Submitted:
29 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Workflow of INAA-LFT
2.1. Amplification of the Target Nucleic Acid Fragments from Pathogens
2.2. Labeling of the Target Nucleic Acid Fragments from Pathogens
2.3. Visible Detection of Target Nucleic Acid Fragments from Pathogens
3. Application of INAA-LFT in Detection of Plant Viruses
3.1. LAMP-LFT Detection of Plant Viruses
3.2. RPA-LFT Detection of Plant Viruses
3.3. RAA-LFT Detection of Plant Viruses
3.4. CRISPR-CAS System-Integrated LFT Detection of Plant Viruses
4. Factors Influencing the Implementation of INAA-LFT for On-Site Detection of Plant Viruses
4.1. Sensitivity and Specificity
4.2. Detection Duration
4.3. Ease of Operation
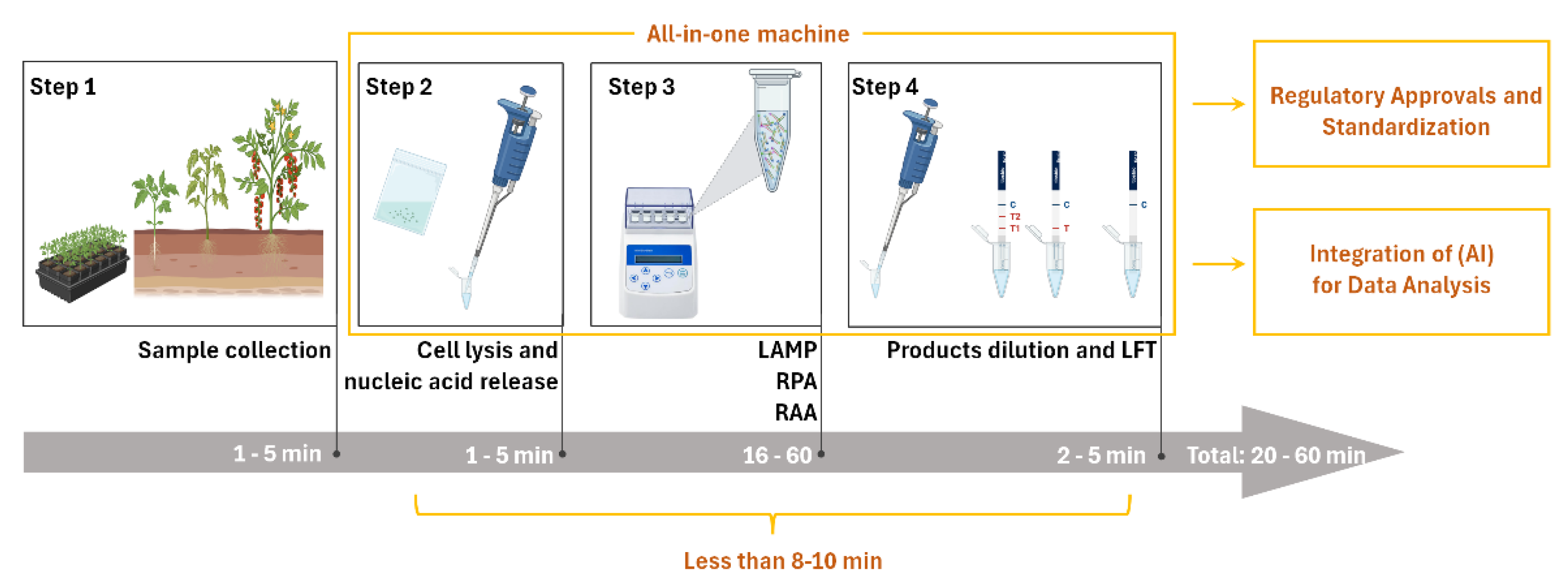
5. The Future Trajectory of INAA-LFT in Detection of Plant Virus
5.1. Expedited Detecting Duration
5.2. Operational Simplification and Automation
5.3. Portable Devices
5.4. Regulatory Approvals and Standardization
5.5. Integration of Artificial Intelligence (AI) for Data Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhat, A.I.; Aman, R.; Mahfouz, M. Onsite detection of plant viruses using isothermal amplification assays. Plant Biotechnol J 2022, 20, 1859–1873. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.P.; Othman, S.; Lau, Y.L.; Radu, S.; Chee, H.Y. Loop-mediated isothermal amplification (LAMP): a versatile technique for detection of micro-organisms. J Appl Microbiol 2018, 124, 626–643. [Google Scholar] [CrossRef] [PubMed]
- Boonham, N.; Kreuze, J.; Winter, S.; van der Vlugt, R.; Bergervoet, J.; Tomlinson, J.; Mumford, R. Methods in virus diagnostics: from ELISA to next generation sequencing. Virus Res 2014, 186, 20–31. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J.C. Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of-Care Diagnosis. ACS Nano 2021, 15, 3593–3611. [Google Scholar] [CrossRef] [PubMed]
- Lefrancois, T.; Malvy, D.; Atlani-Duault, L.; Benamouzig, D.; Druais, P.L.; Yazdanpanah, Y.; Delfraissy, J.F.; Lina, B. After 2 years of the COVID-19 pandemic, translating One Health into action is urgent. Lancet 2023, 401, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. Recombinase Polymerase Amplification Assay with and without Nuclease-Dependent-Labeled Oligonucleotide Probe. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Sang, P.; Hu, Z.; Cheng, Y.; Yu, H.; Xie, Y.; Yao, W.; Guo, Y.; Qian, H. Nucleic Acid Amplification Techniques in Immunoassay: An Integrated Approach with Hybrid Performance. J Agric Food Chem 2021, 69, 5783–5797. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 2000, 28, E63. [Google Scholar] [CrossRef]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chemother 2009, 15, 62–69. [Google Scholar] [CrossRef]
- Gill, P.; Ghaemi, A. Nucleic acid isothermal amplification technologies: a review. Nucleosides Nucleotides Nucleic Acids 2008, 27, 224–243. [Google Scholar] [CrossRef]
