Submitted:
29 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Alzheimer’s Disease (AD)
3. Multi-Target Drugs
3.1. Chemical-based Drugs
3.2. Immune system-modulating Drugs
3.3. Nanobodies
3.3.1. Fab Fragments
3.3.2. Domain Antibodies
3.3.3. Single-Chain Variable Fragments (scFv)
3.4. Antibody Targeting
- Select an Antigenic Epitope: Identify a specific sequence or epitope known to be antigenic, crucial for antibody-antigen interaction in AD.
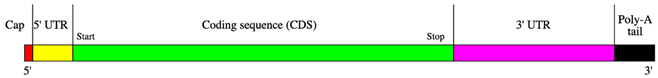
- Design mRNA Sequence: Create an mRNA sequence encoding the chosen epitope, incorporating a 5′ cap and a 3′ poly-A tail to align with transcription, such as starting with a 5′ cap and including a 3′ poly-A tail. Ensure the sequence is in-frame with the ribosome so that translation produces the desired epitope.
- Codon Optimization: Optimize the mRNA sequence for effective translation in the desired host cell, mainly by selecting codons frequently used by the host.
- Consider mRNA Modifications: To boost stability and translation, integrate modified nucleotides such as pseudouridine or 5-methylcytidine into the mRNA sequence, which minimizes immune recognition. Alternatively, the replacement of uridine with pseudouridine is also a practical approach.
- Delivery Method: Decide on the delivery approach for the mRNA to the target cells, such as electroporation, lipid nanoparticles, or viral vectors.
- Expression System: Select an efficient expression system for effective mRNA translation and epitope production, such as a suitable cell line or organism.
- In Vitro Translation: Verify the mRNA's ability to produce the desired epitope through in vitro translation systems.
- Antigen Presentation: Process and present the translated antigenic peptide on the cell surface via major histocompatibility complex (MHC) molecules for immune recognition.
- Immunization: Use the peptide to immunize, stimulating an immune response as part of a vaccine or immunotherapy.
- Immune Response Evaluation: Assess the immune response by measuring antibody or T-cell reactions against the peptide using enzyme-linked immunosorbent assay (ELISA), flow cytometry, or cytokine assays.
4. AI-Driven Multi-Target Drugs
5. Drug Delivery Across the BBB
5.1. Strategies that Aid Drugs Cross the Blood–Brain Barrier
- Invasive techniques include intra-cerebral injection, convection-enhanced delivery, and intra-cerebroventricular infusion [122].
- BBB disruption with bradykinin analogs, ultrasonography, and osmotic pressure [123].
- Physiological procedures involving transporter-mediated delivery, receptor-mediated transcytosis, and adsorptive-mediated transcytosis [124].
- Pharmacological techniques involving liposome-mediated drug delivery or chemically modifying pharmaceuticals to lipophilic molecules [125].
- Opsonization and drug delivery by nanoparticles across the BBB, wherein the drug is adsorbed onto the particles passively [126].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciurea, A.V.; Mohan, A.G.; Covache-Busuioc, R.A.; Costin, H.P.; Glavan, L.A.; Corlatescu, A.D.; Saceleanu, V.M. Unraveling molecular and genetic insights into neurodegenerative diseases: advances in understanding Alzheimer's, Parkinson's, and Huntington's diseases and amyotrophic lateral sclerosis. Int J Mol Sci 2023, 24, 10809. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: current therapeutic approaches and the potential role of nanotherapeutics. Int J Mol Sci 2022, 23, 1851. [Google Scholar] [CrossRef]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O'Connor, K.; Keene, C.D. Traumatic brain injury and risk of neurodegenerative disorder. Biol Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Kakoti, B.B.; Bezbaruah, R.; Ahmed, N. Therapeutic drug repositioning with special emphasis on neurodegenerative diseases: threats and issues. Front Pharmacol 2022, 13, 1007315. [Google Scholar] [CrossRef]
