1. Introduction
Medicinal products on the market must be of appropriate quality to guarantee safety of use and effectiveness. The medicinal substance and product must meet all quality criteria regarding identity, purity, active substance content and suitability. The preferred method for examining the identity of organic compounds is infrared absorption spectrophotometry and chromatographic methods. The most commonly used chromatographic methods are high preformance liquid chromatography (HPLC), thin layer chromatography (TLC) and gas chromatography (GC). In chromatographic methods, confirmation of identity is obtained by comparing the retention times or retardation factors (TLC) of the test substance and the reference substance [
1,
2]. TLC combined with densitometry is also a great tool for investigating the content of biologically active substances in a drug, impurities present in the drug, but also for investigating many physicochemical properties, including lipophilicity of biologically active substances [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13].
Counterfeiting and illegal trade in medicines are global problems. The scale of the phenomenon is becoming more and more common. This poses a threat to the safety and life of patients. Falsified pharmaceuticals do not meet the quality requirements established for given medicinal products. They usually contain ingredients of lower quality, inappropriate proportions, impurities or other unapproved active substances with unknown safety of use - substances dangerous to health and life. Quite often, their composition is completely different from that declared on the packaging [
14,
15]. Hence, there is a need to constantly develop new analytical methods for qualitative and quantitative testing of drugs. One of such methods may be thin-layer chromatography combined with densitometry. Scientific literature describes methods using thin-layer chromatography for the determination of selected 5-nitoimidazole in the presence of another drug, e.g. metronidazole and di-iodohydroxychinoline [
16], diiodohydroxyquine [
17], spiramycin [
18], furazolidone [
19,
20], loperamide [
19], tetracycline hydrochloride [
21], ciprofloxacin [
22], diloxamide furoate [
23], clotrimazole [
24] as well as tinidazole and clotrimazole [
25,
26], omeprazole [
27], clarithromycin [
27], fluconazole [
28,
29], norfloxacin [
30,
31,
32], ciprofloxacin [
33].
So far the scientific literature has not described the simultaneous chromatographic separation using the TLC technique of metronidazole (M), secnidazole (S), ornidazole (O), tinidazole (T) and 2-methyl-5-nitroimidazole (IMP). Therefore, the aim of this work was to develop a fast and cheap thin-layer chromatography method allowing the separation of M, S, O, T, in the presence of potential IMP contamination. Only pharmaceutical preparations containing M and T are available on the Polish pharmaceutical market. Therefore, the developed chromatographic conditions were used to determine M and T in tablets. The proposed method has been fully validated. The motivation to develop another method was to obtain a quick and effective determination, with a low detection limit and at the same time low costs.
2. Materials and Methods
2.1. Chemicals and Reference Standards
Silica gel 60F254 (E. Merck, #1.05554, and #1.05570) plates were used for the study. The solvents used were: benzene, methanol, toluene, acetone, chloroform, ammonia 25%, n-hexane, ethyl acetate, ethanol (99.8%), acetic acid (80%), glacial acetic acid, diethylamine, acetonitrile. The solvents mentioned were produced by POCh Gliwice, Chempur or Merck and showed analytical purity. They were components of the mobile phases used. Methanol was also used to extract the M and T present in the tablets and to dissolve the standards. M, S, O and T as well as IMP were supplied by Sigma-Aldrich (USA). M and T were pharmaceutical primary standards with purity according with United States Pharmacopeia and European Pharmacopoeia, respectively. IMP was British Pharmacopoeia (BP) reference standard. S and O were analytical standards with quality level equal 100. Metronidazole and Tinidazolum tablets (Polpharma, Poland) contained 500 mg of M and 500 mg T, respectively. Anhydrous acetic acid (analytical purity, Chempur, Poland) and chloric acid (VII) (analytical purity, POCh, Poland) were used to determine M and T using pharmacopeia method.
2.2. Preparation of Standard Solutions of APIs
Standard solutions of M and T were prepared by dissolving their standard substances in methanol . Following M and T solutions were obtained, respectively: 0.60, 0.55, 0.50, 0.45, 0.40, 0.35, 0.30, 0.25, 0.20, 0.15, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01 mg⋅mL-1. Methanol solution of mixture of M, S, O, T, and IMP were prepared at a concentration of 0.20 mg⋅mL-1.
Five µL of standard solutions prepared in this way were taken and applied to chromatographic plates.
2.3. Preparation of Solutions of Metronidazole and Tinidazolum Drugs
After weighing ten tablets of Metronidazole Polpharma and Tinidazolum Polpharma, they were crushed for 25 min using a three ball mill at 6000 rpm. Then, the equivalent of 100 mg of M and 100 mg of T was weighed from the obtained powdered tablet masses. Extraction of M and T from tablet masses was carried out using 15 ml of methanol using a three ball mill at 6000 rpm for 20 minutes. The drug extracts obtained in this way were filtered through paper filters into volumetric flasks and supplemented with methanol to a volume of 50 ml, obtaining solutions with a concentration of 100 mg/50 mL. In the next step, a series of dilutions were made to obtain solutions with the following concentrations of M and T: 0.3 mg/5mL, 1.0 mg/5mL, 1.75 mg/5mL.
Five µL of the above-mentioned solutions were taken and applied to chromatographic plates.
2.4. TLC Combined with Densitometry
TLC analysis was performed using 10 cm x 20 cm aluminum plates coated with silica gel 60 F
254 (#1.05554). The chromatographic plates were activated at 120°C by 30 min. Standard solutions of M, T, mixture of M, S, O, T, and IMP as well as
Metronidazole and
Tinidazolum drug extracts were applied using 5 µL micropipettes. The tests were performed using the mobile phase: chloroform + methanol + diethylamine in a volume composition of 9: 1: 1. It was selected experimentally from among the 19 mobile phases tested (
Table S1). The chromatographic chamber was saturated for 30 min. The plates were developed in a chromatographic chamber to a height of approx. 7.5 cm, and then dried in a fume hood for 2 hours.
Using the Camag TLC 3 densitometer, in which the radiation source is a deuterium lamp, spectrodensitometric and densitometric analysis were performed. The parameters of the first of these analyzes were: wavelength 200÷400nm, slit size 12.00x0.40 mm, Macro, scanning speed - 20 nm/s, resolution 1 nm/step. Densitometric scanning parameters were: λmax=313 nm, slit size 12.00x0.40 mm, macro, resolution 100 µm/step and scanning speed 20 mm/s.
2.5. TLC Method Validation
Range and linearity, precision, accuracy, specificity, robustness, limit of detection and quantification were determined according to validation guides [
34,
35], which allowed validation of the TLC method for the determination of M and T. The accuracy of the method was additionally checked by comparison with the pharmacopeial method recognized as accurate [
1]. Validation details are provided in
Tables S2 and S3.
2.6. Quantitative Determination of Metronidazole and Tinidazole in Tablets and Comparison with Pharmacopoeial Method
The comparison of the proposed TLC-densitometric method (method A) with the pharmacopeia method (method B) to determine M, and T in pharmaceutical preparations was studied by the use of ten independently repeated different analyses. The samples about concentrations 1 mg/mL described in section 2.3 were investigated by method A. Method B involves potentiometric titration of samples [
1]. Powdered tablet samples containing 150 mg of M and T, respectively, were dissolved in 50 mL of anhydrous acetic acid. The samples were titrated with chloric acid (VII) at a concentration of 0.1 mol/L, and the end point of the titration was determined using combined pH electrode type EPS (Elmetron, Zabrze, Poland). Students t-test and the F-Snedecor value were used to check the significance of the differences between the two analytical methods.
2.7. Statistical Analysis
Statistical studies of the analysis results were made using the Statistica v. 13 PL program (StatSoft, Kraków, Poland), and the charts using Microsoft Office Excel 2016.
3. Results and Discussion
3.1. Validation
A TLC-densitometric method was developed that allows the separation of M, S, O, T, and IMP. The developed chromatographic conditions were used to determine M and T in Metronidazole Polpharma and Tinidazolum Polpharma tablets. The method has been fully validated (
Table 1,
Table 2,
Table 3,
Table 4 and
Table 5,
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8, S1–S10).