- Tomlinson, J.A.; Ostoja-Starzewska, S.; Adams, I.P.; Miano, D.W.; Abidrabo, P.; Kinyua, Z.; Alicai, T.; Dickinson, M.J.; Peters, D.; Boonham, N. , et al. Loop-mediated isothermal amplification for rapid detection of the causal agents of cassava brown streak disease. J Virol Methods 2013, 191, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Edgu, G.; Freund, L.J.; Hartje, S.; Tacke, E.; Hofferbert, H.R.; Twyman, R.M.; Noll, G.A.; Muth, J.; Prufer, D. Fast, Precise, and Reliable Multiplex Detection of Potato Viruses by Loop-Mediated Isothermal Amplification. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tang, J.; Sun, K.; Yu, X. Identification of a New Badnavirus in the Chinaberry (Melia azedarach) Tree and Establishment of a LAMP-LFD Assay for Its Rapid and Visual Detection. Viruses 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Easley, C.J. Isothermal DNA amplification in bioanalysis: strategies and applications. Bioanalysis 2011, 3, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Daher, R.K.; Stewart, G.; Boissinot, M.; Bergeron, M.G. Recombinase Polymerase Amplification for Diagnostic Applications. Clin Chem 2016, 62, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Gao, Z. Bioanalytical applications of isothermal nucleic acid amplification techniques. Anal Chim Acta 2015, 853, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.Y.; Botella, J.R. Advanced DNA-Based Point-of-Care Diagnostic Methods for Plant Diseases Detection. Front Plant Sci 2017, 8, 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ravelonandro, M.; Russell, P.; McOwen, N.; Briard, P.; Bohannon, S.; Vrient, A. Rapid diagnostic detection of plum pox virus in Prunus plants by isothermal AmplifyRP((R)) using reverse transcription-recombinase polymerase amplification. J Virol Methods 2014, 207, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, F.; Li, X.; Lan, Y.; Du, L.; Zhou, T.; Zhou, Y. Reverse transcription-recombinase polymerase amplification combined with lateral flow strip for detection of rice black-streaked dwarf virus in plants. J Virol Methods 2019, 263, 96–100. [Google Scholar] [CrossRef]
- Cao, Y.; Yan, D.; Wu, X.; Chen, Z.; Lai, Y.; Lv, L.; Yan, F.; Chen, J.; Zheng, H.; Song, X. Rapid and visual detection of milk vetch dwarf virus using recombinase polymerase amplification combined with lateral flow strips. Virol J 2020, 17, 102. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, R.D.; Choi, S.; Ju, H.J.; Yoon, J.Y. Application of Rapid and Reliable Detection of Cymbidium Mosaic Virus by Reverse Transcription Recombinase Polymerase Amplification Combined with Lateral Flow Immunoassay. Plant Pathol J 2022, 38, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.K.; Lee, H.J.; Kim, S.M.; Jeong, R.D. Rapid and Visual Detection of Barley Yellow Dwarf Virus by Reverse Transcription Recombinase Polymerase Amplification with Lateral Flow Strips. Plant Pathol J 2022, 38, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Zhao, X.X.; Wang, D.; Zhang, P.J.; Hu, X.N.; Wei, S.; Liu, J.Y.; Ye, Z.H.; Yu, X.P. A reverse transcription-cross-priming amplification method with lateral flow dipstick assay for the rapid detection of Bean pod mottle virus. Sci Rep 2022, 12, 681. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, H.Y.; Jiang, D.M.; Liu, M.; Zhang, W.; Yan, J.Y. A rapid detection of tomato yellow leaf curl virus using recombinase polymerase amplification-lateral flow dipstick assay. Lett Appl Microbiol 2022, 74, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Greeshma, M.; Bhat, A.I.; Jeevalatha, A. Rapid onsite detection of piper yellow mottle virus infecting black pepper by recombinase polymerase amplification-lateral flow assay (RPA-LFA). J Virol Methods 2023, 315, 114695. [Google Scholar] [CrossRef]