- Han, C.; Chaineau, M.; Chen, C.X.; Beitel, L.K.; Durcan, T.M. Open science meets stem cells: a new drug discovery approach for neurodegenerative disorders. Front Neurosci 2018, 12, 47. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer's disease drug development pipeline: 2022. Alzheimers Dement (N Y) 2022, 8, e12295. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.; Yao, Z.; Khleifat, A.A.L.; Tantiangco, H.; Tamburin, S.; Albertyn, C.; Thakur, L.; Llewellyn, D.J.; Oxtoby, N.P.; Lourida, I.; et al. Artificial intelligence for dementia drug discovery and trials optimization. Alzheimers Dement 2023, 19, 5922–5933. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.; Bose, N.; Nisenbaum, L.; Partrick, K.A.; Fillit, H.M. The critical role of biomarkers for drug development targeting the biology of aging. J Prev Alzheimers Dis 2023, 10, 729–742. [Google Scholar] [CrossRef]
- Li, S.; Yi, Y.; Cui, K.; Zhang, Y.; Chen, Y.; Han, D.; Sun, L.; Zhang, X.; Chen, F.; Zhang, Y.; Yang, Y. A single-chain variable fragment antibody inhibits aggregation of phosphorylated tau and ameliorates tau toxicity in vitro and in vivo. J Alzheimer's Dis 2021, 79, 1613–1629. [Google Scholar] [CrossRef]
- Medeiros, R.; Baglietto-Vargas, D.; LaFerla, F.M. The role of tau in Alzheimer's disease and related disorders. CNS Neurosci Ther 2011, 17, 514–524. [Google Scholar] [CrossRef]
- Kumari, S.; Mehta, S.L.; Li, P.A. Glutamate induces mitochondrial dynamic imbalance and autophagy activation: preventive effects of selenium. PLOS One 2012, 7, e39382. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Song, C.; Qin, T.; et al. Moschus ameliorates glutamate-induced cellular damage by regulating autophagy and apoptosis pathway. Sci Rep 2023, 13, 18586. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G. The pathobiology of Alzheimer's disease. Annu Rev Med 1989, 40, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Roher, A.E.; Lowenson, J.D.; Clarke, S.; Woods, A.S.; Cotter, R.J.; Gowing, E.; Ball, M.J. beta-amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci U S A 1993, 90, 10836–10840. [Google Scholar] [CrossRef] [PubMed]
- Ow, S.Y.; Dunstan, D.E. A brief overview of amyloids and Alzheimer's disease. Protein Sci 23, 1315–1331. [CrossRef]
- Mathur, S.; Gawas, C.; Ahmad, I.Z.; Wani, M.; Tabassum, H. Neurodegenerative disorders: assessing the impact of natural vs drug-induced treatment options. Aging Med (Milton). 2023, 6, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol Neurodegener 2022, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.L.; Tiew, J.K.; Fong, Y.H.; Leong, H.W.; Chan, Y.M.; Chan, Z.L.; Kong, E.W.J. Current pharmacotherapy and multi-target approaches for Alzheimer's disease. Pharmaceuticals (Basel) 2022, 15, 1560. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L.; Giulianotti, M.A.; Welmaker, G.S.; Houghten, R.A. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov Today 2013, 18, 495–501. [Google Scholar] [CrossRef]
- Talevi, A. Multi-target pharmacology: possibilities and limitations of the "skeleton key approach" from a medicinal chemist perspective. Front Pharmacol 2015, 6, 205. [Google Scholar] [CrossRef]
- Makhoba, X.H.; Viegas, C., Jr.; Mosa, R.A.; Viegas, F.P.D.; Pooe, O.J. Potential impact of the multi-target drug approach in the treatment of some complex diseases. Drug Des Devel Ther 2020, 14, 3235–3249. [Google Scholar] [CrossRef]
- Löscher, W. Single-target versus multi-target drugs versus combinations of drugs with multiple targets: preclinical and clinical evidence for the treatment or prevention of epilepsy. Front Pharmacol 2021, 12, 730257. [Google Scholar] [CrossRef]
- Kieburtz, K. Treating neurodegenerative disease before illness: a challenge for the 21st century. Lancet Neurol 2016, 15, 540–541. [Google Scholar] [CrossRef]
- Sheikh, S.; Safia; Haque, E.; Mir, S.S. Neurodegenerative diseases: multifactorial conformational diseases and their therapeutic interventions. J Neurodegener Dis 2013, 2013, 563481. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhang, G.; Qin, Z.; Yin, M.; Chen, W.; Zhang, Y.; Liu, X. Safinamide protects against amyloid β (Aβ)-induced oxidative stress and cellular senescence in M17 neuronal cells. Bioengineered 2022, 13, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Dodge, H.H.; Arnold, S.E. One step forward to personalized medicine? Alzheimers Dement (N Y) 2023, 9, e12435. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, T.; Deore, S.L.; Kide, A.A.; Shende, B.A.; Sharma, R.; Dadarao Chakole, R.; et al. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis: an updated review. Mitochondrion 2023, 71, 83–92. [Google Scholar] [CrossRef]
