Submitted:
29 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
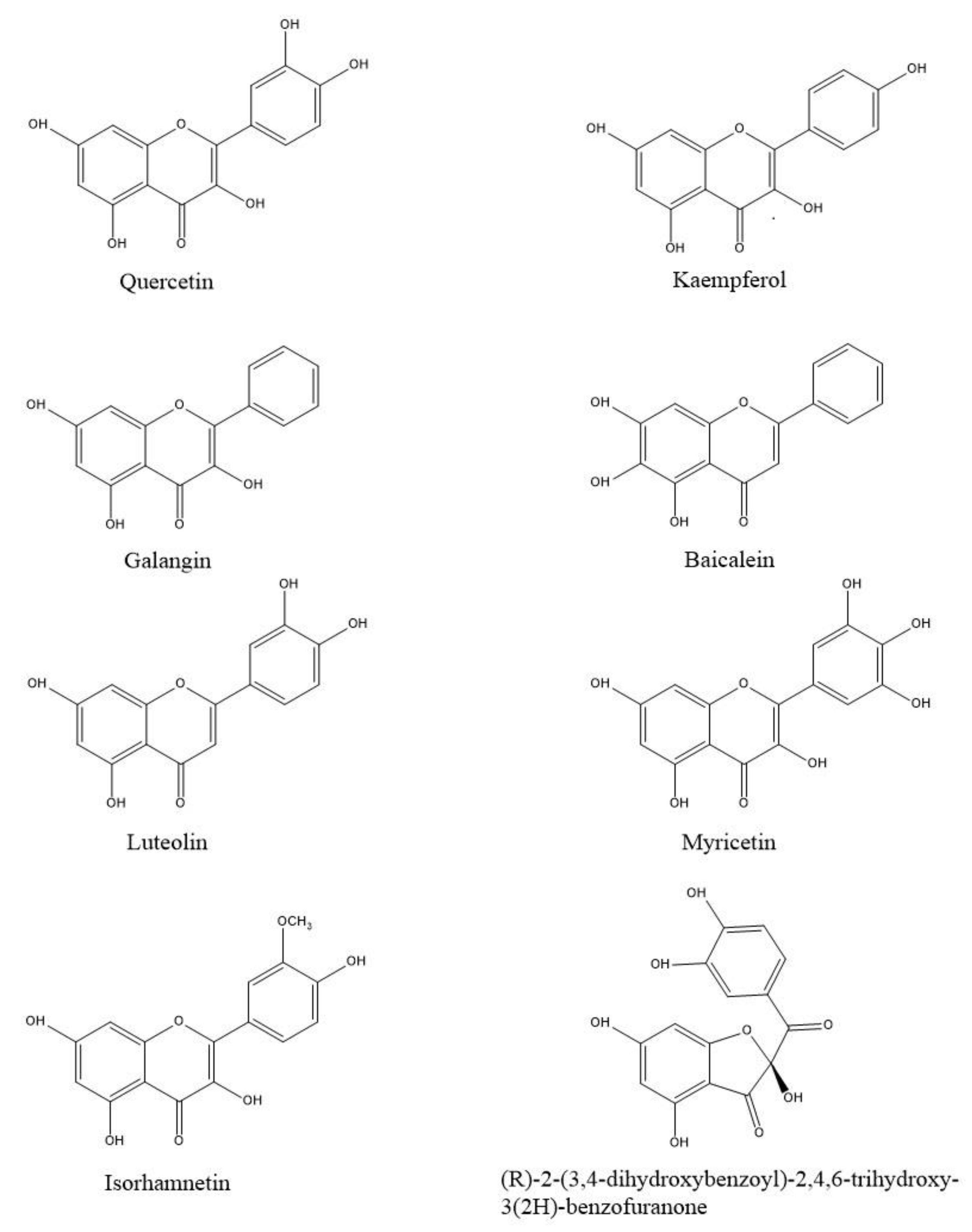
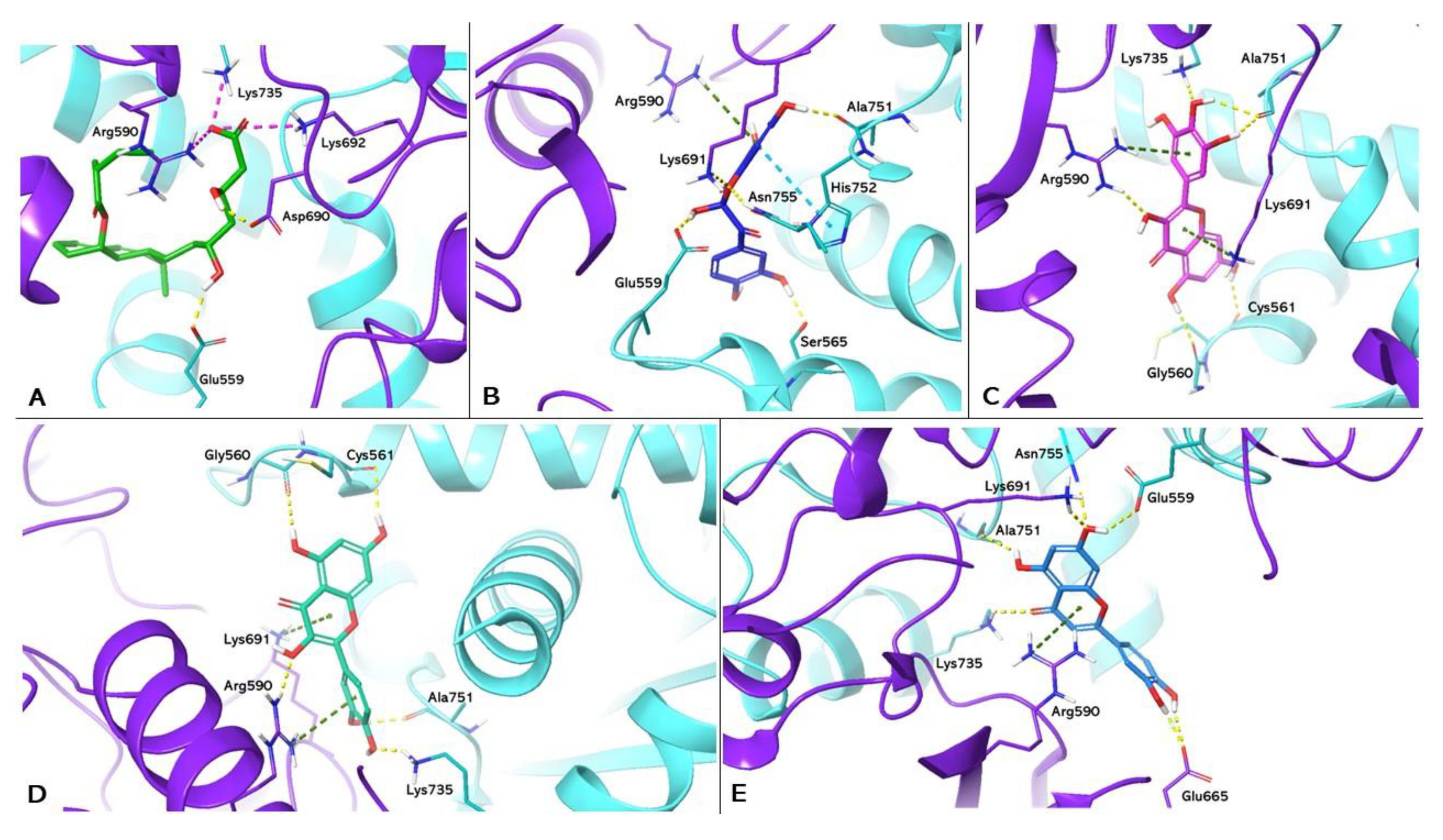
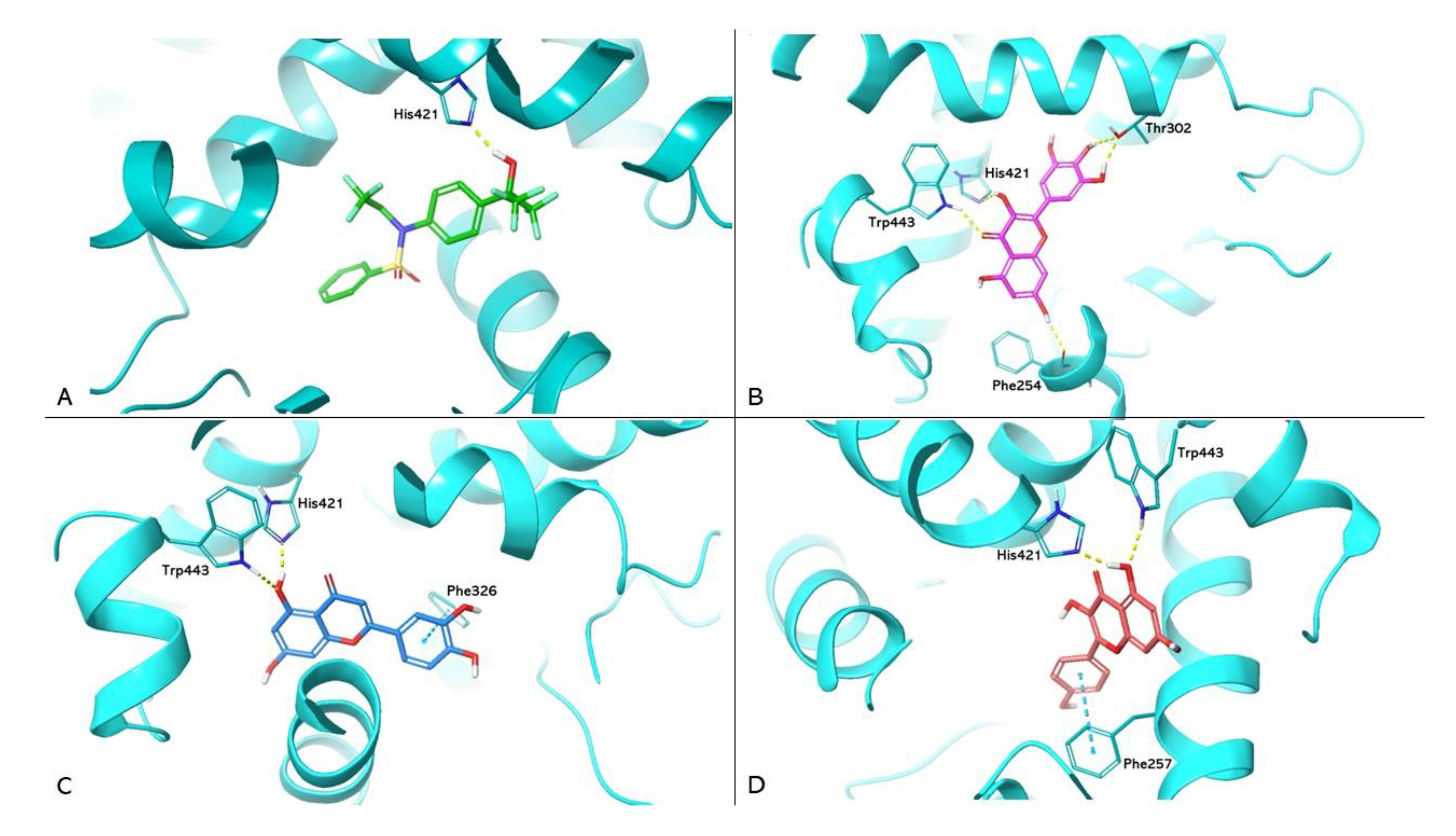
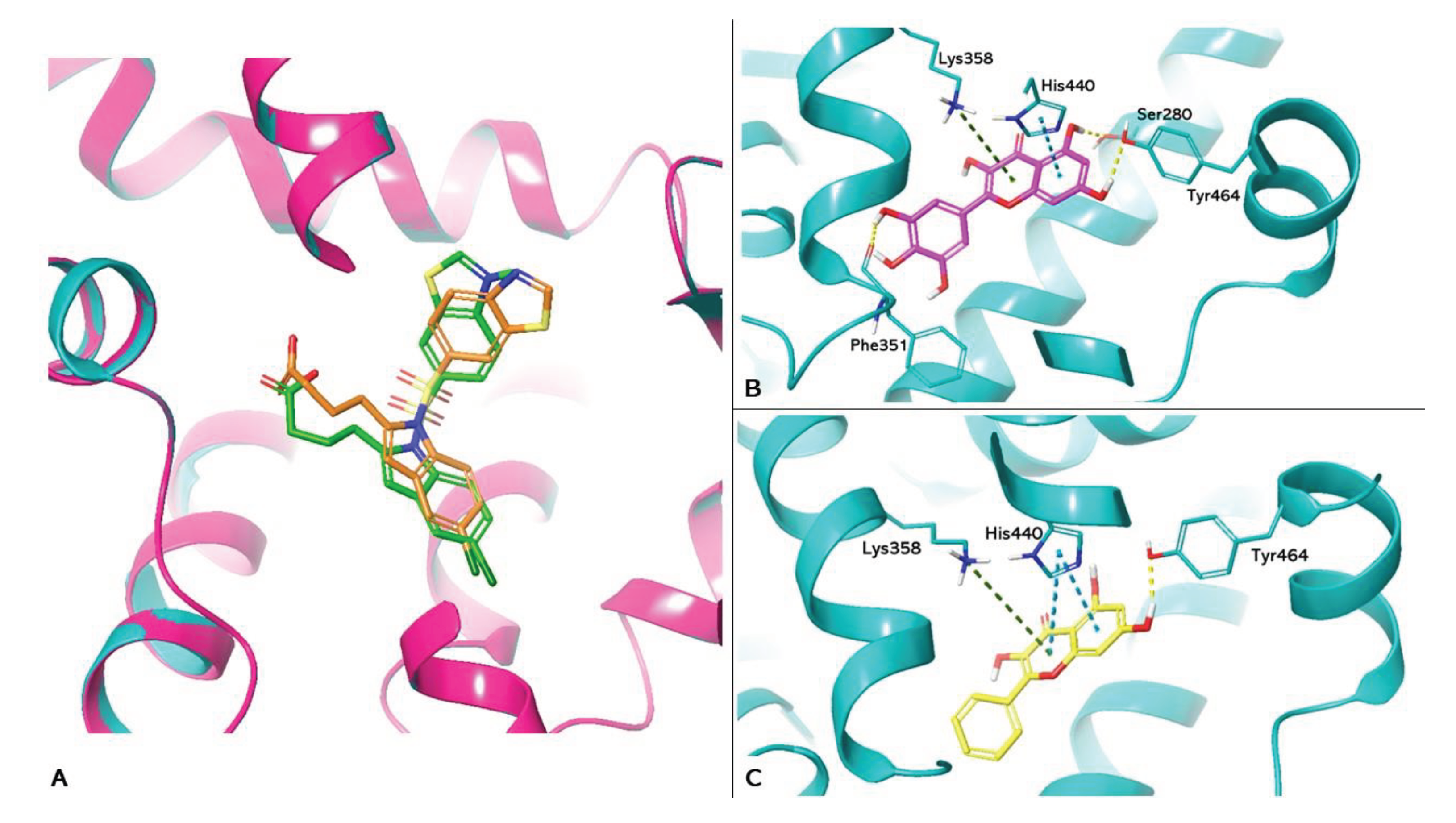
3. Results
4. Discussion
5. Conclusions
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaffner, F.; Thaler, H. Nonalcoholic fatty liver disease. Prog Liver Dis 1986, 8, 283–298. [Google Scholar]
- Abenavoli, L.; Giubilei, L.; Procopio, A.C.; Spagnuolo, R.; Luzza, F.; Boccuto, L.; Scarpellini, E. Gut Microbiota in Non-Alcoholic Fatty Liver Disease Patients with Inflammatory Bowel Diseases: A Complex Interplay. Nutrients 2022, 14, 5323. [Google Scholar] [CrossRef]