3.1.1. Optimization of Chromatographic Conditions
Chromatographic analyzes were performed on plates precoated with silica gel 60F
254. Nineteen mobile phases (
Table S1) were tested for their ability to separate five substances, namely M, S, O, T and the potential contamination of IMP. In this, mobile phases that were previously used for studies on metronidazole and tinidazole were tested [
3,
16,
20,
21,
36,
37,
38]. Attempts were also made to optimize the developing distance. It turned out that increasing the chromatogram development distance had an adverse effect on the blurring of the chromatographic bands (the chromatographic bands of the substance were more blurred when a development distance greater than 8.0 cm was used). This is due to the fact that in thin-layer chromatography without forced flow of the mobile phase (analysis in a regular chromatographic chamber), when ordinary or high-performance plates are used, the values of the theoretical plate height (H) increase over a longer development distance. It is known that the smaller the blurring of the chromatographic bands, the better the separation of the bands and the lower the value of the theoretical shelf height (H), i.e. the more efficient the chromatographic system is. Moreover, increasing the chromatogram development distance also contributes to extending the analysis time. The tested mobile phase was chloroform+methanol 9:1 (phase no. 11), which proved to be effective for testing the degradation products of M,S,O,T carried out in separate samples [
36]. Using this mobile phase, four chromatographic bands are obtained on the densitogram, i.e. no separation of metronidazole from secnidazole is achieved. On the densitograms using mobile phase 18, only two chromatographic bands were obtained, using mobile phases 10 and 17, three chromatographic bands were obtained, while using mobile phases 1,3-7 four chromatographic bands were obtained. Five bands from individual tested biologically active substances were obtained using mobile phases number 2 (
Figure S1), 8 (
Figure S2), 9 (
Figure S3), 12 (
Figure S4), 13 (
Figure S5), 14 (
Figure S6), 16 (
Figure S7), and 19 (
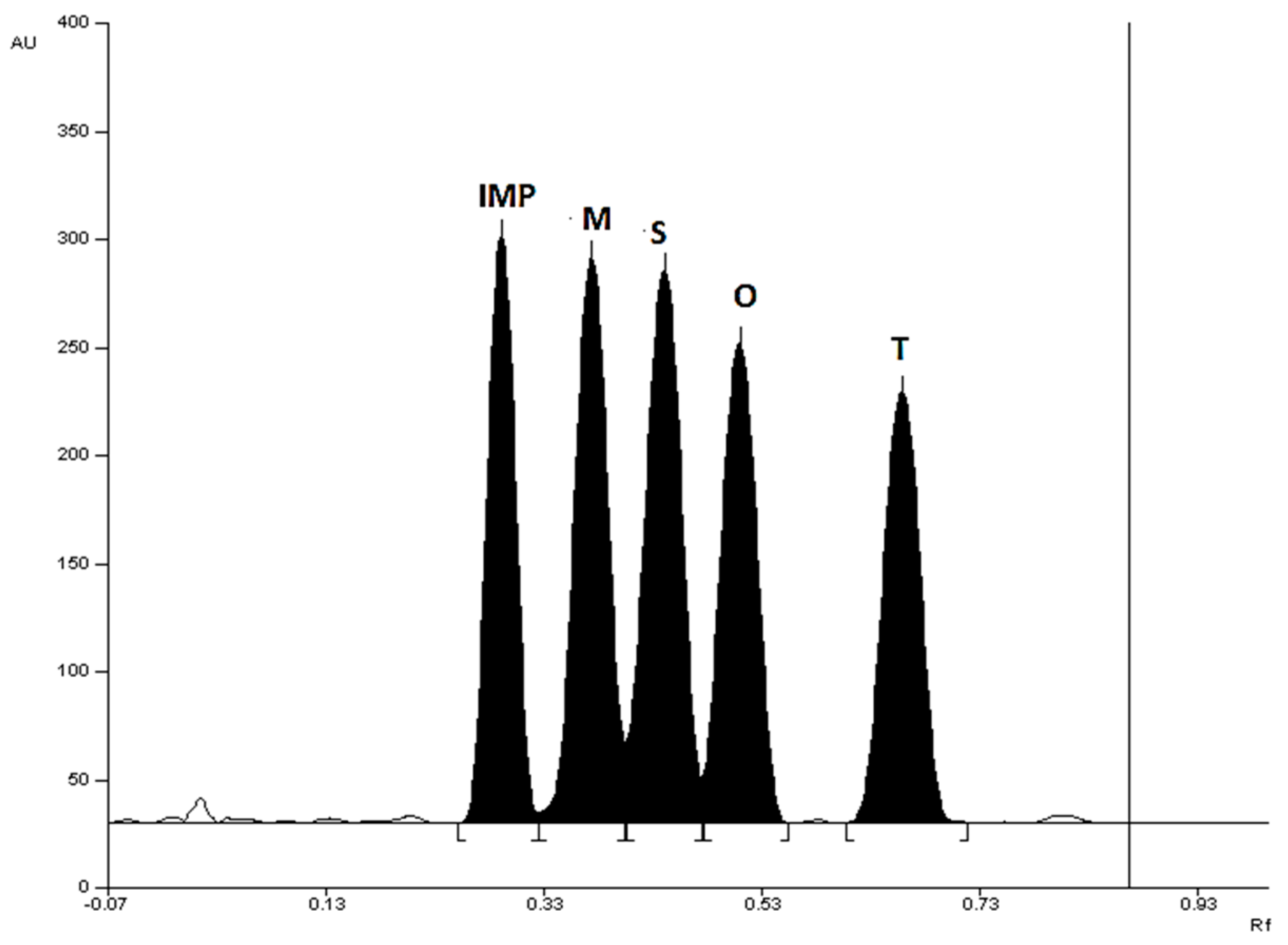
Figure 1).
The order of elution of the substances depends on the mobile phase used. Also, the quality of separation of chromatographic bands varies depending on the mobile phase used. The R
S separation coefficient was used to assess the quality of chromatographic separation.
Table 1 lists the R
F and R
S values for the best M, S, O, T and IMP separations. The presented comparison shows that mobile phase chloroform + methanol + diethylamine (9:1:1, v/v) proposed in this work is the best. When using this mobile phase, all R
S values are greater than 1. Using this mobile phase, the following R
F values were obtained: R
F(IMP) = 0.30±0.02, R
F(M) = 0.38±0.03, R
F(S) = 0.44±0.03, R
F(O) = 0.51±0.03, R
F(T) = 0.70±0.04. The resolution factor (R
S) calculated had the following values: R
S(IMP/M)=1.33, R
S(M/S)=1.22, R
S(S/O)=1.29, R
S(O/T)=2.10. Spectrodensitometric analysis indicates that the maximum absorption of all five investigated compounds occurs at 313 nm (
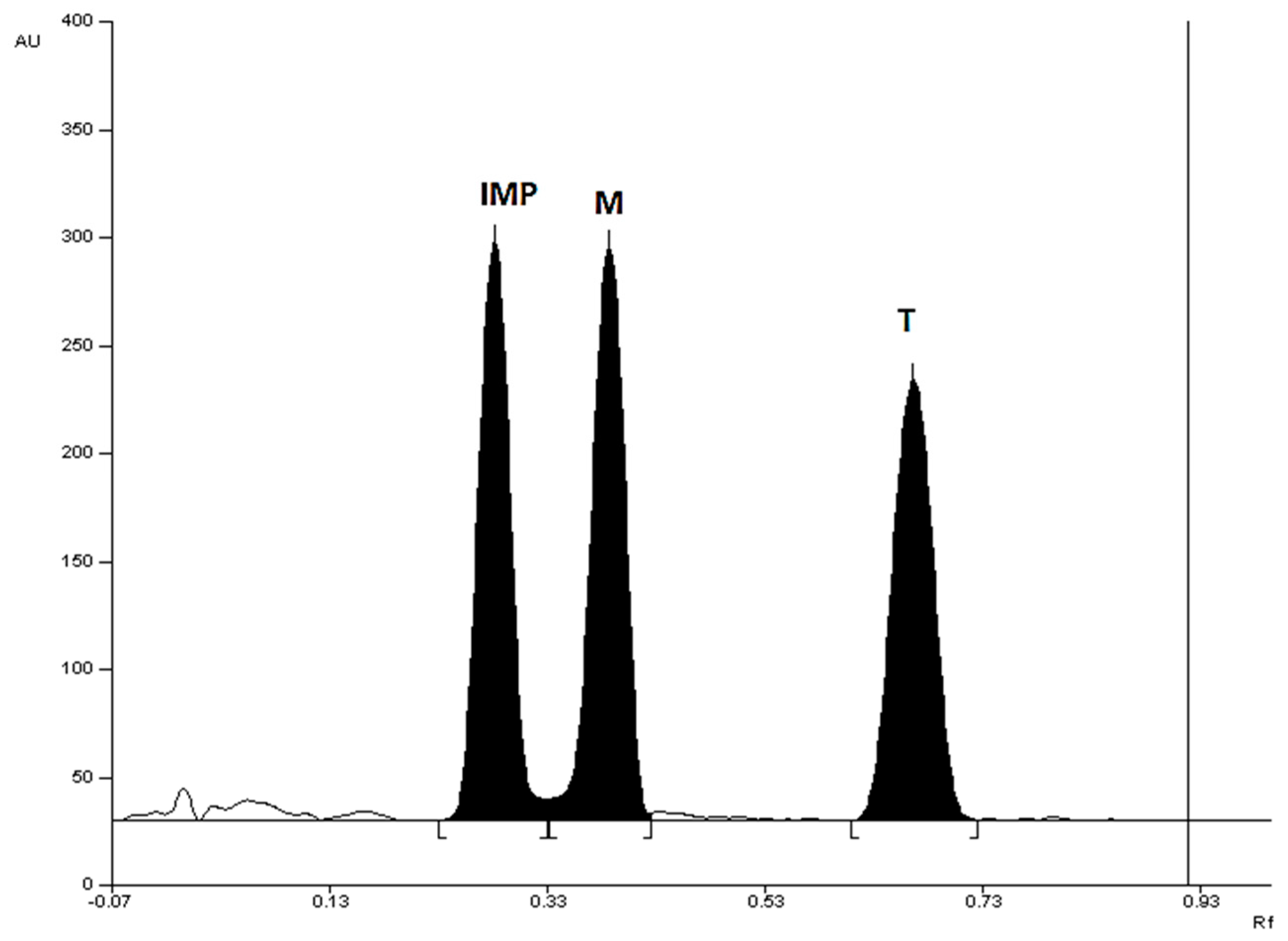
Figure S8). Densitogram of a mixture of standard substances: metronidazole (M), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP) made at 313 nm, using silica gel 60F
254 plate and mobile phase: chloroform + methanol + diethylamine (9:1:1, v/v) was presented in
Figure 2.
