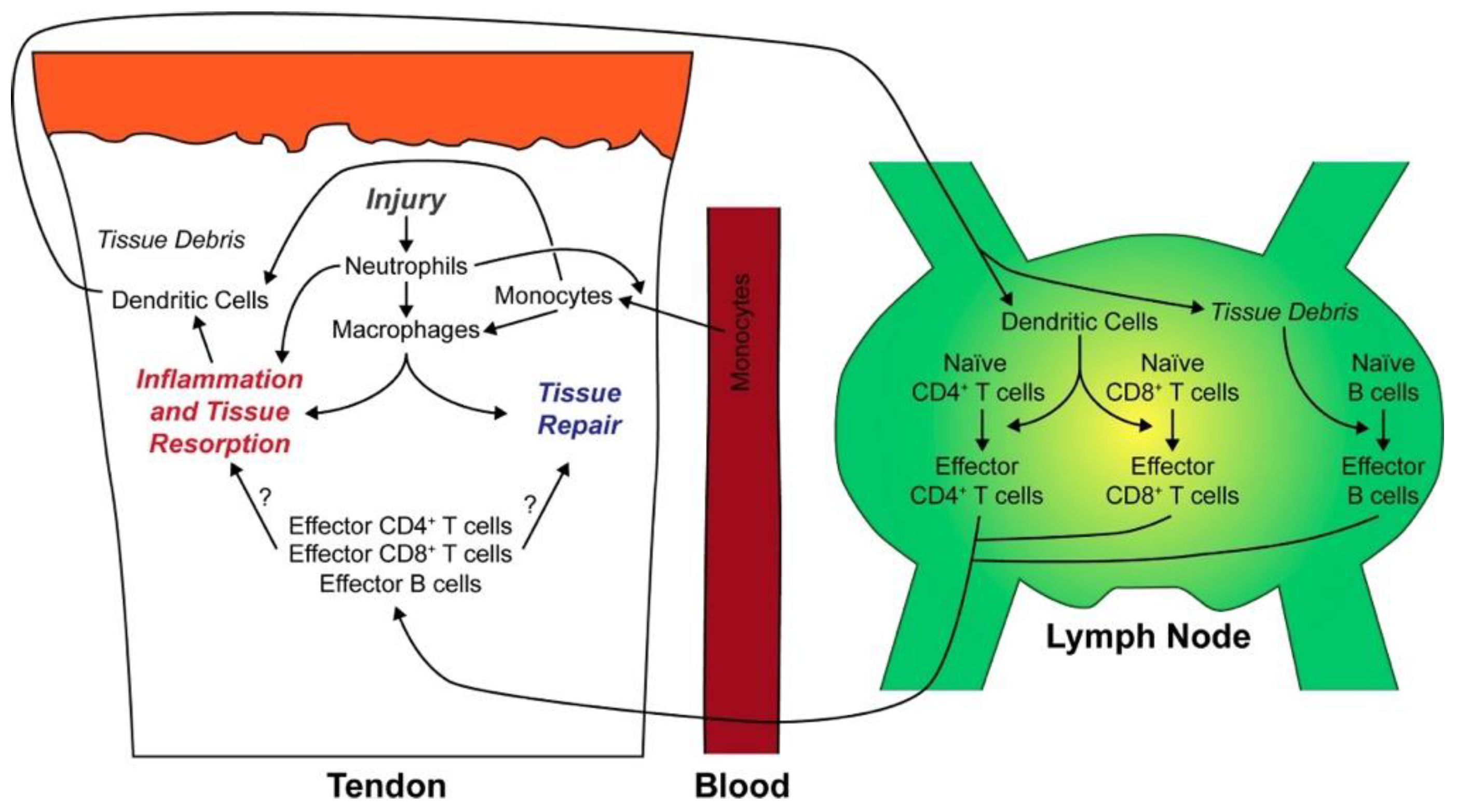
Noah et. al., whilst studying tendon injury and repair in rats, observed the presence of mo-DCs. They proposed that mo-DCs, on being stimulated by locally released inflammatory cytokines, migrated from tendons to lymph nodes, where they primed naïve CD4+ and CD8+ T cells; this led to the production of an adaptive immune response mediated by the primed T cells (
Figure 2) [
40].
The work outlined above supports the hypothesis that IL-17-producing Th17 cells are involved in the pathogenesis of tendinopathy, and that dendritic cells play a crucial role in priming Th17. Whilst this pathway of dendritic cell-mediated immune response (
Figure 3) has been developed and hypothesised in acute tendon injury in rats, to our knowledge this cascade of events has not been explored in a human tendinopathy model. Specifically, to our knowledge, there are no studies that have determined if there is an upregulation of mo-DCs in human tendinopathy.
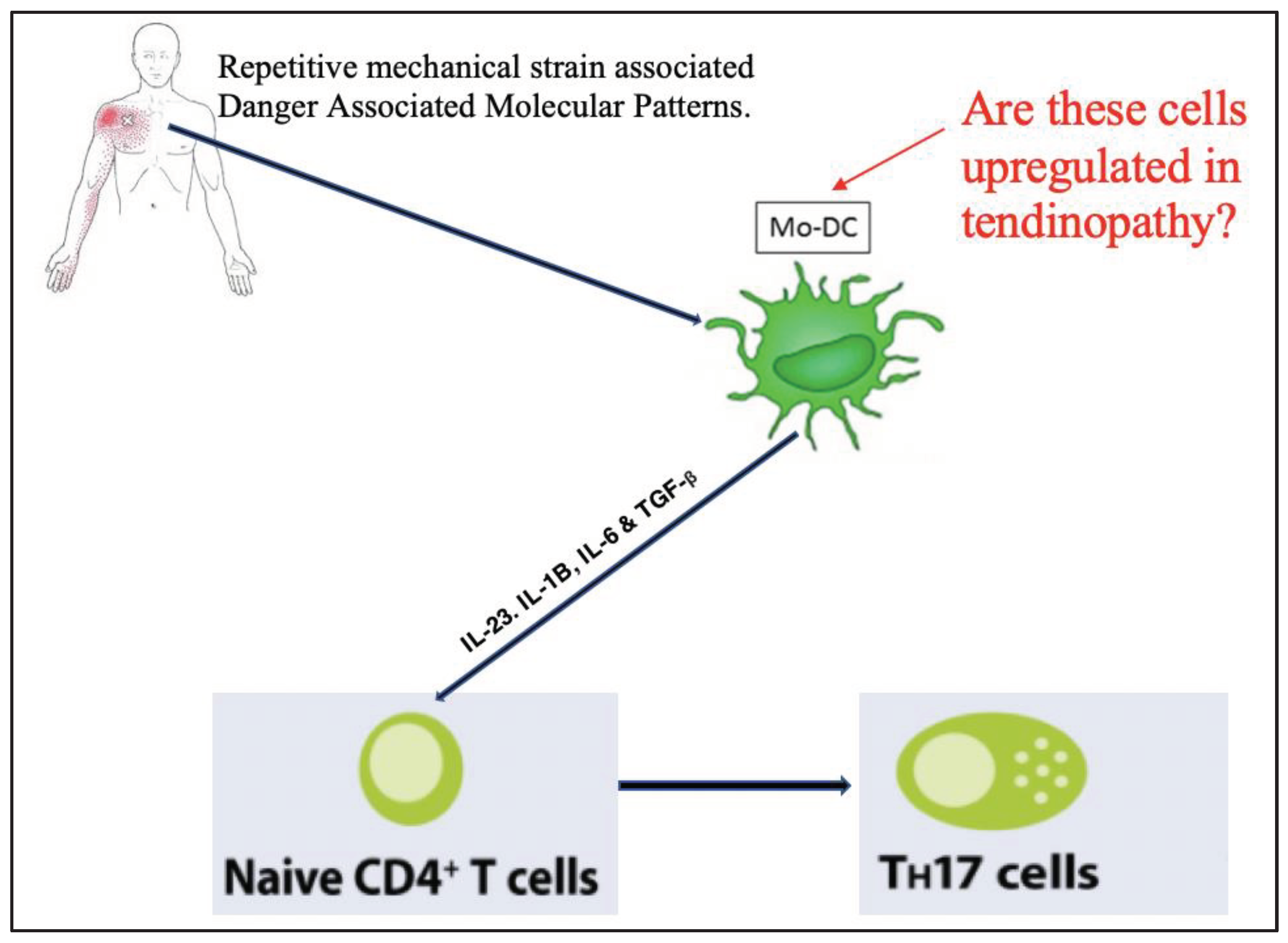
We hypothesised that when tendon tissue encounters repetitive mechanical strain and cell damage, alarmins and tenocytes favour the development of a Th17 inflammatory response, through the activation of monocyte derived dendritic cells (mo-DC). Therefore, the purpose of this study was to determine if mo-DCs are upregulated in a human tendinopathy model (
Figure 3).