- Cummings, J.; Aisen, P.S.; DuBois, B.; Frölich, L.; Jack, C.R., Jr.; Jones, R.W.; Morris, J.C.; Raskin, J.; Dowsett, S.A.; Scheltens, P. Drug development in Alzheimer's disease: the path to 2025. Alzheimers Res Ther 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.T.; Sufian, M.A.; Uddin, M.S.; Begum, M.M.; Akhter, S.; Islam, A.; Amran, M.S., Md; Ashraf, G. NMDA receptor antagonists: repositioning of memantine as a multitargeting agent for Alzheimer's therapy. Curr Pharm Des 2019, 25, 3506–3518. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Kabir, M.T.; Tewari, D.; Mathew, B.; Aleya, L. Emerging signal regulating potential of small molecule biflavonoids to combat neuropathological insults of Alzheimer's disease. Sci Total Environ 2020, 700, 134836. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, W.J.; Grossberg, G.T. A fixed-dose combination of memantine extended-release and donepezil in the treatment of moderate-to-severe Alzheimer's disease. Drug Des Devel Ther 2016, 10, 3267–3279. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Mamun, A.A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination drug therapy for the management of Alzheimer's disease. Int J Mol Sci 2020, 21, 3272. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov Today 2004, 9, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.H.; Shi, Z.; Tan, C.; Jiang, Y.; Go, M.L.; Low, B.C.; Chen, Y.Z. In-silico approaches to multi-target drug discovery: computer aided multi-target drug design, multi-target virtual screening. Pharm Res 2010, 27, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.W.; Savonenko, A.V.; Melnikova, T.; Kim, H.; Price, D.L.; Li, T.; Wong, P.C. Modeling an anti-amyloid combination therapy for Alzheimer's. Sci Transl Med 2010, 2, 13ra1. [Google Scholar] [CrossRef] [PubMed]
- Pepeu, G.; Giovannini, M.G. Cholinesterase inhibitors and beyond. Curr Alzheimer Res 2009, 6, 86–96. [Google Scholar] [CrossRef]
- Pohanka, M. Acetylcholinesterase inhibitors: a patent review (2008 - present). Expert Opin Ther Pat 2012, 22, 871–886. [Google Scholar] [CrossRef]
- Zheng, H.; Fridkin, M.; Youdim, M. From single target to multitarget/network therapeutics in Alzheimer's therapy. Pharmaceuticals (Basel) 2014, 7, 113–135. [Google Scholar] [CrossRef]
- Plazas, E.; Hagenow, S.; Avila Murillo, M.; Stark, H.; Cuca, L.E. Isoquinoline alkaloids from the roots of Zanthoxylum rigidum as multi-target inhibitors of cholinesterase, monoamine oxidase A and Aβ(1-42) aggregation. Bioorg Chem 2020, 98, 103722. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, H.; Gu, Q.; Xu, J. Synthesis and evaluation of tetrahydroisoquinoline-benzimidazole hybrids as multifunctional agents for the treatment of Alzheimer's disease. Eur J Med Chem 2019, 167, 133–145. [Google Scholar] [CrossRef]
- González-Naranjo, P.; Pérez-Macias, N.; Pérez, C.; Roca, C.; Vaca, G.; Girón, R.; Sánchez-Robles, E.; Martín-Fontelles, M.I.; de Ceballos, M.L.; Martin-Requero, A.; et al. Indazolylketones as new multitarget cannabinoid drugs. Eur J Med Chem 2019, 166, 90–107. [Google Scholar] [CrossRef]
- Ivanova, L.; Karelson, M.; Dobchev, D.A. Multitarget approach to drug candidates against Alzheimer's disease related to AChE, SERT, BACE1 and GSK3β protein targets. Molecules 2020, 25, 1846. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and astrocyte function and communication: what do we know in humans? Front Neurosci 2022, 16, 824888. [Google Scholar] [CrossRef]