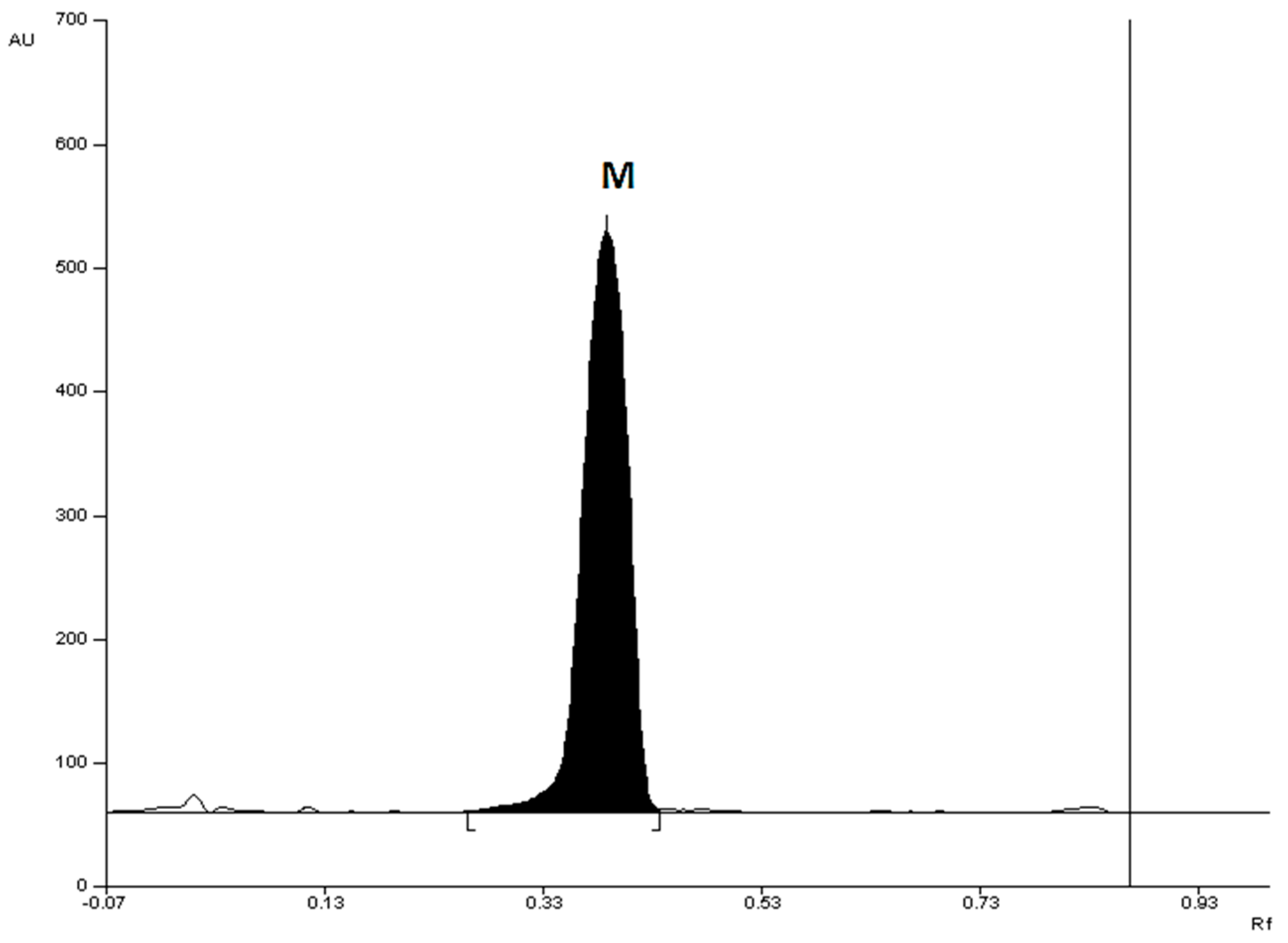
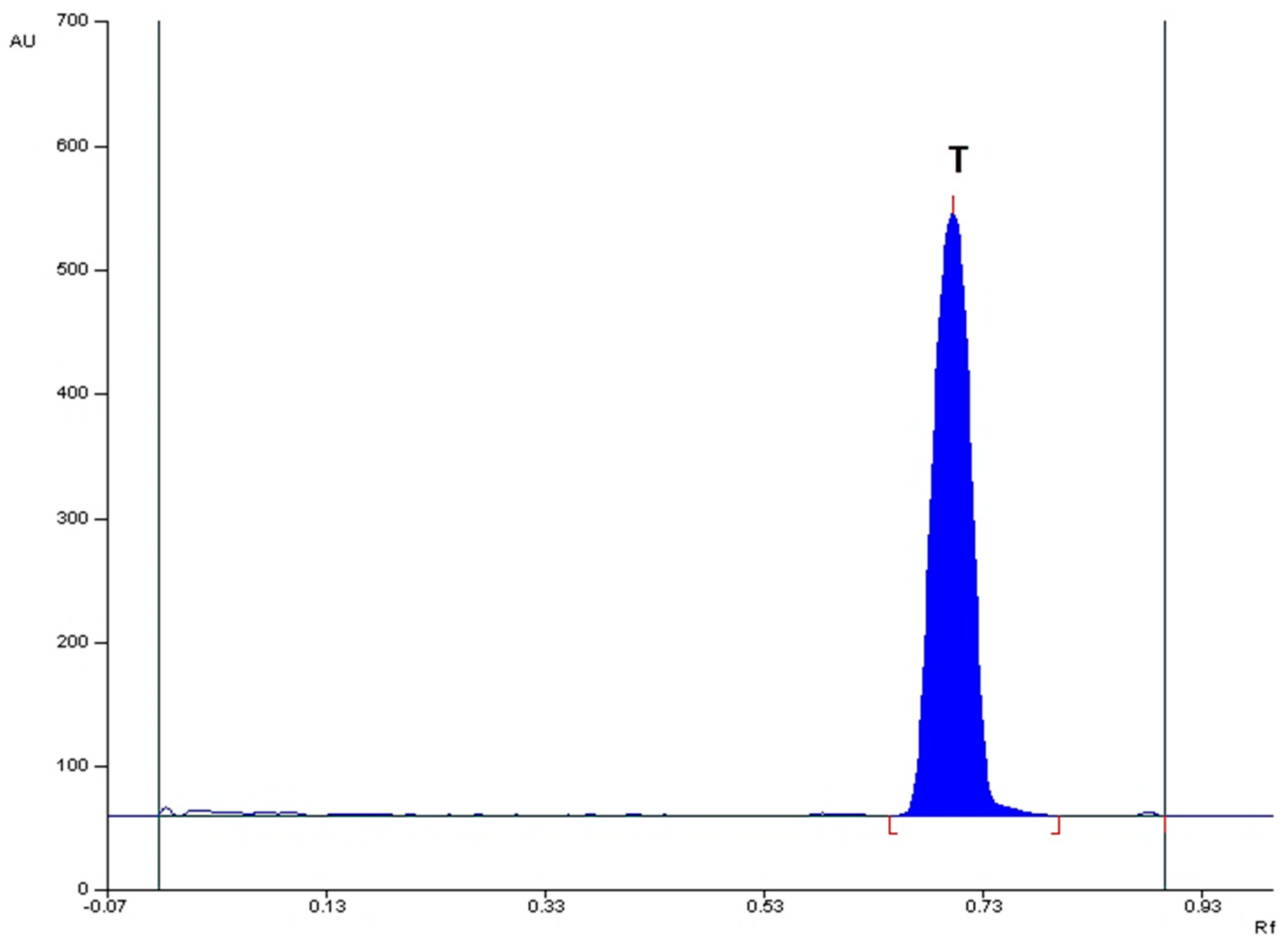
The densitograms obtained from the
Metronidazole Polpharma tablet and
Tinidazolum Polpharma tablet extracts for the optimal chromatographic conditions, shown in
Figure 3 and
Figure 4, respectively and indicate that there are no additional chromatographic bands from the analyzed tablets. This means that no impurities, including 2-methyl-5-nitroimidazole, were found in drug samples. The R
F values of the reference substances metronidazole and tinidazole are consistent with the R
F values of metronidazole and tinidazole from the tablet samples. The spectrodensitograms of metronidazole and tinidazole standards were also found to be consistent with the spectrodensitograms of metronidazole and tinidazole from tablet samples (
Figure 5 and
Figure 6).
3.1.2. Linearity and Range
It was found that the linear range of M and T determined was from 0.20 to 2.00 µg/spot. Linear equations showing the dependence of the area of the chromatographic band on the amount of micrograms/spot of metronidazole and tinidazole determined are presented in
Table 2 and on
Figures S9A and S10A. The differences between the real chromatographic band area values and those calculated from the correlation equations, presented on Figures S9B and S10B, indicate that construction of the correlation equations is correct.
3.1.3. Precision
The intra- and inter-day precisions were described using the coefficient of variation (CV, %) by measuring the area of the chromatographic bands of metronidazole and tinidazole samples with concentrations of 0.30, 1.00 and 1.75 µg/spot. The values of the coefficients CV ranged from 0.99% to 1.12% and from 1.33% to 1.48% for metronidazole and from 0.76% to 1.28% and from 0 .99% to 1.76% for tinidazole, respectively for intraday and interday precisions (
Table 2). These results indicate that proposed method is precise.
3.1.4.. Accuracy
The accuracy of the method was tested by recovery. The average recovery for metronidazole was: 103.8%, 104.3% and 101.2%, and for tinidazole: 101.8%, 99.1% and 100.9%, respectively for 50%, 100% and 150% of standard substances added to the
Metronidazole Polpharma and
Tinidazolum Polpharma samples (
Table 2). The low values of the coefficient of variation, which are less than 3% for both metronidazole and tinidazole, indicate that the proposed method is accurate.
3.1.5. Limit of Detection (LOD) and Limit of Quantification (LOQ)
The detection limit values calculated on the basis of the calibration curves are equal 0.012 and 0.022 µg/spot for metronidazole and tinidazole, respectively. The average values of the limit of quantification are equal 0.036 and 0.066 µg/spot for metronidazole and tinidazole, respectively. Low LOD and LOQ values indicate that the proposed TLC-densitometric method is sensitive.