- Wu, X.; Chen, S.; Zhang, Z.; Zhang, Y.; Li, P.; Chen, X.; Liu, M.; Lu, Q.; Li, Z.; Wei, Z. , et al. Development of Recombinase Polymerase Amplification Combined with Lateral Flow Strips for Rapid Detection of Cowpea Mild Mottle Virus. Plant Pathol J 2023, 39, 486–493. [Google Scholar] [CrossRef]
- Yilmaz, S.; Batuman, O. Development of a reverse transcription recombinase polymerase amplification combined with lateral flow assay for equipment-free on-site field detection of tomato chlorotic spot virus. Virol J 2023, 20, 136. [Google Scholar] [CrossRef]
- Zhang, A.L.; Shi, X.; Xie, C.; Yu, F.; Gao, Z.; Xu, Y.; Liu, Z. Rapid and Visual Detection of Actinidia Chlorotic Ringspot-Associated Virus Using One-Step Reverse-Transcription Recombinase Polymerase Amplification Combined with Lateral Flow Dipstick Assay. Plant Dis, 1094. [Google Scholar] [CrossRef]
- Hammond, R.W.; Zhang, S. Development of a rapid diagnostic assay for the detection of tomato chlorotic dwarf viroid based on isothermal reverse-transcription-recombinase polymerase amplification. J Virol Methods 2016, 236, 62–67. [Google Scholar] [CrossRef]
- Ghosh, D.K.; Kokane, S.B.; Gowda, S. Development of a reverse transcription recombinase polymerase based isothermal amplification coupled with lateral flow immunochromatographic assay (CTV-RT-RPA-LFICA) for rapid detection of Citrus tristeza virus. Sci Rep 2020, 10, 20593. [Google Scholar] [CrossRef]
- Mekuria, T.A.; Zhang, S.; Eastwell, K.C. Rapid and sensitive detection of Little cherry virus 2 using isothermal reverse transcription-recombinase polymerase amplification. J Virol Methods 2014, 205, 24–30. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, I.S.; Ju, H.J.; Jeong, R.D. Rapid and visual detection of tomato spotted wilt virus using recombinase polymerase amplification combined with lateral flow strips. Mol Cell Probes 2021, 57, 101727. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. Multiplex Assay of Viruses Integrating Recombinase Polymerase Amplification, Barcode-Anti-Barcode Pairs, Blocking Anti-Primers, and Lateral Flow Assay. Anal Chem 2021, 93, 13641–13650. [Google Scholar] [CrossRef] [PubMed]
- Redinbaugh, M.G.; Stewart, L.R. Maize Lethal Necrosis: An Emerging, Synergistic Viral Disease. Annu Rev Virol 2018, 5, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.; Frey, T.S.; Barriball, K.; Paul, P.A.; Willie, K.; Mezzalama, M.; Kimani, E.; Mugambi, C.; Wangai, A.; Prasanna, B.M. , et al. Detection of Diverse Maize Chlorotic Mottle Virus Isolates in Maize Seed. Plant Dis 2021, 105, 1596–1601. [Google Scholar] [CrossRef]
- Duan, X.; Ma, W.; Jiao, Z.; Tian, Y.; Ismail, R.G.; Zhou, T.; Fan, Z. Reverse transcription-recombinase-aided amplification and CRISPR/Cas12a-based visual detection of maize chlorotic mottle virus. Phytopathol Res 2022, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, X.; Chen, S.; Chen, J.; Liang, Z.; Chen, B.; Yang, X.; Zhou, G.; Zhang, T. On-site and visual detection of sorghum mosaic virus and rice stripe mosaic virus based on reverse transcription-recombinase-aided amplification and CRISPR/Cas12a. Front Genome Ed 2023, 5, 1124794. [Google Scholar] [CrossRef]