3. Discussion
We hypothesised that when tenocytes encounter repetitive mechanical strain associated cell damage, danger associated molecular patterns (DAMPs) favour the development of a Th17 inflammatory response, through the activation of monocyte derived DCs. Our data supports this hypothesis by demonstrating a significantly higher number of positively stained cells for mo-DC protein markers in the tendinopathic tendon when compared to healthy tendon samples.
This study is the first to determine the upregulation of mo-DCs in the human model of tendinopathy using a rotator cuff tendinopathy model and mo-DC protein markers- CD 206 and CD 11c. Our study’s findings corroborate with our previous observations of increased inflammatory changes in the early stages of tendinopathy[
4,
24].
The positive controls of this study, namely human tonsil tissue, known to express CD 11c surface protein [
49], and human placental tissue, known to express CD 206 surface protein [
50], displayed positive staining. This served to verify that the immunohistochemistry procedure was both working and optimised, and that any negative results were valid. Furthermore, incubation of the tissue with negative isotypes (IgG and IgG Kappa B), which being non-immune antibodies of the same isotype as the primary antibodies, served as negative controls for this study. The absence of positive staining in the negative isotype tendon control samples validates the specificity of the primary antibody staining by demonstrating that the observed staining was not caused by non-specific interactions of the antibody such as Fc receptor interactions and endogenous biotic reactions[
51].
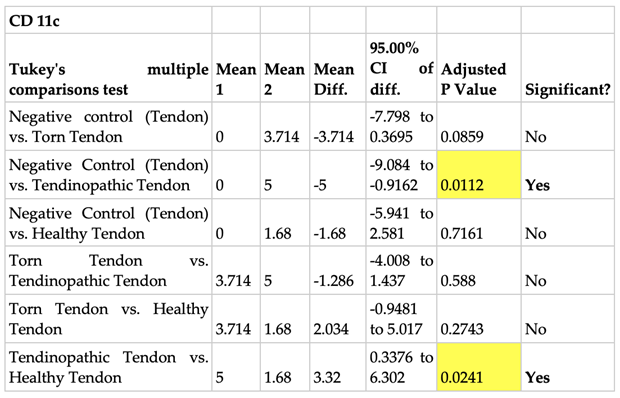
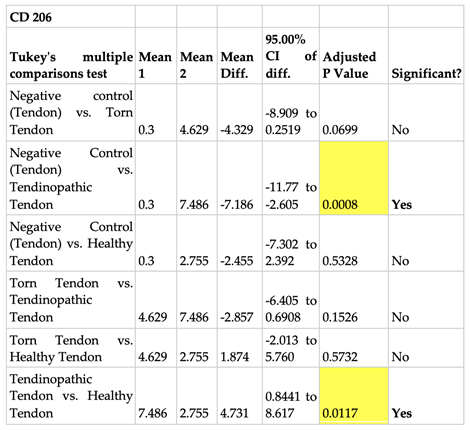
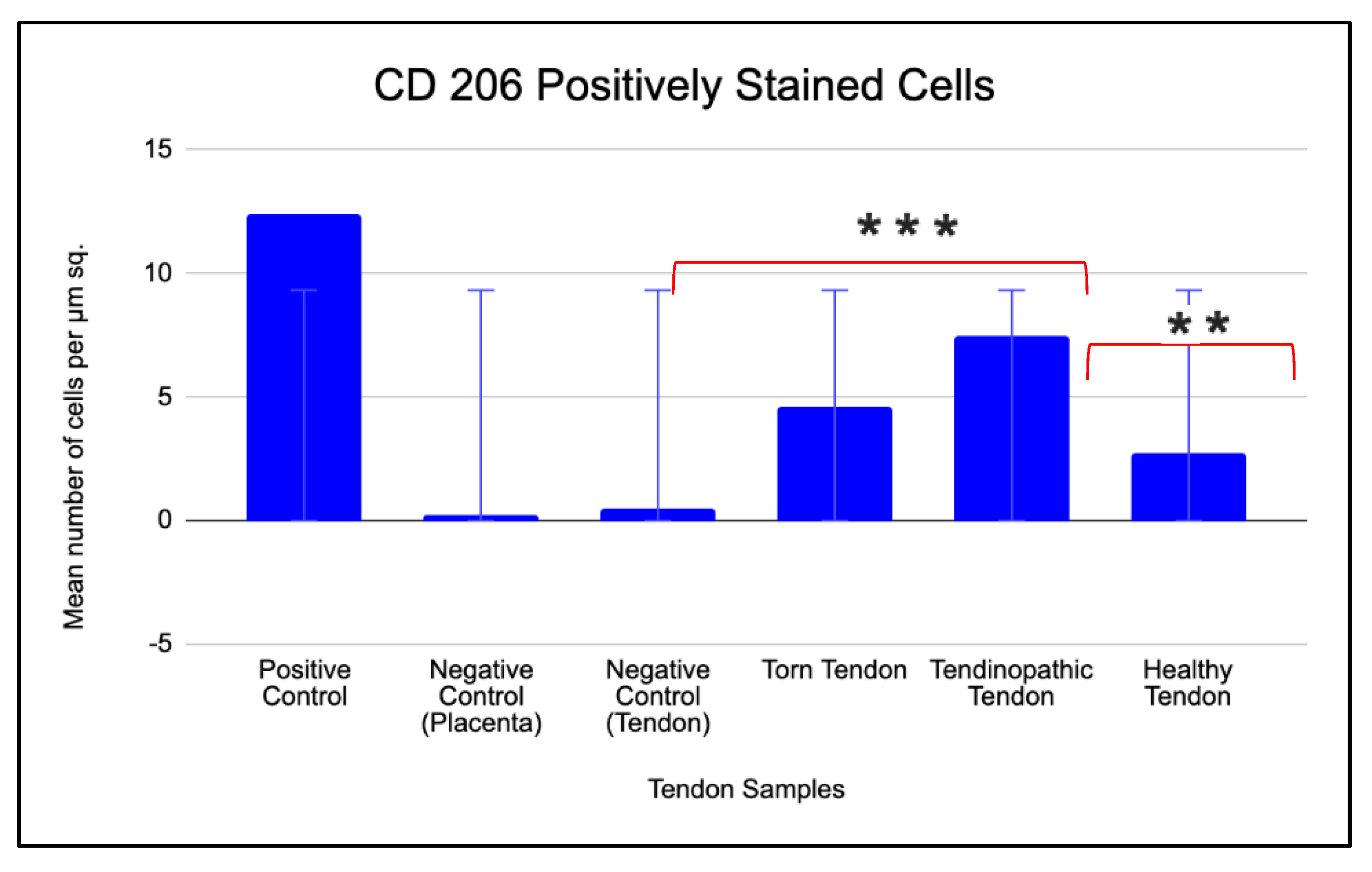
The prevalence of upregulated positively stained cells in the torn tendon when compared to negative tendon control for both CD 11c and CD 206 surface protein markers, borders very close to being statistically significant (p=.08 for CD 11c and p=.06 for CD 206). This trend may be attributed to a small sample size resulting in a low statistical power of the study’s findings.