After reviewing the publicly available literature on the determination of metronidazole and tinidazole using TLC methods, the obtained LOD and LOQ results were compared with those obtained by exemplary authors of other studies (
Table 3). The presented comparison shows that the TLC-densitometric method developed in this study provides lower LOD and LOD values for metronidazole and comparable or lower LOD and LOQ values for tinidazole in relation to those previously described in the scientific literature. The value of the obtained LOD and LOQ values of metronidazole and tinidazole is influenced by the chromatographic conditions used (chromatographic plates and the qualitative and quantitative composition of the mobile phase).
A very important element of determining LOD is checking whether solutions with appropriately selected concentrations were used for testing. Because the LOD results obtained must meet the following criteria [
39]: 10 x LOD > C and LOD < C, where: C are a concentrations of metronidazole and tinidazole used.
3.1.6. Robustness
The robustness of the method [
35,
40,
41] was checked by spotting sample solutions on the plate and developing the plate after altering the conditions (
Table S3). The conditions changed were the sorbent type, development distance, the temperature of plate activation, extraction time, saturation time of the chamber, wavelength in densitometric analysis at λ, and the volume of chloroform in mobile phase. The method conditions and the selected factors which the values of their (+) and (-) levels are summarized in
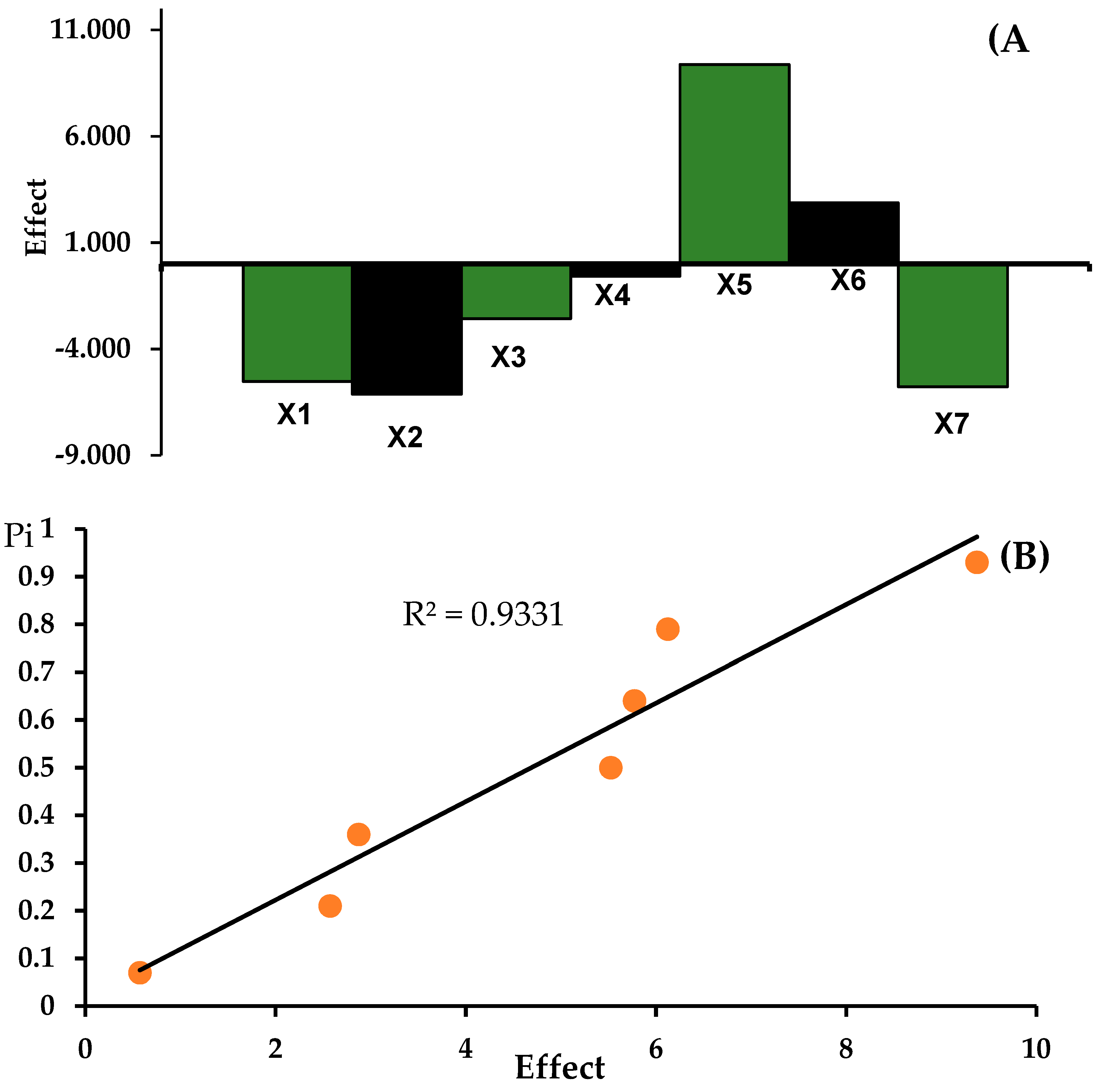
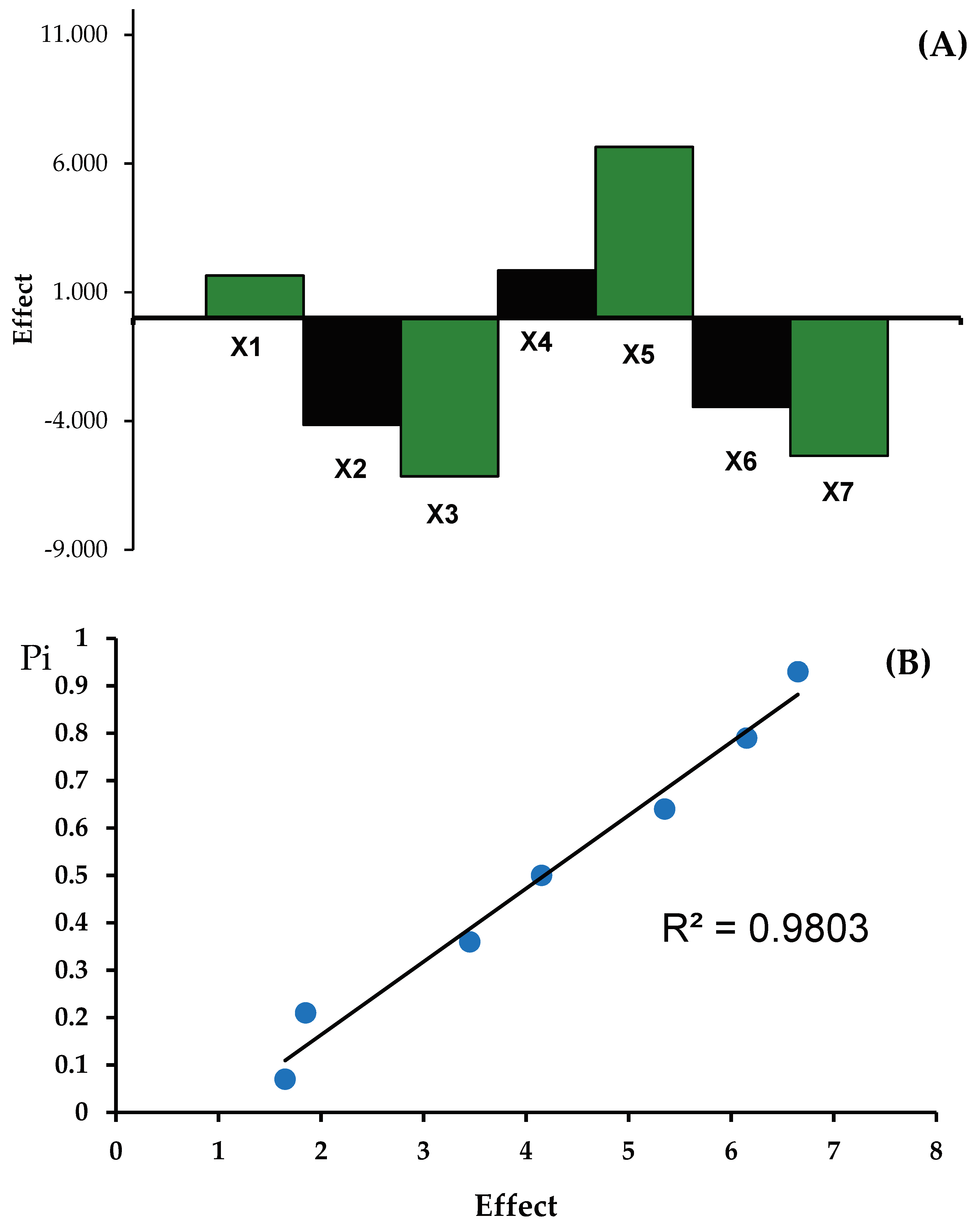
Table 4. A high level is represented by “+” and a low level by “-“. The effects (E) characterizing the particular individual factors and rank probabilities were calculated.
Table 4 shows the results regarding the determination of the content of metronidazole and tinidazole in
Metronidazole Polpharma and
Tinidazolum Polpharma tablets with changed analysis conditions. The results of the analyzes were interpreted with a coefficient of variance that was less than or equal to 1.5% and presented in
Figure 7 and
Figure 8 the effects of factors (A), and half-normal probability plot of effects (B) for determination of metronidazole (M) in Metronidazole Polpharma tablets and for determination of tinidazole (T) in
Tinidazolum Polpharma tablets indicate that the method is robust.