- Calabrò, A.; Procopio, A.C.; Primerano, F.; Larussa, T.; Luzza, F.; Di Renzo, L.; De Lorenzo, A.; Gualtieri, P.; Abenavoli, L. Beneficial effects of coffee in non-alcoholic fatty liver disease: A narrative review. Hepatoma Res 2020, 6, 69. [Google Scholar] [CrossRef]
- Elsayed, A.; Ismaiel, A.; Procopio, A.C.; Luzza, F.; Abenavoli, L.; Dumitrascu, D.L. Noninvasive biochemical markers and surrogate scores in evaluating nonalcoholic steatohepatitis. Minerva Med. 2022, 113, 864–874. [Google Scholar] [CrossRef]
- Larussa, T.; Abenavoli, L.; Procopio, A.C.; Iannelli, C.; Polimeni, N.; Spagnuolo, R.; Doldo, P.; Luzza, F. The role of gluten-free diet in nonalcoholic fatty liver disease development. Eur Rev Med Pharmacol Sci. 2021, 25, 6613–6618. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Procopio, A.C.; Paravati, M.R.; Costa, G.; Milić, N.; Alcaro, S.; Luzza, F. Mediterranean Diet: The Beneficial Effects of Lycopene in Non-Alcoholic Fatty Liver Disease. J Clin Med. 2022, 11, 3477. [Google Scholar] [CrossRef]
- Abenavoli, L.; Maurizi, V.; Boccuto, L.; Di Berardino, A.; Giostra, N.; Santori, P.; Scarcella, M.L.; Procopio, A.C.; Rasetti, C.; Scarpellini, E. Nutritional Support in Acute Liver Failure. Diseases 2022, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Kianian, F.; Marefati, N.; Boskabady, M.; Ghasemi, S.Z.; Boskabady, M.H. Pharmacological Properties of Allium cepa, Preclinical and Clinical Evidences; A Review. Iran J Pharm Res. 2021, 20, 107–134. [Google Scholar] [CrossRef]
- Istvan, E.S. Structural mechanism for statin inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase. American heart journal 2002, 144, S27–S32. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Moeed, A.; Waqar, E.; Atif, A.R.; Kamran, A.; Rizvi, H.; Suri, N.F.; Haider, H.; Shuja, S.H.; Khalid, M.; Minhas, A.M.K. Efficacy of statins in treatment and development of non-alcoholic fatty liver disease and steatohepatitis: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2022, 46, 101816. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, S.G. Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction. Diabetes Metab J. 2020, 44, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; You, H.; Qiu, S.; Yu, D.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. A new perspective on NAFLD: Focusing on the crosstalk between peroxisome proliferator-activated receptor alpha (PPARα) and farnesoid X receptor (FXR). Biomed Pharmacother. 2022, 154, 113577. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, C.; Kim, TH. Targeting Liver X Receptors for the Treatment of Non-Alcoholic Fatty Liver Disease. Cells 2023, 12, 1292. [Google Scholar] [CrossRef] [PubMed]
- The Metabolomics Innovation Centre: FooDB (Version 1). Computer software, Canada: The Metabolomics Innovation Centre. 2017. Available online: http://foodb.ca/ (accessed on 6 November 2023).