Histological findings of the tendon samples are consistent with our previous studies [
20,
27,
41,
42,
43,
44,
45,
46] and supports the hypothesis that matched subscapularis is a good human model of early-stage tendinopathy.
Monocytes play a vital role in the innate response to both danger and pathogen associated molecular patterned alarmins [
52]. They make up 10% of leukocytes in human blood, and can differentiate into specialised antigen presenting dendritic cells (mo-DCs) in the presence of IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF), making them distinct from the innate (polymorphonuclear leukocytes, natural killer cells) and lymphoid (T and B cells) arms of the immune system [
52,
53].
Mo-DCs are identified by the following cell surface markers: CD 206, CD 86, CD11a, CD 11c, CD11b, MHC-II, CD24, and CD172a [
53]. This study used the protein markers - CD 206 and CD 11c, to conduct the investigation. Further evaluation of other mo-DC specific surface protein markers in the human tendinopathy model would be helpful in validating this study’s hypothesis.
Qu et al, León et al and Le Borgne et al demonstrated in infectious diseases such as Leishmania that monocytes in the presence of a cytokine enriched microenvironment differentiate into mo-DCs, which then prime naïve CD4 T cells with antigens in order to facilitate a reparative adaptive immune response [
53,
54,
55,
56].
Mo-DCs play a crucial role in various inflammatory conditions, wherein monocytes upon being recruited to areas of inflammation differentiate into mo-DCs [
53,
54,
55,
56]. Crowe et al [
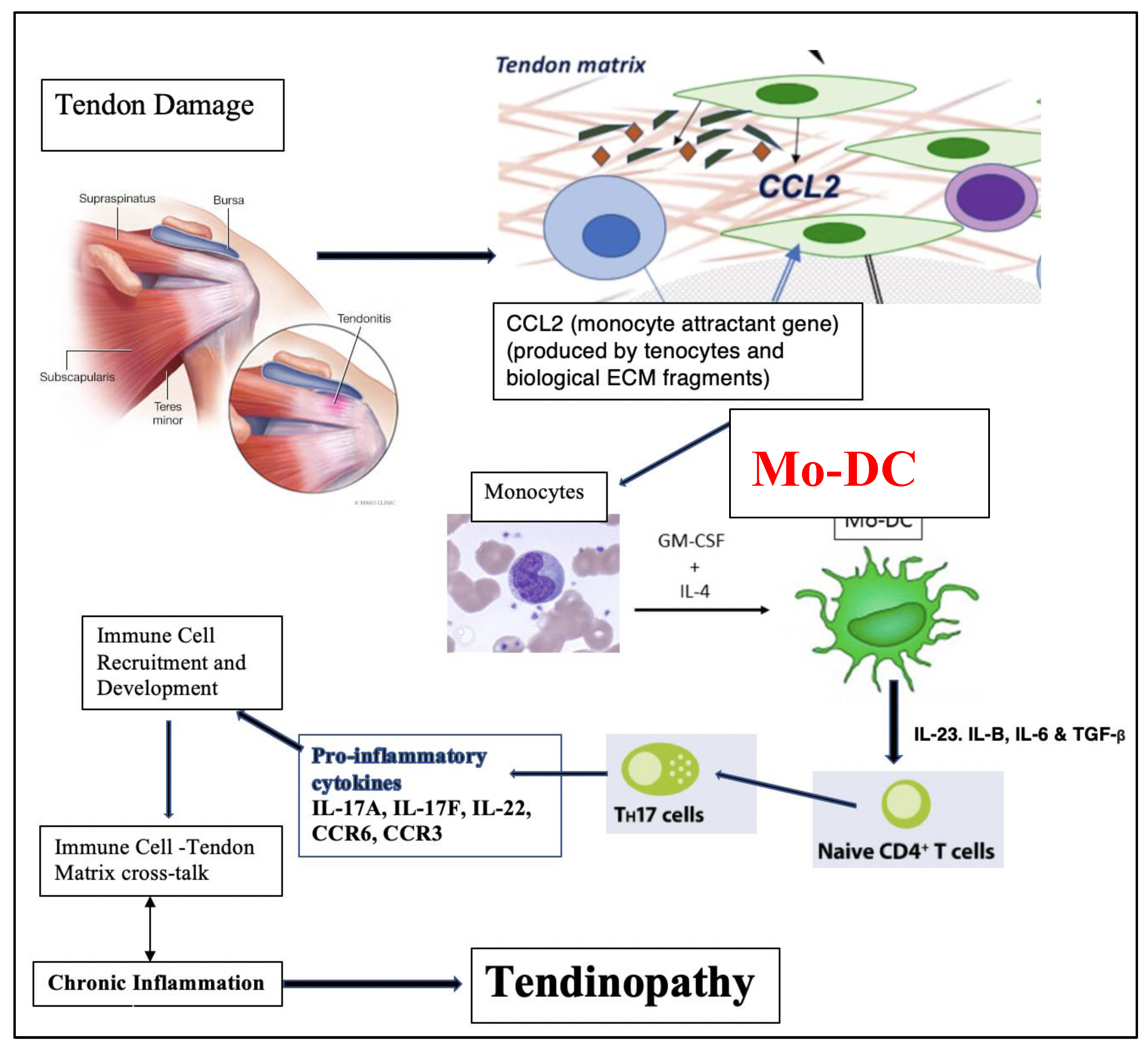
41] proposed that upon cell damage, tenocytes produce chemokine (C-C motif) ligand 2 (CCL2) which recruits monocytes to the site of injury. These monocytes subsequently release S100A8 and S100A9 which bind to tenocyte surface receptors and induces the release of cytokines which mediates further immune cell infiltration. This pro-inflammatory cytokine rich microenvironment enables immune cell-tendon matrix crosstalk and facilitates a chronic inflammatory state (
Figure 10) [
41].
Sugg et al [
57] and Noah et al [
40] similar to Crowe et al observed there to be an upregulation of CCL2 monocyte attractant gene in the first week after tendon injury in a rat Achilles tenotomy and repair model. Noah et al based on their observations hypothesised that upon tendon injury in a rat model, neutrophils accumulate at the injury site and initiate the inflammatory response. Consequently, recruited monocytes undergo differentiation into dendritic cells and macrophages. Finally, both these cells upon processing antigens from the site of injury present it to naïve lymphocytes. Proinflammatory M1 macrophages govern the initial response during the first two weeks of the injury. Progressively over the following weeks, tissue regenerative anti-inflammatory M2 macrophages take over. Concurrently, dendritic cells, upon being stimulated by a milieu of locally produced inflammatory cytokines, journey to neighbouring lymph nodes to prime naïve CD4+ and CD8+ T cells. These differentiated T cells then migrate to the tendon site, where they engage in orchestrating tissue repair [
40]. Our study’s findings corroborate this model in human tendinopathy by evidencing a significant upregulation of mo-DCs in the early stages of the pathology (tendinopathic tendon).