3.2. Quantitative Determination of Metronidazole and Tinidazole in Tablets and Comparison with Pharmakopeial Method
Table 5 shows the results regarding the determination of metronidazole and tinidazole in
Metronidazole Polpharma and
Tinidazolum Polpharma tablets. The content of metronidazole and tinidazole in tablets determined by TLC-densitometry was 506.5 and 499.4 mg, respectively. The content of metronidazole and tinidazole in tablets in relation to the content declared by the manufacturer was 101.3% and 99.8%, respectively. These results are consistent with pharmacopoeial requirements as they range from 95% to 105% [
1,
2]. The obtained results were verified using the pharmacopoeial method. The comparison of both methods is summarized in
Table 5. The results obtained with both methods are similar. This is confirmed by the calculated statistical parameters
t and
F. The calculated
t and
F values also confirm that the proposed TLC-densitometric method is accurate.
4. Conclusions
An easy and short-time TLC-densitometric method was developed for the separation of metronidazole, tinidazole, secnidazole, ornidazole and 2-methyl-5-nitroimidazole and for the determination of metronidazole and tinidazole in pharmaceutical preparations. Analyzes were performed on chromatographic plates precoated with silica gel 60F254 using the optimal mobile phase: chloroform + methanol + diethylamine in a volume ratio of 9:1:1. The method has been validated. The intraday and interday precision values for the three different concentrations ranged from 0.99% to 1.48% and 0.89% to 1.76%, and the precision values ranged from 1.13% to 2.48% and 0.95% to 2.49% for metronidazole and tinidazole, respectively. The limit of quantification (LOQ) was 0.036 and 0.066 µg/spot for metronidazole and tinidazole, respectively. The mean recovery was 103.1% and 100.6% for metronidazole and tinidazole, respectively. The content of metronidazole and tinidazole in tablets in relation to the content declared by the manufacturer was 101.3% and 99.8%, respectively. These results are consistent with pharmacopoeial requirements as they range from 95% to 105%. The presented method turned out to be fast, sensitive, selective, accurate and robust. The obtained results were verified using the pharmacopoeial method. Comparison of both the proposed and pharmacopoeial methods shows that the proposed method is accurate. Elaborated method allows for the analysis of several samples on one chromatography plate at the same time. This method is suitable for quick and routine testing of substance content in pharmaceutical preparations and for routine quality control of products in the pharmaceutical industry.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Mobile phases tested, Table S2: Details of the validation of the proposed TLC-densitometric method, Figure S1: Densitogram of a mixture of standard substances: metronidazole (M), secnidazole (S), ornidazole (O), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP) made at 313 nm, using silica gel 60F254 plate and mobile phase: acetone + chloroform + ethyl acetate (4:4:1, v/v), Figure S2: Densitogram of a mixture of standard substances: metronidazole (M), secnidazole (S), ornidazole (O), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP) made at 313 nm, using silica gel 60F254 plate and mobile phase: chloroform + methanol + ammonia (9:1:0.06, v/v), Figure S3: Densitogram of a mixture of standard substances: metronidazole (M), secnidazole (S), ornidazole (O), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP) made at 313 nm, using silica gel 60F254 plate and mobile phase: chloroform + methanol + ammonia (9:1:0.1, v/v), Figure S4: Densitogram of a mixture of standard substances: metronidazole (M), secnidazole (S), ornidazole (O), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP) made at 313 nm, using silica gel 60F254 plate and mobile phase: chloroform + methanol + glacial acetic acid (9:1:0.1, v/v), Figure S5: Densitogram of a mixture of standard substances: metronidazole (M), secnidazole (S), ornidazole (O), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP) made at 313 nm, using silica gel 60F254 plate and mobile phase: chloroform + methanol + glacial acetic acid (9:1:0.05, v/v), Figure S6: Densitogram of a mixture of standard substances: metronidazole (M), secnidazole (S), ornidazole (O), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP) made at 313 nm, using silica gel 60F254 plate and mobile phase: acetone + chloroform + ethyl acetate + glacial acetic acid (4:4:1:0.05, v/v), Figure S7: Densitogram of a mixture of standard substances: metronidazole (M), secnidazole (S), ornidazole (O), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP) made at 313 nm, using silica gel 60F254 plate and mobile phase: acetone + chloroform + ethyl acetate + acetonitrile (3:4:1:1, v/v), Figure S8: Comparison of spectrodensitograms of M, S, O, T, and IMP, Figure S9: Calibration plot (A) and plot of residuals (B) for metronidazole (M) in the linear working range mobile phase: chloroform + methanol + diethylamine in a volume ratio of 9:1:1, Figure S10: Calibration plot (A) and plot of residuals (B) for tinidazole (T) in the linear working range mobile phase: chloroform + methanol + diethylamine in a volume ratio of 9:1:1.
Funding
This research was funded by the Medical University of Silesia under grant number PCN-1-040/K/2/F and BNW-1-005/K/3/F.
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The author declares no conflicts of interest
References
- Polish Pharmaceutical Society. Polish Pharmacopoeia X; Polish Pharmaceutical Society: Warsaw, Poland, 2014 (In Polish).
- United States Pharmacopeial Convention. The United States Pharmacopoeia, 34th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2011. [Google Scholar]
- Komsta, Ł.; Waksmundzka-Hajnos, M.; Sherma, J. Thin Layer Chromatography in Drug Analysis. 1st ed.; CRC Press, Boca Raton, USA, 2014.
- Sherma, J.; Fried, B. Handbook of Thin Layer Chromatography. Third Edition, revise and expanded. Marcel Dekker, Inc., New York, USA, 2003.
- Bober, K.; Bębenek, E.; Boryczka, S. Application of TLC for evaluation of the lipophilicity of newly synthetized esters: betulin derivatives. J Anal Methods Chem 2019, 2019, 1297659. [Google Scholar] [CrossRef] [PubMed]
- 6. Bębenek, E; Bober-Majnusz, K.; Siudak, S.; Chrobak, E.; Kadela-Tomanek, M.; Wietrzyk, J.; Boryczka, S. Application of TLC to evaluate the lipophilicity of newly synthesized betulin derivatives. J Chromatogr Sci, 2020; 58, 323–333. [CrossRef]
- Wicha-Komsta, K.; Komsta, Ł. Unconventional TLC systems in lipophilicity determination: A review, J Liq Chromatogr Rel Technol 2017, 40, 219-225. [CrossRef]
- Pastewska, M.; Bednarczyk-Cwynar, B.; Kovačević, S.; Buławska, N.; Ulenberg, S.; Georgiev, P.; Kapica, H.; Kawczak, P.; Bączek, T.; Sawicki, W.; Ciura, K. Multivariate assessment of anticancer oleanane triterpenoids lipophilicity. J Chromatogr A 2021, 1656, 462552. [Google Scholar] [CrossRef] [PubMed]
- Nagi, D.M.; Abdelgaleel, M.; Derayea, S.M.; Khashaba, P.Y. Studying the kinetic of midodrine degradations using TLC stability approach: Application to dosage form and human plasma. J Pharm Biomed Anal 2023, 229, 115322. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.A.; Gondalia, I.I.; Patel, V.B.; Mahajan, A.; Chhalotiya, U.; Nagda, D.C. Stability indicating thin-layer chromatographic method for estimation of antidiabetic drug Remogliflozin etabonate. Futur J Pharm Sci 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Soudi, A.T.; Hussein, O.G.; Elzanfaly, E.S.; Zaazaa, H.E.; Abdelkawy, M. Stability indicating TLC–densitometric method for determination of alcaftadine in presence of its degradation products and dosage form preservatives. RJPT 2020, 13(11), 5171–5176. [Google Scholar] [CrossRef]
- Yeniceli Uğur, D.; Uğur, A. Analysis of anticancer drugs using thin layer chromatography- A review. Marmara Pharm J. 2018, 22(3), 334–346. [Google Scholar] [CrossRef]
- Sherma, J.; Rabel, F. Advances in the thin layer chromatographic analysis of counterfeit pharmaceutical products: 2008–2019. J Liq Chromatogr Rel Technol 2019, 42, 367–379. [Google Scholar] [CrossRef]
- Cvetanovski, F.; Brezovska, K.; Poceva Panovska, A.; Tonic Ribarska, J.; Sterjev, Z.; Grozdanova, A.; Netkovska, K. . Counterfeiting of medicines as an infringement of the intellectual property rights. Macedonian Pharm. Bulletin 2016, 62, 85–89. [Google Scholar] [CrossRef]
- Pathak, R.; Gaur, V.; Sankrityayan, H.; ·Gogtay, J. Tackling counterfeit drugs: the challenges and possibilities. Pharm Med 2023, 37, 281–290. [Google Scholar] [CrossRef]
- Ali, N.W.; Gamal, M.; Abdelkawy, M. Chromatographic methods for simultaneous determination of di-iodohydroxyquinoline and metronidazole in their binary mixture. Pak J Pharm Sci 2013, 26(5), 865–871. [Google Scholar]
- Salem, H.; Riad, S.; Rezk, M. Simultaneous determination of metronidazole and diiodohydroxyquine in bulk powder and aaramibe compound tablets by TLC-densitometry and HPLC. Pharm Anal Acta 2012, 10, 2153–2423. [Google Scholar]
- Maher HM, Youssef RM. Development of validated chromatographic methods for the simultaneous determination of metronidazole and spiramycin in tablets. Chromatographia 2009, 69, 345–350. [Google Scholar] [CrossRef]
- Kavitha, J.; Kishore, C.H.; Lakshmi, K.S. Simultaneous estimation of metronidazole, furazolidone and loperamide by HPTLC in veterinary formulation. Int J Pharm Sci 2013, 5, 620–625. [Google Scholar]
- Tendolkar, N.M.; Desai, B.S.; Gaudh, J.S.; Shinde, V.M. Simultaneous determination of tinidazole and furazolidone in suspension by HPTLC and HPLC. Anal Lett 1995, 28(9), 1641–1653. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, M.C. Development and validation of densitometric method for metronidazole and tetracycline hydrochloride in capsule dosage form. Int J Pharma Tech Res 2011, 3, 1169–1173. [Google Scholar]
- Elkady, E.F.; Mahrouse, M.A. Reversed-phase ion-pair HPLC and TLC-densitometric methods for the simultaneous determination of ciprofloxacin hydrochloride and metronidazole in tablets. Chromatographia 2011, 73, 297–305. [Google Scholar] [CrossRef]
- Morcoss MM, Abdelwahab NA. Different chromatographic methods for simultaneous determination of diloxanide furoate, metronidazole and its toxic impurity. J Iran Chem Soc. 2016; 13, 1643–1651.