- Ji, K.; Miyauchi, J.; Tsirka, S.E. Microglia: an active player in the regulation of synaptic activity. Neural Plast 2013, 2013, 627325. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal Transduct Target Ther 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Dobrin, N.; Brehar, F.M.; Popa, C.; Covache-Busuioc, R.A.; Glavan, L.A.; Costin, H.P.; Bratu, B.G.; Corlatescu, A.D.; Popa, A.A.; Ciurea, A.V. From recognition to remedy: the significance of biomarkers in neurodegenerative disease pathology. Int J Mol Sci 2023, 24, 16119. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine 2016, 1, 1003. [Google Scholar]
- Doty, K.R.; Guillot-Sestier, M.V.; Town, T. The role of the immune system in neurodegenerative disorders: adaptive or maladaptive? Brain Res 2015, 1617, 155–173. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Schafer, D.; Vincent, A.; Blachère, N.E.; Bar-Or, A. Neuroinflammation: ways in which the immune system affects the brain. Neurotherapeutics 2015, 12, 896–909. [Google Scholar] [CrossRef]
- Strzelec, M.; Detka, J.; Mieszczak, P.; Sobocińska, M.K.; Majka, M. Immunomodulation-a general review of the current state-of-the-art and new therapeutic strategies for targeting the immune system. Front Immunol 2023, 14, 1127704. [Google Scholar] [CrossRef]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer's disease: pathogenesis, mechanisms, and therapeutic potentials. Front Aging Neurosci 2023, 15, 1201982. [Google Scholar] [CrossRef]
- Yeh, F.L.; Hansen, D.V.; Sheng, M. TREM2, microglia, and neurodegenerative diseases. Trends Mol Med 2017, 23, 512–533. [Google Scholar] [CrossRef]
- Gratuze, M.; Leyns, C.E.G.; Holtzman, D.M. New insights into the role of TREM2 in Alzheimer's disease. Mol Neurodegener 2018, 13, 66. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Marriott, I. The interleukin-10 family of cytokines and their role in the CNS. Front Cell Neurosci 2018, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the immune response in the brain: IL-10 and its regulation. J Neuroinflammation 2016, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. FDA Grants Accelerated Approval for Alzheimer’s Disease Treatment https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment2023.
- Silva, J.D.; Taglialatela, G.; Jupiter, D.C. Reduced prevalence of dementia in patients prescribed tacrolimus, sirolimus, or cyclosporine. J Alzheimers Dis 2023, 95, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Zawia, N.H. Fenamates as potential therapeutics for neurodegenerative disorders. Cells 2021, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shi, J.; Zhang, P.; Zhang, Y.; Xu, J.; Zhao, L.; Zhang, R.; Wang, H.; Chen, H. Immunotherapy for Alzheimer's disease: targeting β-amyloid and beyond. Transl Neurodegener 2022, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Khorassani, F.; Hilas, O. Bapineuzumab, an investigational agent for Alzheimer's disease. PT 2013, 38, 89–91. [Google Scholar]
- Abushouk, A.I.; Elmaraezy, A.; Aglan, A.; Salama, R.; Fouda, S.; Fouda, R.; AlSafadi, A.M. Bapineuzumab for mild to moderate Alzheimer's disease: a meta-analysis of randomized controlled trials. BMC Neurol 2017, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Role of vitamin E in the treatment of Alzheimer's disease: evidence from animal models. Int J Mol Sci 2017, 18, 2504. [Google Scholar] [CrossRef] [PubMed]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer's disease: what do we know so far? Clin Interv Aging 2019, 14, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Grundman, M. Vitamin E and Alzheimer disease: the basis for additional clinical trials. Am J Clin Nutr 2000, 71, 630s–636s. [Google Scholar] [CrossRef]
- Numakawa, T.; Kajihara, R. Neurotrophins and other growth factors in the pathogenesis of Alzheimer's disease. Life (Basel) 2023, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer's disease. Aging Dis 2015, 6, 331–341. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer's disease and its pharmaceutical potential. Transl Neurodegener 2022, 11, 4. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Abskharon, R.; Pan, H.; Sawaya, M.R.; Seidler, P.M.; Olivares, E.J.; Chen, Y.; Murray, K.A.; Zhang, J.; Lantz, C.; Bentzel, M.; et al. Structure-based design of nanobodies that inhibit seeding of Alzheimer's patient-extracted tau fibrils. Proc Natl Acad Sci U S A 2023, 120, e2300258120. [Google Scholar] [CrossRef]