Segura et. al. observed that mo-DCs primed Th17 cells by secreting high levels of IL1β, IL-6, IL-23 and TNF-α in synovial fluid from rheumatoid arthritis patients and ascitic fluid from untreated ovary and breast cancer patients [
30]. These cytokines have been found to be potent inducers of Th17 cells [
58,
59,
60,
61].
Dhodapkar et al demonstrated that mature human mo-DCs when loaded with Candida antigen and apoptotic tumour cells, effectively induced T cells to form Th17 cells in human cell culture; this DC-mediated Th17 cells expansion depended on, the presence of polarizing cytokines (TNF, IL1β, IL-6, and IL-23), and cell-contact [
33].
Zaba et al demonstrated that the pathogenesis of psoriasis in humans, entails complex interactions between DCs, keratinocytes, Th1 cells, and Th17 cells, and that mo-DCs play a central role in inducing a mixed Th 1/17 pro-inflammatory response [
62].
Santini et al demonstrated that in conditions such as psoriasis and carcinomas, IFN-alpha conditioned monocyte-derived DCs upon taking up apoptotic cell-derived antigens efficiently primed autologous IL-17 secreting Th17 cells, mainly through the cytokine- IL-23 in human cell cultures (
Figure 11) [
63].
Farkas and Kemény upon investigating the pathogenesis of psoriasis suggested that interferon-alpha produced by pDCs along with GM-CSF, TNF-α, IL-6, and IL-1β, cumulatively induce monocyte-derived DC differentiation. Mo-DCs, in turn, promote Th1 and/or Th17 cell development. These T helper cells then stimulate further mo-DC differentiation by facilitating the secretion of GM-CSF and TNF-α thereby generating an unrestrained augmentation loop, which results in the accentuation of inflammatory processes [
64]. It is possible that similar processes occur in the pathogenesis of tendinopathy.
Upon investigating the specific nature of the lymphocytic response in human tendinopathy, our research group previously showed that the cytokine IL-17A produced by Th17 cells, is present in early tendinopathy (tendinopathic subscapularis tendon). Additionally, using human tenocyte cultures, they observed that the IL-17A cytokine stimulated endothelial cells, macrophages, and fibroblasts to release several cytokines, such as matrix metalloproteinases, NO synthase, TNF-α, IL-6, and IL-1, resulting inflammatory and apoptotic changes, all of which have been demonstrated to be present in tendinopathy [
18]. As aforementioned, Mo-DCs are known to prime naïve T cells to formulate a pro-inflammatory Th 17 cell response [
29,
30,
33,
35,
37] [
39]. We, therefore, hypothesise that in the early stages tendinopathy, mo-DCs induce Th17 cell polarisation through the selective secretion of Th17 cell polarising cytokines, notably IL-23 [
52]. To test this hypothesis, we require in-vitro studies that evaluate gene expression and cytokine production, specifically IL-23 by mo-DCs in the presence of conditioned media from stimulated tenocytes, and its subsequent effect on naïve T cells. Our study adds the first block of evidence to substantiate this hypothesis by demonstrating a 2-3-fold increase of mo-DC cell prevalence in early tendinopathy compared to healthy tendon samples.
The strengths and weaknesses of this study are similar to our previous studies [
18,
20,
27,
41]. Having subscapularis samples from the same patient served to strengthen the reliability of our findings by eliminating the possibility of biases that may arise from variation between individuals. Furthermore, excluding patients with metabolic diseases and smoking habits serves to eliminate other possible variables that may account for monocyte-derived dendritic cell upregulation and tendon disease. Another strength is that the tissue sample collection in our study was performed by a single experienced orthopaedic surgeon thereby increasing the likelihood of there being greater uniformity in the technique. Finally, the use positive and negative isotype controls ensured the validity of the study’s findings [
51].
We also note several limitations in our study. First, the sample size was relatively small. A larger cohort would have had a greater statistical power. Secondly, the disparity between the ages of our study groups may affect the results. Thirdly, the overall degenerative picture and cell expression seen in the matched subscapularis tendons may be due to age related changes within the tendon samples. However, the lack of degenerative changes on ultrasound and arthroscopic examinations may suggest that the differences are truly at the cellular level as suggested by our previous work [
27]. Fourthly, the subscapularis tendon being organizationally and functionally different from the supraspinatus tendon, responds differently to mechanical loading, which may impact its cellular profile. It is reassuring, however, that previous studies demonstrated the presence of same inflammatory cell subtypes in both torn supraspinatus and matched subscapularis tissue [
18,
20,
27,
41]. This suggests that the inflammatory response is uniform within joints subjected to tendon degeneration [
24]. Fifthly, healthy tendon control samples from subscapularis undergoing stabilisation may not be truly ‘normal’ controls but are currently the best available control tendon sample and this is reflected by a Bonar score of 1. Sixthly, gender and body mass index could impact on both tendon health and (local and systemic) inflammatory status (and could result in monocyte-derived dendritic cell upregulation) are not described for the two groups. Finally, the strength of the study may be affected by the analysis of dendritic cell (DC) density by a single observer only. However, the analysis was undertaken under the guidance of a senior cell biologist on two separate occasions, blinded, and spaced out by a duration of 2 weeks to minimise bias.
4. Materials and Methods
This was a prospective controlled laboratory study designed to investigate the phenotype and quantification of inflammatory monocyte-derived dendritic cells in torn, tendinopathic and healthy control tendon samples. All procedures and protocols were approved by the South East Area Sydney Area Ethics Committee (HREC Ref No. 14/130). The study design for this experiment was based on previous studies done by our research group [
20,
27,
41,
42,
43,
44,
45,
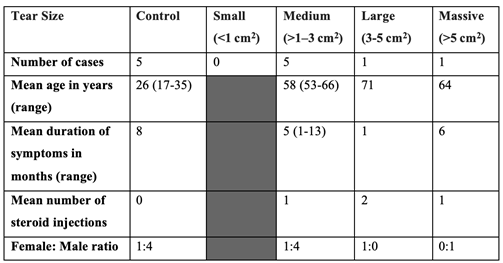
46]. Seven supraspinatus tendon samples were collected from patients with rotator cuff tears undergoing shoulder surgery. Samples of the subscapularis tendon were also collected from the same patients. Patients were only included if there was no clinically detectable damage to the subscapularis tendon at the time of arthroscopy. In this cohort all patients fulfilled the following criteria: 1) a history of shoulder pain and dysfunction, 2) no previous surgery on the affected shoulder, 3) no radiographic sign of fracture of the shoulder, 4) no history of rheumatoid, osteoarthritis, 5) no history of metabolic or hormone-dependent diseases, 6) no history of smoking. Exclusion criteria for this cohort included: 1) revision rotator cuff repair, 2) irreparable rotator cuff tears, 3) tears repaired with synthetic patches, 4) shoulder replacements, fractures, and tumours, 5) arthroscopic damage (tear or partial tear) to the subscapularis tendon.
A healthy tendon control group was obtained comprising subscapularis tendon samples collected from 5 patients undergoing arthroscopic surgery for shoulder stabilization. In this cohort all patients fulfilled the following criteria: 1) no rotator cuff tears, 2) no previous shoulder surgery, 3) no radiographic signs of shoulder fracture and 4) no history of rheumatoid, osteoarthritis or diabetes, 5) no history of metabolic or hormone-dependent diseases, 6) no history of smoking. The absence of rotator cuff tears was confirmed by arthroscopic examination.