- Meshram, D.B.; Bagade, S.B.; Tajne, M.R. TLC-densitometric analysis of clotrimazole and metronidazole in combined dosage forms. J Planar Chromatogr - Modern TLC 2008, 21, 277–282. [Google Scholar] [CrossRef]
- Meshram, D.; Patel, D.; Rohit, M.; Desai, S.; Tajne, M.R. Simultaneous determination of clotrimazole and tinidazole in tablet and cream by HPTLC. Int J Adv Res 2014, 2(7), 855–863. [Google Scholar]
- Patel, S.K.; Kapupara, P.P.; Shah, K.V. Simultaneous estimation of clotrimazole and tinidazole in pharmaceutical formulation by HPTLC. Int. J. Res. Dev. Pharm. Life Sci. 2015, 4(4), 1635–1640. [Google Scholar]
- Salem, H.; Riad, S.; Reda, M.; Ahmed, K. Simultaneous determination of omeprazole, tinidazole and clarithromycin in bulk powder and Helicure tablets by TLC- densitometric technique. J Pharm Educ Res 2013, 4(1), 34–40. [Google Scholar]
- Meshram, D.B.; Mishra, P.; Desai, S.D.; Tajne, M.R. Simultaneous determination of fluconazole and tinidazole in combined dose tablet using high performance thin layer chromatography. Der Chemica Sinica 2017, 8(1), 133–137. [Google Scholar]
- Nethra, K.; Shaik Mohammed, Z.; Kavitha, J.; Seetharaman, R.; Kokilambigai, K.S.; Lakshmi, K.S. Deve;opment and validation of stability indicating HPTLC method for the simultaneous estimation of tinidazole and fluconazole and its applicability in marketed dosage form. Int J Appl Pharm 2022, 14, 153–160. [Google Scholar]
- Mohammad, M.A. , Zawilla, N.H.; El-Anwar, F.M.; El-Moghazy Aly, S.M. Stability indicating methods for the determination of norfloxacin in mixture with tinidazole. Chem Pharm Bull (Tokyo) 2007, 55(1), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Naguib, I.A.; Abdelaleem, E.A.; Hassa, E.S.; Ali, N.W. HPTLC method for simultaneous determination of norfloxacin and tinidazole in presence of tinidazole impurity. J Chromatogr Sci 2019, 57, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Abou-Taleb, N.H.; El-Enany, N.M.; El-Sherbiny, D.T.; El-Subbagh, H.I. Digitally enhanced thin layer chromatography for simultaneous determination of norfloxacin and tinidazole with the aid of Taguchi orthogonal array and desirability function approach: Greenness assessment by analytical Eco-Scale. J Sep Sci 2020, 43, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Saraya, R.E.; Hassan, Y.F.; Eltoukhi, W.E.; Salman, B.I. Application of the green analytical procedure index to the simultaneous analysis of co-formulated tinidazole and ciprofloxacin in pure form, tablet dosage form, and human plasma using an environmentally friendly micellar high-performance thin-layer chromatographic technology. J Planar Chromatogr – Modern TLC 2023, 36, 21–30. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology, Q2(R1); ICH: Geneva, Switzerland, 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 14 December 2023).
- Ferenczi-Fodor, K.; Renger, B.; Végh, Z. The frustrated reviewer – recurrent failures in manuscripts describing validation of quantitative TLC/HPTLC procedures for analysis of pharmaceuticals. J Planar Chromatogr – Modern TLC, 2010; 23, 173–179. [Google Scholar] [CrossRef]
- Pyka-Pająk, A. TLC–densitometric analysis of selected 5-nitroimidazoles. Processes 2023, 11, 170. [Google Scholar] [CrossRef]
- Agbaba, D.; Djurkovic, M.; Brboric, J.; Zivanov-Stakic, D. Simultaneous HPTLC determination of metronidazole and its impurity 2-methyl-5-nitroimidazole in pharmaceuticals. J Planar Chromatogr - Modern TLC 1998, 11, 447–449. [Google Scholar]
- Sanyal, S.N.; Datta, A.K.; Chakrabarti, A. Stability indicating TLC method for the quantification of tinidazole in pharmaceutical dosage form - I.V. Fluid. Drug Dev Ind Pharm 1992, 18, 2095–2100. [Google Scholar] [CrossRef]
- Konieczka, P.; Namiesnik, J. Validation of analytical procedures. In Evaluation and Quality Control of Analytical Measurement Results; Konieczka, P., Namiesnik, J., Eds.; WNT: Warsaw, Poland, 2007. (In Polish) [Google Scholar]
- Hendix, C.D. What every technologist should know about experiment design. Chem Technol 1979, 9, 167–174. [Google Scholar]
- Nagy-Turák, A.; Végh, Z.; Ferenczi-Fodor, K. Validaton of the quantitative planar chromatographic analysis of drug substances. III. Robustness testing in OPLC. J Planar Chromatogr – Modern TLC. 1995; 8, 188–193.
Figure 1.
Densitogram of a mixture of standard substances: metronidazole (M), secnidazole (S), ornidazole (O), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP) made at 313 nm, using silica gel 60F254 plate and mobile phase: chloroform + methanol + diethylamine (9:1:1, v/v).
Figure 1.
Densitogram of a mixture of standard substances: metronidazole (M), secnidazole (S), ornidazole (O), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP) made at 313 nm, using silica gel 60F254 plate and mobile phase: chloroform + methanol + diethylamine (9:1:1, v/v).
Figure 2.
Densitogram of a mixture of standard substances: metronidazole (M), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP) made at 313 nm, using silica gel 60F254 plate and mobile phase: chloroform + methanol + diethylamine (9:1:1, v/v).
Figure 2.
Densitogram of a mixture of standard substances: metronidazole (M), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP) made at 313 nm, using silica gel 60F254 plate and mobile phase: chloroform + methanol + diethylamine (9:1:1, v/v).
Figure 3.
Densitogram of a Metronidazole Polpharma drug sample made at 313 nm, using silica gel 60F254 plate and mobile phase: chloroform + methanol + diethylamine (9:1:1, v/v); where M – metronidazole.
Figure 3.
Densitogram of a Metronidazole Polpharma drug sample made at 313 nm, using silica gel 60F254 plate and mobile phase: chloroform + methanol + diethylamine (9:1:1, v/v); where M – metronidazole.
Figure 4.
Densitogram of a Tinidazolum Polpharma drug sample made at 313 nm, using silica gel 60F254 plate and mobile phase: chloroform + methanol + diethylamine (9:1:1, v/v); where T – tinidazole.
Figure 4.
Densitogram of a Tinidazolum Polpharma drug sample made at 313 nm, using silica gel 60F254 plate and mobile phase: chloroform + methanol + diethylamine (9:1:1, v/v); where T – tinidazole.