- Zheng, F.; Pang, Y.; Li, L.; Pang, Y.; Zhang, J.; Wang, X.; Raes, G. Applications of nanobodies in brain diseases. Front Immunol 2022, 13, 978513. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; et al. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Moos, T.; Thomsen, M.S.; Burkhart, A.; Hede, E.; Laczek, B. Targeted transport of biotherapeutics at the blood-brain barrier. Expert Opin Drug Deliv 2023, 20, 1823–1838. [Google Scholar] [CrossRef]
- Harmsen, M.M.; De Haard, H.J. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol 2007, 77, 13–22. [Google Scholar] [CrossRef]
- Van Audenhove, I.; Gettemans, J. Nanobodies as versatile tools to understand, diagnose, visualize and treat cancer. EBioMedicine 2016, 8, 40–48. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Y.; Ruan, S.; Hu, Y. Current anti-amyloid-β therapy for Alzheimer's disease treatment: from clinical research to nanomedicine. Int J Nanomedicine 2003, 18, 7825–FF. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M.F. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s disease (AD): from risk factors to therapeutic targeting. Cells 2020, 9, 383. [Google Scholar] [CrossRef]
- Saeed, A.F.; Wang, R.; Ling, S.; Wang, S. Antibody engineering for pursuing a healthier future. Front Microbiol 2017, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Nilvebrant, J.; Tessier, P.M.; Sidhu, S.S. Engineered autonomous human variable domains. Curr Pharm Des 2016, 22, 6527–6537. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.J.; Lazar, G.A. Next generation antibody drugs: pursuit of the 'high-hanging fruit'. Nat Rev Drug Discov 2018, 17, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Amano, A.; Sanjo, N.; Araki, W.; Anraku, Y.; Nakakido, M.; Matsubara, E.; Tomiyama, T.; Nagata, T.; Tsumoto, K.; Kataoka, K.; Yokota, T. Peripheral administration of nanomicelle-encapsulated anti-Aβ oligomer fragment antibody reduces various toxic Aβ species in the brain. J Nanobiotechnol 2023, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Tammer, A.H.; Coia, G.; Cappai, R.; Fuller, S.; Masters, C.L.; Hudson, P.; Underwood, J.R. Generation of a recombinant Fab antibody reactive with the Alzheimer's disease-related Abeta peptide. Clin Exp Immunol 2002, 129, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Krah, S.; Schröter, C.; Zielonka, S.; Empting, M.; Valldorf, B.; Kolmar, H. Single-domain antibodies for biomedical applications. Immunopharmacol Immunotoxicol 2016, 38, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.T.; Ma, C.; Li, G.J.; Zheng, X.Y.; Hao, Y.T.; Yang, Y.; Wang, X. Application of antibody fragments against Aβ with emphasis on combined application with nanoparticles in Alzheimer's disease. Front Pharmacol 2021, 12, 654611. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.Z.; Zou, C.J.; Mei, X.; Li, X.F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.X.; Wu, L.; Liu, Y. Targeting neuroinflammation in Alzheimer's disease: from mechanisms to clinical applications. Neural Regen Res 2023, 18, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Satheeshkumar, P.K. Expression of single chain variable fragment (scFv) molecules in plants: a comprehensive update. Mol Biotechnol 2020, 62, 151–167. [Google Scholar] [CrossRef]
- Montoliu-Gaya, L.; Villegas, S. Production of therapeutic single-chain variable fragments (ScFv) in Pichia pastoris. Methods Mol Biol 2022, 2313, 151–167. [Google Scholar] [CrossRef]
- Fan, X.; Xu, L.; Zhang, J.; Wang, Y.; Wu, Z.; Sun, W.; Yao, X.; Wang, X.; Guan, S.; Shan, Y. Mechanism exploration of amyloid-β-42 disaggregation by single-chain variable fragments of Alzheimer’s disease therapeutic antibodies. Int J Mol Sci 2023, 24, 8371. [Google Scholar] [CrossRef]
- Martin-Peña, A.; Rincon-Limas, D.E.; Fernandez-Funez, P. Anti-Aβ single-chain variable fragment antibodies restore memory acquisition in a Drosophila model of Alzheimer’s disease. Sci Rep 2017, 7, 11268. [Google Scholar] [CrossRef]