Tissue Collection and Preparation
Arthroscopic repair of the rotator cuff was carried out using the standard three-portal technique as described by Wu et al [
47], while the cross-sectional size of the rotator cuff tear was estimated and recorded. The subscapularis tendon was biopsied arthroscopically from the superior border of the tendon 1 cm lateral to the glenoid labarum representing mid-body tendon structure using a meniscal biter. The supraspinatus tendon was harvested from within 1.5 cm of the edge of the tear prior to surgical repair.
All tissue samples that were to be analysed histologically were immediately fixed in 10% (v/v) formalin for 24 hours and then processed and embedded in paraffin. Sections were cut to 5 μm thickness using a Leica-LM microtome (Leica Microsystems, Germany) and placed onto Superfrost Ultra Plus glass slides (Gerhard Menzel, Germany).
Histology and Immunohistochemistry Techniques
A hematoxylin and eosin (H&E) stain was performed on tissue samples to assess for the presence or absence of oedema and degeneration, together with the degree of fibroblast cellularity and chondroid metaplasia. The degree of tendinopathy was assessed by a modified version of the Bonar score (Grade 4 = marked tendinopathy, Grade 3 = advanced tendinopathy, 2 = moderate degeneration 1 = mild degeneration 0 = normal tendon)[
48].
For immunohistochemical analysis, the slide-mounted tissue sections were deparaffinated with xylene and rehydrated through graded ethanol steps (100% to 50%). The slides were then washed twice in Tris Buffered Saline 0.05% Tween (TBST buffer) for three minutes. Any epitopes that had been masked during processing were retrieved by heat-induced epitope retrieval using a 0.01M citrate buffer pH 6.0 (EnVision FLEX Target Retrieval Solution K8005, Dako, NSW, Australia). The slides were placed in citrate buffer and the buffer was allowed to boil for 30 minutes. The slides were then cooled to room temperature and then washed in TBST buffer for 3 minutes.
The slides were then transferred to a staining tray and a wax ring was drawn around the sections. The endogenous peroxidase activity of the tissue was quenched by incubating the sections for 15 minutes in a freshly prepared hydrogen peroxide in methanol solution (3% v/v). Non-specific binding was blocked by incubating sections for 20 minutes at room temperature in 10% normal goat serum (ab7481, Abcam, NSW, Australia) in PBS. The blocking serum was tapped off and the sections were incubated overnight at 4 degrees C with the primary antibodies (MMR/CD206/Mannose Receptor Antibody [NBP1-90020] and CD11c Antibody (ITGAX/1242) [NBP2- 44598], Novus Invitro Technologies Ltd, Colorado, United States) or the negative isotype control (Rabbit IgG Isotype Control [NOVNBP224891] and Mouse IgG2b Isotype Control [NOVNBP196980], Novus Invitro Technologies Ltd, Colorado, United States) diluted in the blocking solution used above.
The next day, the slides were brought to room temperature, the antibody was drained from the slides and washed twice in the TBST buffer for 3 minutes. The tissue sections were incubated with the species-specific secondary antibody reagent (Dako REAL EnVision HRP Rabbit/Mouse K5007 Dako, NSW, Australia) for about 60 minutes. They were then washed twice for 3 minutes in the TBST buffer. The sections were incubated in 20 microliter of DAB chromogen solution (DAB Substrate Kit ab64238, Abcam, NSW, Australia) until the desired stain intensity developed. The slides were washed for 3 minutes in TBST, then the sections were rinsed in distilled water for 3 minutes. Sections were counter-stained with filtered Hematoxylin (1 minute) and excess solution was washed off in running tap water. The sections were then alcohol dehydrated to xylene and mounted in Dibutylphthalate Polystyrene Xylene mounting medium.
Control Staining
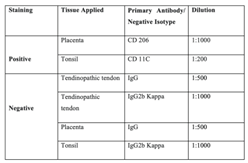
To demonstrate the specificity of the primary antibody staining, the following controls were included (
Table 1):
(a) Positive control: Human placental & tonsil tissue were stained with primary antibody – CD 206 and CD 11C respectively as suggested by the manufacturers.
(b) Negative control: specific negative control isotypes- IgG (CD 206) and IgG2b Kappa (CD 11C) on tendinopathic tendon tissue and positive control tissue (placenta and tonsil tissue respectively).
Table 1.
Details of tissue samples used, and antibody applied.
Table 1.
Details of tissue samples used, and antibody applied.
Image Analysis
Slides were examined with the patient/control identity code masked with black tape. For quantification of positive stained cells, five random high-power fields (x400) of the sample were evaluated by a single assessor and the number of cells per µm² was counted twice.
Data Analysis
Results are reported as mean values ± standard error of the mean (SEM). Dendritic cell (DC) density was counted on two separate occasions by a single observer (SW) and the average count of cells per µm2 was obtained. DC density in torn, tendinopathic and healthy tendon samples was compared using One-way ANOVA with Tukey correction for multiple corrections. All statistical tests were carried out using Prism software (version 8.0, GraphPad Inc, CA). A value of p < 0.05 was considered to be significant.
Figure 1.
Naïve CD 4 T cell differentiation into specialised T helper cells
, adapted from Kimura and Kishimoto, 2011 [
32].
Figure 1.
Naïve CD 4 T cell differentiation into specialised T helper cells
, adapted from Kimura and Kishimoto, 2011 [
32].
Figure 2.
Summary of immune cell changes after tendon injury in a rat tendinopathy model, adapted from Noah et. al., 2020 [
40].
Figure 2.
Summary of immune cell changes after tendon injury in a rat tendinopathy model, adapted from Noah et. al., 2020 [
40].
Figure 3.
Monocyte-derived Dendritic Cells prime naïve CD4+ T cells to form T helper 17 cells in Tendinopathy.
Figure 3.
Monocyte-derived Dendritic Cells prime naïve CD4+ T cells to form T helper 17 cells in Tendinopathy.
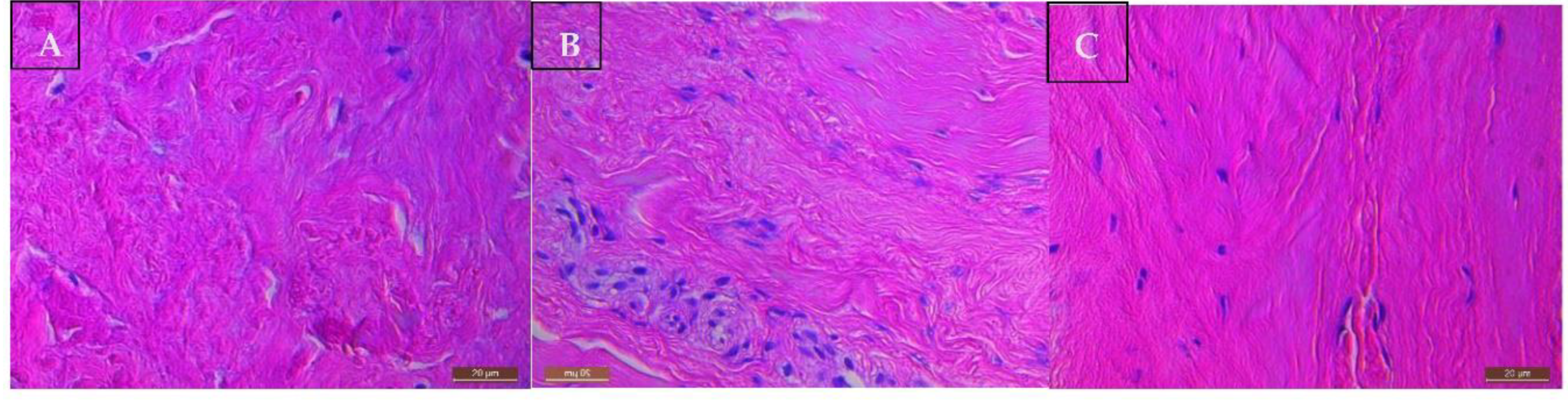
Figure 4.