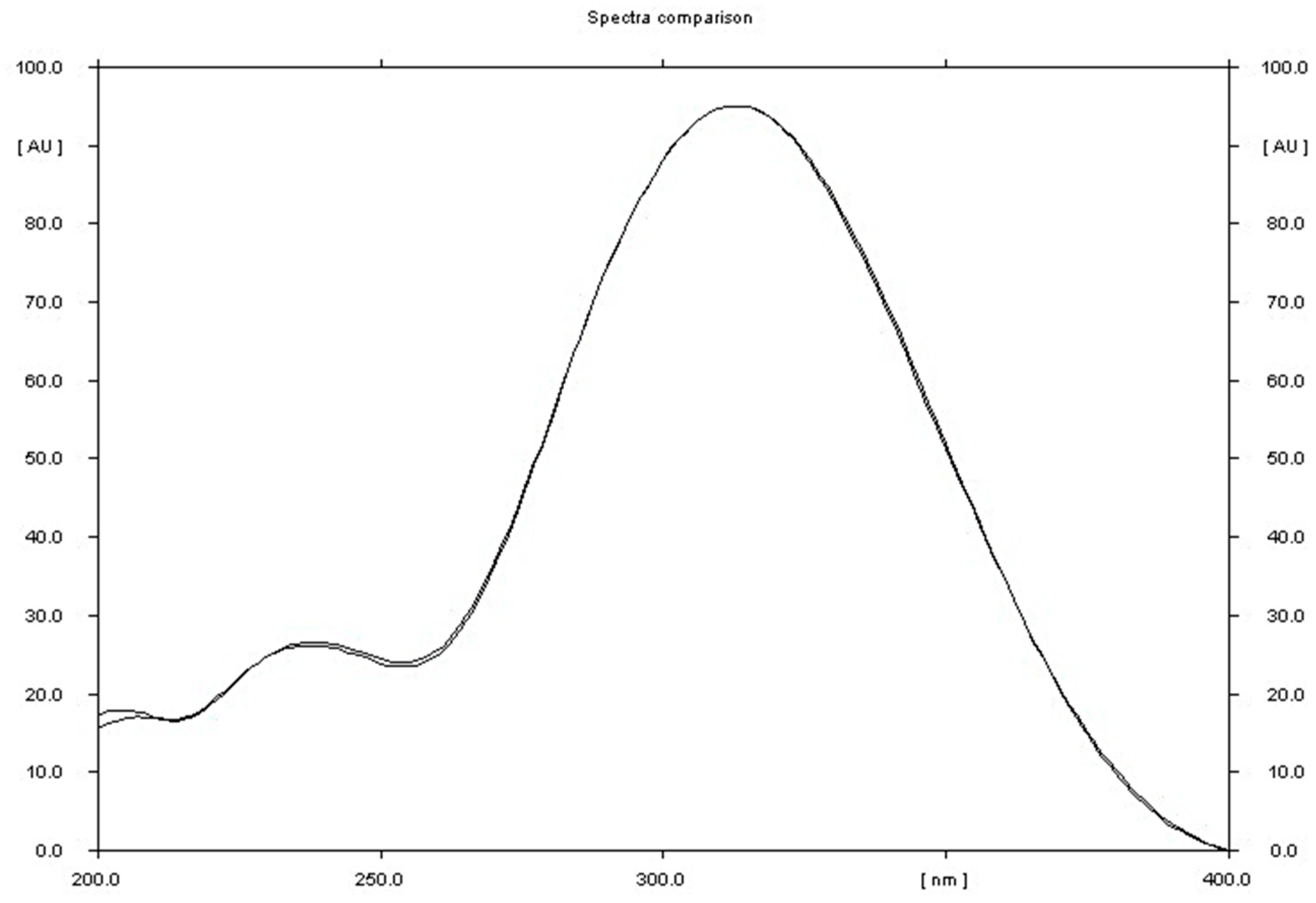
Figure 5.
Comparison of the spectrodensitogram obtained for the standard substance metronidazole with the spectrodensitogram obtained for metronidazole, the source of which was sample of Metronidazole Polpharma tablets.
Figure 5.
Comparison of the spectrodensitogram obtained for the standard substance metronidazole with the spectrodensitogram obtained for metronidazole, the source of which was sample of Metronidazole Polpharma tablets.
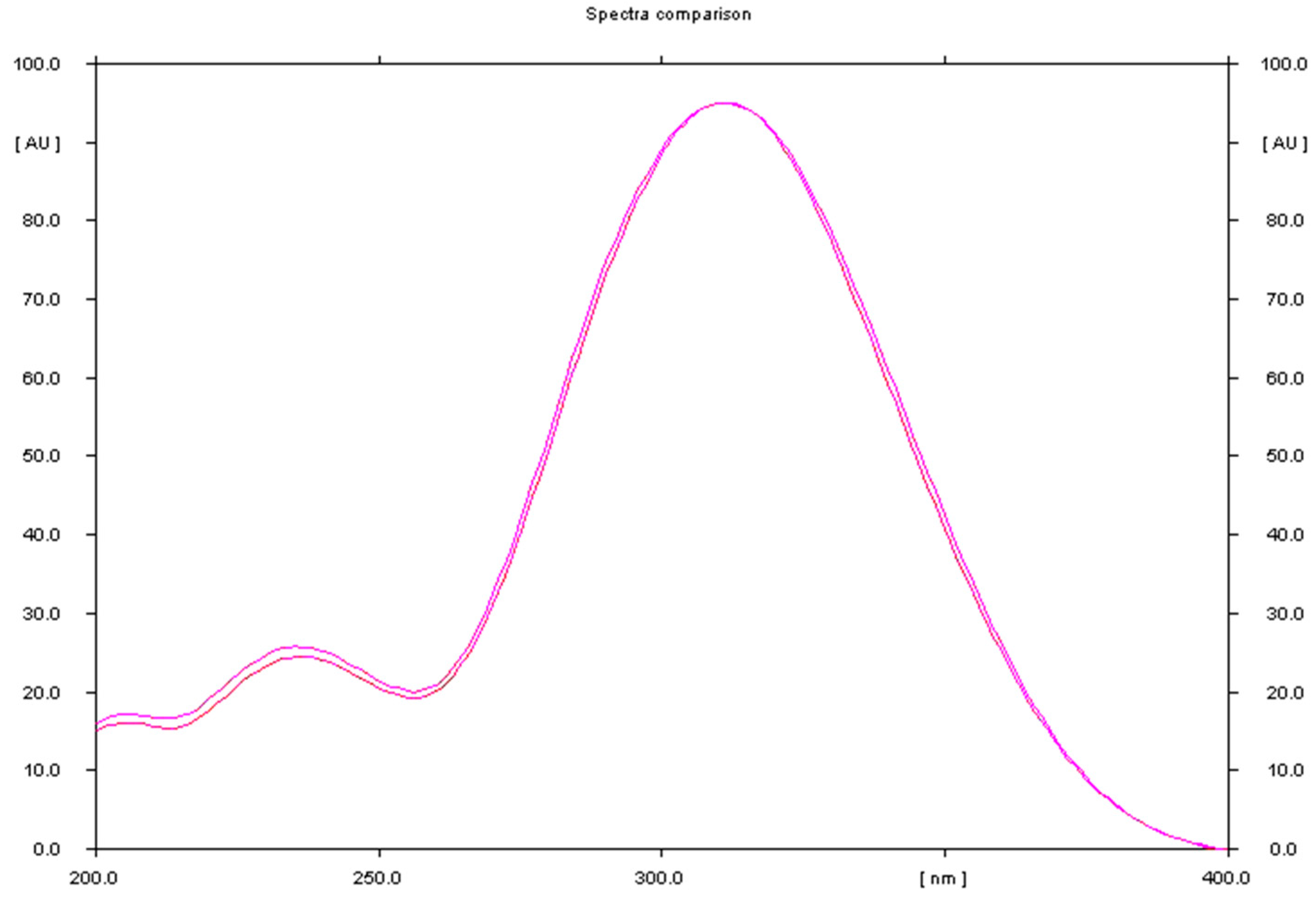
Figure 6.
Comparison of the spectrodensitogram obtained for the standard substance tinidazole with the spectrodensitogram obtained for tinidazole, the source of which was sample of Tinidazolum Polpharma tablets.
Figure 6.
Comparison of the spectrodensitogram obtained for the standard substance tinidazole with the spectrodensitogram obtained for tinidazole, the source of which was sample of Tinidazolum Polpharma tablets.
Figure 7.
Robustness test: the effects of factors (A), and half-normal probability plot of effects (B) for determination of metronidazole (M) in Metronidazol Polpharma tablets.
Figure 7.
Robustness test: the effects of factors (A), and half-normal probability plot of effects (B) for determination of metronidazole (M) in Metronidazol Polpharma tablets.
Figure 8.
Robustness test: the effects of factors (A), and half-normal probability plot of effects (B) for determination of tinidazole (T) in Tinidazolum Polpharma tablets.
Figure 8.
Robustness test: the effects of factors (A), and half-normal probability plot of effects (B) for determination of tinidazole (T) in Tinidazolum Polpharma tablets.
Table 1.
RF and Rs values for the best separations of metronidazole (M), secnidazole (S), ornidazole (O), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP).
Table 1.
RF and Rs values for the best separations of metronidazole (M), secnidazole (S), ornidazole (O), tinidazole (T), and 2-methyl-5-nitroimidazole (IMP).
No
of mobile phasea)
|
|
RF and Rs values |
| 2 |
RF
|
RF(M) = 0.35±0.02, RF(O) = 0.43±0.02, RF(T) = 0.50±0.02, RF(S) = 0.57±0.02, RF(IMP) = 0.64±0.03; |
| RS
|
RS(M/O)=0.78, RS(O/T)=0.77, RS(T/S)=0.83, RS(S/IMP)=0.80 |
| 8 |
RF
|
RF(IMP) = 0.36±0.02, RF(M) = 0.41±0.02, RF(O) = 0.46±0.03, RF(S) = 0.51±0.03, RF(T) = 0.59±0.03; |
| RS
|
RS(IMP/M)=0.75, RS(M/O)=1.07, RS(O/S)=1.05, RS(S/T)=1.29 |
| 9 |
RF
|
RF(IMP) = 0.31±0.02, RF(M) = 0.34±0.02, RF(S) = 0.39±0.02, RF(O) = 0.47±0.03, RF(T) = 0.59±0.03; |
| RS
|
RS(IMP/M)=0.83, RS(M/S)=1.27, RS(S/O)=1.43, RS(O/T)=1.78 |
| 12 |
RF
|
RF(M) = 0.45±0.02, RF(IMP) = 0.49±0.02, RF(O) = 0.55±0.03, RF(S) = 0.65±0.02, RF(T) = 0.74±0.03; |
| RS
|
RS(M/ IMP)=0.83, RS(IMP/O)=1.33, RS(O/S)=1.33, RS(S/T)=1.33 |
| 13 |
RF
|
RF(M) = 0.39±0.02, RF(IMP) = 0.42±0.02, RF(O) = 0.50±0.03, RF(S) = 0.61±0.02, RF(T) = 0.67±0.02; |
| RS
|
RS(M/ IMP)=0.53, RS(IMP/O)=1.18, RS(O/S)=1.58, RS(S/T)=1.13 |
| 14 |
RF
|
RF(M) = 0.28±0.02, RF(S) = 0.35±0.02, RF(T) = 0.41±0.03, RF(O) = 0.47±0.03, RF(IMP) = 0.56±0.03; |
| RS
|
RS(M/S)=0.88, RS(S/T)=1.07, RS(T/O)=1.07, RS(O/IMP)=1.33 |
| 16 |
RF
|
RF(M) = 0.35±0.02, RF(S) = 0.41±0.02, RF(T) = 0.51±0.02, RF(O) = 0.54±0.02, RF(IMP) = 0.63±0.03 |
| RS
|
RS(M/S)=1.05, RS(S/T)=1.44, RS(T/O)=0.30, RS(O/IMP)=1.20 |
| 19 |
RF
|
RF(IMP) = 0.30±0.02, RF(M) = 0.38±0.03, RF(S) = 0.44±0.03, RF(O) = 0.51±0.03, RF(T) = 0.70±0.04 |
| RS
|
RS(IMP/M)=1.33, RS(M/S)=1.22, RS(S/O)=1.29, RS(O/T)=2.10 |
Table 2.