- Lei, R.; Kuang, R.; Peng, X.; Jiao, Z.; Zhao, Z.; Cong, H.; Fan, Z.; Zhang, Y. Portable rapid detection of maize chlorotic mottle virus using RT-RAA/CRISPR-Cas12a based lateral flow assay. Front Plant Sci 2023, 14, 1088544. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, S.; Dong, Z.; Fan, Q.; Lei, R.; Kuang, R.; Zhang, Y. One-Step Reverse-Transcription Recombinase-Aided Amplification CRISPR/Cas12a-Based Lateral Flow Assay for Fast Field Screening and Accurate Differentiation of Four Major Tobamoviruses Infecting Tomato and Pepper. J Agric Food Chem, 1021. [Google Scholar] [CrossRef]
- Marques, M.C.; Sanchez-Vicente, J.; Ruiz, R.; Montagud-Martinez, R.; Marquez-Costa, R.; Gomez, G.; Carbonell, A.; Daros, J.A.; Rodrigo, G. Diagnostics of Infections Produced by the Plant Viruses TMV, TEV, and PVX with CRISPR-Cas12 and CRISPR-Cas13. ACS Synth Biol 2022, 11, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, R.; Zhang, H.; Wang, J.; Lu, Y.; Zhang, D.; Yang, L. PAM-free loop-mediated isothermal amplification coupled with CRISPR/Cas12a cleavage (Cas-PfLAMP) for rapid detection of rice pathogens. Biosens Bioelectron 2022, 204, 114076. [Google Scholar] [CrossRef]
- Cao, Y.; Weng, H.; Rao, S.; Li, J.; Yan, F.; Song, X. Rapid and visual field diagnosis of tomato brown rugose fruit virus using reverse transcription recombinase aided amplification (RT RAA) combined with lateral flow strips (LFS). Crop Protection 2023, 173, 106355. [Google Scholar] [CrossRef]
| Type of Amplification | Virus | Type of Viral Genome | Testing Duration | Ref |
|---|---|---|---|---|
| LAMP | cassava brown streak virus | +ssRNA | 40 min | [11] |
| ugandan cassava brown streak virus | +ssRNA | 40 min | [11] | |
| tobacco rattle virus | +ssRNA | <50 min | [12] | |
| Chinaberry tree badnavirus 1 | dsRNA | 45 min | [13] | |
| rice stripe virus | ±RNA | 50 min | [42] | |
| rice black-streaked dwarf virus | dsRNA | 50 min | [42] | |
| RPA | alfalfa mosaic virus | +ssRNA | 30 min | [6] |
| plum pox virus | +ssRNA | 20 min | [18] | |
| rice black-streaked dwarf virus | dsRNA | 20 min | [19] | |
| milk vetch dwarf virus | ssDNA | 30 min | [20] | |
| cymbidium mosaic virus | +ssRNA | 30 min | [21] | |
| barley yellow dwarf virus | +ssRNA | 20 min | [22] | |
| bean pod mottle virus | +ssRNA | <90 min | [23] | |
| tomato yellow leaf curl virus | ssDNA | 30 min | [24] | |
| piper yellow mottle virus | dsDNA | 30 min | [25] | |
| tomato chlorotic spot virus | ±RNA | 15 min | [27] | |
| actinidia chlorotic ringspot-associated virus | +ssRNA | <40 min | [28] | |
| citrus tristeza virus | +ssRNA | 15-20 min | [30] | |
| little cherry virus 2 | +ssRNA | - | [31] | |
| tomato spotted wilt virus | ±RNA | 15 min | [32] | |
| potato virus Y (PVY) | +ssRNA | 30 min | [33] | |
| potato virus S (PVS) | +ssRNA | 30 min | [33] | |
| potato leafroll virus (PLRV) | +ssRNA | 30 min | [33] | |
| tobacco mosaic virus | +ssRNA | 40 min | [41] | |
| tobacco etch virus | +ssRNA | 40 min | [41] | |
| potato virus X | +ssRNA | 40 min | [41] | |
| RAA | maize chlorotic mottle virus | +ssRNA | 45 min | [36,38] |
| sorghum mosaic virus | +ssRNA | 30 min | [37] | |
| rice stripe mosaic virus | -ssRNA | 30 min | [37] | |
| tomato brown rugose fruit virus | +ssRNA | 20 min | [39] | |
| pepper mild mottle virus | +ssRNA | <1 h | [40] | |
| tomato mosaic virus | +ssRNA | < 1 h | [40] | |
| tomato mottle mosaic virus | +ssRNA | < 1 h | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