Histological Images of Tendinopathic and Healthy tendon tissue using H&E stain at 200x magnification showing: A) Supraspinatus torn tendon sample reveals severe tendinopathic changes- increased glycosaminoglycan, collagen disorientation, vascular hyperplasia, and tenocyte rounding. B) Subscapularis tendinopathic tendon sample reveals both areas wherein collagen fibres are disorganised due to elevated mucoid ground substance, and areas of normal dense homogeneous polarisation of collagen. C) Subscapularis healthy tendon sample reveals intact fibrocollagenous tissue architecture with very little myxoid changes.
Figure 4.
Histological Images of Tendinopathic and Healthy tendon tissue using H&E stain at 200x magnification showing: A) Supraspinatus torn tendon sample reveals severe tendinopathic changes- increased glycosaminoglycan, collagen disorientation, vascular hyperplasia, and tenocyte rounding. B) Subscapularis tendinopathic tendon sample reveals both areas wherein collagen fibres are disorganised due to elevated mucoid ground substance, and areas of normal dense homogeneous polarisation of collagen. C) Subscapularis healthy tendon sample reveals intact fibrocollagenous tissue architecture with very little myxoid changes.
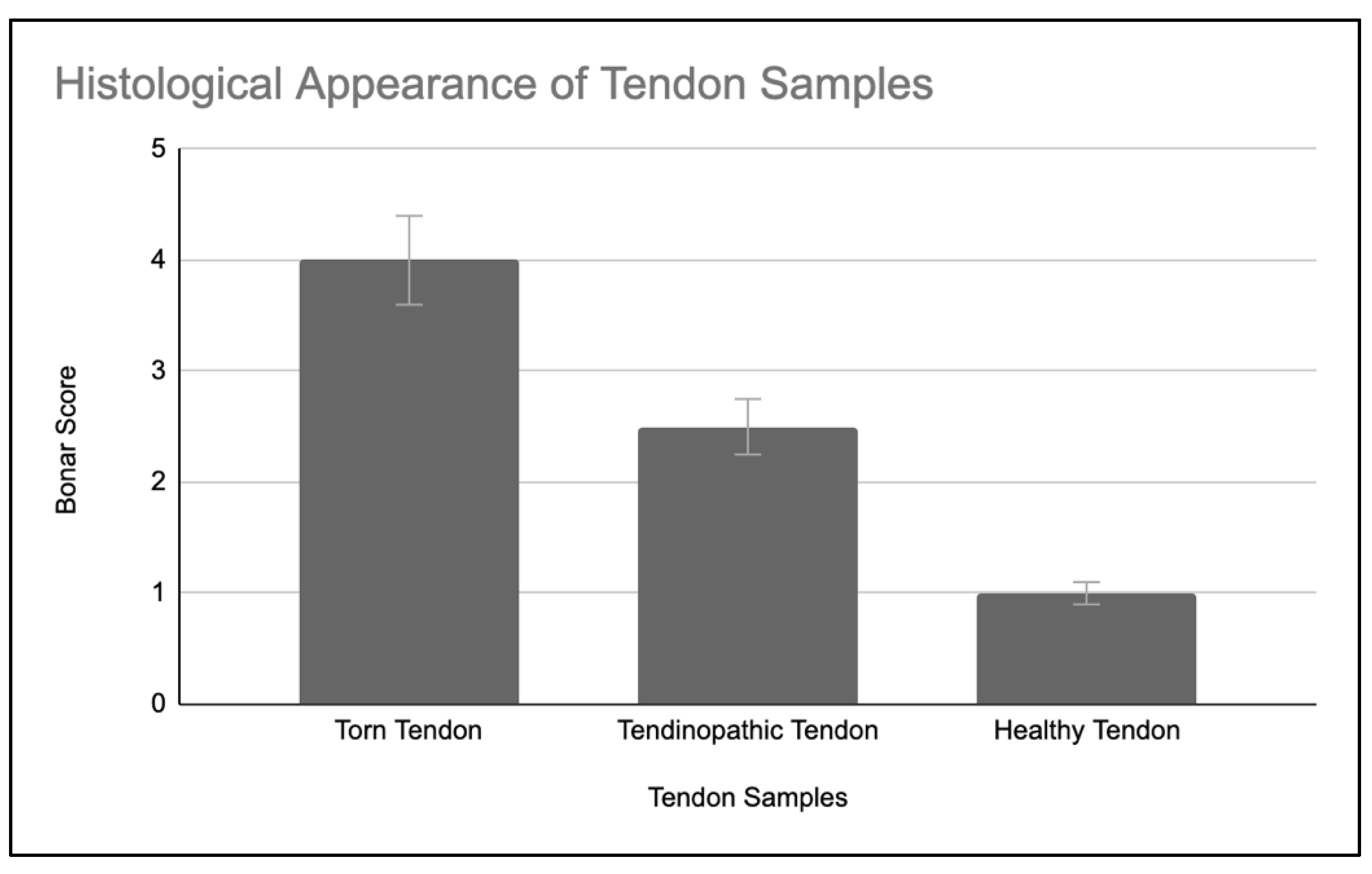
Figure 5.
Histological Appearance of Tendon Samples with SEM shown; n = 5 for control (healthy) tendon, n = 7 for early (tendinopathic tendon) and late (torn tendon) tendinopathy.
Figure 5.
Histological Appearance of Tendon Samples with SEM shown; n = 5 for control (healthy) tendon, n = 7 for early (tendinopathic tendon) and late (torn tendon) tendinopathy.