Method-validation data for the quantitative determination of metronidazole (M) and tinidazole (T) by TLC with densitometry.
Table 2.
Method-validation data for the quantitative determination of metronidazole (M) and tinidazole (T) by TLC with densitometry.
| Method Characteristic |
5-Nitroimidazole |
| Metronidazole |
Tinidazole |
| Retardation factor (Rf) |
0.38 ± 0.03 |
0.70 ± 0.04 |
| Range [μg/spot] |
0.2–2.0 |
0.2–2.0 |
Linearity [μg/spot]
A=a⋅X+b |
a |
7340.4(±133.1) |
7828.0(±114.2) |
| b |
3071.5(±140.2) |
5567.9(±130.1) |
| n |
10 |
10 |
| r |
0.9989 |
0.9992 |
| s |
235.6 |
218.6 |
| F |
3557 |
4701 |
| LOD [µg/spot] |
0.012 |
0.022 |
| LOQ [µg/spot] |
0.036 |
0.066 |
| |
For tablets |
|
| |
Accuracy (n=6) |
|
for 50% standard added
|
R = 103.8%; CV = 1.96% |
R = 101.8%; CV = 0.95% |
for 100% standard added
|
R = 104.3%; CV = 1.13% |
R = 99.1%; CV = 2.31% |
for 150% standard added
|
R = 101.2 %; CV = 2.48% |
R = 100.9%; CV = 2.49% |
| Average recovery |
103.1% |
100.6% |
| |
Precission (CV, [%]) |
|
| Intraday (n=3) |
|
|
| for 1.75 µg/spot |
1.08 |
0.76 |
| for 1.00 µg/spot |
1.12 |
0.89 |
| for 0.30 µg/spot |
0.99 |
1.28 |
| Interday (n=3) |
|
|
| for 1.75 µg/spot |
1.33 |
0.99 |
| for 1.00 µg/spot |
1.39 |
1.44 |
| for 0.30 µg/spot |
1.48 |
1.76 |
| Robustness (CV, [%]) |
robust |
robust |
Table 3.
Comparison of LOD and LOQ of metronidazole and tinidazole obtained by other authors.
Table 3.
Comparison of LOD and LOQ of metronidazole and tinidazole obtained by other authors.
| Method |
Mobile phase |
LOD and LOQ
[µg/plamkę] |
Ref |
| Metronidazole |
| HPTLC |
Methanol + chloroform (9:1, v/v) |
LOD = 0.61
LOQ = 0.95 |
[18] |
| HPTLC |
Toluene + ethyl acetate + methanol + ammonia (3 : 1.5 : 0.5 : 0.1, v/v) |
LOD = 0.046
LOQ = 0.116 |
[19] |
| HPTLC |
Benzene + ethyl acetate + toluene + methanol + glacial acetic acid (9.5 : 2 : 5 : 1.5 : 0.5, v/v) |
LOD = 0.88
LOQ = 1.93 |
[21] |
| TLC |
Acetonitryle + ammonia + methanol + methylene chloride + hexane (1.3 : 1.1 : 2 : 3 : 1, v/v) |
LOD = 0.32
LOQ = 0.96 |
[22] |
| TLC |
Ethyl acetate + acetone + hexane + ammonia (9.5 : 0.5 : 0.3 : 0.3, v/v) |
LOD = 0.13
LOQ = 0.38 |
[23] |
| TLC |
Chloroform + acetone + glacial acetic acid
(7.5 : 2.5 : 0.1, v/v) |
LOD = 0.51
LOQ = 1.55 |
[16] |
| TLC |
Chloroform+methanol (9:1, v/v ) |
LOD = 0.052
LOQ = 0.159 |
[36] |
| Tinidazole |
| HPTLC |
toluene + ethyl acetate + methanol + triethyl amine (5.5:1.0:1.0:0.1, v/v) |
LOD = 0.011
LOQ = 0.037 |
[25] |
| HPTLC |
Acetone + ethanol + 2% watery sodium dodecyl sulfate
(3:4:2, v/v) |
LOD = 0.0067
LOQ = 0.0203 |
[33] |
| HPTLC |
30% Trifluoroacetic acid |
LODa = 0.01
LOQa = 0.03
LODb = 0.12
LOQb = 0.36 |
[32] |
| TLC |
Isopropanol + butanol + ammonia + water (25:50:5:25, v/v) |
LOD = 0.1
LOQ = 0.3 |
[30] |
| TLC |
methylene chloride + isopropyl alcohol + acetonitrile + ammonia (11: 1.2: 5: 0.2, v/v) |
LOD = 0.2
LOQ = 0.6 |
[27] |
| TLC |
Chloroform+methanol (9:1, v/v) |
LOD = 0.058
LOQ = 0.174 |
[36] |
Table 4.
Experimental design matrix (23) for robustness test for metronidazole (M) and tinidazole (T) ingredients in tablets.
Table 4.
Experimental design matrix (23) for robustness test for metronidazole (M) and tinidazole (T) ingredients in tablets.
Experiment
No |
X1
|
X2
|
X3
|
X4
|
X5
|
X6
|
X7
|
Active pharmaceutical ingredienta content (yi)
[mg⋅tablet-1] |
| M |
T |
| 1 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
493.8 |
494.2 |
| 2 |
+ |
+ |
- |
+ |
- |
- |
- |
489.9 |
502.5 |
| 3 |
+ |
- |
+ |
- |
- |
+ |
- |
496.9 |
495.2 |
| 4 |
+ |
- |
- |
- |
+ |
- |
+ |
500.2 |
506.1 |
| 5 |
- |
+ |
+ |
- |
+ |
- |
- |
502.8 |
499.5 |
| 6 |
- |
+ |
- |
- |
- |
+ |
+ |
493.1 |
490.2 |
| 7 |
- |
- |
+ |
+ |
- |
- |
+ |
493.2 |
493.5 |
| 8 |
- |
- |
- |
+ |
+ |
+ |
- |
513.8 |
508.2 |
| Size of effect |
M |
-3.025 |
-8.625 |
-5.075 |
-3.075 |
11.875 |
0.375 |
-3.275 |
|
|
| T |
1.650 |
-4.150 |
-6.150 |
1.850 |
6.650 |
-3.450 |
-5.350 |
| The label claim [mg] |
|
|
|
|
|
|
|
|
500 |
500 |
| Average amount [mg] |
|
|
|
|
|
|
|
|
498.0 |
498.7 |
| Variance |
|
|
|
|
|
|
|
|
58.4 |
41.7 |
| Standard devitation (SD) |
|
|
|
|
|
|
|
|
7.64 |
6.46 |
| Coefficient of variation [CV, %] |
|
|
|
|
|
|
|
|
1.5 |
1.3 |
Table 5.
Comparison of metronidazole, and tinidazole assays [mg/tablet] obtained from ten repeated different analysis of by proposed TLC-densitometric (A) and pharmakopeial (B) methods.
Table 5.
Comparison of metronidazole, and tinidazole assays [mg/tablet] obtained from ten repeated different analysis of by proposed TLC-densitometric (A) and pharmakopeial (B) methods.
| |
Metronidazole |
Tinidazole |
| Method |
| A |
B |
A |
B |
| Number of analysis |
10 |
10 |
10 |
10 |
| 1 |
510.3 |
528.6 |
489.9 |
492.5 |
| 2 |
523.0 |
506.4 |
495.2 |
488.9 |
| 3 |
520.0 |
521.5 |
492.3 |
512.6 |
| 4 |
495.6 |
516.2 |
487.6 |
501.8 |
| 5 |
503.6 |
508.3 |
502.6 |
481.7 |
| 6 |
488.4 |
498.2 |
509.9 |
479.6 |
| 7 |
520.1 |
501.3 |
487.1 |
509.9 |
| 8 |
515.3 |
496.3 |
512.3 |
508.1 |
| 9 |
491.7 |
517.8 |
507.8 |
505.5 |
| 10 |
496.7 |
497.5 |
508.8 |
491.8 |
| Average |
506.5 |
509.2 |
499.4 |
497.2 |
| Label claimed |
500 |
500 |
500 |
500 |
| Amount of metronidazole and tinidazole (%) in relations to the label claim |
101.3 |
101.8 |
99.8 |
99.4 |
| Standard deviation (SD) |
12.9 |
11.3 |
10.0 |
11.9 |
| Coefficient of variation [CV, %] |
2.55 |
2.22 |
2.00 |
2.39 |
Confidence interval of
arithmetic mean with
confidence level equal 95% |
µ=506.5±9.2 |
µ=509.2±8.1 |
µ=499.4±7.2 |
µ=497.2±8.5 |
| t calculated |
0.497 |
0.447 |
| t(95%.18) tabulated |
2.101 |
2.101 |
| F calculated |
1.30 |
1.42 |
| F(95%.f1 = f2 = 9) tabulated |
3.18 |
3.18 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).