- Logovinsky, V.; Satlin, A.; Lai, R.; Swanson, C.; Kaplow, J.; Osswald, G.; Basun, H.; Lannfelt, L. Safety and tolerability of BAN2401--a clinical study in Alzheimer's disease with a protofibril selective Abeta antibody. Alzheimers Res Ther 2016, 8, 14. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Im, D.; Heo, C.E.; Son, M.K.; Park, C.R.; Kim, H.I.; Choi, J.M. Kinetic modulation of amyloid-beta (1-42) aggregation and toxicity by structure-based rational design. J Am Chem Soc 2022, 144, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Thacker, D.; Willas, A.; Dear, A.J.; Linse, S. Role of hydrophobicity at the N-terminal region of Abeta42 in secondary nucleation. ACS Chem Neurosci 2022, 13, 3477–3487. [Google Scholar] [CrossRef] [PubMed]
- Ravetch, J.V.; Bolland, S. IgG Fc receptors. Annu Rev Immunol 2001, 19, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.; Janeway, C. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Pub: New York, USA, 2001; pp: xviii. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J Mol Biol 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res 2013, 41, D36–D42. [Google Scholar] [CrossRef] [PubMed]
- Mooers, B.H.M. Shortcuts for faster image creation in PyMOL. Protein Sci 2020, 29, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Pal, A.; Pyne, N.; Paul, S. In-silico designing of a multi-epitope vaccine against SARS-CoV2 and studying the interaction of the vaccine with alpha, beta, delta and Omicron variants of concern. Curr Drug Discov Technol 2023, 20, e090922208713. [Google Scholar] [CrossRef]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; et al. The ClusPro web server for protein-protein docking. Nat Protoc 2017, 12, 255–278. [Google Scholar] [CrossRef]
- Mauger, D.M.; Cabral, B.J.; Presnyak, V.; Su, S.V.; Reid, D.W.; Goodman, B.; et al. mRNA structure regulates protein expression through changes in functional half-life. Proc Natl Acad Sci U S A 2019, 116, 24075–24083. [Google Scholar] [CrossRef]
- Wu, Y.; Mao, M.; Wang, L.J. Integrated clustering signature of genomic heterogeneity, stemness and tumor microenvironment predicts glioma prognosis and immunotherapy response. Aging (Albany NY) 2023, 15, 9086–9104. [Google Scholar] [CrossRef]
- Lührs, T.; Ritter, C.; Adrian, M.; Riek-Loher, D.; Bohrmann, B.; Döbeli, H.; et al. 3D structure of Alzheimer's amyloid-beta(1-42) fibrils. Proc Natl Acad Sci 2005, 102, 17342–17347. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 1998, 95, 6448–6453. [Google Scholar] [CrossRef] [PubMed]
- Hartley, D.M.; Walsh, D.M.; Ye, C.P.; Diehl, T.; Vasquez, S.; Vassilev, PM.; et al. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci 1999, 19, 8876–8884. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.M.; González Díaz, A.; Fuxreiter, M.; Pun, F.W.; Zhavoronkov, A.; Vendruscolo, M. Multiomic prediction of therapeutic targets for human diseases associated with protein phase separation. Proc Natl Acad Sci U S A 2023, 120, e2300215120. [Google Scholar] [CrossRef]
- Merchant, J.P.; Zhu, K.; Henrion, M.Y.R.; Zaidi, S.S.A.; Lau, B.; Moein, S.; Alamprese, M.L.; Pearse, R.V., 2nd; Bennett, D.A.; Ertekin-Taner, N.; et al. Predictive network analysis identifies JMJD6 and other potential key drivers in Alzheimer's disease. Commun Biol 2023, 6, 503. [Google Scholar] [CrossRef]
- Silva-Spínola, A.; Baldeiras, I.; Arrais, J.P.; Santana, I. The road to personalized medicine in Alzheimer's disease: the use of artificial intelligence. Biomedicines 2022, 10, 315. [Google Scholar] [CrossRef]
- Arrué, L.; Cigna-Méndez, A.; Barbosa, T.; Borrego-Muñoz, P.; Struve-Villalobos, S.; Oviedo, V.; Martínez-García, C.; Sepúlveda-Lara, A.; Millán, N.; Márquez Montesinos, J.C.E.; et al. New drug design avenues targeting Alzheimer’s disease by pharmacoinformatics-aided tools. Pharmaceutics 2022, 14, 1914. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chowdhury, S.; Kumar, S. In silico repurposing of antipsychotic drugs for Alzheimer's disease. BMC Neurosci 2017, 18, 76. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Whittlesey, K.J.; Shea, L.D. Nerve growth factor expression by PLG-mediated lipofection. Biomaterials 2006, 27, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, A.; Collin, L.; Lalli, G. Nucleofection of primary neurons. Methods Enzymol 2006, 406, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Saltzman, W.M. Enhancement of transfection by physical concentration of DNA at the cell surface. Nat Biotechnol 2000, 18, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug targeting to the brain. Pharm Res 2007, 24, 1733–1744. [Google Scholar] [CrossRef]