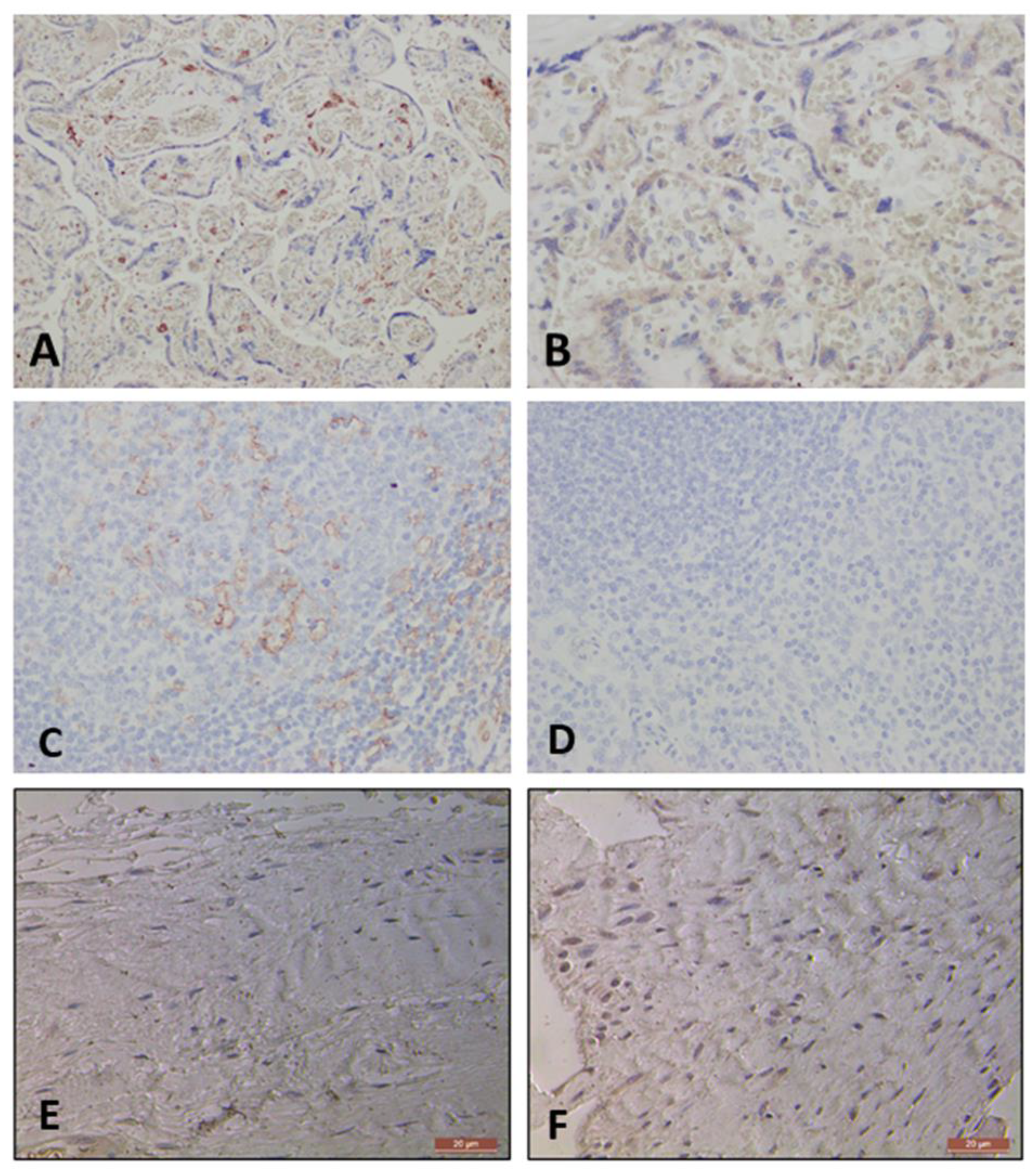
Figure 6.
Images showing Control Placental Tissue: CD 206 staining (A) and negative IgG control Isotype (B), at 400x magnification. Images showing Control Tonsil Tissue: CD 11c antibody applied and positive (C) and negative control IgG2b Kappa Isotype (D), at 200x magnification. Negative staining shown upon applying specific Isotype to Tendon Samples, IgG kappa b for CD 206 (E) and IgG for CD 11c (F), at 400x magnification.
Figure 6.
Images showing Control Placental Tissue: CD 206 staining (A) and negative IgG control Isotype (B), at 400x magnification. Images showing Control Tonsil Tissue: CD 11c antibody applied and positive (C) and negative control IgG2b Kappa Isotype (D), at 200x magnification. Negative staining shown upon applying specific Isotype to Tendon Samples, IgG kappa b for CD 206 (E) and IgG for CD 11c (F), at 400x magnification.
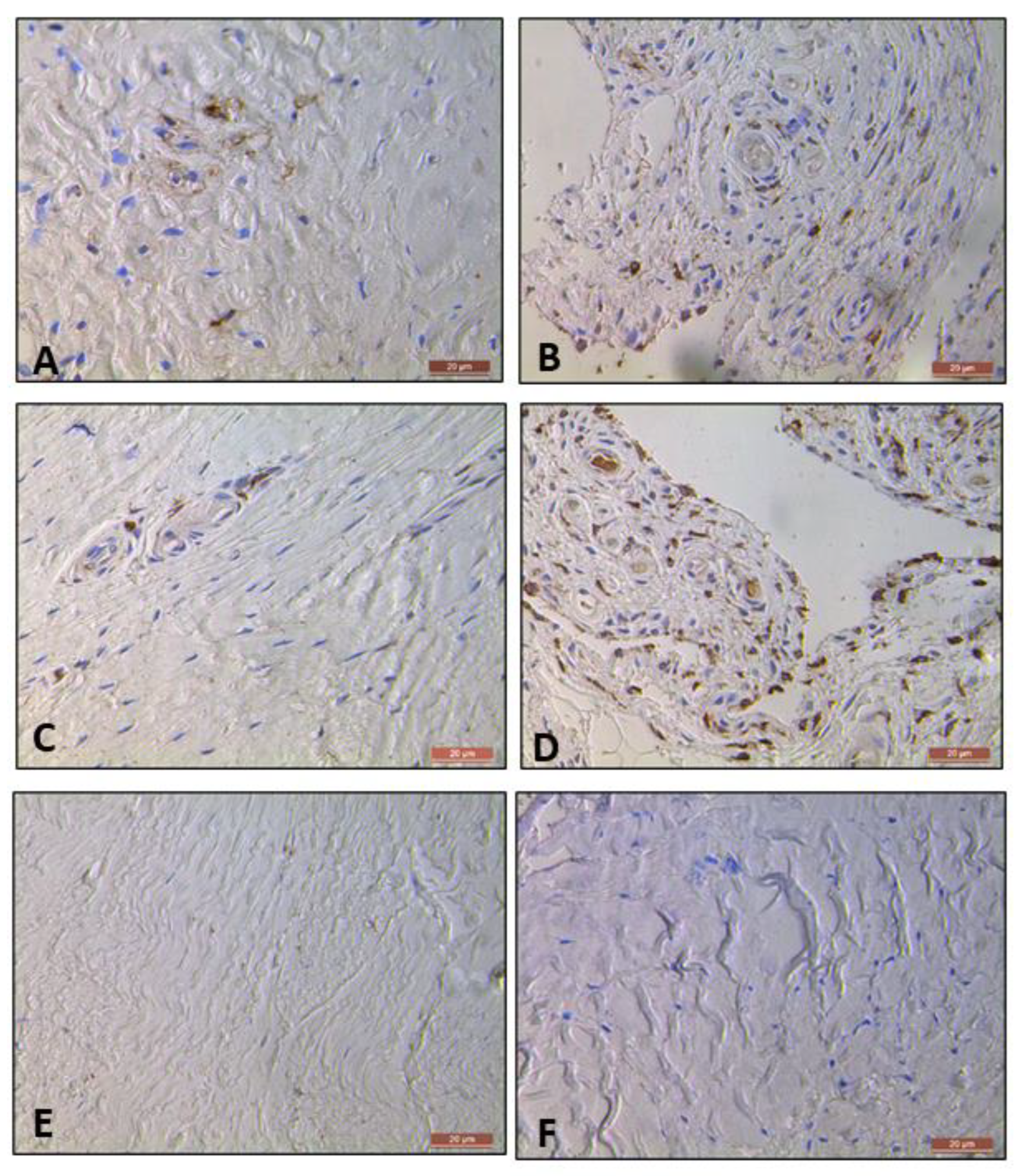
Figure 7.
Representative image of positively stained CD 11c cells in Torn Tendon Samples (A) and Tendinopathic Tendon Samples (B); Representative image of positively stained CD 206 cells in Torn Tendon Sample (C) and Tendinopathic Tendon Samples (D). Images of Healthy Tendon Samples for stained CD 11c cells (E) and CD 206 cells (F), at 400x magnification.