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; Wang, J.; Yu, B.; Zhang, J.; Bryant, S.H. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202-13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; Tomar, M.; Satankar, V.; Senapathy, M.; Anitha, T.; Dey, A.; Sayed, A.A.S.; Gadallah, F.M.; Amarowicz, R.; Mekhemar, M. Onion (Allium cepa L.) peels: A review on bioactive compounds and biomedical activities. Biomed Pharmacother 2022, 146, 112498. [Google Scholar] [CrossRef] [PubMed]
- ChemDraw Professional. ChemDraw Professional, Version 16 0.0.82 (68); Perkin Elmer Informatics, Inc.: Cambridge, MA, USA, 2019. [Google Scholar]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. Journal of computational chemistry 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J Mol Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Free Maestro Academic Version v13.4 Package; Schrödinger Release 2022-4; Maestro, Schrödinger, LLC: New York, NY, USA, 2022.
- da Costa, R.F.; Freire, V.N.; Bezerra, E.M.; Cavada, B.S.; Caetano, E.W.; de Lima Filho, J.L.; Albuquerque, E.L. Explaining statin inhibition effectiveness of HMG-CoA reductase by quantum biochemistry computations. Physical Chemistry Chemical Physics 2012, 14, 1389–1398. [Google Scholar] [CrossRef]
- Svensson, S.; Ostberg, T.; Jacobsson, M.; Norström, C.; Stefansson, K.; Hallén, D.; Johansson, I.C.; Zachrisson, K.; Ogg, D.; Jendeberg, L. Crystal structure of the heterodimeric complex of LXRalpha and RXRbeta ligand-binding domains in a fully agonistic conformation. EMBO J. 2003, 22, 4625–4633. [Google Scholar] [CrossRef]
- Kamata, S.; Honda, A.; Ishikawa, R.; Akahane, M.; Fujita, A.; Kaneko, C.; Miyawaki, S.; Habu, Y.; Shiiyama, Y.; Uchii, K.; Machida, Y.; Oyama, T.; Ishii, I. Functional and Structural Insights into the Human PPARα/δ/γ Targeting Preferences of Anti-NASH Investigational Drugs, Lanifibranor, Seladelpar, and Elafibranor. Antioxidants 2023, 12, 1523. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V. MASLD treatment-a shift in the paradigm is imminent. Front Med 2023, 10, 1316284. [Google Scholar] [CrossRef] [PubMed]
- Istvan, E.S.; Deisenhofer, J. The structure of the catalytic portion of human HMG-CoA reductase. Biochim Biophys Acta. 2000, 1529, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.B.; Taft, C.A.; Silva, C.H. Use of virtual screening, flexible docking, and molecular interaction fields to design novel HMG-CoA reductase inhibitors for the treatment of hypercholesterolemia. J Phys Chem A. 2008, 112, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Waiz, M.; Alvi, S.S.; Khan, M.S. Potential dual inhibitors of PCSK-9 and HMG-R from natural sources in cardiovascular risk management. EXCLI J. 2022, 21, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Khamis, A.A.; Salama, A.F.; Kenawy, M.E.; Mohamed, T.M. Regulation of hepatic hydroxy methyl glutarate—CoA reductase for controlling hypercholesterolemia in rats. Biomed Pharmacother. 2017, 95, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Tice, C.M.; Noto, P.B.; Fan, K.Y.; Zhuang, L.; Lala, D.S.; Singh, S.B. The medicinal chemistry of liver X receptor (LXR) modulators. J Med Chem. 2014, 57, 7182–7205. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, K.; Kim, S.H.; Hong, M.J.; Jeong, N.J.; Kim, M.S. Luteolin improves hypercholesterolemia and glucose intolerance through LXRα-dependent pathway in diet-induced obese mice. J Food Biochem 2020, 44, e13358. [Google Scholar] [CrossRef]
- Dhoke, G.V.; Gangwal, R.P.; Sangamwar, A.T. A combined ligand and structure based approach to design potent PPAR-alpha agonists. Journal of Molecular Structure 2012, 1028, 22–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