- Fortin, D.; Gendron, C.; Boudrias, M.; Garant, M.P. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in the treatment of cerebral metastasis. Cancer 2007, 109, 751–760. [Google Scholar] [CrossRef]
- Jones, A.R.; Shusta, E.V. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res 2007, 24, 1759–1771. [Google Scholar] [CrossRef]
- Bradley, M.O.; Swindell, C.S.; Anthony, F.H.; Witman, P.A.; Devanesan, P.; Webb, N.L.; et al. Tumor targeting by conjugation of DHA to paclitaxel. J Control Release 2001, 74, 233–236. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Szebeni, J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res 2003, 42, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med 2011, 3, 84ra44. [Google Scholar] [CrossRef] [PubMed]
- Niewoehner, J.; Bohrmann, B.; Collin, L.; Urich, E.; Sade, H.; Maier, P.; et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 2014, 81, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Biden, J. FACT SHEET: The United States Announces New Investments and Resources to Advance President Biden’s National Biotechnology and Biomanufacturing Initiative. https://www.whitehouse.gov/briefing-room/statements-releases/2022/09/14/fact-sheet-the-united-states-announces-new-investments-and-resources-to-advance-president-bidens-national-biotechnology-and-biomanufacturing-initiative/; White House; 2022.

| Drug name | Target(s) | Function(s) | Stage of development |
|---|---|---|---|
| Aβ oligomer inhibitors (e.g., BAN2401, aducanumab) | Amyloid-β oligomers | Prevent or disassemble toxic clumps of amyloid-β | Clinical trials (aducanumab recently received FDA approval) |
| BACE1 inhibitors (e.g., verubecestat, MK-8931) | β-Secretase 1 (BACE1) | Reduce production of amyloid-β by inhibiting the enzyme that cleaves its precursor | Clinical trials (some promising results, others halted due to lack of efficacy) |
| Tau aggregates inhibitors (e.g., P-tau217 PET tracers, LMTX) | Tau protein aggregates | Prevent or remove tangles of misfolded tau protein | Preclinical/early clinical trials (imaging agents more advanced than therapeutic agents) |
| Cholinesterase inhibitors (donepezil, rivastigmine, galantamine) | Acetylcholinesterase (AChE) | Increase levels of the neurotransmitter acetylcholine, which is depleted in AD | Approved for symptomatic treatment of mild-to-moderate AD |
| NMDA receptor modulators (memantine) | N-methyl-D-aspartate (NMDA) receptors | Protect neurons from excitotoxicity and improve cognitive function | Approved for moderate-to-severe AD |
| Multi-target drugs (e.g., J147, AV-1750, CTS-5559) | Combinations of targets from above (e.g., AChE + NMDA, BACE1 + tau) | Address multiple aspects of AD pathology for potentially greater efficacy | Preclinical/early clinical trials (potentially more effective but require careful design and validation) |
| Type of nanobodies | Description | Mechanism of action | Advantage | Disadvantage |
|---|---|---|---|---|
| Fab fragments | Modified antigen-binding fragments of conventional antibodies | Bind to specific targets, trigger immune response | High affinity, good specificity | Large size, limited tissue penetration |
| Domain antibodies | Single variable domains from antibodies with only the heavy chain (VH) | Bind to specific targets, inhibit specific pathways | Smaller than Fab fragments, they have potentially better tissue penetration | Less potent than Fab fragments, limited repertoire |
| Single-chain variable fragments (scFv) | Engineered fusion of heavy and light chain variable domains | Bind to specific targets, can be engineered for additional functions | Smaller than Fab fragments, customizable | Lower affinity than Fab fragments, limited potential stability |
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).