Figure 7.
Representative image of positively stained CD 11c cells in Torn Tendon Samples (A) and Tendinopathic Tendon Samples (B); Representative image of positively stained CD 206 cells in Torn Tendon Sample (C) and Tendinopathic Tendon Samples (D). Images of Healthy Tendon Samples for stained CD 11c cells (E) and CD 206 cells (F), at 400x magnification.
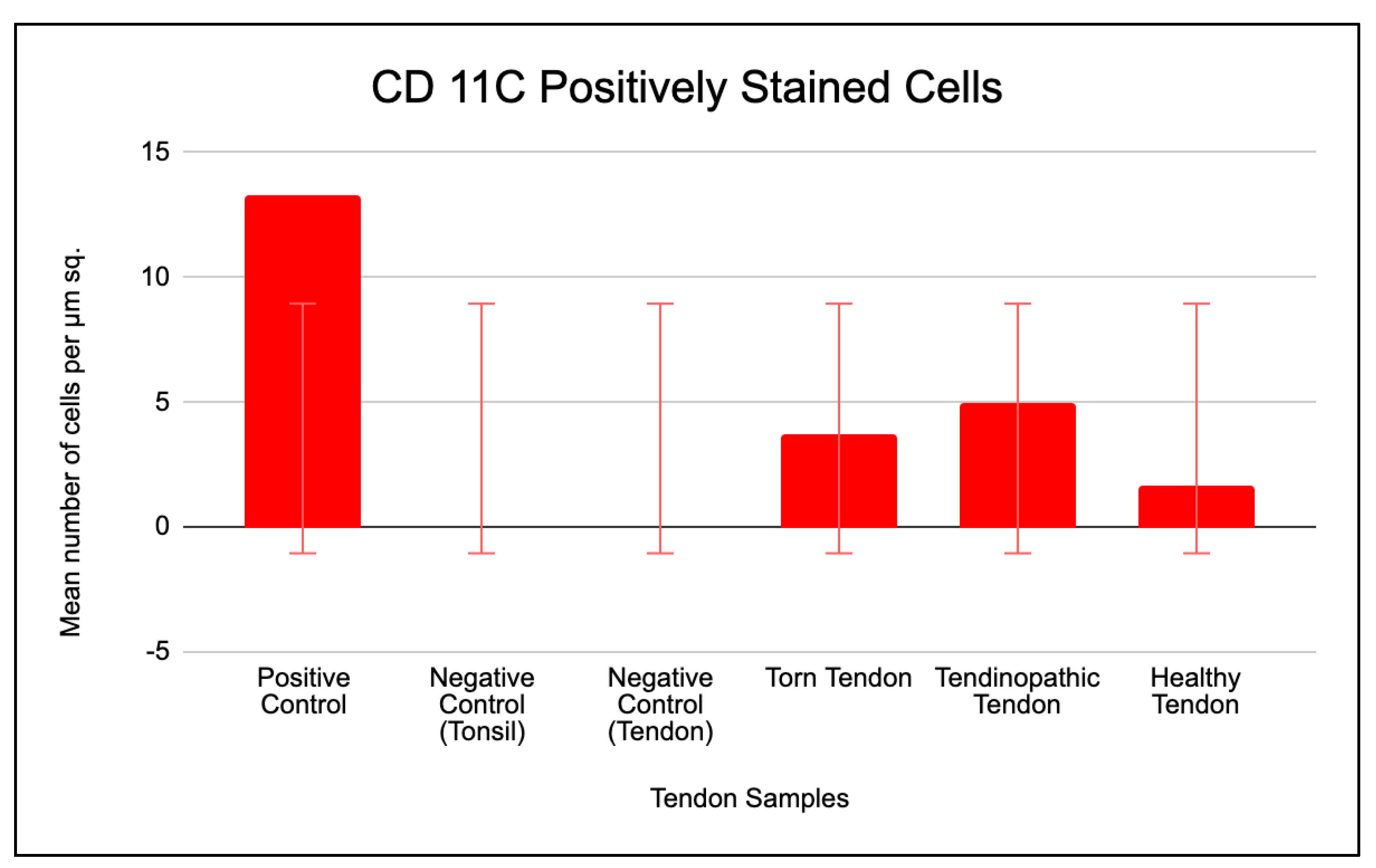
Figure 8.
Expression of CD 11c positively stained cells in control tissue, torn, tendinopathic and healthy tendon samples. Mean (SD) positively stained number of cells per µm2 area of tissue (one field view) for CD 11c surface protein markers for samples of positive and negative controls and human tendon biopsies with SEM shown; n = 5 for control (healthy) tendon, n = 7 for early (tendinopathic tendon) and late (torn tendon) tendinopathy. *P <.05; **P <.01; ***P <.001.
Figure 8.
Expression of CD 11c positively stained cells in control tissue, torn, tendinopathic and healthy tendon samples. Mean (SD) positively stained number of cells per µm2 area of tissue (one field view) for CD 11c surface protein markers for samples of positive and negative controls and human tendon biopsies with SEM shown; n = 5 for control (healthy) tendon, n = 7 for early (tendinopathic tendon) and late (torn tendon) tendinopathy. *P <.05; **P <.01; ***P <.001.
Figure 9.
Expression of CD 206 positively stained cells in control tissue, torn, tendinopathic and healthy tendon samples. Mean (SD) positively stained number of cells per µm2 area of tissue (one field view) for CD 206 surface protein markers for samples of positive and negative controls and human tendon biopsies with SEM shown; n = 5 for control (healthy) tendon, n = 7 for early (tendinopathic tendon) and late (torn tendon) tendinopathy. *P <.05; **P <.01; ***P <.001.
Figure 9.
Expression of CD 206 positively stained cells in control tissue, torn, tendinopathic and healthy tendon samples. Mean (SD) positively stained number of cells per µm2 area of tissue (one field view) for CD 206 surface protein markers for samples of positive and negative controls and human tendon biopsies with SEM shown; n = 5 for control (healthy) tendon, n = 7 for early (tendinopathic tendon) and late (torn tendon) tendinopathy. *P <.05; **P <.01; ***P <.001.
Figure 10.
Alarmin mediated tendon injury model of tendinopathy, adapted from Crowe et. al., 2019 [
41].
Figure 10.
Alarmin mediated tendon injury model of tendinopathy, adapted from Crowe et. al., 2019 [
41].
Figure 11.
Induction of Th17 cell development by IFN-α-conditioned mo-DCs, adapted from Santini et. al., 2011 [
63].
Figure 11.
Induction of Th17 cell development by IFN-α-conditioned mo-DCs, adapted from Santini et. al., 2011 [
63].
Figure 12.
The role of mo-DCs in inflammatory mechanisms in tendinopathy.Schematic diagram illustrating the manner in which early tendinopathy may arise due to Th 17 associated IL-17 cytokine induction mediated via monocyte-derived DCs. Increased stress that a tendon cell experiences results in the release of various inflammatory mediators which interact to drive the tendon matrix toward a degenerative of reparative process.
Figure 12.
The role of mo-DCs in inflammatory mechanisms in tendinopathy.Schematic diagram illustrating the manner in which early tendinopathy may arise due to Th 17 associated IL-17 cytokine induction mediated via monocyte-derived DCs. Increased stress that a tendon cell experiences results in the release of various inflammatory mediators which interact to drive the tendon matrix toward a degenerative of reparative process.